Version Changes
Revised. Amendments from Version 1
The main modification to the text based on comments from the reviewers. 1. Abstract: the words of some sentences have been changed. 2. Introduction: added explanation for the use of vit B12 as antioxidant. 3. Method: rephrased group II in the experimental animal, moved figure 1 to the result section and added explanation to it. 4. Result: I added this explanation (Values expressed in non-identical letters (a, b, c, d)are significantly difference (p<0.05) ) to the figures 1,2,3,4, added missing words to the( effect of cyanocobalamin on Malondialdehyde (MDA) Levels within the kidney tissue Homogenate of Rat) and corrected the wrong words in the (effect of cyanocobalamin on Glutathione (GSH) Levels within the kidney tissue Homogenate of Rat). 5. Discussion: added definition to the MDA, GSH. 6. References: changed the wrong references and rearrange them. 7. Grammar and punctuation of the article were corrected.
Abstract
Background: Methotrexate (MTX) is a chemotherapeutic drug, used mainly in many cancerous stages, inflammatory and auto-immune diseases, but its use has been limited by its nephrotoxicity. Cyanocobalamin is a water-soluble vitamin possessing nephro-protective properties. The aim of this study was to investigate the effect of cyanocobalamin on the nephrotoxicity of methotrexate.
Methods: In this study 42 albino adult female rats were used, divided into six groups each containing seven rats (n=7). First group: Control group (Negative control), 7 rats were injected intraperitoneally with 0.5ml/kg/day NS. Second group: 7 rats were injected intraperitoneal with a single dose of methotrexate (20 mg/kg) for 4 days. Third Group: 7 rats were given intraperitoneal cyanocobalamin at a dose (1.5 mg/kg/day) for two weeks, fourth, fifth, sixth group: 7 rats from each group were injected intraperitoneal with different concentrations of cyanocobalamin (0.5, 1, 1.5 mg/kg /day) respectively for two weeks and MTX (20 mg/kg) which was injected only on day 11. On day 15, rats from all groups were euthanized, and blood samples were taken for biochemical tests, including evaluating serum urea and creatinine. The kidneys were extracted for histological investigation and evaluation of antioxidant (GSH) and oxidative stress (MDA) by using kidney tissue homogenates.
Results: This study revealed that kidney damage produced by the MTX (group II) is manifested by significantly elevated (P<0.05) urea and creatinine. On the contrary, the cyanocobalamin groups (IV, V, VI) significantly (P<0.05) reduced urea and creatinine. Renal antioxidant defense systems, such as reduced glutathione depleted by MTX therapy, were restored to normal levels by cyanocobalamin. Furthermore, cyanocobalamin reduced oxidative stress (MDA) and histologically reduced renal tissue injury induced by MTX.
Conclusions: In conclusion, the study revealed that cyanocobalamin has a nephroprotective action upon MTX-induced renal damage in rats; cyanocobalamin may offer a protective effect, such as antioxidant action.
Keywords: Methotrexate, nephrotoxicity, cyanocobalamin, antioxidants, rat, histopathology
Introduction
Methotrexate (MTX) is known as an antifolate drug and has been prescribed for over 60 years to treat different auto-immune disorders 1 and numerous types of cancers, also used in combination with other anticancer drugs 2 and remains of great interest for researchers all over the world. 2
However, some adverse effects including nephrotoxicity triggered by MTX are severe, thus, assessment of effective drugs to overcome this problem are necessary. 3
MTX is one of the most common drugs induced nephrotoxicity when used at high doses, having molecular features that favor crystal precipitation in the tubular lumens in a manner of slowing urine flow and decreasing urine pH. 4 , 5
Researchers reported that the mechanism of MTX inducing nephrotoxicity is a result of oxidative damage by the formation of reactive oxygen species (ROS), then creating an imbalance between oxidants and antioxidants that are responsible for the adverse effect of MTX, such as nephrotoxicity. 6
Histologically in MTX–treated rats, kidneys showed: enlargement of Bowman capsule cavity, infiltration of lymphocytes, diminish of glomeruli size, an increase in blood cells count and degeneration of tubules. 7 , 8
Like other drugs with nephrotoxic effects, MTX-induced renal function declination is clinically tested by observation of hematuria and deterioration in serum urea and creatinine level. Reproduction of such effects is done in research studies in animals after a single dose of MTX. 9
Many studies show that dietary antioxidants play a role in the protection of the kidney against MTX-induced nephrotoxicity. 6
Cyanocobalamin is considered a synthetic compound of vitamin B12. Vitamin B12 is known as a water-soluble vitamin which is synthesized by microorganisms in nature, and in the chemical aspect it is related to a class known as corrinoids. This name is derived from the cyanide group that is attached to molecule. 10
Furtheremore , vitamin B12 has several functions in methylation reactions in the body, that acts as a cofactor in the form of methylcoblamin by the conversion of homocysteine to methionine and in the form of adenosylcobalamin in the transformation of methylmalonyl-CoA to succinyl-CoA. Both of these chemical reactions are important for cell division and growth, 11 in addition vitamin B12 plays a role in erythropoiesis and healthy neurological functions, 12 and it proved useful in the treatment of many diseases associated with inflammatory and oxidative stress. 13
The FDA have stated that cyanocobalamin is safe, there is no toxicity reported when cyanocobalamin given to animals, even at several thousand times their nutritional requirements. 14
In this study, vit B12 was selected due to antioxidant action according to previous animal studies, in which vitamin B12 can lower oxidative stress when used with different drugs such as gentamycin and tamoxifen. 13 , 15
Vit B12 act as anti-oxidant by different mechanism: as a direct superoxide scavenger; in addition, it may indirectly enhance ROS scavenging by protecting glutathione, which probably involves a complex network of processes that is not yet fully understood. 16
The objective of this study is to determine the protective effects of cyanocobalamin administration on methotrexate-induced nephrotoxicity in rats using biochemical and histopathological studies in renal tissue of rats.
Methods
Ethical approval
We have received ethical approval for work on experimental animals from the Researcher Ethics Committees at Department of Pharmacology and College of Medicine/University of Baghdad. On 7 November 2021 via ethical letter (approval number 1455). This study is reported in line with the ARRIVE guidelines. 40
Chemicals
All chemicals used in this experimental study were of the highest available purity, and no purification was necessary. Vitamin B12 GERDA (1000 mcg/4 ml) (GERDA ®1000, batch: H060) was purchased from GERDA, France. Methotrexate ampule (50 mg/2 ml) Methotrexate Mylan 50 mg/2 ml (batch/LOT 5103) was purchased from Mylan – Merck generiques, Italy, pellet food patch number 3218 from Mazuri ® food pellets (Mazuri Primate Diet, Special Diet Foods Ltd., UK).
