Abstract
α-2,3-Sialyltransferase mutants of three genetically and phenotypically diverse Neisseria meningitidis strains were compared with regard to resistance to human serum and systemic spread in the infant rat. Lipopolysaccharide sialylation was found to be of minor importance for the resistance of serogroup B and C meningococcal disease isolates to complement attack.
The complement system serves as a first line of defense against meningococci (Neisseria meningitidis) (2, 9, 12, 21, 26). Meningococci protect themselves against complement attack by the expression of polysaccharide capsules (13, 19, 20, 28, 30). In the case of serogroup B and C meningococci, the polysaccharide capsules consist of homopolymers of sialic acid (N-acetyl-neuraminic acid [NANA]) (3, 9). Sialic acid also occurs in pathogenic neisseriae as a terminal substitution of lipopolysaccharide (LPS) (16, 17, 22, 23, 31). In gonococci, sialylated LPS contributes to serum resistance (4, 6, 8, 31), probably by enhancing the binding of the regulatory factor H (24). The role of LPS sialylation in serum resistance of encapsulated meningococci, however, is a matter of debate. We demonstrated that truncation of the LPS molecule by mutation of the galE gene renders meningococci serum sensitive despite the presence of a polysialic acid capsule (28, 30). The galE gene encodes the UDP-glucose 4-epimerase, which is necessary in meningococci and gonococci for the production of galactose, a residue of the LPS of virulent meningococci and gonococci (11, 14, 15, 25). Furthermore, we showed that, in contrast to gonococcal serum resistance, LPS sialylation is dispensable for meningococcal serum resistance (27). These experiments were performed with the serogroup B strain B1940, whose isogenic α-2,3-sialyltransferase (lst) mutant showed a serum-resistant phenotype. The α-2,3-sialyltransferase catalyzes the terminal linkage of sialic acid to the lacto-N-neotetraose (LNnT) epitope of the meningococcal LPS with immunotype L3,7,9, which is often found in virulent neisserial strains (10, 16, 18). This enzymatic activity requires activated N-acetyl-neuraminic acid (CMP-NANA), which is endogenously synthesized or exogenously supplied (16, 17). Knockout of the lst gene resulted in the exclusive expression of unsialylated LNnT. In contrast to our findings regarding the role of meningococcal LPS sialylation, a recent analysis by Estabrook et al. (7) of a set of serogroup C meningococcal isolates which exhibited varying degrees of LPS sialylation suggested that the amount of free LNnT expressed by meningococci is negatively correlated with serum resistance. These conflicting results prompted us to analyze the serum resistance of isogenic lst mutants of meningococcal strains, which were different from strain B1940, previously studied by our group (27), in order to rule out the possibility of strain- or serogroup-specific effects. We continued our recent approach with isogenic lst mutants because we wanted to study LPS sialylation in a genetically defined background and because we wanted to analyze the interaction of the complement system with meningococcal surfaces devoid of sialylated LPS.
The serogroup B and C meningococcal strains used in this study are listed in Table 1. The strains exhibited different sero- (sub)types. Strains MC58 and 2120 were derived from clonal lineages frequently causing meningococcal disease, i.e., from electrophoretic types belonging to the ET-5 and ET-37 complexes, respectively (1). Expression of the Opc protein and of pilin differed among the three strains (Table 1). galE mutants were constructed as previously described (11). For the construction of lst mutants, a PCR product of the lst gene of strain B1940 was obtained by using primers UV21 (5′-ATGGGCTTGAAAAAGGCTTGT-3′; positions 573 to 593 [GenBank accession no. U60660]) and UV23 (5′-CCGCGCACTGCCCGCC-3′; positions 2039 to 2024). This PCR product was 350 bp larger than the UV21-UV22 PCR product used recently for the same purpose (27), which greatly facilitated the final transformation of meningococci with the plasmid harboring the mutated lst gene. The UV21-UV23 PCR product was cloned into the vector pCR-Script Amp SK(+) (Stratagene, Heidelberg, Germany). The kanamycin resistance gene of plasmid pUC4K (Pharmacia, Freiburg, Germany) was then inserted into the HincII site of the lst gene (position 1549), resulting in plasmid pCR-Script-lst/Kan, which was used to transform meningococci. The genotype of the meningococcal lst mutants was controlled by PCR with primers UV21 and UV22 (27) and by Southern blot hybridizations of EcoRI/ClaI-digested chromosomal DNA with the kanamycin resistance gene or the lst gene used as probes. Both tests demonstrated that correct allelic exchange of the lst gene had been achieved (data not shown). The lst mutants of strains B1940, MC58, and 2120 retained binding of the monoclonal antibody (MAb) 9-2-L379, specific for immunotype L3,7,9 (kindly provided by W. D. Zollinger), as evidenced by immunofluorescence techniques (data not shown). Western blot analysis revealed that Opa, Opc, and pilin expression was not altered in the lst mutants, compared to the wild-type strains (Table 1).
