Abstract
The balance between oxidation and antioxidant is crucial for maintaining homeostasis. Once disrupted, it can lead to various pathological outcomes and diseases, such as depression. Oxidative stress can result in or aggravate a battery of pathological processes including mitochondrial dysfunction, neuroinflammation, autophagical disorder and ferroptosis, which have been found to be involved in the development of depression. Inhibition of oxidative stress and related pathological processes can help improve depression. In this regard, the nuclear factor erythroid 2-related factor 2 (Nrf2) in the antioxidant defense system may play a pivotal role. Nrf2 activation can not only regulate the expression of a series of antioxidant genes that reduce oxidative stress and its damages, but also directly regulate the genes related to the above pathological processes to combat the corresponding alterations. Therefore, targeting Nrf2 has great potential for the treatment of depression. Activation of Nrf2 has antidepressant effect, but the specific mechanism remains to be elucidated. This article reviews the key role of Nrf2 in depression, focusing on the possible mechanisms of Nrf2 regulating oxidative stress and related pathological processes in depression treatment. Meanwhile, we summarize some natural and synthetic compounds targeting Nrf2 in depression therapy. All the above may provide new insights into targeting Nrf2 for the treatment of depression and provide a broad basis for clinical transformation.
Keywords: Depression, Nrf2, Oxidative stress, Neuroinflammation, Autophagy, Ferroptosis
Abbreviations
- ARE
antioxidant response elements
- AA
arachidonic acid
- bZIP
basic-region leucine zipper
- BDNF
brain-derived neurotrophic factor
- BTB
bric-a-brac
- β-TrCP
β-transducin repeat-containing protein
- CREB
cAMP response element-binding protein
- CBP
CREB-binding protein
- CNC
Cap'n’collar
- CK2
Casein kinase II
- CAT
catalase
- CSF
cerebrospinal fluid
- CSDS
chronic social defeat stress
- CUMS
chronic unpredictable mild stress
- JNK
c-Jun N-terminal kinase
- CRP
C-reactive protein
- Cul3
Cullin 3
- DAMPs
damage-associated molecular patterns
- DMF
dimethyl fumarate
- DGR
double glycine repeat
- Drp1
dynamin-related protein 1
- ETC
electron transport chain
- ERK
extracellular regulated kinases
- FTH1
ferritin heavy chain 1
- FTL1
ferritin light chain 1
- GCL
glutamate-cysteine ligase
- GSH
glutathione
- GPX
glutathione peroxidase
- GR
glutathione reductase
- GST
glutathione S-transferase
- GSS
glutathione synthetase
- GSK-3β
glycogen synthase kinase-3β
- HO-1
heme oxygenase 1
- IDO
indoleamine-pyrrole 2,3-dioxygenase
- IL
interleukin
- Keap1
Kelch-like ECH-associated protein 1
- LIP
labile iron pools
- LC3
light chain 3
- LH
learned helplessness
- L-OH
lipid alcohols
- L-OOH
lipid hydroperoxides
- LPS
lipopolysaccharide
- MDD
major depressive disorder
- MDA
malondialdehyde
- mtDNA
mitochondrial DNA
- mPFC
medial prefrontal cortex
- MPTP
mitochondrial permeability transition pores
- MAO
monoamine oxidase
- MAOI
monoamine oxidase inhibitor
- NQO1
NAD(P)H dehydrogenase quinone 1
- NF-κB
nuclear factor-κB
- Nrf2
nuclear factor erythroid 2-related factor 2
- PRDX
peroxiredoxin
- PPARγ
Peroxisome proliferator-activated receptor gamma
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator alpha
- PUFA
polyunsaturated fatty acids
- PFC
prefrontal cortex
- PKC
Protein kinase C
- PKR
protein kinase R
- PERK
PKR-like endoplasmic reticulum kinase
- PINK1
PTEN-induced putative kinase 1
- MAPK
p38 mitogen-activated protein kinases
- RLS
reactive lipid species
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- rTMS
Repetitive transcranial magnetic stimulation
- RXRα
retinoic X receptor α
- SSRIs
selective serotonin reuptake inhibitors
- Sirt1
sirtuin-1
- sMaf
small musculoaponeurotic fibrosarcoma
- Srx1
sulfiredoxin 1
- SOD
Superoxide dismutase
- TRX
thioredoxin
- TrxR
thioredoxin reductase
- TFR1
Transferrin receptor 1
- TSPO
translocator protein
- TNF-α
tumor necrosis factor-α
- ULK1
unc-51-like kinase 1
- XO
xanthine oxidase
- 4-HNE
4-hydroxy-2-nonenal
- 5-HT
5-hydroxytryptamine
- 8-OHdG
8-OH 2-deoxyguanosine
1. Introduction
Depression is a mental disorder characterized by persistent low mood, decreased interest, cognitive impairment, sleep disorders, decreased appetite and suicidal tendencies, which severely limits the patients' psychosocial function and quality of life [1]. With the increasing pressure of modern life and work, a growing number of people suffer from mental illness, with about 350 million depressed patients worldwide. The World Health Organization ranks depression as the third leading cause of the global burden of disease in the world [2]. People with depression also have a significantly increased risk of cardiovascular disease, stroke, autoimmune disease, diabetes and cancer and are less responsive to treatment for these conditions.
Currently, antidepressants are the mainstay of treatment for depression. The commonly used first-line antidepressants, including serotonin or norepinephrine reuptake inhibitors and monoamine oxidase inhibitor (MAOI), aligns well with the monoamine theory of depression. This hypothesis states that the underlying pathophysiology of depression is a decrease in the levels of 5-hydroxytryptamine (5-HT), norepinephrine, and/or dopamine in the central nervous system [3]. However, traditional antidepressants act slowly and have multiple side effects, with a third of patients failing to respond, indicating that other factors may be involved in the pathogenesis of depression [4]. Therefore, it is an urgent need to further investigate the pathophysiology of depression and explore novel targets.
Oxidative stress is defined as an imbalance between the generation of reactive oxygen species (ROS) and the antioxidant defences [5]. Abundant evidence suggests that oxidative stress plays a critical role in the pathophysiology of depression [[6], [7], [8]]. ROS/reactive nitrogen species (RNS) refer to free radicals (superoxide, hydroxyl radical) or nonradical molecules (hydrogen peroxide) and their derivatives [9]. Physiological levels of ROS/RNS are involved in many metabolic processes in the organism [6,7,10]. However, overproduction of ROS/RNS can cause extensive damages to DNA, proteins and lipids [11]. The brain is a major consumer of oxygen and is rich in oxidative lipids, making it more vulnerable to damage from oxidative stress. Oxidative stress markers, such as 8-OH 2-deoxyguanosine (8-OHdG) and F2-isoprostanes are found to be significantly increased in major depressive disorder (MDD) [8].
Oxidative stress is intimately linked to a variety of pathophysiological processes, including neuroinflammation, dysfunction of mitochondria and autophagical disorder. These pathological processes have also been found to be involved in depression. Oxidative stress and inflammation may mutually promote under pathological conditions and form a co-activation state [12]. Elevated levels of proinflammatory cytokines have been found both peripherally and centrally in patients with depression [[13], [14], [15]]. Activation of inflammatory pathways, such as nuclear factor-κB (NF-κB) signaling, are found in rodent models of depression [16]. Mitochondria, which are responsible for energy production, are the main source of ROS (mtROS) [17]. An increased level of mtROS has been found in depression, indicating mitochondrial dysfunction [18]. Damaged electron transport chain (ETC), ATP production and mitochondrial DNA have been reported in depressed patients [19]. Additionally, ROS can induce autophagy by mediating multiple signaling pathways [20]. Autophagy is a process that degrades cellular waste and maintains tissue homeostasis. Altered autophagy-related genes and signaling have been observed in patients with MDD and animal models of depression, indicating dysfunction of autophagy [21,22]. Recently, the role of ferroptosis in depression has received attention. Ferroptosis, which is first proposed in 2012, is an iron- and lipid peroxidation-dependent form of cell death [23]. By using quantitative proteomics, the study by our team identifies activation of ferroptosis in mice models of chronic unpredictable mild stress (CUMS)-induced depression [24].
The main way to combat oxidative stress in the body is the antioxidant defense system,in which the nuclear factor erythroid 2-related factor 2 (Nrf2) acts as a master regulator [11,25]. Nrf2 is a transcription factor that can regulate a large number of antioxidant and cytoprotective genes [25,26]. By promoting the expression of these genes, Nrf2 is not only involved in the regulation of redox homeostasis, but also in the regulation of other processes, such as neuroinflammation, mitochondrial dysfunction, autophagical disorder and ferroptosis, making Nrf2 the hub for regulating these pathological processes. Dysregulation of the Nrf2 pathway may contribute to the development of a series of pathologies or diseases including psychiatric disorders and neurodegenerative diseases [26,27]. In fact, clinical and preclinical studies have demonstrated decreased expression of Nrf2 in the prefrontal cortex (PFC) of MDD patients and rodent models of depression [28,29]. Decreased protein levels of Nrf2 have been found in the postmortem brain from depressed patients compared with control subjects [30]. In the rodent models of depression, including learned helplessness (LH) paradigm and chronic social defeat stress (CSDS), the protein levels of Nrf2 in the medial prefrontal cortex (mPFC) and hippocampus are lower than those of control and LH/CSDS-resilient mice, which indicate that Nrf2 may contribute to stress resilience [31,32]. It is reported that Nrf2 knockout mice present depression-like phenotypes, which is associated with increased inflammation and decreased BDNF-TrkB signaling [29,33]. A large amount of studies suggest a crucial role of Nrf2 in the treatment of depression. The therapeutic effects of several antidepressants have been found to be strongly associated with Nrf2 [34,35]. Fluoxetine, a commonly-used SSRI, has anti-inflammatory and antioxidant properties in depression driven by alterations in Nrf2-dependent apoptosis and autophagy [34]. A new fast-acting antidepressant, (R)-ketamine, is shown to produce long-lasting antidepressant effects in Nrf2 KO mice via BDNF-TrkB activation [36]. Moreover, some Nrf2 activators, such as sulforaphane and dimethyl fumarate (DMF), exhibit significant antidepressant effects [37,38]. Therefore, targeting Nrf2 may represent a promising strategy for the treatment and prevention of depression.
In this review, we aim to highlight the critical role of Nrf2 in depression, in particular the possible mechanisms by which Nrf2 regulates related pathological processes that may be involved in the pathogenesis of depression, and further discuss natural and synthetic compounds that target Nrf2 in the treatment of depression.
2. The structure and regulation of Nrf2
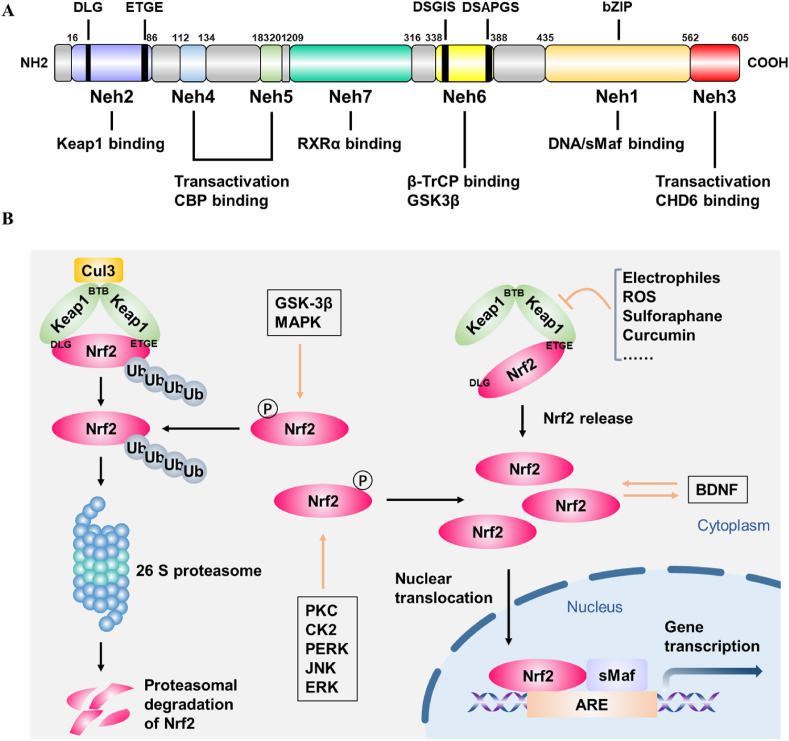
Nrf2,encoded by NFE2L2 gene, belongs to the Cap'n’collar (CNC) transcription factor family [39]. The Nrf2 protein is composed of 605 amino acids and contains seven highly conserved functional domains, named Nrf2-ECH homology 1 (Neh1)-Neh7 [39]. Neh1 has a basic-region leucine zipper (bZIP) motif which is required for combination with small musculoaponeurotic fibrosarcoma (sMaf) proteins and mediates Nrf2-antioxidant response elements (ARE) binding in the nucleus, thus promoting the transcription of various antioxidant enzymes [25]. Neh2 contains two motifs ETGE and DLG that are responsible for interaction with Kelch-like ECH-associated protein 1 (Keap1) and subsequent Keap1-dependent ubiquitination and proteasomal degradation of Nrf2 [40]. Neh3 modulates the activation of ARE-dependent genes by binding to the transcription coactivator, CHD6 [25]. Analogously, Neh4 and Neh5 can interact with cAMP response element-binding protein (CREB)-binding protein (CBP) and facilitate transcription activation [41]. Neh6 has DSGIS and DSAPGS motifs that can bind with β-transducin repeat-containing protein (β-TrCP) and is involved in the keap1-independent degradation of Nrf2 [40]. Neh6 also mediates Nrf2 phosphorylation by glycogen synthase kinase-3β (GSK-3β) [42]. Neh7 is responsible for inhibition of Nrf2-ARE signaling pathway through binding to the retinoic X receptor α (RXRα) [43] (Fig. 1A).