Experimental animals
The study was carried out on 42 adult female albino rats. The sample size of n=7 per group was calculated based on previous research, inclusion criteria include (ages ranged from 2-3 months, sex: female and body weight ranged from 150-250 grams. In this study there was no exclusion criteria, the animals used were obtained from the same source, at the same time, there is no previous procedure performed on these rats before the experimental study.
The rats were obtained from the Animal House of the Iraqi center of cancer and genetic research/Almustansria University. They remained for one week to be adapted without intervention in a climate-controlled environment with appropriate temperature (22-25 c) and synthetic 12:12 hours in the light-dark cycle, rats received pellet food and tap water/ad libitum.
The human care of the animals was according to international guidelines for the care and use of laboratory animals, All of these efforts were made to reduce the number of rats and their suffering, These efforts included: 1. constant checking of animal house air conditioning with a standard free access to tap water/ad libitum; 2. cleaning the animal house and cages; 3. During the experiment careful handling was given into account and injection dose of the drug was as quickly as possible and given the dose to each rat separated from other; 4. used the effective anesthetic dose.
When the period of adaptation was over, the weight of the rats was recorded and they were randomly divided and placed in one cage per group, the name of the group was then recorded in each cage.
In total, 42 rats were randomly (simple randomization) divided into six group (n=7). All groups of animal treated in the same house under same environment. The experimental procedure was done blindly, daily between 8 am -12 pm for 15 days.
Six animal groups were made in this study (7 animals/each group) as follows:
Group I (control): 7 rats were treated with 0.5 ml normal saline once daily by intraperitoneal (I.P.) injection for 14 days and it was served as negative control.
Group II (MTX) : 7 rats were treated with a single IP dose of methotrexate (MTX) (20 mg/kg) followed by single IP injection of 0.5 ml normal saline for 4 days. This group was served as positive control. 17
Group III (1.5 mg of vit B12): 7 rats were treated with vitamin B12 (1.5 mg/kg/rat/day) once daily by I.P. for two weeks.
Group IV (0.5 mg vit B12 +MTX): 7 rats were treated with vitamin B12 (0.5 mg/kg/rat/day)) once daily by I.P. for two weeks + 20 mg/kg I.P. of MTX injected only at day 11. 18
Group V (1 mg vit B12 +MTX): 7 rats were treated with vitamin B12 (1 mg/kg/rat/day) once daily by I.P. for two week + 20 mg/kg I.P. of MTX injected only at day 11.
Group VI (1.5 mg vit B12 +MTX): 7 rats were treated with vitamin B12 (1.5 mg/kg/rat/day) once daily by I.P.for two week + 20 mg/kg by I.P. of MTX injected only at day 11. 18
At the end of this experiment and on day 15 the rats were euthanized by the use of xylazine (10 mg/kg) and ketamine (75 mg/kg) 19 both are commonly used for this purpose (this is followed by the ethical standards and ARRIVE guidelines, and this type of euthanization is normally performed in most acute toxicity tests). Kidney tissue samples were taken for biochemical 37 and histopathological studies. 38
Sample collection and tissue preparation
After euthanasian of the animals, blood was collected by intracardiac puncture. The clot was isolated with a glass rod and then centrifuged (CGOLDENWALL 80-2 Electric Lab Centrifuge) at 3000 rpm for 15 minutes, the supernatant was used to evaluate urea and creatinine as parameters of kidney function. 37 The abdomen was cut with a scalpel blade, then the kidney was extracted and stored in 10% formalin (Pan Reac APPliChem, Cat NO:A0877.100).
A small portion of the kidney was cut and weighed on an electrical weighing scale (Redwag, model: AS220.R1). A sample of almost 0.4 gram weight was converted to homogenizer (Omni TH, Cat NO, 51-001) and mixed with 1 ml of ice cold phosphate buffered saline and centrifuged for 15 minutes at 5000 rpm. Subsequently, the resulting supernatant was isolated and stored at -20° C until used for the determination of the MDA and GSH biomarkers. 37
Investigated parameters
Histological examination of the kidneys
Histopathological studies were performed, kidney tissue samples were fixed in 10% formalin, then graded in ethanol, dehydrated, and embedded in paraffin. Kidney sections were cut with a microtome (HHQ-1508R Rotary Microtome, China) set at a thickness of 3-4 μm; mounted on clear glass, then the kidney tissue was stained with Hematoxylin and Eosin (H&E). Then the kidney tissue sections were examined and evaluated by the histopathologist using light microscopy at 10× and 40× (Viola Mc 20i, Micros ® Austria). 20 An overall score of the severity of damage to kidney tissue was assessed in stained tissue sections by scoring each of the following histopathological observations A. congestion of glomeruli B. congestion of interstitial C. cast and degeneration D. Inflammatory cell infiltrate E. Increased urinary space F. Inflammation of the Pelvicalyceal area.
Where, 0=none (no damage); 1=mild; 2=moderate; 3=severe. A total of field of section was examined for all animals in each group. 19 , 38
Serum analysis
Determination of serum creatinine and urea level
The quantitative assay of creatinine using the jaffe method was used. The quantitative assay of creatinine by the jaffe method is based on the reaction of creatinine with sodium picrate. Creatinine reacts with alkaline picrate forming a red-color complex. The intensity of the formed color is proportional to the creatinine concentration within the sample and the creatinine level can be calculated by measuring the absorbance at 490 nm. 21 This method was performed using a creatinine kit (Cat. NO D500, Spinreact, Espana), while urea was measured quantitatively using the enzyme method described below, 22 using a urea kit (Cat.NO p406, Spinreact, Espana).
Enzymatic method
Quantitative determination of serum urea depend on enzymatic reaction in which the urea within the sample is hydrolyzed enzymatically into ammonia (NH4 +) and carbon dioxide (CO2). Ammonia ions formed reacts with α-ketoglutarate in a reaction catalyzed by glutamate dehydrogenase (GLDH) with simultaneous oxidation of NADH to NAD+:
The decrease in NADH concentration is proportional to the concentration of urea within the samples, and the level of urea can be calculated by measuring the absorbance at 340 nm. Calculation of urea (mg/dl) within serum samples = (∆A) Sample × 50 (Standard conc.) = mg/dL urea in the sample.