TABLE 1.
Meningococcal strains
| Strain | Source (year of isolation) | Isolate type (location) | Sero-group | Sero(sub)type | Clustera | Reactivity with MAbb:
|
Refer-ence | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 9-2-L379 | B306 | 4B12/C11 | SM1 | |||||||
| B1940 | U. Berger (?) | Clinical (Germany) | B | NT:P1.3,6,15 | — | + | + | + | + | 9 |
| MC58 | E. R. Moxon (1985) | Clinical (United Kingdom) | B | 15:P1.17,16b | ET-5 complex | + | + | + | + | 5 |
| 2120 | Our clinical isolate (1997) | Clinical (Germany) | C | NT:P1.2,5 | ET-37 complex | + | − | + | − | 29 |
ET, electrophoretic type; —, the ET of strain B1940 does not correspond to any well-known clone associated with a high incidence of disease.
Reactivities with MAbs were determined by indirect immunofluorescence (for 9-2-L379) and by Western blot analysis (for B306, 4B12/C11, and SM1). 9-2-L379 is reactive with LPS of immunotype L3,7,9 and was kindly provided by W. D. Zollinger (Washington, D.C.); B306 and 4B12/C11 bind to meningococcal Opc and Opa, respectively, and were provided by M. Achtman (Berlin, Germany); and SM1 binds to neisserial pilin of class I pili (donation of M. Virji, Bristol, United Kingdom).
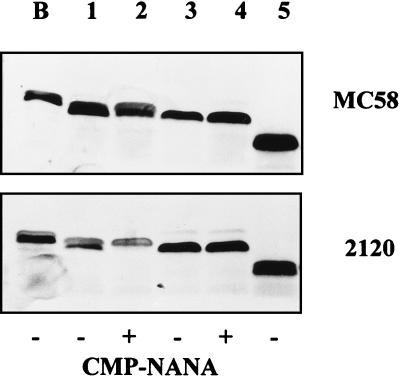
The LPS of wild-type MC58 and 2120 and their lst and galE mutants was analyzed by Tricine gel electrophoresis of partially purified LPS, as described previously (27). The analysis of the set of mutants of strain B1940 has been shown recently (27). LPS of the wild-type strains was partially endogenously sialylated and accessible to exogenous sialylation during growth in culture medium supplemented with CMP-NANA, as previously described (27). The LPS of the lst mutants migrated as fast as the unsialylated LPS fraction of the wild-type meningococci (Fig. 1). No endogenous sialylation was observed. Moreover, due to the knockout of the lst gene, the LPS of the mutants was not exogenously sialylated. The LPS of strain B1940 migrated slightly slower than that of MC58 and 2120, indicating a chemical modification of the B1940 LPS. Truncation of the majority of the LPS population by galE mutation was demonstrated by faster migration in the Tricine gel compared to wild-type LPS, and visual inspection of the Tricine gels suggested a similar appearance of the LPS of the galE mutants of all three strains. One should nevertheless keep in mind that structural analysis of a galE mutant of the meningococcal strain NMB revealed a heterogeneity in the LPS population which was most likely caused by a glycosyltransferase polymerizing glucose moieties (15) and which might depend on the background of the strain used.
FIG. 1.
Tricine gel electrophoresis of partially purified LPS of strains MC58 and 2120 and their derivatives grown in the absence or presence of CMP-NANA. Lane B, LPS of wild-type strain B1940 shown as a control (27); lanes 1 and 2, wild-type strains; lanes 3 and 4, lst mutant; lane 5, galE mutant. Double bands result from the different migrations of sialylated LPS (upper band) and unsialylated LPS (lower band) in Tricine gels (27).
