Abstract
Exosomes are extracellular vesicles implicated in cell-to-cell communication. The objective was to investigate the effect of exosomes in macrophages under hypoxia. Exosomes were isolated from skim milk using differential centrifugation and was characterized by particle size and exosomal markers TSG101, CD81, and ALIX. The effect of exosomes on macrophages under hypoxia was investigated by assessing proliferation, cytokine and reactive oxygen species (ROS) production, and cell cycle. Exosomes treatment increased the cell viability under hypoxia while ROS production was significantly reduced. The production of TNF-α was not affected by hypoxia alone but increased in a dose-dependent manner in cells treated with exosomes under hypoxic condition. Hypoxia arrested cells in the G0/G1 phase whereas exosome treatment reduced the cell in this phase. Our study found that bovine milk exosomes affect the proliferation of macrophages under hypoxia and are able to reverse the adverse effects of hypoxia on cell viability.
Keywords: Cell cycle, Exosomes, Hypoxia, Macrophage, Proliferation, Reactive oxygen species
Graphical abstract
Highlights
-
•
Bovine milk exosomes affected proliferation of macrophages under hypoxia.
-
•
Bovine milk exosomes showed immunomodulating property under hypoxia.
-
•
Bovine milk exosomes affected cell cycle progression of macrophages under hypoxia.
1. Introduction
Hypoxia is a condition characterized by low oxygen concentrations caused by the low blood supply to the body's tissue. Not all forms of hypoxia are deleterious to body tissue. In some cells and tissue, such as intestinal epithelial cells, low oxygen concentrations are considered physiological, hence the term physiological hypoxia (Zheng et al., 2015). However, when an environment of low oxygen causes the impaired function of the cells and tissues, hypoxia can become pathological. Often, the reduction of blood supply and consequent low oxygen concentrations can be seen in the inflamed and injured tissue. This is due to the inability of blood vesicles to grow and supply oxygen to the growing affected tissue caused by infiltration of the cells (Murdoch et al., 2019). Consequently, hypoxia may cause immune cell impairment and dysfunction.
Macrophages are the cells of the innate immune system and their primary function is phagocytosis. They exist in our body in two different forms: tissue-resident and monocyte-derived macrophages (Zhao et al., 2018). When a pathogen gets sensed, one of the functions of the tissue-resident macrophage is to start initiating inflammatory response (Davies et al., 2013). In an environment of low oxygen tension, macrophages upregulate some genes in order to perform their function in hypoxic environment. Hypoxia-inducible factor family is one of the genes that is upregulated in low oxygen conditions and upregulation of HIF-1α is implicated in hypoxia-induced apoptosis (Krzywinska and Stockmann, 2018; Greijer and Wall, 2004). Furthermore, when macrophage cells were infected with Escherichia coli and Staphylococcus aureus and exposed to hypoxic conditions, the ability of macrophages to eliminate these two pathogens was significantly reduced even though these pathogens are readily killed by macrophages under normoxic conditions (Wiese et al., 2012). Interestingly, some natural compounds and compounds synthesized based on natural compounds are capable of modulating the activity of macrophages. For instance, the immunomodulatory activities of polysaccharides from Ganoderma is related to their chemical composition and structure, conformation, and physical properties (Huang and Nie, 2015; Ren et al., 2021). Further, we previously observed that exosomes derived from bovine milk can protect macrophages against cisplatin-induced cytotoxicity (Matic, D'Souza, Wu, Pangloli and Dia, 2020).
Exosomes are extracellular vesicles with sizes ranging from 50 to 150 nm (Niel et al., 2018). Their synthesis and secretion have been well documented in almost all types of cells (Vlassov et al., 2012). The presence and isolation of exosomes from different foods including milk and edible plants and their effect on human health are being investigated (Pieters et al., 2015; Mu et al., 2014). It has been documented that bovine milk exosomes contain miRNAs that are resistant to in vitro digestion and are taken up by the human macrophage cell line THP-1 (Benmoussa and Lee, 2016; Izumi et al., 2015). Moreover, bovine milk exosome treatment prevented the development of necrotizing enterocolitis in vivo (Id et al., 2019). The effect of food derived exosomes on hypoxia-induced changes has not been extensively investigated. A previous study reported that bovine milk exosomes increased the proliferation of intestinal epithelial cells in vitro under hypoxia (Gao et al., 2019). Therefore, the objective of this study was to isolate bovine milk exosomes from commercially available milk and investigate its effect on the proliferation of murine-like macrophages RAW 264.7 under hypoxic conditions.
