Abstract
An extracellular exopolysaccharide (slime) is produced by Vibrio cholerae O139 MO10 in response to nutrient starvation. The presence of this slime layer on the cell surface and its subsequent release have been shown to be associated with biofilm formation and the change from a normal smooth colony morphology to a rugose one. An immunoelectron microscopic examination demonstrated that there is an epitope common to the exopolysaccharide antigen of V. cholerae O1 and that of O139 MO10.
Vibrio cholerae is the causative agent of cholera, which in its most severe form is characterized by profuse diarrhea, vomiting, and muscle cramps. V. cholerae strains have been divided into two groups, O1 and non-O1, based on their ability to cause cholera epidemics. To date, there have been seven recorded pandemics of this severe dehydrating diarrheal disease caused by V. cholerae strains of serotype O1, and it was therefore assumed that only this serotype has epidemic potential. The new serogroup, designated O139 synonym Bengal, is the first recorded serogroup other than O1 to cause epidemic cholera. V. cholerae O139 closely resembles V. cholerae O1 biotype El Tor strains of the seventh pandemic (5, 12, 21, 40). The major differences between V. cholerae O139 and O1 are the composition and lengths of the O side chains of the cell wall lipopolysaccharide (LPS) and the presence of a capsular polysaccharide (CPS) in O139 strains that is not found in V. cholerae O1 strains (7, 15, 20). Serological and genetic studies suggested that CPS of O139 V. cholerae has the same repeating unit as the O antigen (8, 39).
V. cholerae strains are natural inhabitants of brackish water and estuarine systems (6). As a response to nutrient depletion, copiotrophic (32) heterotrophic bacteria may undergo considerable morphological, physiological, and chemical changes (11, 22, 23, 26, 27, 29). In fact, to survive energy- and nutrient-deprived conditions, non-spore-forming, heterotrophic bacteria are known to undergo an active adaptation program (29). Wai et al. (38) reported that V. cholerae O1 TSI-4 can shift to a rugose colony morphology associated with the expression of an amorphous exopolysaccharide (EPS) that promotes biofilm formation, and they also indicated that rugose strains displayed resistance to osmotic and oxidative stress.
Many microorganisms produce EPSs which are located outside the cell wall, either attached to it in the form of capsules or secreted into the extracellular environment in the form of slime. Extracellular polysaccharide excretion (slime or capsule) is a common phenomenon for many bacteria following the exhaustion of the nitrogen supply under otherwise nutrient-sufficient conditions (28). Bacterial cells initiate the process of irreversible adhesion by binding to the surface by using EPSs, glycocalyx polymers, and the development of biofilms. A biofilm is a functional consortium of microorganisms organized within an extensive exopolymer matrix comprised mainly of hydrated polysaccharides (43). Biofilms are produced by a wide variety of environmentally and medically important microorganisms, including Staphylococcus, Pseudomonas, Desulfovibrio, Thermococcus, and Methanosarcina (4, 9, 10, 18, 35, 36). The production of biofilm may enhance the survival of cells in dynamic environments by allowing the formation of colonies containing thousands of cells.
This study demonstrates that V. cholerae O139 MO10 is able to shift to a phenotype having a rugose colony morphology associated with the excretion of slime in response to starvation. This form promotes biofilm formation. Interestingly, the antiserum against V. cholerae O1 TSI-4 EPS (38) is reactive with the slime produced by V. cholerae O139 rugose strains. It may support the hypothesis that V. cholerae O139 arose from an O1 El Tor strain.
Isolation of the rugose strain of V. cholerae O139 MO10.
