Abstract
Background
Transurethral resection of bladder tumor (TURBT) combined with intravesical Bacillus Calmette-Guérin (BCG) perfusion is a widely accepted treatment for moderate or high risk non-muscular-invasive bladder cancer (BCa). Despite its effectiveness, the recurrence and progression rate of tumor are still high. We evaluated the predictive role of fluorescence in situ hybridization (FISH) for the response to BCG perfusion in BCa.
Methods
Patients with BCa who underwent BCG perfusion and FISH test in our hospital were selected. Logistic regression and Kaplan-Meier methods were used to evaluate the relationship between FISH results and tumor recurrence or progression. COX proportional hazards regression analysis was used to identify risk factors for recurrence or progression. SPSS (version 24.0, IBM Corporation, USA) was used for statistical analysis.
Results
Seventy-six patients were included in this study, with a median age of 63.0 (55.0–70.0) years, and a median follow-up time of 19.0 (7.5–29.0) months. Fifteen patients relapsed after BCG perfusion. Before TURBT, 39 patients were positive for FISH and 20 were negative. There was no significant difference in the recurrence rate of BCG after perfusion predicted by preoperative FISH positive (10.3% vs. 10%, P=0.675). No association was found between preoperative FISH and tumor recurrence (P=0.955) or disease progression (P=0.186). After BCG perfusion, 10 patients were FISH positive and 14 patients were FISH negative. There was significant difference in recurrence rate of BCa predicted by positive FISH (100% vs. 7.1%, P<0.001). FISH results were significantly associated with tumor recurrence (P<0.001) and disease progression (P=0.001). Kaplan-Meier and univariate COX proportional hazards regression analysis clarified that FISH positive after BCG perfusion, tumor-node-metastasis (TNM) stage, and multiple tumors were risk factors for tumor recurrence and progression (P<0.05). Tumor TNM stage and FISH positive after BCG perfusion were independent risk factors for recurrence.
Conclusions
Positive FISH after BCG perfusion can well predict the risk of recurrence and progression of BCa, and recurrence within six months is more likely. After BCG perfusion, it is better to recommend close follow up in patients with positive FISH.
Keywords: Fluorescence in situ hybridization (FISH), bladder cancer (BCa), Bacillus Calmette-Guérin (BCG) perfusion
Introduction
In recent decades, with the change of environment, the continuous improvement of living and medical standards, the transformation of people’s health awareness and the continuous promotion of cancer screening, the overall incidence and mortality of bladder cancer (BCa) in China has been on the rise (1,2). Non-muscle invasive bladder cancer (NMIBC) is a group of superficial bladder tumors (Ta, T1, and carcinoma in situ) that account for 70% to 75% of new BCas (3,4). The European Urological Association and American Urological Association recommend transurethral resection of bladder tumor (TURBT) combined with intravesical Bacillus Calmette-Guérin (BCG) perfusion for moderate or high risk NMIBC (5-7). Despite its effectiveness, BCG treatment can lead to local side effects in 62.8% of patients and 30.6% of patients with systemic side effects. Post-treatment tumor recurrence and progression rates remain high, with failure rates as high as 40% (8-10). The gold standard of postoperative follow-up of TURBT is regular cystoscopy and urine cytology, but cystoscopy depends on subjective visible changes and the actual tumor recurrence, so it cannot early predict whether intravesical BCG therapy is effective. Urine cytology has high sensitivity for high-grade tumors and low sensitivity for low-grade tumors, and it is difficult to distinguish inflammatory responses from tumor recurrence, especially in patients receiving intravesical BCG perfusion (11-14).
Fluorescence in situ hybridization (FISH) uses fluorescent-labeled DNA probes to identify chromosomal aberrations associated with BCa, such as amplification on chromosomes 3, 7, and 17 and deletion of the 9p21 gene locus (15-17). It is more sensitive to urothelial carcinoma than urine cytology, and not affected by hematuria, urinary tract infection, and BCG induced inflammation (11). Genetic mutation can be identified at an early stage of cancer development and become an important indicator for clinical testing as cancer progresses (18,19). A recent update to the American Urological Association guidelines mentions that FISH tests can be used to assess response to BCG therapy in BCa, but the evidence is limited to expert opinion (5). Given that there is currently no definitive tool for early prediction of the efficacy of BCG therapy, we evaluated the predictive role of FISH for the efficacy of BCG perfusion in BCa. We present the following article in accordance with the TRIPOD reporting checklist (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1367/rc).