2. Materials and methods
2.1. Materials
Murine macrophage RAW 264.7 cell line (American Type Culture Collection, Manassas, VA) was grown in DMEM 1X (Corning Inc., Corning, NY) containing 10% fetal bovine serum (FBS, Life Technologies Carlsbad, CA). Primary antibodies (p53 10442-1-AP, p21 60214-1-Ig, HIF-1α 20960-1-AP, and GAPDH 60004-1-Ig) were purchased from Proteintech (Rosemont, IL). Secondary antibodies (goat anti-mouse and goat anti-rabbit) were purchased from Thermo Fisher Scientific (Waltham, MA).
2.2. Isolation and characterization of bovine milk exosomes and particle size analysis
Exosomes were isolated from commercially available fat-free milk by differential centrifugation under sterile conditions following previously described protocol with some modifications (Munagala et al., 2016). Briefly, milk was centrifuged at 13,000×g at 4 °C for 30 min in 250-mL centrifuge bottles using SS-34 rotor and SORVALL® RC-5B PLUS centrifuge. The whey was collected, transferred into polycarbonate 26.3-mL Beckman centrifuge tubes, and centrifuged at 100,000×g at 4 °C for 60 min in Type 50.2 Ti rotor using Optima XL-80K Ultracentrifuge (Beckman Coulter, Inc.). The final supernatant was collected, transferred to 26.3-mL centrifuge tubes and centrifuged at 135,000×g at 4 °C for 90 min. The supernatant was discarded, and the pellet was washed with PBS twice. The exosome pellet was collected and resuspended in PBS. The protein concentration of exosomes was determined by Pierce ™ BCA Protein Assay Kit (Thermo Scientific, Rockford, IL U.S.A). Exosomes were diluted to 1 mg/mL concentration for particle size and zeta potential analysis. The size and zeta potential were measured by Zetasizer (Malvern Instruments Ltd., Malvern, Worcestershire, UK). Exosomal marker TSG101, CD81, and ALIX were determined by Western blot. Isolated exosomes were sterile filtered through 0.22 μm filter and stored at −80 °C until further use.
2.3. Cell culture and cell proliferation assay
Murine macrophages RAW 264.7 were cultured in DMEM containing 10% FBS and 1% penicillin/streptomycin and at 37 °C in 5% CO2 until confluency. For proliferation assay, 5 × 103 cells/well were seeded into a 96-well plate in the total volume of 200 μL and incubated overnight to attach at 37 °C in 5% CO2. Then, the cells were treated with different exosomes concentration (0, 100 and 200 μg/mL) and placed in a hypoxia chamber for 24 h. The composition of the gas in the hypoxia chamber was 94% N2, 5% CO2 and 1% O2. Untreated cells were incubated under normoxia. After the treatment, the growth media was replaced with 100 μL fresh plain growth media containing 10% MTS (Promega, Madison WI) reagent and incubated for 3 h under normoxia. The absorbance was read at 490 nm and the percent of viable cells was calculated with respect to untreated cells under normoxia.
2.4. Whole cell lysate preparation and supernatant collection
Briefly, 2 × 105 cells/well were seeded into a 6-well plate in the total volume adjusted to 2 mL and incubated overnight to attach at 37 °C in 5% CO2. After overnight incubation, the cells were treated with 0, 100 and 200 μg/mL exosomes and placed in a hypoxia chamber containing 94% N2, 5% CO2 and 1% O2 for 24 h. Untreated cells were incubated under normoxia. After 24 h of treatment, the 6-well plates were placed on ice and the growth media was collected for cytokine analysis. The cells were washed twice with ice-cold PBS to remove cell debris. After washing, 100 μL radioimmunoprecipitation assay (RIPA) lysis buffer containing 1% protease inhibitor complex was added to each well and incubated for 5 min. Cells were harvested by scraping and transferred to 1.5 mL microfuge tubes and vortexed at 14, 000×g for 10 min at 4 °C. The supernatant was collected, and protein concentration was measured using Bradford reagent and bovine serum albumin as the standard. The supernatant was mixed with Laemmli buffer containing 5% β-mercaptoethanol in equal volumes and boiled for 5 min. The samples were stored in −80 °C until use.