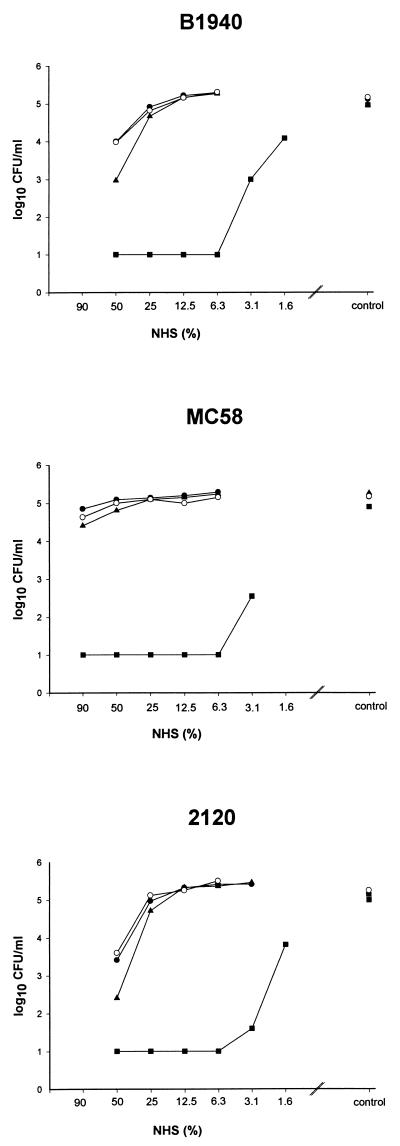
Bactericidal assays were performed as previously described (27) in Veronal-buffered saline by using 105 CFU/ml of buffer and normal human serum (NHS). In the case of wild-type strains B1940 and 2120, a 10-fold reduction in bacterial counts was achieved by 20 to 40% NHS after 1 h of incubation in NHS, whereas >80% NHS was necessary for a comparable reduction of the bacterial counts in the case of strain MC58 (data not shown). In all three strains, exogenous sialylation did not result in enhanced serum resistance (Fig. 2), although LPS sialylation was enhanced by exogenous supplementation with CMP-NANA, as evidenced by silver-stained Tricine gels (Fig. 1). This suggests that the degree of endogenous sialylation exhibited by the disease isolates investigated here was sufficient for the maximum possible serum resistance. The following experiments were therefore designed to study whether endogenous sialylation contributes to serum resistance of the disease isolates. In dose titration curves, lst mutants of all strains retained the serum-resistant phenotype of the parental strain (Fig. 2). A reduction in serum resistance by lst mutation was observed only at high serum concentrations (i.e., 50% NHS in the case of the lst mutants of strains B1940 and 2120 and 90% NHS in the case of strain MC58). This reduction was significant, as determined by Student’s t test, in the cases of B1940 (P = 0.0001) and 2120 (P = 0.0009) but not in the case of MC58 (P = 0.27). Differences between the experimental groups (wild-type strains and respective lst mutants) were calculated by using Student’s t test from at least four independent experiments and for all serum concentrations applied. Differences were considered significant at P of <0.01. In all strains used, a galE mutation resulted in a complete loss of serum resistance, because a 16- to 32-fold higher dilution of NHS had to be applied to achieve bacterial survival comparable to that of the wild-type strain. Therefore, a galE, but not an lst, mutation resulted in a modification of the LPS structure which was incompatible with survival in NHS.
FIG. 2.
Bactericidal assays. Bacteria (105/ml) were incubated in twofold NHS dilutions or in buffer alone (control) for 1 h at 37°C. The strains are given above each graph. The experiments were repeated at least four times, yielding comparable results. A representative experiment is shown. •, wild type; ○, wild type grown in the presence of CMP-NANA; ▴, lst mutant; ■, galE mutant.
We recently demonstrated that the lst mutant of strain B1940 induces bacteremia in the infant rat (27). The same held true for the lst mutants of strains MC58 and 2120, indicating that LPS sialylation is dispensable for survival in the infant rat (Table 2). The animals were infected intraperitoneally with 106 CFU, and the number of CFU/ml of blood was determined at 8 h postinfection. There was a significant reduction in the bacterial counts induced by the lst mutant of strain 2120, but not of strain MC58, compared to the wild-type strain. However, the effect of lst mutation in 2120 was relatively small in contrast to the effect of the galE mutation, which completely abrogated the capability to survive immune attack in the infant rat.
TABLE 2.
