Abstract
We investigated the effects of finely ground wheat bran on the nutrient digestibility, digesta passage rate, and gut microbiota structure in sows. A 3 × 3 Latin square design with 3 test periods and 3 experimental diets was used. Six non-pregnant sows (parity: 5 to 7) were randomly assigned to 3 experimental diets with 2 replicates per treatment in each period. Each period lasted 19 d (12 d for adaptation and 7 d for experiment). The experimental diets included (a) a basal corn and soybean meal diet (CON), (b) a basal diet with 20% coarse wheat bran (CWB; particle size: 605 μm), and (c) a basal diet with 20% fine wheat bran (FWB; particle size: 438 μm). The results demonstrated that the apparent total tract digestibility of neutral detergent fiber, acid detergent fiber and energy were reduced (P < 0.05) in the FWB and CWB groups compared with those in the CON group. Viscosity of digesta increased (P < 0.001) in FWB-fed sows. The passage rate of digesta from the mouth to the ileum decreased (P < 0.001) in FWB-fed sows. Peptide YY (PYY) concentration increased (P = 0.01) in FWB-fed sows after 30 min of feeding. In the FWB group, the relative abundance of Lactobacillaceae at the family level increased (P < 0.05) in the ileal digesta. At the class level, the relative abundance of Clostridia in feces decreased (P < 0.05) in FWB-fed sows. FWB enhanced the concentration of butyrate in feces compared with CON and CWB (P = 0.04). These results suggest that dietary supplementation with finely ground wheat bran reduces the passage rate of digesta, increases the abundance of beneficial microorganisms, and elevates the concentration of short-chain fatty acids and PYY in sows. These findings indicate that the addition of finely-ground wheat bran to the diets of sows is more effective than using coarse wheat bran for improving their satiety and intestinal microbial composition.
Keywords: Finely ground wheat bran, Digestive physiology, Digesta passage rate, Gut microbiota, Sow
Highlights
-
•
Nutrient digestibility and digesta characteristics between bran types were compared
-
•
Finely-ground wheat bran increases digesta viscosity, reduces its passage rate and increases retention time in the total intestine
-
•
Finely-ground wheat bran increases short-chain fatty acids and relative abundance of beneficial microorganisms
-
•
Diets enriched in finely-ground wheat bran improve digestive physiology of sows
1. Introduction
Dietary fiber (DF) refers to plant polysaccharides and lignin that cannot be hydrolyzed by endogenous enzymes in the mammalian intestine (Knudsen, 1997). The DF that is not digested by endogenous enzymes in the small intestine enters the large intestine, facilitating microbial growth and production of short-chain fatty acids (SCFA) (Serena et al., 2008). Gut microbiota regulate the intestinal tract physiology of a host. An important function of microbiota is to degrade DF into SCFA (e.g., acetate, propionate, and butyrate). These SCFA affect the gut microbial ecology, physiology and overall health of the host (Ríos-Covián et al., 2016).
Gastric emptying rate, passage rate of digesta and total transit time are affected by the type of DF (Serena et al., 2008) and physicochemical properties of DF can influence the passage kinetics of digesta (Schop et al., 2020). Further, different DF types possess varying physicochemical properties. Soluble dietary fiber (SDF) can delay gastric emptying due to its high water-holding capacity (WHC) and high viscosity. Compared with SDF, insoluble dietary fiber (IDF) can increase fecal excretion due to reduced microbial degradation of fiber within the large intestine (Serena, 2005). Therefore, the passage rate of digesta, nutrient digestibility, microbial community structure and physiological metabolic processes are influenced by DF characteristics (Knudsen et al., 1993; Owusu-Asiedu et al., 2006). The passage rate of digesta in the gastrointestinal tract impacts transit time and total time of digestion by the endogenous digestive juice and microbes, and influences the degree of digestion (Owusu-Asiedu et al., 2006).
Wheat bran primarily contains IDF (Messia et al., 2016) which is not easily fermented by large intestine microorganisms, reducing the nutritional value of wheat bran. Further, its physiological effects in the host are limited (Lin et al., 2021). If the wheat bran can be reasonably processed, for example through fine grinding, the value of wheat bran can be used to improve the digestive physiology of sows and utilization of feed. Many in vitro studies have demonstrated that fine grinding can improve the physicochemical properties of wheat bran by decreasing particle size, increasing porosity and increasing specific surface area (Silva and Bloom, 2012; Santala et al., 2014). Feed processing of fine grinding not only increases the content of SDF, WHC and fermentability of wheat bran, but also improves lipid metabolism and inflammatory response by modulating the gut microbiota structure in pregnant sows (Wang et al., 2022). We hypothesized that finely ground wheat bran will reduce the passage rate of digesta and increase digestion and decomposition of large intestinal microorganisms. Therefore, the current study aimed to investigate the effects of finely ground wheat bran on the 1) ileal and fecal nutrient digestibility and microbial population composition; 2) digesta passage rate through the small intestine and total tract; 3) plasma concentrations of hormones; and 4) SCFA concentrations in the ileum and feces.
2. Materials and methods
2.1. Ethical approval
All experimental protocols in the current study were approved by the Animal Care and Use Committee of China Agricultural University (Beijing, China). Protocols were based on the National Research Council's Guide for the Care and Use of Laboratory Animals (AW42022202-1-1).
2.2. Wheat bran samples
Wheat bran was purchased from Hebei Flour Mill (Hebei, China), and a shredding machine equipped with a 1.0 mm screen (Jiangyin Hongda powder equipment limited company, Jiangsu, China) was used to grind wheat bran into a fine sample.
2.3. Experimental diets
Three experimental diets were used in this experiment. The experimental diets included a basal corn and soybean meal diet (CON; basal diet), a basal diet with 20% coarse wheat bran (CWB; particle size, 605 μm) and a basal diet with 20% fine wheat bran (FWB; particle size, 438 μm). The particle size of wheat bran was determined using the fourteen-sieve method of the ANSI/ASAE S319.4-2008 standard (Stark and Chewning, 2011). All experimental diets met or exceeded the nutrient requirements of pregnant sows, based on the nutrient requirements of swine (NRC, 2012). The composition and nutrient content of the experimental diets are shown in Table 1.
Table 1.
Ingredient composition and nutrient concentrations of experimental diets (%, as-fed basis).
| Item | CON 1 | CWB 2 | FWB 3 |
|---|---|---|---|
| Corn | 77.30 | 59.35 | 59.35 |
| Soybean meal | 17.55 | 15.50 | 15.50 |
| Coarse wheat bran 4 | 20.00 | ||
| Fine wheat bran 5 | 20.00 | ||
| Soybean oil | 2.00 | 2.00 | 2.00 |
| Dicalcium Phosphate | 1.30 | 1.30 | 1.30 |
| Limestone | 1.05 | 1.05 | 1.05 |
| Salt | 0.30 | 0.30 | 0.30 |
| Vitamin and mineral premix 6 | 0.50 | 0.50 | 0.50 |
| Calculated composition | |||
| ME 7, kcal/kg | 3,285 | 3,159 | 3,159 |
| SID lysine | 0.58 | 0.57 | 0.57 |
| Calcium | 0.78 | 0.80 | 0.80 |
| Total phosphorus | 0.70 | 0.72 | 0.72 |
| Analyzed composition | |||
| GE8, kcal/kg | 3,729 | 3,662 | 3,662 |
| Crude protein | 12.68 | 15.31 | 15.30 |
| Neutral detergent fiber | 15.70 | 18.30 | 18.28 |
| Total dietary fiber | 10.74 | 15.19 | 15.21 |
| Soluble dietary fiber | 1.49 | 2.20 | 2.90 |
| Insoluble dietary fiber | 9.25 | 12.99 | 12.31 |
CON = a corn-soybean meal diet.
