Highlights
-
•
Myeloma bone disease is a challenging complication of multiple myeloma and one of increasing treatment interest.
-
•
Over 90% of patients develop local osteolytic lesions and skeletal-related events at some point during the progression of the disease.
-
•
Bone lesions can induce severe pain and immobility and can also increase the risk of fractures and osteomyelitis.
-
•
Denosumab can reduce skeletal-related events and bortezomib/1D11 can reduce bone destruction and pathological fractures in multiple myeloma patients.
Keywords: Multiple myeloma, Myeloma bone disease, Osteolytic bone infections, Osteomyelitis, Cancer
Abstract
Multiple myeloma is a hematological malignancy affecting the plasma cells. It is the second most common hematologic cancer in adults. Over 90% of patients develop local osteolytic lesions and skeletal-related events at some point during the progression of the disease. Bone lesions can induce severe pain and immobility and can also increase the risk of fractures and osteomyelitis. Skeletal complications are associated with poor clinical outcomes, affecting quality of life and mortality. Current standards of care for myeloma, e.g., autologous stem-cell transplantation (ASCT) and chemotherapy, do not lessen the risk of adverse events in bone. Once bone lesions are present, bone-targeted interventions are limited, with bone antiresorptive drugs being a mainstay of treatment. This review highlights the growing literature surrounding osteolytic lesions and bone infections associated with multiple myeloma and assesses current and emerging treatments. Emerging evidence from clinical trials suggests that denosumab can reduce skeletal-related events, and the potential application of bortezomib/1D11 can reduce bone destruction and pathological fractures in MM patients. Once established, bone lesions are prone to develop osteomyelitis – especially in immunocompromised individuals. Antibiotics and surgical interventions have been used to manage bone infections in most reported cases. As the bone infection risk associated with MM bone lesions become more evident, there is scope to improve patient management by mitigating this risk with prophylactic antimicrobial therapy.
1. The disease burden of multiple myeloma
Multiple myeloma (MM) is a currently incurable hematological malignancy affecting the plasma cells. It is the second most common hematologic cancer in adults, accounting for 10 % of hematologic malignancies and 1 % of all cancers [1]. The incidence rate of MM worldwide is estimated to be 21 per 1,000,000, and ∼150,000 individuals are newly diagnosed every year worldwide [2], [3]. The mortality of MM accounts for 1 % of all cancer-related deaths and an estimated 72,000 people die from MM annually [4].
Multiple myeloma features clonal proliferation of B-lymphocyte-derived plasma cells in the bone marrow that leads to progressive immune dysfunction. MM typically starts as a monoclonal gammopathy of undetermined significance (MGUS) or smouldering multiple myeloma (SMM), both of which can be asymptomatic [5]. At present, MM is still considered a single disease, but clinically, it is a collection of several different cytogenetically distinct plasma cell malignancies. Patients with MM can develop a range of complications including anemia, immune dysfunction, renal impairment and osteolytic bone lesions before ultimately succumbing to their disease [6].
MM is often identified coincidently following blood testing for general symptoms, such as bone pain. A diagnostic confirmation is achieved through serum biochemistry, blood cell counts, serum or urine tests or a bone marrow biopsy for monoclonal M−protein production or free light chains [7]. The criteria for the diagnosis of multiple myeloma requiring therapy are 10 % or more plasma cells in the bone marrow, abnormal immunoglobulins in the blood/urine and the presence of one or more myeloma defining events (MDE), including hypercalcemia, renal failure, anemia, and lytic bone lesions [8].
In general, the prognosis of multiple myeloma is poor despite the advancement of anti-myeloma therapy. According to the revised international staging system (RISS), there are three stages of multiple myeloma. In stage one, patients’ levels of albumin, beta-2-microglobulin (B2M) and lactate dehydrogenase (LDH) are normal, and it is most treatable at this stage. However, most patients are diagnosed in stage two, in which case, their albumin level is low, B2M level may increase, and more than half of these patients will live seven years or more past the start of treatment. In the advanced stage three, patients will have high B2M and/or LDH levels, suggesting the disease is widespread and more than half of patients in this stage will survive for another three and a half years [9], [10]. The rate of SRE and bone lytic lesions is also high, and it is often lytic disease or fracture that provokes a late-stage diagnosis. Overall, only 50.7 % of MM patients were alive five years post diagnosis, mostly due to the under detection and difficulties to diagnose MM until it has progressed. In addition, only approximately 5 % of MM patients are diagnosed at an early stage. Their five-year survival rate is higher (71 %) than later-stage diagnosis (48 %).
2. Clinical interventions for multiple myeloma
Conventional chemotherapy for MM uses melphalan, an alkylating agent, and prednisone [11], and it has been the gold standard conditioning regimen for decades. Currently, MM patients are typically treated with approximately-three to four cycles of induction therapy with bortezomib (an antineoplastic agent), lenalidomide (an immunomodulatory drug), and dexamethasone (VRd) prior to stem cell harvest. In the presence of acute renal failure, other bortezomib-containing regimens such as bortezomib-thalidomide-dexamethasone (VTd) or bortezomib-cyclophosphamide-dexamethasone (VCd) can be used instead of VRd. However, the low‐dose dexamethasone regimen (40 mg once a week) is generally preferred with chemotherapies to minimize toxicity [12].
After harvest, patients can either undergo frontline autologous stem cell transplantation (ASCT) or resume induction therapy delaying ASCT until first relapse. ASCT can prolong patient survival and has become the standard of care for treating newly diagnosed multiple myeloma in young and select, fit, elderly patients [13]. Still, not all MM patients are suitable candidates for ASCT. One of the key clinical challenges is to critically assess the patient’s overall health to ensure a balance between risks and benefits [14].
Since patients can become refractory to their initial treatment, multiple therapeutic options have been developed and can improve long-term outcomes. Combined therapies with thalidomide, lenalidomide, bortezomib and dexamethasone have been shown to significantly improve the five-year related overall survival from 29 % to 35 % over the past 20 years [15], [16], [17], [18], [19]. Meanwhile, trials with immunomodulatory drugs (e.g., pomalidomide), proteasome inhibitors (e.g., carfilzomib and ixazomib), histone deacetylase inhibitor, CD38-targeting monoclonal antibodies and B-lymphocyte mutation antigen CAR T-lymphocyte therapy have also shown promising outcomes [20], [21], [22], [23], [24].
Thalidomide slows blood vessel growth around the abnormal plasma cells (anti-angiogenesis), and it is an effective and well-tolerated front-line chemotherapy for MM [25]. As an initial treatment to prepare patients for autologous stem-cell transplantation, thalidomide in combination with dexamethasone resulted in higher response rates than the combination of vincristine, doxorubicin, and dexamethasone or dexamethasone alone [25], [26]. Clinical trials have shown prolonged progression-free survival rate and improved depth of response when combinations of conventional (e.g. melphalan and prednisone) and novel therapies (e.g. thalidomide or lenalidomide) are applied [15], [27], [28], [29], [30], [31].
Subsequently, daratumumab (16 mg/kg), an IgG-kappa monoclonal antibody targeting CD38, is approved as monotherapy in patients with heavily pre-treated relapsed or refractory multiple myeloma (RRMM) and in combination with bortezomib/dexamethasone or lenalidomide/dexamethasone [32].
Since many MM patients become refractory to treatment and relapses are likely to occur in all cases, clinical trials are constantly focusing on testing new chemotherapeutics and refining immunotherapies. Although the treatment of multiple myeloma is rapidly evolving, and there are many options available, daratumumab is to some extent the final treatment option for MM. In contrast to the extensive developments in anti-tumor therapeutics, therapeutic management of myeloma-induced skeletal-related events remain under-researched. Given the increased survival rates we have achieved with tumor targeted therapies, this is becoming more pertinent as this increases the time frame during which skeletal related events such as fracture can occur. Therefore, there is significant potential to reduce the burden of disease and improve the patient experience by specifically addressing the osseous lesions and resulting complications [33].
3. Osseous lesions and associated complications in myeloma bone disease
Myeloma bone disease (MBD) is a common and devastating complication of multiple myeloma and a major cause of increased morbidity and mortality [34], [35]. The clinical features of MM are enhanced bone loss mainly associated with the axial skeleton and pelvis, such as diffuse osteopenia, focal lytic bone lesions, spinal cord compression, pathological fractures, hypercalcemia, renal insufficiency, and bony pain [36]. Indeed, at least 85 % of MM patients show some degree of osteopenia at diagnosis, and the severity of bone destruction typically correlates with tumor burden and prognosis [35], [37]. Moreover, 80–90 % of patients will develop osteolytic bone lesions as their cancer progresses, which can negatively affect quality of life and worsen survival prospects.[38], [39].
As the low sensitivity of skeletal survey in identifying lytic bone lesions in MM patients has necessitated the use of more sophisticated imaging approach, whole-body low-dose CT (WBLDCT) is recommended as the imaging modality of choice for the initial assessment of MM-related lytic bone lesions [40]. Meanwhile, MRI is the gold standard imaging modality for detecting bone marrow involvement and to rule out spinal cord compression in MM patients [41]. In contrast, XR, PET/CT and MIBI imaging are not recommended for routine use in the management of myeloma patients but can still provide valuable prognostic data to assess the response to therapy and warrant clarification of previous imaging findings in selected cases [42].
Clinical data suggest that bone pain is common in MM patients (63 %) and most patients (74 %) had two or more bone lesions at initiation of first-line treatment [43]. Pain can be intense, but the focal lytic lesions can also lead to bone deformation, spinal cord compression and concomitant height loss, and pathological fractures. However, in the early stage, MM patients were often asymptomatic and underdiagnosed until initial orthopedic symptoms associated with bone pain occur. In some cases, the pain and fractures precede osteoclastic-activating growth factors, driving further osteopenia and lesions. The cytokine release and the lack of osteoblastic response are also contributed to deterioration [44].
Mechanistically, osteolytic lesions and bone pain are caused by bone marrow infiltration by malignant plasma cells and expansion within the bone microenvironment [45]. Most lesions are localized to the spine, spinal discs, ribs, skull, or pelvis. Bone destruction in MM is multifactorial, resulting from an interaction of bone marrow stromal cells and myeloma tumour cells within the microenvironment of the bone marrow [44]. MM is often characterized by a loss of synchronization between bone formation and resorption, associated with an excess osteoclasts and reduced osteoblast activity [35], [36].