Determination of malondialdehyde (MDA) and reduced glutathione (GSH) within the kidney homogenate of the rat
Malondialdehyde and reduced glutathione levels were measured by the enzyme-linked immunosorbent assay (ELISA) sandwich technique. 23
The MDA content in the kidney tissue homogenate was quantitatively estimated by the MDA kit based on the ELISA method according to the manufacturer's instructions (Cat. No. E0156Ra, Bioassay Technology Laboratory, China) and Rat reduced glutathione ELISA kit (Cat. No E1443Ra, Bioassay technology laboratory, China), as written on the manufacturing protocol.
The content of MDA and GSH in kidney tissue homogenate samples can be measured by comparing the optical density of the samples, with the standard curve. Level of MDA is expressed as nmol/mL, and level of GSH is expressed in Ng/ml.
Elisa MDA kit procedure
Preparation was done to all reagents and chemicals to kit in the kit box (and stored at room temperature, then 40 μl sample added to sample wells and after that add 10 μl anti-MDA antibody to sample wells, then 50 μl streptavidin-HRP to sample wells. Then mixed them well and Incubated for 60 minutes at 37°C. The sealer was removed and the plate was cleaned 5 times with washing buffer. Then the product was placed in automated washing (biotek ® ELX 600-biotek Co. – USA), then the absorption of the plate was determined using Elisa reader equipment (biotek ® ELX 600-biotek Co. – USA) the absorption is directly proportional to the concentration, which is then estimated by comparing the reading with the control group.
GSH procedure
This was the same as the MDA procedure.
Statistical analysis
Data was expressed statistically as the mean±SEM, (standard error of mean) and the significance of the differences among various groups was decided by one-way analysis of variance (ANОVA), then by least significant difference (LSD) test. The statistical package for the social sciences (SPSS) version 26 was used. Differences were deemed statistically significant at the P-value less than 0.05 level. 24
Results
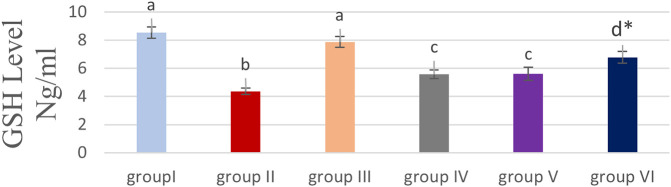
Effect of cyanocobalamin on GSH contents within kidney tissue homogenate of rats
A non-significant difference was observed between the cyanocobalamin (group III) and control groups (group I). 37
While the level of GSH in the renal tissue homogenate significantly increased (P<0.05) in both groups IV and V, it was highly significantly increased in group VI compared to the group treated with MTX treated group (P<0.01) as shown in Figure 1.
Figure 1. Showing the effect of cyanocobalamin on glutathione (GSH) contents in the kidney tissue homogenate of rats.
-
-Each value represents mean ± standard error of means (SEM) (N=7/Group),
-
-Values expressed in non-identical letters (a, b,c ,d) are significantly difference (P<0.05),
-
-*Highly significant difference from MTX P<0.01.
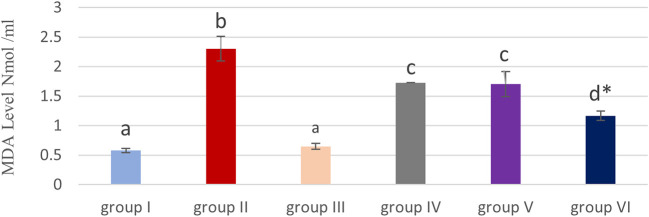
Effect of cyanocobalamin on Malondialdehyde (MDA) Levels within the kidney tissue homogenate of rat
Table 1 and Figure 2 show that, the MDA level was significantly (P<0.05) elevated in the methotrexate group (II) compared to the control (group I) and cobalamin (group III).
Table 1. Effect of cyanocobalamin on levels of malondialdehyde (MDA) and glutathione (GSH) within the kidney tissue homogenate of rats.
| Group N=7/group | Malondialdehyde (MDA) nmol/ml | Glutathione Ng/ml |
|---|---|---|
| Group I (control) /I.P. injected with 0.5 ml/kg/day normal saline | 0.57829±0.038840 a | 8.53457±0.411815 a |
| Group II/MTX I.P. 20 mg/kg | 2.30229±0.208563 b | 4.35600±0.224845 b |
| Group III/B12 1.5 mg/kg for 14 days | 0.64900±0.049109 a | 7.87243+0.396563 a |
| Group IV (20 mg of MTX +0.5 mg/kg of VIT B12 for 14 days) | 1.72586±O.285667 c | 5.55929±0.308215 c |
| Group V(20 mg of MTX +1 mg/kg of VIT B12 for 14 days) | 1.70429±0.211100 c | 5.59300±0.463932 c |
| Group VI (20 mg/kg of MTX +1.5 mg/kg of vit b12 for 14 days) | 1.16757±0.077618 d * | 6.77829±0.414523 d * |
Each value represents the mean±standard error of the means (SEM) (N=7/Group). Values expressed in non-identical letters (a, b,c,d) are significantly difference (P<0.05).
Highly significant difference from MTX P<0.01.
Figure 2. Showing effect of cyanocobalamin on malondialdehyde (MDA) contents in the kidney tissue homogenate of rats.
-
-Each value represents mean ± standard error of means (SEM) (N=7/Group),
-
-Values expressed in non-identical letters (a, b,c ,d) are significantly difference (P<0.05),
-
-*Highly significant difference from MTX P<0.01.
Non-significant differences were detected among the cyanocobalamin (group III) and control groups (group I).
Although MDA levels in renal tissue homogenate (P<0.05) significantly decrease in the IV & V groups, and highly significant decrease in group VI compared to the MTX treated group (P<0.01).