CWB = diet contained coarse wheat bran with particle size of 605 μm.
FWB = diet contained fine wheat bran with particle size of 438 μm.
Coarse wheat bran, with particle size of 605 μm. Analyzed coarse wheat bran (as fed-basis): dry matter, 87.37%; ash, 5.02%; crude protein, 16.93%; neutral detergent fiber, 51.65%; acid detergent fiber, 13.23%; total dietary fiber, 43.77%; soluble dietary fiber, 4.01%; insoluble dietary fiber, 39.76%.
Fine wheat bran, with particle size of 438 μm. Analyzed fine wheat bran (as fed-basis): dry matter, 86.98%; ash, 4.55%; crude protein, 16.79%; neutral detergent fiber, 49.11%; acid detergent fiber, 13.01%; total dietary fiber, 43.92%; soluble dietary fiber, 5.98%; insoluble dietary fiber, 37.94%.
The vitamin and mineral premix provided the following per kilogram of the diet: 12,000 IU vitamin A; 3,000 IU vitamin D3; 15 IU vitamin E; 1.8 mg vitamin K3; 1.0 mg thiamine; 3.0 mg riboflavin; 1.5 mg pyridoxine; 0.015 mg vitamin B12; 15 mg pantothenic acid; 30 mg nicotinic acid; 0.2 mg biotin; 1.5 mg folic acid; 100 mg Zn; 85 mg Fe; 12 mg Mn; 20 mg Cu; 1.2 mg I; 0.4 mg Se.
ME = metabolizable energy.
GE = gross energy.
2.4. Experimental design
This experiment was conducted at the Feng Ning Swine Research Unit of China Agricultural University (Chengdejiuyun Agricultural and Livestock Co., Ltd., Hebei, China). A 3 × 3 Latin square design with 3 test periods and 3 experimental diets was used.
2.5. Animals and experimental procedures
Six Yorkshire × Landrace non-pregnant sows (average initial BW = 250 kg; parity = 5 to 7) were fed a standard diet 1 wk before the experiment began. Sows were housed in a metabolism stall (1.80 m × 0.65 m) equipped with a feeder, nipple drinker and slatted floor. The average ambient temperature was maintained at 22 °C. After a week-long adaptation period, a T-cannula was installed in the distal ileum of each sow. Installation of the cannula and postoperative care of the sows was performed according to a previous study (Stein et al., 1998). During the 10-d recovery period, the sows were allowed ad libitum access to water and a standard diet. After the recovery period, the sows were assigned randomly to 1 of the 3 experimental diets, and each diet was fed to 2 sows during each of the 3 periods. Each experimental period included 12 d of acclimatization to their assigned test diet, followed by 2 d of ileal digesta collection (d 13 and d 14), 4 d of fecal sampling (from d 15 to 18), and 1 d of blood collection (d 19). The feeding levels were equal among diets and set at 152 kcal/kg BW0.75 per day based on the BW recorded on d 0 and 19 of each period. These feeding levels were equal to 1.5 times the metabolizable energy maintenance requirements of pregnant sows (NRC, 2012). Daily feed rations were offered in 2 equal meals at 08:00 and 17:00.
2.6. Sample collection
Feed samples were collected during each phase of the experiment and stored at −20 °C until analysis. On d 13, the digesta samples were collected every 15 min for 4 h by fastening a plastic bag on the cannula. Digesta samples were stored at −80 °C to determine microbial composition and SCFA concentrations. On d 14, 0.2% Cr2O3 was added to the morning meal (08:00). Digesta samples were collected every 30 min (from 08:00 to 17:00), weighed and then stored at −20 °C for further analysis. Cr2O3 was detected in the subsample of each digesta sample. The rest of the digesta samples were thawed, homogenized, subsampled and dried for further analysis. On d 15, fresh feces were collected and stored at −80 °C to analyze microbial communities and SCFA concentrations. On d 16 and d 17, the morning meal was supplemented with 1% ferric oxide, and we recorded the time ferric oxide first appeared in the feces. On d 18, 0.2% Cr2O3 was added to the morning meal (08:00), and feces were collected during excretion at any time until 17:00. The collected feces were stored immediately at −20 °C to prevent microbial fermentation. At the end of each fecal collection period, all collected feces were thawed, homogenized, sub-sampled and dried for further analysis. Blood samples were collected from the anterior vena cava in tubes without anticoagulant 30 min before feeding, and 30, 60 and 90 min after feeding on d 19. All blood samples were centrifuged for 15 min at 3,000 × g at 4 °C. Plasma samples were collected and stored at −20 °C for analysis of glucose, insulin, glucagon-like peptide-1 (GLP-1), ghrelin and peptide YY (PYY).
2.7. Chemical analysis
The viscosities of the diets and digesta were analyzed using a digital viscometer (Bangxi Instrument Technology Co., Ltd, Shanghai, China). Briefly, ileal digesta (30 g) were weighed into 50-mL tubes. The samples were centrifuged at 4,000 × g for 30 min at 4 °C, 5 mL supernatant was added into the sample container, and CEP-40 rotator was selected to determine the viscosity. At 37 °C and 60 rpm, the value was read after stabilizing for 30 s. The WHC of the diets and digesta was measured using a centrifugation approach (Owusu-Asiedu et al., 2006). Briefly, the diet (2 g) and ileal digesta (7 g) were weighed into 50-mL tubes. Then, 30 mL 0.9% NaCl containing 0.2% NaN3 was added and the samples were placed in a shaking water bath at 39 °C for overnight culture. The samples were centrifuged at 4,000 × g for 20 min at 25 °C, the supernatant was removed, dried at 103 °C for 20 h and weighed. The results were expressed as gram water retained per gram dry residue.
Diets, digesta and fecal samples were analyzed for dry matter (DM) (Method 934.01; AOAC, 2007), crude protein (CP) (Method 976.05; AOAC, 2007), ether extract (EE) (Thiex et al., 2003), neutral detergent fiber (NDF) and acid detergent fiber (ADF) (Van Soest et al., 1991). Wheat bran and diet samples were analyzed for total dietary fiber, SDF and IDF using the methods described by Prosky et al. (1998). Cr2O3 content in digesta and fecal samples was detected using an atomic absorption spectrophotometer (Fan and Sauer, 2002). The gross energy of the diets, digesta and fecal samples was determined using an isoperibol calorimeter (Parr 6300 Calorimeter, Moline, IL, USA).
2.8. Analyses of glucose, insulin, GLP-1, ghrelin, and PYY levels
The plasma glucose concentrations at different time points were analyzed using an automatic biochemical analyzer (Thermo Fisher Scientific, Waltham, MA, US). Plasma concentrations of GLP-1 (KDEIA00105D, the minimum test dose: 0.125 mol/L), ghrelin (KDEIA00112D, the minimum test dose: 15 pg/mL) and PYY (KDEIA00093D, the minimum test dose: 5 pg/mL) were detected using an enzyme-linked immunosorbent assay (ELISA) kit (Beijing Kangjia Hongyuan Biotechnology Co., Ltd., Beijing, China). Plasma insulin concentration was quantified via radioimmunoassay using a GC-2010 immune counter (Anhui Zhongke Zhongjia Scientific Instrument Co., Ltd., Hefei, China).