V. cholerae O139 opaque encapsulated MO10 (40) was used in this study. The original isolate of strain MO10 had a smooth colony morphology. Cells of MO10 were routinely grown at 37°C with shaking or in a static condition in Luria (L) broth (25). MO10 cells were incubated to mid-log phase, which corresponded to an A600 of 0.4. The cells were then harvested by centrifugation (13,000 × g for 10 min), washed three times with cold M9 salts (37), resuspended in starvation medium (M9 salts) to give a final concentration of approximately 5 × 107 cells per ml, and incubated at 4°C without shaking. Strain MO10 exhibits a shift of colony morphology to the rugose form under starvation conditions at 2 weeks after inoculation. To establish the criteria for slime production and rugose colony morphology, V. cholerae O139 MO10 strains have been classified as either slime-producing rugose type (MO10/SPR) or non-slime-producing smooth type (MO10/NSPS). MO10/SPR grown overnight with shaking in L broth produced MO10/NSPS colonies at a frequency of 1.5 × 10−5. Colony counts for rugose strains represent the number of particles not the number of cells (34). This makes the determination of a frequency of phase variation difficult. Two distinct colony morphologies are shown in Fig. 1. The larger size of the smooth colonies is due to the difference of the growth rates of smooth and rugose colonies. Both colony types were tested for agglutination with anti-O139 Bengal sera (Denka, Seiken, Co. Ltd., Tokyo, Japan) and showed positive reactions. The antiserum against V. cholerae O1 TSI-4 EPS (38) agglutinated MO10/SPR, whereas it did not agglutinate MO10/NSPS.
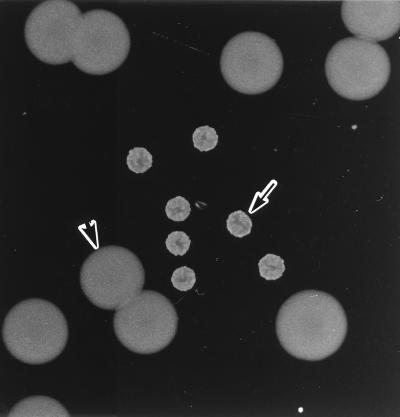
FIG. 1.
Photomicrograph of V. cholerae O139 MO10/SPR (arrow) and MO10/NSPS (arrow head) colonies. Bacteria were incubated on an L agar plate at 37°C for 18 h.
Like the O1 TSI-4 rugose strain (38), V. cholerae MO10/SPR was much more resistant to osmotic, oxidative, and acidic stress than MO10/NSPS (data not shown).
Thin-section electron microscopy.
To determine the nature of the colony morphology differences, bacterial pellets were stained with polycationic ferritin, and thin sections were observed by electron microscopy as described previously (38). Both strains were surrounded by relatively thin electron-dense capsule (Fig. 2). Slime materials released by MO10/SPR were recognized as a heavy, electron-dense ferritin-stained layer surrounding the cell in addition to a thin electron-dense layer of capsule (Fig. 2A and B), but MO10/NSPS did not appear to have this slime layer surrounding its cells (Fig. 2C).
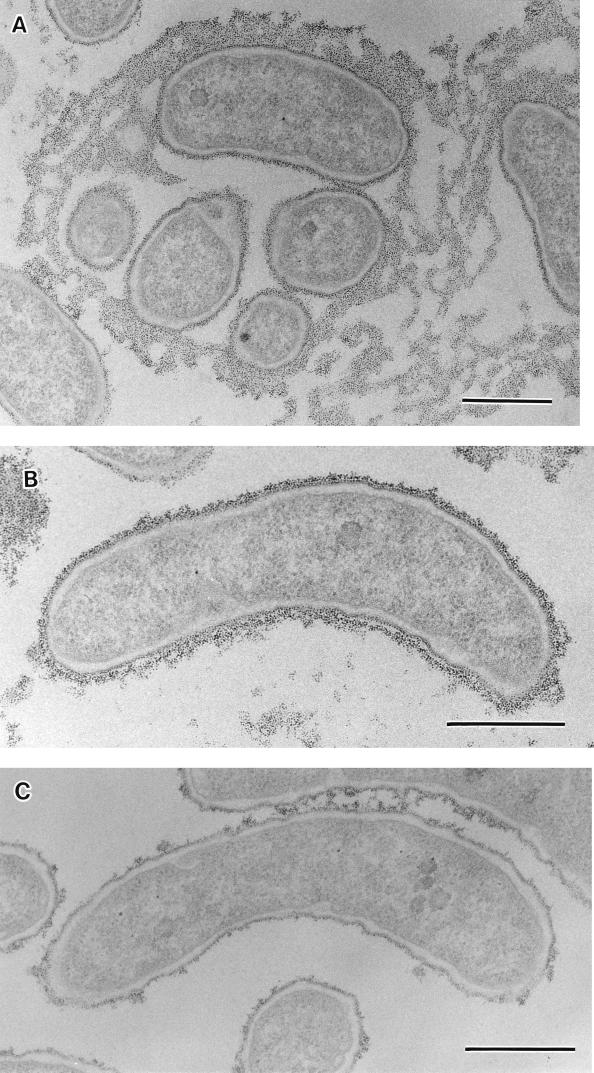
FIG. 2.