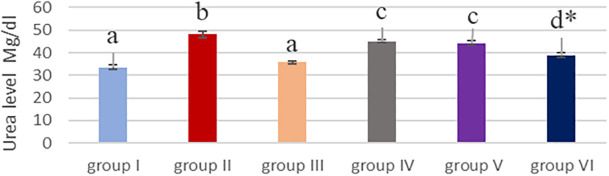
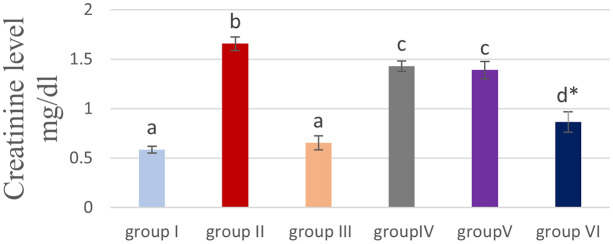
Effect of cyanocobalamin on plasma urea (U) and creatinine (CR) level
Table 2 and Figures 3 & 4 showed that the methotrexate-treated rat group (II) produced a significant elevation (P<0.05) of the plasma level of urea and creatinine, compared to the corresponding levels of the control group (I) and the cobalamin group (III).
Table 2. Effect of cyanocobalamin on plasma urea and creatinine level.
| Group N=7/group | urea mg/dl | Creatinine mg/dl |
|---|---|---|
| Group I (control) /I.P. injected with o.5 ml/kg/day normal saline | 33.557±0.9198 a | 0.586±0.0340 a |
| Group II/MTX I.P. 20 mg/kg | 48.071±1.4276 b | 1.657±0.0685 b |
| Group III/B12 1.5 mg/kg for 14 days | 35.757±0.5871 a | 0.653±0.0706 a |
| Group IV (20 mg of MTX +0.5 mg/kg of VIT B12 for 14 days) | 45.143±0.5084 c | 1.429±0.0522 c |
| Group V (20 mg of MTX +1 mg/kg of VIT B12 for 14 days) | 44.286±1.0169 c | 1.390±0.0879 c |
| Group VI (20 mg/kg of MTX +1.5 mg/kg of vit b12 for 14 days) | 38.886±1.1365 d * | 0.864±0.1036 d * |
Each value represents mean ± standard error of means (SEM) (N=7/Group), Values expressed in non-identical letters (a, b, c, d) are significantly difference (p<0.05).
Highly significant difference from MTX P<0.01.
Figure 3. Showing effect of cyanocobalamin on plasma urea level.
-
-Each value represents mean ± standard error of means (SEM) (N=7/Group),
-
-Values expressed in non-identical letters (a, b,c ,d) are significantly difference (P<0.05),
-
-*Highly significant difference from MTX P<0.01.
Figure 4. Showing the effect of cyanocobalamin on plasma creatinine level.
-
-Each value represents mean ± standard error of means (SEM) (N=7/Group),
-
-Values expressed in non-identical letters ( a, b,c ,d) are significantly difference (P<0.05),
-
-*Highly significant difference from MTX P<0.01.
Non-significant difference (P>0.05) of the serum of urea and creatinine was found between the control group (I) and cobalamin group (III).
Rats treated with different concentrations of cyanocobalamins in group IV and group V produced significantly decreased serum urea and creatinine levels (P<0.05), while group VI produces highly significant decreased serum urea levels compared to MTX-treated group II (P<0.01).
Histological findings
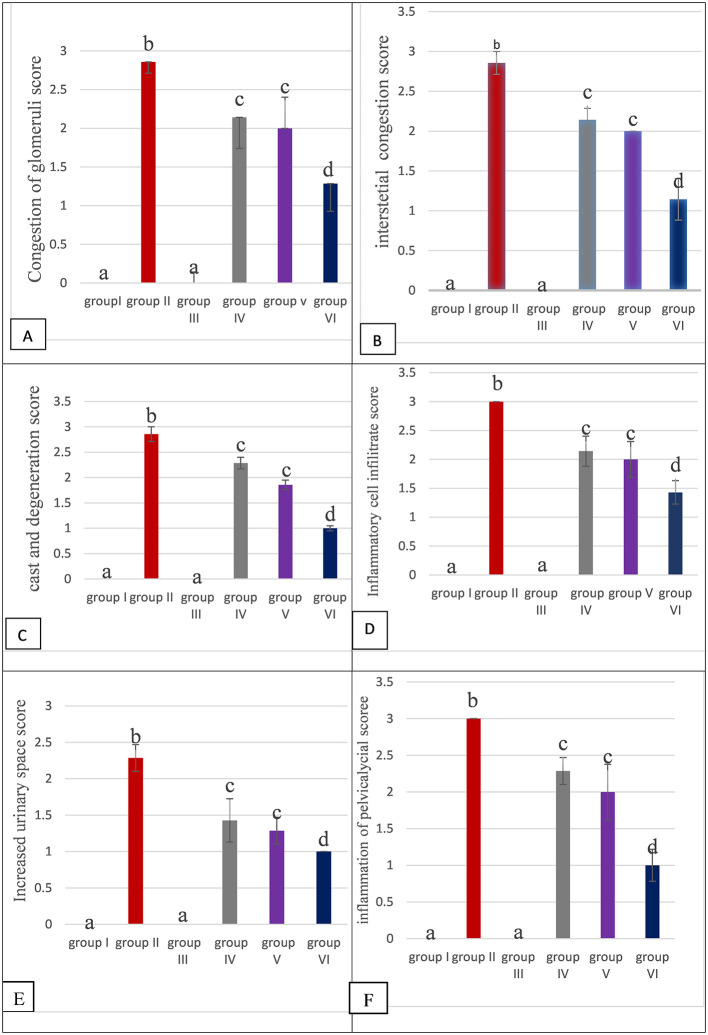
Histopathological changes were examined to assist the finding of biochemical markers. 38 , 39 Figure 5 showed the scoring severity of the histopathological parameters of kidney tissue (Congestion of glomeruli, Congestion of interstitial, Cast and degeneration, inflammatory cell infiltrate, increased urinary space, Inflammation of pelvicalyceal system).
Figure 5. The histological scores of all the groups. Values are mean ±SEM for seven rats in each group, values expressed in non-identical letters (a, b c, d) are significantly difference at P value (P<0.05) (N=7).
There were non-significant difference (P>0.05) of histopathological parameters in rats kidney section of group I (CONTROL) compared to group III.
While morphological damage (histopathological) are observed within the kidney section of rats treated with MTX group II, caused significant damage (P<0.05) in rats kidney tissue in all histopathological parameters compared to each histopathological parameter in each of Group I, III, IV, V , VI as found in Figure 5.
Moreover, there is significant decrease in damage in all histological parameters in group IV, V, VI compared to group II.
In addition there is significant decrease in damage in all histological parameters (P<0.05) in the Group VI compared to Group IV and Group V.
