Abstract
Background
Selecting a medicine has a significant impact on the quality of therapy including efficacy and safety. P-glycoprotein and CYP3A4 share several common substrates known as bi-substrates. Both play major role in the pharmacokinetics and pharmacodynamics when over or under expressed.
Objective
The study aimed to assess the Drug–Drug Interaction (DDI) related to P-glycoprotein (P-gp) and Cytochrome P450-3A4 (CYP3A4), to predict their clinical outcomes and also to discover prospective predictors of pDDIs.
Methods
The subjects in this retrospective study ranged in age from 18 to 95 years with polypharmacy prescriptions. Information was gathered through patient medical records. Based on Micromedex and previous literature studies, medications prescribed to the patients were observed for pDDIs according to risk rating scale for drug interactions.
Results
A total of 504 patients (160 males and 344 females) were included in the study. The mean of pDDI seen in the patients was 1.66 ± 1.48 and total 825 pDDIs were discovered. The factors significantly associated with having ≥1 pDDIs included: taking ≥5 medicines (OR 1.747), increased age (OR 1.026) increased comorbidities (OR 1.73).
Conclusion
In prescriptions, a considerable number of probable DDI were discovered. Therefore, careful selection of drugs and identification of mechanisms for DDI is needed to lower the frequency of pDDI.
Keywords: MDR1 gene, CYP3A4, Bi-substrates, Drug–drug interaction (DDI), Pharmacology, Outpatient, P-Glycoprotein (P-gp)
Graphical abstract
P-glycoprotein; MDR1 gene; P-gp; CYP3A4; Bi-substrates; Drug–drug interaction (DDI); Pharmacology; Outpatient.
1. Introduction
Adverse drug reactions significantly increases burden of healthcare cost and inpatient care. The most common cause of adverse drug reactions, increased length of hospitalization and death begins with drug–drug interaction. Drug–drug interaction (DDI) is defined as when one drug interferes with the pharmacological action and effectiveness of another drug [1, 2]. When two or more drugs interacts with each other, it usually results into either reduced pharmacological action and effectiveness or increased toxicity of the object drug [3].
Drug–Drug interactions can be either pharmacodynamic or pharmacokinetic in nature [4]. A pharmacokinetic interaction can alter a medication's absorption, distribution, metabolism, and excretion (ADME), resulting in a change in serum drug concentration and potentially differing clinical effects. Thereby, resulting in altered pharmacological effect of the drug. The most common cause of pharmacokinetic interaction entails the role of drug metabolizing enzymes, mainly phase I enzymes (cytochrome P450 superfamily) and drug transporters such as ABCB1 (P-glycoprotein), ABCG2 (BCRP), SLC22A6 (OAT1 or NKT), SLC22A8 (OAT3 or ROCT), SLC22A2 (OCT2), SLCO1B1 (OATP1B1) and SLCO1B3 (OATP1B3) [5, 6]. About 75% of total drug metabolism is carried out by CYP enzymes and about 60% of drugs are metabolized by CYP3A superfamily [5, 7]. When drugs that are metabolized by the same enzyme are given together, possibility of DDIs can increase. Moreover, drug efflux, notably through P-gp, is increasingly recognized as being important as many drug's disposition is influenced by the presence of this protein [8]. Studies have reported that the drug transporter P-gp and the metabolizing enzyme CYP3A shares an extensive range of substrates and/or modulators and they are often found together in cells. As a result, the disposition of drugs is greatly influenced by both transport and metabolism [9]. Moreover, co-administered drugs that interact with P-gp and CYP3A at the gut/liver axis, may increase the possibility of notable drug–drug interactions [10].
P-glycoprotein (P-gp), a 170 kDa membrane glycoprotein, product of the Multi-Drug Resistant 1 gene (MDR1) was first identified as an efflux pump in 1976 by Juliano and Ling [11]. It is a member of the adenosine triphosphate–binding cassette (ABC) superfamily of cellular efflux drug transporters which are very well known for their role in drug transport and chemoresistance [12, 13]. It has the capacity to transport a broad range of cationic, hydrophobic, endogenous, and exogenous compounds across the cell membrane. P-glycoprotein is extensively found in human body, in the intestinal epithelium, in liver cells, in the cells of proximal tubule of kidney and in capillary endothelial cells of blood-brain barrier and blood-testis barrier. P-gp has also been discovered to be localized on the cells of Placenta [14]. As a result of which, P-glycoprotein is considered to be one of the important transporter proteins facilitating the removal of toxic metabolites and xenobiotic out of the body cells.
The proportion of P-gp expression, regulation, and activity can have a direct impact on the pharmacokinetics and pharmacodynamics of drugs that are P-gp substrates [15, 16, 17]. P-gp expression is variable for number of individuals and organ to organ with least expression in stomach and prominent expression in colon. As the drug reaches the systemic circulation, P-gp expression prevents it from penetrating into a number of sensitive tissues ultimately resulting in transporting drugs out of the system [14, 18]. With P-gp having varied substrate specificity, many drugs function as P-gp inhibitors or inducers [19]. pDDI should be considered when P-gp inducers or inhibitors are co-administered with substrates [20]. Therefore, the role of P-gp in causing clinically significant drug–drug interaction is worth considering.
Of the Cytochrome 450 family, CYP3A is an important metabolizing enzyme responsible for the breakdown of more than 50% of drugs prescribed in clinical settings [21]. It serves as an integral part of intestinal as well as hepatic first-pass effect. In the adult human liver and small intestine, CYP3A4 accounts for 40% and 80% of the total respectively [7, 22, 23]. It is found in enterocytes at the tips of villi and is most abundant in the jejunum and ileum [22]. Presence of P-glycoprotein in the GI-tract accounts for the collaborative role of P-gp and CYP3A4 in altering the systemic exposure of the substrate drug. It has been reported that when transported in the secretory, detoxifying basolateral-to-apical route, P-gp increased the degree of CYP3A4-mediated loss of parent drugs. Data further confirmed the broad concept that P-gp activity can enhance the degree of CYP3A4 metabolism of drugs which are also substrate for P-gp efflux [24].
The overlapping substrates of P-gp and CYP3A4 are referred as “bi-substrates” [19]. The presence of P-gp and CYP3A4 together in the body can cause alterations in absorption, distribution, elimination, availability, and therapeutic efficacy of substrate drugs. Studies have also confirmed that P-gp and CYP3A4 share a variety of common inducers, and inhibitors which increases the probability of drug–drug interaction when prescribed simultaneously with substrates [25, 26, 27]. Considering the possible interaction of P-gp and CYP3A substrates, inducers, and inhibitors, recognizing how much each aspect of drug detoxification is a part of a drug interaction is vital. Drug absorption and delivery are particularly complex due to the overlapping tissue distribution of CYP3A4 and P-gp [10]. The relative affinities of the substrates are likely to influence interactions between P-gp and CYP3A4 during the absorption of P-gp/CYP3A4 substrates, leading to various consequences, ranging from P-gp's lack of impact on parent compound metabolism to almost total dependence on apical P-gp-mediated recycling activity [24].