Methods
Study design and cohorts
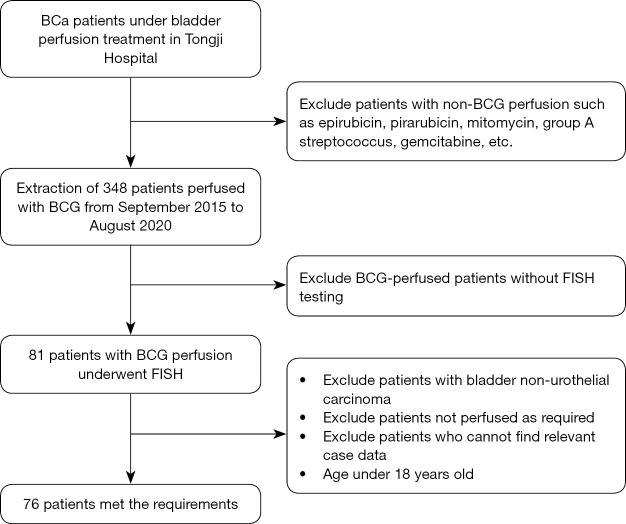
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (Approval No. TJ-IRB20210521) and individual consent for this retrospective analysis was waived. We extracted the relevant data of BCG perfusion patients from September 2015 to August 2020 from the urology treatment room, and a total of 348 patients were extracted. Then, the FISH-related information of these patients was retrospectively searched in the FISH detection database of the Institute of Urology of our hospital, and a total of 81 patients who had undergone FISH detection were screened. The electronic medical record system of our hospital was used to query the relevant clinical data of the patients, and 76 patients meeting the requirements were screened out (Figure 1). Inclusion criteria: (I) age over 18 years old; (II) postoperative pathologically confirmed bladder urothelial carcinoma; (III) regular BCG perfusion according to the process requirements; (IV) relevant clinical data can be queried in the electronic medical record system.
Figure 1.
Flow chart of case collection. BCa, bladder cancer; BCG, Bacillus Calmette-Guérin; FISH, fluorescence in situ hybridization.
The primary endpoints of this study were recurrence and progression, as until the last follow-up, only two patients had died, one due to BCa and the other due to a sudden cerebrovascular accident. Recurrence was defined as any tumor found after bladder BCG perfusion (cystoscopy biopsy or postoperative pathology), regardless of grade or stage, but tumors found by secondary resection within 2~6 weeks were not counted, because it was impossible to determine whether the tumor was completely removed in the first operation. Progression was defined as an increase in non-invasive to invasive staging, or histological grade from low-grade malignant potential and low-grade to high-grade, or the occurrence of adjacent tissue and organ invasion and metastasis.
Research methods
Detection method of urine FISH
The specific results of urine FISH in 76 patients were obtained directly from the Institute of Urology in our hospital. The UroVysion kit used was provided by Beijing Jinpujia Medical Technology Co., Ltd. and consisted of two combinations of chromosome-specific centromeric probe (CSP)3 (green)/CSP7 (red) and gene locus-specific probe (GLP) p16 (red)/CSP17 (green). Since it was a retrospective study, the time interval of FISH detection was not fixed. However, FISH detection after BCG perfusion was performed after at least 6 perfusions. All patients with positive FISH after BCG perfusion were confirmed by cystoscopy at that time.
BCG perfusion protocol
Dosage of BCG (Bisegi, Chengdu Institute of Biological Products Co., Ltd., China, S20123007) for therapeutic use: two doses (120 mg) each time, shaken with 40–50 mL saline, were introduced through catheter. Treatment course: once a week for 6 consecutive times, once every two weeks for 3 consecutive times, once a month for 10 consecutive times. The intravesical residence time of each perfusion was 2 hours, and four positions of supine, prone, left lateral and right lateral positions were exchanged.
Data collection
The collected data included basic demographic characteristics, FISH test results before TURBT and after BCG perfusion, histological grade, pathological stage, multiple tumors, history of bladder cancer, history of intravesical perfusion with other drugs, BCG perfusion, recurrence and progression after BCG perfusion, etc. Follow-up data were obtained mainly through electronic medical record system and telephone communication.
Statistical analysis
SPSS 24.0 statistical software was used to analyze the data. Measurement data conforming to normal distribution were expressed as mean ± standard deviation, and t-test was used for comparison between groups. Measurement data that did not conform to normal distribution were expressed as median (M) and interquartile range (Q1, Q3), and nonparametric tests were used for comparison between groups. Counting data are expressed as percentages, and the χ2 test was used for comparison between groups. When the amount of data was limited, Fisher’s exact test was used. Logistic regression and Kaplan-Meier methods were used to evaluate the relationship between FISH results and tumor recurrence or progression. COX proportional hazards regression analysis was used to identify risk factors for recurrence or progression.