Fig. 1.
The structure and regulation of Nrf2. (A) The fundamental structure of Nrf2. (B) Keap1 -dependent and -independent regulation of Nrf2.
The activity of the NRF2-ARE signaling pathway is mainly regulated by Keap1, a major negative regulator of Nrf2 [44]. Keap1 relies on the bric-a-brac (BTB) domain to form homodimer, which then binds to the ETGE and DLG motifs of Nrf2 [25]. Under physiological conditions, Keap1-Nrf2 combines with E3 ubiquitin ligase complex Cullin 3 (Cul3) via the Neh6 domain of Nrf2, leading to the ubiquitination and proteasomal degradation of Nrf2 by the 26 S proteasome [41]. Under stressed conditions such as ROS accumulation, the double glycine repeat (DGR) domain of Keap1 undergoes conformational changes and the DLG motif dissociates from Keap1. This process inhibits the ubiquitination and degradation of Nrf2 protein. Nrf2 is released and translocates into the nucleus where it binds to sMaf and ARE and further increases the transcriptional activation of a series of antioxidant enzymes [45]. Apart from the canonical Keap1-Nrf2 regulation, there exists Keap1-independent regulation of Nrf2. Several kinases can phosphorylate Nrf2 and regulate its activity. Protein kinase C (PKC), Casein kinase II (CK2), protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK), c-Jun N-terminal kinase (JNK) and extracellular regulated kinases (ERK) phosphorylate Nrf2 and facilitate its translocation into the nucleus [[46], [47], [48], [49]]. Furthermore, BDNF can act as a steady-state regulator of Nrf2 activity and promote the nuclear translocation of Nrf2 [50]. On the contrary, Nrf2 phosphorylation by GSK-3β and p38 mitogen-activated protein kinases (MAPK) promote its degradation [42,48]. In addition, BACH1 and Hrd1 play negative regulatory roles on Nrf2 and repress the transcription of NRF2 target genes [43,51] (Fig. 1B).
3. The role of Nrf2 in depression
Although studies have shown that activation of Nrf2 can play an antidepressant role, the specific mechanisms of Nrf2 regulation in depression has not been fully elucidated. Since the target genes regulated by Nrf2 are involved in multiple pathological processes, its role in depression may also be multifaceted. Consequently,the role of Nrf2 in regulating oxidative stress, neuroinflammation, autophagy and ferroptosis in depression will be discussed separately.
3.1. Nrf2 and oxidative stress in depression
Studies have confirmed the dysregulation of redox homeostasis in the pathophysiology of depression [[52], [53], [54]]. Meta-analyses also indicate increased oxidative stress production and impaired antioxidant defenses in depression [55]. ROS, including superoxide radicals, hydrogen peroxide, hydroxyl radicals and singlet oxygen, are byproducts of aerobic metabolism [10]. Normal physiological levels of ROS are essential in regulation of cellular functions, such as neurogenesis, neuronal activities and cellular signaling pathways [6,7,10]. However, high levels of ROS cause damage to lipids, proteins and DNA, a process known as oxidative stress, and ultimately lead to cell disruption and apoptosis [11,56]. The production of ROS also increases the secretion of inflammatory mediators [57]. Chronic psychological or physiological stress may increase generation of ROS [58]. Moreover, RNS, which refers to various nitric oxide–derived compounds, often act together with ROS to damage cells and exacerbate the deleterious effects [10]. The cause of oxidative stress in depression has not been elucidated. Stress-induced hyperfunction of the HPA-axis may increase blood cortisol level and accelerate ROS production [57]. Oxidative stress markers, F2-isoprostanes and 8-OHdG, increased in the plasma and urine of patients with depression [[59], [60], [61], [62]]. The 8-OHdG is a derivative of deoxyguanosine and represents oxidative damage to DNA [59]. In addition to F2-isoprostanes, oxidative damage to lipids in depression is also indicated by increased concentration of malondialdehyde (MDA) [63]. MDA is a byproduct of polyunsaturated fatty acid peroxidation and an important feature of ferroptosis (see further). Elevated levels of xanthine oxidase (XO) and monoamine oxidase (MAO), enzymes involved in ROS production, have been detected in depressed patients and in postmortem depressed patients [9].
Under oxidative stress, the antioxidant defense system is activated to combat the cellular damage caused by ROS/RNS and maintain redox homeostasis [64,65]. Nrf2 is a major endogenous regulator of antioxidant defense [11]. When translocated into the nucleus, Nrf2 binds to ARE and induces the expression of a series of antioxidant enzymes [25]. These antioxidant enzymes involve multiple systems and are strongly linked to depression. The Nrf2 targets involved in the glutathione (GSH) system consist of GSH, glutamate-cysteine ligase (GCL), glutathione S-transferase (GST), glutathione synthetase (GSS), glutathione peroxidase (GPX) and glutathione reductase (GR) [66]. Decreased levels of GSH, GPX and GST Mu have been found in the post-mortem PFC from patients with MDD [67,68]. Reduced GSH level has also been observed in rodent brain exposed to chronic stress [69,70]. It is reported that administration of GSH centrally produces antidepressant-like effects in mice [71]. The thioredoxin (TRX)-based antioxidant system is another Nrf2-dependent system that maintains protein thiols in a reduced state [26]. This redoxin system contains TRX, peroxiredoxin (PRDX), thioredoxin reductase (TrxR) and sulfiredoxin 1 (Srx1). The level of TRX is significantly decreased in the brain of rats exposed to CUMS, which can be reversed by fluoxetine [72]. Studies have shown a reduced level of PRDX6 in the PFC of suicide victims and CUMS-induced rats [73]. In addition, the level of Srx1 is downregulated in depressed mice [74]. These results indicate that the TRX-PRDX system is compromised in depression. The expression of two antioxidant enzymes targeted by Nrf2, NAD(P)H dehydrogenase quinone 1 (NQO1) and heme oxygenase 1 (HO-1), is markedly decreased in rats under CUMS [75]. Administration of curcumin, an Nrf2 activator, can significantly increase the mRNA expression of NQO-1 and HO-1 and relieve depression-like state of rats [76]. Superoxide dismutase (SOD) and catalase (CAT) that belong to free radical scavengers are Nrf2 targets as well [66]. SOD breaks down the superoxide into oxygen molecules and H2O2, which is then scavenged by CAT to form water and oxygen [77]. Several studies have found lowered SOD activity in depressed patients [54,78]. Decreased SOD and CAT activities have also been found in the CUMS models [54]. The decreased activity of Nrf2 and changes of its regulated antioxidant enzyme profile indicate that Nrf2-mediated antioxidant stress defense was impaired in depression. In mice models of depression, Nrf2 translocation and activation of antioxidant enzymes can be prevented by the decreased levels of BDNF, leading to sustained oxidative stress [79]. Studies have demonstrated that various classes of antidepressants can lower the levels of oxidative stress and some antioxidants, especially Nrf2 activator, have potential antidepressant efficacy in depression [54,80,81].
ROS are mainly produced by mitochondria and accumulated ROS can in turn cause damage to mitochondria [82]. An increasing number of evidence indicates that there exists mitochondrial dysfunction in depression [83,84]. A series of mitochondrial disorders have been observed in patients with depression and animal models of depression [85]. For example, the levels of ATP production are lower in the brains and peripheral blood mononuclear cells of depressed patients compared to the controls [86,87]. Research from animals demonstrate the inhibition of oxidative phosphorylation and complexes I, III and IV of ETC as well as decreased ATP production [85,88]. Secondly, reduced activity of mitochondrial enzyme, damaged mitochondrial membrane potential, altered mitochondrial ultrastructure are found in mice with chronic mild stress-induce depression [88,89]. Increased amount of mtDNA deletions and mutations and decreased mtDNA copy number are detected in patients with depression [90,91]. These changes may result in reduced mitochondrial biogenesis and increased membrane permeability and mitochondrial ROS production, consequently, leading to impaired neurogenesis and synaptic plasticity and elevated apoptosis [85].
Nrf2 is closely related to mitochondrial function. Decreased Nrf2 levels in depression may contribute to mitochondrial dysfunction as evidenced by studies demonstrating that impaired mitochondrial function is observed after Nrf2 knockout in vivo and in vitro [11,92]. Through multiple pathways, Nrf2 plays a protective role in mitochondrial structure and function. Nrf2 promotes mitochondrial biogenesis by regulating the expression of sirtuin-1/peroxisome proliferator-activated receptor gamma coactivator alpha (SIRT1/PGC-1α) [93,94]. Nrf2 activation can increase proteasomal activity which induces decreased dynamin-related protein 1 (Drp1) protein levels and ultimately result in mitochondrial hyperfusion [95]. Nrf2 also affects mitochondrial anti-apoptotic proteins Bcl-2 and Bcl-xL [96]. Downregulation of Bcl-2 and Bcl-xL can lead to increased mitochondrial permeability transition pores (MPTP), which perpetuates mitochondrial damage and loss of function [97].
3.2. Nrf2 and autophagy in depression
Autophagy is a process that removes cytosolic pathogens or damaged cell components to maintain cellular survival and homeostasis [98]. Macroautophagy, also known as autophagy, is the most studied form of autophagy [99]. Cytosolic materials are first enwrapped by autophagosome, a double-membrane vesicular structure. Then this complex fuses with lysosome to form autolysosome, where the cytosolic materials are degraded and recycled [100]. Under normal conditions, autophagy is beneficial and plays an important roles in promoting neuronal growth, differentiation and synaptic plasticity [101,102]. Whereas autophagy disorders can affect neuronal development and synaptic plasticity, induce accumulation of cellular wastes such as misfolded proteins and exacerbate redox imbalances and inflammatory responses, leading to cell dysfunction and death [103]. In fact, dysregulation of autophagy have been demonstrated in depression. Patients with MDD exhibit increased expression of autophagy genes like microtubule associated protein 1 light chain 3 (LC3), ATG12, and Beclin 1 in the peripheral blood mononuclear cells [21]. The mTOR signaling pathway which is associated with autophagy is comprised in the postmortem brains of MDD patients [104]. Reduced expression of autophagy markers and increased autophagosome accumulation have been found in the prefrontal cortex and hippocampus of depressed mice [105]. P62, a Ub-binding autophagy receptor, is shown to be increased in the PFC of mice exposed to CUMS [106]. Meanwhile, studies have found that antidepressants have an impact on autophagy. Classic antidepressants amitriptyline and fluoxetine can induce autophagic flux in the hippocampus, which can be abolished by inhibition of Beclin 1 [107,108]. mTOR-dependent signaling pathway and beclin pathway may mediate the regulation of autophagy by antidepressant drugs [103]. Non-pharmacological treatment such as electroconvulsive therapy has also been shown to enhance autophagy [109]. Moreover, interventions that promote autophagy are reported to have antidepressant effects, suggesting that targeting autophagy may be a promising strategy for treating depression [110].
There is a direct crosstalk between Nrf2 and autophagy due to the fact that Nrf2 can regulate the expression of autophagy genes. Nrf2 can induce the expression of autophagy-related genes, including Atg5, p62, LC3B and unc-51-like kinase 1 (ULK1) [111]. Nrf2 can induce the expression of Sestrin2, which can inhibit mTORC1 expression, thereby indirectly activating selective autophagy [112,113]. Nrf2 knockdown reduces autophagic flux whereas Nrf2 activation promotes autophagy, which is intimately related to changes of p62/SQSTM1 levels [45]. P62 can compete with Nrf2 in binding to Keap1, resulting in the release and nucleus translocation of Nrf2 [25,114]. Therefore, the mutual regulation of p62 and Nrf2 constitutes a positive feedback loop that is associated with cytoprotection.