Hence to understand the extent of bioavailability, disposition and elimination of object drug, it is important to identify potential interactions with inducers and inhibitors. The prevalence potential DDI's involving the role of p-gp and CYP3A4 have been documented very little in different population of Gujarat and relatively few from India. To the best of our knowledge, we provide evidence for the first time of potential DDI's involving the role of P-glycoprotein and CYP3A4.
The present retrospective study analysis was carried out to assess prevalence, clinical significance, and association of P-gp and CYP3A4 with potential DDI in a large outpatient population of tertiary care hospital in Gujarat, India.
2. Methodology
2.1. Study design
This retrospective observational study was performed after the approval from the Institutional Ethics Committee (IEC) (Protocol no: LMIEC/2021-22/PD/006) and the superintendent of Sheth Vadilal Sarabhai General Hospital. The study was carried out for duration of three months in the Medicine outpatient department (OPD) of the hospital. A total of 504 patients with polypharmacy (having five or more medications) and having two or more drugs working through P-gp or/and CYP3A4 in their prescription were included in the study. Necessary information such as age, sex, concomitantly existing disease, prescribed drugs were obtained from patient medical records and prescription. Data from all the prescriptions were entered in case report form. Based on Micromedex and Literature studies, the medications prescribed to the patients were analysed for interactions according to the risk rating scale for drug–drug interactions (Table 1).
Table 1.
Drug–drug interaction risk rating scale.
| Rating | Category | Action | Explanation |
|---|---|---|---|
| X | Contraindicated | Avoid combination | The drugs are contraindicated for concurrent use |
| D | Major | Consider therapy modification | The interaction may be life threatening and/or require medical intervention to minimize or prevent serious adverse events |
| C | Moderate | Monitor therapy | The interaction may result in exacerbation of the patient's condition and/or require an alteration in therapy |
| B | Minor | No action needed | The interaction would have limited clinical effects. May include an increase in the frequency or severity of side effects but generally would not require a major alteration in therapy. |
| A | Unknown | No known interaction | Unknown |
Note: Data from Armahizer et al [28] and Micromedex (MICROMEDEX, 2022).
2.2. Subjects
The subjects 18–95 years of age or older group, diagnosed with Cardiovascular (CVS), Gastrointestinal tract system (GIT), Endocrine, Neurologic or/and Chronic obstructive pulmonary disorder COPD disease along with medication regimen complexity and polypharmacy were included in the study. Patients were excluded if they had less than five oral medications and less than two drugs working through P-gp axis CYP3A4 in their prescription.
2.3. Ethics
The study was a retrospective observational register study as confirmed by the Institutional Ethics Committee (LMIEC Ahmedabad, Gujarat, India) (Protocol no. LMIEC/2021-22/PD/006). Permission to access and process data was given by Sheth Vadilal Sarabhai General Hospital, Ahmedabad, Gujarat, India.
2.4. Data collection
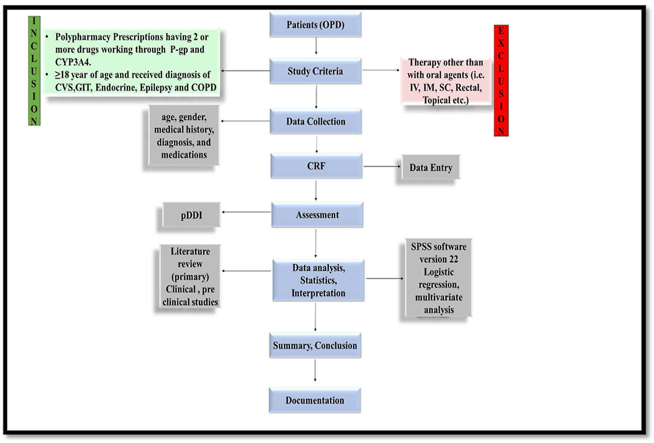
Eligible cases were identified through medical records of the patient. We reviewed each case file, and using a standard form purposely designed for the study – extracted data on sex, age, concomitantly existing disease, therapy regimen prescribed for each patient (Figure 1).
Figure 1.
Data collection and category of drug drug interaction.
GIT: Gastro-Intestinal; CVS: Cardiovascular; DDI: Drug drug interaction.
2.5. Assessment of drug–drug interaction
Potential drug–drug interactions are classified based on severity. DDI severity was classified as unknown (A), minor (B), moderate (C), or major (D), contraindicated (X). They were analysed for severity through Micromedex and Literature sources. As you proceed from A to X urgency in the action to be taken increases (A < B < C < D < X). In general, A and B are theoretically significant but not clinically important, whereas C and D always need to be managed. The risk category “A” indicates that there is no evidence of a pharmacological interaction, whereas the risk category “B” indicates that there is evidence of potential interactions but not enough to cause clinical concern. As a result, no action is required in either of these groups. When there is evidence of a potential interaction that is clinically significant, treatment monitoring is advised and are classified as category “C”, it means that what can be gained is much more significant than what might be risked. Dosage modifications are rarely necessary. Therapeutic modification, which may include dose modifications, alternative therapy considerations, and close supervision to reduce toxicities, is recommended for category “D”. Whereas, “X” represents the interactions where the co-administration should be avoided since the risks outweigh the benefits.
2.6. Statistical analysis of data
The unit of analysis was the individual subjects. Descriptive analysis was performed to assess absolute and relative frequencies of categorical variables. Continuous variables were represented as mean ± SD and categorical variables using frequencies/percentages. To evaluate the risk factors related to the presence of drug–drug interactions in prescription, the binary logistic regression analysis was performed; and investigation of the relationship between the occurrence of dependent variable that is drug–drug interaction and several independent risks factors such as gender, age, and diagnosis and number of drugs prescribed. Only statistically significant associations and plausible variables were considered for the logistic regression model. We used the adjusted odds ratio produced with 95% confidence interval as the result of the logistic regression. All the p values were obtained from two tailed test with a significance level of 0.05. All statistical analyses were accomplished using Statistical Package for the Social Sciences (SPSS version 22) for Windows.
3. Results
3.1. P-gp and CYP3A4 substrates, inhibitors, inducers observed in prescriptions
504 prescriptions were analysed for potential drug–drug interactions and in the prescription following list of P-gp and CYP3A4 substrates, inhibitors, inducers were observed (Table 2 and 3).
Table 2.
List of P-gp and CYP3A4 substrates, inhibitors and inducers observed in prescriptions.
| Inducers | Inhibitors | Substrates |
|---|---|---|
| P-GP | ||
| Aspirin | Atorvastatin | Clopidogrel |
| Diltiazem | Metformin | Pantoprazole |
| Spironolactone | Pioglitazone | Omeprazole |
| Carvedilol | Losartan | |
| Diltiazem | Digoxin | |
| Sodium valproate | Amitriptyline | |
| Pantoprazole | Teneligliptin | |
| Omeprazole | Propranolol | |
| Carvedilol | ||
| Acetaminophen | ||
| CYP3A4 | ||
| Phenytoin | Amlodipine | Atorvastatin |
| Losartan | ||
| Amitriptyline | ||
| Clopidogrel | ||
| Phenytoin | ||
| Amlodipine | ||
Table 3.