2.5. Production of reactive oxygen species (ROS) by flow cytometry
Briefly, RAW 264.7 cells were seeded, treated, and incubated in the 6-well plates under normoxia and hypoxia following the above-mentioned protocol. After treatment, the plates were placed on ice and the media was removed. The cells were washed with ice-cold PBS twice. Two hundred μL of growth media was added to each well and the cells were scraped. Additional 800 μL of growth media was added to each well and cells were transferred to 1.5 mL microfuge tubes. The cells were centrifuged at 1000×g for 5 min at 4 °C. After centrifugation, the supernatant was removed, and the cells were resuspended in 1 mL PBS. The cells were again centrifuged at 1000×g for 5 min at 4 °C and supernatant was removed. Finally, the cells were resuspended in 500 μL PBS containing 10 μM 2′,7′-dichlorofluorescein diacetate and incubated at 37 °C for 30 min in a CO2 incubator. The number of cells with green fluorescence was measured using the FL 1 channel of a MACSQuant flow cytometer and data was analyzed with MACSQuantify software (Miltenyi Biotec, Somerville MA).
2.6. Enzyme-linked immunosorbent assay (ELISA) for measurement cytokine production (IL-1β, IL-6, IL-10 and TNF-α)
Production of IL-1β, IL-6, IL-10, and TNF-α was measured using commercially available ELISA kits (BioLegend, San Diego CA). The cytokine detection was performed using the supernatant collected after the cell treatment with exosomes. Briefly, uncoated plates were incubated with capture antibodies overnight at 4 °C. After incubation, the plates were washed with washing buffer 4 times and blocked with assay diluent for 1 h with shaking at room temperature. After blocking, the plates were washed, and standards and samples were plated and incubated with shaking at room temperature for 2 h. The plates were washed and detection antibodies were added and incubated for 1 h with shaking. After washing, the avidin-HRP solution was added and incubated for 30 min with shaking. Finally, after washing, the substrate solution was added and incubated in the dark for 15 or 20 min. The stop solution was added, and absorbance was read at 450 nm. The concentration of cytokines was calculated based on the standard curve for each cytokine.
2.7. Western blot for hypoxia marker HIF-1α and proliferation proteins p21 and p53
Approximately 10 μg of proteins of the cell lysate was loaded in 8–16% SurePAGE (GenScript) gels. The gel electrophoresis was run in Tris-MOPS-SDS (GenScript) running buffer at 140 V for 50 min. Proteins were transferred into the Trans-Blot® Turbo™ PVDF membrane (Bio-Rad) using Trans-Blot®Turbo™ Transfer System (Bio-Rad). After the transfer, the membrane was blocked with 5% non-fat dry milk in TBST for 1 h at room temperature. After blocking, the membrane was washed in TBST three times for 5 min each. The membrane was incubated with primary antibodies against cyclin-dependent kinase inhibitor 1, p21, at dilution 1:1000, tumor protein p53 at dilution 1:3000, hypoxia-inducible factor-1α, HIF-1α, at 1:1000 dilution, and glyceraldehyde 3-phosphate dehydrogenase, GAPDH, (house-keeping protein; for normalization of data) at dilution 1:5000 overnight at 4 °C. After incubation, the membrane was washed as described above and secondary antibodies (anti-rabbit for p53 and HIF-1α and anti-mouse for p21 and GAPDH) at dilution 1:5000 were incubated for 1.5 h at room temperature. Finally, the membrane was washed, saturated with Bio-Rad ClarityTM Western ECL Substrate, and imaged by a C-Digit blot scanner (Li-Cor Biosciences, U.S.).
2.8. Cell-cycle analysis by flow cytometry
The cells were fixed and stained following the previously described protocol (Distelhorst, 2009). Briefly, the cells were seeded in 6-well plates and treated with exosomes (0, 100 and 200 μg/mL) under hypoxia following above-described conditions. Also, untreated cells were incubated under normoxia. After treatment, the plates were placed on ice and the media was removed. The cells were washed with ice-cold PBS twice. Two hundred μL of growth media was added to each well and the cells were scraped. Additional 800 μL of growth media was added to each well and cells were transferred to 1.5 mL microfuge tubes. The cells were centrifuged at 1000×g for 5 min at 4 °C. After centrifugation, the supernatant was removed, and the cells were resuspended in 60 μL PBS. Then, 70% ethanol was added to the cell suspension drop by drop with constant vortexing. The cells were left overnight at 4 °C for fixation. After fixation, cells were centrifuged at 300×g at room temperature, the supernatant was removed and 1 mL staining mixture containing propidium iodide and RNase was added. The cells were vortexed and incubated for 1 h at 37 °C protected from light. Cell cycle analysis was performed using MACSQuant flow cytometer and data analyzed with MACSQuantify software (Miltenyi Biotec, Somerville MA).
2.9. Statistical analysis
All experiments were performed in at least three independent replicates. All results were expressed as mean ± SD or mean ± SEM. Analysis of variance (ANOVA) with Tukey's test was done to determine a significant difference (p < 0.05) between different treatment groups using Minitab statistical software (Minitab Inc., Pennsylvania, USA).