2.9. Microbiota composition of digesta and feces
The microbiota of digesta and feces was assayed using 16 S rRNA high-throughput sequencing. Microbial genomic DNA was recovered from the digesta and fecal samples using a DNA extraction kit (Omega Bio-tek, Norcross, GA, US). Amplification of the V3–V4 hypervariable region of bacterial 16 S rRNA was achieved using primers F338 (5′-ACTCCTACGGGAGGCAGCAG-3′) and R806 (5′-GGACTACHVGGGTWTCTAAT-3′). The PCR reaction conditions were as follows: 27 cycles of pre-denaturation at 95 °C for 3 min, denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 30 s. Amplification products were sequenced on an Illumina MiSeq platform (Majorbio Pharm Technology, Shanghai, China). Data were analyzed using the online platform Majorbio Cloud Platform (www.majorbio.com).
2.10. SCFA analyses
SCFA concentrations in digesta and feces were determined using the gas chromatography method (Shang et al., 2019). Briefly, 0.5 g of feces or 1.0 g of digesta were weighed into a tube (10 mL). Then, a 2-ethylbutyric acid (200 μL of 1.0 mg/mL) solution and a mixed hydrochloric acid-formic acid solution (50:50, vol:vol) (5 mL) were added to the centrifuge tube and mixed well. The tube was vibrated ultrasonically for 5 min and then centrifuged at 1,000 × g at 4 °C for 10 min. The supernatant was transferred into a centrifuge tube (1.5 mL) and centrifuged at 14,000 × g at 4 °C for 10 min. The supernatant was filtered through a membrane and placed in a gas chromatograph for further analysis.
2.11. Calculations
Apparent total tract digestibility (ATTD) and apparent ileal digestibility (AID) values were calculated using the indirect marker method (Fan and Sauer, 2002). The equations used for digestibility calculation were the following:
| AID = [1 - (Crdiet/Crileal digesta) × (Nileal digesta/Ndiet)] × 100% , |
| ATTD = [1 - (Crdiet/Crfeces) × (Nfeces/Ndiet)] × 100% , |
where Cr represents the content of Cr2O3 in diet, digesta and feces, and N represents the contents of nutrient in diet, digesta and feces.
The Cr2O3 concentrations at each time point were calculated according to the linear relationship of first-order kinetics (Owusu-Asiedu et al., 2006): Y = a + bX, where Y is the Cr2O3 concentration (g of Cr2O3/g of DM), and X is the time (h). The slope (b) of the line is the Cr2O3 excretion rate, which describes the rate of digesta passage. The mean retention time was determined from the concentration of Cr2O3 using the formula (Faichney, 1975): mean retention time = ΣCiti/ΣCi, where i stands for 1, 2, 3 …, Ci is the concentration of Cr2O3 at time ti.
2.12. Statistical analyses
Data were analyzed using the general linear model (GLM) procedures in SAS (version 9.2; SAS Inst. Inc., Cary, NC, USA) with each sow as the experimental unit. Diets were treated as the fixed effect, while period and parity were considered as the random effect. The least squares procedure was employed to calculate mean values, and Tukey's test was used to calculate the differences among dietary treatments. Data are presented as mean ± standard error of the mean (SEM). Figures were created using GraphPad Prism (version 5; GraphPad Software Inc., San Diego, CA, USA). The composition and diversity of the microbiota community expressed as standardized operational taxonomic unit (OTU) readings were analyzed using R software (version 3.3.1; R Software Inc., Auckland, New Zealand). The relative abundance of microbial species at different levels was analyzed using the Kruskal Wallis H test. Differences were considered to be statistically signiflcant when P < 0.05, and tendencies were declared at 0.05 ≤ P < 0.10.
3. Results
3.1. Diets and digesta characteristics
Diets containing wheat bran (CWB and FWB) exhibited higher WHC than the CON group (P < 0.001; Table 2). The FWB diet had a higher WHC (P < 0.001), compared with the CWB diet. The FWB diet had a higher viscosity than the CON and CWB diets (P = 0.01).
Table 2.
Effects of finely ground wheat bran on feed and digesta characteristics of sows1.
| Item | CON 2 | CWB 3 | FWB 4 | SEM 5 | P-value |
|---|---|---|---|---|---|
| Feed | |||||
| Water holding capacity, g/g | 1.18c | 1.45b | 2.45a | 0.02 | <0.001 |
| Viscosity, mPa·s | 1.13b | 1.38b | 1.66a | 0.10 | 0.01 |
| Ileal digesta | |||||
| Quantity collected, g/kg of feed DM | 953.60b | 1,065.00b | 1,860.60a | 170.40 | 0.02 |
| Water holding capacity, g/g | 2.47b | 2.98b | 3.68a | 0.24 | 0.02 |
| Viscosity, mPa·s | 1.38b | 1.48b | 2.15a | 0.08 | <0.001 |
| Passage rate, % of Cr2O3/h | 1.38a | 1.32a | 1.05b | 0.03 | <0.001 |
| Mean retention time, h | 4.43b | 4.40b | 4.88a | 0.05 | <0.01 |
| Total tract retention time, h | 33.83b | 28.33c | 36.83a | 0.52 | <0.001 |
a, b, c Different superscripts represents a significant difference, P < 0.05.
Data are shown as mean ± SEM (n = 6).
CON = a corn soybean meal diet.
CWB = diet contained coarse wheat bran with particle size of 605 μm.
FWB = diet contained fine wheat bran with particle size of 438 μm.
SEM = standard error of the mean.
The quantity of digesta collected at the end of the ileum was greater in the FWB group than in the CON and CWB groups (P = 0.02; Table 2). Similarly, digesta viscosity in FWB-fed sows was greater (P < 0.001) than that in CON or CWB-fed sows. Finely ground wheat bran supplementation increased the WHC of digesta in the ileum (P = 0.02) compared with the CWB. Supplementation of finely ground wheat bran to the diet decreased the digesta passage rate in the small intestine (P < 0.001), corresponding with increased digesta retention time (P < 0.01) compared to CON and CWB. There were no significant differences in digesta characteristics between the CON and CWB-fed sows. Dietary supplementation with finely ground wheat bran increased the total tract retention time in sows (P < 0.001) compared with CON and CWB-fed sows. However, dietary incorporation of coarse wheat bran reduced the total tract retention time in sows (P < 0.001) compared with CON and FWB-fed sows.
3.2. DM feed intake and nutrient digestibility
CON-fed sows had lower levels of total DM intake and daily DM intake than CWB or FWB-fed sows (P < 0.001; Table 3), however, there were no significant differences between the CWB and FWB groups. Dietary treatment had no effect on the AID of DM, CP, NDF and ADF (P > 0.05; Table 3). AID of EE (P = 0.03) and energy (P < 0.001) was lower in the CWB- and FWB-fed sows than in the CON-fed sows. Finely ground wheat bran consumption resulted in a lower AID of EE and energy than CWB (P < 0.05). Dietary treatment had no effect on the ATTD of DM, CP and EE (P > 0.05). Dietary wheat bran (CWB and FWB) reduced the ATTD of energy compared with the CON diet (P < 0.05). Control sows demonstrated the greatest ATTD of NDF (P = 0.02) and ADF (P < 0.01) compared to CWB or FWB sows, but there was no difference between CWB and FWB sows.