Thin sections of V. cholerae O139 stained with polycationic ferritin showing a thick, electron-dense slime layer in addition to a thin electron-dense layer of capsule surrounding MO10/SPR cells (A and B) and no slime layer surrounding MO10/NSPS (C). Bars, 0.5 μm.
Immunoelectron microscopy.
Immunoelectron microscopy was performed with anti-EPS serum of the rugose form of V. cholerae O1 TSI-4 as described previously (38). The antiserum against V. cholerae O1 TSI-4 EPS (38) was reactive only with V. cholerae O139 MO10/SPR and not with MO10/NSPS (Fig. 3A and B). The gold particles were specifically bound to the slime layer surrounding MO10/SPR cells and at the intercellular spaces (Fig. 3A).
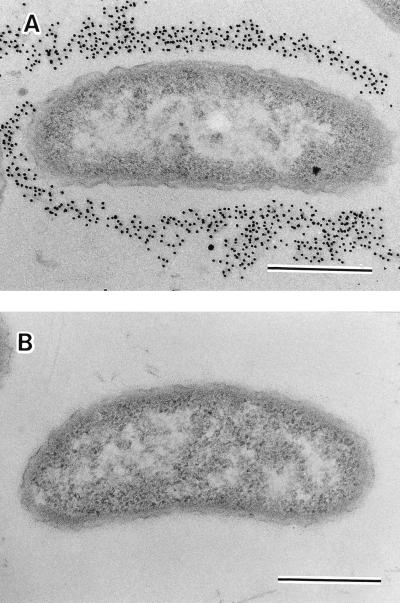
FIG. 3.
Immunoelectron micrographs of the surface labeling of V. cholerae O139 MO10/SPR (A) and MO10/NSPS (B) with antiserum against EPS of rugose V. cholerae O1 TSI-4. Bars, 0.5 μm.
Outer membrane and LPS profiles.
The outer membrane was prepared from a broth culture of V. cholerae O139 MO10/NSPS or MO10/SPR according to the method of Filip et al. (13). LPS was prepared from 1 ml of an overnight culture (11). LPS and outer membrane samples were electrophoresed and detected by silver staining as previously described (16). No outer membrane protein or LPS differences between cell types were detected (data not shown).
Biofilm growth of V. cholerae O139 MO10/SPR and scanning electron microscopy.
V. cholerae O139 MO10/SPR was cultured overnight in L broth at 37°C without shaking. The biofilms growing on the upper surface of the L broth and on the wall of a culture tube were sampled and prepared for scanning electron microscopy as described previously (38). The specimens were examined with a scanning image-observing device (ASID) equipped with a JEOL JEM 2000EX electron microscope. Figure 4 shows a biofilm examined by scanning electron microscopy; the surface of the film was completely covered with a layer of rod cells, rounded cells, and filamentous cells embedded within a polymeric matrix. Throughout the biofilm, cells were interconnected by a finger-like glycocalyx matrix that extended from the substratum to the outer boundaries of the biofilm. Interestingly, some of the surface of the biofilm was covered by a twisting long filamentous growth of bacteria.