PDDI alter Bioavailability as well as enhance risk of toxicity.
| Drug Combinations | Clinical significance | Magnitude | Frequency observed | Possible Effects |
|---|---|---|---|---|
| Increased Bioavailability | ||||
| ∗Carvedilol + Digoxin (P-gp inhibitor) (P-gp substrate) | Moderate | AUC of Digoxin increased by 14% | 4 | Increased Bioavailability of Digoxin. |
| ∗Amlodipine + Atorvastatin (CYP3A4 inhibitor) (CYP3A4 substrate) | Moderate | AUC of atorvastatin increased by 15% | 76 | Increased Bioavailability of Atorvastatin. |
| ∗PPI's + Atorvastatin (P-gp inhibitors) (P-gp substrate) | Minor | AUC increased of Atorvastatin. | 28 | Increased Bioavailability of Atorvastatin. |
| PPI's + Digoxin (P-gp inhibitors) (P-gp substrate) | Minor | AUC of digoxin increased by at most 10% | 3 | Increased Bioavailability of Digoxin |
| Telmisartan + Digoxin (P-gp inhibitor) (P-gp substrate) | Minor | AUC of digoxin increased by 50% | 1 | Increased Bioavailability of Digoxin |
| Spironolactone + Digoxin (P-gp inhibitor) (P-gp substrate) | Minor | Renal Clearance of Digoxin of digoxin was reduced by 13% | 3 | Increased Bioavailability of Digoxin |
| Reduced Bioavailability | ||||
| Aspirin + Clopidogrel (P-gp inducer) (P-gp substrate) | Moderate | AUC of Clopidogrel decreased by 14% | 63 | Reduced Bioavailability of Clopidogrel |
| Phenytoin + Amlodipine (CYP3A4 inducer) (CYP3A4 substrate) | Major | A 10-fold decrease in AUC | 5 | Reduced Bioavailability of Amlodipine |
| DDIs enhance risk of toxicity | ||||
| PPI's + Atorvastatin (P-gp inhibitors) (P-gp substrate) | Minor | AUC increased of Atorvastatin. | 28 | Risk of myalgia, myotoxicity, myositis, or rhabdomyolysis |
| Amlodipine + Atorvastatin (CYP3A4 inhibitor) (CYP3A4 substrate) | Moderate | AUC of atorvastatin increased by 15% | 76 | Risk of myalgia, myotoxicity, myositis or rhabdomyolysis and acute kidney injury, hyperkalaemia, and myocardial infarction |
| Carvedilol + Digoxin (P-gp inhibitor) (P-gp substrate) | Moderate | AUC of Digoxin increased by 14% | 4 | Risk of Bradycardia |
Abbreviations: AUC: Area under curve, DDI: Drug–drug interactions.
Remarks: Some of DDI's also increase the risk of toxicity.
3.2. Demographic characteristics alters pDDIs
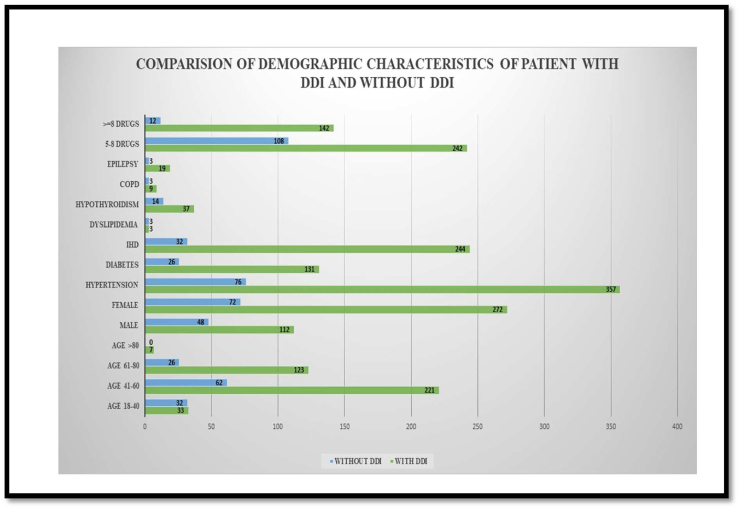
Medical and medication profiles of 504 patients were evaluated during the study period. Among them, 160 (31.7%) were males and 344 (68.3%) were females. The mean age of the study population was 54.6 ± 11.9 years, with majority of patients belonging to 41–60 age group. Most common comorbidities present in the study population were Hypertension (85.5%), IHD (54.6%), Diabetes mellitus (31.5%), Hypothyroidism (11.1%), and Epilepsy (4.4%). The mean number of comorbidities was 1.96 ± 0.87 per patient. The total number of medicines prescribed to the 504 patients was 3,384 with a mean number of medications received was 6.71 ± 1.60 per prescription and around 154 (30.6 %) patients received more than seven medications (Figure 4; Refer Supplementary material). Comparison of Demographic characteristic of patients with and without DDI are shown in Figure 2.
Figure 2.
Comparison of Demographic Characteristics in a Patient receiving P-gp and CYP3A4 modulators.
3.3. Possible pDDIs observed based on severity scale
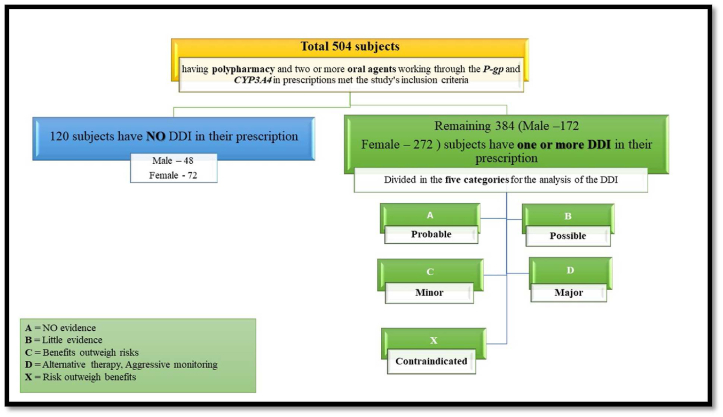
Out of the 504 prescriptions analysed, 384 (76.2%) prescriptions comprised of one or more potential drug interactions and it was found that 825 drug interactions were present, with male having 112 encounters and female having 272 encounters. Potential drug interactions detected within a single prescription were expressed as either the number of interacting drug pairs and frequency, shown in (Table 5; Refer Supplementary material, Figure 3). From the severity point of view, 1 of the potential DDI was major (D), 3 interactions were moderate (C), 4 were minor (B) and 19 were considered unknown, where future investigations are required (A).
Figure 3.
Drug–drug Interactions observed in the patients.
