Abstract
The biochemical features that distinguish human M cells from other intestinal epithelial cell types are important for understanding microbial pathogenesis and for targeting vaccines to the mucosal immune system. We applied a large panel of carbohydrate-specific monoclonal antibodies and lectins to Peyer’s patch and cecum biopsy specimens from three normal individuals and a patient with inflammatory bowel disease. The results show that human M-cell glycosylation patterns are distinct from those of other species examined and that human M cells preferentially display the sialyl Lewis A antigen. This carbohydrate epitope is also present in a small subpopulation of enterocytes in the follicle-associated epithelium and in goblet cell mucins.
The mucosal surface of the gastrointestinal tract is lined by a single layer of epithelial cells that serves as a delicate barrier to foreign antigens and microbial pathogens in the intestinal lumen. An important component in protection of this vulnerable surface is the mucosal immune system, an assembly of cells in the lamina propria which responds to luminal antigens by producing secretory antibodies and other local immune effectors (37). Sampling of luminal antigens occurs at specialized local inductive sites, the organized mucosa-associated lymphoid tissues, that appear as single or aggregated mucosal lymphoid follicles in the small intestine, cecum, appendix, colon, and rectum. Transport of antigens and microorganisms across the epithelial barrier at these sites is accomplished by a specialized follicle-associated epithelium (FAE). The FAE contains M cells, a unique, relatively rare epithelial cell type specialized for transepithelial transport of macromolecules, particles, and microorganisms (43). Because of their importance in microbial pathogenesis and their potential in targeting of vaccines to the mucosal immune system, there is great current interest in elucidating the functional and biochemical features that distinguish M cells from other intestinal epithelial cell types.
M cells in many species, including humans (15, 23, 44, 48), can be identified by morphological features such as their flattened apical surfaces and intraepithelial pockets containing lymphoid cells. Immunocytochemical studies of experimental animals have revealed other distinguishing M-cell features, including reduced surface expression of brush border hydrolases (47, 55); expression of the intermediate filament proteins vimentin, cytokeratin 8, and cytokeratin 18 in rabbit, rat, and pig M cells, respectively (16, 18, 25, 51); diffuse cytoplasmic distribution of the actin-bundling protein villin (30); and apical expression of β1 integrin, a protein that is basolateral on other epithelial cells (9, 36). With the possible exception of β1 integrin, however, none of these components can serve to explain the selective binding of certain pathogens to M-cell surfaces or can be exploited to target antigens to these cells.
Although M cells generally lack the uniform thick glycocalyx seen on enterocytes, their apical membranes do display abundant glycoconjugates (3, 14, 41) that may serve as binding sites for microorganisms. Recent lectin-binding studies have established that M cells in experimental animals have glycosylation patterns that differ from those of their epithelial neighbors. For example, in Peyer’s patches of BALB/c mice, lectins that recognize a range of carbohydrate structures containing α(1-2)-fucose selectively stained M cells in the FAE (6, 11, 19). We observed such lectin binding sites not only on M-cell apical membranes but also on intracellular vesicles and basolateral membranes, including the pocket domain (19). These fucose-containing carbohydrate structures have not proven to be universal M-cell markers, however. Glycoconjugates expressed on M cells in other intestinal regions (cecum, colon, and rectum) of BALB/c mice (7, 19) and in other species such as rabbit (17, 26) were found to differ from those in BALB/c mouse Peyer’s patches. Despite these species and regional differences, the animal data suggest that M-cell-specific glycoconjugates might also exist in humans.
Panels of lectins and monoclonal antibodies have been extensively used to survey epithelial and mucin glycoconjugate expression patterns in human biopsy specimens. Such studies have demonstrated clear differences between glycoconjugates of humans and other species (34, 38, 64), between normal and neoplastic human mucosae (4, 35, 52), and between normal mucosa and mucosa affected by inflammatory bowel disease (IBD) (24, 50, 53, 65). A few studies have applied lectins to normal human mucosa containing organized lymphoid tissues but have failed to identify a marker for human M cells in Peyer’s patches (27, 56) or appendix (2). These studies were limited by the fact that many lectins are capable of recognizing multiple related carbohydrate structures. In the present study, we applied a panel of monoclonal antibodies specific for single carbohydrate epitopes, as well as a large panel of lectins, to intestinal biopsy specimens from both normal individuals and one patient with IBD. We report here that human M cells can indeed display glycosylation patterns that distinguish them from other intestinal epithelial cell types.
Histochemistry of human intestinal tissue.
Biopsy specimens were obtained from the Endoscopy Suite at Children’s Hospital, Boston, Mass., as part of endoscopic examinations. Ileal, cecal, and rectal biopsies were typically taken from each individual. Peyer’s patch samples from normal individuals of blood group type O (age of individual, 16 years) and type A (age, 13 years) as well as an individual diagnosed with ulcerative colitis (UC) of blood group O (age, 10 years) were analyzed. Cecal tissue from a type O normal individual (age, 6 years) was also examined. The research protocol was followed with prior approval by the Children’s Hospital Committee on Clinical Investigation and informed consent from patient guardians. Pinch biopsies were immediately placed in 3% paraformaldehyde–0.1% glutaraldehyde in 0.1 M cacodylate (pH 7.2) and fixed for 3 h at 4°C. Samples were processed, embedded in Epon-Araldite, and sectioned at a 0.5-μm thickness as described previously (19).
For brevity, the complex oligosaccharide specificities of 28 of the lectins that were previously listed and referenced (19) are not given here. We have included in Table 1 the complete specificities of the lectins not listed in reference 19 grouped by major inhibiting sugar. Table 2 lists all probes tested in this study. Biotinylated lectins were purchased from Vector Labs (Burlingame, Calif.), except AAA and LFA (E-Y Labs, San Mateo, Calif.), HPA (Helix pomatia) and GS I-A4 (Griffonia simplicifolia type I isolectin-A4) (Sigma Chemical Co., St. Louis, Mo.), and OPA (Boehringer Mannheim, Indianapolis, Ind.). Mouse monoclonal immunoglobulin M (IgM) antibodies specific for blood group antigens A, B, and H-2 were purchased from DAKO Corp. (Carpinteria, Calif.). Mouse monoclonal antibodies specific for blood group antigen H-1, Lewis B, Lewis X, and Lewis Y were purchased from Signet Labs (Dedham, Mass.). Mouse monoclonal antibodies specific for sialyl Lewis A and sialyl Lewis X were purchased from Kamiya Biomedical (Thousand Oaks, Calif.). Mouse monoclonal antibody specific for Lewis A was acquired from Immucor (Norcross, Ga.). Tetramethylrhodamine isothiocyanate (TRITC)-labeled goat anti-mouse IgM was obtained from Kirkegaard and Perry Labs (Gaithersburg, Md.). TRITC-labeled goat anti-mouse IgG was purchased from Hyclone Labs (Logan, Utah). TRITC-labeled streptavidin was obtained from Molecular Probes (Eugene, Oreg.). None of the TRITC-conjugated anti-mouse Ig or streptavidin preparations bound to sections of human intestine when they were applied without their cognate primary reagent (data not shown).