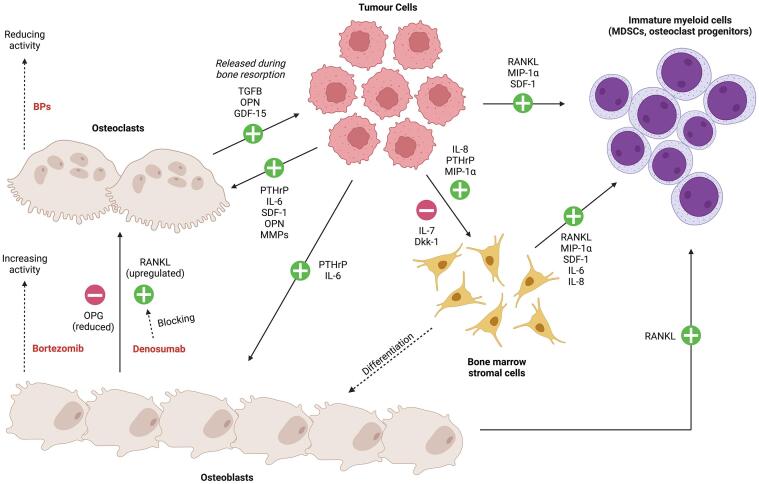
The pathogenic mechanism of MM involves the interaction between multiple signaling pathways and cell types (Fig. 1). In brief, tumor cells that enter and colonize the bone microenvironment propagate osteolytic lesions by increasing osteoclast activation and differentiation [46]. Tumor cells express receptor activator of NF-κB ligand (RANKL) and macrophage inflammatory protein-1α (MIP-1α, CCL3), which stimulate differentiation of osteoclast progenitors in the bone marrow into mature osteoclasts. Local expression of parathyroid hormone-related protein (PTHrP), IL-6, along with stromal cell-derived factor-1 (SDF-1 or CXCL12), osteopontin, and matrix metalloproteinase 13 (MMP13) can further promote bone turnover by osteoblasts and osteoclasts. MIP-1α, PTHrP and IL-8 upregulate RANKL in stromal cells, further stimulating osteoclastogenesis indirectly. Additionally, Dkk1 and IL-7 from multiple myeloma cells can suppress osteoblast differentiation from mesenchymal stromal progenitors. These alterations in bone turnover led to the initiation of the well described vicious cycle in which osteoclast resorption of bone matrix releases pro-tumorigenic factors such as TGFβ, osteopontin and GDF15, stimulating further MM cell expansion in bone [47], [48]. Overall, this results in a positive feedback loop of tumor proliferation and bone destruction.
Fig. 1.
Interactions of tumor cells and drug activities with osteoblasts, osteoclasts bone marrow stromal cells and immature myeloid cells. The schematic incorporates points of drug intervention including bisphosphonates (BPs) to reduce osteoclastic activity [49]; Denosumab a neutralizing antibody inhibitor of RANKL that suppresses osteoclastogenesis [50]; and first-in-class proteasome inhibitor (PI) bortezomib that can increase vitamin D receptor (VDR) signaling and markers of osteoblast differentiation [51]. Notably, no therapeutic approach will eliminate the lytic bone lesions caused by MM and can still have progression of skeletal disease if the osteolytic lesions are left untreated [36].
4. Conventional and emerging treatments for myeloma bone disease
Nearly 30 % of patients with multiple myeloma have pathological fractures in the spine and long bones [52]. If the patients are not treated in a timely fashion, these can lead to long-term or permanent disability and severely impact their quality of life. Patients with a single bone lesion, a negative bone marrow biopsy, and no paraproteinemia in serum had a better survival probability [53]. In these conditions, surgical intervention can strengthen and support the weak section of the bone. However, surgical treatment in patients with multiple myeloma is mostly limited to a palliative approach. Although osteolytic lesions and skeletal diseases are common in multiple myeloma patients, studies focusing on treating osteolytic lesions in multiple myeloma are limited. Only several clinical trials in the past twenty years focused on treatment in MM patients with orthopedic measures. To examine the clinical evidence for the current therapies to treat bone lesions or osteomyelitis, we conducted a semi-systematic search for clinical trials in the English literature using the PubMed database was conducted. All searches were limited to January 2000-Decemeber 2021. We used medical subject headings (MeSH) and keyword terms in the title/abstract, and the final search used the terms “multiple myeloma AND bone lesion OR osteolytic lesions OR osteomyelitis OR bone infection” and filtered for “clinical trials” only. Additional clinical trials that were found relevant to the subject were manually added to the list. Clinical trial findings are summarized chronologically in Table 1.
Table 1.
Summary of clinical trials focused on treatment in MM patients with bone defect measures (Jan 2000- Dec 2021).
| References | Cohort | Phase | Study design | Treatment groups and medications | Outcomes |
|---|---|---|---|---|---|
| Barlogie et al. 2008 [54] | 76 adult patients with smoldering multiple myeloma SMM) | II | Survival surveillance and cohort study | Thalidomide (200 mg/d) with monthly pamidronate (PAM) | No SMM patients had hypercalcemia more than 2.625 mM (10.5 mg/dL). No patients developed hypercalcemia or bone lesions after treatment. 36 % of patients required reduced dosing due to peripheral neuropathy.Other side effects include neutropenia and dizziness (8 %), fatigue, thrombocytopenia, and cardiovascular events (7 %). |
| Rajkumar et al. 2010 [15] | 445 adult patients with MM | ND | Open label, randomized controlled trial | High dose group: lenalidomide (25 mg) on days 1–21 plus dexamethasone 40 mg on days 1–4, 9–12, and 17–20 of a 28-day cycle Low dose group: lenalidomide (25 mg) given on the same schedule with dexamethasone 40 mg on days 1, 8, 15, and 22 of a 28-day cycle |
149 patients (67 %) in the high-dose group had bone disease at baseline compared with 127 (57 %) of 222 in the low-dose group. No improvement in bone disease incidence between the high-dose (67 %) and low-dose (57 %) groups. Lenalidomide plus low-dose dexamethasone is associated with better short-term survival, and with lower toxicity than lenalidomide plus high-dose dexamethasone. |
| Gimsing et al. 2010 [55] | 504 patients of any age with untreated symptomatic multiple myeloma | III | Double-blind, multi-center, randomized controlled trial | Group 1: received pamidronate (30 mg) monthlyGroup 2: pamidronate (90 mg) monthly |
Median time to first skeletal-related event in patients was 9.2 month in the 90 mg and 10.2 months in the 30 mg group. Eight patients in the 90 mg group developed osteonecrosis of the jaw compared to only two patients in the 30 mg group. Pamidronate 90 mg per month was not superior to a dose of 30 mg for prevention of skeletal events or for improvement of QOL in patients with NDMM. No further inhibition of the osteoclast by bisphosphonates can be achieved by higher doses. |
| Morgan et al. 2012 [56] | 1960 patients with NDMM | ND | Randomized trial | Intensive treatment pathway: Group 1: CLO + CVAD Group 2: ZA + CVAD Group 3: CLO + CTD Group 4: ZA + CTD Non-intensive treatment pathway: Group 5: CLO + MP Group 6: ZA + MP Group 7: CLO + CTDa Group 8: ZA + CTDa |
Patients who received attenuated oral cyclophosphamide, thalidomide, and dexamethasone (CTDa) had better responses and lower SRE rates than melphalan and prednisolone (MP). Zoledronic acid (ZA) improved the overall survival (OS) compared with clodronate (CLO) independently of gender, stage, or myeloma subtype, most profoundly in patients with baseline bone disease or other SREs. In patients treated for more than two years, ZA improved OS compared with CLO from randomization. Thalidomide-containing regimens had better efficacy than traditional regimens, and ZOL demonstrated greater benefits than CLO. |
| Witzig et al. 2013 [57] | 68 adult MM patients | III | Randomized trial | Group 1: Thalidomide (Thal)/Zoledronic acid (ZA) Group 2: Zoledronic acid only |
The risk of progression to active MM significantly reduced with a combination of Thal and ZA. The time to progression (TTP) was superior for Thal/ZA (n = 35) patients compared with ZA alone (n = 33). In the first year, 86 % of Thal/ZA patients were progression free compared with 55 % on ZA alone. The overall response rate after the first year was 37 % for Thal/ZA with a median duration of response of 3.3 years, but there were no confirmed responses to ZA alone. The addition of Thal to standard ZA treatment produces anti-tumor responses whereas ZA alone does not. No significant difference of osteolytic bone lesions (2 %) in both groups. |
| Miguel et al. 2013 [58] | 455 adult patients with refractory or relapsed multiple myeloma | III | Multicentre, open label, randomized trial | Group 1: pomalidomide + low-dose dexamethasone Group 2: high-dose dexamethasone |
Pomalidomide plus low-dose dexamethasone resulted in significantly longer progression-free survival and overall survival, and a greater number of responses compared with high-dose dexamethasone in patients. The median PFS was 4.0 months in the low-dose dexamethasone group compared with 1.9 months in the high-dose group. Clinical data showed that high-dose dexamethasone resulted fewer infections and infestations (53 % vs 68 %), back pain (16 % vs 20 %) and bone pain (13 % vs 17 %). |
| García-Sanz et al. 2015 [59] | 100 adult patients with MM | IV | Prospective, open-label, randomized trial | Group 1: Zoledronic acid (4 mg, IV, every-four weeks) Group 2: No zoledronic acid |
ZA shows no anti-tumor effect but reduces the risk of progression with symptomatic bone disease and skeletal complications. There were fewer skeletal-related events in the treated group than in the untreated control group. Progressive osteolytic bone lesions, spinal cord compression and hypercalcemia were observed in 16 % of patients in the ZA group, and 41 % in the control group (P = 0.005). The pattern of relapses was different for treated versus control patients, including progressive bone disease (8 vs 20), anemia (24 vs 18), renal dysfunction (1 vs 2), and plasmacytomas (1 vs 1). |
| Diel et al. 2015 [60] | 3822 adult patients with multiple myeloma | III | Double-blind, active-controlled, randomized trial | Group 1: Denosumab (120 mg, IV) every-four weeksGroup 2: Zoledronic Acid (4 mg, IV) every-four weeks |
Denosumab delayed hypercalcaemia of malignancy (HCM), representing a 37 % reduction in the hazard ratio (HR) compared with zoledronic acid and reduced the risk of developing recurrent HCM by 52 %. Fewer patients receiving denosumab compared with zoledronic acid experienced an HCM event. Denosumab treatment was more efficacious than treatment with zoledronic acid in delaying or preventing HCM in advanced MM and other cancers. |
| Moreau et al. 2018 [61] | 578 adult patients with relapsed and refractory multiple myeloma | III | Multi-center, randomized trial | Group 1: Carfilzomib once a week (70 mg/m2) with dexamethasoneGroup 2: Carfilzomib twice a week (27 mg/m2) with dexamethasone |
Once weekly carfilzomib and dexamethasone had significantly improved progression-free survival, higher overall response, and deeper responses compared with patients who received twice weekly carfilzomib with dexamethasone. No difference in the incidence of bone lesions (77 %) presence. |
| Raje et al. 2018 [33] | 1718 adult patients with NDMM | III | Multi-center, double-blind, randomized, controlled trial | Group 1: subcutaneous denosumab (120 mg) with intravenous placebo every 4 weeksGroup 2: intravenous zoledronic acid (4 mg; dose adjusted for renal function at baseline) with subcutaneous placebo every 4 weeks |
Denosumab was non-inferior to zoledronic acid in the prevention of skeletal-related events. The greater progression-free survival with denosumab than with zoledronic acid. Denosumab had an improved renal adverse event profile. The risk of osteonecrosis of the jaw is an adverse reaction of denosumab and zoledronic acid, there was no significant difference in incidence between the two groups. The most common grade 3 or higher treatment-emergent adverse events for denosumab and ZA were neutropenia, thrombocytopenia, anemia, febrile neutropenia, and pneumonia. Denosumab could be an additional option for the standard of care for patients with MM-related bone diseases. |
| Terpos et al. 2019 [62] | 59 adult patients with MM | I | Open-label prospective study | Lenalidomide (VR) (25 mg)Bortezomib (0.7–1.3 mg/m2)Valacyclovir (500 mg)Trimethoprim/sulfamethoxazole (800 mg/160 mg) |