3.4. No. of drug–drug interactions based on prescription analysis during outpatient department
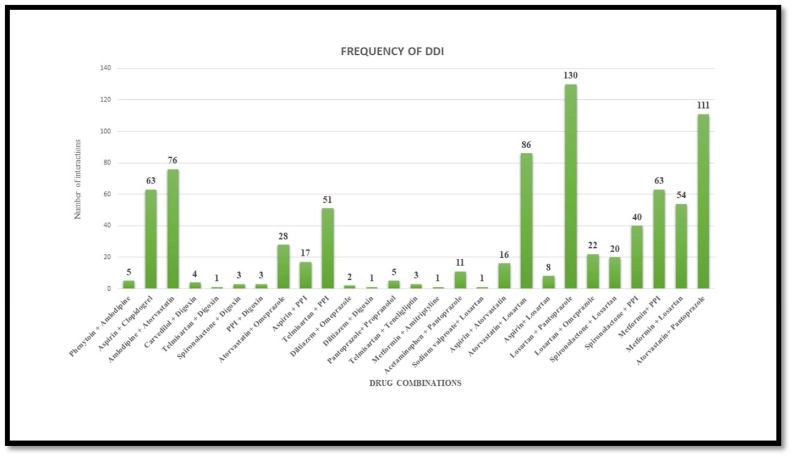
103 (23.4 %) patients were having 2 drug interactions in their prescription, 69 (13.7%) patients were having 3 drug interactions and 51 (10.1%) patients were having 4 or more interactions. 22 (4.4%) patients had ≥5 drug interactions in their prescription. The mean number of potential drug interactions seen in the prescription of these patients were 1.66 ± 1.48 per prescription. The category-wise number of drug interactions observed among all patients are shown in (Figure 5; Refer Supplementary material).
Most common drug pair to cause interaction of clinical concern was identified as Atorvastatin with Losartan (Category A, n = 86). 5 patients were prescribed drugs with one potential type D interaction Phenytoin and Amlodipine. Other important drug–drug interaction observed were Digoxin + Carvedilol (n = 4), Aspirin + Clopidogrel (n = 63), Amlodipine + Atorvastatin (n = 76) which belonged to category C (Table 5; Refer Supplementary material).
Significant correlations of the frequency of drug interactions with age, the number of medications, and the number of comorbidities (Pearson correlation, p < 0.01) were observed. Among the comorbidities, Hypertension and IHD was significantly correlated with the frequency of drug interactions.
Hypertension was predominantly associated with Drug–drug interaction followed by ischemic heart disease and Diabetes Mellitus (Figure 6; Refer Supplementary material). 51–60 age group was associated with the occurrence of more drug interactions followed by 41–50 age group (Figure 7; Refer Supplementary material).
3.5. Predictors related to drug–drug interactions OR associated factors for pDDIs
Multivariate logistic regression analysis showed that increased age is associated with the presence of drug interactions (OR 1.026; CI: 1.005–1.047; p = 0.016). Increased number of medications was independently associated with the occurrence of drug interactions (OR 1.747; CI: (1.401–2.179); p = 0.000). The presence of drug interactions was associated with the increased number of comorbidities (OR 1.735; CI: (1.247–2.414); p < 0.001). Among the comorbidities, Hypertension and Ischemic heart disease was independently associated with the occurrence of interactions. The presence of drug interactions was not associated with gender (Table 4).
Table 4.
Predictors of DDI's (Multivariate logistic analysis).
| Variables | Group | Patient with DDI | Patient without DDI | Adjusted Odds ratio | P value |
|---|---|---|---|---|---|
| Age | 18-≤40 | 33 | 32 | 1.026 (1.005–1.047) | 0.016 |
| 41–≤60 | 221 | 62 | |||
| 61–≤80 | 123 | 26 | |||
| >80 | 7 | 0 | |||
| Gender | Male | 112 | 48 | 0.614 (0.379–0.994) | 0.047 |
| Female | 272 | 72 | |||
| No of Disease Condition | <3 | 270 | 85 | 1.735 (1.247–2.414) | 0.001 |
| ≥3 | 111 | 15 | |||
| Type of Disease | Hypertension | 357 | 76 | 7.514 (4.159–13.574) | 0.000 |
| Diabetes | 131 | 26 | 2.216 (1.299–3.781) | 0.004 | |
| IHD | 244 | 32 | 5.315 (3.223–8.763) | 0.000 | |
| Number of medications | 5–<8 | 242 | 108 | 1.747 (1.401–2.179) | 0.000 |
| ≥8 | 142 | 12 |
Values of p < 0.05 were considered statistically significant.
Significant levels were seen in most of the variables except between the gender.
OR: Odds ratio, CI: Confidence interval.
4. Discussion
Potential drug–drug and drug-disease interactions are considered as major cause of adverse drug reactions (ADRs). These interactions frequently occurs when patients receive multiple medications. This is true for both ambulatory as well as hospitalized patients. In many cases, these DDIs may lead to several or untoward adverse effects and/or changes in therapeutic efficacies of the combined medicines, and thereby poor control of the diseases under treatment.
Despite of DDIs being modifiable drug induced problem, they could still induce clinico-pathological symptoms up to 11%, out of which 2.8% may require hospitalization [30]. In this study, 30.4% (n = 384) of prescriptions had one or more pDDIs, ranging from 1 to 8 pDDIs per prescription, which is supported by previously published retrospective studies carried out in outpatients showing the DDIs ranges from 8.3% to 63% [31, 32]. Having observed the prevalence of DDIs in outpatients’ population, we observed that the prevalence of pDDIs per patient was 1.66, which is similar to the findings reported in Ethiopia 1.63 and India 1.68 [33, 34].
This study revealed the mean number of medications received was 6.71 ± 1.60 per prescription with 30.6% of patients having more than seven medications in their prescription. This was in accordance to a study carried out in United States, which showed majority (82%) of the prescriptions for potentially interacting medications occurred in visits involving 5 or more medications [30].
According to the findings from this study, the relationship between the prevalence of probable DDIs per patient and increasing age prevailed. Consistent with the findings from previous reports of potential DDIs, older patients presented higher odds of exposure to DDI [35, 36]. Outpatients aged 65–74, 75–84, and ≥85 years showed increasing odds of exposure to potential DDIs than younger patients in a study [37]. Conclusively, as the patient age increases, likelihood of comorbidities increases resulting in a greater number of drugs prescribed, predisposing to higher number of pDDIs [34].
In the univariate analysis, we found there was no difference in the prevalence of probable pDDIs per patient pertaining to the gender, which was in agreement with the findings from previous studies [35, 36]. Patients in this study with higher number of diagnosed diseases were at an increased risk of the occurrence of DDI. Previous studies showed similar findings of a higher risk of developing probable DDIs in patients having more number of diagnosed diseases [35].
A relationship was also observed between the prevalence of potential DDIs per patient and increasing number of drugs prescribed [OR: 1.747; 95 percent CI: (1.401–2.179); p = 0.000], which is in accordance with the findings from other studies [31, 33].