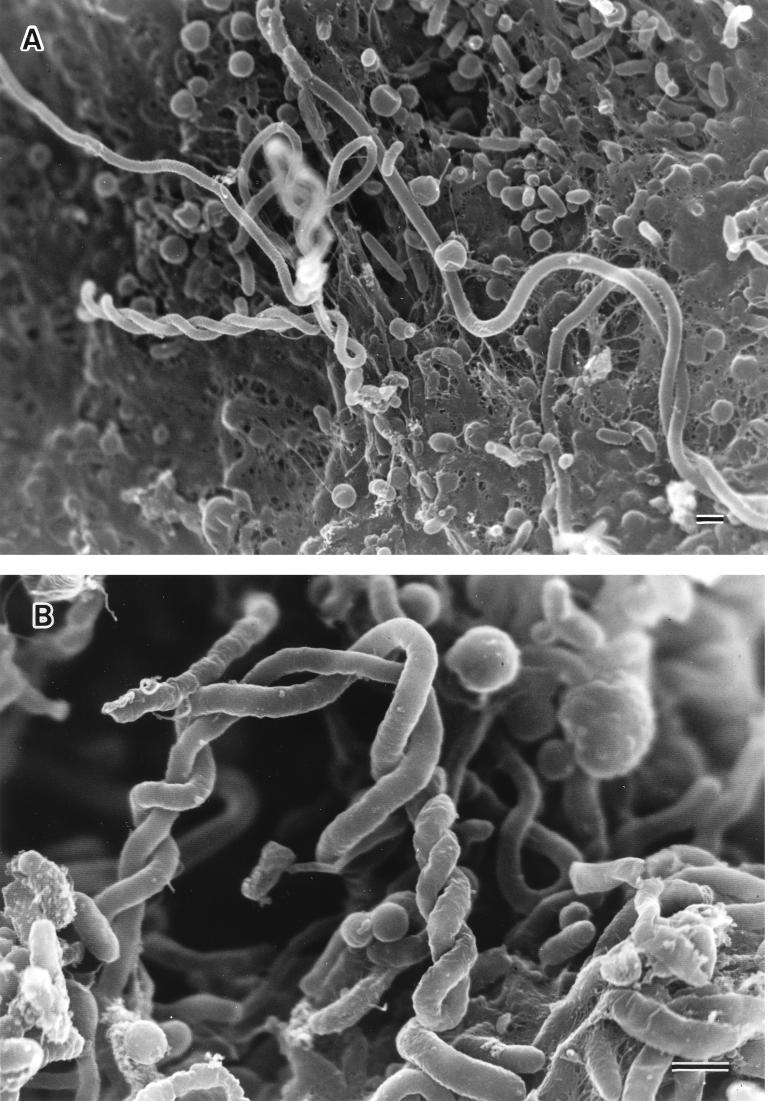
FIG. 4.
Scanning electron micrographs of biofilm formation by V. cholerae O139 MO10/SPR. (A) Most of the surface has been colonized by rod cells, rounded cells, and twisting filamentous cells, and finger-like projections of extracellular polymeric material are present. Bar, 1 μm. (B) High magnification shows extracellular polymeric materials on the surface of bacterial cells and long twisting filamentous cells. Bar, 1 μm.
The rugose form of V. cholerae was first described in 1938 by Bruce White, who recognized that it might be a survival form of the organism (42). Rice et al. (33, 34) suggested that the V. cholerae rugose phenotype represents a fully virulent survival form of the organism that can persist in the presence of free chlorine and that this phenotype may limit the usefulness of chlorination in blocking the endemic and epidemic spread of cholera. Morris et al. (30) have supported and confirmed that rugose strains appear to produce an EPS that promotes cell aggregation and causes human disease. Recently, Wai et al. (38) reported that V. cholerae O1 TSI-4 can shift to a rugose colony morphology from its normal translucent colony morphology in response to nutrient starvation. They also observed that EPS material on the surface of the V. cholerae O1 TSI-4 rugose strain promoted biofilm formation and resistance to the effects of osmotic and oxidative stress, as in the case of the O139 rugose strain. These observations suggest that the persistence of this type of setting may, in turn, contribute to the further spread of the infection in human populations. It is suggested that an improved understanding of starvation survival and nongrowth biology is an essential goal in microbiology, with far-reaching implications for bacterial physiology and ecology, as well as for applied bacteriology and biotechnology.
V. cholerae O139 is replacing O1 strains in some areas, and it has been suggested that the O139 strain may cause the eighth cholera pandemic (14, 19). V. cholerae O139 Bengal is the second most common etiologic agent of cholera, and the disease caused by this organism has now become endemic in the Indian subcontinent and neighboring countries (1). Prior infection with V. cholerae O1, the traditional causative agent of cholera, does not cross-protect against infection with V. cholerae O139 (2, 3), since the LPS antigens of the two vibrios are different (15). In addition, unlike V. cholerae O1, V. cholerae O139 possesses a CPS (20, 21, 39, 41), and it is likely that this CPS can potentially mask certain critical surface antigens, with a resulting decrease in the host immune response (31). Effective vaccines against O1 strains have been developed and are being tested in field trials (17, 24), and they do not cross-protect against V. cholerae O139 infection.