Recently, another particular form of autophagy, mitophagy, has also been found to play an important role in depression [18,115]. Mitophagy is a method for mitochondria to achieve self-control and renewal. By removing damaged or redundant mitochondria through autophagy, mitophagy maintained the stability of mitochondrial number and energy metabolism [115]. Damage of mitochondrial DNA (mtDNA) has been reported in patients with MDD [18]. Mice treated with chronic mild stress exhibit damaged mitochondrial ultrastructure in the hippocampus, cortex and hypothalamus [18]. Recent evidence suggests that the upregulated levels of Mfn-2, short Opa-1 and Fis-1 in MDD patients, indicating increased mitochondrial fragments [116]. Besides, increased Pink-1 levels and decreased Parkin levels of MDD patients suggest impaired clearance of damaged mitochondria since PTEN-induced putative kinase 1 (PINK1)/Parkin pathway can specifically recognize defective mitochondria and regulate mitophagy [116]. Redundant mitochondrial fragments can lead to apoptosis under stress conditions through excess ROS production, impaired bioenergetics and dysfunction of endogenous respiration. Release of mitochondrial fragments especially mtDNA can act as damage-associated molecular patterns (DAMPs) and further induce inflammatory response via interacting with TLR9 and activating NF-κB and NLRP3 inflammasome [117,118].
Intriguingly, mitophagy is directly regulated by Nrf2. Functional sequence analysis demonstrated the presence of ARE in the PINK1 promoter, which is the binding site of NRF2 [119]. Activation of endogenous Nrf2 can induce an increase of Pink1 expression, while silencing Nrf2 reversed the upregulated Pink1 level triggered by Nrf2 inducer tBHQ [119]. On the other hand, p62 is also involved in mitophagy. P62 can interacts with ubiquitinated molecules on the autophagosome [120]. Studies have shown that p62 can mediate autophagy with or without dependence on the Pink1/Parkin pathway [121]. P62 knockdown thoroughly blocked the clearance of damaged mitochondria [122]. These observations point out the potential involvement of Nrf2 in controlling mitophagy and mitochondrial integrity through modulating Pink1 and p62.
3.3. Nrf2 and neuroinflammation in depression
Numerous studies have emphasized the strong link between depression and inflammation. Elevated levels of pro-inflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) have been reported in the serum and cerebrospinal fluid (CSF) of patients with MDD [123]. Increased mRNA and protein levels of IL-1β, IL-6 and TNF-α have also been observed in the PFC of depressed individuals who died by suicide compared to controls [124]. Meanwhile, elevations of C-reactive protein (CRP), an acute-phase protein, and translocator protein (TSPO), a marker of neuroinflammation, have been found in patients with depression [125,126]. In animal studies, chronic stress can induce depression-like behaviors accompany with upregulation of pro-inflammatory cytokines levels. Administration of lipopolysaccharide (LPS) that activates the immune system and inflammatory responses can produce depressive-like behaviors in rodents [127]. Pro-inflammatory cytokines can also reduce brain serotonin through inducing indoleamine-pyrrole 2,3-dioxygenase (IDO), which metabolizes tryptophan [123]. Further evidence supporting the relationship of depression and inflammation come from the high comorbidity between depression and inflammation-related diseases, such as cancer, diabetes, cardiovascular diseases and inflammatory bowel disease [128,129].
Modulation of neuroinflammation may be one of the targets of antidepressant therapy. Classic antidepressants including selective serotonin reuptake inhibitors (SSRIs) have an impact on peripheral inflammation and can reduce inflammatory cytokines [130,131]. Several studies show that ketamine, a fast-acting antidepressant, also has immunomodulatory ability [128]. On the other hand, the antidepressant effects of anti-inflammatory agents have been studied. A meta-analysis comprised of 30 RCTs with 1610 participants suggest that anti-inflammatory agents, whether acting as adjunctive treatments or monotherapy, have significant antidepressant effects in comparison with placebo for patients with MDD [132].
The exact mechanisms by which inflammation plays a role in depression are not fully understood. Studies strongly indicate that microglia, the brain-resident immune cells, may play a central role in neuroinflammation of depression [133]. An increase of microglia activation has been found in both MDD patients and depressed animals [134,135]. Activated microglia are major source of inflammatory cytokines. It is assumed that the production of pro-inflammatory cytokines is mainly regulated by NF-κB signaling. NF-κB is an transcription factor belonging to Rel family and participates in regulating immune and inflammatory processes. Upon stress, IκBα is phosphorylated at serine residues and then becomes ubiquitinated and degraded. Subsequently NF-κB is phosphorylated by activated IκB and translocates into the nucleus, where it promotes the expression of its target genes, including IL-6, IL-1β, TNF-α, COX-2 and iNOS [125,136]. The pro-inflammatory cytokines in turn activate NF-κB and amplify inflammatory responses [137,138]. Antidepressants including SSRIs and SNRIs have been shown to alleviate the neuroinflammation through NF-κB inflammatory pathway and reducing cytokines in blood or tissue [139].
The finding that there exists a crosstalk between Nrf2 and NF-κB has established the anti-inflammatory role of Nrf2. Nrf2 can inhibit oxidative stress-mediated NF-κB activation through the mechanism of redox regulation. HO-1, one of the target genes of Nrf2, has been reported to inhibit NF-κB activity [140,141]. It is also shown that Nrf2 can restrain NF-κB-induced inflammatory response by competing with NF-κB to bind to CBP [39]. And NF-κB can in turn repress the transcriptional level of Nrf2 through this competition [142]. Nrf2-deficient mice have higher sensitivity to LPS-induced inflammation and enhanced levels of TNF-α and MMP-9 which are regulated by NF-κB, as well as depressive-like behaviors which can be reversed by the anti-inflammatory drug rofecoxib [33,143]. In addition, Nrf2 is reported to directly inhibit LPS-induced upregulation of proinflammatory cytokines, including IL-6 and IL-1β, through the ROS-independent transcriptional inhibition [144]. Studies also suggest that Nrf2 directly regulate certain anti-inflammatory mediators such as IL-17D and CD36 [145,146]. Several compounds that activate Nrf2 signaling have been shown to exert antidepressant effects via suppressing NF-κB signaling and lowering proinflammatory cytokines [45,147].
Recently, studies have found that Nrf2 inducers can activate the anti-inflammatory phenotype and inhibit the pro-inflammatory phenotype of microglia through regulating BDNF [50,148]. BDNF is a well-known neurotrophin that plays an important role in depression [149,150]. Emerging evidence suggest a close relationship between BDNF and neuroinflammation in depression [151]. Nrf2 KO mice showed decreased levels of BDNF-TrkB signaling and enhanced inflammation [29]. Activation of Nrf2 can transcriptionally activate BDNF through binding to the bdnf exon I promoter and decreasing transcriptional suppressors of BDNF (HDAC2, mSin3a, and MeCP2) [32]. BDNF can in turn activate the antioxidant defense mechanism by increasing the nuclear translocation of Nrf2 [50]. Hence, there seems to be a positive feedback loop between Nrf2 and BDNF in depression and the underlying mechanisms need further research.
3.4. Nrf2 and ferroptosis in depression
Ferroptosis is a novel form of regulated cell death characterized by iron-dependent lipid peroxidation which is distinct from other cell death processes in morphology and mechanism [152]. The classical pathologic processes of ferroptosis include excessive free iron, glutathione peroxidase 4 (GPX4) inactivation, and phospholipid oxidation of polyunsaturated fatty acids (PUFA) [23]. Excessive free iron acts as a critical initiator of ferroptosis although its precise role is unclear [153,154]. Ferroptosis can be inhibited by iron chelators such as deferoxamine and deferiprone [154,155]. GPX4 is a major negative regulator of ferroptosis. As a lipid repairing enzyme, GPX4 can transform the toxic lipid hydroperoxides (L-OOH) on the cell membrane into non-toxic lipid alcohols (L-OH) [154]. Deletion of GPX4 or inhibition of GPX4 expression can trigger ferroptosis [11,[153], [154], [155]]. The function of GPX4 requires GSH as a substrate, while GSH synthesis needs GCL and GSS and is regulated by the glutamate-cystine antiporter system xc- [152,154,155]. Inhibition of glutamate synthesis or XC - can induce ferroptosis as well [155]. Lipid peroxidation is the main characteristic of ferroptosis. The PUFA-containing phospholipids such as arachidonic acid (AA) and epinephrine are oxidized preferentially [156,157]. Lipid peroxidation alters the structure and function of the cell membrane, leading to cell dysfunction and extensive tissue damage. 4-hydroxy-2-nonenal (4-HNE) and MDA are reactive lipid species (RLS) generated by lipid peroxidation, which can initiate lipid peroxidation themselves.
Since it was defined in 2012, emerging evidence suggest that ferroptosis is involved in many diseases from cancer to a series of neurological diseases including stroke and AD [158,159]. Recent studies have shown a link between depression and ferroptosis. The study by our team using proteomics have found activation of ferroptosis in mice exposed to CUMS [24]. Further analysis demonstrates increased levels of MDA and ferritin light chain 1 (FTL1) and decreased levels of GPX4 and GSH in depressed mice which result in neuronal loss [24]. Mice exposed to chronic restraint stress exhibit iron homeostasis imbalance and iron accumulation [160]. Increased levels of lipid peroxidation have been repeatedly reported in patients with depression as evidenced by high levels of MDA and 4-HNE in the plasma [161,162]. High lipid peroxidation increases the risk of treatment-resistant depression [162]. Antidepressants including fluoxetine and citalopram can reduce MDA levels in depressed patients [163]. Post-mortem results of MDD patients showed decreased levels of GSH and GPX in the prefrontal cortex [67]. The single-nucleotide polymorphisms of GPX4 are found to be associated with the risk of depression [164]. As previously mentioned, the mitochondrial dysfunction observed in depression may also result from ferroptosis. Taken together, these studies provide evidence of ferroptosis existing in depression and regulating ferroptosis may be a promising treatment for depression.
Numerous studies indicate that Nrf2 can regulate ferroptosis through multiple aspects. Actually, almost all ferroptosis-related genes are directly or indirectly regulated by Nrf2. Nrf2 targets genes that are related to iron homeostasis such as HO-1, ferritin heavy chain 1 (FTH1), FTL, FPN1 (SLC40A1) and Transferrin receptor 1 (TFR1) [[165], [166], [167], [168]]. FTH1 and FTL are two subunits of the cytosolic iron storage protein ferritin, which sequesters excess free iron and maintains labile iron pools (LIP) homeostasis [169]. FPN1 and TFR1 are responsible for exporting excess iron from cells and importing iron into cells respectively. NRF2 regulates genes involved in heme biosynthesis and transport and thus indirectly regulates iron metabolism [170]. The enzymes that are essential for GSH synthesis including GCLC, GCLM and GSS, as well as SLC7A11, an important subunit of xc-, all of which promotes the synthesis of GPX4 and further mediates suppression of ferroptosis, are targets of Nrf2 [171]. Moreover, Nrf2 is required for the basal expression of GPX4 [172]. Nrf2 also regulates a number of genes involved in lipid metabolism in ferroptosis. Peroxisome proliferator-activated receptor gamma (PPARγ), a major regulator of lipid metabolism, activates with Nrf2 mutually and initiates ferroptosis [171]. Although studies on Nrf2 regulation of ferroptosis in depression are very rare, a recent study have shown that edaravone, a free radical scavenger, can reduce oxidative stress and ferroptosis and alleviate depressive symptoms through the Sirt1/Nrf2/HO-1/Gpx4 pathway in chronic social defeat stress (CSDS) depression model [173].
4. Targeting Nrf2 to treat depression
Since Nrf2 activation can play a neuroprotective role by modulating a variety of pathological processes, many natural and synthetic compounds have been shown to activate the Nrf2 pathway and used to treat various neurological diseases, some of which have also been reported in the treatment of depression.
4.1. Natural NRF2 activators
Sulforaphane, an organosulfur compound isolated from Brassicaceae plants, is a potent natural NRF2 activator. Sulforaphane has been reported to increase Nrf2 expression and nuclear localization as well as interact with Cys151 residue of KEAP1 to inhibit Nrf2 degradation [174]. Several studies have evaluated the role of sulforaphane in depression. It is reported that sulforaphane exerts antidepressant- and anxiolytic-like activities and inhibits HPA axis and inflammatory response in chronic stress-induced depression models [37]. Another study have found that sulforaphane has both therapeutic and prophylactic effects on inflammation-related depression [175]. It is also found that sulforaphane can promote the binding of Nrf2 and BDNF in CSDS mice models, indicating that sulforaphane may confer stress resilience [32]. Moreover, sulforaphane has also been found to protect neurons via autophagy and promote mitochondrial biogenesis by activating Nrf2 [176].