Results
Demographic and clinical characteristics of the research objects
Since 3 cases were not bladder urothelial carcinoma and 2 cases did not receive BCG perfusion as required, only 76 patients were finally included in the study. As shown in Table 1, there were 65 (85.5%) males and 11 (14.5%) females, with a median age of 63.0 (55.0–70.0) years. Twenty-nine (38.2%) patients had a history of bladder tumor, of which 18 had a history of receiving intravesical non-BCG perfusion therapy, such as epirubicin, pirarubicin, mitomycin, group A streptococcus, gemcitabine, etc. The main adverse reactions after BCG perfusion were urinary tract irritation symptoms, including urinary frequency, urgency, dysuria, and hematuria, of which frequent urination and urgent urination were the most common, accounting for 51.3% (39/76), followed by dysuria and urethral burning 27.6% (21/76), and only 11.8% (9/76) of hematuria. According to TNM stage, the tumors were divided into Ta 21 (27.6%) cases, T1 47 (61.8%) cases, and T2a 7 (9.2%) cases, of which the staging data of 1 case were unknown. According to the histological differentiation, there were 8 (10.5%) cases in G1, 19 (25.0%) cases in G2, and 49 (64.5%) cases in G3. There were 24 (31.6%) cases with multiple bladder tumors. The median follow-up time for the entire cohort was 19.0 (7.5–29.0) months, during which 15 (19.7%) patients relapsed after BCG perfusion, with a median relapse-free time of 6 (3.0–11.0) months, and disease progression occurred in 12 patients.
Table 1. Demographic and clinical characteristics of bladder cancer patients with BCG perfusion (N=76).
| Parameters | Value |
|---|---|
| Sex, n (%) | |
| Male | 65 (85.5) |
| Female | 11 (14.5) |
| Median age, (interquartile range, years) | 63.0 (55.0–70.0) |
| Median follow-up time, (interquartile range, months) | 19.0 (7.5–29.0) |
| History of bladder cancer, n (%) | 29 (38.2) |
| Relapse after BCG perfusion, n (%) | 15 (19.7) |
| History of intravesical non-BCG perfusion, n (%) | 18 (23.7) |
| TNM stage, n (%) | |
| Ta | 21 (27.6) |
| T1 | 47 (61.8) |
| T2 | 7 (9.2) |
| Unknown | 1 (1.3) |
| Grade, n (%) | |
| G1 | 8 (10.5) |
| G2 | 19 (25.0) |
| G3 | 49 (64.5) |
| Tumor number, n (%) | |
| Multiple | 24 (31.6) |
| Single | 52 (68.4) |
| BCG perfusion side effects, n (%) | |
| Frequent urination | 39 (51.3) |
| Dysuria | 21 (27.6) |
| Hematuria | 9 (11.8) |
BCG, Bacillus Calmette-Guérin; TNM, tumor-node-metastasis.
Analysis of risk factors for recurrence and progression of bladder cancer after BCG perfusion by FISH
Figure 2 shows the FISH test results of 76 patients before TURBT and after BCG perfusion. A total of 83 FISH test results were collected, including 59 before TURBT and 24 after BCG perfusion.
Figure 2.
Analysis of risk factors for recurrence and progression of bladder cancer after BCG perfusion by FISH. TURBT, transurethral resection of bladder tumor; BCG, Bacillus Calmette-Guérin; FISH, fluorescence in situ hybridization.