Four cycles of VR consolidation without dexamethasone after ASCT in NDMM patients improved the depth of response and survival outcomes with a manageable safety profile. 58 % of patients improved their response status after ASCT, but 39 % patients following VR consolidation had further deepened response. Stringent complete response rate increased to 51 % after VR from 24 % post-ASCT. A favorable effect on bone remodeling and skeletal-related events (SRE) incidence was observed in the absence of bisphosphonates. VR consolidation is an effective, dexamethasone- and bisphosphonate-free approach that offers long overall survival with improvements on bone metabolism and no SREs. |
| Fazzi et al. 2020 [63] | 44 adult patients with MM | II | Multi-center, single arm trial | IL-2 (SC, 2×106 IU)Zoledronic acid (IV, 4 mg) |
The median time to progression was 22.5 months. Treatment was well-tolerated without grade 3 or 4 toxicities. IL-2 and zoledronate may have activity against myeloma possibly through the activation of γδ T-lymphocytes. The clinical benefit does not support the use of maintenance treatment with IL-2/zoledronate in myeloma patients after autologous bone marrow transplantation. |
| Huang, et al. 2020 [64] | 1718 adult patients with MM | III | Double-blinded, randomized trial | Group 1: Denosumab 120 mg (SC) Group 2: Zoledronic acid 4 mg (IV) |
The most common adverse events reported in either group (denosumab, zoledronic acid) were diarrhea, nausea, and pyrexia.Treatment-emergent renal toxicity occurred in 9/102 (denosumab) and 20/92 (zoledronic acid) patients. Fewer patients in the denosumab group developed first on-study SRE compared with the zoledronic acid group. Results support denosumab as an additional treatment for standard of care for NDMM Asian patients. |
| Diamond et al. 2021 [65] | 108 adult patients with MM | II | Single-arm, single center | Oral lenalidomide (10 mg) was given on days 1–21 of a 28-day cycle continuously, until progression or intolerance, for up to 5 years on protocol Aspirin was required for thrombophylaxis |
Median follow-up was 40.7 months. At 60 months, progression-free survival was 64 %. The most common adverse events of grade 3 or higher were decreased lymphocyte count in 48 (44 %) patients and decreased neutrophil count in 47 (44 %) patients. One death occurred on study due to sepsis and heart failure but was unrelated to the treatment. The treatment had several adverse reactions, including fractures (3–6 %) and bone/other infections (5–34 %). |
4.1. Bisphosphonates can prevent bone disease in NDMM patients
Bisphosphonates (BPs) are bone antiresorptives with a long history of clinical use, particularly for treating cancers that have metastasized to the bone [66]. Pamidronate and zoledronic acid (ZA) are third-generation nitrogen-containing BPs with a high potency [67]. When given by intravenous infusion, these agents can preserve skeletal integrity and minimize the impact of MBD [68]. This can reduce the risk of complications caused by pathologic fracture, spinal cord compression and hypercalcemia due to persistent osteolytic lesions [69]. Numerous clinical trials have demonstrated the efficacy of bisphosphonates (BPs) in preventing skeletal-related events in newly diagnosed multiple myeloma (NDMM) patients [33], [56], [57], [59], [60], [64]. The application of high-dose BPs has been linked to osteonecrosis of the jaw and are contraindicated when dental procedures are required [70]. However, they can represent an invaluable tool for the management of osteolytic lesions.
One of the more notable studies to highlight the utility of BPs in the management of MM was the comprehensive and randomized MRC Myeloma IX trial [56]. A cohort of nearly 2000 patients were dosed with IV ZA versus oral Clodronate in combination with a variety of other drugs (see Table 1). The primary outcome of overall survival was significantly improved with ZA as a preferred agent over clodronate (the historical treatment of choice [71]). A secondary conclusion was that the combination of ZA with thalidomide yielded better efficacy than other traditional regimens.
Gimsing et al (2010) cited a pre-publication of these findings to justify their efforts to refine a BP dose given by monthly infusion to prevent bone disease in NDMM patients [55]. Their study was double-blinded and compared the incidence of skeletal-related events in patients who received 30 mg and 90 mg of pamidronate. While there was a small difference in median time to the first skeletal event, they concluded overall the safety benefits of the lower pamidronate dose made it the superior treatment. Critically, the higher dose was linked to an increase in osteonecrosis of the jaw and a lack of improvement in quality of life. A limitation to this trial was the reliance on historical placebo control data showing the efficacy of BP treatment alone.
Another notable study showed that BPs can prevent bone disease in MM patients presenting with an asymptomatic biochemical relapse after a prior response to standard therapy [59]. While early monotherapy with ZA produced no anti-tumor effects, it nevertheless reduced the risk of progression to symptomatic bone disease and skeletal complications. Only 16 % of ZA-treated patients (4 mg IV/4 weeks, 12 doses) developed osteolytic bone lesions, spinal cord compression and hypercalcemia – significantly lower than the control group (41 %, p = 0.005). The pattern of relapses was also different for treated versus control patients, including progressive bone disease, anemia, renal dysfunction, and plasmacytomas. However, follow-up was limited in patients that remained asymptomatic and did not show progression after one year of ZA treatment.
BPs are currently listed as a non-therapeutic intervention for pain management in MM and the International Myeloma Working Group has recommended that BPs should be considered in all MM patients receiving first-line antimyeloma therapy regardless of the presence of osteolytic bone lesions on conventional radiography [72]. Co-morbidities associated with chronic kidney disease or dental complications can contraindicate their use, although this is not unique to MM and is a consideration for all patients potentially receiving bisphosphonates [70], [73]. Under such circumstances, other emerging agents such as denosumab may have considerable potential.
4.2. Denosumab can prevent skeletal-related events in MM patients
Denosumab is a monoclonal antibody that binds and blocks the activity of RANKL. It is approved by the FDA for the management of MBD and prevention of skeletal-related events (e.g. bone pain and fractures) secondary to MM [74]. It is now considered as an alternative therapy for MM patients with renal impairment where BPs may be contraindicated [75].
The efficacy of denosumab has been tested in multiple clinical trials [33], [60], [64]. Overall, these studies showed that denosumab (120 mg) was more effective than ZA (4 mg) in preventing skeletal-related events (SRE) and controlling hypercalcemia of malignancy (HCM). However, the cost and accessibility of the antibody therapy must be considered in contrast to more conventional BP therapies.
Among MM patients, hypercalcemia of malignancy (HCM) is common (30–80 %) and associated with a poor prognosis. Diel et al. (2015) conducted a phase III randomized trial (n = 3822) to compare the activity of denosumab and ZA for delaying and preventing HCM in patients with MM [60]. Denosumab treatment significantly delayed the time to first onset HCM compared with ZA treatment. The higher efficacy of denosumab treatment was observed as early as six months and continued through to the end of the study. Denosumab also reduced the risk of recurrent HCM by 52 %, suggesting it may be superior to BPs to manage hypercalcemia in advanced MM patients. However, the overall rates of adverse events (AEs) and serious adverse events (SAEs) were similar between the denosumab and ZA treatment groups. This study did include non-MM patients (e.g., breast cancer and other solid tumors), which could have confounded these findings.
Still, the superiority of denosumab over ZA was further supported by additional studies in terms of preventing skeletal-related events (SRE) and controlling hypercalcemia of malignancy (HCM) [33], [64]. As previously noted, chronic kidney disease is a contraindication for BP use, and it was speculated that denosumab may be a better treatment option under such conditions. Indeed, Raje et al. (2018) reported an improved renal adverse event profile and greater progression-free survival rate with denosumab [33]. Similarly, Huang et al. (2020) found that there were fewer patients with treatment-emergent renal toxicity in the denosumab group versus the ZA group, however, the absolute numbers were low (9/102, 9 % vs 20/92, 22 %) [64]. In contrast, the risk of osteonecrosis of the jaw not significantly different between denosumab and ZA arms. While the study was well designed and powered, recruitment was highly skewed towards Asian patients with MM. It also excluded patients with compromised kidney function (a creatinine clearance of less than 30 mL/min) as treatment assignment was blinded, although this might represent a key target patient subgroup in terms of future clinical treatment. Both studies concluded that denosumab could be considered an additional option for managing patients with MM-related bone diseases.
As a treatment option, denosumab may be limited by its so-called “rebound phenomenon” that occurs upon discontinuation [76]. This is associated with a sharp increase in osteoclast number and activity that can lead to a profound increase in localized or systemic bone turnover. Thus, the suspension of denosumab in the absence of any alternative antiresorptive treatment may lead to adverse outcomes in some patients.
4.3. Bortezomib stimulates osteoblast growth and differentiation
Bortezomib is an anti-cancer medication used to treat MM and some lymphomas and acts on the proteosome (a mechanism important for maintaining the immortality of myeloma cells). It is often used in combination with other agents, such as lenalidomide, dexamethasone, melphalan and/or prednisone [77]. There is potential for bortezomib to improve outcomes after autologous stem cell transplantation and it has been tested in combination with nalidomide, valacyclovir, and trimethoprim/sulfamethoxazole [62]. However, bortezomib has also been suggested to upregulate vitamin D receptor signaling, which can directly stimulate osteoblast growth and differentiation [51]. Bortezomib has also be indicated to inhibit osteoclastogenesis and osteoclast activity, the other side of bone metabolism equation [62], [78].
The bone anabolic effects of bortezomib have been suggested to lead to repair of lytic lesions in some patients, even in the absence of BPs. Following stem cell transplantation, one study reported a favorable effect of bortezomib-based induction and lenalidomide (VR) consolidation on bone remodeling and SREs [62]. This featured a normalization in circulating bone markers including RANKL/OPG rations and serum sclerostin. Notably, this trial eschewed the use of other classical bone-affecting therapies such as BPs and dexamethasone but was limited to only transplant-eligible NDMM patients.
It is unclear whether other next-generation proteasome inhibitors (such as carfilzomib and ixazomib [79]) will produce similar bone-benefits to bortezomib in the bone compartment. While there is potential for these drugs to be effective MM therapeutics [80], [81], [82], there is a critical lack of mechanistic studies addressing effects on myeloma progression versus direct impact on osteolytic lesions.
4.4. Chemotherapy does not reduce the formation of osteolytic lesions
Although chemotherapy remains the frontline treatment for MM, there is little evidence that it positively impacts on the progression of MBD and any established osteolytic lesions.