TABLE 1.
Partial listing of lectins and antibodies used in this studya
| Lectin or antibody | Specificity | Epitope(s) | Reference |
|---|---|---|---|
| HPA | d-GalNAc | GalNAcα, GalNAcβ(1-3)Gal, GalNAc(1-4)Gal | 21 |
| WFA | GalNAcα, GalNAcβ | 1 | |
| WBA I (Psophocarpus tetragonolobus I) | GalNAcα | 28 | |
| GS I-A4 | GalNAcα | 39 | |
| MAL I (Maackia amurensis I) | d-Galactose | Galβ(1-3)GlcNAc, Neu5Acα(2-3)Gal | 32 |
| BPA (Bauhinia purpurea) | Galβ(1-3)GlcNAc | 1 | |
| GNA (Galanthus nivalis) | d-Mannose | Manα | 62 |
| SNA | Sialic acid | Neu5Acα(2-6)Gal, Neu5Acα(2-6)GalNAc | 57 |
| MAL II (Maackia amurensis II) | Neu5Acα(2-3)Gal, Neu5Acα(2-3)GalNAc | 29 | |
| Anti-Lewis A (IgM-clone LM112 or LM161) | MAb | Galβ(1-3)GlcNAc[Fucα(1-4)] | 54 |
| Anti-Lewis X (IgM-clone P12) | Galβ(1-4)GlcNAc[Fucα(1-3)] | 54 | |
| Anti-Sialyl Lewis A (IgG-clone KM-231) | Neu5Acα(2-3)Galβ(1-3)GlcNAc[Fucα(1-4)] | 22 | |
| Anti-sialyl Lewis X (IgM-clone KM-93) | Neu5Acα(2-3)Galβ(1-4)GlcNAc[Fucα(1-3)] | 58 |
Refer to Giannasca et al. (19) for additional probes and specificities. Abbreviations: GlcNAc, N-acetylglucosamine; GalNAc, N-acetylgalactosamine; Neu5Ac, N-acetylneuraminic acid; MAb, monoclonal antibody.
TABLE 2.
Lectin and antibody binding patterns in human Peyer’s patch and cecum
| Specificity | Lectin or antibodya | Reactivityb of apical membranes of:
|
|||||
|---|---|---|---|---|---|---|---|
| Normal PPc
|
UC PP
|
Normal cecum
|
|||||
| M cells | Enterocytese | M cells | Enterocytes | M cells | Enterocytes | ||
| l-Fucose | UEA I, AAA, LTA | − | + | + | + | + | + |
| OPA | + | + | + | + | + | + | |
| d-GalNAc | VVA | − | −/+f | + | + | + | + |
| DBA | − | − | +g | +h | − | − | |
| SBA | − | −/+f | + | + | + | + | |
| HPA | − | −/+f | − | − | − | − | |
| WFA, GS I-A4 | − | + | NDd | ND | + | + | |
| WBA I | − | − | ND | ND | − | − | |
| d-GlcNAc | GS II | − | −/+i | + | + | + | + |
| LEA, UEA II | − | −/+f | + | + | + | + | |
| STA | −/+f | + | + | + | + | + | |
| WGA | − | −/+f | − | − | + | + | |
| d-Galactose | Jacalin | + | + | + | + | + | + |
| RCA I, GS I-B4 | +/−j | −/+f | + | + | + | + | |
| PNA | − | −/+f | + | + | + | + | |
| ECA, BPA | − | + | + | + | + | + | |
| MAL I | − | − | ND | ND | + | + | |
| d-Mannose, d-glucose | LCA | +k | +k | + | + | + | + |
| GNA | +k | +k | ND | ND | + | + | |
| Sialic acid | LFA, MAL II | − | − | − | − | + | + |
| SNA | − | +l | − | +l | + | + | |
| Complex | EEA, WBA II | − | − | − | − | + | + |
| SJA | − | −/+f | − | −h | − | − | |
| Monoclonal antibodies | Anti-blood group A | − | −m | − | − | − | − |
| Anti-blood groups B, H-1 | − | − | − | − | − | − | |
| Anti-blood group H-2 | − | − | − | − | + | + | |
| Anti-Lewis A | + | + | ND | ND | + | + | |
| Anti-Lewis X | − | + | ND | ND | + | + | |
| Anti-Lewis B | − | +/−n | − | − | + | + | |
| Anti-Lewis Y | − | − | − | − | + | + | |
| Anti-sialyl Lewis A | + | −o | + | − | +p | − | |
| Anti-sialyl Lewis X | − | − | ND | ND | − | − | |
See lectin abbreviations and probe specificities in the text.
Differences in reactivities are explained in footnotes.
PP, Peyer’s patch.
ND, not determined.
Refers to villus and FAE enterocytes.
Negative in type O individual, positive in type A individual.
Few (<20%) M cells were stained.
Few villus enterocytes were stained.
Few (<20%) enterocytes were stained in the type A individual.
Positive in type O individual, negative in type A individual.
Not tested on type O individual.
A subpopulation (<90%) of FAE enterocytes was positive; few (<20%) villus enterocytes were stained.
Few (<5%) enterocytes were stained in the type A individual.
Few (<20%) enterocytes were stained in the type O individual.
Few (<20%) FAE enterocytes were positive.
Few (≤5%) M cells were stained.