Table 3.
Effects of finely ground wheat bran on DM feed intake and nutrient digestibility of sows1.
| Item | CON 2 | CWB 3 | FWB 4 | SEM 5 | P-value |
|---|---|---|---|---|---|
| DM feed intake, kg | |||||
| Total DM feed intake | 43.66b | 46.68a | 46.70a | 0.05 | <0.001 |
| Daily DM feed intake | 2.30b | 2.46a | 2.46a | 0.003 | <0.001 |
| Apparent ileal digestibility, % | |||||
| DM | 78.34 | 77.80 | 78.18 | 0.58 | 0.80 |
| Energy | 78.43a | 68.28b | 63.14c | 0.30 | <0.001 |
| CP | 66.61 | 63.56 | 63.56 | 1.32 | 0.30 |
| EE | 74.34a | 69.17b | 64.76c | 1.77 | 0.03 |
| NDF | 41.69 | 46.50 | 50.05 | 4.85 | 0.50 |
| ADF | 62.05 | 56.48 | 66.04 | 2.90 | 0.11 |
| Apparent total tract digestibility, % | |||||
| DM | 85.33 | 85.61 | 85.38 | 0.74 | 0.96 |
| Energy | 89.21a | 84.95b | 84.51b | 0.78 | <0.05 |
| CP | 86.61 | 86.75 | 86.32 | 0.90 | 0.94 |
| EE | 69.02 | 68.71 | 65.42 | 1.90 | 0.37 |
| NDF | 75.88a | 58.16b | 63.03b | 3.72 | 0.02 |
| ADF | 84.39a | 71.25b | 74.31b | 2.42 | <0.01 |
ADF = acid detergent fiber; CF = crude fiber; CP = crude protein; DM = dry matter; EE = ether extract; NDF = neutral detergent fiber.
a, b, c Different superscripts represents a significant difference, P < 0.05.
Data are shown as mean ± SEM (n = 6).
CON = a corn soybean meal diet.
CWB = diet contained coarse wheat bran with particle size of 605 μm.
FWB = diet contained fine wheat bran with particle size of 438 μm.
SEM = standard error of the mean.
3.3. Plasma glucose and hormones
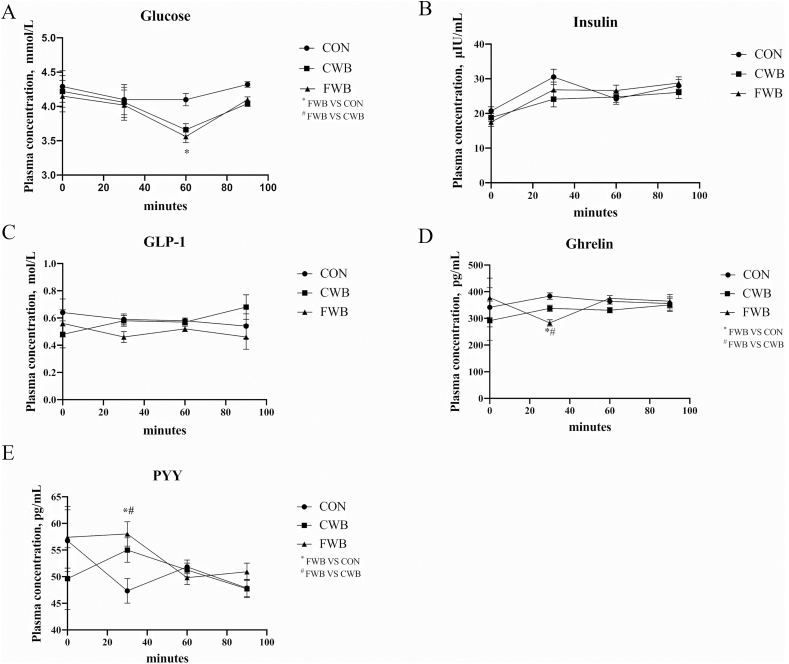
The concentration of glucose in the plasma was not affected by dietary treatment before feeding, 30 min after feeding, or 90 min after feeding (Fig. 1A). However, 60 after feeding, the concentration of plasma glucose was higher in CON sows than in CWB and FWB-fed sows (P < 0.01; Fig. 1A). The plasma insulin concentration pattern was similar to that of the plasma glucose; however, the difference was not significant (Fig. 1B). Dietary treatment exhibited no effect on GLP-1 concentration (Fig. 1C). In FWB-fed sows, the concentration of ghrelin decreased (P < 0.01; Fig. 1D) 30 min after feeding compared to CON- and CWB-sows; however, the concentration of PYY increased (P = 0.01; Fig. 1E).
Fig. 1.
Effects of finely ground wheat bran on plasma glucose, insulin, GLP-1, ghrelin and PYY at different times in sows. (A) Plasma glucose concentrations at different time points. (B) Plasma insulin concentrations at different time points. (C) Plasma GLP-1 concentrations at different time points. (D) Plasma ghrelin concentrations at different time points. (E) Plasma PYY concentrations at different times points. The x-axis represents minutes after a meal. ∗ Denotes a significant difference (P < 0.01) from the CON group; # denotes a significant difference (P < 0.01) from the CWB group; n = 6. CON = a corn soybean meal diet; CWB = diet contained coarse wheat bran with particle size of 605 μm; FWB = diet contained fine wheat bran with particle size of 438 μm; GLP-1 = glucagon-like peptide-1; PYY = peptide tyrosine.
3.4. Ileal bacterial populations
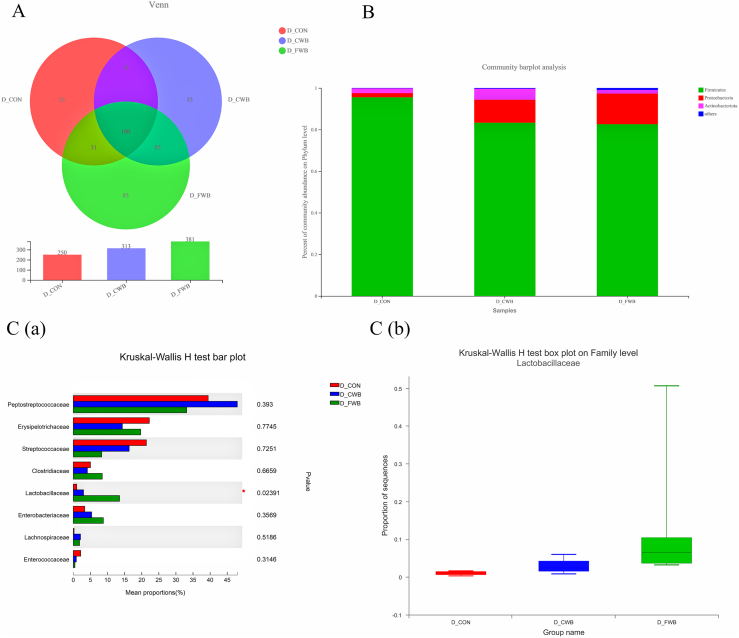
Overall, 250, 313 and 381 OTUs were recorded in the CON, CWB and FWB groups, respectively, of which 180 OTUs were shared among the 3 groups (Fig. 2A). At the phylum level, Firmicutes and Proteobacteria were the most abundant, followed by Actinobacteriota (Fig. 2B). At the family level, the relative abundance of Lactobacillaceae in the FWB group was higher than that in CON- or CWB-fed sows (P < 0.05; Fig. 2C).