Miguel et al. (2013) recommended pomalidomide plus low-dose dexamethasone to be used in patients with refractory or relapsed and refractory MM who had failed previous treatments of bortezomib and lenalidomide [58]. This was based on their findings that median progression-free survival was 4.0 months in the low-dose dexamethasone group compared with 1.9 months in the high-dose group. While the high vs low-dose dexamethasone did not significantly affect many bone-related adverse events (e.g., back pain and bone pain), the overall rate of infections and infestations was extremely high (68 %). The study did not report what percentage affected the bone, but it does point to an overall higher infection risk associated with MM.
A small but randomized trial reported the progression to active MM was significantly reduced with thalidomide treatment [57]. However, unless combined with ZA, thalidomide on its own did not prevent the acquisition of osteolytic bone lesions. While the sample size was small, the findings are consistent with the concept that many anti-cancer agents are ineffective against MBD. In another trial, comparison of high-dose versus low-dose lenalidomide and dexamethasone showed no improvement in the incidence of bone disease [15]. This study had greater numbers and showed difference in short-term survival with low-dose treatment such that the high-dose group was transitioned to the lower therapy after the 1-year interim analysis. While the MBD analysis was limited, the early mortality in the first four months with high-dose therapy suggests that even short courses carry significant risk, though the study did not mandate thromboprophylaxis or antibiotic prophylaxis.
Diamond et al. (2021) also tested oral lenalidomide (10 mg) given on days 1–21 of a 28-day cycle continuously until progression or intolerance for up to five years in patients with multiple myeloma. It was evident that the treatment had several adverse reactions, which may associate with fractures (3–6 %) and bone or other infections (5–35 %) [65]. The most common adverse events of grade 3 or worse were decreased lymphocyte count in 48 (44 %) patients and decreased neutrophil count in 47 (44 %) patients. At 60 months, progression-free survival was 64 %. One death occurred on study due to sepsis and heart failure; though this was unrelated to treatment, it further illustrates the vulnerability of MM patients to infections. While this was a single-center study it achieved sufficient recruitment to adequately power its primary endpoint.
In another randomized trial, Moreau et al. (2018) tested the efficacies of once (70 mg/m2) and twice weekly (27 mg/m2) carfilzomib with dexamethasone (40 mg, weekly) in patients with relapsed and refractory MM to bortezomib or ixazomib. The trial found no difference in the incidence of bone lesions between both groups, and the treatments did not reduce bone pain or skeletal events in NDMM patients [61]. However, once weekly carfilzomib and dexamethasone had significantly improved progression-free survival, higher overall response, and deeper responses compared with patients who received twice weekly carfilzomib with dexamethasone. Nonetheless, this study was not double-blinded and allowed for the possibility of selection bias.
4.5. Interleukin-2 does not prevent myeloma bone disease
In 2020, a phase II trial determined the efficacy of interleukin-2 (IL-2) combined with zoledronate (4 mg). It shows that IL-2/zoledronate activates T-lymphocytes against myeloma cells in MM patients. Whilst the treatment is feasible in terms of adverse events, it is challenging for patients to have numerous visits to the hospital. The clinical benefits observed in terms of time to progression also did not support the use of maintenance treatment with IL-2/zoledronate as a treatment option in myeloma patients after autologous bone marrow transplantation [63]. No clinical data indicated that IL-2 could prevent bone diseases in MM patients.
However, the idea of immunotherapy for myeloma bone disease may still be valid as multiple preclinical in vivo studies have identified some proteins (e.g., sclerostin, parathyroid hormone, and BMP) that play a crucial role in myeloma bone disease progression, and are prone to be therapeutic targets [83], [84], [85], [86], [87], [88]. Clinical studies exploring such potential will be discussed later in this review.
5. MM lytic lesions as a risk factor for osteomyelitis
MM patients that are immunocompromised by chemotherapy, transplantation or steroid medications can be susceptible to infections. However, the medical literature rarely highlights the risks of bone infection associated with myeloma bone disease. While the literature to date chiefly consists of case reports rather than rigorous prospective trials, the anecdotal evidence would suggest that this may be an area of clinical concern that justifies further investigation. Moreover, when osteomyelitis effects a cancerous bone lesion, the clinical outcomes are often poor.
Consequently, we conducted a literature search on PubMed database looking for clinical case reports of osteomyelitis in multiple myeloma patients. All searches were limited to January 2000- December 2021. We used medical subject headings (MeSH) and keyword terms in the title/abstract, and the final search used the terms “multiple myeloma AND bone lesion OR osteolytic lesions OR osteomyelitis OR bone infection” and filtered for “case reports” only. Additional reports that were found relevant to the subject were manually added to the list. All case reports were summarized in Table 2.
Table 2.
Summary of case reports focused on treatment of bone infections in MM patients (Jan 2000- Dec 2021).
| References | Patient details | Medical history, sign, and symptoms | Infection site and causative pathogens | Treatments and clinical outcomes |
|---|---|---|---|---|
| Desikan, et al. (2003) [89] | 72, Male | Stage IIA kappa light-chain disease, received 140 mg/m2 melphalan after induction therapy with 40 mg of dexamethasone Had a history of oxacillin-resistant Staphylococcus aureus infection, treated with intravenous vancomycin Three months later, readmitted with significant right shoulder pain and required intravenous morphine |
Spondylodiscitis with prevertebral and epidural abscesses causing impingement of the cervical cord Blood culture was positive for S. aureus |
Received discectomy and corpectomy of C4 and C5 vertebrae along with arthrodesis of the C3-C6 vertebrae. |
| Desikan, et al. (2003) [89] | 56, Male | Stage IIIA IgG lambda multiple myeloma Received induction therapy and tandem transplants Recurrence of MM with severe low back pain |
An MRI scan revealed spondylodiscitis of disc L4-L5 with an associated epidural abscess. Culture of the epidural abscess showed positive for Streptococcus pneumoniae with intermediate resistance to penicillin. |
A partial laminectomy of L4 was performed to evacuate the epidural abscess. Received intravenous vancomycin for six weeks, and no recurrence of vertebral infection was observed. The patient suffered respiratory infections and died of progressive disease one year later. |
| Desikan, et al. (2003) [89] | 61, Male | VAD refractory stage IIIB kappa light-chain myeloma received combination chemotherapy with dexamethasone, thalidomide, cisplatinum, adriamycin, cyclophosphamide and etoposide (DT-PACE) for stem cell procurement. Developed fever, complained of localized pain and tenderness of the lower back before admission for stem cell transplant. The back pain worsened during the post-transplant neutropenic period. |
An MRI revealed spondylodiscitis of the L5-S1 disc space. A CT-guided aspirate was positive for coagulase-negative Staphylococci. |
Received intravenous vancomycin for one month. The patient had no recurrence of pain. Evaluations were normal for the next three years. |
| Yu et al. (2010) [90] | 57, Female | IgG kappa gammopathy (subsequent diagnosis of myeloma), had 3-week history of chill and low back pain. No history of trauma. The patient also had mild tenderness and knocking pain over the lumbar area. The MRI of spine demonstrated hyperintensity at the L2 body with a pre-vertebral abscess, suspected spondylitis. |
Blood culture revealed Escherichia coli, and a CT-guided biopsy at the L2 vertebra confirmed infectious spondylitis. | First treated with intravenous oxacillin, then switched to cefazolin and cefuroxime. Neurological deficit was alleviated after eight weeks of antibiotic therapy. The patient was free of recurrent back pain and fever six months after discharge, and was subsequently treated with melphalan, dexamethasone and thalidomide for MM. |
| Mohan et al. (2016) [91] | 69, Male | Relapsed refractory MM, admitted for chemotherapy and autologous stem cell transplant (ASCT). He was diagnosed with MM and had been heavily treated in the past including three prior ASCTs. He was admitted for velcade, dexamethasone, thalidomide, adriamycin, cytoxan, and etoposide administration days 1 to 4, with one dose of melphalan and ASCT on day 6. He developed new onset atrial fibrillation with acute renal failure, and the neutropenic phase was further complicated with sepsis caused by vancomycin- and daptomycin-resistant Enterococcus faecium bacteremia. The infection was successfully treated with quinapristin-dalfopristin. |
Lasiodiplodia On day 6 post ASCT, the patient reported new swelling and erythema of the third right toe. Physical examination showed hemorrhagic bullae around the nail with reddish discoloration of the entire third right toe and minimal oedema. |
Successfully treated with amputation and antifungal medications (oral voriconazole alone after 14 days of liposomal amphotericin B). |
| Park et al. (2016) [92] | 74, Female, Korean | Fever and diffuse abdominal pain and septic shock Multiple myeloma, had 2 cycles of chemotherapy with thalidomide-cyclophosphamide-dexamethasone for relapsed MM after previous chemotherapy with bortezomib-melphalan-prednisolone and lenalidomide-dexamethasone |
Escherichia coli in blood culture Chest computed tomography (CT) showed incidental intraosseous gas in her sternum and T6 vertebra, suggesting emphysematous osteomyelitis |
Meropenem and supportive treatment The patient recovered and was discharged 20 days later. |
| Webber et al. (2017) [93] | 25, Male | Femoral pyomyositis, hypercalcemia, mild anaemia, and elevated inflammatory markers. Diagnosed with IgG kappa multiple myeloma. |
Streptococcus pneumoniae Femoral pyomyositis |
Received zoledronic acid therapy for hypercalcemia. Completed a 4-week course of IV ceftriaxone.Received bortezomib-lenalidomide-dexamethasone (VRd) therapy and autologous bone marrow transplant. One year later, the swelling and the leg/thigh pain had resolved. |
| Cohen et al. (2019) [94] | 56, Male | Undiagnosed multiple myeloma with severe sepsis associated with pneumonia, meningitis, polyarthritis, and osteomyelitis | Haemophilus quentini | Not determined. |
| Sassine, et al. (2021) [95] | 77, Male | Had multiple myeloma treated with lenalidomide, developed a slowly progressive right upper thigh pain with no antecedent trauma or known history of osteolytic lesions. PET-CT showed a right proximal femoral diaphysis lesion with cortical destruction and intensely avid FDG uptake. |
Tissue cultures positive for Cryptococcus neoformans. Bone histology was consistent with cryptococcosis. Serum positive for cryptococcal antigen. |
Received intravenous liposomal amphotericin B (5 mg/kg daily) for one week and was discharged on a high dose (800 mg/d) of oral fluconazole. Had nailing of the femur to prevent fracture, following a switch to oral voriconazole (300 mg twice daily) for three months. |
| Roque et al. (2021) [96] | 57, Male | Had lumbar pain, paraplegia, and fever. Diagnosed with spondylodiscitis. MRI identified a mass extending from D9 to the vertebral canal with numerous adjacent osteolytic lesions. A plasmacytoma was confirmed by C9′s biopsy. |
B. melitensis | A short course of radiation therapy and high-dose corticosteroids were used to treat the patient. |
Various bacterial pathogens have been reported to cause osteomyelitis in multiple myeloma patients, including Staphylococcus aureus and coagulase-negative Staphylococci [89], Streptococcus pneumoniae [89], [93], Escherichia coli [90], [92], Haemophilus quentini [94]. Most reported infections were associated with vertebra, particularly in the lumbar region.