Lectin and antibody histochemistry was performed on 0.5-μm-thick sections after extraction of the epoxy with melting solution as described previously (19). Briefly, lectin-biotin conjugates were prepared in gelatin–phosphate-buffered saline (PBS) at a concentration of 10 μg/ml, except for LFA lectin, which was used at 50 μg/ml. All antibodies were used at a concentration of 2 to 5 μg/ml in gelatin-PBS. Extracted tissue sections were overlaid with lectin or antibody solutions, incubated for 60 min at room temperature, and washed three times for 5 min each in gelatin-PBS. Bound lectins or antibodies were labeled with one of the following secondary reagents: (i) TRITC-labeled streptavidin, (ii) TRITC-labeled goat anti-mouse IgM antibodies, or (iii) TRITC-labeled goat anti-mouse IgG, all diluted to 2 μg/ml in gelatin-PBS. Slides were briefly rinsed with PBS and distilled H2O, and coverslips were mounted. Photography was performed with a Zeiss Axiophot microscope equipped for epifluorescence and with Kodak T-Max 400 film.
Glycoconjugates on normal human Peyer’s patch M cells.
The binding and cellular localizations of 31 lectins and 10 anticarbohydrate monoclonal antibodies were analyzed. In Peyer’s patches of the two normal and the UC individual, cells displaying the morphological features of M cells represented only a small percentage (<5%) of FAE cells, consistent with the frequency reported by others (10, 33). M-cell morphology in Peyer’s patches varied depending on the position of the M cells in the dome epithelia. M cells emerging from crypts and on the lateral portion of a dome were intact and contained several leukocytes within small intraepithelial pockets. M cells positioned closer to the dome apexes contained larger pockets with many immune cells and often displayed ruptured apical membranes, with cells extruding into the lumens. We did not observe M cells at or near the apexes of the domes, confirming previous observations (10) and suggesting that M-cell exfoliation occurs prior to this point.
Lectin and antibody binding revealed a more limited range of carbohydrate epitopes on M cells than on enterocytes in the FAE or on villi (Table 2). Many of the probes bound to enterocyte membranes, and binding sites were abundant in the highly glycosylated apical brush borders as expected. By contrast, fewer probes labeled M cells. For example, UEA-I lectin recognizes several carbohydrate structures containing α(1-2) fucose and is a marker for BALB/c mouse Peyer’s patch M cells (6, 19). This lectin did not bind to M cells in our human specimens but strongly bound to all enterocytes (Fig. 1A and B). While many probes showed similar reactivities with epithelial cells from the two normal individuals of distinct blood groups (A versus O), others showed clear differences. For example, enterocyte binding by RCA I (Ricinus communis type I), GS I-B4, and other probes was observed in the type A specimen but was absent in the type O specimen.
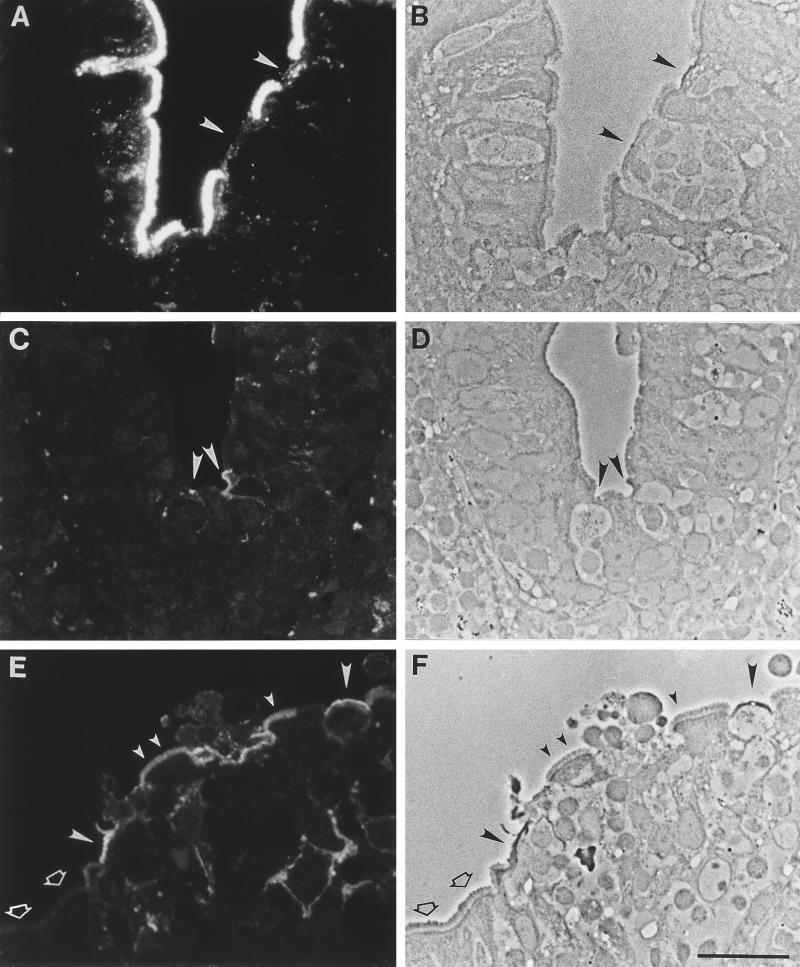
FIG. 1.
Lectin and antibody binding patterns in normal human Peyer’s patch. Sections were labeled with lectin or antibody and viewed by fluorescence (A, C, and E) and phase-contrast (B, D, and F) microscopy. (A to D) A dome with FAE is on the right, and a villus is on the left. (A and B) UEA I lectin, which selectively labels BALB/c mouse PP M cells, has abundant binding sites expressed uniformly on human enterocytes. Conversely, little or no label is seen on M cells in the FAE (arrowheads). (C and D) Monoclonal antibody specific for sialyl Lewis A antigen shows selective binding to apical membranes of M cells (arrowheads) as well as weaker staining of internal and basolateral membranes. (E and F) On the FAE, anti-sialyl Lewis A strongly labels most M cells (large filled arrowheads). A small (<20%) population of FAE enterocytes (small filled arrowheads) are also labeled. However, most enterocytes are negative (open arrowheads). Bar, 20 μm.