Curcumin is naturally occurring polyphenolic compound derived from rhizomes of Curcuma longa and has anti-inflammatory and anti-oxidant properties [177]. Curcumin can also bind to Cys151 residue of KEAP1, leading to the Nrf2-ARE binding and an increase of HO-1 expression [[178], [179], [180]]. It is reported recently that curcumin can activate Nrf2 through PKCδ-mediated p62 phosphorylation at S351 [181]. Meta-analysis reveals that curcumin is effective in improving depressive and anxiety symptoms [182]. Curcumin exerts antidepressant and antioxidant effects in CUMS model of depression [183]. In a LPS-induced rat model of depression, curcumin is found to inhibit oxidative stress, inflammation and neuronal apoptosis [184].
Quercetin is a natural polyphenol flavonoid compound abundantly present in fruits and vegetables. Quercetin manifests potent free-radical scavenging activity and can reduce ROS levels [185]. In vitro, the administration of quercetin was able to promote Nrf2 nuclear translocation, upregulate the expression of GCLC and increase total GSH levels [186]. In mice subjected to CUMS, quercetin at 40 mg/kg relieves depressive-like behaviors and reverses the abnormalities of Nrf2, HO-1, SOD, GST, MDA and NO, indicating the antioxidant and anti-inflammatory role of quercetin [187].
Resveratrol is a naturally polyphenolic compound found in grapes, berries, and nuts. Studies have shown that resveratrol can alleviate cell oxidative damage by reducing MDA levels through activating Nrf2/HO-1 [188]. Anti-inflammatory and anti-apoptotic effects of resveratrol have also been reported in several studies [[189], [190], [191], [192]]. Resveratrol is reported to alleviate depression-like symptoms and accompanied oxidative stress via reducing MDA and increasing SOD in rats exposed to CUMS. In addition, resveratrol can reduce the expression of NF-κB and pro-inflammatory cytokines in LPS-induced depression [193].
Melatonin,mainly produced by the pineal gland, is an endogenous neuro-hormone regulating circadian and seasonal rhythms. Numerous studies have shown that melatonin activates Nrf2 and its target phase-2 antioxidative enzymes including HO-1, NQO-1 and SOD [194]. It is shown that melatonin ameliorates LTP-induced depression-like behaviors in mice and suppress the production of mitochondrial and cytosolic ROS and NF-κB signaling through Sirt2/Nrf2 pathway [195]. Using the same depression model, another study have found that melatonin can exert pro-autophagy and anti-inflammatory effects [196].
Lycopene is an aliphatic hydrocarbon carotenoid extracted from tomatoes, watermelons and papayas. Lycopene has been reported to exert prophylactic and therapeutic effects in multiple neurological diseases including depression [197]. Lycopene can upregulate the expressions of HO-1 and NQO-1, and downregulate inflammatory cytokines IL-1β and TNF-α through activation of Nrf2 signaling and inactivation of NF-κB translocation [198]. Pretreatment of lycopene ameliorates LPS-induced depression-like behaviors, reduces the levels of IL-6 and TNF-α in the plasma and alleviates neuronal injury in the hippocampus [199]. In vitro, lycopene inhibits LPS-induced COX-2 expression through HO-1 activation in microglia [200]. However, the role of lycopene activating Nrf2 in depression needs more research.
4.2. Synthetic NRF2 activators
TBHQ is a synthetic phenolic antioxidant and commonly used as a food preservative. TBHQ activates Nrf2 through electrophilic modification of Keap1 [201]. Through inducing Nrf2, TBHQ activates enzymes of glutathione system such as GST, GCL, GSH-Px and GR and inhibits the NOX4 transcription, thereby decreasing the levels of ROS and MDA [202]. It has been shown that TBHQ ameliorates methamphetamine-induced depression-like behaviors, which is associated with reduced ROS and apoptosis levels resulted from activation of Nrf2/HO-1 and PI3K/AKT [203]. TBHQ is also found to promote autophagy in an Nrf2-dependent pathway in depressed mice [34]. Recently another study demonstrates that TBHQ alleviates LPS-induced depression-like behaviors by suppressing neuroinflammation in mice [204].
DMF is an Nrf2 activator approved by US Food and Drug Administration used for the treatment of relapsing multiple sclerosis. DMF has been found to promote Nrf2 transcription by modifying the Cys151 residue resides in the BTB domain of the Keap1 [205]. It is shown recently that DMF can also bind to the β-propeller domain (Keap1-DC) of Keap1 [206]. DMF was reported to mitigate CUMS induced anxiety- and depressive-like behaviors as well as neuinflammation through Nrf2 signaling [38]. DMF is also found to promote mitochondrial biogenesis in vitro and in vivo [207]. Recent study suggests that DMF can inhibit ferroptosis in chronic cerebral hypoperfusion model [208]. The multiple mechanisms of DMF in the treatment of depression need to be further investigated.
Edaravone is a free radical scavenger. Through activating Nrf2 pathway, edaravone plays a protective role in a variety of neurological diseases such as acute cerebral infarction, traumatic brain injury, amyotrophic lateral sclerosis and epilepsy [[209], [210], [211]]. Edaravone is found to have significant effects on corticosterone-, LPS-, and CRS-induced mice models of depression [[212], [213], [214]]. In a recent study, it is shown that edaravone ameliorates CSDS-induced depression and anxiety-related behaviors, prevents oxidative stress and mitochondrial damage as well as neuroinflammation and these effects can be abolished by Nrf2 and GPX4 inhibitor, indicating that Gpx4-mediated ferroptosis via Sirt1/Nrf2/HO-1 pathway may be involved [173].
TBE-31 and MCE-1 TBE-31 is a tricyclic compound containing two cyano enones, both of which can bind with cysteine residues of Keap1, making it a highly potent Nrf2 activator [215,216]. In an LPS-induced inflammatory model of depression, TBE-31 is found to significantly attenuate depression-like behaviors and decrease the serum level of TNF-α in mice [217].
In addition, some non-pharmacologic modulation have been found to exert beneficial effects by targeting Nrf2 pathway. Repetitive transcranial magnetic stimulation (rTMS) is a noninvasive and safe treatment for depression, but its underlying mechanism has not been fully elucidated. rTMS is proved to regulate synaptic plasticity, neurogenesis and inflammatory response in mice models of depression [218,219]. In the CUMS-induced rat depression model, rTMS increases Nrf2 nuclear translocation and reduces levels of inflammatory cytokines in the hippocampus [220]. Physical exercise is an effective intervention for depression but its mechanism is still unclear. Previous studies indicate that physical activity can improve depressive symptoms though regulating inflammation, oxidative stress and neuroplasticity [221]. Moreover, exercise has been shown to activate Nrf2 signaling in multiple tissues including brain in rodent models [222,223]. It is reasonable to speculate that Nrf2 activation may mediate the antidepressant effects of physical exercise, which still need further research.
5. Conclusion
A large amount of evidence indicate that oxidative stress and impaired antioxidant defense system induced by the production of ROS/RNS play an important role in the pathogenesis of depression. Oxidative stress can lead to or aggravate a series of pathological processes including mitochondrial dysfunction, neuroinflammation, autophagical disorder and ferroptosis. Boosting antioxidant defenses has beneficial effects in the treatment of depression. NRF2 is a key endogenous regulator in the protection against oxidative stress. Activation of Nrf2 holds great promise in depression therapy. On the one hand, activation of Nrf2 promotes the transcription of antioxidant genes to combat oxidative stress. On the other hand, Nrf2 activation can affect the transcription of genes that directly regulate mitochondrial dysfunction, neuroinflammation, autophagical disorder and ferroptosis. Therefore, Nrf2 plays a neuroprotective role via regulating multiple pathways (Fig. 2). Targeting the Nrf2 pathway has shown exciting results in various depression models and in vitro experiments. However, since Nrf2 regulates multiple processes, the potential of Nrf2 activators in the treatment of depression needs to be further carefully investigated. Some natural and synthetic compounds acting as Nrf2 activators have shown significant antidepressant effects in preclinical experiments and have great potential for clinical translation. Although some fundamental questions remain to be addressed before these novel compounds targeting Nrf2 can be further used in clinical trials. Moreover, several non-pharmacological interventions such as rTMS and physical exercise have been shown to exert antidepressant effects by activating Nrf2 signaling.
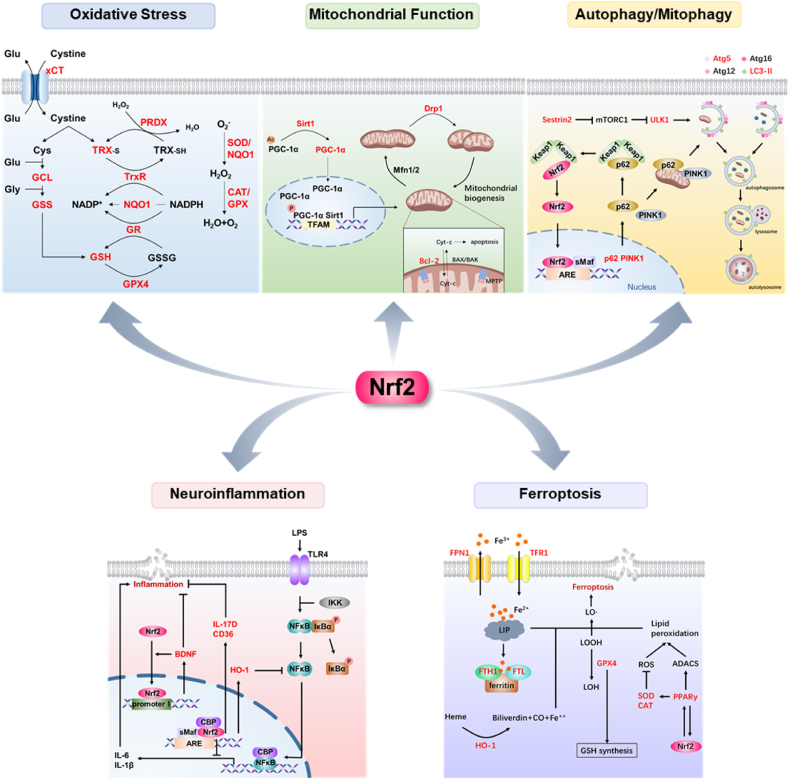
Fig. 2.
Schematic representation of the multifaceted role of Nrf2 in depression. The target molecules of Nrf2 (marked in red) are invovled in oxidative stress, mitochondrial function, autophagy/mitophagy, neuroinflammation and ferroptosis. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Funding
This work was supported by the National Key Research and Development Program of Hubei Province (2020BCA089), the National Key Research and Development Program of China (2020YFC2006001), the National Natural Science Foundation of China (81974218, 81671064).
Declaration of competing interest
None.
Data availability
No data was used for the research described in the article.