Fig. 2.
Effects of finely ground wheat bran on ileal digesta microbiota composition of sows. (A) OTU Venn of 3 dietary treatments. (B) Percent of community abundance at the phylum level. The results were analyzed by Student's t-test and presented as mean values of different bacteria, n = 6. (C-a) The difference of microbiota at the family level; the results analyzed by Kruskal–Wallis H test, n = 6; the y-axis represents the microbiota name at a certain classification level; the x-axis represents the average relative abundance in different groups of species; and the columns with different colors represent different groups. The rightmost is the P-value, ∗P ≤ 0.05. (C-b) The relative abundance of Lactobacillaceae in different groups, the x-axis represents different samples, columns of different colors represent different groups, and the y-axis represents the abundance proportion of a microbiota in different samples. OTU = operational taxonomic unit.
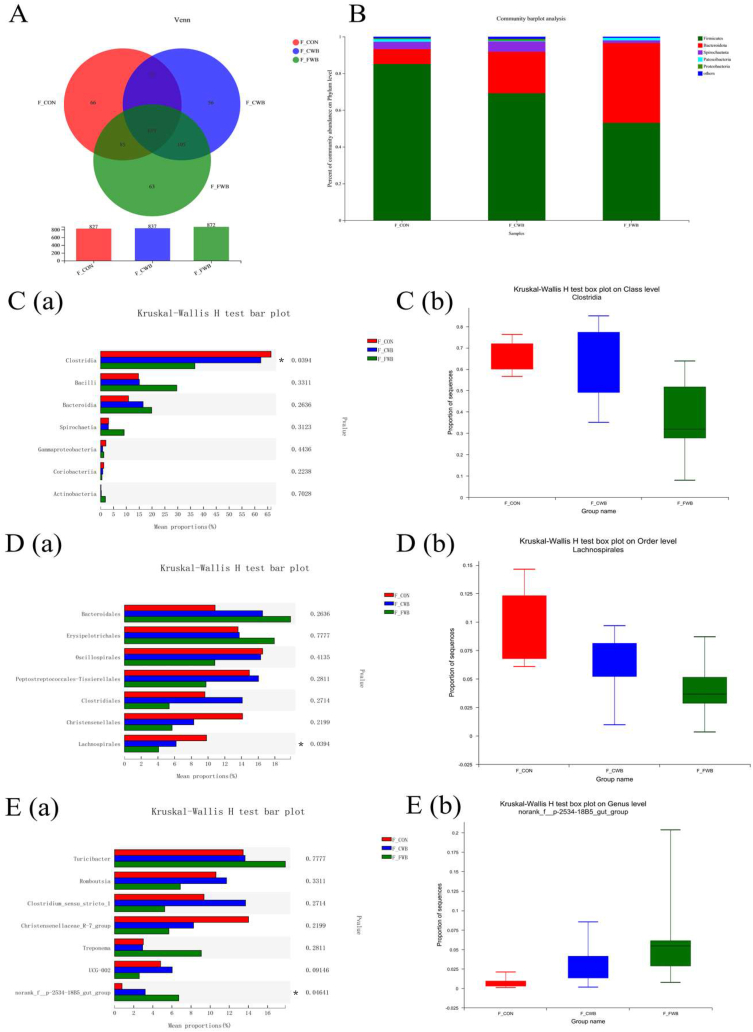
3.5. Fecal microbiota composition
Overall, 827, 837 and 872 OTUs were identified in the CON, CWB and FWB groups, respectively, of which 619 OTUs were shared among the 3 groups (Fig. 3A). At the phylum level, Firmicutes and Bacteroidetes were the dominant bacteria, followed by Spirochaetes (Fig. 3B). At the class level, the relative abundance of Clostridia was lower in the FWB group than in the CON group (P < 0.05), but not in the CWB group (Fig. 3C). At the order level, the relative abundance of Lachnospirales decreased in the CWB- and FWB-fed sows (P < 0.05; Fig. 3D). At the genus level, the relative abundance of norank_f_p-2534-18B5_gut_group increased in CWB- and FWB-fed sows (P < 0.05) compared to CON-fed sows. However, compared with FWB-fed sows, the relative abundance of norank_f_p-2534-18B5_gut_group reduced in CWB-fed sows (P < 0.05; Fig. 3E).
Fig. 3.
Effects of finely ground wheat bran on fecal microbiota composition of sows. (A) OTU Venn of 3 dietary treatments. (B) Percent of community abundance at the phylum level, the results were analyzed by Student's t-test and presented as mean values of different bacteria, n = 6. (C-a) The difference of microbiota at the class level. (C-b) The relative abundance of Clostridia in different groups. (D-a) The difference of microbiota at the order level. (D-b) The relative abundance of Lachnospirales in different groups. (E-a) The difference of microbiota at the genus level. (E-b) The relative abundance of norank_f_p-2534-18B5_gut_group in different groups. The results of (C-a), (D-a), and (E-a) analyzed by Kruskal–Wallis H test, n = 6; the y-axis represents the microbiota name at a certain classification level; the x-axis represents the average relative abundance in different groups of species; and the columns with different colors represent different groups. The rightmost is the P-value, ∗P ≤ 0.05. (C-b), (D-b) and (E-b) The x-axis represents different samples; columns of different colors represent different groups; and the y-axis represents the abundance proportion of a microbiota in different samples. OTU = operational taxonomic unit.
3.6. SCFA concentration in ileal digesta and feces
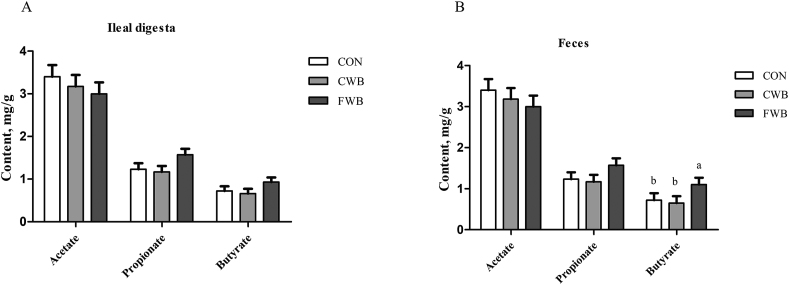
Dietary treatment had no effect on the acetate, propionate and butyrate contents in the ileal digesta (Fig. 4A). Acetate and propionate concentrations in feces were not affected by the dietary treatment (Fig. 4B). The concentration of butyrate in feces was higher in FWB-fed sows than in CON or CWB sows (P = 0.04); however, there was no difference between CON and CWB sows.
Fig. 4.
Effects of finely ground wheat bran on ileal digesta and fecal short-chain fatty acids of sows. (A) Acetate, propionate, and butyrate concentrations in ileal digesta. (B) Acetate, propionate and butyrate concentrations in feces. Values are presented as mean ± SEM (n = 6). a, b Means without common letters differ at P < 0.05. CON = a corn soymeal diet; CWB = diet contained coarse wheat bran with particle size of 605 μm; FWB = diet contained fine wheat bran with particle size of 438 μm; SEM = standard error of the mean.