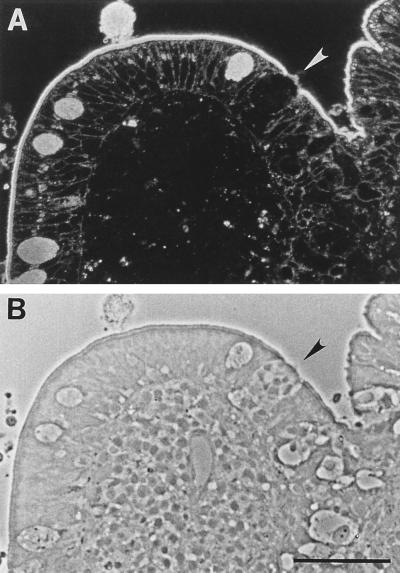
None of the 31 lectins tested selectively labeled M cells (Table 2), confirming previous observations. However, a monoclonal antibody (anti-sialyl Lewis A) selectively recognized a subpopulation (∼80%) of M cells in the FAE, staining apical and subcellular membranes of the cells (Fig. 1C to F). It also bound weakly to infrequent (<20%) FAE enterocytes of both individuals. Limited recognition of certain FAE enterocytes by M-cell-specific probes has also been observed in animal studies and may reflect the relative plasticity of the epithelial cell phenotypes in this epithelium (31, 40). Villus enterocytes were not stained by this antibody, although goblet cell mucins were strongly stained. Interestingly, the related Lewis A antigen was not M cell specific; it was abundantly expressed on apical and basolateral membranes of both M cells and enterocytes (Fig. 2).
FIG. 2.
Anti-Lewis A binding pattern in normal human Peyer’s patch. Sections were labeled with anti-Lewis A antibody and viewed by fluorescence (A) and phase-contrast (B) microscopy. Anti-Lewis A has abundant binding sites expressed uniformly on enterocytes. Binding is also observed on M cells (arrowheads). Bar, 50 μm.
Gene expression in the entire FAE is known to be distinct from that of the villus epithelium (43, 55) as evidenced by the absence of polymeric Ig receptors (49), reduced numbers of goblet cells (46), and reduced expression of cell surface hydrolases (47, 60). Although the levels of expression of some lectin and antibody binding sites on FAE enterocytes were similar to those on villus enterocytes in the human small intestine, differences were observed. For example, SNA (Sambucus nigra) binding sites were present on the apical surfaces of a majority of FAE enterocytes but were largely absent from villus enterocytes, confirming results of previous reports (27, 56). In the Peyer’s patch specimen from the blood type A individual, villus enterocytes showed a very high density of terminal GalNAc-containing epitopes, as evidenced by intense staining with lectins that recognize carbohydrate structures containing terminal GalNAc: WFA (Wisteria floribunda) and GS I-A4. This is consistent with αGalNAc being part of the blood group A determinant. In this specimen, however, the same lectins stained FAE enterocytes more weakly. Since α(1-3)-N-acetylgalactosyltransferase is required to synthesize this determinant on epithelial cells, endothelial cells, and erythrocytes (45), the expression of this enzyme is probably lower in the FAE than in the villus epithelium.
Glycoconjugate expression by normal cecal M cells.
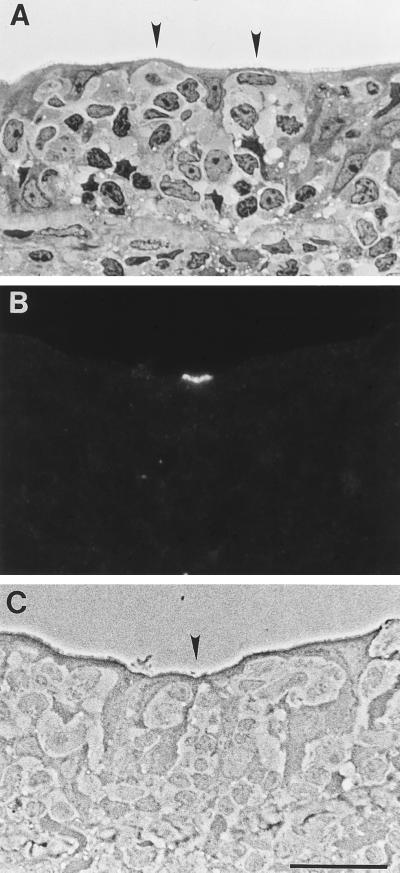
It is known that intestinal epithelial glycosylation patterns are distinct in different regions of the digestive tracts of animal and humans. For example, the transition from the small to the large intestine is accompanied by substantial changes in the profiles and abundance of glycoconjugates (reviewed in reference 61). Similarly, M cells exhibit glycosylation patterns specific to the small or large intestine in mice (7, 19) and rabbits (17, 26). Thus, we examined the glycosylation profile of epithelial cells in a cecal biopsy containing mucosal lymphoid tissue from a type O normal individual. The abundance of M cells as determined morphologically was higher in cecum than in Peyer’s patch; in some areas identifiable M cells represented >50% of FAE cells. Cecal M cells were largely intact, with large intraepithelial pockets containing multiple leukocytes (Fig. 3A). Lectin- and antibody-binding assays revealed that cecal M cells displayed more abundant glycoconjugates with greater epitope diversity than M cells in the normal Peyer’s patch tissues examined (Table 2). Most probes bound to both M cells and enterocytes, consistent with results obtained with human appendix (2). The intensity of labeling was often more varied on M-cell apical membranes than on enterocytes, however, perhaps reflecting variations in the thickness of the M-cell glycocalyx. In the cecum, as in Peyer’s patch, only the anti-sialyl Lewis A monoclonal antibody was selective for M cells (Fig. 3B and C); however, only ∼5% of M cells were labeled and binding was restricted to apical membranes.
FIG. 3.
Anti-sialyl Lewis A binding pattern in normal human cecum. Sections were stained with toluidine blue and viewed by brightfield microscopy (A) or labeled with anti-sialyl Lewis A and viewed by fluorescence (B) and phase-contrast (C) microscopy. In cecal specimens which contained lymphoid follicles, identifiable M cells were more abundant in the FAE than in Peyer’s patch. (A) M cells (arrowheads) were clustered in certain regions of the FAE. (B and C) Anti-sialyl Lewis A labeled the apical membranes of a small subpopulation of M cells (arrowhead). No other cell types were recognized by this probe. Bar, 20 μm.