To facilitate the development of vaccines effective against both V. cholerae O1 and O139, many researchers have been studying the genes encoding O antigen and capsular synthesis in O1 and O139 strains. In our study, interestingly, antiserum against the EPS of V. cholerae O1 TSI-4 showed a cross-reaction with EPS materials on the surface of rugose V. cholerae O139 MO10. We suggest that the study of the genes encoding the EPS (slime) in V. cholerae O1 and O139 may facilitate the development of vaccines effective against both V. cholerae O1 and O139.
Acknowledgments
We thank K. Ohga for her technical assistance.
This work was supported by a grant from the Ministry of Education, Science, Sports, and Culture of Japan.
REFERENCES
- 1.Albert M J. Epidemiology and molecular biology of V. cholerae O139 Bengal. Indian J Med Res. 1996;104:14–27. [PubMed] [Google Scholar]
- 2.Albert M J, Alam K, Ansaruzzaman M, Quadri F, Sack R B. Lack of cross-protection against diarrhea due to Vibrio cholerae O139 synonym Bengal after oral immunization of rabbits with V. cholerae O1 vaccine strain, CVD 103-HgR. J Infect Dis. 1994;169:230–231. doi: 10.1093/infdis/169.1.230. [DOI] [PubMed] [Google Scholar]
- 3.Albert M J, Siddique A K, Islam M S, Faruque A S G, Ansaruzzaman M, Faruque S M, Sack R B. Large outbreak of clinical cholera due to Vibrio cholerae non-O1 in Bangladesh. Lancet. 1993;341:704. doi: 10.1016/0140-6736(93)90481-u. [DOI] [PubMed] [Google Scholar]
- 4.Antón J, Meseguer I, Rodríguez-Valera F. Production of an extracellular polysaccharide by Haloferax mediterranei. Appl Environ Microbiol. 1988;54:2381–2386. doi: 10.1128/aem.54.10.2381-2386.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berche P, Poyart C, Abachin E, Lelievre H, Vandepitte J, Dodin A, Fournier J. The novel epidemic strain O139 is closely related to the pandemic strain O1 of Vibrio cholerae. J Infect Dis. 1994;170:701–704. doi: 10.1093/infdis/170.3.701. [DOI] [PubMed] [Google Scholar]
- 6.Colwell R R, Huq A. Vibrios in the environment:viable but nonculturable Vibrio cholerae. In: Wachsmuth I K, Blake P A, Olsvik Ø, editors. Vibrio cholerae and cholera:molecular to global perspectives. Washington, D.C: ASM Press; 1994. pp. 117–133. [Google Scholar]
- 7.Comstock L E, Johnson J A, Michalsky J M, Morris J G, Jr, Kaper J B. Cloning and sequence of a region encoding a surface polysaccharide of Vibrio cholerae O139 and characterization of the insertion site in the chromosome of Vibrio cholerae O1. Mol Microbiol. 1996;19:815–826. doi: 10.1046/j.1365-2958.1996.407928.x. [DOI] [PubMed] [Google Scholar]
- 8.Comstock L E, Maneval D, Jr, Panigrahi P, Joseph A, Levine M M, Kaper J B, Morris J G, Jr, Johnson J A. Capsule and O antigen in Vibrio cholerae O139 Bengal are associated with a genetic region not present in Vibrio cholerae O1. Infect Immun. 1995;63:317–323. doi: 10.1128/iai.63.1.317-323.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costerton J W, Lewandowski Z, Caldwell D E, Lorber D R, Lappin-Scott H M. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 10.Costerton J W, Marrie T J. The role of the bacterial glycocalyx in resistance to antimicrobial agents. In: Easmon C S F, Jelijaszewicz J, Brown M R W, Lambert P A, editors. Role of the envelope in the survival of bacteria in infection. London, England: Academic Press; 1983. pp. 63–85. [Google Scholar]
- 11.Dawson M P, Humphrey B, Marshall K C. Adhesion:a tactic in the survival strategy of a marine Vibrio during starvation. Curr Microbiol. 1981;6:195–201. [Google Scholar]