Infant rat model of meningococcal infection
| Parameter | MC58
|
2120
|
||||
|---|---|---|---|---|---|---|
| Wild type | lst | galE | Wild type | lst | galE | |
| Infective dose [log10(CFU)/ animal] | 6.32 | 6.37 | 5.58 | 6.67 | 6.49 | 6.66 |
| n | 9 | 8 | 4 | 7 | 7 | 4 |
| Log10(CFU)/ ml of blooda | 4.97 ± 0.28 | 4.80 ± 0.29 | <1.0 | 4.63 ± 0.70 | 3.39 ± 0.49 | <1.0 |
| Pb | 0.25 | 0.002 | ||||
The limit of detection was 1.0 CFU/ml of blood.
Log10(CFU)/ml of blood values were compared by Student’s t test. Differences were considered significant at P of <0.01.
In conclusion, we constructed lst mutants from two genetically diverse serogroup B and C meningococcal disease isolates. This approach was chosen to obtain isogenic derivatives expressing a maximum amount of unsialylated LPS. For all strains tested, the lst mutants almost retained a serum-resistant phenotype. Only at very high concentrations of NHS was the serum resistance of strains B1940 and 2120, but not of MC58, significantly reduced by lst mutation. In contrast to the lst mutation, all galE mutants were serum sensitive. These findings confirm our recent report that LPS sialylation is almost dispensable for serum resistance of meningococci, and, even more important, they show that our earlier conclusions hold true for serogroup B and C disease isolates of genetically diverse clonal groupings.
We cannot support the finding of Estabrook et al. (7) that the amount of free (unsialylated) LNnT expressed on the meningococcal surface is negatively correlated to serum resistance. Two differences in study design, the methods applied and the selection of strains, may account for this discrepancy. In contrast to Estabrook et al., we constructed isogenic mutants of meningococcal strains devoid of LPS sialylation. Thus, we took a methodological viewpoint on LPS sialylation opposite that of Estabrook et al., who analyzed natural variations in the degree of LPS sialylation in different strains and the effects of further exogenous sialylation. Estabrook et al. used a collection of both carrier and disease serogroup C isolates. In contrast, the purpose of our study was to analyze well-characterized disease isolates derived from different clonal groupings which frequently cause serogroup B and C meningococcal disease (i.e., ET-5 and ET-37 complexes). It is tempting to speculate that encapsulated immunotype L3,7,9 carrier isolates frequently depend on exogenous sialylation of LPS for survival in NHS and thus require the function of the α-2,3-sialyltransferase. These isolates, however, must differ in factors other than LPS from serum-resistant disease isolates, which require neither exogenous nor endogenous sialylation of LPS to survive in NHS. A possible mechanism might be that in serum-sensitive carrier isolates, sialylated LPS works better than unsialylated LPS to protect vulnerable sites on the bacterial surface from complement attack. These sites may not be present or accessible in serum-resistant disease isolates, which therefore require LNnT but not its sialylation for survival in NHS.
Acknowledgments
We thank M. Achtman, M. Virji, and W. D. Zollinger for the donation of monoclonal antibodies and for helpful advice. R. E. Moxon is acknowledged for providing strain MC58. A. W. Friedrich is thanked for critically reading the manuscript.