Diversity among M cells has also been observed in mice, where lectin and antibody probes revealed variations in glycosylation patterns of individual M cells within a single FAE. We proposed that this diversity might expand the possible microbial lectin–M-cell surface carbohydrate interactions of the local M-cell population and allow the M cells to “sample” a wider variety of microorganisms (19, 42). It is possible that the human M cells not recognized by the anti-sialyl Lewis A monoclonal antibody in this study display distinct carbohydrate epitopes that might be detected with other antibody probes.
Glycoconjugate expression by Peyer’s patch M cells in an IBD patient.
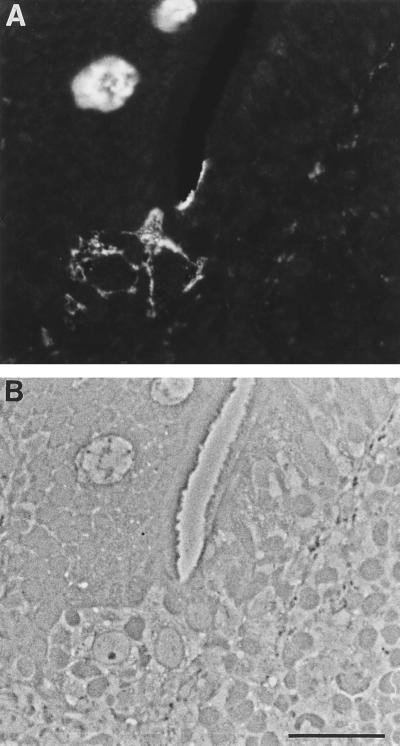
Others have reported changes in intestinal epithelial cell glycosylation in IBD specimens (2, 24, 50, 53, 65). We therefore surveyed lectin and antibody binding on tissue biopsies from a patient diagnosed with UC, analyzing in detail a Peyer’s patch specimen from an area unaffected by the disease. The numbers of M cells in the FAE were comparable to those in normal tissue, unlike the findings for specimens from adults with spondylarthropathy reported previously (10). We observed that Peyer’s patch M cells from this colitis patient displayed more diverse and abundant oligosaccharide epitopes than those from the two normal individuals tested, irrespective of blood group (Table 2). In particular, many lectin probes recognizing terminal fucose- or galactose-containing glycoconjugates showed binding to M cells in this specimen but not in the normal samples tested. Nevertheless, we again observed that expression of sialyl Lewis A antigen was largely restricted to M cells (Fig. 4). In summary, the sialyl Lewis A antigen is selectively expressed by M cells in both small and large intestines of normal individuals of distinct blood groups and in small intestine of a patient with IBD.
FIG. 4.
Anti-sialyl Lewis A binding pattern in human Peyer’s patch from a patient with UC. Sections were labeled with anti-sialyl Lewis A antibody and viewed by fluorescence (A) and phase-contrast (B) microscopy. A dome with FAE is on the right, and a villus is on the left. Anti-sialyl Lewis A shows selective binding to M-cell apical membranes. There was no recognition of enterocyte membranes in this specimen. Mucins of virtually all goblet cells were labeled with this antibody. Bar, 20 μm.
M-cell glycosylation in microbial pathogenesis and mucosal immunity.
The expression of distinct glycoconjugates by M cells in various tissues and species suggests an important role for carbohydrate epitopes in the function of this unique cell type. The structural modifications of the M-cell apical surface and the display of particular oligosaccharides together would allow M cells to present a conspicuously unique biochemical face to the lumen which might facilitate adherence, uptake, and immunological sampling of microorganisms. The transepithelial transport of microbes from the external environment as part of routine mucosal immune surveillance renders the host vulnerable to invasive pathogens, however, and the tropism that many pathogens demonstrate for M cells (42, 59) supports this concept.
Our results also raise the possibility that M cell-specific glycoconjugates can be exploited to enhance delivery of mucosal vaccines in humans. M-cell-specific glycoconjugates have been shown to be capable of mediating delivery to organized mucosal lymphoid tissues in mice and hamsters. Incubation of M-cell-selective lectins in mouse ligated intestinal loops containing Peyer’s patches (8, 19) or feeding of lectin intragastically (unpublished results) resulted in M-cell adherence and transcytosis into Peyer’s patch lymphoid follicles, and an M-cell-selective lectin given intranasally to hamsters targeted M cells overlying nasal lymphoid tissue (20). M-cell-selective lectins have also been used to target particles such as polymerized liposomes (5) and latex beads (13) to mouse small-intestinal M cells.
The results from the two normal individuals studied here indicate that human Peyer’s patch M cells display a more restricted array of carbohydrate epitopes than do enterocytes, suggesting that under normal conditions, expression of certain glycosyltransferases is reduced in M cells. In contrast, a previous lectin-binding survey of human Peyer’s patch tissue (56) found that more lectins recognized M-cell glycoconjugates than did lectins in the present study. Whether this discrepancy reflects normal variation in the human population cannot be resolved with the limited specimens available to us. It should be noted that the specimens examined in the previous study were taken from colon carcinoma patients and that epithelial glycosylation patterns in the gut are known to be altered in colon cancer (4, 35, 52). Analysis of a larger number of normal tissue specimens will be required to define the diversity of glycoconjugate expression in human M cells.
Consistent findings in this study were that the sialyl Lewis A antigen was largely restricted to M cells but that the Lewis A antigen was ubiquitous on epithelial cell surfaces. The monosialyl Lewis A antigen, defined as Neu5Acα(2-3)Galβ(1-3)GlcNAc[Fucα(1-4)], differs from the Lewis A antigen only by the presence of a single α(2-3)-linked sialic acid residue. The sialyl Lewis A antigen has been identified as a ligand for selectins expressed by lymphocytes and endothelial cells (reviewed in reference 63). Lymphocytes selectively enter the M-cell intraepithelial pocket to interact closely with the basolateral M-cell membrane, and we have observed that luminal lymphocytes can interact with M-cell apical surfaces in experimental animals (66). It is tempting to propose a role for sialyl Lewis A in the observed interactions of lymphocytes with M cells. Indeed, B and T lymphocytes within M-cell pockets were shown to express L-selectin in human biopsies (12), supporting this hypothesis. Further studies will be needed to comprehensively define the oligosaccharide repertoire on human M cells and to elucidate their role in the biology of this unique epithelial cell type.