4. Discussion
The structure and physicochemical characteristics of DF, such as WHC and viscosity, influence digestion and metabolism of feedstuffs in the digestive tract (Owusu-Asiedu et al., 2006). In the current study, a simple T-cannula was used to investigate the effects of finely ground wheat bran on the digestive physiology, gut hormones, and gut microbiota in sows.
Finely ground wheat bran increased digesta viscosity, slowed digesta passage rate and increased total tract retention time compared to corn soybean meal and CWB diets. Hydration of DF will enhance viscosity, but this depends upon the chemical structure of DF and other cell wall compounds connected to DF (Wenk, 2001). In general, the viscosity of SDF is greater than IDF (Brockman, 2014). A small quantity of SDF, such as guar gum or β-glucan, has very high viscosity when hydrated in the digestive tract (Dikeman et al., 2006). However, wheat bran contains high IDF, which has low viscosity even when hydrated in the digestive tract (Gallaher et al., 2000). In our previous study, finely ground wheat bran exhibited higher SDF and β-glucan content than coarse wheat bran (Wang et al., 2022). In the current study, dietary supplementation with finely ground wheat bran increased the viscosity of the feed. This demonstrated that enhanced SDF concentrations increased the feed viscosity, resulting in enhanced digesta viscosity. Sugar beet pulp is rich in SDF and can reduce the passage rate of digesta in the small intestine due to higher digesta viscosity (Knudsen and Hansen, 1991). Finely ground wheat bran may act in a manner similar to sugar beet pulp in decreasing the passage rate of digesta. Cereal bran is rich in IDF and can increase digesta passage rate throughout the entire gastrointestinal tract owing to the decrease in digesta viscosity (Jenkins et al., 2000). Conversely, the increased viscosity of finely ground wheat bran led to the increase in total tract retention time. Thus, dietary supplementation with finely ground wheat bran may have important effects on the apparent digestibility of nutrients in the small intestine and degradation of nutrients in the large intestine. Physicochemical properties of DF affect digesta quantity in terminal ileum. The increased quantity of digesta in the distal ileum may be due to the increase in WHC and viscosity caused by SDF (Smulikowska et al., 2002). In the present study, finely ground wheat bran increased the quantity of digesta in the distal ileum. The diet containing finely ground wheat bran increased WHC and viscosity, suggesting that fine wheat bran plays a crucial role in increasing digesta quantity.
Dietary supplementation with coarse wheat bran and fine wheat bran decreased the AID and ATTD of energy in the current study. Dietary fiber itself has a low energy concentration, which reduces the energy density of diets and daily energy intake of animals (Slavin, 2005), thereby reducing the AID and ATTD of energy compared to the control diet. The inclusion of 20% coarse wheat bran and 20% fine wheat bran in the diet decreased the ATTD of NDF and ADF. Coarse wheat bran increased the total tract passage rate. An increase in total tract passage rate reduces the effective time for digestion and absorption of nutrients (Hooda et al., 2001) in the gastrointestinal tract, resulting in reduced digestibility. Hydration of DF increases the viscosity of digesta, hinders contact between digesta and digestive enzymes, and therefore decreases the digestibility of nutrients (Murray et al., 1999). In the current study, increased digesta viscosity caused by finely ground wheat bran likely hindered the contact between digesta and digestive enzymes, which reduced the digestibility of NDF, ADF and energy. The interaction between digesta viscosity and passage rate of digesta is suggested to influence the digestion characteristics of diets.
Dietary fiber can modify some gut hormones such as ghrelin, GLP-1 and PYY, which regulate satiety and energy intake (Sánchez et al., 2012). Ghrelin is mainly produced in the gastric mucosa and regulates satiety (Tarini and Wolever, 2010). Fermentable fiber polysaccharides are rapidly and widely fermented in the proximal colon and may regulate ghrelin levels (Tarini and Wolever, 2010). GLP-1 and PYY are hormones secreted by hindgut L cells that signal satiety after food intake and participate in short-term regulation of food intake (Silva and Bloom, 2012). Fermentable DF increased the secretion of PYY and GLP-1 in a rodent model (Chaudhri et al., 2006). A diet containing 3% barley β-glucan reduced the concentration of ghrelin and increased the concentration of PYY in the plasma, which resulted in increased satiety after eating (Vitaglione et al., 2009). In the current study, dietary supplementation with fine wheat bran decreased plasma ghrelin concentration and enhanced plasma PYY concentration 30 min after feeding, indicating that fine wheat bran elicited enhanced satiety after feeding compared with coarse wheat bran. These changes in gut hormone levels may be attributed to the following aspects: 1) Increasing the fiber content of a diet increases the amount of digesta in the intestinal tract, influencing the secretion of gut hormones; 2) finely ground wheat bran reduces the passage rate of digesta and increases the retention time of digesta in the gastrointestinal tract, indirectly promoting the sense of satiety and reducing the secretion of ghrelin; and 3) the concentration of butyrate is involved in increasing the secretion of PYY and decreasing the secretion of ghrelin in the intestine (Klosterbuer et al., 2012). Finely ground wheat bran consumption increased fecal butyrate content in the current study, which may be the primary reason for the changes in ghrelin and PYY concentrations.
In the present study, fine wheat bran increased the relative abundance of Lactobacillaceae in the ileal digesta of the sows. The relative abundance of Clostridia in the feces of the sows declined when they consumed FWB. Bacteria prefer carbohydrates with specific chemical structures as fermentation substrates (Owusu-Asiedu et al., 2006). Thus, feed components which differ in the types of DF and speed of digestion (Serena et al., 2008) could facilitate the growth of specific microbiota. A study on humans suggested that wheat bran extract, a food-grade soluble fiber preparation, had prebiotic activity and could increase the abundance of Lactobacillus and Bifidobacterium (François et al., 2012). The stimulation of Bifidobacterium species after the intake of FWB demonstrates that the high SDF of finely-ground wheat bran has prebiotic effects that coarse wheat bran with a higher IDF content does not possess. Clostridia are generally regarded as pathogenic bacteria (Yang et al., 2019), and decreased relative abundance of Clostridia can attenuate inflammation of the gut in pigs (Shang et al., 2019). Dietary supplementation with finely-ground wheat bran reduced the relative abundance of Clostridia, suggesting that finely-ground wheat bran could prevent intestinal inflammation to some extent.
The enhanced relative abundance of Lactobacillaceae caused by finely-ground wheat bran may be due to the combined effects of increased digesta viscosity and decreased nutrient digestibility. Increased digesta viscosity elicited by SDF supplementation leads to changes in the physiology and ecosystem of the gut (Bedford and Classen, 1992), thereby hindering the contact between digesta and digestive enzymes and reducing the effective absorption of nutrients. Decreased energy digestibility increases the undigested energy and amount of digesta in the distal ileum, which serves as a substrate for the microbiome, thus providing a stable environment for microbes to proliferate. Alternatively, a slower digesta passage rate with low oxygen tension ensures a relatively stable environment for colonization and proliferation of microbiota in the small intestine (De Boever et al., 2000). The decreased relative abundance of Clostridia in feces caused by finely-ground wheat bran may be due to the elevated content of SDF and β-glucan, which are not preferred substrates for fermentation by Clostridia (Owusu-Asiedu et al., 2006).