3. Results and discussion
3.1. Identification of bovine milk exosomes isolated from commercially available milk using particle size
Bovine milk exosomes isolated from commercially available milk have an average particle size of 152.2 nm ± 4.25 (Fig. 1a) which is consistent with the literature (Samuel et al., 2017). Polydispersity index (PDI) was 0.289 ± 0.024. In the previously mentioned article, the size of bovine milk exosomes depends on the maturation stage of milk (Samuel et al., 2017). Mature milk has a larger particle size distribution when compared with exosomes isolated from colostrum. In addition, the amount of exosomal miRNA isolated from commercially available milk did not significantly differ from the amount of exosomal miRNA from raw milk suggesting that standard heat treatment does not affect exosomes (Pieters et al., 2015). The average zeta potential of bovine milk exosomes was −10.9 mV ± 0.834 (Fig. 1b) which would suggest short-term stability. Furthermore, the stability of exosomes also depends on storage conditions, pH, and thaw-freeze cycles (Cheng et al., 2019). Consequently, the refrigeration temperatures are not recommended for long-term storage and can reduce exosomal proteins and RNA (Lee et al., 2016). Bovine milk exosomes isolated from commercially available milk were positive for exosomal markers ALIX, TSG101, and CD81 (Fig. 1c). The identification of exosomes has been relying on the presence of ALIX, TSG101, and CD81 (Munagala et al., 2016).
Fig. 1.
Characterization of bovine milk exosomes: a) particle size analysis, b) zeta potential, and c) Western blot analysis of exosomal markers.
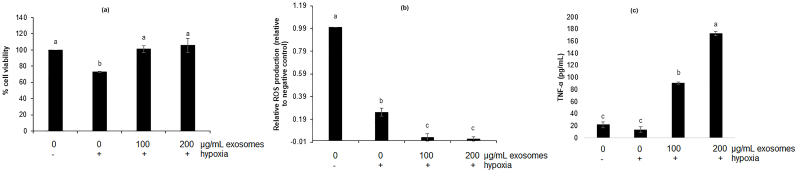
3.2. Bovine milk exosomes increased the proliferation of RAW 264.7 cell under hypoxia
Macrophages are fast acting phagocytic cells of the innate immune system (Romo, 2016). In Fig. 2a, exosomes treatment significantly increased the proliferation of RAW 264.7 cells when compared to untreated cells under hypoxia. The cell viability decreased by 27% when untreated cells were incubated under hypoxia compared to untreated cells incubated under normal oxygen conditions. Moreover, exosomes treatment at 100 and 200 μg/mL significantly increased the proliferation by 27.9% and 32.6%, respectively. The results showed that exosomes treatment reversed the anti-proliferative effect of hypoxia in macrophages and macrophage proliferation when treated with exosomes under hypoxia is the same as proliferation of macrophages in normoxia. The macrophage cells exist in two states: tissue-resident and monocyte-derived macrophages (Zhao et al., 2018). Since they are the cells of the innate immunity, they are the first line of defense when an infection happens. Often, in the case of inflamed and damaged tissue, the levels of oxygen may decrease which may cause a decrease in macrophage function and finally apoptosis (Lewis et al., 1999). Decreased function or apoptosis can lead to a reduced ability of macrophages to fight an infection. Furthermore, to our knowledge, this is the first report on the effect of bovine milk exosomes on RAW 264.7 cell proliferation under hypoxia. Previous reports on bovine milk exosomes and yak milk exosomes have shown increased cell survival of mouse small intestine epithelial cells (IEC-6) under hypoxia (Gao et al., 2019). The effect of exosomes on macrophage cell proliferation is limited. Our previous study reported that under normoxic condition, bovine milk exosomes can increase the proliferation of RAW 264.7 macrophages accompanied by changes in the protein levels of β-catenin, p21, p53, and cyclin D1 (Matic et al., 2020). On the other hand, several studies have reported the effect of milk exosomes on the proliferation of other cells, especially intestinal cells. For instance, porcine milk-derived exosomes promoted proliferation of IPEC-J2 cells by significantly affecting the protein levels and gene expression of CDX2, IGF-1R, PCNA, and p53 (Chen et al., 2016). Similar results were observed by Gao et al. (2019) on the proliferation of IEC-6 intestinal epithelial cells treated with yak milk-derived exosomes by affecting protein levels of p53 and PHD-1.
Fig. 2.