Acknowledgments
This work was supported by NIH research grants HD17557 and AI34757 and NIH center grant DK-34854 to the Harvard Digestive Diseases Center.
REFERENCES
- 1.Allen H J, Johnson E A Z, Matta K L. Binding site specificity of lectins from Bauhinia purpurea alba, Sophora japonica, and Wisteria floribunda. Carbohydr Res. 1980;86:123–131. doi: 10.1016/s0008-6215(00)84587-5. [DOI] [PubMed] [Google Scholar]
- 2.Brinck U, Bosbach R, Korabiowska M, Schauer A, Gabius H-J. Lectin-binding sites in the epithelium of normal human appendix veriformis and in acute appendicitis. Histol Histopathol. 1995;10:61–70. [PubMed] [Google Scholar]
- 3.Bye W A, Allan C H, Trier J S. Structure, distribution, and origin of M cells in Peyer’s patches of mouse ileum. Gastroenterology. 1984;86:789–801. [PubMed] [Google Scholar]
- 4.Caldero J, Campo E, Ascaso C, Ramos J, Panades M J, Rene J M. Regional distribution of glycoconjugates in normal, transitional and neoplastic human colonic mucosa. A histochemical study using lectins. Virchows Arch Abt A Pathol Anat Histopathol. 1989;415:347–356. doi: 10.1007/BF00718637. [DOI] [PubMed] [Google Scholar]
- 5.Chen H, Torchilin V, Langer R. Lectin-bearing polymerized liposomes as potential oral vaccine carriers. Pharm Res. 1996;13:1378–1383. doi: 10.1023/a:1016030202104. [DOI] [PubMed] [Google Scholar]
- 6.Clark M A, Jepson M A, Simmons N L, Hirst B H. Differential expression of lectin-binding sites defines mouse intestinal M cells. J Histochem Cytochem. 1993;41:1679–1687. doi: 10.1177/41.11.7691933. [DOI] [PubMed] [Google Scholar]
- 7.Clark M A, Jepson M A, Hirst B H. Lectin binding defines and differentiates M-cells in mouse small intestine and cecum. Histochem Cell Biol. 1995;104:161–168. doi: 10.1007/BF01451575. [DOI] [PubMed] [Google Scholar]
- 8.Clark M A, Jepson M A, Simmons N L, Hirst B H. Selective binding and transcytosis of Ulex europaeus I lectin by mouse Peyer’s patch M-cells in vivo. Cell Tissue Res. 1995;282:455–461. doi: 10.1007/BF00318877. [DOI] [PubMed] [Google Scholar]
- 9.Clark M A, Hirst B H, Jepson M A. M-cell surface β1 integrin expression and invasin-mediated targeting of Yersinia pseudotuberculosis to mouse Peyer’s patch M cells. Infect Immun. 1998;66:1237–1243. doi: 10.1128/iai.66.3.1237-1243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuvelier C A, Quatacker J, Mielants H, De Vos M, Veys E, Roels H J. M-cells are damaged and increased in number in inflamed human ileal mucosa. Histopathology. 1994;24:417–426. doi: 10.1111/j.1365-2559.1994.tb00550.x. [DOI] [PubMed] [Google Scholar]
- 11.Falk P, Roth K A, Gordon J I. Lectins are sensitive tools for defining the differentiation programs of epithelial cell lineages in the developing and adult mouse gastrointestinal tract. Am J Physiol. 1994;266:G987–G1003. doi: 10.1152/ajpgi.1994.266.6.G987. [DOI] [PubMed] [Google Scholar]
- 12.Farstad I N, Halstensen T S, Fausa O, Brandtzaeg P. Heterogeneity of M cell-associated B and T cells in human Peyer’s patches. Immunology. 1994;83:457–464. [PMC free article] [PubMed] [Google Scholar]
- 13.Foster M, Clark M A, Jepson M A, Hirst B H. Ulex europaeus 1 lectin targets microspheres to mouse Peyer’s patch M cells in vivo. Vaccine. 1998;16:536–541. doi: 10.1016/s0264-410x(97)00222-3. [DOI] [PubMed] [Google Scholar]
- 14.Frey A, Giannasca K T, Weltzin R, Giannasca P J, Reggio H, Lencer W I, Neutra M R. Role of the glycocalyx in regulating access of microparticles to apical plasma membranes of intestinal epithelial cells: implications for microbial attachment and oral vaccine targeting. J Exp Med. 1996;184:1045–1059. doi: 10.1084/jem.184.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujimura Y, Hosobe M, Kihara T. Ultrastructural study of M cells from colonic lymphoid nodules obtained by colonoscopic biopsy. Dig Dis Sci. 1992;37:1089–1098. doi: 10.1007/BF01300292. [DOI] [PubMed] [Google Scholar]
- 16.Gebert A, Hach G, Bartels H. Co-localization of vimentin and cytokeratins in M cells of rabbit gut-associated lymphoid tissue. Cell Tissue Res. 1992;269:331–340. doi: 10.1007/BF00319625. [DOI] [PubMed] [Google Scholar]
- 17.Gebert A, Hach G. Differential binding of lectins to M cells and enterocytes in the rabbit cecum. Gastroenterology. 1993;105:1350–1361. doi: 10.1016/0016-5085(93)90139-4. [DOI] [PubMed] [Google Scholar]
- 18.Gebert A, Rothkotter H J, Pabst R. Cytokeratin 18 is an M cell marker in porcine Peyer’s patches. Cell Tissue Res. 1994;276:213–221. doi: 10.1007/BF00306106. [DOI] [PubMed] [Google Scholar]
- 19.Giannasca P J, Giannasca K T, Falk P, Gordon J I, Neutra M R. Regional differences in glycoconjugates of intestinal M cells in mice: potential targets for mucosal vaccines. Am J Physiol. 1994;267:G1108–G1121. doi: 10.1152/ajpgi.1994.267.6.G1108. [DOI] [PubMed] [Google Scholar]
- 20.Giannasca P J, Boden J A, Monath T P. Targeted delivery of antigen to hamster nasal lymphoid tissue with M-cell-directed lectins. Infect Immun. 1997;65:4288–4298. doi: 10.1128/iai.65.10.4288-4298.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammarstrom S, Westoo A, Bjork I. Subunit structure of Helix pomatia hemagglutinin. Scand J Immunol. 1972;1:295–309. doi: 10.1111/j.1365-3083.1972.tb03295.x. [DOI] [PubMed] [Google Scholar]