Desikan et al. (2003) reported three cases of spondylodiscitis and epidural abscesses in MM patients. Staphylococci and Streptococci were found in culture [89]. One 72-year-old male patient received discectomy and corpectomy of C4-C5 vertebrae along with arthrodesis of the C3-C6 vertebrae. Another 56-year-old male patient received a partial laminectomy of L4-L5 to evacuate the epidural abscess, and then received intravenous vancomycin for six weeks with no recurrence of vertebral infections. The third case, a 61-year-old male patient had spondylodiscitis of the L5-S1 disc space. The patient received intravenous vancomycin for one month and had no recurrence of pain. Evaluations were normal for the next three years.
Roque et al. (2021) reported a 57-year-old man with lumbar pain, paraplegia and fever was diagnosed with spondylodiscitis. MRI identified a mass extending from the intervertebral disc D9 to the vertebral canal with a numerous adjacent osteolytic lesion. A plasmacytoma was later confirmed by a biopsy of D9, suggesting skeleton osteolytic lesions caused by multiple myeloma [96]. In the end, a short course of radiation therapy and high-dose corticosteroids were used to treat the patient.
Yu et al. (2010) revealed a case of a 57-year-old female patient with IgG kappa gammopathy with tenderness and knocking pain over the lumbar area (L2) [90]. Blood culture later confirmed an E. coli infection and a CT-guided biopsy at the L2 vertebra confirmed infectious spondylitis. The patient was first treated with intravenous oxacillin, and then switched to cefazolin and cefuroxime. The patient was free of recurrent back pain and fever six months after, and was subsequently treated with melphalan, dexamethasone and thalidomide for MM [90]. Park et al. (2016) also reported a 74-year-old female patient with Fever and diffuse abdominal pain and septic shock [92]. Chest CT showed emphysematous osteomyelitis on her T6 vertebra, and blood culture was positive for E. coli.
Although most MM patients with osteomyelitis were above 50 years of age, osteomyelitis sometimes occurred in younger patients. Webber et al. (2017) reported a 25-year-old male patient with femoral pyomyositis, hypercalcemia, mild anemia [93]. The patient was later diagnosed with IgG kappa multiple myeloma, and Streptococcus pneumoniae was identified. The patient received zoledronate for hypercalcemia and completed a four-week course of IV ceftriaxone for the infection. He also received bortezomib-lenalidomide-dexamethasone therapy and autologous bone marrow transplant for MM. One year later, the swelling and the pain were resolved.
Apart from bacteria, other invasive fungal pathogens can also cause bone infections in MM patients. Mohan et al. (2016) and Sassine et al. (2021) reported two individual cases of osteomyelitis associated with Lasiodiplodia and Cryptococcus neoformans in MM patients [91], [95]. A 69-year-old male patient had relapsed refractory MM and was admitted for chemotherapy and ASCT was infected with Lasiodiplodia. In the end, the infection was treated with amputation and antifungal medications (oral voriconazole alone after 14 days of liposomal amphotericin B). In the other case, a 77-year-old male patient had multiple myeloma treated with lenalidomide developed a slowly progressive right upper thigh pain with no antecedent trauma or known history of osteolytic lesions. Tissue cultures and bone histology later identified Cryptococcosis. The patient had then received intravenous liposomal amphotericin B (5 mg/kg daily) for one week and was discharged on a high dose (800 mg/day) of oral fluconazole. Nailing of the femur was conducted to prevent fracture and following a switch to oral voriconazole (300 mg twice daily) for three months.
Despite the high patient impact of these complications, it must be recognized that only ten published case reports of osteomyelitis associated with MBD were identified and most were single cases only. Moreover, many did not fully address the management or outcomes. In the absence of unbiased clinical data longitudinally tracking infection comorbid with MBD it is not possible to gauge the relative infection risk associated with osteolytic lesions.
6. Discussion
Multiple myeloma is a malignant tumor of plasma cells that involves the bone marrow and can cause severe lytic bone damage in the axial skeleton and pelvis. Primary bone tumors and lesions are commonly found in the spine, pelvis, skull, sternum, and ribs [97]. Skeletal complications caused by multiple myeloma are associated with considerable pain in patients, increased mortality, and low quality of life.
Infection is the leading cause of morbidity and mortality in patients with multiple myeloma (MM) [98]. Although osteomyelitis is not the most common form of infections among MM patients, osteolytic bone lesions and profound immunodeficiency can increase the risk of developing bone infections in immunosuppressive MM patients. In severe cases, infection results in life-threatening complications, including bacteremia, organ failures, septic shock, or even death [99]. While bone infections are manageable in most reported cases with broad spectrum antibiotics, prolonged hospitalization, antibiotic treatment, and additional surgeries could significantly increase the burden of disease and severely affect the prognosis with increased mortality. Also, drug-resistant bacterial infection has been reported [91], and can potentially be a major threat to MM patients in the future.
Subsequently, clinical trials focusing on antimicrobial prophylaxis in multiple myeloma are limited, and the data are inconsistent. Oken et al. (1996) showed that administering trimethoprim-sulfamethoxazole (TMP-SMX) for the first two months of initial chemotherapy is effective, inexpensive prophylaxis for early bacterial infection in multiple myeloma [100]. However, Vesole et al. (2012) contradictorily reported that prophylactic treatment with TMP-SMX and Ciprofloxacin (500 mg) or Ofloxacin (400 mg) did not decrease the incidence of serious bacterial infections (⩾grade 3) within the first two months of treatment. Later, the clinical trials of Drayson et al. (2019) tested the addition of prophylactic levofloxacin to active myeloma treatment during the first 12 weeks of therapy, and the results showed reduction of febrile episodes and mortality compared with the placebo group without increasing health care-associated infections, suggesting that prophylactic levofloxacin may be beneficial for patients with NDMM undergoing antimyeloma therapy [99]. However, none of the clinical trials focused on preventing osteomyelitis in MM patients. There is also a knowledge gap in the efficacy of prophylactic antimicrobial use for reducing morbidity and mortality of bone infections in MM patients.
Pain induced by vertebral fracture in multiple myeloma are common with additional causes such as spondylosis deformans, osteochondrosis, stenosis of the spinal canal and intervertebral nerve compression [101]. IV antibiotic and surgical interventions, such as discectomy, decompression, and debridement, are used to manage spondylodiscitis and epidural abscesses. Whereas amputation is used to manage osteonecrosis in the long bones [89], [93]. Multiple case reports have shown that the vertebra and vertebral discs are highly susceptible to infections in patients aged over 50 [89], [90], [96]. Therefore, future studies should focus on etiology of vertebral infections in MM patients to reduce disease burden, improve prognosis and prevent severe complications.
While fungal osteomyelitis is rarely encountered, it is often difficult to culture and diagnose. The clinical case reports highlighted that there were certain risks factors associated with immunocompromised patients and fungal osteomyelitis that can lead to severe consequences (e.g., amputation and length antifungal treatment) [91], [95]. The studies of fungal osteomyelitis in multiple myeloma patients are minority. Nevertheless, fungal pathogens should not be overlooked in MM patients when diagnosing osteomyelitis.
Multiple myeloma patients frequently develop tumor-induced bone destruction, but no therapy eliminates the tumor or fully reverses bone loss. Agents that prevent bone resorption like BPs and denosumab can effectively reduce the risk of skeletal-related events and myeloma bone disease in NDMM patients. Conventionally, zoledronate was widely prescribed as a prophylaxis to prevent local osteolytic lesions, but recent trials suggested that denosumab is a better alternative with high efficacy. However, cumulative doses of BPs and denosumab are associated with serious adverse reactions and medication-related osteonecrosis of the jaw (MRONJ) [102]. Therefore, administration and treatment must be carefully monitored, and dentist consultation is warranted.
On the other hand, Wnt signaling pathway such as Dkk-1 may be responsible for inhibiting osteoblast activities [103], [104]. Fulciniti, et al. (2009) identified anti-Dkk-1 monoclonal antibody (BHQ880) as a potential therapeutic agent for multiple myeloma. The pro-anabolic effect and anti-myeloma activity of anti-Dkk-1 neutralizing antibodies was determined in in vitro and in vivo preclinical trials [105], [106], [107]. Later, a phase 1B clinical trial showed that BHQ880 in combination with zoledronate and anti-myeloma therapy was well tolerated and may be eligible for patients with relapsed or refractory multiple myeloma [108].
Furthermore, studies show that sclerostin, a glycoprotein that is exclusively secreted by osteocytes, is also involved in the regulation of bone metabolism. It affects the activity of BMPs and inhibits Wnt/β-catenin metabolic pathway in bone cells [86]. Sclerostin is also an early marker of relapse in multiple myeloma and can be targeted for therapies [83], [84]. In preclinical models, the deletion of SOST gene (encoding sclerostin) prevented MM-induced bone disease, and the administration of anti-sclerostin antibody (Scl-Ab) increased bone mass and decreases osteolysis in immune-competent mice with established MM [85]. Subsequently, treatment with anti-sclerostin antibody combined with zoledronic acid also displayed higher bone mass and fracture resistance than zoledronic acid alone [87]. The combination therapy of anti-sclerostin antibody and the proteasome inhibitor carfilzomib also show potent anti-myeloma activity with positive effects on bone disease [109].
Preclinical trials also indicated that transforming growth factor-β (TGF-β) inhibition can induce the repair of lytic lesions in mice bearing myeloma [110]. Such findings have raised the clinical potential for combined therapy with bortezomib/1D11 with zoledronate [111], [112], [113]. Nyman et al. (2016) found that although monotherapy with TGF-β inhibitors is unlikely to be beneficial, a combined therapy of 1D11 (an anti-TGF-β antibody inhibiting TGF-β signaling) with bortezomib (a proteasome inhibitor) can reduce bone destruction and pathological fractures in MM patients [114]. Substantial clinical trials should continue to optimize its efficacy and establish clinical guidelines for this therapy.
Although bone morphogenetic protein (BMP) signaling was not reported to be dysregulated in myeloma bone disease previously, a study found that BMP upregulated signaling in stromal progenitor cells [88], and the in vivo murine model later confirmed that inhibiting BMP signaling using a small molecule BMP receptor antagonist or a BMPR1a-FC receptor ligand trap could prevent trabecular and cortical bone loss caused by myeloma without increasing the tumor burden. It was hypothesized that BMP inhibition can directly reduce osteoclastogenesis, increase osteoblasts and bone formation and suppress bone marrow sclerostin levels. This study highlighted the possibility of targeting the BMP pathway to prevent myeloma-induced bone disease.
Finally, there are many other factors released by bone resorption further promote MM cell growth perpetuating the vicious cycle of malignant cell expansion and bone destruction. For example, the receptor activator of NF-κB ligand (RANKL), may influence osteoclast activation [115], [116]. Serum parathyroid hormone (PTH) level was also found to be associated with risk factors and clinical outcome in MM patients with extensive bone disease [117]. As primary hyperparathyroidism (PHPT) is the most common cause of non-neoplastic hypercalcemia in MM patients, it was suspected that high secretion of PTH may have a negative impact on MM associated bone disease and MM progression [118]. Future trials and continuous studies investigating these pathological pathways may be critical for finding novel interventions to treat myeloma bone diseases.