- 12.Faruque S M, Alim A R M A, Roy S K, Khan F, Nair B G, Sack R B, Albert M J. Molecular analysis of rRNA and cholera toxin genes carried by the new epidemic strain of toxigenic Vibrio cholerae O139 synonym Bengal. J Clin Microbiol. 1994;32:1050–1053. doi: 10.1128/jcm.32.4.1050-1053.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filip C, Fletcher G, Wulff J L, Earhart C F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973;115:717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garg S, Saha P K, Ramamurthy T, Deb B C, Nair G B, Shimada T, Takeda Y. Nationwide prevalence of the new epidemic strain of Vibrio cholerae O139 Bengal in India. J Infect. 1993;27:108–109. doi: 10.1016/0163-4453(93)94398-u. [DOI] [PubMed] [Google Scholar]
- 15.Hisatsune K, Kondo S, Isshiki Y, Iguchi T, Kawamata Y, Shimada T. O-antigenic lipopolysaccharide of Vibrio cholerae O139 Bengal, a new epidemic strain for recent cholera in the Indian subcontinent. Biochem Biophys Res Commun. 1993;196:1309–1315. doi: 10.1006/bbrc.1993.2395. [DOI] [PubMed] [Google Scholar]
- 16.Hitchcock P J, Brown T M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-staining polyacrylamide gels. J Bacteriol. 1983;154:269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmgren J, Osek J, Svennerholm A-M. Protective oral cholera vaccine based on a combination of cholera toxin B subunit and inactivated cholera vibrios. In: Wachsmuth I K, Blake P A, Olsvik Ø, editors. Vibrio cholerae and cholera:molecular to global perspectives. Washington, D.C: ASM Press; 1994. pp. 415–424. [Google Scholar]
- 18.Hoyle B D, Jass J, Costerton J W. The biofilm glycocalyx as a resistance factor. J Antimicrob Chemother. 1990;26:1–6. doi: 10.1093/jac/26.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Jesudason M V, John T J. Major shift in prevalence of non-O1 and El Tor Vibrio cholerae. Lancet. 1993;341:1090–1091. doi: 10.1016/0140-6736(93)92447-2. [DOI] [PubMed] [Google Scholar]
- 20.Johnson G, Osek J, Svennerholm A-M, Holmgren J. Immune mechanisms and protective antigens of Vibrio cholerae serogroup O139 as a basis for vaccine development. Infect Immun. 1996;64:3778–3785. doi: 10.1128/iai.64.9.3778-3785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson J A, Salles C A, Panigrahi P, Albert M J, Wright A C, Johnson R J, Morris J G., Jr Vibrio cholerae O139 synonym Bengal is closely related to Vibrio cholerae El Tor but has important differences. Infect Immun. 1994;62:2108–2110. doi: 10.1128/iai.62.5.2108-2110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kjelleberg S, Hermansson M, Mården P, Jones G W. The transient phase between growth and nongrowth of heterotrophic bacteria, with emphasis on the marine environment. Annu Rev Microbiol. 1987;41:25–49. doi: 10.1146/annurev.mi.41.100187.000325. [DOI] [PubMed] [Google Scholar]
- 23.Kjelleberg S, Humphrey B A, Marshall K C. Effect of interfaces on small, starved marine bacteria. Appl Environ Microbiol. 1982;43:1166–1172. doi: 10.1128/aem.43.5.1166-1172.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levine M M, Tacket C O. Recombinant live cholera vaccines. In: Wachsmuth I K, Blake P A, Olsvik Ø, editors. Vibrio cholerae and cholera:molecular to global perspectives. Washington, D.C: ASM Press; 1994. pp. 395–413. [Google Scholar]
- 25.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning, a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 26.Mården P, Nyström T, Kjelleberg S. Uptake of leucine by a marine gram-negative heterotrophic bacterium during exposure to starvation conditions. FEMS Microbiol Ecol. 1987;45:233–241. [Google Scholar]
- 27.Mården P, Tunlid A, Malmcrona-Friberg K, Odham G, Kjelleberg S. Physiological and morphological changes during short term starvation of marine bacteria isolate. Arch Microbiol. 1985;149:326–332. [Google Scholar]
- 28.Mason C A, Egli T. Dynamics of microbial growth in the deceleration and stationary phase of batch culture. In: Kjelleberg S, editor. Starvation in bacteria. New York, N.Y: Plenum Publishing Corp.; 1993. pp. 92–93. [Google Scholar]