SCFA are the final products of the anaerobic fermentation of DF by the gut microbiome. These end products have multiple beneficial effects on mammalian energy metabolism (Besten et al., 2013). Butyrate can provide energy to colonic epithelial cells, promote the proliferation of probiotics, reduce inflammation and maintain intestinal function, all of which have a positive impact on gut health (Cornick et al., 2015; Makki et al., 2018). In the present study, dietary supplementation with finely-ground wheat bran improved butyrate concentration and finely-ground wheat bran positively affected gut health. The degree and speed of DF degradation by gut microorganisms is related to water solubility, chemical structure, particle size and other factors of the fiber (Anguita et al., 2006). Structural characteristics, such as type, quantity and bonding mode of monosaccharides in polysaccharide molecules, largely determine the fermentation of fibers in the large intestine (Henningsson et al., 2001). The smallest size fraction of corn bran is the most extensively fermented fraction and produces the highest levels of butyrate (Thakkar et al., 2020). The increased concentration of butyrate in FWB-fed sows may be due to the decrease in particle size of wheat bran, increase in specific surface area, and enzymes secreted by microorganisms which degrade the fiber content of the wheat bran more adequately. In addition, finely-ground wheat bran prolonged the total tract retention time, which enables microorganisms to fully interact with the fiber. Finally, the quantity of digesta in the terminal ileum increased in FWB-fed sows, suggesting that it provides a sufficient amount of substrate for the microorganisms residing in the large intestine.
5. Conclusions
Dietary supplementation with finely-ground wheat bran decreased digesta passage rate, increased the abundance of beneficial microorganisms and enhanced the concentrations of SCFA and PYY in sows. Therefore, dietary supplementation with fine wheat bran exhibited a better effect on improving satiety and intestinal microbial composition of sows.
Author contributions
Zijie Wang conducted the animal experiment and prepared the first manuscript draft. Wenhui Wang and Song Xu assisted in collection of samples. Xiangfang Zeng and Jian Ding assisted in preparing the manuscript. Hu Liu assisted in preparing the manuscript and data curation. Fenglai Wang contributed to designing the studies and providing financial support. All authors have read and approved the final manuscript.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, and there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the content of this paper.
Acknowledgments
This study was financially supported by the National Key Research and Development Program of China (Project No: 2021YFD1300202) and the National Natural Science Foundation of China (Project No: 32102587).
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Anguita M., Canibe N., Pérez J.F., Jensen B.B. Influence of the amount of dietary fiber on the available energy from hindgut fermentation in growing pigs: use of cannulated pigs and in vitro fermentation. J Anim Sci. 2006;84:2766–2778. doi: 10.2527/jas.2005-212. [DOI] [PubMed] [Google Scholar]
- Aoac International . 18th ed. AOAC Int.; Gaithersburg, MD: 2007. Official method of analysis of AOAC international. [Google Scholar]
- Bedford M.R., Classen H.L. Reduction of intestinal viscosity through manipulation of dietary rye and pentosanase concentration is affected through changes in the carbohydrate composition of the intestinal aqueous phase and results in improved growth rate and food conversion efficiency of broiler chicks. J Nutr. 1992;122:560–569. doi: 10.1093/jn/122.3.560. [DOI] [PubMed] [Google Scholar]
- Besten G.D., Eunen K.V., Groen A.K., Venema K., Reijngoud D.J., Bakker B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54:2325–2340. doi: 10.1194/jlr.R036012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman D.A. The effect of viscous and fermentable dietary fiber consumption on adiposity, insulin resistance and fuel utilization in rats. J Nutr. 2014;144:1415–1422. [Google Scholar]
- Chaudhri O., Small C., Bloom S. Gastrointestinal hormones regulating appetite. Philos Trans R Soc Lond B Biol Sci. 2006;361:87–209. doi: 10.1098/rstb.2006.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornick S., Tawiah A., Chadee K. Roles and regulation of the mucus barrier in the gut. Tissue Barriers. 2015;3 doi: 10.4161/21688370.2014.982426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boever P., Deplancke B., Verstraete W. Fermentation by gut microbiota cultured in a simulator of the human intestinal microbial ecosystem is improved by supplementing a soygerm powder. J Nutr. 2000;30:2599–2606. doi: 10.1093/jn/130.10.2599. [DOI] [PubMed] [Google Scholar]
- Dikeman C.L., Murphy M.R., Fahey G.C. Dietary fibers affect viscosity of solutions and simulated human gastric and small intestinal digesta. J Nutr. 2006;136:913–919. doi: 10.1093/jn/136.4.913. [DOI] [PubMed] [Google Scholar]
- Fan M.Z., Sauer W.C. Determination of true ileal amino acid digestibility and the endogenous amino acid outputs associated with barley samples for growing-finishing pigs by the regression analysis technique. J Anim Sci. 2002;6:1593–1605. doi: 10.2527/2002.8061593x. [DOI] [PubMed] [Google Scholar]
- Faichney G.J. The effect of formaldehyde treatment of a concentrate diet on the passage of solute and particular markers through the gastrointestinal tract of sheep. Aust J Agric Res. 1975;26:319–327. [Google Scholar]
- François I.E., Lescroart O., Veraverbeke W.S., Marzorati M., Possemiers S., Evenepoel P. Effects of a wheat bran extract containing arabinoxylan oligosaccharides on gastrointestinal health parameters in healthy adult human volunteers: a double-blind, randomised, placebo-controlled, cross-over trial. Br J Nutr. 2012;108:2229–2242. doi: 10.1017/S0007114512000372. [DOI] [PubMed] [Google Scholar]
- Gallaher C.M., Munion J., Hesslink R.J., Wise J., Gallaher D.D. Cholesterol reduction by glucomannan and chitosan is mediated by changes in cholesterol absorption and bile acid and fat excretion in rats. J Nutr. 2000;130:2753–2759. doi: 10.1093/jn/130.11.2753. [DOI] [PubMed] [Google Scholar]
- Henningsson A.M., Margareta E., Nyman G., BjRck I.M. Content of short-chain fatty acids in the hindgut of rats fed processed bean (Phaseolus vulgaris) flours varying in distribution and content of indigestible carbohydrates. Br J Nutr. 2001;86:379–389. doi: 10.1079/bjn2001423. [DOI] [PubMed] [Google Scholar]
- Hooda S., Metzler-Zebeli B.U., Vasanthan T., Zijlstra R.T. Effects of viscosity and fermentability of dietary fibre on nutrient digestibility and digesta characteristics in ileal-cannulated grower pigs. Br J Nutr. 2001;106:664–674. doi: 10.1017/S0007114511000985. [DOI] [PubMed] [Google Scholar]
- Jenkins D.J., Axelsen M., Kendall C.W., Augustin L.S., Vuksan V., Smith U. Dietary fibre, lente carbohydrates and the insulin-resistant diseases. Br J Nutr. 2000;83:S157.$63. doi: 10.1017/s0007114500001100. [DOI] [PubMed] [Google Scholar]