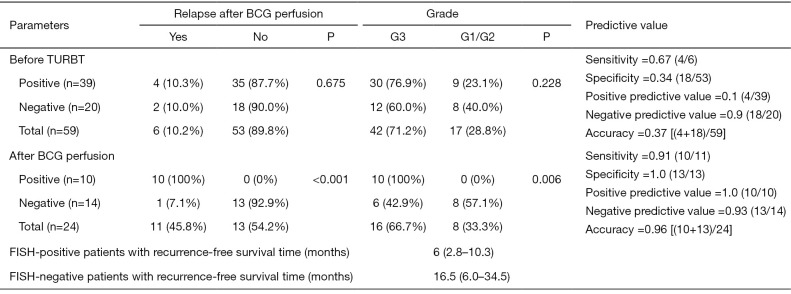
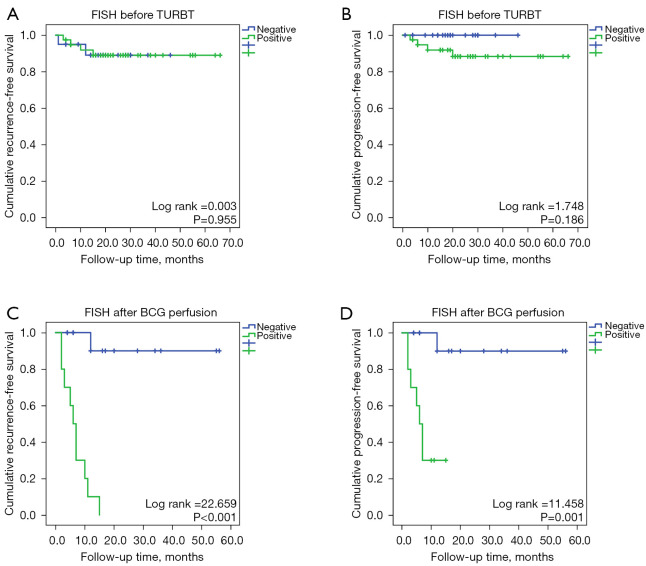
Before TURBT, there were 39 patients with positive FISH, of which 76.9% (30/39) had tumor tissue differentiated into G3, 23.1% (9/39) with G1/G2, and 4 (10.3%) after BCG perfusion relapse. There were 20 patients with negative FISH preoperatively, G3 accounted for 60.0% (12/20), G1/G2 accounted for 40.0% (8/20), and 2 (10.0%) patients recurred after BCG perfusion. The sensitivity, specificity, and accuracy of preoperative FISH in predicting recurrence after BCG perfusion were 67%, 34%, and 37%, respectively. There was no significant difference in preoperative FISH positive prediction of recurrence rate after BCG perfusion (10.3% vs. 10%, P=0.675). No association was found between FISH results and tumor recurrence (P=0.955) or disease progression (P=0.186) (Figure 3A,3B).
Figure 3.
Predictive effect of fluorescence in situ hybridization on BCG perfusion in bladder cancer. (A,B) Prediction effect of preoperative FISH for recurrence and progression of bladder cancer; (C,D) Prediction effect of FISH after BCG perfusion for recurrence and progression of bladder cancer. FISH, fluorescence in situ hybridization; TURBT, transurethral resection of bladder tumor; BCG, Bacillus Calmette-Guérin.
After BCG perfusion, there were 10 patients with positive FISH, of which 100% (10/10) had tumor tissue differentiated into G3, with a recurrence rate of 100% (10/10) and a recurrence rate of 70% (7/10) within six months. There were 14 negative FISH patients after BCG perfusion, G3 accounted for 42.9% (6/14), G1/G2 accounted for 57.1% (8/14), and only 1 (7.1%) relapsed after one year. After BCG perfusion, the sensitivity, specificity, and accuracy of FISH in predicting recurrence were 91%, 100%, and 96%, respectively. FISH positivity was significantly different in predicting bladder cancer recurrence (100% vs. 7.1%, P<0.001). The recurrence-free survival time was 6 (2.8–10.3) months in patients with positive FISH and 16.5 (6.0–34.5) months in patients with negative FISH. FISH results were significantly associated with tumor recurrence (P<0.001) and disease progression (P=0.001) (Figure 3C,3D).
Kaplan-Meier and univariate COX proportional hazards regression analysis clarified that FISH positive after BCG perfusion, TNM stage, and multiple tumors were risk factors for tumor recurrence and progression (P<0.05). No correlation was found between histological grade and disease progression (P=0.083), but was associated with recurrence (P=0.025) (Table 2). Multivariate COX proportional risk regression confirmed TNM stage [hazard ratio (HR) =5.599, 95% confidence interval (CI): 1.230–25.494, P=0.026] and FISH positive after BCG perfusion (HR =13.889, 95% CI: 1.634–19.257, P=0.002) were independent risk factors for recurrence (Table 3).
Table 2. Univariate analysis of recurrence and progression of bladder cancer after BCG perfusion.
| Parameters | Recurrence | Progress | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| FISH positive before TURBT | 0.953 | 0.174–5.207 | 0.955 | 36.062 | 0.004–293228.920 | 0.435 | |
| FISH positive after BCG perfusion | 33.136 | 4.028–272.613 | 0.001 | 17.491 | 1.979–154.618 | 0.010 | |
| Age >65 years old | 1.152 | 0.418–3.179 | 0.784 | 1.020 | 0.329–3.164 | 0.973 | |
| Sex (male) | 26.014 | 0.059–11,541.871 | 0.259 | 0.499 | 0.064–3.871 | 0.499 | |
| G3 | 4.653 | 1.048–20.665 | 0.043 | 3.506 | 0.767–16.033 | 0.106 | |
| TNM stage (T2) | |||||||
| Ta | 0.045 | 0.005–0.390 | 0.005 | 0.046 | 0.005–0.393 | 0.005 | |
| T1 | 0.210 | 0.070–0.629 | 0.005 | 0.137 | 0.041–0.455 | 0.001 | |
| Multiple tumor | 4.338 | 1.482–12.692 | 0.007 | 6.670 | 1.805–24.653 | 0.004 | |
BCG, Bacillus Calmette-Guérin; HR, hazard ratio; CI, confidence interval; FISH, fluorescence in situ hybridization; TURBT, transurethral resection of bladder tumor; TNM, tumor-node-metastasis.