- 29.Matin A, Auger E A, Blum P H, Schultz J E. Genetic basis of starvation survival in nondifferentiating bacteria. Annu Rev Microbiol. 1989;42:293–316. doi: 10.1146/annurev.mi.43.100189.001453. [DOI] [PubMed] [Google Scholar]
- 30.Morris J G, Jr, Sztein M B, Rice E W, Nataro J P, Losonsky G A, Panigrahi P, Tacket C O, Johnson J A. Vibrio cholerae O1 can assume a chlorine-resistant rugose survival form that is virulent for humans. J Infect Dis. 1996;174:1364–1368. doi: 10.1093/infdis/174.6.1364. [DOI] [PubMed] [Google Scholar]
- 31.Panigrahi P, Srinivas S, Morris J G., Jr . Program and abstracts of the 32nd Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1992. In vivo modulation of non-O1 Vibrio cholerae virulence, abstr. 616; p. 213. [Google Scholar]
- 32.Poindexter J S. The caulobacters:ubiquitous unusual bacteria. Microbiol Rev. 1981;45:123–179. doi: 10.1128/mr.45.1.123-179.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rice E W, Johnson C J, Clark R M, Fox K R, Reasoner D J, Dunnigan M E, Panigrahi P, Johnson J A, Morris J G., Jr Chlorine and survival of “rugose” Vibrio cholerae. Lancet. 1992;340:740. doi: 10.1016/0140-6736(92)92289-r. [DOI] [PubMed] [Google Scholar]
- 34.Rice E W, Johnson C J, Clark R M, Fox K R, Reasoner D J, Dunnigan M E, Panigrahi P, Johnson J A, Morris J G., Jr Vibrio cholerae O1 can assume a ’rugose’ survival form that resists killing by chlorine, yet retains virulence. Int J Environ Health Res. 1993;3:89–98. [Google Scholar]
- 35.Rinker K D, Kelly R M. Growth physiology of the hyperthermophilic archaeon Thermococcus litoralis:development of a sulfur-free defined medium, characterization of an exopolysaccharide, and evidence of biofilm formation. Appl Environ Microbiol. 1996;62:4478–4485. doi: 10.1128/aem.62.12.4478-4485.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sowers K R, Gunsalus R P. Adaptation for growth at various saline concentrations by the archaebacterium Methanosarcina thermophila. J Bacteriol. 1988;170:998–1002. doi: 10.1128/jb.170.2.998-1002.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wai S N, Moriya T, Kondo K, Misumi H, Amako K. Resuscitation of Vibrio cholerae O1 strain TSI-4 from a viable but nonculturable state by heat shock. FEMS Microbiol Lett. 1996;136:187–191. doi: 10.1111/j.1574-6968.1996.tb08047.x. [DOI] [PubMed] [Google Scholar]
- 38.Wai S N, Mizunoe Y, Takade A, Kawabata S, Yoshida S. Vibrio cholerae O1 strain TSI-4 produces the exopolysaccharide materials that determine colony morphology, stress resistance, and biofilm formation. Appl Environ Microbiol. 1998;64:3648–3655. doi: 10.1128/aem.64.10.3648-3655.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waldor M K, Colwell R, Mekalanos J J. The Vibrio cholerae O139 serogroup antigen includes capsular and lipopolysaccharide virulence determinants. Proc Natl Acad Sci USA. 1994;91:11388–11392. doi: 10.1073/pnas.91.24.11388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Waldor M K, Mekalanos J J. Emergence of a new cholera pandemic:molecular analysis of virulence determinants in Vibrio cholerae O139 and development of a live vaccine prototype. J Infect Dis. 1994;170:278–283. doi: 10.1093/infdis/170.2.278. [DOI] [PubMed] [Google Scholar]
- 41.Weintraub A, Widmalm G, Jansson P-E, Hansson M, Hultenby K, Albert M J. Vibrio cholerae O139 Bengal possesses a capsular polysaccharide which may confer increased virulence. Microb Pathog. 1994;16:235–241. doi: 10.1006/mpat.1994.1024. [DOI] [PubMed] [Google Scholar]
- 42.White P B. The rugose variant of Vibrios. J Pathol Bacteriol. 1938;46:1–6. [Google Scholar]
- 43.Whitfield C, Keenleyside W J. Regulation of expression of group IA capsular polysaccharides in Escherichia coli and related extracellular polysaccharides in other bacteria. J Ind Microbiol. 1995;15:361–371. doi: 10.1007/BF01569992. [DOI] [PubMed] [Google Scholar]