Although not all pDDIs were equally detrimental, determining the severity of each pDDI was critical in determining its clinical significance and proper care. In this study, from the severity point of view, 1 of the DDI was major (D), 3 interactions were minor (C), 4 were possible (B) and 19 were considered probable (A). A tiny fraction of drug interactions was observed as risk category D which might be readily avoided by utilizing other drugs that are not linked to P-gp/CYP3A4 (Table 3).
The most common drug pair involved in DDI having clinical relevance was identified as Atorvastatin with Losartan. Statins such as Atorvastatin, Lovastatin, and Simvastatin have been found to act as both inhibitors and substrates of P-glycoprotein [38]. Losartan being the substrate of P-glycoprotein, an increase in the bioavailability of Losartan can be suspected when administered along with statins. Studies have shown that co-administration of Simvastatin with Losartan has significantly increased the AUC and Cmax of Losartan by 59.6% and 45.8% respectively in rats. The absolute bioavailability of Losartan also showed a remarkable increment of 59.6%. Since this interaction lacks any clinical evidence, the clinical outcomes cannot be anticipated in humans.
Other Important Drug–drug Interaction observed, having clinical relevancy as supported by previous clinical findings were, PHENYTOIN + AMLODIPINE (CATEGORY D). Studies have shown that potent inducers of CYP3A4 may significantly decrease the plasma concentration of Calcium Channel Blockers. In a study published by Maideen, the interaction between Dihydropyridine CCB's (Nifedipine, Amlodipine, Felodipine, Nicardipine, etc) and Phenytoin concluded that the potent inducer of CYP3A4 was found to decrease the bioavailability of dihydropyridine CCB, altering their therapeutic efficacy [39]. Michelucci et al. (1996) in their study to assess the effect of phenytoin on the pharmacokinetics of dihydropyridine CCB (Nisoldipine) have reported that phenytoin did increase the first-pass metabolism of Nisoldipine at a clinically significant level. Epileptic patients showed exceptionally low levels of nisoldipine even after doubling the dose of CCB. The observed Cmax of Nisoldipine in patients was 0.19 μg/L while in the control group, the Cmax was 1.06 μg/L (p < 0.002). The mean AUC was 10 fold lower in patients with epilepsy as compared to the control group [40]. Similar responses can be anticipated when other CYP3A4 inducers such as phenobarbital and carbamazepine are co-administered with dihydropyridine CCB's. The therapeutic efficacy of CCB's is compromised when administered with anti-epileptics which are potent inducers of CYP3A4.
CARVEDILOL + DIGOXIN (CATEGORY C), studies have confirmed that the administration of Carvedilol with Digoxin leads to increased bioavailability of Digoxin. Carvedilol's suppression of the intestinal and renal P-glycoprotein efflux transporters may result in increased absorption and decreased renal excretion of digoxin. De Mey et al. (1990) have reported that, on co-administration of 0.5 mg Digoxin with 25 mg Carvedilol, a subsequent increase in Cmax and AUC of Digoxin was observed. The increase in Cmax was by an average of 0.97 ng ml-1 but the plasma concentration of Digoxin after 24h was decreased to a lesser extent. The study concluded that the clinical relevance of the interaction with such dosing of both the drugs is likely to be less but the increased bioavailability of Digoxin was possibly due to increased vasodilation [41]. Wermeling et al. (1994) has reported that the AUC and Cmax of Digoxin were increased by 14% and 32% respectively, when administered together, with no change in Tmax when the given dose of Digoxin was 0.25 mg once daily and 25 mg once daily of Carvedilol. Hence, in conclusion, the concomitant use of Carvedilol with Digoxin may increase the therapeutic efficacy of Digoxin and is likely to cause bradycardia. Close monitoring is advised when the combination of these drugs is used. However, the benefits of the combination in treatment of heart failure and persistent atrial fibrillation are undeniable. Since, in some cases the benefits outweigh the risk, the combination can be prescribed in clinical settings along with periodical monitoring of the same.
ASPIRIN + CLOPIDOGREL (CATEGORY C), studies have shown that Aspirin increases the expression of P-glycoprotein both in-vitro (CaCo-2 cells) and in-vivo (rat intestine). When given in combination with Clopidogrel, Aspirin significantly reduced its absorption in CaCo-2 cells and rat intestine. Since the regulation of Clopidogrel is mediated by P-glycoprotein in the intestine, administration of Aspirin leads to overexpression of P-glycoprotein in the intestine leading to alterations in the absorption of Clopidogrel [42]. Jung et al. (2011) in their study concluded that prolonged use of Aspirin may cause decreased absorption of Clopidogrel when prescribed together, which was proven when aspirin-induced the expression of P-gp in CaCo-2 cells in-vitro and rat intestine in-vivo further decreasing Clopidogrel absorption remarkably in aspirin-treated CaCo-2 cells and in rat intestine [42]. In a clinical study by Liang et al. (2014), the effect of Aspirin 100 mg once daily was observed on Clopidogrel 75 mg before and after 2 and 4 weeks of administration. The results after aspirin pretreatment showed a 7.67-fold increase in the P-gp microRNA mir-27a (p = 0.004) and the AUC of Clopidogrel was decreased by 14%. However, the AUC of active metabolite remained unchanged and a 15% increase in relative platelet inhibition was observed (p = 0.002) [43]. These findings suggest that taking low-dose aspirin with Clopidogrel reduces its bioavailability but has no effect on its efficacy [44].
AMLODIPINE + ATORVASTATIN (CATEGORY C), Kellick et al. (2014) have reported that the concomitant use of Amlodipine (10 mg) with Atorvastatin (80 mg) caused the atorvastatin change in AUC by 15% [45]. Wang et al. (2016) examined the patients, who received CCBs with statins and have reported that, CCB (more likely, Amlodipine) increased acute kidney injury, hyperkalaemia, and myocardial infarction in patients who received metabolized statins (like simvastatin, lovastatin, and atorvastatin) [46]. Hence, it can be concluded that the concomitant use of Amlodipine along with Atorvastatin has significant pharmacokinetic as well as pharmacodynamic effects. A newer aspect with the same combination lies in the role of P-glycoprotein in drug–drug interaction. Zhou et al. (2014) reported that the interaction between both drugs occurs mainly due to hepatic inhibition of CYP3A4 and the combination can be prescribed in clinical settings keeping in mind accurate dosing and frequency of both the drugs with little to moderate monitoring [47].
STATINS + PPIs (CATEGORY B), when Atorvastatin or Simvastatin is co-prescribed with PPI's (mainly Omeprazole and Lansoprazole) there is a possibility of the increased bioavailability of statins owing to the inhibitory actions of proton pump inhibitor on P-glycoprotein. Atorvastatin acts as both substrate and inhibitor at different doses [38]. Syafhan et al. (2018) have reported that the competitive inhibitors of P-glycoprotein such as Lansoprazole and Omeprazole when co-administered with statins, may decrease the drug transportation into intestinal lumen thus increasing the bioavailability of statins like Atorvastatin and Simvastatin. In such cases, the chances of myalgia, myotoxicity, myositis, or rhabdomyolysis increase indicating statin toxicity [48]. Hence, patients on long-term therapy with statins and PPI's should be monitored for possible drug toxicity.