REFERENCES
- 1.Achtman M. Global epidemiology of meningococcal disease. In: Cartwright K, editor. Meningococcal disease. Chichester, England: John Wiley & Sons, Ltd.; 1995. pp. 159–175. [Google Scholar]
- 2.Cartwright K. Meningococcal carriage and disease. In: Cartwright K, editor. Meningococcal disease. Chichester, England: John Wiley and Sons, Ltd.; 1995. pp. 115–146. [Google Scholar]
- 3.Claus H, Vogel U, Mühlenhoff M, Gerardy-Schahn R, Frosch M. Molecular divergence of the sia locus in different serogroups of Neisseria meningitidis expressing polysialic acid capsules. Mol Gen Genet. 1997;257:28–34. doi: 10.1007/pl00008618. [DOI] [PubMed] [Google Scholar]
- 4.de la Paz H, Cooke S J, Heckels J E. Effect of sialylation of lipopolysaccharide of Neisseria gonorrhoeae on recognition and complement-mediated killing by monoclonal antibodies directed against different outer-membrane antigens. Microbiology. 1995;141:913–920. doi: 10.1099/13500872-141-4-913. [DOI] [PubMed] [Google Scholar]
- 5.Dunn K L, Virji M, Moxon E R. Investigations into the molecular basis of meningococcal toxicity for human endothelial and epithelial cells: the synergistic effect of LPS and pili. Microb Pathog. 1995;18:81–96. doi: 10.1016/s0882-4010(95)90085-3. [DOI] [PubMed] [Google Scholar]
- 6.Elkins C, Carbonetti N H, Varela V A, Stirewalt D, Klapper D G, Sparling P F. Antibodies to N-terminal peptides of gonococcal porin are bactericidal when gonococcal lipopolysaccharide is not sialylated. Mol Microbiol. 1992;6:2617–2628. doi: 10.1111/j.1365-2958.1992.tb01439.x. [DOI] [PubMed] [Google Scholar]
- 7.Estabrook M M, Griffiss J M, Jarvis G A. Sialylation of Neisseria meningitidis lipooligosaccharide inhibits serum bactericidal activity by masking lacto-N-neotetraose. Infect Immun. 1997;65:4436–4444. doi: 10.1128/iai.65.11.4436-4444.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frangipane J V, Rest R F. Anaerobic growth and cytidine 5′-monophospho-N-acetylneuraminic acid act synergistically to induce high-level serum resistance in Neisseria gonorrhoeae. Infect Immun. 1993;61:1657–1666. doi: 10.1128/iai.61.5.1657-1666.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frosch M, Weisgerber C, Meyer T F. Molecular characterization and expression in Escherichia coli of the gene complex encoding the polysaccharide capsule of Neisseria meningitidis group B. Proc Natl Acad Sci USA. 1989;86:1669–1673. doi: 10.1073/pnas.86.5.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert M, Watson D C, Cunningham A M, Jennings M P, Young N M, Wakarchuk W W. Cloning of the lipooligosaccharide alpha-2,3-sialyltransferase from the bacterial pathogens Neisseria meningitidis and Neisseria gonorrhoeae. J Biol Chem. 1996;271:28271–28276. doi: 10.1074/jbc.271.45.28271. [DOI] [PubMed] [Google Scholar]
- 11.Hammerschmidt S, Birkholz C, Zähringer U, Robertson B D, van Putten J, Ebeling O, Frosch M. Contribution of genes from the capsule gene complex (cps) to lipooligosaccharide biosynthesis and serum resistance in Neisseria meningitidis. Mol Microbiol. 1994;11:885–896. doi: 10.1111/j.1365-2958.1994.tb00367.x. [DOI] [PubMed] [Google Scholar]
- 12.Jarvis G A. Analysis of C3 deposition and degradation on Neisseria meningitidis and Neisseria gonorrhoeae. Infect Immun. 1994;62:1755–1760. doi: 10.1128/iai.62.5.1755-1760.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarvis G A, Vedros N A. Sialic acid of group B Neisseria meningitidis regulates alternative complement pathway activation. Infect Immun. 1987;55:174–180. doi: 10.1128/iai.55.1.174-180.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jennings M P, van der Ley P, Wilks K E, Maskell D J, Poolman J T, Moxon E R. Cloning and molecular analysis of the galE gene of Neisseria meningitidis and its role in lipopolysaccharide biosynthesis. Mol Microbiol. 1993;10:361–369. [PubMed] [Google Scholar]
- 15.Lee F K N, Stephens D S, Gibson B W, Engstrom J J, Zhou D, Apicella M A. Microheterogeneity of Neisseria lipooligosaccharide: analysis of a UDP-glucose 4-epimerase mutant of Neisseria meningitidis NMB. Infect Immun. 1995;63:2508–2515. doi: 10.1128/iai.63.7.2508-2515.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandrell R E, Apicella M A. Lipo-oligosaccharides (LOS) of mucosal pathogens: molecular mimicry and host-modification of LOS. Immunobiology. 1993;187:382–402. doi: 10.1016/S0171-2985(11)80352-9. [DOI] [PubMed] [Google Scholar]