Table 3. Multivariate analysis of recurrence of bladder cancer after BCG perfusion.
| Parameters | β | S | P value | HRs for recurrence (95% CI) |
|---|---|---|---|---|
| TNM stage | 1.723 | 0.773 | 0.026 | 5.599 (1.230–25.494) |
| FISH positive after BCG perfusion | 1.358 | 0.442 | 0.002 | 13.889 (1.634–19.257) |
HRs of each variable were obtained using separate proportional hazard Cox models after adjustment for preoperative FISH positive, age, sex, grade and multiple tumor. BCG, Bacillus Calmette-Guérin; HR, hazard ratio; CI, confidence interval; TNM, tumor-node-metastasis; FISH, fluorescence in situ hybridization.
Discussion
Urothelial carcinoma is one of the common urological cancers, and bladder urothelial carcinoma is the most common in clinical practice, accounting for 90% of bladder cancer (4,20). Worldwide, the incidence of bladder cancer ranks 9th among malignant tumors, ranking 7th among men and 10th among women (4). In 2015, the incidence of bladder cancer in my country was 5.80/100,000, and the mortality rate was 2.37/100,000 (1,2). Bladder cancer is characterized by high incidence, easy recurrence and poor prognosis (3,4,21,22). It is a cancer that directly and seriously threatens the quality of life and survival time of patients. Therefore, researchers pay special attention to the early diagnosis and prognosis monitoring of bladder cancer.
There were 59 FISH tests before TURBT, and the positive rate was only 66.1% (39/59), which was significantly lower than the 87.18% sensitivity of FISH for urothelial carcinoma as shown by Hu et al. (23). This may be due to insufficient shedding of cancerous cells into the urine in some cases, or the inability of fluorescent probes to specifically recognize aberrant chromosomes. This is not surprising since chromosomes 1, 5, 8, and 11 are also associated with bladder cancer, but they do not belong to the group of FISH probes used in this study (24). In addition, due to the limitations of retrospective study, relatively few patients, some heterogeneity in the patient population, and not all patients underwent FISH testing before TURBT, these factors will reduce the sensitivity of FISH testing.
Studies have shown that preoperative FISH positivity were directly related to the number of chromosomally aberrant cells in urine, corresponding to high-grade, high-burden bladder cancer (12-14). Compared with low-grade and low-burden bladder cancers, high-grade and high-burden bladder cancers are more likely to relapse without chemotherapy or with chemotherapy drugs. However, this study found that preoperative FISH-positive patients and FISH-negative patients had similar recurrence rates after BCG perfusion (10.3% vs. 10%, P=0.675), indicating that BCG can effectively reduce the risk of recurrence in FISH-positive bladder cancer (high-grade, high-burden).
Since this study is a retrospective study, the FSIH results collected before BCG perfusion were preoperative, while the mainstream literature published so far is the correlation analysis using FISH before the first BCG perfusion after TURBT. The role of FISH before the first BCG perfusion after TURBT in predicting the efficacy of BCG perfusion in bladder cancer is still controversial. A retrospective study published in European Journal of Urology by Mengual et al. (25) found no association between FISH results before BCG perfusion and the risk of tumor recurrence (P=0.44) or progression to muscle-invasive disease (P=0.97). Savic et al. (11) prospectively evaluated 68 patients with NMIBC and determined that FISH positivity before BCG perfusion was not significantly associated with an increased risk of recurrence. Liem et al. (26) found in a multicenter prospective study that there was no significant correlation between positive FISH before BCG perfusion and the risk of recurrence (P=0.79). The author’s review and meta-analysis summary published in Journal of Urology in 2019 also mentioned that positive FISH before BCG perfusion was not associated with a higher risk of recurrence (27). However, Kamat et al. (28) conducted a prospective study of 126 bladder cancer patients and found that patients with positive FISH before BCG perfusion had a significantly higher risk of recurrence and disease progression than those with negative results (38.3% vs. 17.8%, P=0.020; 19.8% vs. 4.4%, P=0.032). This inconsistency can be explained by differences in the patient cohort. Eighty-nine percent of patients in the cohort assessed by Kamat et al. (28) had previously been treated for bladder cancer and 48% had carcinoma in situ as a secondary discovery, compared to 38.2% in our cohort. In addition, there are interferences from factors such as secondary resection and immediate postoperative chemotherapy.