DIGOXIN + PPIs (CATEGORY B), similarly, when PPIs are co-prescribed with Digoxin which is a substrate of P-glycoprotein, increase in plasma levels of digoxin are reported. Pantoprazole undergoes both phase I metabolism of the parent drug and phase II conjugation of the active metabolite, whereas omeprazole is nearly fully metabolized by a phase I metabolic pathway, leaving almost no unaltered drug to be excreted. This discrepancy explains why pantoprazole is less likely than omeprazole to cause drug interactions [49]. Oosterhuis et al. (1991) have reported that the absorption of Digoxin (1 mg) did increase when co-administered with Omeprazole (20 mg). The observed increase in AUC and Cmax of Digoxin was at most 10%, indicating that the combination of both the drugs caused a minor increase in drug absorption of Digoxin. However, for the vast majority of patients, the intensity of this effect is not regarded as clinically important [50]. Pauli-Magnus et al. (2001) have reported that Omeprazole, Pantoprazole and Lansoprazole efficiently inhibited the transport of Digoxin into the intestinal lumen with IC50 values 17.7, 17.9, and 62.8 Μm respectively. The study proved that PPIs do inhibit the P-glycoprotein activity and result in increased plasma concentration of Digoxin [51].
TELMISARTAN + DIGOXIN (CATEGORY B), the Angiotensin II receptor blockers like Telmisartan, Losartan, Irbesartan, and Candesartan have the potential to inhibit intestinal P-gp, thus affecting the transport of substrate drugs [52]. Hence, when co-administered together there are chances of increased plasma concentrations of Digoxin. Stangier et al. (2000) have reported that when a single high dose of Telmisartan (120 mg once daily) is used with Digoxin (0.5 mg once followed by 0.25 mg daily for a week), the AUC, Cmax and trough concentration (Cmin) of Digoxin were increased by 50%, 22%, and 13% respectively. But since the trough plasma levels of Digoxin were not remarkably increased, it was concluded that the drug interaction was of little clinical significance [53]. Kamiyama et al. (2010) have reported that the interaction of ARBs with P-gp substrate- Digoxin in CaCo-2 cells increased the Cmax of Digoxin by 1.5-fold as compared to when Telmisartan was not added. The IC50 of Telmisartan was 2.19 μM. The findings suggested that the changes in the pharmacokinetics of Digoxin were mainly due to inhibition of P-glycoprotein in the intestine by Telmisartan [52].
SPIRONOLACTONE + DIGOXIN (CATEGORY B), various studies have confirmed the inhibitory action of Spironolactone on P-glycoprotein. When co-administered with Digoxin, it increased the plasma concentration of Digoxin. Hedman A. et al. (1992) showed that AUC of Digoxin increased and renal clearance of Digoxin was reduced by 13% however, biliary clearance of Digoxin was not affected [54]. Although no strong clinical evidence regarding the clinical outcome from this combination is available.
This study successfully identified the prevalence, pattern, and factors associated with pDDIs mediated by P-gp and CYP3A4 in the outpatient department of a tertiary care hospital. The overall prevalence of pDDIs was found to be 76% and the majority of the pDDIs were probable in severity. Furthermore, patients may also use over-the-counter drugs in addition to prescribed prescriptions, increasing the risk of drug interactions, therefore, healthcare professionals must educate patients and counsel them. Drug interactions can have an impact on one's quality of life, and clinically undetected drug interactions can lead to an increase in morbidity. Potential drug interactions should be checked for medications used to treat the most prevalent comorbidities, such as diabetes and hypertension.
4.1. Study limitations
The limitations of the study should not be overlooked. To begin with, the drug–drug interaction discovered were simply possible (it is unclear whether they caused any harm to patients). The study did not attempt to determine whether or not this was the case. It was not a multi-center study; we could not collect data from other hospital settings hence we are not sure of the prescribing patterns from other clinical settings. Lastly, only prescribed medications were included in this study, and most illicit, OTC, and herbal medicines were not included. Potential DDIs could greatly depend on the dose of each medicine provided. For example, the combination of Carvedilol and Digoxin caused an increase in the bioavailability of Digoxin due to the inhibitory actions of Carvedilol on P-gp. This effect was observed when Digoxin was administered at a dose of 0.25 mg once daily and Carvedilol, 25 mg once daily. However, in the data collected from observation at the hospital, patients have been prescribed Digoxin 0.25 mg once daily and Carvedilol 3.125 mg twice daily. Hence, the change in the pharmacokinetics of Digoxin may be subject to the doses prescribed.
This study was successful in identifying the frequency and pattern of pDDIs related to P-gp and CYP3A4 in Outpatient Department. The findings demonstrated that future investigations on pDDIs and actual incidence DDIs are possible. Apart from the known substrates, inducers, and inhibitors available, various newer drugs continue to add on the list. Many in-vitro and in-vivo studies are carried out to confirm numerous drugs and the role of P-glycoprotein/CYP3A associated with them. Such data serves as a starting point for investigating pharmacokinetic and pharmacodynamic effects of drugs on humans. A clinical study can further help to predict long term outcomes and also warn us about combinations of drugs to be avoided while prescribing.
5. Conclusions
The findings of the study give knowledge on DDIs related to efflux protein P-gp and CYP3A4 in outpatient setting. Small fraction of outpatient visits resulted in prescription combinations with the potential for clinically significant DDIs. More than four drug–drug interactions were found in a considerable number of patient prescriptions. The prescriber's understanding of P-gp and CYP3A4 related DDI and the identification of harm by that is the most effective strategy for reducing patient's suffering followed by appropriate intervention. Pharmacists can also help to reduce clinically significant DDIs by managing their medication therapy more effectively through periodical monitoring and patient education.
Declarations
Author contribution statement
Patel Krupa, Bhatt Masumi: Conceived and designed the experiments, Performed the experiments, Analyzed and interpreted the data.
Hirani Rajvi, Patel Vidheesha: Conceived and designed the experiments, Performed the experiments, Wrote the paper.
Patel Vishvas, Chorawala Mehul, Shah Gaurang: Conceived and designed the experiments, Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to express heartfelt gratitude to various physicians and residents of Sheth Vadilal Sarabhai General Hospital, for giving us the opportunity to conduct the research. Acknowledgment also extends to L. M. College of Pharmacy, Ahmedabad, Gujarat, India for its generous support throughout this project and to Dr. Raju Chaudhari, Associate Professor, M.G. Science Institute, Ahmedabad, for helping out with statistical plan.
Contributor Information
Krupa A. Patel, Email: krupapatel91223@gmail.com.
Masumi H. Bhatt, Email: bhattmasumi31@gmail.com.