References
- 1.McCarron R.M., Shapiro B., Rawles J., Luo J. Depression. Ann Intern Med. 2021;174:ITC65–ITC80. doi: 10.7326/AITC202105180. [DOI] [PubMed] [Google Scholar]
- 2.Suicide Worldwide in 2019: Global Health Estimates. World Health Organization; Geneva: 2021. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 3.Cowen P.J., Browning M. What has serotonin to do with depression? World Psychiatr. : Off. J. World. Psychiatr. Assoc.(WPA). 2015;14:158–160. doi: 10.1002/wps.20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hindmarch I. Beyond the monoamine hypothesis: mechanisms, molecules and methods. Eur. Psychiatr. : Off. J. Assoc. Eur. Psychiatr. 2002;17(Suppl 3):294–299. doi: 10.1016/s0924-9338(02)00653-3. [DOI] [PubMed] [Google Scholar]
- 5.Betteridge D.J. What is oxidative stress? Metab. Clin. Exp. 2000;49:3–8. doi: 10.1016/s0026-0495(00)80077-3. [DOI] [PubMed] [Google Scholar]
- 6.Bhatt S., Nagappa A.N., Patil C.R. Role of oxidative stress in depression. Drug Discov. Today. 2020;25:1270–1276. doi: 10.1016/j.drudis.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 7.Moylan S., et al. Oxidative & nitrosative stress in depression: why so much stress? Neurosci. Biobehav. Rev. 2014;45:46–62. doi: 10.1016/j.neubiorev.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Lindqvist D., et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology. 2017;76:197–205. doi: 10.1016/j.psyneuen.2016.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vavakova M., Durackova Z., Trebaticka J. Markers of oxidative stress and neuroprogression in depression disorder. Oxid. Med. Cell. Longev. 2015 doi: 10.1155/2015/898393. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pizzino G., et al. Oxidative stress: harms and benefits for human health. Oxid. Med. Cell. Longev. 2017 doi: 10.1155/2017/8416763. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qu Z., Sun J., Zhang W., Yu J., Zhuang C. Transcription factor NRF2 as a promising therapeutic target for Alzheimer's disease. Free Radic. Biol. Med. 2020;159:87–102. doi: 10.1016/j.freeradbiomed.2020.06.028. [DOI] [PubMed] [Google Scholar]
- 12.Cao H., et al. High frequency repetitive transcranial magnetic stimulation alleviates cognitive deficits in 3xTg-AD mice by modulating the PI3K/Akt/GLT-1 axis. Redox Biol. 2022;54 doi: 10.1016/j.redox.2022.102354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Syed S.A., et al. Defective inflammatory pathways in never-treated depressed patients are associated with poor treatment response. Neuron. 2018;99:914–924. doi: 10.1016/j.neuron.2018.08.001. e913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Köhler C.A., et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr. Scand. 2017;135:373–387. doi: 10.1111/acps.12698. [DOI] [PubMed] [Google Scholar]
- 15.Miller A.H., Raison C.L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rawdin B.J., et al. Dysregulated relationship of inflammation and oxidative stress in major depression. Brain Behav. Immun. 2013;31:143–152. doi: 10.1016/j.bbi.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dan Dunn J., Alvarez L.A., Zhang X., Soldati T. Reactive oxygen species and mitochondria: a nexus of cellular homeostasis. Redox Biol. 2015;6:472–485. doi: 10.1016/j.redox.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tripathi A., Scaini G., Barichello T., Quevedo J., Pillai A. Mitophagy in depression: pathophysiology and treatment targets. Mitochondrion. 2021;61:1–10. doi: 10.1016/j.mito.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Czarny P., Wigner P., Galecki P., Sliwinski T. The interplay between inflammation, oxidative stress, DNA damage, DNA repair and mitochondrial dysfunction in depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2018;80:309–321. doi: 10.1016/j.pnpbp.2017.06.036. [DOI] [PubMed] [Google Scholar]
- 20.Gao Q. Oxidative stress and autophagy. Adv. Exp. Med. Biol. 2019;1206:179–198. doi: 10.1007/978-981-15-0602-4_9. [DOI] [PubMed] [Google Scholar]
- 21.Alcocer-Gómez E., Casas-Barquero N., Núñez-Vasco J., Navarro-Pando J.M., Bullón P. Psychological status in depressive patients correlates with metabolic gene expression. CNS Neurosci. Ther. 2017;23:843–845. doi: 10.1111/cns.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Z., et al. Rosiglitazone exerts an anti-depressive effect in unpredictable chronic mild-stress-induced depressive mice by maintaining essential neuron autophagy and inhibiting excessive astrocytic apoptosis. Front. Mol. Neurosci. 2017;10:293. doi: 10.3389/fnmol.2017.00293. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Dixon S.J., et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao H., et al. Hippocampal proteomic analysis reveals activation of necroptosis and ferroptosis in a mouse model of chronic unpredictable mild stress-induced depression. Behav. Brain Res. 2021;407 doi: 10.1016/j.bbr.2021.113261. [DOI] [PubMed] [Google Scholar]
- 25.Zhang W., Feng C., Jiang H. Novel target for treating Alzheimer's Diseases: crosstalk between the Nrf2 pathway and autophagy. Ageing Res. Rev. 2021;65 doi: 10.1016/j.arr.2020.101207. [DOI] [PubMed] [Google Scholar]
- 26.Zgorzynska E., Dziedzic B., Walczewska A. An overview of the Nrf2/ARE pathway and its role in neurodegenerative diseases. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22179592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hashimoto K. Essential role of keap1-nrf2 signaling in mood disorders: overview and future perspective. Front. Pharmacol. 2018;9:1182. doi: 10.3389/fphar.2018.01182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martín-Hernández D., et al. Intracellular inflammatory and antioxidant pathways in postmortem frontal cortex of subjects with major depression: effect of antidepressants. J. Neuroinflammation. 2018;15:251. doi: 10.1186/s12974-018-1294-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao W., et al. Role of Keap1-Nrf2 signaling in depression and dietary intake of glucoraphanin confers stress resilience in mice. Sci. Rep. 2016;6 doi: 10.1038/srep30659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J.C., et al. Keap1-Nrf2 signaling pathway confers resilience versus susceptibility to inescapable electric stress. Eur. Arch. Psychiatr. Clin. Neurosci. 2018;268:865–870. doi: 10.1007/s00406-017-0848-0. [DOI] [PubMed] [Google Scholar]
- 31.Li S., et al. Role of keap1-nrf2 signaling in anhedonia symptoms in a rat model of chronic neuropathic pain: improvement with sulforaphane. Front. Pharmacol. 2018;9:887. doi: 10.3389/fphar.2018.00887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao W., et al. Activation of BDNF by transcription factor Nrf2 contributes to antidepressant-like actions in rodents. Transl. Psychiatry. 2021;11:140. doi: 10.1038/s41398-021-01261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martín-de-Saavedra M.D., et al. Nrf2 participates in depressive disorders through an anti-inflammatory mechanism. Psychoneuroendocrinology. 2013;38:2010–2022. doi: 10.1016/j.psyneuen.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 34.Ghosh S., et al. Inflammation-induced behavioral changes is driven by alterations in Nrf2-dependent apoptosis and autophagy in mouse hippocampus: role of fluoxetine. Cell. Signal. 2020;68 doi: 10.1016/j.cellsig.2019.109521. [DOI] [PubMed] [Google Scholar]
- 35.Martín-Hernández D., et al. Modulation of the antioxidant nuclear factor (erythroid 2-derived)-like 2 pathway by antidepressants in rats. Neuropharmacology. 2016;103:79–91. doi: 10.1016/j.neuropharm.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 36.Qu, Y. et al. Rapid-acting and long-lasting antidepressant-like action of (R)-ketamine in Nrf2 knock-out mice: a role of TrkB signaling. Eur. Arch. Psychiatr. Clin. Neurosci. 271, 439-446, doi:10.1007/s00406-020-01208-w(2021). [DOI] [PubMed]
- 37.Wu S., et al. Sulforaphane produces antidepressant- and anxiolytic-like effects in adult mice. Behav. Brain Res. 2016;301:55–62. doi: 10.1016/j.bbr.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 38.de Souza, A. G. et al. Neuroprotective effects of dimethyl fumarate against depression-like behaviors via astrocytes and microglia modulation in mice: possible involvement of the HCAR2/Nrf2 signaling pathway. Naunyn-Schmiedeberg’s Arch. Pharmacol. 395, 1029-1045, doi:10.1007/s00210-022-02247-x(2022). [DOI] [PubMed]
- 39.Saha S., Buttari B., Panieri E., Profumo E., Saso L. An overview of Nrf2 signaling pathway and its role in inflammation. Molecules. 2020;25 doi: 10.3390/molecules25225474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohan S., Gupta D. Crosstalk of toll-like receptors signaling and Nrf2 pathway for regulation of inflammation. Biomed. Pharmacother. 2018;108:1866–1878. doi: 10.1016/j.biopha.2018.10.019. [DOI] [PubMed] [Google Scholar]
- 41.Sivandzade F., Prasad S., Bhalerao A., Cucullo L. NRF2 and NF-B interplay in cerebrovascular and neurodegenerative disorders: molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019;21 doi: 10.1016/j.redox.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chowdhry S., et al. Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene. 2013;32:3765–3781. doi: 10.1038/onc.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Osama A., Zhang J., Yao J., Yao X., Fang J. Nrf2: a dark horse in Alzheimer's disease treatment. Ageing Res. Rev. 2020;64 doi: 10.1016/j.arr.2020.101206. [DOI] [PubMed] [Google Scholar]
- 44.Saha S., Buttari B., Profumo E., Tucci P., Saso L. A perspective on Nrf2 signaling pathway for neuroinflammation: a potential therapeutic target in alzheimer's and Parkinson's diseases. Front. Cell. Neurosci. 2021;15 doi: 10.3389/fncel.2021.787258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dinkova-Kostova A.T., Kostov R.V., Kazantsev A.G. The role of Nrf2 signaling in counteracting neurodegenerative diseases. FEBS J. 2018;285:3576–3590. doi: 10.1111/febs.14379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang H.C., Nguyen T., Pickett C.B. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J. Biol. Chem. 2002;277:42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- 47.Pi J., et al. Molecular mechanism of human Nrf2 activation and degradation: role of sequential phosphorylation by protein kinase CK2. Free Radic. Biol. Med. 2007;42:1797–1806. doi: 10.1016/j.freeradbiomed.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Keum Y.S., et al. Mechanism of action of sulforaphane: inhibition of p38 mitogen-activated protein kinase isoforms contributing to the induction of antioxidant response element-mediated heme oxygenase-1 in human hepatoma HepG2 cells. Cancer Res. 2006;66:8804–8813. doi: 10.1158/0008-5472.Can-05-3513. [DOI] [PubMed] [Google Scholar]
- 49.Cullinan S.B., Diehl J.A. PERK-dependent activation of Nrf2 contributes to redox homeostasis and cell survival following endoplasmic reticulum stress. J. Biol. Chem. 2004;279:20108–20117. doi: 10.1074/jbc.M314219200. [DOI] [PubMed] [Google Scholar]
- 50.Yu Y., Li Y., Qi K., Xu W., Wei Y. Rosmarinic acid relieves LPS-induced sickness and depressive-like behaviors in mice by activating the BDNF/Nrf2 signaling and autophagy pathway. Behav. Brain Res. 2022;433 doi: 10.1016/j.bbr.2022.114006. [DOI] [PubMed] [Google Scholar]
- 51.Wu T., et al. Hrd1 suppresses Nrf2-mediated cellular protection during liver cirrhosis. Gene Dev. 2014;28:708–722. doi: 10.1101/gad.238246.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sies H., Berndt C., Jones D.P. Oxidative stress. Annu. Rev. Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 53.Pandya C.D., Howell K.R., Pillai A. Antioxidants as potential therapeutics for neuropsychiatric disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2013;46:214–223. doi: 10.1016/j.pnpbp.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maes M., Galecki P., Chang Y.S., Berk M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2011;35:676–692. doi: 10.1016/j.pnpbp.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 55.Palta P., Samuel L.J., Miller E.R., 3rd, Szanton S.L. Depression and oxidative stress: results from a meta-analysis of observational studies. Psychosom. Med. 2014;76:12–19. doi: 10.1097/psy.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Michel T.M., Pülschen D., Thome J. The role of oxidative stress in depressive disorders. Curr. Pharmaceut. Des. 2012;18:5890–5899. doi: 10.2174/138161212803523554. [DOI] [PubMed] [Google Scholar]
- 57.Juszczyk G., et al. Chronic stress and oxidative stress as common factors of the pathogenesis of depression and alzheimer's disease: the role of antioxidants in prevention and treatment. Antioxidants. 2021;10 doi: 10.3390/antiox10091439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Subba R., Ahmad M.H., Ghosh B., Mondal A.C. Targeting NRF2 in Type 2 diabetes mellitus and depression: efficacy of natural and synthetic compounds. Eur. J. Pharmacol. 2022;925 doi: 10.1016/j.ejphar.2022.174993. [DOI] [PubMed] [Google Scholar]