After BCG perfusion, FISH positive was significantly associated with the risk of tumor recurrence and progression (P<0.001), which is consistent with the findings of many studies (Table S1). Mengual et al. (25) found that patients with positive FISH after BCG perfusion had a 2.7 times higher risk of tumor recurrence than patients with negative FISH (P=0.017, 95% CI: 1.18–6.15). Lotan et al. (29) validated a 3.3-fold increased risk of recurrence in FISH-positive patients after BCG treatment in NMIBC patients in a prospective multicenter trial. Liem et al. (27) mentioned in a meta-analysis that the hazard ratio for FISH-positive recurrence after BCG treatment was 2.23 (95% CI: 1.31–3.62) at 6 weeks, 3.70 (95% CI: 2.34–5.83) at 3 months, 23.44 (95% CI: 5.26–104.49) at 6 months. Kamat et al. (28) found that patients 6 weeks or 3 or 6 months after starting BCG treatment, FISH-positive patients were 3–5 times more likely to relapse and 5–13 times more likely to have disease progression than controls. Since this study is a retrospective study, the number of patients reviewing FISH after TURBT is small and irregular, so it is not possible to conduct stratified analysis of FISH test results by time. Therefore, the risk ratio obtained is the result of the overall analysis. The recurrence rate of FISH-positive patients was 100%, and the recurrence-free survival time was only 6 (2.8–10.3) months, which meant that the recurrence rate of FISH-positive patients within six months after BCG treatment was very high. Liem et al. (26) also mentioned in their study that the median recurrence-free time of FISH-positive patients after BCG treatment was 6 [3–28] months, Therefore, patients with positive FISH should be followed up closely after BCG perfusion and the follow-up interval should be appropriately shortened. In conclusion, regular FISH tests (6 weeks, 3 months, 6 months and 9 months after BCG perfusion) can be performed to determine whether BCG perfusion is effective. How to formulate the treatment plan for FISH-positive patients after BCG treatment needs further research and discussion, because it is necessary to balance tumor prognosis, quality of life and complications caused by surgery. If they all receive radical cystectomy, there is a great risk of overtreatment.
In addition to FISH, non-invasive follow-up monitoring of bladder cancer includes mini chromosome maintenance 5 (MCM5) urine expression (ADXBLADDER) (30), Bladder EpiCheck Test (31), mRNA-based urine test (Xpert Bladder Cancer Monitor) (32), etc. These new bladder cancer biomarkers can monitor the risk of tumor recurrence with better sensitivity than cytology. FISH also has some potential deficiencies. FISH is less sensitive to detect low-grade tumors, which may be explained by the fact that the chromosomes of low-grade tumors are usually diploid or nearly diploid, without obvious genetic abnormalities, and similar to normal cells. Secondly, the locus probe 9p21 has the smallest volume, and it is also the most common genetic abnormal locus, so it is not easy to observe.
As for the risk factors for bladder cancer recurrence, this study found that positive FISH after BCG perfusion, TNM stage, G3 and multiple tumors were closely related to tumor recurrence, which was consistent with the conclusion of Whitson et al. (33). These indicators (except positive FISH after BCG perfusion) are similarly described in the 2019 Chinese Urology and Andrology Guidelines (34).
Our study also had some limitations. First of all, this was a single-center retrospective study with a small sample size and no multi-center large data for mutual verification. Secondly, because the cost of FISH detection was more expensive than cystoscopy, the data of patients undergoing FISH detection after BCG perfusion was less and the detection time was very irregular, so it was impossible to perform stratified analysis according to the detection time of FISH. The follow-up time of some patients was short, and the observation endpoint event did not occur.
Conclusions
Preoperative FISH positive can provide early diagnosis of bladder cancer, but cannot predict the risk of recurrence and progression after BCG perfusion. FISH positive after BCG perfusion, TNM stage, and multiple tumors were risk factors for bladder cancer recurrence and progression. After BCG perfusion, it is better to recommend close follow up in patients with positive FISH. Periodic FISH testing (6 weeks, 3 months, 6 months, and 9 months after perfusion) can be attempted to determine whether BCG perfusion is effective.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by National Natural Science Foundation of China (No. 81702989).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Medical Ethics Committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (Approval No. TJ-IRB20210521) and individual consent for this retrospective analysis was waived.
Footnotes
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1367/rc
Data Sharing Statement: Available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1367/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tcr.amegroups.com/article/view/10.21037/tcr-22-1367/coif). The authors have no conflicts of interest to declare.