Mehul R. Chorawala, Email: mehul.chorawala@lmcp.ac.in.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Ahmad A., et al. Evaluation of potential drug - drug interactions in general medicine ward of teaching hospital in Southern India. J. Clin. Diagn. Res. 2015;9 doi: 10.7860/JCDR/2015/11264.5608. FC10–FC13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Létinier L., et al. Risk of drug–drug interactions in out-hospital drug dispensings in France: results from the Drug–drug interaction prevalence study. Front. Pharmacol. 2019;10:1–9. doi: 10.3389/fphar.2019.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shetty V., et al. Evaluation of potential drug–drug interactions with medications prescribed to geriatric patients in a tertiary care hospital. J. Aging Res. 2018;2018 doi: 10.1155/2018/5728957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rana D., Suthar J., Malhotra S., Patel V., Patel P. A study of potential adverse drug–drug interactions among prescribed drugs in medicine outpatient department of a tertiary care teaching hospital. J. Basic Clin. Pharm. 2014;5:44. doi: 10.4103/0976-0105.134983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallo P., et al. Drug–drug interactions involving CYP3A4 and p-glycoprotein in hospitalized elderly patients. Eur. J. Intern. Med. 2019;65:51–57. doi: 10.1016/j.ejim.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Nigam S.K. What do drug transporters really do? Nat. Rev. Drug Discov. 2014;14:29–44. doi: 10.1038/nrd4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ince I., Knibbe C.A.J., Danhof M., De Wildt S.N. Developmental changes in the expression and function of cytochrome p450 3a isoforms: evidence from in vitro and in vivo investigations. Clin. Pharmacokinet. 2013;52:333–345. doi: 10.1007/s40262-013-0041-1. [DOI] [PubMed] [Google Scholar]
- 8.Cascorbi I. Arzneimittelinteraktionen: Prinzipien, Beispiele und klinische Folgen. Dtsch. Arztebl. Int. 2012;109:546–556. doi: 10.3238/arztebl.2012.0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y.T., Hao H.P., Liu C.X., Wang G.J., Xie H.G. Drugs as CYP3A probes, inducers, and inhibitors. Drug Metab. Rev. 2007;39:699–721. doi: 10.1080/03602530701690374. [DOI] [PubMed] [Google Scholar]
- 10.Yasuda K., et al. Interaction of cytochrome P450 3A inhibitors with P-glycoprotein. J. Pharmacol. Exp. Therapeut. 2002;303:323–332. doi: 10.1124/jpet.102.037549. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira R.J., Dos Santos D.J., Ferreira M.J.U. P-glycoprotein and membrane roles in multidrug resistance. Future Med. Chem. 2015;7:929–946. doi: 10.4155/fmc.15.36. [DOI] [PubMed] [Google Scholar]
- 12.Wandel C., Kim R., Wood M., Ch M.B.B., Wood A. Interaction of Morphine, Fentanyl, Sufentanil, Alfentanil, and Loperamide with the efflux drug transporter P-glycoprotein. Anesthesiology. 2002;96 doi: 10.1097/00000542-200204000-00019. [DOI] [PubMed] [Google Scholar]
- 13.Sharom F.J. Shedding light on drug transport: Structure and function of the P-glycoprotein multidrug transporter (ABCB1) Biochem. Cell. Biol. 2006;84:979–992. doi: 10.1139/o06-199. [DOI] [PubMed] [Google Scholar]
- 14.Prachayasittikul V., Prachayasittikul V. P-glycoprotein transporter in drug development. EXCLI J. 2016;15:113–118. doi: 10.17179/excli2015-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodges L. Vol. 21. 2011. Very important phamacogene summary ABCB1; pp. 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrew Finch P.P. P-glycoprotein and its role in drug–drug interactions. Aust. Prescr. 2014;37:137–139. [Google Scholar]
- 17.Bachmakov I., Werner U., Endress B., Auge D., Fromm M.F. Characterization of β-adrenoceptor antagonists as substrates and inhibitors of the drug transporter P-glycoprotein. Fundam. Clin. Pharmacol. 2006;20:273–282. doi: 10.1111/j.1472-8206.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- 18.Lin Y.C., Ellingrod V.L., Bishop J.R., Miller D.D. The relationship between P-glycoprotein (PGP) polymorphisms and response to olanzapine treatment in schizophrenia. Ther. Drug Monit. 2006;28:668–672. doi: 10.1097/01.ftd.0000246761.82377.a6. [DOI] [PubMed] [Google Scholar]
- 19.Silva R., et al. Modulation of P-glycoprotein efflux pump: induction and activation as a therapeutic strategy. Pharmacol. Therapeut. 2015;149 doi: 10.1016/j.pharmthera.2014.11.013. 1–14123. [DOI] [PubMed] [Google Scholar]
- 20.Ghanem C.I., et al. Induction of rat intestinal P-glycoprotein by Spironolactone and its effect on absorption of orally administered digoxin. J. Pharmacol. Exp. Therapeut. 2006;318:1146–1152. doi: 10.1124/jpet.106.105668. [DOI] [PubMed] [Google Scholar]
- 21.Zhou S., et al. Mechanism-based inhibition of cytochrome P450 3A4 by therapeutic drugs. Clin. Pharmacokinet. 2005;44:279–304. doi: 10.2165/00003088-200544030-00005. [DOI] [PubMed] [Google Scholar]
- 22.Wilson A., et al. Crohn’s disease is associated with decreased CYP3A4 and P-glycoprotein protein expression. Mol. Pharm. 2019;16:4059–4064. doi: 10.1021/acs.molpharmaceut.9b00459. [DOI] [PubMed] [Google Scholar]
- 23.Xie F., Ding X., Zhang Q.Y. An update on the role of intestinal cytochrome P450 enzymes in drug disposition. Acta Pharm. Sin. B. 2016;6:374–383. doi: 10.1016/j.apsb.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan L.M.S., Cooper A.E., Dudley A.L.J., Ford D., Hirst B.H. P-glycoprotein Potentiates CYP3A4-mediated drug disappearance during Caco-2 intestinal secretory detoxification. J. Drug Target. 2004;12:405–413. doi: 10.1080/10611860412331285224. [DOI] [PubMed] [Google Scholar]
- 25.Marquez B., Van Bambeke F. ABC multidrug transporters: target for modulation of drug pharmacokinetics and drug–drug interactions. Curr. Drug Targets. 2011;12:600–620. doi: 10.2174/138945011795378504. [DOI] [PubMed] [Google Scholar]
- 26.Ieiri I. Functional significance of genetic polymorphisms in P-glycoprotein (MDR1, ABCB1) and breast cancer resistance protein (BCRP, ABCG2) Drug Metabol. Pharmacokinet. 2012;27:85–105. doi: 10.2133/dmpk.dmpk-11-rv-098. [DOI] [PubMed] [Google Scholar]
- 27.Eagling V.A., Profit L., Back D.J. Inhibition of the CYP3A4-mediated metabolism and P-glycoprotein-mediated transport of the HIV-I protease inhibitor saquinavir by grapefruit juice components. Br. J. Clin. Pharmacol. 1999;48:543–552. doi: 10.1046/j.1365-2125.1999.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armahizer M.J., Kane-gill S.L., Smithburger P.L., Anthes A.M., Seybert A.L. 1–7. 2013. Comparing Drug–drug Interaction Severity Ratings between Bedside Clinicians and Proprietary Databases. 2013. [Google Scholar]