- 59.Forlenza M.J., Miller G.E. Increased serum levels of 8-hydroxy-2'-deoxyguanosine in clinical depression. Psychosom. Med. 2006;68:1–7. doi: 10.1097/01.psy.0000195780.37277.2a. [DOI] [PubMed] [Google Scholar]
- 60.Irie M., Miyata M., Kasai H. Depression and possible cancer risk due to oxidative DNA damage. J. Psychiatr. Res. 2005;39:553–560. doi: 10.1016/j.jpsychires.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 61.Chung C.P., Schmidt D., Stein C.M., Morrow J.D., Salomon R.M. Increased oxidative stress in patients with depression and its relationship to treatment. Psychiatr. Res. 2013;206:213–216. doi: 10.1016/j.psychres.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Savage K., et al. The relationship between F(2)-isoprostanes plasma levels and depression symptoms in healthy older adults. Antioxidants. 2022;11 doi: 10.3390/antiox11050822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ayala A., Muñoz M.F., Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014 doi: 10.1155/2014/360438. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Villavicencio Tejo F., Quintanilla R.A. Contribution of the Nrf2 pathway on oxidative damage and mitochondrial failure in Parkinson and alzheimer's disease. Antioxidants. 2021;10 doi: 10.3390/antiox10071069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang Y., et al. An overview of the molecular mechanisms and novel roles of Nrf2 in neurodegenerative disorders. Cytokine Growth Factor Rev. 2015;26:47–57. doi: 10.1016/j.cytogfr.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 66.He F., Ru X., Wen T. NRF2, a transcription factor for stress response and beyond. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21134777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gawryluk J.W., Wang J.F., Andreazza A.C., Shao L., Young L.T. Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int. J. Neuropsychopharmacol. 2011;14:123–130. doi: 10.1017/s1461145710000805. [DOI] [PubMed] [Google Scholar]
- 68.Gawryluk J.W., et al. Prefrontal cortex glutathione S-transferase levels in patients with bipolar disorder, major depression and schizophrenia. Int. J. Neuropsychopharmacol. 2011;14:1069–1074. doi: 10.1017/s1461145711000617. [DOI] [PubMed] [Google Scholar]
- 69.Madrigal J.L., et al. Glutathione depletion, lipid peroxidation and mitochondrial dysfunction are induced by chronic stress in rat brain. Neuropsychopharmacology. 2001;24:420–429. doi: 10.1016/s0893-133x(00)00208-6. official publication of the American College of Neuropsychopharmacology. [DOI] [PubMed] [Google Scholar]
- 70.Sahin E., Gümüşlü S. Alterations in brain antioxidant status, protein oxidation and lipid peroxidation in response to different stress models. Behav. Brain Res. 2004;155:241–248. doi: 10.1016/j.bbr.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 71.Rosa J.M., Dafre A.L., Rodrigues A.L.S. Antidepressant-like responses in the forced swimming test elicited by glutathione and redox modulation. Behav. Brain Res. 2013;253:165–172. doi: 10.1016/j.bbr.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 72.Song Y., et al. Perilla aldehyde attenuates CUMS-induced depressive-like behaviors via regulating TXNIP/TRX/NLRP3 pathway in rats. Life Sci. 2018;206:117–124. doi: 10.1016/j.lfs.2018.05.038. [DOI] [PubMed] [Google Scholar]
- 73.Liao, J., Zhang, Y., Chen, X. & Zhang, J. The roles of peroxiredoxin 6 in brain diseases. Mol. Neurobiol. 58, 4348-4364, doi:10.1007/s12035-021-02427-5(2021). [DOI] [PubMed]
- 74.Kwatra M., et al. Lipopolysaccharide exacerbates chronic restraint stress-induced neurobehavioral deficits: mechanisms by redox imbalance, ASK1-related apoptosis, autophagic dysregulation. J. Psychiatr. Res. 2021;144:462–482. doi: 10.1016/j.jpsychires.2021.10.021. [DOI] [PubMed] [Google Scholar]
- 75.Liao D., et al. Salvianolic acid B improves chronic mild stress-induced depressive behaviors in rats: involvement of AMPK/SIRT1 signaling pathway. J. Inflamm. Res. 2020;13:195–206. doi: 10.2147/jir.S249363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liao D., et al. Curcumin attenuates chronic unpredictable mild stress-induced depressive-like behaviors via restoring changes in oxidative stress and the activation of Nrf2 signaling pathway in rats. Oxid. Med. Cell. Longev. 2020 doi: 10.1155/2020/9268083. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shaw P., Chattopadhyay A. Nrf2-ARE signaling in cellular protection: mechanism of action and the regulatory mechanisms. J. Cell. Physiol. 2020;235:3119–3130. doi: 10.1002/jcp.29219. [DOI] [PubMed] [Google Scholar]
- 78.Herken H., et al. Adenosine deaminase, nitric oxide, superoxide dismutase, and xanthine oxidase in patients with major depression: impact of antidepressant treatment. Arch. Med. Res. 2007;38:247–252. doi: 10.1016/j.arcmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 79.Bouvier E., et al. Nrf2-dependent persistent oxidative stress results in stress-induced vulnerability to depression. Mol. Psychiatr. 2017;22:1701–1713. doi: 10.1038/mp.2016.144. [DOI] [PubMed] [Google Scholar]
- 80.Eren I., Naziroğlu M., Demirdaş A. Protective effects of lamotrigine, aripiprazole and escitalopram on depression-induced oxidative stress in rat brain. Neurochem. Res. 2007;32:1188–1195. doi: 10.1007/s11064-007-9289-x. [DOI] [PubMed] [Google Scholar]
- 81.Eren I., et al. Venlafaxine modulates depression-induced oxidative stress in brain and medulla of rat. Neurochem. Res. 2007;32:497–505. doi: 10.1007/s11064-006-9258-9. [DOI] [PubMed] [Google Scholar]
- 82.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shao L., et al. Mitochondrial involvement in psychiatric disorders. Ann. Med. 2008;40:281–295. doi: 10.1080/07853890801923753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fattal O., Budur K., Vaughan A.J., Franco K. Review of the literature on major mental disorders in adult patients with mitochondrial diseases. Psychosomatics. 2006;47:1–7. doi: 10.1176/appi.psy.47.1.1. [DOI] [PubMed] [Google Scholar]
- 85.Allen J., Romay-Tallon R., Brymer K.J., Caruncho H.J., Kalynchuk L.E. Mitochondria and mood: mitochondrial dysfunction as a key player in the manifestation of depression. Front. Neurosci. 2018;12:386. doi: 10.3389/fnins.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moretti A., Gorini A., Villa R.F. Affective disorders, antidepressant drugs and brain metabolism. Mol. Psychiatr. 2003;8:773–785. doi: 10.1038/sj.mp.4001353. [DOI] [PubMed] [Google Scholar]
- 87.Martins-de-Souza D., et al. Identification of proteomic signatures associated with depression and psychotic depression in post-mortem brains from major depression patients. Transl. Psychiatry. 2012;2:e87. doi: 10.1038/tp.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rezin G.T., et al. Inhibition of mitochondrial respiratory chain in brain of rats subjected to an experimental model of depression. Neurochem. Int. 2008;53:395–400. doi: 10.1016/j.neuint.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 89.Gong Y., Chai Y., Ding J.H., Sun X.L., Hu G. Chronic mild stress damages mitochondrial ultrastructure and function in mouse brain. Neurosci. Lett. 2011;488:76–80. doi: 10.1016/j.neulet.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 90.Gardner A., et al. Alterations of mitochondrial function and correlations with personality traits in selected major depressive disorder patients. J. Affect. Disord. 2003;76:55–68. doi: 10.1016/s0165-0327(02)00067-8. [DOI] [PubMed] [Google Scholar]
- 91.Chang C.C., Jou S.H., Lin T.T., Lai T.J., Liu C.S. Mitochondria DNA change and oxidative damage in clinically stable patients with major depressive disorder. PLoS One. 2015;10 doi: 10.1371/journal.pone.0125855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Holmström K.M., Kostov R.V., Dinkova-Kostova A.T. The multifaceted role of Nrf2 in mitochondrial function. Curr. Opin. Toxicol. 2016;1:80–91. doi: 10.1016/j.cotox.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Piantadosi C.A., Carraway M.S., Babiker A., Suliman H.B. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ. Res. 2008;103:1232–1240. doi: 10.1161/01.RES.0000338597.71702.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gureev A.P., Shaforostova E.A., Popov V.N. Regulation of mitochondrial biogenesis as a way for active longevity: interaction between the Nrf2 and PGC-1α signaling pathways. Front. Genet. 2019;10:435. doi: 10.3389/fgene.2019.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sabouny R., et al. The keap1-nrf2 stress response pathway promotes mitochondrial hyperfusion through degradation of the mitochondrial fission protein Drp1. Antioxidants Redox Signal. 2017;27:1447–1459. doi: 10.1089/ars.2016.6855. [DOI] [PubMed] [Google Scholar]
- 96.Dodson M., et al. Modulating NRF2 in disease: timing is everything. Annu. Rev. Pharmacol. Toxicol. 2019;59:555–575. doi: 10.1146/annurev-pharmtox-010818-021856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schmidlin C.J., Dodson M.B., Madhavan L., Zhang D.D. Redox regulation by NRF2 in aging and disease. Free Radic. Biol. Med. 2019;134:702–707. doi: 10.1016/j.freeradbiomed.2019.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tang M., Liu T., Jiang P., Dang R. The interaction between autophagy and neuroinflammation in major depressive disorder: from pathophysiology to therapeutic implications. Pharmacol. Res. 2021;168 doi: 10.1016/j.phrs.2021.105586. [DOI] [PubMed] [Google Scholar]
- 99.Rein T. Is autophagy involved in the diverse effects of antidepressants? Cells. 2019;8 doi: 10.3390/cells8010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pierone B.C., Pereira C.A., Garcez M.L., Kaster M.P. Stress and signaling pathways regulating autophagy: from behavioral models to psychiatric disorders. Exp. Neurol. 2020;334 doi: 10.1016/j.expneurol.2020.113485. [DOI] [PubMed] [Google Scholar]
- 101.Damme M., Suntio T., Saftig P., Eskelinen E.L. Autophagy in neuronal cells: general principles and physiological and pathological functions. Acta Neuropathol. 2015;129:337–362. doi: 10.1007/s00401-014-1361-4. [DOI] [PubMed] [Google Scholar]
- 102.Lee K.M., Hwang S.K., Lee J.A. Neuronal autophagy and neurodevelopmental disorders. Exp. Neurobiol. 2013;22:133–142. doi: 10.5607/en.2013.22.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jia J., Le W. Molecular network of neuronal autophagy in the pathophysiology and treatment of depression. Neurosci. Bull. 2015;31:427–434. doi: 10.1007/s12264-015-1548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jernigan C.S., et al. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2011;35:1774–1779. doi: 10.1016/j.pnpbp.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gassen N.C., Rein T. Is there a role of autophagy in depression and antidepressant action? Front. Psychiatr. 2019;10:337. doi: 10.3389/fpsyt.2019.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhuo J., et al. Patchouli alcohol protects against chronic unpredictable mild stress-induced depressant-like behavior through inhibiting excessive autophagy via activation of mTOR signaling pathway. Biomed. Pharmacother. 2020;127 doi: 10.1016/j.biopha.2020.110115. [DOI] [PubMed] [Google Scholar]
- 107.Alcocer-Gómez E., et al. Antidepressants induce autophagy dependent-NLRP3-inflammasome inhibition in Major depressive disorder. Pharmacol. Res. 2017;121:114–121. doi: 10.1016/j.phrs.2017.04.028. [DOI] [PubMed] [Google Scholar]
- 108.Li J.R., et al. Fluoxetine-enhanced autophagy ameliorates early brain injury via inhibition of NLRP3 inflammasome activation following subrachnoid hemorrhage in rats. J. Neuroinflammation. 2017;14:186. doi: 10.1186/s12974-017-0959-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pagnin D., de Queiroz V., Pini S., Cassano G.B. Efficacy of ECT in depression: a meta-analytic review. J. ECT. 2004;20:13–20. doi: 10.1097/00124509-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 110.Abelaira H.M., et al. Effects of ketamine administration on mTOR and reticulum stress signaling pathways in the brain after the infusion of rapamycin into prefrontal cortex. J. Psychiatr. Res. 2017;87:81–87. doi: 10.1016/j.jpsychires.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 111.Pajares M., et al. Transcription factor NFE2L2/NRF2 is a regulator of macroautophagy genes. Autophagy. 2016;12:1902–1916. doi: 10.1080/15548627.2016.1208889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dikic I. Proteasomal and autophagic degradation systems. Annu. Rev. Biochem. 2017;86:193–224. doi: 10.1146/annurev-biochem-061516-044908. [DOI] [PubMed] [Google Scholar]
- 113.Pasha M., Eid A.H., Eid A.A., Gorin Y., Munusamy S. Sestrin2 as a novel biomarker and therapeutic target for various diseases. Oxid. Med. Cell. Longev. 2017 doi: 10.1155/2017/3296294. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lau A., et al. A noncanonical mechanism of Nrf2 activation by autophagy deficiency: direct interaction between Keap1 and p62. Mol. Cell Biol. 2010;30:3275–3285. doi: 10.1128/mcb.00248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Guo L., et al. Repeated social defeat stress inhibits development of hippocampus neurons through mitophagy and autophagy. Brain Res. Bull. 2022;182:111–117. doi: 10.1016/j.brainresbull.2022.01.009. [DOI] [PubMed] [Google Scholar]