Proliferation (a), relative production of reactive oxygen species(ROS, b) and TNF-α production of RAW 264.7 cell treated with bovine milk exosomes under hypoxia. Results are presented as mean ± SD. Means that do not share a letter are significantly different (p < 0.05).
3.3. Bovine milk exosomes reduced production of ROS in RAW 264.7 cell under hypoxic conditions
Reactive oxygen species are generated under oxidative stress. The oxidative stress may be defined as an imbalance between free radicals and antioxidant (Clanton, 2007). In our study, hypoxia caused a significant 4- fold ROS reduction in untreated cells when compared to the untreated cell under normoxic conditions (Fig. 2b). Furthermore, the exosome treatment significantly reduced the ROS production when compared to untreated cells in hypoxic conditions. However, there was no significant difference in ROS production between the cell treated with 100 μg/mL and 200 μg/mL exosomes. It is still controversial whether hypoxic conditions increase ROS production. Our findings are not consistent with current literature (Lee et al., 2017; Srinivasan and Avadhani, 2007). For instance, when RAW 264.7 cells were incubated in hypoxic conditions (1% O2) for 20 h, there was a significant increase in ROS production when compared to the cell incubated in normoxic conditions (Lee et al., 2017) and when RAW 264.7 cells were grown under hypoxia (0.5% O2) for 10 h, there was a significant increase in ROS production when compared to the cells grown under normoxia (Srinivasan and Avadhani, 2007). Consequently, differences in exposure time and oxygen concentrations may have affected the production of ROS.
Cellular hypoxia is defined as a state of reductive stress where reducing equivalents such as NADH and FADH2 build up if oxygen is not available (Clanton, 2007). Moreover, the buildup of reducing equivalents makes the electrons more available to reduce the available oxygen to superoxide. However, even if reducing equivalents are available, the formation of ROS is not possible when oxygen is not present (Dawson et al., 1993). It was reported that the formation of ROS depends on constant cycles of anoxia and reoxygenation (Dawson et al., 1993). Another study repeated that reoxygenation increased the formation of ROS in RAW 264.7 cell with fast reoxygenation producing more ROS when compared to slow reoxygenation (Lee et al., 2017). In our study, we have seen a decrease in ROS when the cells were exposed to hypoxia and it may be due to exposing the cells to hypoxia for a longer period of time where no more available oxygen was present to produce ROS. Moreover, ROS are very short-living molecules with unpaired electrons that are transformed and eliminated in the variety of cellular processes (Panieri and Santoro, 2016). The reduction in ROS generation in our study may be explained by possible transformation or elimination of ROS over a prolonged period under hypoxia when reoxygenation was not present.
3.4. Bovine milk exosomes increased TNF-α in RAW 264.7 under hypoxia
Production of TNF-α was increased in the cells treated with exosomes under hypoxia in a dose-dependent manner (Fig. 2c). When the cells were treated with 100 μg/mL and 200 μg/mL exosomes, the concentration of TNF-α was 90.8 pg/mL and 172.4 pg/mL, respectively. However, there was no significant difference in the TNF-α concentration between untreated cell under hypoxia when compared to the untreated cell under normoxia. Clearly, hypoxia itself was not enough to affect TNF-α production in RAW 264.7 cells which is consistent with the literature where TNF-α was not induced in RAW 264.7 cells in hypoxic conditions even after 48 h (Xiong et al., 1998). TNF-α is a proinflammatory cytokine that plays an important role in host defense against pathogens (Pfeffer, 2003). Previously published study found that the treatment with human TNF-α increased the resistance against Listeria infection in in vivo mouse model (Kato et al., 1989). Immunomodulatory effect of bovine milk exosomes in RAW 264.7 cells under hypoxia that was observed in our study may have a beneficial effect in fighting an infection in a low oxygen environment. IL-1β, IL-6, and IL-10 were not affected by hypoxia and exosome treatment.