7. Conclusion
Immunocompromised multiple myeloma patients with bone defects are susceptible to fractures and substantial osteomyelitis, increasing mortality and the burden of disease. For antiresorptive agents, denosumab has advantages over conventional zoledronate therapy in preventing myeloma bone disease, particularly in some patient subgroups. Chemotherapy agents like lenalidomide and carfilzomib do not reduce bone pain and osteolytic lesions, whereas a robust anti-myeloma agent like bortezomib can reduce the tumor area, and the anti-TGF-β antibody 1D11 can improve bone repair and bone quality in multiple myeloma. Emerging clinical data suggest that trimethoprim-sulfamethoxazole and levofloxacin can be used as a prophylaxis for bone infections, although further clinical trials are needed. Despite these advances, MM remains incurable, and patients continue to suffer from bone lesions and fractures. While bone infection is not the most common complication of MBD, persistent local osteolytic lesions possess an underlying risk of progression to osteomyelitis. Indeed, the lack of studies testing the connection between MBD and bone infection represent an opportunity to undertake retrospective reviews of the clinical data and prospective trials. This may identify a need for new prophylaxis strategies to prevent bone infection as well as improved clinical guidelines for the treatment of infected bone lesions.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Neuse C.J., Lomas O.C., Schliemann C., et al. Genome instability in multiple myeloma. Leukemia. 2020;34(11):2887–2897. doi: 10.1038/s41375-020-0921-y. [DOI] [PubMed] [Google Scholar]
- 2.Shah U.A., Mailankody S. Emerging immunotherapies in multiple myeloma. Bmj. 2020;370 doi: 10.1136/bmj.m3176. [DOI] [PubMed] [Google Scholar]
- 3.Terpos E., Raje N., Croucher P., et al. Denosumab compared with zoledronic acid on PFS in multiple myeloma: exploratory results of an international phase 3 study. Blood Adv. 2021;5(3):725–736. doi: 10.1182/bloodadvances.2020002378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson P.G., Laubach J.P., Schlossman R.L., et al. The Medical Research Council Myeloma IX trial: the impact on treatment paradigms. Eur J Haematol. 2012;88(1):1–7. doi: 10.1111/j.1600-0609.2011.01721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van de Donk N.W.C.J., Pawlyn C., Yong K.L. Multiple myeloma. The Lancet. 2021;397(10272):410–427. doi: 10.1016/S0140-6736(21)00135-5. [DOI] [PubMed] [Google Scholar]
- 6.Gay F., Palumbo A. Management of disease- and treatment-related complications in patients with multiple myeloma. Med Oncol. 2010;27(Suppl 1):S43–S52. doi: 10.1007/s12032-010-9542-z. [DOI] [PubMed] [Google Scholar]
- 7.Rajkumar S.V. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am J Hematol. 2020;95(5):548–567. doi: 10.1002/ajh.25791. [DOI] [PubMed] [Google Scholar]
- 8.Landgren O., Rajkumar S.V. New Developments in Diagnosis, Prognosis, and Assessment of Response in Multiple Myeloma. Clin Cancer Res. 2016;22(22):5428–5433. doi: 10.1158/1078-0432.CCR-16-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palumbo A., Avet-Loiseau H., Oliva S., et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J Clin Oncol. 2015;33(26):2863–2869. doi: 10.1200/JCO.2015.61.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowan A.J., Green D.J., Kwok M., et al. Diagnosis and Management of Multiple Myeloma: A Review. Jama. 2022;327(5):464–477. doi: 10.1001/jama.2022.0003. [DOI] [PubMed] [Google Scholar]
- 11.Rajkumar S.V. Treatment of multiple myeloma. Nat Rev Clin Oncol. 2011;8(8):479–491. doi: 10.1038/nrclinonc.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajkumar S.V. Multiple myeloma: Every year a new standard? Hematol Oncol. 2019;37 Suppl 1(Suppl 1):62–65. doi: 10.1002/hon.2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al Hamed R., Bazarbachi A.H., Malard F., Harousseau J.L., Mohty M. Current status of autologous stem cell transplantation for multiple myeloma. Blood Cancer J. 2019;9(4):44. doi: 10.1038/s41408-019-0205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ricciuti G., Falcone A., Cascavilla N., Martinelli G., Cerchione C. Autologous stem cell transplantation in multiple myeloma. Panminerva Med. 2020;62(4):220–224. doi: 10.23736/S0031-0808.20.04114-2. [DOI] [PubMed] [Google Scholar]
- 15.Rajkumar S.V., Jacobus S., Callander N.S., et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. 2010;11(1):29–37. doi: 10.1016/S1470-2045(09)70284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singhal S., Mehta J., Desikan R., et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341(21):1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 17.Dimopoulos M., Spencer A., Attal M., et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357(21):2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 18.Richardson P.G., Barlogie B., Berenson J., et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348(26):2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 19.Eda H., Santo L., David Roodman G., Raje N. Bone Disease in Multiple Myeloma. Cancer treatment and research. 2016;169:251–270. doi: 10.1007/978-3-319-40320-5_14. [DOI] [PubMed] [Google Scholar]
- 20.Ntanasis-Stathopoulos I., Gavriatopoulou M., Kastritis E., Terpos E., Dimopoulos M.A. Multiple myeloma: Role of autologous transplantation. Cancer Treat Rev. 2020;82 doi: 10.1016/j.ctrv.2019.101929. [DOI] [PubMed] [Google Scholar]
- 21.Choi T. Is autologous stem cell transplantation still relevant for multiple myeloma? Curr Opin Hematol. 2019;26(6):386–391. doi: 10.1097/MOH.0000000000000538. [DOI] [PubMed] [Google Scholar]
- 22.Parrondo R.D., Ailawadhi S., Sher T., Chanan-Khan A.A., Roy V. Autologous Stem-Cell Transplantation for Multiple Myeloma in the Era of Novel Therapies. JCO Oncol Pract. 2020;16(2):56–66. doi: 10.1200/JOP.19.00335. [DOI] [PubMed] [Google Scholar]
- 23.Gentile M., Morabito F., Martino M., et al. Chemotherapy-based regimens in multiple myeloma in 2020. Panminerva Med. 2021;63(1):7–12. doi: 10.23736/S0031-0808.20.04145-2. [DOI] [PubMed] [Google Scholar]
- 24.Petrucci M.T., Vozella F. The Anti-CD38 Antibody Therapy in Multiple Myeloma. Cells. 2019;8(12) doi: 10.3390/cells8121629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cavallo F., Boccadoro M., Palumbo A. Review of thalidomide in the treatment of newly diagnosed multiple myeloma. Ther Clin Risk Manag. 2007;3(4):543–552. [PMC free article] [PubMed] [Google Scholar]
- 26.Cavo M., Zamagni E., Tosi P., et al. First-line therapy with thalidomide and dexamethasone in preparation for autologous stem cell transplantation for multiple myeloma. Haematologica. 2004;89(7):826–831. [PubMed] [Google Scholar]
- 27.Dimopoulos M., Quach H., Mateos M.V., et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open-label, phase 3 study. Lancet. 2020;396(10245):186–197. doi: 10.1016/S0140-6736(20)30734-0. [DOI] [PubMed] [Google Scholar]
- 28.Attal M., Richardson P.G., Rajkumar S.V., et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet. 2019;394(10214):2096–2107. doi: 10.1016/S0140-6736(19)32556-5. [DOI] [PubMed] [Google Scholar]
- 29.Voorhees P.M., Kaufman J.L., Laubach J., et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood. 2020;136(8):936–945. doi: 10.1182/blood.2020005288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mateos M.V., Bladé J., Bringhen S., et al. Melflufen: A Peptide-Drug Conjugate for the Treatment of Multiple Myeloma. J Clin Med. 2020;9(10) doi: 10.3390/jcm9103120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zweegman S., van der Holt B., Mellqvist U.H., et al. Melphalan, prednisone, and lenalidomide versus melphalan, prednisone, and thalidomide in untreated multiple myeloma. Blood. 2016;127(9):1109–1116. doi: 10.1182/blood-2015-11-679415. [DOI] [PubMed] [Google Scholar]
- 32.Weisel K., Spencer A., Lentzsch S., et al. Daratumumab, bortezomib, and dexamethasone in relapsed or refractory multiple myeloma: subgroup analysis of CASTOR based on cytogenetic risk. J Hematol Oncol. 2020;13(1):115. doi: 10.1186/s13045-020-00948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raje N., Terpos E., Willenbacher W., et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: an international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018;19(3):370–381. doi: 10.1016/S1470-2045(18)30072-X. [DOI] [PubMed] [Google Scholar]
- 34.Sonmez M., Akagun T., Topbas M., et al. Effect of pathologic fractures on survival in multiple myeloma patients: a case control study. J Exp Clin Cancer Res. 2008;27(1):11. doi: 10.1186/1756-9966-27-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panaroni C., Yee A.J., Raje N.S. Myeloma and Bone Disease. Current osteoporosis reports. 2017;15(5):483–498. doi: 10.1007/s11914-017-0397-5. [DOI] [PubMed] [Google Scholar]
- 36.Yeh H.S., Berenson J.R. Treatment for myeloma bone disease. Clin Cancer Res. 2006;12(20 Pt 2):6279s–s6284. doi: 10.1158/1078-0432.CCR-06-0681. [DOI] [PubMed] [Google Scholar]
- 37.Hjertner Ø., Standal T., Børset M., Sundan A., Waage A. Bone disease in multiple myeloma. Med Oncol. 2006;23(4):431–441. doi: 10.1385/mo:23:4:431. [DOI] [PubMed] [Google Scholar]
- 38.Terpos E., Ntanasis-Stathopoulos I., Dimopoulos M.A. Myeloma bone disease: from biology findings to treatment approaches. Blood. 2019;133(14):1534–1539. doi: 10.1182/blood-2018-11-852459. [DOI] [PubMed] [Google Scholar]
- 39.Mai E.K., Miah K., Bertsch U., et al. Bortezomib-based induction, high-dose melphalan and lenalidomide maintenance in myeloma up to 70 years of age. Leukemia. 2021;35(3):809–822. doi: 10.1038/s41375-020-0976-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moulopoulos L.A., Koutoulidis V., Hillengass J., et al. Recommendations for acquisition, interpretation and reporting of whole body low dose CT in patients with multiple myeloma and other plasma cell disorders: a report of the IMWG Bone Working Group. Blood Cancer Journal. 2018;8(10):95. doi: 10.1038/s41408-018-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Treitl K.M., Ricke J., Baur-Melnyk A. Whole-body magnetic resonance imaging (WBMRI) versus whole-body computed tomography (WBCT) for myeloma imaging and staging. Skeletal Radiology. 2021 doi: 10.1007/s00256-021-03799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dimopoulos M., Terpos E., Comenzo R.L., et al. International myeloma working group consensus statement and guidelines regarding the current role of imaging techniques in the diagnosis and monitoring of multiple Myeloma. Leukemia. 2009;23(9):1545–1556. doi: 10.1038/leu.2009.89. [DOI] [PubMed] [Google Scholar]