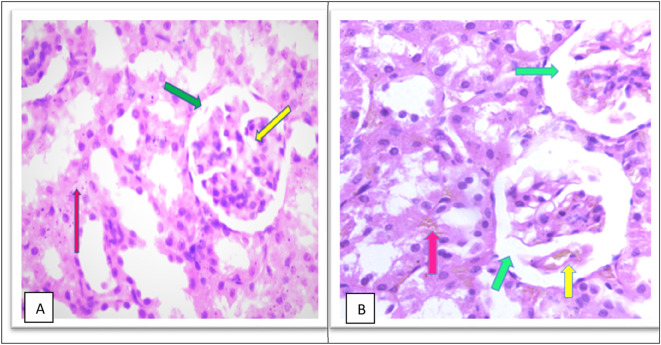
The MTX –treated group (II) kidneys revealed obvious variations and injuries, characterized by severe congestion of both interstitial and glomeruli, moderate increased urinary space causing shrinkage in the glomerular size as shown in Figure 6B and a high number of hyaline casts in the medullary region with degeneration of tubules as shown in Figure 6C, severe inflammation of inflammatory cell infiltrate as shown in Figure 6D and pelvicalyceal system as shown in the as shown in Figure 6E in the Extended data. 39
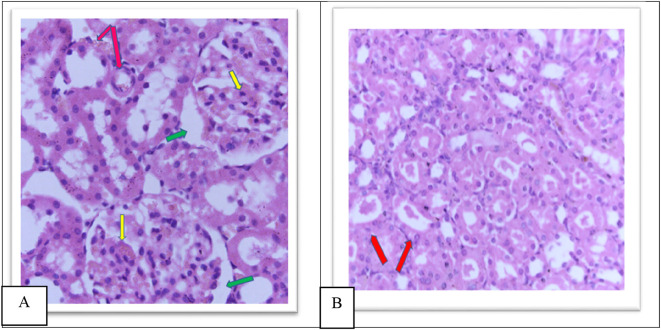
Figure 6. (A) section of kidney control group (Group I) of rat shows a Normal looking cortex, the glomeruli are with in normal (yellow arrow) no congestion of their capillaries, the urinary space is not dilated (green arrow), and the interstitial shows no congestion (pink arrow) (H&E ×40). (B) The kidney tissue section of rat treated with methotrexate for 4 days (group II) shows severe glomeruli congestion (Yellow arrow) and atrophy with widening of the urinary space (green arrow), (green arrow), severe congestion is also observed in the interstitial (pink arrow) (H&E ×40).
Furthermore, the cobalamins group (group III) show a normal glomerular cellularity (no congestion) and the urinary space is within normal limits. The interstitial does not show congestion; the tubule does not show degeneration and no inflammatory infiltrate as shown in Figure 7, similar to the control group (group I), which shows a normal looking cortex; the glomeruli have normal cellularity, no capillary congestion, the urinary space is not dilated; the interstitial does not show congestion, no inflammatory infiltrate, there is no tubular cell degeneration, and no casts. The pelvicalyceal system is not shown here, as shown in Figure 6A.
Figure 7. Section of the kidney tissue of rat treated Cyanocobalamin for 14 days (Group III) show A normal glomerulus (yellow arrow) and the urinary space is within normal limits (Green arrow), the interstitial shows no congestion (Pink arrow) (H&E, ×40).
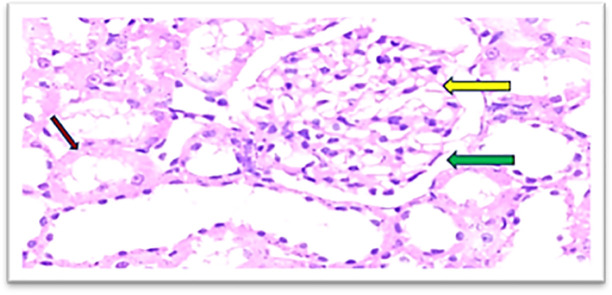
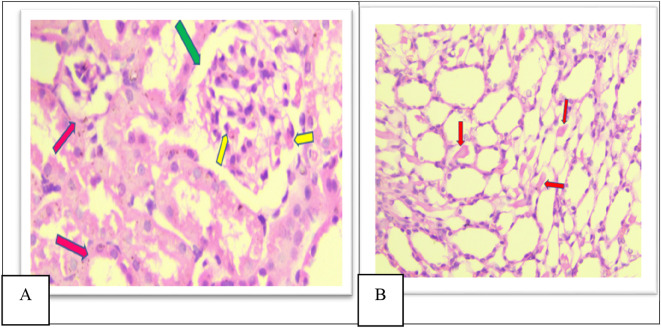
Group IV showed a moderate increase in urinary space, moderate congestion of the glomeruli and interstitial as shown in Figure 8A, a moderate amount of hyaline cast formation and degeneration as shown in Figure 8B, moderate inflammation of the pelvicalyceal system Figure 8c, moderate infiltrate of inflammatory cells of the interstitial Figure 8d as shown in the Extended data. 39
Figure 8. A. Section of the kidney tissue of rat (Group IV) show moderate congestion of interstitial (Pink arrow), moderate congestion of glomeruli (yellow arrow) and moderate increased of urinary space (Green arrow) (H&E, ×40). B. The section of rat kidney tissue (Group IV) shows degeneration and moderate amount of hyaline cast formation (Red arrow) (H&E, ×40).
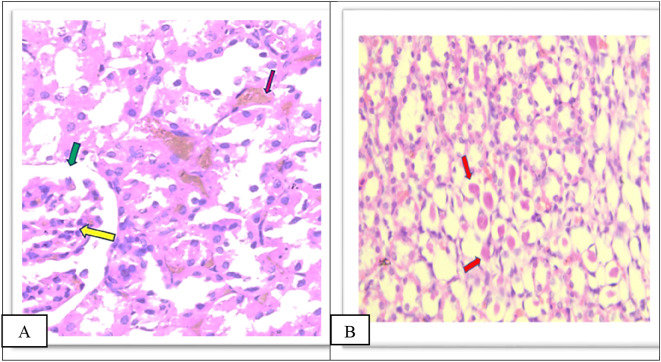
Group V showed a moderate increase in urinary space, moderate congestion of glomeruli and interstitial as shown in Figure 9A, moderate amount of hyaline cast in the tubular lumen and degeneration as shown in Figure 9B, moderate inflammation of the pelvicalyceal system 9C, moderate inflammatory cell infiltrate of interstitial as shown in Figure 9D as shown in the Extended data. 39
Figure 9. A. Section of the kidney tissue of rat (Group V) Show moderate congestion of interstitial (Pink arrow), moderate congestion of glomeruli (yellow arrow) and moderate increased of urinary space (Green arrow) (H&E, ×40). B. The section of rat kidney tissue (Group V) shows a moderate tubular degeneration and a moderate formation of hyaline casts with in tubular lumens (red arrow) (H&E, ×40).