- Klosterbuer A.S., Greaves K.A., Slavin J. Fiber intake inconsistently alters gut hormone levels in humans following acute or chronic intake. J Food Res. 2012;1:255–273. [Google Scholar]
- Knudsen K.E.B. Carbohydrate and lignin contents of plant materials used in animal feeding. Anim Feed Sci Technol. 1997;67:319–338. [Google Scholar]
- Knudsen K.E.B., Hansen Gastrointestinal implications in pigs of wheat and oat fractions. 1. Digestibility and bulking properties of polysaccharides and other major constituents. Br J Nutr. 1991;65:217–232. doi: 10.1079/bjn19910082. [DOI] [PubMed] [Google Scholar]
- Knudsen K.E.B., Jensen B.B., Hansen I. Digestion of polysaccharides and other major components in the small and large intestine of pigs fed on diets consisting of oat fractions rich in beta-d-glucan. Br J Nutr. 1993;70:537–556. doi: 10.1079/bjn19930147. [DOI] [PubMed] [Google Scholar]
- Lin X.X., Zhang X.X., Zhang R., Ni Z.J., Elam E., Thakur K. Gut modulation based anti-diabetic effects of carboxymethylated wheat bran dietary fiber in high-fat diet/streptozotocin-induced diabetic mice and their potential mechanisms. Food Chem Toxicol. 2021;152 doi: 10.1016/j.fct.2021.112235. [DOI] [PubMed] [Google Scholar]
- Makki K., Deehan E.C., Walter J., Bckhed F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe. 2018;23:705–715. doi: 10.1016/j.chom.2018.05.012. [DOI] [PubMed] [Google Scholar]
- Messia M.C., Reale A., Maiuro L., Candigliota T., Sorrentino E., Marconi E. Effects of pre-fermented wheat bran on dough and bread characteristics. J Cereal Sci. 2016;69:138–144. [Google Scholar]
- Murray S.M., Patil Ar G., Jr., Merchen N.R., Wolf B.W., Lai C.S. Apparent digestibility and glycaemic responses to an experimental induced viscosity dietary fibre incorporated into an enteral formula fed to dogs cannulated in the ileum. Food Chem Toxicol. 1999;37:47–56. doi: 10.1016/s0278-6915(98)00097-0. [DOI] [PubMed] [Google Scholar]
- Owusu-Asiedu A., Patience J.F., Laarveld B., Kessel A., Zijlstra R.T. Effects of guar gum and cellulose on digesta passage rate, ileal microbial populations, energy and protein digestibility, and performance of grower pigs. J Anim Sci. 2006;84:843–852. doi: 10.2527/2006.844843x. [DOI] [PubMed] [Google Scholar]
- Prosky L., Nils-Georg A., Schweizer T.F., Devries J.W., Furda I. Determination of soluble dietary fiber in foods and food products: collaborative study. J AOAC Int. 1998;77:690–694. [PubMed] [Google Scholar]
- Ríos-Covián D., Ruas-Madiedo P., Margolles A., Gueimonde M., Reyes-Gavilán C.G., Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol. 2016;7:185. doi: 10.3389/fmicb.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez D., Miguel M., Aleixandre A. Dietary fiber, gut peptide, and adipocytokines. J Med Food. 2012;15:223–230. doi: 10.1089/jmf.2011.0072. [DOI] [PubMed] [Google Scholar]
- Santala O., Kiran A., Sozer N., Poutanen K., Nordlund E. Enzymatic modification and particle size reduction of wheat bran improves the mechanical properties and structure of bran-enriched expanded extrudates. J Cereal Sci. 2014;60:448–456. [Google Scholar]
- Schop M., Jansman A., Vries S.D., Gerrits W. Increased diet viscosity by oat β-glucans decreases the passage rate of liquids in the stomach and affects digesta physicochemical properties in growing pigs. Animals. 2020;14:269–276. doi: 10.1017/S1751731119001824. [DOI] [PubMed] [Google Scholar]
- Serena A., Jrgensen H., Knudsen K.E.B. Digestion of carbohydrates and utilization of energy in sows fed diets with contrasting levels and physicochemical properties of dietary fiber. J Anim Sci. 2008;86:2208–2216. doi: 10.2527/jas.2006-060. [DOI] [PubMed] [Google Scholar]
- Serena A. Danish Institute of Agricultural Sciences, Department of Animal Health, Welfare and Nutrition, Research Centre Foulum; Tjele, Denmark: 2005. Physiological properties of dietary carbohydrates for sows. PhD Thesis. [Google Scholar]
- Shang Q., Liu H., Liu S., He T., Piao X. Effects of dietary fiber sources during late gestation and lactation on sow performance, milk quality, and intestinal health in piglets. J Anim Sci. 2019;97:4922–4933. doi: 10.1093/jas/skz278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva A.D., Bloom S.R. Gut hormones and appetite control: a focus on pyy and glp-1 as therapeutic targets in obesity. Gut Liver. 2012;6:10–20. doi: 10.5009/gnl.2012.6.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavin J.L. Dietary fiber and body weight. Nutrition. 2005;21:411–418. doi: 10.1016/j.nut.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Smulikowska S., Mieczkowska A., Nguyen C.V., Babelewska M. The influence of digesta viscosity on the development of the stomach, on in vitro small intestinal motility and on digestion of nutrients in broiler chickens. J Anim Feed Sci. 2002;11:683–694. [Google Scholar]
- Stark C.R., Chewning C.G. The effect of sieve agitators and dispersing agent on the method of determining and expressing fineness of feed materials by sieving. Anim Prod Sci. 2011;52:69. [Google Scholar]
- Stein H.H., Shipley C.F., Easter R.A. Technical note: a technique for inserting a T-cannula into the distal ileum of pregnant sows. J Anim Sci. 1998;76:1433–1436. doi: 10.2527/1998.7651433x. [DOI] [PubMed] [Google Scholar]
- Tarini J., Wolever T. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Appl Physiol Nutr Metab. 2010;35:9–16. doi: 10.1139/H09-119. [DOI] [PubMed] [Google Scholar]
- Thakkar R.D., Tuncil Y.E., Hamaker B.R., Lindemann S.R. Maize bran particle size governs the community composition and metabolic output of human gut microbiota in in vitro fermentations. Front Microbiol. 2020;11:1009. doi: 10.3389/fmicb.2020.01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiex Nancy J., Anderson Shirle, Gildemeister Bryan. Crude fat, diethyl ether extraction, in feed, cereal Grain, and forage (Randall/Soxtec/Submersion method): collaborative study. J AOAC Int. 2003;86:888–898. [PubMed] [Google Scholar]
- Van Soest P.J., Robertson J.B., Lewis B.A. Methods for dietary fiber neutral detergent fiber and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- Vitaglione P., Lumaga R.B., Stanzione A., Scalfi L., Fogliano V. β-Glucan-enriched bread reduces energy intake and modifies plasma ghrelin and peptide YY concentrations in the short term. Appetite. 2009;3:338–344. doi: 10.1016/j.appet.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Wang Z.J., Chen Y.F., Wang W.H., Huang C.Y., Hu Y.F., Lee J., Wang F.L. Dietary supplementation with fine-grinding wheat bran improves lipid metabolism and inflammatory response via modulating the gut microbiota structure in pregnant sow. Front Microbiol. 2022;3 doi: 10.3389/fmicb.2022.835950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk C. The role of dietary fibre in the digestive physiology of the pig. Anim Feed Sci Technol. 2001;90:21–33. [Google Scholar]
- Yang W., Lee Y., Lu H., Chou C., Wang C. Analysis of gut microbiota and the effect of lauric acid against necrotic enteritis in Clostridium perfringens and Eimeria side-by-side challenge model. PLoS One. 2019;14 doi: 10.1371/journal.pone.0205784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC . 11th ed. National Academy Press; Washington, DC, USA: 2012. Nutrient requirements of swine. [Google Scholar]