References
- 1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 2.Chen W. Cancer statistics: updated cancer burden in China. Chin J Cancer Res 2015;27:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cumberbatch MGK, Jubber I, Black PC, et al. Epidemiology of Bladder Cancer: A Systematic Review and Contemporary Update of Risk Factors in 2018. Eur Urol 2018;74:784-95. 10.1016/j.eururo.2018.09.001 [DOI] [PubMed] [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 5.Flaig TW, Spiess PE, Agarwal N, et al. Bladder Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2020;18:329-54. 10.6004/jnccn.2020.0011 [DOI] [PubMed] [Google Scholar]
- 6.Babjuk M, Burger M, Compérat EM, et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ) - 2019 Update. Eur Urol 2019;76:639-57. 10.1016/j.eururo.2019.08.016 [DOI] [PubMed] [Google Scholar]
- 7.Chang SS, Boorjian SA, Chou R, et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J Urol 2016;196:1021-9. 10.1016/j.juro.2016.06.049 [DOI] [PubMed] [Google Scholar]
- 8.Brausi M, Oddens J, Sylvester R, et al. Side effects of Bacillus Calmette-Guérin (BCG) in the treatment of intermediate- and high-risk Ta, T1 papillary carcinoma of the bladder: results of the EORTC genito-urinary cancers group randomised phase 3 study comparing one-third dose with full dose and 1 year with 3 years of maintenance BCG. Eur Urol 2014;65:69-76. 10.1016/j.eururo.2013.07.021 [DOI] [PubMed] [Google Scholar]
- 9.Pansadoro V, Emiliozzi P, de Paula F, et al. Long-term follow-up of G3T1 transitional cell carcinoma of the bladder treated with intravesical bacille Calmette-Guérin: 18-year experience. Urology 2002;59:227-31. 10.1016/S0090-4295(01)01603-X [DOI] [PubMed] [Google Scholar]
- 10.Shelley MD, Kynaston H, Court J, et al. A systematic review of intravesical bacillus Calmette-Guérin plus transurethral resection vs transurethral resection alone in Ta and T1 bladder cancer. BJU Int 2001;88:209-16. 10.1046/j.1464-410x.2001.02306.x [DOI] [PubMed] [Google Scholar]
- 11.Savic S, Zlobec I, Thalmann GN, et al. The prognostic value of cytology and fluorescence in situ hybridization in the follow-up of nonmuscle-invasive bladder cancer after intravesical Bacillus Calmette-Guérin therapy. Int J Cancer 2009;124:2899-904. 10.1002/ijc.24258 [DOI] [PubMed] [Google Scholar]
- 12.Sarosdy MF, Schellhammer P, Bokinsky G, et al. Clinical evaluation of a multi-target fluorescent in situ hybridization assay for detection of bladder cancer. J Urol 2002;168:1950-4. 10.1016/S0022-5347(05)64270-X [DOI] [PubMed] [Google Scholar]
- 13.Halling KC, King W, Sokolova IA, et al. A comparison of BTA stat, hemoglobin dipstick, telomerase and Vysis UroVysion assays for the detection of urothelial carcinoma in urine. J Urol 2002;167:2001-6. 10.1016/S0022-5347(05)65072-0 [DOI] [PubMed] [Google Scholar]
- 14.Halling KC, King W, Sokolova IA, et al. A comparison of cytology and fluorescence in situ hybridization for the detection of urothelial carcinoma. J Urol 2000;164:1768-75. 10.1016/S0022-5347(05)67104-2 [DOI] [PubMed] [Google Scholar]
- 15.Levsky JM, Singer RH. Fluorescence in situ hybridization: past, present and future. J Cell Sci 2003;116:2833-8. 10.1242/jcs.00633 [DOI] [PubMed] [Google Scholar]
- 16.Sokolova IA, Halling KC, Jenkins RB, et al. The development of a multitarget, multicolor fluorescence in situ hybridization assay for the detection of urothelial carcinoma in urine. J Mol Diagn 2000;2:116-23. 10.1016/S1525-1578(10)60625-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beijing Jin Pu Jia Medicial Technology Co. L. Available online: http://www.gpmedical.com.cn
- 18.Marín-Aguilera M, Mengual L, Ribal MJ, et al. Utility of fluorescence in situ hybridization as a non-invasive technique in the diagnosis of upper urinary tract urothelial carcinoma. Eur Urol 2007;51:409-15; discussion 415. 10.1016/j.eururo.2006.08.045 [DOI] [PubMed] [Google Scholar]
- 19.Miyazaki A, Tadokoro H, Drury JK, et al. Retrograde coronary venous administration of recombinant tissue-type plasminogen activator: a unique and effective approach to coronary artery thrombolysis. J Am Coll Cardiol 1991;18:613-20. 10.1016/0735-1097(91)90621-F [DOI] [PubMed] [Google Scholar]