- 22.Hanai N, Shitura K, Furuya A, Yoshida H, Dohi T, Nudelman E, Hakomori S-I, Satoh S. Detailed characterization of reactivities of anti-gastric cancer monoclonal antibodies to carbohydrate antigen. Anticancer Res. 1990;10:1579–1586. [PubMed] [Google Scholar]
- 23.Jacob E, Baker S J, Swaminathan S P. ‘M’ cells in the follicle-associated epithelium of the human colon. Histopathology. 1987;11:941–952. doi: 10.1111/j.1365-2559.1987.tb01900.x. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs L R, Huber P W. Regional distribution and alterations of lectin binding to colorectal mucosal biopsies from controls and subjects with inflammatory bowel disease. J Clin Investig. 1985;75:112–118. doi: 10.1172/JCI111662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jepson M A, Mason C M, Bennett M K, Simmons N L, Hirst B H. Co-expression of vimentin and cytokeratins in M cells of rabbit intestinal lymphoid follicle-associated epithelium. Histochem J. 1992;24:33–39. doi: 10.1007/BF01043285. [DOI] [PubMed] [Google Scholar]
- 26.Jepson M A, Clark M A, Simmons N L, Hirst B H. Epithelial M cells in the rabbit caecal lymphoid patch display distinctive surface characteristics. Histochemistry. 1993;100:441–447. doi: 10.1007/BF00267824. [DOI] [PubMed] [Google Scholar]
- 27.Jepson M A, Clark M A, Foster N, Mason C M, Bennett M K, Simmons N L, Hirst B H. Targeting to intestinal M cells. J Anat. 1996;189:507–516. [PMC free article] [PubMed] [Google Scholar]
- 28.Kahn M I, Sastry M V, Surolia A. Thermodynamic and kinetic analysis of carbohydrate binding to the basic lectin from winged bean (Psophocarpus tetragonolobus) J Biol Chem. 1986;261:3013–3019. [PubMed] [Google Scholar]
- 29.Kawaguchi T, Matsumoto I, Osawa T. Studies on hemagglutinins from Maackia amurensis seeds. J Biol Chem. 1974;249:2786–2792. [PubMed] [Google Scholar]
- 30.Kerneis S, Bogdanova A, Colucci-Guyon E, Kraehenbuhl J-P, Pringault E. Cytosolic distribution of villin in M cells from mouse Peyer’s patches correlates with the absence of a brush border. Gastroenterology. 1996;110:515–521. doi: 10.1053/gast.1996.v110.pm8566599. [DOI] [PubMed] [Google Scholar]
- 31.Kerneis S, Bogdanova A, Kraehenbuhl J-P, Pringault E. Conversion by Peyer’s patch lymphocytes of human enterocytes into M cells that transport bacteria. Science. 1997;277:949–952. doi: 10.1126/science.277.5328.949. [DOI] [PubMed] [Google Scholar]
- 32.Knibbs R N, Goldstein I J, Ratcliffe R M, Shibuya N. Characterization of the carbohydrate binding specificity of the leukoagglutinating lectin from Maackia amurensis. Comparison with other sialic acid-specific lectins. J Biol Chem. 1991;266:83–88. [PubMed] [Google Scholar]
- 33.Langman J M, Rowland R. The number and distribution of lymphoid follicles in the human large intestine. J Anat. 1986;194:189–194. [PMC free article] [PubMed] [Google Scholar]
- 34.Lee Y S. Lectin reactivity in human large bowel. Pathology. 1987;19:397–401. doi: 10.3109/00313028709103890. [DOI] [PubMed] [Google Scholar]
- 35.Lee Y S. Lectin expression in neoplastic and non-neoplastic lesions of the rectum. Pathology. 1988;20:157–165. doi: 10.3109/00313028809066627. [DOI] [PubMed] [Google Scholar]
- 36.Marra A, Isberg R R. Invasin-dependent and invasin-independent pathways for translocation of Yersinia pseudotuberculosis across the Peyer’s patch intestinal epithelium. Infect Immun. 1997;65:3412–3421. doi: 10.1128/iai.65.8.3412-3421.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGhee J R, Mestecky J, Dertzbaugh M T, Eldridge J H, Hirasawa M, Kiyono H. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine. 1992;10:75–88. doi: 10.1016/0264-410x(92)90021-b. [DOI] [PubMed] [Google Scholar]
- 38.McMahon R F T, Panesar M J R, Stoddard R W. Glycoconjugates of the normal human colorectum: a lectin histochemical study. Histochem J. 1994;26:504–518. doi: 10.1007/BF00157896. [DOI] [PubMed] [Google Scholar]
- 39.Murphy L A, Goldstein I J. Physical-chemical characterization and carbohydrate-binding activity of the A and B subunits of the Bandeiraea simplifolica I isolectins. Biochemistry. 1979;18:4999–5005. doi: 10.1021/bi00589a030. [DOI] [PubMed] [Google Scholar]
- 40.Neutra M R. The role of M cells in transepithelial transport of antigens and pathogens to the mucosal immune system. Am J Physiol. 1998;274:G785–G791. doi: 10.1152/ajpgi.1998.274.5.G785. [DOI] [PubMed] [Google Scholar]
- 41.Neutra M R, Phillips T L, Mayer E L, Fishkind D J. Transport of membrane-bound macromolecules by M cells in follicle-associated epithelium of rabbit Peyer’s patch. Cell Tissue Res. 1987;247:537–546. doi: 10.1007/BF00215747. [DOI] [PubMed] [Google Scholar]
- 42.Neutra M R, Giannasca P J, Giannasca K T, Kraehenbuhl J-P. M cells and microbial pathogens. In: Blaser M, Smith P, Ravdin J, Greenberg H, Guerrant R, editors. Infections of the GI tract. New York, N.Y: Raven Press; 1995. pp. 163–178. [Google Scholar]
- 43.Neutra M R, Pringault E, Kraehenbuhl J-P. Antigen sampling across epithelial barriers and induction of mucosal immune responses. Annu Rev Immunol. 1996;14:275–300. doi: 10.1146/annurev.immunol.14.1.275. [DOI] [PubMed] [Google Scholar]