- 43.Mateos M.V., Fink L., Koneswaran N., et al. Bone complications in patients with multiple myeloma in five European countries: a retrospective patient chart review. BMC cancer. 2020;20(1):170. doi: 10.1186/s12885-020-6596-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miceli T.S., Colson K., Faiman B.M., Miller K., Tariman J.D. Maintaining bone health in patients with multiple myeloma: survivorship care plan of the International Myeloma Foundation Nurse Leadership Board. Clin J Oncol Nurs. 2011;15 Suppl(0):9–23. doi: 10.1188/11.S1.CJON.9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valentin-Opran A., Charhon S.A., Meunier P.J., Edouard C.M., Arlot M.E. Quantitative histology of myeloma-induced bone changes. British journal of haematology. 1982;52(4):601–610. doi: 10.1111/j.1365-2141.1982.tb03936.x. [DOI] [PubMed] [Google Scholar]
- 46.Kovacic N., Croucher P.I., McDonald M.M. Signaling between tumor cells and the host bone marrow microenvironment. Calcified tissue international. 2014;94(1):125–139. doi: 10.1007/s00223-013-9794-7. [DOI] [PubMed] [Google Scholar]
- 47.Croucher P.I., McDonald M.M., Martin T.J. Bone metastasis: the importance of the neighbourhood. Nat Rev Cancer. 2016;16(6):373–386. doi: 10.1038/nrc.2016.44. [DOI] [PubMed] [Google Scholar]
- 48.Weilbaecher K.N., Guise T.A., McCauley L.K. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11(6):411–425. doi: 10.1038/nrc3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tella S.H., Gallagher J.C. Prevention and treatment of postmenopausal osteoporosis. The Journal of steroid biochemistry and molecular biology. 2014;142:155–170. doi: 10.1016/j.jsbmb.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deeks E.D. Denosumab: A Review in Postmenopausal Osteoporosis. Drugs & aging. 2018;35(2):163–173. doi: 10.1007/s40266-018-0525-7. [DOI] [PubMed] [Google Scholar]
- 51.Kaiser M.F., Heider U., Mieth M., Zang C., von Metzler I., Sezer O. The proteasome inhibitor bortezomib stimulates osteoblastic differentiation of human osteoblast precursors via upregulation of vitamin D receptor signalling. Eur J Haematol. 2013;90(4):263–272. doi: 10.1111/ejh.12069. [DOI] [PubMed] [Google Scholar]
- 52.Guzik G. Oncological and functional results of the surgical treatment of vertebral metastases in patients with multiple myeloma“. BMC Surg. 2017;17(1):92. doi: 10.1186/s12893-017-0288-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Utzschneider S., Schmidt H., Weber P., Schmidt G.P., Jansson V., Dürr H.R. Surgical therapy of skeletal complications in multiple myeloma. Int Orthop. 2011;35(8):1209–1213. doi: 10.1007/s00264-010-1127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barlogie B., van Rhee F., Shaughnessy J.D., Jr, et al. Seven-year median time to progression with thalidomide for smoldering myeloma: partial response identifies subset requiring earlier salvage therapy for symptomatic disease. Blood. 2008;112(8):3122–3125. doi: 10.1182/blood-2008-06-164228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gimsing P., Carlson K., Turesson I., et al. Effect of pamidronate 30 mg versus 90 mg on physical function in patients with newly diagnosed multiple myeloma (Nordic Myeloma Study Group): a double-blind, randomised controlled trial. Lancet Oncol. 2010;11(10):973–982. doi: 10.1016/S1470-2045(10)70198-4. [DOI] [PubMed] [Google Scholar]
- 56.Morgan G.J., Davies F.E., Gregory W.M., et al. Effects of induction and maintenance plus long-term bisphosphonates on bone disease in patients with multiple myeloma: the Medical Research Council Myeloma IX Trial. Blood. 2012;119(23):5374–5383. doi: 10.1182/blood-2011-11-392522. [DOI] [PubMed] [Google Scholar]
- 57.Witzig T.E., Laumann K.M., Lacy M.Q., et al. A phase III randomized trial of thalidomide plus zoledronic acid versus zoledronic acid alone in patients with asymptomatic multiple myeloma. Leukemia. 2013;27(1):220–225. doi: 10.1038/leu.2012.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miguel J.S., Weisel K., Moreau P., et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(11):1055–1066. doi: 10.1016/S1470-2045(13)70380-2. [DOI] [PubMed] [Google Scholar]
- 59.García-Sanz R., Oriol A., Moreno M.J., et al. Zoledronic acid as compared with observation in multiple myeloma patients at biochemical relapse: results of the randomized AZABACHE Spanish trial. Haematologica. 2015;100(9):1207–1213. doi: 10.3324/haematol.2015.128439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diel I.J., Body J.J., Stopeck A.T., et al. The role of denosumab in the prevention of hypercalcaemia of malignancy in cancer patients with metastatic bone disease. Eur J Cancer. 2015;51(11):1467–1475. doi: 10.1016/j.ejca.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 61.Moreau P., Mateos M.V., Berenson J.R., et al. Once weekly versus twice weekly carfilzomib dosing in patients with relapsed and refractory multiple myeloma (A.R.R.O.W.): interim analysis results of a randomised, phase 3 study. Lancet Oncol. 2018;19(7):953–964. doi: 10.1016/S1470-2045(18)30354-1. [DOI] [PubMed] [Google Scholar]
- 62.Terpos E., Kastritis E., Ntanasis-Stathopoulos I., et al. Consolidation therapy with the combination of bortezomib and lenalidomide (VR) without dexamethasone in multiple myeloma patients after transplant: Effects on survival and bone outcomes in the absence of bisphosphonates. Am J Hematol. 2019;94(4):400–407. doi: 10.1002/ajh.25392. [DOI] [PubMed] [Google Scholar]
- 63.Fazzi R., Petrini I., Giuliani N., et al. Phase II Trial of Maintenance Treatment With IL2 and Zoledronate in Multiple Myeloma After Bone Marrow Transplantation: Biological and Clinical Results. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.573156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang S.Y., Yoon S.S., Shimizu K., et al. Denosumab Versus Zoledronic Acid in Bone Disease Treatment of Newly Diagnosed Multiple Myeloma: An International, Double-Blind, Randomized Controlled Phase 3 Study-Asian Subgroup Analysis. Adv Ther. 2020;37(7):3404–3416. doi: 10.1007/s12325-020-01395-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diamond B., Korde N., Lesokhin A.M., et al. Dynamics of minimal residual disease in patients with multiple myeloma on continuous lenalidomide maintenance: a single-arm, single-centre, phase 2 trial. Lancet Haematol. 2021;8(6):e422–e432. doi: 10.1016/S2352-3026(21)00130-7. [DOI] [PubMed] [Google Scholar]
- 66.Mbese Z., Aderibigbe B.A. Bisphosphonate-Based Conjugates and Derivatives as Potential Therapeutic Agents in Osteoporosis, Bone Cancer and Metastatic Bone Cancer. International journal of molecular sciences. 2021;22(13) doi: 10.3390/ijms22136869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee O.L., Horvath N., Lee C., et al. Bisphosphonate guidelines for treatment and prevention of myeloma bone disease. Internal medicine journal. 2017;47(8):938–951. doi: 10.1111/imj.13502. [DOI] [PubMed] [Google Scholar]
- 68.Alegre A., Gironella M., Bailén A., Giraldo P. Zoledronic acid in the management of bone disease as a consequence of multiple myeloma: a review. Eur J Haematol. 2014;92(3):181–188. doi: 10.1111/ejh.12239. [DOI] [PubMed] [Google Scholar]
- 69.Guzdar A., Costello C. Supportive Care in Multiple Myeloma. Curr Hematol Malig Rep. 2020;15(2):56–61. doi: 10.1007/s11899-020-00570-9. [DOI] [PubMed] [Google Scholar]
- 70.Snowden J.A., Ahmedzai S.H., Ashcroft J., et al. Guidelines for supportive care in multiple myeloma 2011. British journal of haematology. 2011;154(1):76–103. doi: 10.1111/j.1365-2141.2011.08574.x. [DOI] [PubMed] [Google Scholar]
- 71.Lahtinen R., Laakso M., Palva I., Virkkunen P., Elomaa I. Randomised, placebo-controlled multicentre trial of clodronate in multiple myeloma. Finnish Leukaemia Group. Lancet. 1992;340(8827):1049–1052. doi: 10.1016/0140-6736(92)93075-x. [DOI] [PubMed] [Google Scholar]
- 72.Terpos E., Morgan G., Dimopoulos M.A., et al. International Myeloma Working Group Recommendations for the Treatment of Multiple Myeloma-Related Bone Disease. Journal of Clinical Oncology. 2013;31(18):2347–2357. doi: 10.1200/JCO.2012.47.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Robinson D.E., Ali M.S., Pallares N., et al. Safety of Oral Bisphosphonates in Moderate-to-Severe Chronic Kidney Disease: A Binational Cohort Analysis. J Bone Miner Res. 2021;36(5):820–832. doi: 10.1002/jbmr.4235. [DOI] [PubMed] [Google Scholar]
- 74.Zamagni E., Cavo M., Fakhri B., Vij R., Roodman D. Bones in Multiple Myeloma: Imaging and Therapy. American Society of Clinical Oncology Educational Book. 2018;38:638–646. doi: 10.1200/EDBK_205583. [DOI] [PubMed] [Google Scholar]
- 75.Rasch S., Lund T., Asmussen J.T., et al. Multiple Myeloma Associated Bone Disease. Cancers (Basel) 2020;12:8. doi: 10.3390/cancers12082113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anastasilakis A.D., Makras P., Yavropoulou M.P., Tabacco G., Naciu A.M., Palermo A. Denosumab Discontinuation and the Rebound Phenomenon: A Narrative Review. Journal of Clinical Medicine. 2021;10(1):152. doi: 10.3390/jcm10010152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Piechotta V., Jakob T., Langer P., et al. Multiple drug combinations of bortezomib, lenalidomide, and thalidomide for first-line treatment in adults with transplant-ineligible multiple myeloma: a network meta-analysis. Cochrane Database Syst Rev. 2019;2019(11) doi: 10.1002/14651858.CD013487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mohty M., Malard F., Mohty B., Savani B., Moreau P., Terpos E. The effects of bortezomib on bone disease in patients with multiple myeloma. Cancer. 2014;120(5):618–623. doi: 10.1002/cncr.28481. [DOI] [PubMed] [Google Scholar]
- 79.Jayaweera S.P.E., Wanigasinghe Kanakanamge S.P., Rajalingam D., Silva G.N. Carfilzomib: A Promising Proteasome Inhibitor for the Treatment of Relapsed and Refractory Multiple Myeloma. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.740796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roussel M, Lauwers-Cances V, Robillard N, et al. Frontline Therapy with Carfilzomib, Lenalidomide, and Dexamethasone (KRd) Induction Followed By Autologous Stem Cell Transplantation, Krd Consolidation and Lenalidomide Maintenance in Newly Diagnosed Multiple Myeloma (NDMM) Patients: Primary Results of the Intergroupe Francophone Du MyéLome (IFM) Krd Phase II Study. Blood 2016; 128: 1142-.