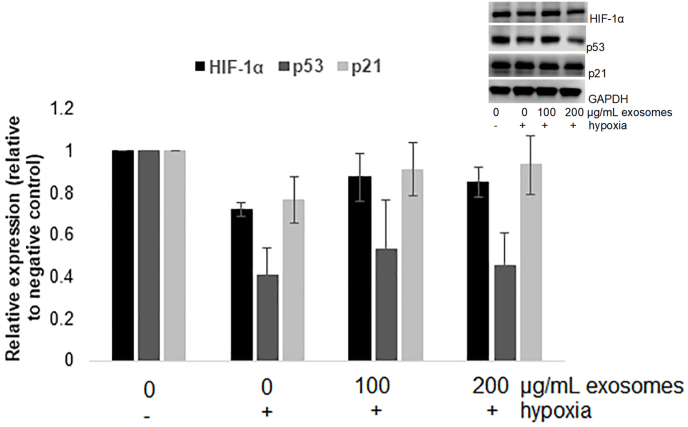
3.5. Bovine milk exosomes did not affect the expression of HIF-1α, p21 and p53
As shown in Fig. 3, HIF-1α, p21, and p53 were not affected by hypoxia or exosomes treatment under hypoxia. Although a low oxygen environment is considered to be one of the most important factors affecting HIF-1α expressions, there is evidence that prolonged hypoxia may decrease expression of HIF-1α (Uchida et al., 2004). In the previously mentioned study, when the human lung adenocarcinoma cells (A549) were exposed to 0.5%O2 for 12 h, HIF-1α expression significantly decreased when compared to the expressions at the exposure time of 4 h. The expression of p21 is regulated by p53 (Elbendary et al., 1996), so it is not surprising that both proteins were not affected.
Fig. 3.
Effect of milk exosomes on RAW 264.7 expression of hypoxia protein HIF-1α and proliferation proteins p53 and p21. Results are presented as mean ± SE.
3.6. Bovine milk exosomes affect the cell cycle of RAW 264.7 cells under hypoxia
The cell cycle of RAW 264.7 cells under hypoxia and the effect of bovine milk exosomes on the cell cycle are shown in Fig. 4. Our results showed that exposing RAW 264. 7 cells to hypoxia caused cell arrest in the G0/G1 phase of the cell cycle when compared to the cell under normoxia. The percent number of cells in G0/G1 phase significantly decreased from 61.3% in untreated cells under hypoxia to 56.7% and 56.9% in the cell treated with 100 μg/mL and 200 μg/mL exosomes, respectively. There was no significant difference (p < 0.05) between untreated cells under normoxia and the cells treated with exosomes under hypoxia suggesting that bovine exosomes treatment is capable of reversing the effect of hypoxia on cell cycle of RAW 264.7 macrophages. The number of cells in pre-apoptotic phase did not significantly differ between different treatments. A significant reduction of cells in the S phase can be seen in untreated cells exposed to the hypoxic condition when compared to the cells exposed to normoxic conditions. Moreover, when the cells were treated with 100 μg/mL and 200 μg/mL exosomes, the percent of untreated cells under hypoxia in S phase increased from 10.9% to 15.5% and 16.9%, respectively, which was not statistically different from the number of untreated cells under normoxia in S phase. It is well documented that exposing the cells to a low oxygen environment affects the cell cycle progression. Not many studies have been done on the effect of hypoxia on RAW 264.7 cell. One of the previously published articles found that exposing RAW 264.7 cells to hypoxia increased the number of apoptotic cells in 24 h (Apetina, 1997). While our study did not find any difference in the number of apoptotic cells, the arrest in G0/G1 and reduction of cells in S phase was consistent with the current literature (Fong et al., 2007). To our knowledge, this is the first study that investigates the effect of bovine milk exosomes on the cell cycle progression in RAW 264.7 cells under hypoxia. Clearly, bovine milk exosomes were able to reverse the effect of hypoxia on the cell cycle of RAW 264.7 cells.
Fig. 4.
Effect of hypoxia and bovine milk treatment on the cell cycle of RAW 264.7 cells. Results are presented as mean ± SD. Means that do not share a letter are significantly different (p < 0.05).
4. Conclusion
Our study found that bovine milk exosomes are present in commercially available bovine milk suggesting that the pasteurization process does not destroy them. This is the first report on the ability of bovine milk exosomes to reverse hypoxia-induced reduction in macrophage cell proliferation associated with cell cycle arrest. This could have an impact on the ability of bovine milk exosomes to act as immunomodulatory molecules in human during cases of infection. Further investigation on a deeper understanding by which mechanism bovine milk exosomes reverse the effect of hypoxia in immune cells is guaranteed.
CRediT authorship contribution statement
Svjetlana Matic: Investigation, Formal analysis, Methodology, Writing – original draft, Formal analysis. Vermont P. Dia: Conceptualization, Methodology, writing – review, revision, Validation, Supervision, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
The work was partially supported by the University of Tennessee, Institute of Agriculture AgResearch and USDA HATCH TEN00585 to VPD.
Data availability
Data will be made available on request.