- 116.Scaini G., et al. Dysregulation of mitochondrial dynamics, mitophagy and apoptosis in major depressive disorder: does inflammation play a role? Mol. Psychiatr. 2022;27:1095–1102. doi: 10.1038/s41380-021-01312-w. [DOI] [PubMed] [Google Scholar]
- 117.Zhang Q., et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu G., et al. Extracellular mitochondrial DNA promote NLRP3 inflammasome activation and induce acute lung injury through TLR9 and NF-κB. J. Thorac. Dis. 2019;11:4816–4828. doi: 10.21037/jtd.2019.10.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Murata H., et al. NRF2 regulates PINK1 expression under oxidative stress conditions. PLoS One. 2015;10 doi: 10.1371/journal.pone.0142438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gureev A.P., Sadovnikova I.S., Starkov N.N., Starkov A.A., Popov V.N. p62-Nrf2-p62 mitophagy regulatory loop as a target for preventive therapy of neurodegenerative diseases. Brain Sci. 2020;10 doi: 10.3390/brainsci10110847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yamada T., Dawson T.M., Yanagawa T., Iijima M., Sesaki H. SQSTM1/p62 promotes mitochondrial ubiquitination independently of PINK1 and PRKN/parkin in mitophagy. Autophagy. 2019;15:2012–2018. doi: 10.1080/15548627.2019.1643185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Geisler S., et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 123.Hayley S., Hakim A.M., Albert P.R. Depression, dementia and immune dysregulation. Brain. 2021;144:746–760. doi: 10.1093/brain/awaa405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pandey G.N., Rizavi H.S., Zhang H., Bhaumik R., Ren X. Abnormal protein and mRNA expression of inflammatory cytokines in the prefrontal cortex of depressed individuals who died by suicide. J. Psychiatry Neurosci. : JPN (J. Psychiatry Neurosci.) 2018;43:376–385. doi: 10.1503/jpn.170192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhu L., et al. Esculetin attenuates lipopolysaccharide (LPS)-induced neuroinflammatory processes and depressive-like behavior in mice. Physiol. Behav. 2016;163:184–192. doi: 10.1016/j.physbeh.2016.04.051. [DOI] [PubMed] [Google Scholar]
- 126.Li H., Sagar A.P., Kéri S. Translocator protein (18kDa TSPO) binding, a marker of microglia, is reduced in major depression during cognitive-behavioral therapy. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2018;83:1–7. doi: 10.1016/j.pnpbp.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 127.Franklin T.C., Xu C., Duman R.S. Depression and sterile inflammation: essential role of danger associated molecular patterns. Brain Behav. Immun. 2018;72:2–13. doi: 10.1016/j.bbi.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 128.Cui W., et al. Crosstalk between inflammation and glutamate system in depression: signaling pathway and molecular biomarkers for ketamine's antidepressant effect. Mol. Neurobiol. 2019;56:3484–3500. doi: 10.1007/s12035-018-1306-3. [DOI] [PubMed] [Google Scholar]
- 129.Beurel E., Toups M., Nemeroff C.B. The bidirectional relationship of depression and inflammation: double trouble. Neuron. 2020;107:234–256. doi: 10.1016/j.neuron.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kohler C.A., et al. Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta-analysis. Mol. Neurobiol. 2018;55:4195–4206. doi: 10.1007/s12035-017-0632-1. [DOI] [PubMed] [Google Scholar]
- 131.Wang L., et al. Effects of SSRIs on peripheral inflammatory markers in patients with major depressive disorder: a systematic review and meta-analysis. Brain Behav. Immun. 2019;79:24–38. doi: 10.1016/j.bbi.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 132.Bai S., et al. Efficacy and safety of anti-inflammatory agents for the treatment of major depressive disorder: a systematic review and meta-analysis of randomised controlled trials. J. Neurol. Neurosurg. Psychiatr. 2020;91:21–32. doi: 10.1136/jnnp-2019-320912. [DOI] [PubMed] [Google Scholar]
- 133.Deng S.L., Chen J.G., Wang F. Microglia: a central player in depression. Curr. Med. Sci. 2020;40:391–400. doi: 10.1007/s11596-020-2193-1. [DOI] [PubMed] [Google Scholar]
- 134.Steiner J., et al. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J. Psychiatr. Res. 2008;42:151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 135.Pan Y., Chen X.Y., Zhang Q.Y., Kong L.D. Microglial NLRP3 inflammasome activation mediates IL-1β-related inflammation in prefrontal cortex of depressive rats. Brain Behav. Immun. 2014;41:90–100. doi: 10.1016/j.bbi.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 136.O'Neill L.A., Kaltschmidt C. NF-kappa B: A crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997;20:252–258. doi: 10.1016/s0166-2236(96)01035-1. [DOI] [PubMed] [Google Scholar]
- 137.Fan C., et al. Neuroprotective effects of ginsenoside-rg1 against depression-like behaviors via suppressing glial activation, synaptic deficits, and neuronal apoptosis in rats. Front. Immunol. 2018;9:2889. doi: 10.3389/fimmu.2018.02889. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 138.Kim Y.W., et al. Anti-inflammatory effects of liquiritigenin as a consequence of the inhibition of NF-κB-dependent iNOS and proinflammatory cytokines production. Br. J. Pharmacol. 2008;154:165–173. doi: 10.1038/bjp.2008.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dionisie, V., Filip, G. A., Manea, M. C., Manea, M. & Riga, S. The anti-inflammatory role of SSRI and SNRI in the treatment of depression: a review of human and rodent research studies. Inflammopharmacology 29, 75-90, doi:10.1007/s10787-020-00777-5(2021). [DOI] [PubMed]
- 140.Soares M.P., et al. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J. Immunol. 2004;172:3553–3563. doi: 10.4049/jimmunol.172.6.3553. [DOI] [PubMed] [Google Scholar]
- 141.Bellezza I., et al. Inhibition of NF-kappaB nuclear translocation via HO-1 activation underlies alpha-tocopheryl succinate toxicity. J. Nutr. Biochem. 2012;23:1583–1591. doi: 10.1016/j.jnutbio.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 142.Liu G.H., Qu J., Shen X. NF-kappaB/p65 antagonizes Nrf2-ARE pathway by depriving CBP from Nrf2 and facilitating recruitment of HDAC3 to MafK. Biochim. Biophys. Acta. 2008;1783:713–727. doi: 10.1016/j.bbamcr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 143.Innamorato N.G., et al. The transcription factor Nrf2 is a therapeutic target against brain inflammation. J. Immunol. 2008;181:680–689. doi: 10.4049/jimmunol.181.1.680. [DOI] [PubMed] [Google Scholar]
- 144.Kobayashi E.H., et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016;7 doi: 10.1038/ncomms11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Saddawi-Konefka R., et al. Nrf2 induces IL-17d to mediate tumor and virus surveillance. Cell Rep. 2016;16:2348–2358. doi: 10.1016/j.celrep.2016.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ishii T., et al. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: activation by oxidatively modified LDL and 4-hydroxynonenal. Circ. Res. 2004;94:609–616. doi: 10.1161/01.Res.0000119171.44657.45. [DOI] [PubMed] [Google Scholar]
- 147.Peng H., et al. Dimethyl fumarate inhibits dendritic cell maturation via nuclear factor κB (NF-κB) and extracellular signal-regulated kinase 1 and 2 (ERK1/2) and mitogen stress-activated kinase 1 (MSK1) signaling. J. Biol. Chem. 2012;287:28017–28026. doi: 10.1074/jbc.M112.383380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tang, R. et al. Sulforaphane activates anti-inflammatory microglia, modulating stress resilience associated with BDNF transcription. Acta Pharmacol. Sin. 43, 829-839, doi:10.1038/s41401-021-00727-z(2022). [DOI] [PMC free article] [PubMed]
- 149.Björkholm C., Monteggia L.M. BDNF - a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–79. doi: 10.1016/j.neuropharm.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hashimoto K. Brain-derived neurotrophic factor as a biomarker for mood disorders: an historical overview and future directions. Psychiatr. Clin. Neurosci. 2010;64:341–357. doi: 10.1111/j.1440-1819.2010.02113.x. [DOI] [PubMed] [Google Scholar]
- 151.Porter G.A., O'Connor J.C. Brain-derived neurotrophic factor and inflammation in depression: pathogenic partners in crime? World J. Psychiatr. 2022;12:77–97. doi: 10.5498/wjp.v12.i1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dodson M., Castro-Portuguez R., Zhang D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019;23 doi: 10.1016/j.redox.2019.101107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lane D.J.R., Metselaar B., Greenough M., Bush A.I., Ayton S.J. Ferroptosis and NRF2: an emerging battlefield in the neurodegeneration of Alzheimer's disease. Essays Biochem. 2021;65:925–940. doi: 10.1042/EBC20210017. [DOI] [PubMed] [Google Scholar]
- 154.Abdalkader M., Lampinen R., Kanninen K.M., Malm T.M., Liddell J.R. Targeting Nrf2 to suppress ferroptosis and mitochondrial dysfunction in neurodegeneration. Front. Neurosci. 2018;12:466. doi: 10.3389/fnins.2018.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Song X., Long D. Nrf2 and ferroptosis: a new research direction for neurodegenerative diseases. Front. Neurosci. 2020;14:267. doi: 10.3389/fnins.2020.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dixon S.J., et al. Human haploid cell genetics reveals roles for lipid metabolism genes in nonapoptotic cell death. ACS Chem. Biol. 2015;10:1604–1609. doi: 10.1021/acschembio.5b00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kagan V.E., et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 2017;13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhao L., et al. Ferroptosis in cancer and cancer immunotherapy. Cancer Commun. 2022;42:88–116. doi: 10.1002/cac2.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stockwell B.R., et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhang H., Jiao W., Cui H., Sun Q., Fan H. Combined exposure of alumina nanoparticles and chronic stress exacerbates hippocampal neuronal ferroptosis via activating IFN-gamma/ASK1/JNK signaling pathway in rats. J. Hazard Mater. 2021;411 doi: 10.1016/j.jhazmat.2021.125179. [DOI] [PubMed] [Google Scholar]
- 161.Romano A., et al. Linking lipid peroxidation and neuropsychiatric disorders: focus on 4-hydroxy-2-nonenal. Free Radic. Biol. Med. 2017;111:281–293. doi: 10.1016/j.freeradbiomed.2016.12.046. [DOI] [PubMed] [Google Scholar]
- 162.Sowa-Kucma M., et al. Lipid peroxidation and immune biomarkers are associated with major depression and its phenotypes, including treatment-resistant depression and melancholia. Neurotox. Res. 2018;33:448–460. doi: 10.1007/s12640-017-9835-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Khanzode S.D., Dakhale G.N., Khanzode S.S., Saoji A., Palasodkar R. Oxidative damage and major depression: the potential antioxidant action of selective serotonin re-uptake inhibitors. Redox Rep. 2003;8:365–370. doi: 10.1179/135100003225003393. [DOI] [PubMed] [Google Scholar]
- 164.Wigner P., et al. Variation of genes involved in oxidative and nitrosative stresses in depression. Eur. Psychiatr. : Off. J. Assoc. Eur. Psychiatr. 2018;48:38–48. doi: 10.1016/j.eurpsy.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 165.Alam J., et al. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 1999;274:26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- 166.Harada N., et al. Nrf2 regulates ferroportin 1-mediated iron efflux and counteracts lipopolysaccharide-induced ferroportin 1 mRNA suppression in macrophages. Arch. Biochem. Biophys. 2011;508:101–109. doi: 10.1016/j.abb.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 167.Agyeman A.S., et al. Transcriptomic and proteomic profiling of KEAP1 disrupted and sulforaphane-treated human breast epithelial cells reveals common expression profiles. Breast Cancer Res. Treat. 2012;132:175–187. doi: 10.1007/s10549-011-1536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lee J.M., Calkins M.J., Chan K., Kan Y.W., Johnson J.A. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J. Biol. Chem. 2003;278:12029–12038. doi: 10.1074/jbc.M211558200. [DOI] [PubMed] [Google Scholar]
- 169.Kerins M.J., Ooi A. The roles of NRF2 in modulating cellular iron homeostasis. Antioxidants Redox Signal. 2018;29:1756–1773. doi: 10.1089/ars.2017.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Campbell M.R., et al. Novel hematopoietic target genes in the NRF2-mediated transcriptional pathway. Oxid. Med. Cell. Longev. 2013 doi: 10.1155/2013/120305. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lu J., Zhao Y., Liu M., Lu J., Guan S. Toward improved human health: Nrf2 plays a critical role in regulating ferroptosis. Food Funct. 2021;12:9583–9606. doi: 10.1039/d1fo01036k. [DOI] [PubMed] [Google Scholar]
- 172.Thimmulappa R.K., et al. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res. 2002;62:5196–5203. [PubMed] [Google Scholar]