- 17.Mandrell R E, Kim J J, John C M, Gibson B W, Sugai J V, Apicella M A, Griffiss J M, Yamasaki R. Endogenous sialylation of the lipooligosaccharides of Neisseria meningitidis. J Bacteriol. 1991;173:2823–2832. doi: 10.1128/jb.173.9.2823-2832.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandrell R E, Griffiss J M, Macher B A. Lipooligosaccharides (LOS) of Neisseria gonorrhoeae and Neisseria meningitidis have components that are immunochemically similar to precursors of human blood group antigens. Carbohydrate sequence specificity of the mouse monoclonal antibodies that recognize cross reacting antigens on LOS and human erythrocytes. J Exp Med. 1988;168:107–126. doi: 10.1084/jem.168.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masson L, Holbein B E, Ashton F E. Virulence linked to polysaccharide production in serogroup B Neisseria meningitidis. FEMS Microbiol Lett. 1982;13:187–190. [Google Scholar]
- 20.Masson L, Holbein B E. Influence of nutrient limitation and low pH on serogroup B Neisseria meningitidis capsular polysaccharide levels: correlation with virulence for mice. Infect Immun. 1985;47:465–471. doi: 10.1128/iai.47.2.465-471.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moran E E, Brandt B L, Zollinger W D. Expression of the L8 lipopolysaccharide determinant increases the sensitivity of Neisseria meningitidis to serum bactericidal activity. Infect Immun. 1994;62:5290–5295. doi: 10.1128/iai.62.12.5290-5295.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nairn C A, Cole J A, Patel P V, Parsons N J, Fox J E, Smith H. Cytidine-5′-monophospho-N-acetyl neuraminic acid or a related compound is the low Mr factor from human red blood cells which induces gonococcal resistance to killing by human serum. J Gen Microbiol. 1988;134:3295–3306. doi: 10.1099/00221287-134-12-3295. [DOI] [PubMed] [Google Scholar]
- 23.Parsons N J, Andrade J R, Patel P V, Cole J A, Smith H. Sialylation of lipopolysaccharide and loss of absorption of bactericidal antibody during conversion of gonococci to serum resistance by cytidine 5′-monophospho-N-acetyl neuraminic acid. Microb Pathog. 1989;7:63–72. doi: 10.1016/0882-4010(89)90112-5. [DOI] [PubMed] [Google Scholar]
- 24.Ram S, Sharma A K, Simpson S D, Gulati S, McQuillen D P, Pangburn M K, Rice P A. A novel sialic acid binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J Exp Med. 1998;187:743–752. doi: 10.1084/jem.187.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson B D, Frosch M, van Putten J P. The role of galE in the biosynthesis and function of gonococcal lipopolysaccharide. Mol Microbiol. 1993;8:891–901. doi: 10.1111/j.1365-2958.1993.tb01635.x. [DOI] [PubMed] [Google Scholar]
- 26.Ross S C, Densen P. Complement deficiency states and infection: epidemiology, pathogenesis and consequences of neisserial and other infections in an immune deficiency. Medicine. 1984;63:243–273. [PubMed] [Google Scholar]
- 27.Vogel U, Claus H, Heinze G, Frosch M. Functional characterization of an isogenic meningococcal α-2,3-sialyltransferase mutant: the role of lipooligosaccharide for serum resistance in serogroup B meningococci. Med Microbiol Immunol (Berlin) 1997;186:159–166. doi: 10.1007/s004300050059. [DOI] [PubMed] [Google Scholar]
- 28.Vogel U, Hammerschmidt S, Frosch M. Sialic acids of both the capsule and the sialylated lipooligosaccharide of Neisseria meningitidis serogroup B are prerequisites for virulence of meningococci in the infant rat. Med Microbiol Immunol (Berlin) 1996;185:81–87. doi: 10.1007/s004300050018. [DOI] [PubMed] [Google Scholar]
- 29.Vogel U, Morelli G, Zurth K, Claus H, Kriener E, Achtman M, Frosch M. Necessity of molecular techniques to distinguish between Neisseria meningitidis strains isolated from patients with meningococcal disease and from their healthy contacts. J Clin Microbiol. 1998;36:2465–2470. doi: 10.1128/jcm.36.9.2465-2470.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vogel U, Weinberger A, Frank R, Müller A, Köhl J, Atkinson J P, Frosch M. Complement factor C3 deposition and serum resistance in isogenic capsule and lipooligosaccharide sialic acid mutants of serogroup B Neisseria meningitidis. Infect Immun. 1997;65:4022–4029. doi: 10.1128/iai.65.10.4022-4029.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wetzler L M, Barry K, Blake M S, Gotschlich E C. Gonococcal lipooligosaccharide sialylation prevents complement-dependent killing by immune sera. Infect Immun. 1992;60:39–43. doi: 10.1128/iai.60.1.39-43.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]