Furthermore, in group VI there was a significant decrease in damage in all histopathological parameters compared to other groups (II, IV, and V), There is mild glomerular congestion and increased cellularity, the interstitial space also shows mild congestion and degeneration, mild increased urinary space as shown in Figure 10A, Few casts are seen within tubular lumens as shown in Figure 10B, mild inflammatory infiltrate in the pelvicalyceal system as shown in Figure 10c, mild inflammatory cell infiltrate of the interstitial as shown in Figure 10d in the Extended data. 39
Figure 10. A. Section of the kidney tissue of rat (Group VI) Shows mild congestion of glomeruli (Yellow arrow) the interstitial space shows mild congestion and degeneration (Pink arrow) and mild increased urinary space (Green arrow) (H&E, ×40). B. Section of the kidney tissue of the rat (Group VI) Shows that a few casts (Red arrow) are seen within tubular lumens (H&E, ×40).
Discussion
The renal system plays a vital role in maintaining extracellular environment homeostasis, mainly detoxification and elimination of toxic drugs and their metabolites. Nephrotoxicity related to toxic medications, especially chemotherapeutic drugs are common and represents approximately 20% of all renal failure cases. 15
This study focused on the protective effect of cyanocobalamin on MTX-induced renal damage in rats by reducing Cr and urea levels and repairing histopathological damage to the kidney.
The present data established that the activities of vit B12 against MTX-induced renal damage in rats could be successful by suppressing oxidative stress by reducing MDA levels and increasing GSH levels.
There were significant elevations in creatinine and urea levels in the MTX treated group (group II), compared to the levels in the control rats (group I) and the cyanocobalamin treated group (group III) (P<0.05).
Researchers indicated the main pathway for MTX elimination by kidney. Furthermore, the administration of high doses of MTX causes higher drug concentrations in plasma and urine, at an acute and extremely toxic dose the elimination of MTX is delayed execution, then increases the serum creatinine level, and thus is one of the biomarkers of renal failure, this is consistent with the present study. 25 , 26
Group IV, V, and VI resulted in a significant reduction (P <0.05) in plasma urea and creatinine levels, compared to those levels in group II. That is consistent with the finding of Alshanwani et al., 15 which showed that vitamin B12 improved creatinine clearance levels and renal blood flow, thus, this can reduce creatinine and blood urea nitrogen. 15
MDA is highly reactive compound, is formed through the peroxidation of polyunsaturated fatty acids by the action of reactive oxygen species, as an effect of the depletion of antioxidant systems. 27
In the current study, a non-significant difference was found (p >0.05) within the content of MDA of kidney tissue homogenate among the control (group I) and cyanocobalamin (group III), which is in line with a previous animal study. 13
MTX treated rats (group II) demonstrated significant increases in MDA levels in group II, compared to control (group I) and cyanocobalamin (group III) which is consistent with previous studies. 28 , 29
Increased levels of MDA in the methotrexate group might be related to oxidative stress, which likely stores the leukocyte in tissues by increasing the level of ROS. 30 Active leukocytes release many chemical enzymes, such as protease, elastase, and myeloperoxidase, resulting in more free radicals.
In addition, reactive oxygen species produce changes in permeability of both endothelial and epithelial cells. It can produce tissue injury, caused by MTX-prompted OS, by interfering with unsaturated form fatty acids of cell membranes, nucleic acid and sulfhydryl bonds. MTX therapy can indirectly secrete mitochondrial enzymes that lead to damaged mitochondria, and a decrease in antioxidant action. 8
In this study, groups IV, V and VI produced a significant reduction (P<0.05) in the level of MDA, compared to the group II treated with MTX.
These results are due to the antioxidant function of Cyanocobalamin by inducing the activity of methionine synthase, interacting with ROS, and avoiding glutathione. 13 , 31
The body’s tissues contain relatively large concentrations of the reduced glutathione (GSH) peptide, a water-soluble, low-molecular-weight tripeptide made of cysteine, glutamic acid, and glycine. And it is a non-enzymatic antioxidant; preserving reduced glutathione in the cell is essential to improve the immune system and protect the body from diseases. 32 , 33
GSH act as a reducing agent that is important for conserving enzymes and antioxidants in the form of an active state. This mechanism is important to provide protection from cytotoxic agents, free radicals, and oxidative damage. 34
In this study, the MTX group (group II) produced a significant reduction in the level of GSH, compared to the control group (group I) which is in line with a previous study. 3
This result may be related to the mechanism of OS generation by MTX by inhibiting cellular NADPH (nicotinamide adenine dinucleotide phosphate). Through the pentose phosphate cycle, the glutathione reductase enzyme NADPH is used as a reducing agent for cellular GSH (primary antioxidant). The reduction of cellular GSH by MTX decreased the systemic antioxidant action. 35
Group IV, V, and VI produced a significant (P<0.05) raise in GSH levels compared to those levels in groups II, these effects could be explained by the fact that cyanocobalamin has antioxidant properties consistent with the findings of Padmanabhan et al., which showed that supplementation with cyanocobalamin was confirmed by increased GSH, this institutes the main intracellular antioxidant and reduces oxidative stress. 34
However, histological analysis revealed that, unlike the control group (group I), the methotrexate group (group II) had destructive effects, were kidney sections of such rats showed moderate increased urinary space causing shrinkage in the glomerular size, forming a high number of hyaline casts in the medullary region, with degeneration of tubules, congestion of both interstitial and glomeruli. The glomerular show reduced cellularity, severe inflammation of the inflammatory cell infiltrate, and pelvicalyceal system. This is similar to the findings of other previous studies. 7 , 8
The exact mechanism of MTX nephrotoxicity was unclear. However, some studies found that the main factor in MTX-related tissue injury was oxidative damage, with successive generation of more free radicals, the role of oxidative stress has been reported in MTX-induced nephrotoxicity. 36
Moreover, in groups IV, V and VI, there was a significant decrease in damage in all histological parameters (Expansion of bowman space, Congestion of glomeruli, Congestion of interstitial, Cast and degeneration, Inflammatory cell infiltrate, Inflammation of pelvicalyceal system) (P<0.05), as compared to group II. These findings are similar to those of Saeed et al 13 ; where the protective effect of vitamin B12 against nephrotoxicity was observed by histopathological examination. 13
These results were considered an index of the defensive effect of cyanocobalamin on the kidney damaged by the methotrexate drug, the protective action of vitamin b12 has been previously reported in experimental studies. 15
Conclusion
This study demonstrates the beneficial effects of cyanocobalamin on MTX-induced nephrotoxicity. Data suggest that the protective effect of cyanocobalamin is through the amelioration of oxidative markers (MDA) in kidney homogenate; the improvement of antioxidants (GSH), compensating for the increase in urea, creatinine, and the improvement of histopathological lesions of the kidney of rats. These results will make a new technique of protection from nephrotoxicity by MTX therapy, by administration of cyanocobalamin.