- 44.O’Leary A D, Sweeney E C. Lymphoglandular complexes of the colon: structure and distribution. Histopathology. 1986;10:267–283. doi: 10.1111/j.1365-2559.1986.tb02481.x. [DOI] [PubMed] [Google Scholar]
- 45.Oriol R, Le Pendu J, Mallicone R. Genetics of ABO, H, Lewis, X and related antigens. Vox Sang. 1986;51:161–171. doi: 10.1111/j.1423-0410.1986.tb01946.x. [DOI] [PubMed] [Google Scholar]
- 46.Owen R L. Sequential uptake of horseradish peroxidase by lymphoid follicle epithelium of Peyer’s patches in the normal unobstructed mouse intestine: an ultrastructural study. Gastroenterology. 1977;72:440–451. [PubMed] [Google Scholar]
- 47.Owen R L, Bhalla D K. Cytochemical analysis of alkaline phosphatase and esterase activities and of lectin-binding and anionic sites in rat and mouse M cells. Am J Anat. 1983;168:199–212. doi: 10.1002/aja.1001680207. [DOI] [PubMed] [Google Scholar]
- 48.Owen R L, Jones A L. Epithelial cell specialization within human Peyer’s patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology. 1974;66:189–203. [PubMed] [Google Scholar]
- 49.Pappo J, Owen R L. Absence of secretory component expression by epithelial cells overlying rabbit gut-associated lymphoid tissue. Gastroenterology. 1988;95:1173–1177. doi: 10.1016/0016-5085(88)90347-2. [DOI] [PubMed] [Google Scholar]
- 50.Podolsky D K, Fournier D A. Alterations in mucosal content of colonic glycoconjugates in inflammatory bowel disease defined by monoclonal antibodies. Gastroenterology. 1988;95:379–387. doi: 10.1016/0016-5085(88)90494-5. [DOI] [PubMed] [Google Scholar]
- 51.Rautenberg K, Cichon C, Heyer G, Demel M, Schmidt M A. Immunocytochemical characterization of the follicle-associated epithelium of Peyer’s patches: anti-cytokeratin 8 antibody (clone 4.1.18) as a molecular marker for rat M cells. Eur J Cell Biol. 1996;71:363–370. [PubMed] [Google Scholar]
- 52.Rhodes J M, Black R R, Savage A. Glycoprotein abnormalities in colonic carcinomata, adenomata, and hyperplastic polyps shown by lectin peroxidase histochemistry. J Clin Pathol. 1986;39:1331–1334. doi: 10.1136/jcp.39.12.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rhodes J M, Black R R, Savage A. Altered lectin binding by colonic epithelial glycoconjugates in ulcerative colitis and Crohn’s disease. Dig Dis Sci. 1988;33:1359–1363. doi: 10.1007/BF01536988. [DOI] [PubMed] [Google Scholar]
- 54.Sakomoto J, Furukawa K, Cordon-Cardo C, Yin B W T, Rettig W J, Oettgen H F, Old L J, Lloyd K O. Expression of Lewis A, Lewis B, X, and Y blood group antigens in human colonic tumors, normal tissue and in human tumor derived cell lines. Cancer Res. 1986;46:1553–1561. [PubMed] [Google Scholar]
- 55.Savidge T. The life and times of an intestinal M cell. Trends Microbiol. 1996;4:301–306. doi: 10.1016/0966-842x(96)10052-4. [DOI] [PubMed] [Google Scholar]
- 56.Sharma R, van Damme E J M, Peumans W J, Sarsfield P, Schumacher U. Lectin binding reveals divergent carbohydrate expression in human and mouse Peyer’s patches. Histochem Cell Biol. 1996;105:459–465. doi: 10.1007/BF01457659. [DOI] [PubMed] [Google Scholar]
- 57.Shibuya N, Goldstein I J, Broekaert W F, Makuta-Lubaki M, Peeters B, Peumans W J. The elderberry (Sambucus nigra) bark lectin recognizes the Neu5Ac(α2-6)Gal/GalNAc sequence. J Biol Chem. 1987;262:1596–1601. [PubMed] [Google Scholar]
- 58.Shitara K, Hanai N, Yoshida H. Distribution of lung adenocarcinoma-associated antigens in human tissues and sera defined by monoclonal antibodies KM-52 and KM-93. Cancer Res. 1987;47:1267–1272. [PubMed] [Google Scholar]
- 59.Siebers A, Finlay B B. M cells and the pathogenesis of mucosal and systemic infections. Trends Microbiol. 1996;4:22–29. doi: 10.1016/0966-842x(96)81501-0. [DOI] [PubMed] [Google Scholar]
- 60.Smith M W, James P S, Tivey D R, Brown D. Automated histochemical analysis of cell populations in the intact follicle-associated epithelium of the mouse Peyer’s patch. J Histochem. 1988;20:443–448. doi: 10.1007/BF01002430. [DOI] [PubMed] [Google Scholar]
- 61.Taatjes D J, Roth J. Glycosylation in intestinal epithelium. Int Rev Cytol. 1991;126:135–193. doi: 10.1016/S0074-7696(08)60684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Damme E J M, Allen A K, Peumans W J. Isolation and characterization of a lectin with exclusive specificity towards mannose from snowdrop (Galanthus nivalis) bulbs. FEBS Lett. 1987;215:140–144. [Google Scholar]
- 63.Varki A. Selectin ligands. Proc Natl Acad Sci USA. 1994;91:7390–7397. doi: 10.1073/pnas.91.16.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vecchi M, Torgano G, Monti M, Berti E, Agape D, Primignani M, Ronchi G, De Franchis R. Evaluation of structural and secretory glycoconjugates in normal human jejunum by means of lectin histochemistry. Histochemistry. 1987;86:359–364. doi: 10.1007/BF00494993. [DOI] [PubMed] [Google Scholar]
- 65.Yoshioka H, Inada M, Ogawa K, Ohshio G, Yamabe H, Hamashima Y, Miyake T. Lectin histochemistry in ulcerative colitis and Crohn’s disease. J Exp Pathol. 1989;4:69–78. [PubMed] [Google Scholar]
- 66.Zhou, F., and M. R. Neutra. Unpublished data.