- 173.Dang R., et al. Edaravone ameliorates depressive and anxiety-like behaviors via Sirt1/Nrf2/HO-1/Gpx4 pathway. J. Neuroinflammation. 2022;19:41. doi: 10.1186/s12974-022-02400-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Santín-Márquez R., Alarcón-Aguilar A., López-Diazguerrero N.E., Chondrogianni N., Königsberg M. Sulforaphane - role in aging and neurodegeneration. GeroScience. 2019;41:655–670. doi: 10.1007/s11357-019-00061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhang J.C., et al. Prophylactic effects of sulforaphane on depression-like behavior and dendritic changes in mice after inflammation. J. Nutr. Biochem. 2017;39:134–144. doi: 10.1016/j.jnutbio.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 176.Zheng W., Li X., Zhang T., Wang J. Biological mechanisms and clinical efficacy of sulforaphane for mental disorders. Gen. Psychiatr. 2022;35 doi: 10.1136/gpsych-2021-100700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Patel S.S., et al. Cellular and molecular mechanisms of curcumin in prevention and treatment of disease. Crit. Rev. Food Sci. Nutr. 2020;60:887–939. doi: 10.1080/10408398.2018.1552244. [DOI] [PubMed] [Google Scholar]
- 178.Shin J.W., et al. Curcumin induces stabilization of Nrf2 protein through Keap1 cysteine modification. Biochem. Pharmacol. 2020;173 doi: 10.1016/j.bcp.2020.113820. [DOI] [PubMed] [Google Scholar]
- 179.Robledinos-Antón N., Fernández-Ginés R., Manda G., Cuadrado A. Activators and inhibitors of NRF2: a review of their potential for clinical development. Oxid. Med. Cell. Longev. 2019 doi: 10.1155/2019/9372182. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Balogun E., et al. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003;371:887–895. doi: 10.1042/bj20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Park, J. Y., Sohn, H. Y., Koh, Y. H. & Jo, C. Curcumin activates Nrf2 through PKCδ-mediated p62 phosphorylation at Ser351. Sci. Rep. 11, 8430, doi:10.1038/s41598-021-87225-8(2021). [DOI] [PMC free article] [PubMed]
- 182.Fusar-Poli L., et al. Curcumin for depression: a meta-analysis. Crit. Rev. Food Sci. Nutr. 2020;60:2643–2653. doi: 10.1080/10408398.2019.1653260. [DOI] [PubMed] [Google Scholar]
- 183.da Silva Marques J.G., et al. Adaptogenic effects of curcumin on depression induced by moderate and unpredictable chronic stress in mice. Behav. Brain Res. 2021;399 doi: 10.1016/j.bbr.2020.113002. [DOI] [PubMed] [Google Scholar]
- 184.Fan C., et al. Prophylactic treatment of curcumin in a rat model of depression by attenuating hippocampal synaptic loss. Food Funct. 2021;12:11202–11213. doi: 10.1039/d1fo02676c. [DOI] [PubMed] [Google Scholar]
- 185.Costa L.G., Garrick J.M., Roquè P.J., Pellacani C. Mechanisms of neuroprotection by quercetin: counteracting oxidative stress and more. Oxid. Med. Cell. Longev. 2016 doi: 10.1155/2016/2986796. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Arredondo F., et al. After cellular internalization, quercetin causes Nrf2 nuclear translocation, increases glutathione levels, and prevents neuronal death against an oxidative insult. Free Radic. Biol. Med. 2010;49:738–747. doi: 10.1016/j.freeradbiomed.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 187.Guan Y., et al. Quercetin reverses chronic unpredictable mild stress-induced depression-like behavior in vivo by involving nuclear factor-E2-related factor 2. Brain Res. 2021;1772 doi: 10.1016/j.brainres.2021.147661. [DOI] [PubMed] [Google Scholar]
- 188.Farkhondeh T., Folgado S.L., Pourbagher-Shahri A.M., Ashrafizadeh M., Samarghandian S. The therapeutic effect of resveratrol: focusing on the Nrf2 signaling pathway. Biomed. Pharmacother. 2020;127 doi: 10.1016/j.biopha.2020.110234. [DOI] [PubMed] [Google Scholar]
- 189.Gao Y., et al. Resveratrol mitigates the oxidative stress mediated by hypoxic-ischemic brain injury in neonatal rats via Nrf2/HO-1 pathway. Pharmaceut. Biol. 2018;56:440–449. doi: 10.1080/13880209.2018.1502326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rosa P.M., Martins L.A.M., Souza D.O., Quincozes-Santos A. Glioprotective effect of resveratrol: an emerging therapeutic role for oligodendroglial cells. Mol. Neurobiol. 2018;55:2967–2978. doi: 10.1007/s12035-017-0510-x. [DOI] [PubMed] [Google Scholar]
- 191.Kumar A., Singh C.K., Lavoie H.A., Dipette D.J., Singh U.S. Resveratrol restores Nrf2 level and prevents ethanol-induced toxic effects in the cerebellum of a rodent model of fetal alcohol spectrum disorders. Mol. Pharmacol. 2011;80:446–457. doi: 10.1124/mol.111.071126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Shi Z., Qiu W., Xiao G., Cheng J., Zhang N. Resveratrol attenuates cognitive deficits of traumatic brain injury by activating p38 signaling in the brain. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. : Int. Med. J. Exp. Clin. Res. 2018;24:1097–1103. doi: 10.12659/msm.909042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Moore A., Beidler J., Hong M.Y. Resveratrol and depression in animal models: a systematic review of the biological mechanisms. Molecules. 2018;23 doi: 10.3390/molecules23092197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Galano A., Tan D.X., Reiter R.J. Melatonin: a versatile protector against oxidative DNA damage. Molecules. 2018;23 doi: 10.3390/molecules23030530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Arioz B.I., et al. Melatonin attenuates LPS-induced acute depressive-like behaviors and microglial NLRP3 inflammasome activation through the SIRT1/nrf2 pathway. Front. Immunol. 2019;10:1511. doi: 10.3389/fimmu.2019.01511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ali T., et al. Melatonin prevents neuroinflammation and relieves depression by attenuating autophagy impairment through FOXO3a regulation. J. Pineal Res. 2020;69 doi: 10.1111/jpi.12667. [DOI] [PubMed] [Google Scholar]
- 197.Saini R.K., Rengasamy K.R.R., Mahomoodally F.M., Keum Y.S. Protective effects of lycopene in cancer, cardiovascular, and neurodegenerative diseases: an update on epidemiological and mechanistic perspectives. Pharmacol. Res. 2020;155 doi: 10.1016/j.phrs.2020.104730. [DOI] [PubMed] [Google Scholar]
- 198.Zhao B., et al. Supplementation of lycopene attenuates oxidative stress induced neuroinflammation and cognitive impairment via Nrf2/NF-κB transcriptional pathway. Food Chem. Toxicol. : Int. J. Publ. Br. Indust. Biol. Res. Assoc. 2017;109:505–516. doi: 10.1016/j.fct.2017.09.050. [DOI] [PubMed] [Google Scholar]
- 199.Zhang F., et al. Depression-like behaviors and heme oxygenase-1 are regulated by Lycopene in lipopolysaccharide-induced neuroinflammation. J. Neuroimmunol. 2016;298:1–8. doi: 10.1016/j.jneuroim.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 200.Lin H.Y., et al. Antineuroinflammatory effects of lycopene via activation of adenosine monophosphate-activated protein kinase-α1/heme oxygenase-1 pathways. Neurobiol. Aging. 2014;35:191–202. doi: 10.1016/j.neurobiolaging.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 201.Abiko Y., Miura T., Phuc B.H., Shinkai Y., Kumagai Y. Participation of covalent modification of Keap1 in the activation of Nrf2 by tert-butylbenzoquinone, an electrophilic metabolite of butylated hydroxyanisole. Toxicol. Appl. Pharmacol. 2011;255:32–39. doi: 10.1016/j.taap.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 202.Zhao Y.L., Zhao W., Liu M., Liu L., Wang Y. TBHQ-overview of multiple mechanisms against oxidative stress for attenuating methamphetamine-induced neurotoxicity. Oxid. Med. Cell. Longev. 2020 doi: 10.1155/2020/8874304. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Meng X., et al. TBHQ attenuates neurotoxicity induced by methamphetamine in the VTA through the Nrf2/HO-1 and PI3K/AKT signaling pathways. Oxid. Med. Cell. Longev. 2020 doi: 10.1155/2020/8787156. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Bian H., Yan F., Li W., Tu W., Ji X. Tert-butylhydroquinone prevents neuroinflammation and relieves depression via regulation of NLRP3 signaling in mice. Int. Immunopharm. 2022;107 doi: 10.1016/j.intimp.2022.108723. [DOI] [PubMed] [Google Scholar]
- 205.Taguchi K., Yamamoto M. The KEAP1-NRF2 system as a molecular target of cancer treatment. Cancers. 2020;13 doi: 10.3390/cancers13010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Unni S., Deshmukh P., Krishnappa G., Kommu P., Padmanabhan B. Structural insights into the multiple binding modes of Dimethyl Fumarate (DMF) and its analogs to the Kelch domain of Keap1. FEBS J. 2021;288:1599–1613. doi: 10.1111/febs.15485. [DOI] [PubMed] [Google Scholar]
- 207.Hayashi G., et al. Dimethyl fumarate mediates Nrf2-dependent mitochondrial biogenesis in mice and humans. Hum. Mol. Genet. 2017;26:2864–2873. doi: 10.1093/hmg/ddx167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yan N., Xu Z., Qu C., Zhang J. Dimethyl fumarate improves cognitive deficits in chronic cerebral hypoperfusion rats by alleviating inflammation, oxidative stress, and ferroptosis via NRF2/ARE/NF-κB signal pathway. Int. Immunopharm. 2021;98 doi: 10.1016/j.intimp.2021.107844. [DOI] [PubMed] [Google Scholar]
- 209.Liu Z., et al. Neuroprotection of edaravone on the hippocampus of kainate-induced epilepsy rats through Nrf2/HO-1 pathway. Neurochem. Int. 2018;112:159–165. doi: 10.1016/j.neuint.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 210.Wang D., et al. Edaravone promotes nerve function recovery after acute cerebral infarction in rats via targeting Keap1-Nrf2/ARE. Panminerva Med. 2021;63:384–385. doi: 10.23736/s0031-0808.19.03694-2. [DOI] [PubMed] [Google Scholar]
- 211.Ismail H., et al. Traumatic brain injury: oxidative stress and novel anti-oxidants such as mitoquinone and edaravone. Antioxidants. 2020;9 doi: 10.3390/antiox9100943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Herbet M., et al. Edaravone presents antidepressant-like activity in corticosterone model of depression in mice with possible role of Fkbp5, Comt, Adora1 and Slc6a15 genes. Toxicol. Appl. Pharmacol. 2019;380 doi: 10.1016/j.taap.2019.114689. [DOI] [PubMed] [Google Scholar]
- 213.Sriram C.S., Jangra A., Gurjar S.S., Mohan P., Bezbaruah B.K. Edaravone abrogates LPS-induced behavioral anomalies, neuroinflammation and PARP-1. Physiol. Behav. 2016;154:135–144. doi: 10.1016/j.physbeh.2015.10.029. [DOI] [PubMed] [Google Scholar]
- 214.Jangra A., et al. Sodium phenylbutyrate and edaravone abrogate chronic restraint stress-induced behavioral deficits: implication of oxido-nitrosative, endoplasmic reticulum stress cascade, and neuroinflammation. Cell. Mol. Neurobiol. 2017;37:65–81. doi: 10.1007/s10571-016-0344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Honda T., et al. Tricyclic compounds containing nonenolizable cyano enones. A novel class of highly potent anti-inflammatory and cytoprotective agents. J. Med. Chem. 2011;54:1762–1778. doi: 10.1021/jm101445p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kostov R.V., et al. Pharmacokinetics and pharmacodynamics of orally administered acetylenic tricyclic bis(cyanoenone), a highly potent Nrf2 activator with a reversible covalent mode of action. Biochem. Biophys. Res. Commun. 2015;465:402–407. doi: 10.1016/j.bbrc.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yao W., et al. Antidepressant effects of TBE-31 and MCE-1, the novel Nrf2 activators, in an inflammation model of depression. Eur. J. Pharmacol. 2016;793:21–27. doi: 10.1016/j.ejphar.2016.10.037. [DOI] [PubMed] [Google Scholar]
- 218.Zuo C., et al. Neuroprotective efficacy of different levels of high-frequency repetitive transcranial magnetic stimulation in mice with CUMS-induced depression: involvement of the p11/BDNF/Homer1a signaling pathway. J. Psychiatr. Res. 2020;125:152–163. doi: 10.1016/j.jpsychires.2020.03.018. [DOI] [PubMed] [Google Scholar]
- 219.Zuo C., et al. Repetitive transcranial magnetic stimulation exerts anti-inflammatory effects via modulating glial activation in mice with chronic unpredictable mild stress-induced depression. Int. Immunopharm. 2022;109 doi: 10.1016/j.intimp.2022.108788. [DOI] [PubMed] [Google Scholar]
- 220.Tian L., et al. Repetitive transcranial magnetic stimulation elicits antidepressant- and anxiolytic-like effect via nuclear factor-E2-related factor 2-mediated anti-inflammation mechanism in rats. Neuroscience. 2020;429:119–133. doi: 10.1016/j.neuroscience.2019.12.025. [DOI] [PubMed] [Google Scholar]
- 221.Kandola A., Ashdown-Franks G., Hendrikse J., Sabiston C.M., Stubbs B. Physical activity and depression: towards understanding the antidepressant mechanisms of physical activity. Neurosci. Biobehav. Rev. 2019;107:525–539. doi: 10.1016/j.neubiorev.2019.09.040. [DOI] [PubMed] [Google Scholar]
- 222.Mallard A.R., Spathis J.G., Coombes J.S. Nuclear factor (erythroid-derived 2)-like 2 (Nrf2) and exercise. Free Radic. Biol. Med. 2020;160:471–479. doi: 10.1016/j.freeradbiomed.2020.08.024. [DOI] [PubMed] [Google Scholar]
- 223.Done A.J., Traustadóttir T. Nrf2 mediates redox adaptations to exercise. Redox Biol. 2016;10:191–199. doi: 10.1016/j.redox.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.