- 29.Teixeira R., Nascimento Y. de A., Crespo D. Safety aspects of protease inhibitors for chronic hepatitis C: adverse events and drug-to-drug interactions. Braz. J. Infect. Dis. 2013;17:194–204. doi: 10.1016/j.bjid.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aparasu R., Baer R., Aparasu A. Clinically important potential drug–drug interactions in outpatient settings. Res. Soc. Adm. Pharm. 2007;3:426–437. doi: 10.1016/j.sapharm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Lin C.F., Wang C.Y., Bai C.H. Polypharmacy, aging and potential drug–drug interactions in outpatients in Taiwan: a retrospective computerized screening study. Drugs Aging. 2011;28:219–225. doi: 10.2165/11586870-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 32.Mandavi Kashyap, Sanjay D’cruz, Atul Sachdev, P. T. Drug–drug interactions and their predictors: Results from Indian elderly inpatients. [DOI] [PMC free article] [PubMed]
- 33.Safety P. 2021. Assessment of Potential Drug – Drug Interactions and Their Predictors in Chronic Outpatient Department of Dessie Referral Hospital, Dessie, Northeast Ethiopia; pp. 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chavda N.B., Solanky P.P., Baria H., Naik R., Bharti K. Vol. 5. 2015. Study of potential drug – drug interaction between prescribed drugs in patients attending outpatient department of medicine at tertiary-care hospital in south Gujarat region; pp. 236–242. [Google Scholar]
- 35.Obreli-Neto P.R., et al. Adverse drug reactions caused by drug–drug interactions in elderly outpatients: a prospective cohort study. Eur. J. Clin. Pharmacol. 2012;68:1667–1676. doi: 10.1007/s00228-012-1309-3. [DOI] [PubMed] [Google Scholar]
- 36.Janchawee B., Owatranporn T., Mahatthanatrakul W., Chongsuvivatwong V. Clinical drug interactions in outpatients of a university hospital in Thailand. J. Clin. Pharm. Therapeut. 2005;30:583–590. doi: 10.1111/j.1365-2710.2005.00688.x. [DOI] [PubMed] [Google Scholar]
- 37.Nobili A., et al. 2009. Potentially Severe Drug Interactions in Elderly Outpatients : Results of an Observational Study of an Administrative Prescription Database; pp. 377–386. [DOI] [PubMed] [Google Scholar]
- 38.Holtzman C.W., Wiggins B.S., Spinler S.A. Role of P-glycoprotein in statin drug interactions. Pharmacotherapy. 2006;26:1601–1607. doi: 10.1592/phco.26.11.1601. [DOI] [PubMed] [Google Scholar]
- 39.Drug interactions of dihydropyridine Calcium Channel blockers (CCBs) involving CYP3A4 enzymes. Eur. J. Med. 2019;7 [Google Scholar]
- 40.Michelucci R., et al. Reduced plasma nisoldipine concentrations in phenytoin-treated patients with epilepsy. Epilepsia. 1996;37 doi: 10.1111/j.1528-1157.1996.tb01032.x. [DOI] [PubMed] [Google Scholar]
- 41.De Mey C., Brendel E., Enterling D. Carvedilol increases the systemic bioavailability of oral digoxin. Br. J. Clin. Pharmacol. 1990;29:486–490. doi: 10.1111/j.1365-2125.1990.tb03668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jung K.H., et al. Prolonged use of aspirin alters human and rat intestinal cells and thereby limits the absorption of clopidogrel. Clin. Pharmacol. Ther. 2011;90:612–619. doi: 10.1038/clpt.2011.163. [DOI] [PubMed] [Google Scholar]
- 43.Oh J., et al. Aspirin decreases systemic exposure to clopidogrel through modulation of P-glycoprotein but does not alter its antithrombotic activity. Clin. Pharmacol. Ther. 2014;95:608–616. doi: 10.1038/clpt.2014.49. [DOI] [PubMed] [Google Scholar]
- 44.Zhou Q., et al. Pharmacokinetic drug interactions with clopidogrel: updated review and risk management in combination therapy. Therapeut. Clin. Risk Manag. 2015;11:449. doi: 10.2147/TCRM.S80437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kellick K.A., Bottorff M., Toth P.P. A clinician ’ s guide to statin drug–drug interactions. J. Clin. Lipidol. 2014;8 doi: 10.1016/j.jacl.2014.02.010. S30–S46. [DOI] [PubMed] [Google Scholar]
- 46.Neuvonen P.J., Niemi M., Backman J.T. Drug interactions with lipid-lowering drugs: mechanisms and clinical relevance. Clin. Pharmacol. Ther. 2006;80:565–581. doi: 10.1016/j.clpt.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 47.Zhou Y.T., et al. Pharmacokinetic drug–drug interactions between 1,4-dihydropyridine calcium channel blockers and statins: factors determining interaction strength and relevant clinical risk management. Therapeut. Clin. Risk Manag. 2014;10:17–26. doi: 10.2147/TCRM.S55512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Syafhan N.F., Augustine M., Ramadhani U., Hersunaryati Y. Proton-pump inhibitor use and potential drug interactions in outpatients. Int. J. Appl. Pharm. 2018;10:358–363. [Google Scholar]
- 49.Doligalski C.T., Logan A.T., Silverman A. vol. 8. 2012. Drug Interactions: A Primer for the Gastroenterologist. 376 Gastroenterology & Hepatology. [PMC free article] [PubMed] [Google Scholar]
- 50.Oosterhuis B., Jonkman J., Andersson T., Zuiderwijk P., Jedema J. Minor effect of multiple dose omeprazole on the pharmacokinetics of digoxin after a single oral dose. Br. J. Clin. Pharmacol. 1991;32:569–572. doi: 10.1111/j.1365-2125.1991.tb03953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pauli-Magnus C., Rekersbrink S., Klotz U., Fromm M.F. Interaction of omeprazole, lansoprazole and pantoprazole with P-glycoprotein. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2001;364:551–557. doi: 10.1007/s00210-001-0489-7. [DOI] [PubMed] [Google Scholar]
- 52.Kamiyama E., Nakai D., Mikkaichi T., Okudaira N., Okazaki O. Interaction of angiotensin II type 1 receptor blockers with P-gp substrates in Caco-2 cells and hMDR1-expressing membranes. Life Sci. 2010;86:52–58. doi: 10.1016/j.lfs.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 53.Stangier J., et al. The effect of telmisartan on the steady-state pharmacokinetics of digoxin in healthy male volunteers. J. Clin. Pharmacol. 2000;40:1373–1379. [PubMed] [Google Scholar]
- 54.Hedman A., Angelin B., Arvidsson A., Dahlqvist R. Digoxin-interactions in man: Spironolactone reduces renal but not biliary digoxin clearance. Eur. J. Clin. Pharmacol. 1992;42:481–485. doi: 10.1007/BF00314854. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.