Data availability
Underlying data
Zenodo: Study in the protective effects of cyanocobalamin on Methotrexate induced Nephrotoxicity in rats. https://doi.org/10.5281/zenodo.6842130. 37
This project contains the following underlying data:
-
-
Z.xlsx (biochemical test plasma of urea and creatinine, antioxidant enzyme (GHS) and oxidative stress marker (MDA))
Zenodo: Study in the protective effects of cyanocobalamin on Methotrexate induced Nephrotoxicity in rats. https://doi.org/10.5281/zenodo.6970347. 38
This project contains the following underlying data:
-
-
Histopathological score.xlsx
Extended data
Zenodo: Study in the protective effects of Cyanocobalamin on Methotrexate induced Nephrotoxcity in rats. https://doi.org/10.5281/zenodo.7008082. 39
This project contains the following extended data:
-
‐
Supplementary data.docx (histopathological images of kidney section of all six group (n=7))
Reporting guidelines
Zenodo: ARRIVE checklist for ‘Study in the protective effects of cyanocobalamin on Methotrexate induced nephrotoxicity in rats’. https://doi.org/10.5281/zenodo.6982116. 40
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Acknowledgments
The authors would like to thanks department of pharmacology/college of medicine, to providing facilities to bring this study and doctor Ban A. Abdul Majeed, consultant pathologist and PhD Farah Kais Abdul-Wahab for assistance during the study.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 2; peer review: 2 approved]
References
- 1. Yan H, Su R, Xue H, et al. : Pharmacomicrobiology of Methotrexate in Rheumatoid Arthritis: Gut Microbiome as Predictor of Therapeutic Response. Front. Immunol. 2021;12. 10.3389/fimmu.2021.789334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koźmiński P, et al. : Overview of dual-acting drug methotrexate in different neurological diseases, autoimmune pathologies and cancers. Int. J. Mol. Sci. 2020;21(10):3483. 10.3390/ijms21103483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li Y, et al. : Dioscin ameliorates methotrexate-induced liver and kidney damages via adjusting miRNA-145-5p-mediated oxidative stress. Free Radic. Biol. Med. 2021;169:99–109. 10.1016/j.freeradbiomed.2021.03.035 [DOI] [PubMed] [Google Scholar]
- 4. Izzedine H, Perazella MA: Anticancer drug-induced acute kidney injury. Kidney Int. Rep. 2017;2(4):504–514. 10.1016/j.ekir.2017.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Valade S, et al. : High-dose methotrexate in ICU patients: a retrospective study. Ann. Intensive Care. 2020;10(1):1–5. 10.1186/s13613-020-00693-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yuksel Y, et al. : Effects of quercetin on methotrexate-induced nephrotoxicity in rats. Hum. Exp. Toxicol. 2017;36(1):51–61. 10.1177/0960327116637414 [DOI] [PubMed] [Google Scholar]
- 7. Al-Rashidy AH, et al. : Role of erythropoietin in methotrexate-induced nephrotoxicity in adult male albino rats. J. Nephropharmacol. 2018;7(2):156–163. 10.15171/npj.2018.31 [DOI] [Google Scholar]
- 8. Jalili C, et al. : Toxic effects of methotrexate on rat kidney recovered by crocin as a consequence of antioxidant activity and lipid peroxidation prevention. Iran. Biomed. J. 2020;24(1):39–46. 10.29252/ibj.24.1.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stojiljkovic N, et al. : The encapsulation of lycopene in nanoliposomes enhances its protective potential in methotrexate-induced kidney injury model. Oxidative Med. Cell. Longev. 2018;2018:1–11. 10.1155/2018/2627917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Derya K, Nurhan K, Emin K, et al. : Vitamin B12 Regulates the Expression of Methotrexate-Induced Increased Markers of Autophagy: An Immunohistochemical Study. Open Acc. J. Toxicol. 2021;4(5):555648. [Google Scholar]
- 11. Allen LH: Vitamin B-12. Adv. Nutr. (Bethesda, Md.).2012;3(1),54–55. 10.3945/an.111.001370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Greibe E, et al. : Dietary intake of vitamin B12 is better for restoring a low B12 status than a daily high-dose vitamin pill: An experimental study in rats. Nutrients. 2018;10(8):1096. 10.3390/nu10081096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hajihashemi S, et al. : Effects of cobalamin (Vitamin B12) on gentamicin induced nephrotoxicity in rat. Drug Res. 2017;67(12):710–718. 10.1055/s-0043-117418 [DOI] [PubMed] [Google Scholar]
- 14. Gabriele A, Alex B, Vasileios B, et al. : Scientific Opinion on safety and efficacy of vitamin B12 (cyanocobalamin) produced by Ensiferadhaerens when used as a feed additive for all animal species. EFSA J. 2015;13:1–21. [Google Scholar]
- 15. Alshanwani AR, et al. : Cyanocobalamin and/or calcitriol mitigate renal damage-mediated by tamoxifen in rats: Implication of caspase-3/NF-κB signaling pathways. Life Sci. 2021;277: 119512. 10.1016/j.lfs.2021.119512 [DOI] [PubMed] [Google Scholar]
- 16. Ghanim WK, Al-Shawi NN, Al-Moziel MS: Effects of Two Different Doses of Vitamin B2 and a Single Dose of Vitamin B12 Against Cyclophosphamide Induced Nephrotoxicity. Medico-legal Update .2020;20(3):493. [Google Scholar]
- 17. Yucel Y, Tabur S, Gozeneli O, et al. : The effects of lycopene on intestinal injury due to methotrexate in rats. Redox Rep. 2016;21:113–118. 10.1179/1351000215Y.0000000041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salah R, Salama MF, Mahgoub HA, et al. : Antitumor activity of sitagliptin and vitamin B12 on Ehrlich ascites carcinoma solid tumor in mice. J. Biochem. Mol. Toxicol. 2021;35(2):e22645. 10.1002/jbt.22645 [DOI] [PubMed] [Google Scholar]
- 19. Dabak DO, Kocaman N: Effects of silymarin on methotrexate-induced nephrotoxicity in rats. Ren. Fail. 2015;37(4):734–739. 10.3109/0886022X.2015.1012984 [DOI] [PubMed] [Google Scholar]
- 20. Tambağ AK, Kazak F, Peker Akalın P, et al. : The protective effect of rutin against methotrexate-induced nephrotoxicity in rats. Turk. J. Nephrol. 2021;30(3):218–223. 10.5152/turkjnephrol.2021.4731 [DOI] [Google Scholar]
- 21. Young DS: Effects of disease on clinical Lab. Tests. 4th ed. AACC;2001. [Google Scholar]
- 22. Urea KA, Kaplan A, et al. : Clin Chem the C.V. Mosby Co. St Louis. Toronto. Princeton 1984; 1257-1260 and 437 and 418.