References
- Apetina E.D.G.L. Inflammatory mediators are perpetuated in macrophages resistant to apoptosis induced by hypoxia. Proc. Natl. Acad. Sci. U.S.A. 1997;94:13903–13908. doi: 10.1073/pnas.94.25.13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benmoussa A., Lee C.H.C., et al. Commercial dairy cow milk microRNAs resist digestion under simulated gastrointestinal. J. Nutr. 2016;146:2206–2215. doi: 10.3945/jn.116.237651.interspecies. [DOI] [PubMed] [Google Scholar]
- Chen T., Xie M.Y., Sun J.J., Ye R.S., Cheng X., Sun R.P., We L.M., Li M., Lin D.L., Jiang Q.Y., Xi Q.Y., Zhang Y.L. Procine milk-derived exosomes promote proliferation of intestinal epithelial cells. Sci. Rep. 2016;6 doi: 10.1038/srep33862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Zeng Q., Han Q., Xia W. Effect of pH , temperature and freezing-thawing on quantity changes and cellular uptake of exosomes. Protein and Cell. 2019;10(4):295–299. doi: 10.1007/s13238-018-0529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clanton T.L. Hypoxia-induced reactive oxygen species formation in skeletal muscle. J. Appl. Physiol. 2007;102:2379–2388. doi: 10.1152/japplphysiol.01298.2006. [DOI] [PubMed] [Google Scholar]
- Davies L.C., Jenkins S.J., Allen J.E., Taylor P.R. Tissue-resident macrophages. Nat. Immunol. 2013;14(10):986. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson T.L., Gores G.J., Herman B., et al. Mitochondria as a source of reactive oxygen species during reductive stress in rat hepatocytes. Am. J. Physiol. Cell Physiol. 1993;264:C961–C967. doi: 10.1152/ajpcell.1993.264.4.C961. [DOI] [PubMed] [Google Scholar]
- Distelhorst C.W. Apoptosis. Methods Mol. Biol. 2009;559:433–454. [Google Scholar]
- Elbendary A.A., Cirisano F.D., Evans A.C., et al. Relationship between p21 expression and mutation of the p53 tumor suppressor gene in normal and malignant ovarian epithelial cells. Clin. Cancer Res. 1996;2:1571–1575. [PubMed] [Google Scholar]
- Fong C., Zhang Q., Shi Y., Wu R.S.S., Fong W., Yang M. Effect of hypoxia on RAW264.7 macrophages apoptosis and signaling. Toxicology. 2007;235:52–61. doi: 10.1016/j.tox.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Gao H.N., Guo H.Y., Zhang H., Xie X.L., Wen P.C., Ren F.Z. Yak-milk-derived exosomes promote proliferation of intestinal epithelial cells in an hypoxic environment. J. Dairy Sci. 2019;102(2):985–996. doi: 10.3168/jds.2018-14946. [DOI] [PubMed] [Google Scholar]
- Greijer A.E., Wall E Van Der. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J. Clin. Pathol. 2004;57:1009–1014. doi: 10.1136/jcp.2003.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Nie S. The structure of mushroom polysaccharides and their beneficial role in health. Food Funct. 2015;6(10):3205–3217. doi: 10.1039/c5fo00678c. [DOI] [PubMed] [Google Scholar]
- Id B.L., Hock A., Wu R.Y., et al. Bovine milk-derived exosomes enhance goblet cell activity and prevent the development of experimental necrotizing enterocolitis. PLoS One. 2019;14 doi: 10.1371/journal.pone.0211431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi H., Tsuda M., Sato Y., Kosaka N., Ochiya T., Iwamoto H. Bovine milk exosomes contain microRNA and mRNA and are taken up by human macrophages. J. Dairy Sci. 2015;98:2920–2933. doi: 10.3168/jds.2014-9076. [DOI] [PubMed] [Google Scholar]
- Kato K., Nakane A., Minagawa T., et al. Human tumor necrosis factor increases the resistance against Listeria infection in mice. Med. Microbiol. Immunol. 1989;178:337–346. doi: 10.1007/BF00197452. [DOI] [PubMed] [Google Scholar]
- Krzywinska E., Stockmann C. Hypoxia , metabolism and immune cell function. Biomedicines. 2018;6:56. doi: 10.3390/biomedicines6020056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.H., Kim K., Jo Y.H., Hwang E., Chung J., Yang C. Reoxygenation speed and its implication for cellular injury responses in hypoxic RAW 264.7 cells. J. Surg. Res. 2017;227:88–94. doi: 10.1016/j.jss.2017.11.005. [DOI] [PubMed] [Google Scholar]
- Lee M., Ban J.-J., Wooseok Im, Kim M. Influence of storage condition on exosome recovery. Biotechnol. Bioproc. Eng. 2016;21:299–304. [Google Scholar]