- 20.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. 10.3322/caac.20073 [DOI] [PubMed] [Google Scholar]
- 21.Villavicencio H, Rodriguez Faba O, Palou J, et al. Bladder preservation strategy based on combined therapy in patients with muscle-invasive bladder cancer: management and results at long-term follow-up. Urol Int 2010;85:281-6. 10.1159/000316076 [DOI] [PubMed] [Google Scholar]
- 22.Lee R, Droller MJ. The natural history of bladder cancer. Implications for therapy. Urol Clin North Am 2000;27:1-13, vii. 10.1016/S0094-0143(05)70229-9 [DOI] [PubMed] [Google Scholar]
- 23.Hu Z, Ke C, Liu Z, et al. Evaluation of UroVysion for Urachal Carcinoma Detection. Front Med (Lausanne) 2020;7:437. 10.3389/fmed.2020.00437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smeets W, Pauwels R, Laarakkers L, et al. Chromosomal analysis of bladder cancer. III. Nonrandom alterations. Cancer Genet Cytogenet 1987;29:29-41. 10.1016/0165-4608(87)90028-8 [DOI] [PubMed] [Google Scholar]
- 25.Mengual L, Marín-Aguilera M, Ribal MJ, et al. Clinical utility of fluorescent in situ hybridization for the surveillance of bladder cancer patients treated with bacillus Calmette-Guérin therapy. Eur Urol 2007;52:752-9. 10.1016/j.eururo.2007.03.001 [DOI] [PubMed] [Google Scholar]
- 26.Liem EIML, Baard J, Cauberg ECC, et al. Fluorescence in situ hybridization as prognostic predictor of tumor recurrence during treatment with Bacillus Calmette-Guérin therapy for intermediate- and high-risk non-muscle-invasive bladder cancer. Med Oncol 2017;34:172. 10.1007/s12032-017-1033-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liem EIML, Oddens JR, Vernooij RWM, et al. The Role of Fluorescence In Situ Hybridization for Predicting Recurrence after Adjuvant bacillus Calmette-Guérin in Patients with Intermediate and High Risk Nonmuscle Invasive Bladder Cancer: A Systematic Review and Meta-Analysis of Individual Patient Data. J Urol 2020;203:283-91. 10.1097/JU.0000000000000566 [DOI] [PubMed] [Google Scholar]
- 28.Kamat AM, Dickstein RJ, Messetti F, et al. Use of fluorescence in situ hybridization to predict response to bacillus Calmette-Guérin therapy for bladder cancer: results of a prospective trial. J Urol 2012;187:862-7. 10.1016/j.juro.2011.10.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lotan Y, Inman BA, Davis LG, et al. Evaluation of the Fluorescence In Situ Hybridization Test to Predict Recurrence and/or Progression of Disease after bacillus Calmette-Guérin for Primary High Grade Nonmuscle Invasive Bladder Cancer: Results from a Prospective Multicenter Trial. J Urol 2019;202:920-6. 10.1097/JU.0000000000000355 [DOI] [PubMed] [Google Scholar]
- 30.Białek Ł, Czerwińska K, Fus Ł, et al. MCM5 urine expression (ADXBLADDER) is a reliable biomarker of high-risk non- muscle-invasive bladder cancer recurrence: A prospective matched case-control study. Cancer Biomark 2021;30:139-43. 10.3233/CBM-200316 [DOI] [PubMed] [Google Scholar]
- 31.Trenti E, D'Elia C, Mian C, et al. Diagnostic predictive value of the Bladder EpiCheck test in the follow-up of patients with non-muscle-invasive bladder cancer. Cancer Cytopathol 2019;127:465-9. 10.1002/cncy.22152 [DOI] [PubMed] [Google Scholar]
- 32.Cowan B, Klein E, Jansz K, et al. Longitudinal follow-up and performance validation of an mRNA-based urine test (Xpert® Bladder Cancer Monitor) for surveillance in patients with non-muscle-invasive bladder cancer. BJU Int 2021;128:713-21. 10.1111/bju.15418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitson J, Berry A, Carroll P, et al. A multicolour fluorescence in situ hybridization test predicts recurrence in patients with high-risk superficial bladder tumours undergoing intravesical therapy. BJU Int 2009;104:336-9. 10.1111/j.1464-410X.2009.08375.x [DOI] [PubMed] [Google Scholar]
- 34.Huang J. Chinese Guidelines for the Diagnosis and Treatment of Urology and Andrology Diseases (2019 Edition). Beijing: Science Press, 2020;10:27-84. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as