- 81.Zimmerman T, Raje N, Vij R, et al. Final Results of a Phase 2 Trial of Extended Treatment (tx) with Carfilzomib (CFZ), Lenalidomide (LEN), and Dexamethasone (KRd) Plus Autologous Stem Cell Transplantation (ASCT) in Newly Diagnosed Multiple Myeloma (NDMM). Blood 2016; 128: 675-.
- 82.Jasielec J.K., Kubicki T., Raje N., et al. Carfilzomib, lenalidomide, and dexamethasone plus transplant in newly diagnosed multiple myeloma. Blood. 2020;136(22):2513–2523. doi: 10.1182/blood.2020007522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Toscani D., Bolzoni M., Ferretti M., Palumbo C., Giuliani N. Role of Osteocytes in Myeloma Bone Disease: Anti-sclerostin Antibody as New Therapeutic Strategy. Front Immunol. 2018;9:2467. doi: 10.3389/fimmu.2018.02467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McDonald M.M., Delgado-Calle J. Sclerostin: an Emerging Target for the Treatment of Cancer-Induced Bone Disease. Current osteoporosis reports. 2017;15(6):532–541. doi: 10.1007/s11914-017-0403-y. [DOI] [PubMed] [Google Scholar]
- 85.Delgado-Calle J., Anderson J., Cregor M.D., et al. Genetic deletion of Sost or pharmacological inhibition of sclerostin prevent multiple myeloma-induced bone disease without affecting tumor growth. Leukemia. 2017;31(12):2686–2694. doi: 10.1038/leu.2017.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Delgado-Calle J., Sato A.Y., Bellido T. Role and mechanism of action of sclerostin in bone. Bone. 2017;96:29–37. doi: 10.1016/j.bone.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McDonald M.M., Reagan M.R., Youlten S.E., et al. Inhibiting the osteocyte-specific protein sclerostin increases bone mass and fracture resistance in multiple myeloma. Blood. 2017;129(26):3452–3464. doi: 10.1182/blood-2017-03-773341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gooding S., Olechnowicz S.W.Z., Morris E.V., et al. Transcriptomic profiling of the myeloma bone-lining niche reveals BMP signalling inhibition to improve bone disease. Nat Commun. 2019;10(1):4533. doi: 10.1038/s41467-019-12296-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Desikan R., Barlogie B., Sethi R., et al. Infection–an underappreciated cause of bone pain in multiple myeloma. British journal of haematology. 2003;120(6):1047–1050. doi: 10.1046/j.1365-2141.2003.04178.x. [DOI] [PubMed] [Google Scholar]
- 90.Yu S.F., Lui C.C., Pei S.N., Liu J.W., Cheng T.T. Unsuspected multiple myeloma presenting as Escherichia coli infectious spondylitis: a case report. Int J Rheum Dis. 2010;13(4):e55–e58. doi: 10.1111/j.1756-185X.2010.01558.x. [DOI] [PubMed] [Google Scholar]
- 91.Mohan M., Shalin S.C., Kothari A., Rico J.C., Caradine K., Burgess M. Lasiodiplodia species fungal osteomyelitis in a multiple myeloma patient. Transpl Infect Dis. 2016;18(5):761–764. doi: 10.1111/tid.12573. [DOI] [PubMed] [Google Scholar]
- 92.Park S.S., Lee S.E., Min C.K. Emphysematous osteomyelitis due to Escherichia coli in multiple myeloma. Blood Res. 2016;51(4):224. doi: 10.5045/br.2016.51.4.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Webber T., Lawlor M., Balach T. Streptococcus pneumoniae Osteomyelitis in a 25-Year-Old Man as the Initial Presentation of Multiple Myeloma: A Case Report. JBJS Case Connect. 2017;7(3):e72. doi: 10.2106/JBJS.CC.16.00272. [DOI] [PubMed] [Google Scholar]
- 94.Cohen R., Finn T., Babushkin F., et al. Disseminated “Haemophilus quentini” infection in a patient with multiple myeloma – A case report and review of the literature. Diagn Microbiol Infect Dis. 2019;94(3):293–296. doi: 10.1016/j.diagmicrobio.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 95.Sassine J., Kontoyiannis D.P. A 77-Year-Old Man with Multiple Myeloma and a Lytic Bone Lesion. Am J Med. 2021;134(7):860–862. doi: 10.1016/j.amjmed.2021.01.032. [DOI] [PubMed] [Google Scholar]
- 96.Roque R., Machado L., Flor D., Cunha F. Oligosecretory multiple myeloma: a devastating presentation of a difficult diagnosis. BMJ Case Rep. 2021;14(4) doi: 10.1136/bcr-2020-240404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yildirim M., Baykara M. Differentiation of Multiple Myeloma and Lytic Bone Metastases: Histogram Analysis. Journal of Computer Assisted Tomography. 2020;44(6):953–955. doi: 10.1097/RCT.0000000000001086. [DOI] [PubMed] [Google Scholar]
- 98.Kristinsson S.Y., Tang M., Pfeiffer R.M., et al. Monoclonal gammopathy of undetermined significance and risk of infections: a population-based study. Haematologica. 2012;97(6):854–858. doi: 10.3324/haematol.2011.054015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Drayson M.T., Bowcock S., Planche T., et al. Levofloxacin prophylaxis in patients with newly diagnosed myeloma (TEAMM): a multicentre, double-blind, placebo-controlled, randomised, phase 3 trial. The Lancet Oncology. 2019;20(12):1760–1772. doi: 10.1016/S1470-2045(19)30506-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Oken M.M., Pomeroy C., Weisdorf D., Bennett J.M. Prophylactic antibiotics for the prevention of early infection in multiple myeloma. Am J Med. 1996;100(6):624–628. doi: 10.1016/s0002-9343(95)00043-7. [DOI] [PubMed] [Google Scholar]
- 101.Kasperk C., Grafe I. Osteoplastic procedures for the treatment of vertebral complications in multiple myeloma patients. Recent Results Cancer Res. 2011;183:293–306. doi: 10.1007/978-3-540-85772-3_14. [DOI] [PubMed] [Google Scholar]
- 102.Nicolatou-Galitis O., Schiødt M., Mendes R.A., et al. Medication-related osteonecrosis of the jaw: definition and best practice for prevention, diagnosis, and treatment. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;127(2):117–135. doi: 10.1016/j.oooo.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 103.Heath D.J., Chantry A.D., Buckle C.H., et al. Inhibiting Dickkopf-1 (Dkk1) removes suppression of bone formation and prevents the development of osteolytic bone disease in multiple myeloma. J Bone Miner Res. 2009;24(3):425–436. doi: 10.1359/jbmr.081104. [DOI] [PubMed] [Google Scholar]
- 104.Zhou F., Meng S., Song H., Claret F.X. Dickkopf-1 is a key regulator of myeloma bone disease: opportunities and challenges for therapeutic intervention. Blood Rev. 2013;27(6):261–267. doi: 10.1016/j.blre.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pozzi S., Fulciniti M., Yan H., et al. In vivo and in vitro effects of a novel anti-Dkk1 neutralizing antibody in multiple myeloma. Bone. 2013;53(2):487–496. doi: 10.1016/j.bone.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fulciniti M., Tassone P., Hideshima T., et al. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood. 2009;114(2):371–379. doi: 10.1182/blood-2008-11-191577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yaccoby S., Ling W., Zhan F., Walker R., Barlogie B., Shaughnessy J.D., Jr. Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood. 2007;109(5):2106–2111. doi: 10.1182/blood-2006-09-047712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Iyer S.P., Beck J.T., Stewart A.K., et al. A Phase IB multicentre dose-determination study of BHQ880 in combination with anti-myeloma therapy and zoledronic acid in patients with relapsed or refractory multiple myeloma and prior skeletal-related events. British journal of haematology. 2014;167(3):366–375. doi: 10.1111/bjh.13056. [DOI] [PubMed] [Google Scholar]
- 109.Eda H., Santo L., Wein M.N., et al. Regulation of Sclerostin Expression in Multiple Myeloma by Dkk-1: A Potential Therapeutic Strategy for Myeloma Bone Disease. J Bone Miner Res. 2016;31(6):1225–1234. doi: 10.1002/jbmr.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yin J.J., Selander K., Chirgwin J.M., et al. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest. 1999;103(2):197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Paton-Hough J., Tazzyman S., Evans H., et al. Preventing and Repairing Myeloma Bone Disease by Combining Conventional Antiresorptive Treatment With a Bone Anabolic Agent in Murine Models. J Bone Miner Res. 2019;34(5):783–796. doi: 10.1002/jbmr.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kouroukis T.C., Baldassarre F.G., Haynes A.E., Imrie K., Reece D.E., Cheung M.C. Bortezomib in multiple myeloma: systematic review and clinical considerations. Curr Oncol. 2014;21(4):e573–e603. doi: 10.3747/co.21.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Field-Smith A., Morgan G.J., Davies F.E. Bortezomib (Velcadetrade mark) in the Treatment of Multiple Myeloma. Ther Clin Risk Manag. 2006;2(3):271–279. doi: 10.2147/tcrm.2006.2.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nyman J.S., Merkel A.R., Uppuganti S., et al. Combined treatment with a transforming growth factor beta inhibitor (1D11) and bortezomib improves bone architecture in a mouse model of myeloma-induced bone disease. Bone. 2016;91:81–91. doi: 10.1016/j.bone.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Roux S., Mariette X. The high rate of bone resorption in multiple myeloma is due to RANK (receptor activator of nuclear factor-kappaB) and RANK Ligand expression. Leuk Lymphoma. 2004;45(6):1111–1118. doi: 10.1080/10428194310001593193. [DOI] [PubMed] [Google Scholar]
- 116.Giuliani N., Colla S., Rizzoli V. New insight in the mechanism of osteoclast activation and formation in multiple myeloma: focus on the receptor activator of NF-kappaB ligand (RANKL) Exp Hematol. 2004;32(8):685–691. doi: 10.1016/j.exphem.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 117.Kang M.G., Won E.J., Choi H.W., et al. Serum parathyroid hormone is a new potential risk factor in multiple myeloma. Biomed Res Int. 2014;2014 doi: 10.1155/2014/804182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Notarfranchi L., Marchica V., Dalla Palma B., et al. Concomitant Primary Hyperparathyroidism in Patients with Multiple Myeloma: A Possible Link? Acta Haematologica. 2021;144(3):302–307. doi: 10.1159/000509768. [DOI] [PubMed] [Google Scholar]