- Lewis J.S., Lee J.A., Underwood J.C.E., Harris A.L., Lewis C.E. Macrophage responses to hypoxia : relevance to disease mechanisms. J. Leukoc. Biol. 1999;66:889–900. doi: 10.1002/jlb.66.6.889. [DOI] [PubMed] [Google Scholar]
- Matic S., D'Souza D., Wu T., Pangloli P., Dia V.P. Bovine milk exosomes affect proliferation and protect macrophages against cisplatin-induced cytotoxicity. Immunol. Invest. 2020;49(7):711–725. doi: 10.1080/08820139.2020.1769647. [DOI] [PubMed] [Google Scholar]
- Mu J., Zhuang X., Wang Q., et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol. Nutr. Food Res. 2014;58(7):1561–1573. doi: 10.1002/mnfr.201300729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munagala R., Aqil F., Jeyabalan J., Gupta R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016;371:48–61. doi: 10.1016/j.canlet.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch C., Muthana M., Lewis C.E. Hypoxia regulates macrophage functions in inflammation. J. Immunol. 2019;175:6257–6263. doi: 10.4049/jimmunol.175.10.6257. [DOI] [PubMed] [Google Scholar]
- Niel G Van, Angelo G.D., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- Panieri E., Santoro M.M. ROS homeostasis and metabolism : a dangerous liaison in cancer cells. Cell Death Dis. 2016;7(6) doi: 10.1038/cddis.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer K. Biological functions of tumor necrosis factor cytokines and their receptors. Cytokine Growth Factor Rev. 2003;14:185–191. doi: 10.1016/s1359-6101(03)00022-4. [DOI] [PubMed] [Google Scholar]
- Pieters B.C.H., Arntz O.J., Bennink M.B., Broeren M.G.A., Arjan P. Commercial cow milk contains physically stable extracellular vesicles expressing immunoregulatory TGF- β. PLoS One. 2015;10(3):1–14. doi: 10.1371/journal.pone.0121123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L., Zhang J., Zhang T. Immunomodulatories activities of polysaccharides from Ganoderma on immune effector cells. Food Chem. 2021;340 doi: 10.1016/j.foodchem.2020.127933. [DOI] [PubMed] [Google Scholar]
- Romo M.R. Innate immunity in vertebrates : an overview. Immunology. 2016;148:125–139. doi: 10.1111/imm.12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel M., Chisanga D., Liem M., et al. Bovine milk-derived exosomes from colostrum are enriched with proteins implicated in immune response and growth. Sci. Rep. 2017;7(June):5933. doi: 10.1038/s41598-017-06288-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S., Avadhani N.G. Hypoxia-mediated mitochondrial stress in RAW264.7 cells induced Osteoclast-like TRAP-positive cells. Ann. N. Y. Acad. Sci. 2007;1117:51–61. doi: 10.1196/annals.1402.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T., Rossignol F., Matthay M.A., et al. Prolonged hypoxia differentially regulates hypoxia-inducible factor (HIF)-1α and HIF-2α expression in lung epithelial cells. J. Biol. Chem. 2004;279(15):14871–14878. doi: 10.1074/jbc.M400461200. [DOI] [PubMed] [Google Scholar]
- Vlassov A.V., Magdaleno S., Setterquist R., Conrad R. Exosomes : current knowledge of their composition , biological functions , and diagnostic and therapeutic potentials. Biochim. Biophys. Acta. 2012;1820(7):940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Wiese M., Gerlach R.G., Popp I., Matuszak J., Mahapatro M., Castiglione K. Hypoxia-mediated impairment of the mitochondrial respiratory chain inhibits the bactericidal activity of macrophages. Infect. Immun. 2012;80:1455–1466. doi: 10.1128/IAI.05972-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong M., Elson G., Legarda D., Leibovich S.J. Production of vascular endothelial growth factor by murine macrophages. Am. J. Pathol. 1998;153(2):587–598. doi: 10.1016/S0002-9440(10)65601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Zou W., Du J., Zhao Y. The origins and homeostasis of monocytes and tissue-resident macrophages in physiological situation. J. Cell. Physiol. 2018;233:6425–6439. doi: 10.1002/jcp.26461. [DOI] [PubMed] [Google Scholar]
- Zheng L., Kelly C.J., Colgan S.P. Physiologic hypoxia and oxygen homeostasis in the healthy intestine . A review in the theme : cellular responses to hypoxia. Am. J. Physiol. Cell Physiol. 2015;309:350–360. doi: 10.1152/ajpcell.00191.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.