- 23. Dahniar S A’a, et al. : Effect of Brown Algae Extract Sargassum sp on Malondialdehyde Levels in White Rats (Rattus Norvegicus) Pregnant Wistar Strains. Medico Legal Update. 2020;20(3):822–827. [Google Scholar]
- 24. Abdul-Wahab FK, Al-Shawi NN: Effects of Vitamin D3 on Methotrexate-Induced Jejunum Damage in Rats. Iraqi. J. Pharm. Sci. 2020;29(1):260–267. 10.31351/vol29iss1pp260-267 [DOI] [Google Scholar]
- 25. Osman AT, et al. : Empagliflozin and neohesperidin protect against methotrexate-induced renal toxicity via suppression of oxidative stress and inflammation in male rats. Food Chem. Toxicol. 2021;155:112406. 10.1016/j.fct.2021.112406 [DOI] [PubMed] [Google Scholar]
- 26. Patel NN, et al. : Subacute toxicopathological studies of methotrexate in Wistar rats. Vet. World. 2014;7(7):489–495. 10.14202/vetworld.2014.489-495 [DOI] [Google Scholar]
- 27. Kapusta A, Kuczyńska B, Puppel K: Relationship between the degree of antioxidant protection and the level of malondialdehyde in high-performance Polish Holstein-Friesian cows in peak of lactation. PLoS One. 2018;13(3): e0193512. 10.1371/journal.pone.0193512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Al-Abdaly YZ, et al. : Effect of methotrexate and aspirin interaction and its relationship to oxidative stress in rats. Iraqi J. Vet. Sci. 2021;35(1):151–156. 10.33899/ijvs.2020.126490.1335 [DOI] [Google Scholar]
- 29. Moodi H, et al. : Ethanolic extract of Iris songarica rhizome attenuates methotrexate-induced liver and kidney damages in rats. Avicenna J. Phytomed. 2020;10(4):372–383. [PMC free article] [PubMed] [Google Scholar]
- 30. Sener G, Eksioğlu-Demiralp E, Cetiner M, et al. : Beta-glucan ameliorates methotrexate-induced oxidative organ injury via its antioxidant and immunomodulatory effects. Eur. J. Pharmacol. 2006;542(1-3):170–178. 10.1016/j.ejphar.2006.02.056 [DOI] [PubMed] [Google Scholar]
- 31. Salah R, et al. : Antitumor activity of sitagliptin and vitamin B12 on Ehrlich ascites carcinoma solid tumor in mice. J. Biochem. Mol. Toxicol. 2021;35(2):e22645. 10.1002/jbt.22645 [DOI] [PubMed] [Google Scholar]
- 32. Bas Z: Inhibition effect of nicotinamide (vitamin B3) and reduced glutathione (GSH) peptide on angiotensin-converting enzyme activity purified from sheep kidney. Int. J. Biol. Macromol. 2021;189:65–71. 10.1016/j.ijbiomac.2021.08.109 [DOI] [PubMed] [Google Scholar]
- 33. Lucio C d F, et al. : Effect of reduced glutathione (GSH) in canine sperm cryopreservation: in vitro and in vivo evaluation. Cryobiology. 2016;72(2):135–140. 10.1016/j.cryobiol.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 34. Padmanabhan S, et al. : Folate/vitamin B12 supplementation combats oxidative stress-associated carcinogenesis in a rat model of colon cancer. Nutr. Cancer. 2019;71(1):100–110. 10.1080/01635581.2018.1513047 [DOI] [PubMed] [Google Scholar]
- 35. García-Sánchez A, Miranda-Díaz AG, Cardona-Muñoz EG: The role of oxidative stress in physiopathology and pharmacological treatment with pro-and antioxidant properties in chronic diseases. Oxidative Med. Cell. Longev. 2020;2020:1–16. 10.1155/2020/2082145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Çetin ES, et al. : Methotrexate-induced nephrotoxicity in rats: protective effect of mistletoe (Viscum album L.) extract. Complement. Med. Res. 2017;24(6):364–370. 10.1159/000468984 [DOI] [PubMed] [Google Scholar]
- 37. Abdulwahab RQ, Ali SM: Study in the protective effects of cyanocobalamin on Methotrexate induced Nephrotoxicity in rats. [Dataset]. 2022. 10.5281/zenodo.6842130 [DOI] [PMC free article] [PubMed]
- 38. Abdulwahab RQ, Ali SM: Study in the protective effects of cyanocobalamin on Methotrexate induced Nephrotoxicity in rats. [Dataset]. 2022. Reference Source [DOI] [PMC free article] [PubMed]
- 39. Abdulwahab RQ: Study in the protective effects of Cyanocobalamin on Methotrexate induced Nephrotoxcity in rats. 2022. 10.5281/zenodo.7008082 [DOI] [PMC free article] [PubMed]
- 40. Abdulwahab RQ: Study in the protective effects of cyanocobalamin on Methotrexate induced Nephrotoxicity in rats. 2022. 10.5281/zenodo.6982116 [DOI] [PMC free article] [PubMed]