Abstract
Nasopharyngeal carcinoma (NPC) is not only a common malignant disease of the head and neck, but also presented as locoregionally advanced NPC at diagnosis with poor prognosis. The efficacy of current chemoradiotherapy is unsatisfactory; therefore, in this study, we evaluated the safety and efficacy of treating locally advanced NPC using recombinant human endostatin injection (Endostar), combined with a cisplatin plus 5-fluorouracil (PF) regimen and sequential intensity-modulated radiotherapy (IMRT), and compared it with PF plus IMRT regimen. This phase II study included 83 eligible patients with stages III-IVa NPC (8th AJCC/UICC) who were randomized 1:1 into control (n = 42) and experimental (n = 41) groups. The control group received PF chemotherapy and IMRT for locally advanced NPC; One cycle of induction chemotherapy (IC) was administered before IMRT, and three cycles of adjuvant chemotherapy (AC) were administered four weeks post-radiotherapy. The experimental group received additional Endostar therapy. All patients were followed up for at least 5 years. The primary endpoints were progression-free survival (PFS) and the objective response rate. The secondary endpoints included overall survival and treatment-related toxicities. The short-term efficacy was evaluated at the end of the fourth chemotherapy cycle. Our results showed that the complete response rate of nasopharyngeal lesions was not significantly different between the experimental and control groups (80.5 vs. 71.4%, P = 0.335); however, there were significant differences in the complete response rates of cervical metastatic lymph nodes (75.6 vs. 40.5%, P = 0.001), especially for cervical N3 lymph nodes in the experimental group (55.6 vs. 9.5%, P = 0.004). The overall median follow-up time was 69.7 months. Patients in the experimental group showed significantly prolonged PFS by about four months (hazard ratio [HR] = 0.64, 95% CI: 0.41-0.99, P = 0.045). There was no significant difference in the median overall survival (P = 0.374). Furthermore, subgroup analysis indicated that the risk of death in patients with cervical N3 lymph nodes in the experimental group was reduced by 52% (HR = 0.48, 95% CI: 0.23-0.99, P = 0.046). Moreover, the incidence of radiation-induced grades 3-4 oral mucositis was significantly lower in the experimental group (29.3% vs. 54.8%, P = 0.019), while no significant differences in other severe adverse reactions were observed between the two groups (P>0.05). Taken together, our study indicated that, in patients with locally advanced NPC, Endostar in combination with PF chemotherapy and sequential IMRT significantly improved PFS, had tolerable treatment-related toxicities, improved the prognoses of patients with cervical N3 lymph nodes, and reduced the incidence of radiation-related oral mucositis.
Keywords: Locally advanced nasopharyngeal carcinoma, Endostar, chemotherapy, intensity-modulated radiotherapy, efficacy, safety
Introduction
Nasopharyngeal carcinoma (NPC) is a common malignant disease of the head and neck, with a unique and unbalanced endemic distribution, especially in South China [1]. More than 70% of the new cases of NPC are diagnosed as locoregionally advanced NPC and have an unsatisfactory prognosis [2]. Platinum-based concurrent chemoradiotherapy (CCRT) is considered the standard treatment for locoregionally advanced NPC [3]; however, the high incidence of treatment--related toxicities and the poor compliance in CCRT compromise the efficacy of CCRT [4]. In addition, it has been reported that both induction chemotherapy (IC) and adjuvant chemotherapy (AC) fail to reduce the distant metastasis rate or to improve the overall survival (OS) rate, except showing progression-free survival (PFS) benefit [5,6]. Indeed, 30% of patients die of distant metastasis, the primary indicator of treatment failure [7,8]. Therefore, it is imperative to identify more effective combination therapies and treatment regimens.
It has been known that neovascularization is important for the proliferation and metastasis of solid tumors [9]. Vascular endothelial growth factor (VEGF) is a tumor-inducing factor that promotes angiogenesis, tumorigenesis, and metastasis [10]. It is commonly overexpressed in NPC and attributes to the poor survival outcome of patients with NPC [11]. The results from the clinical trial RTOG0615 have indicated that the combination of bevacizumab and the standard chemoradiation treatment is safe and effective for patients with NPC, suggesting the prospect of applying targeted anti-angiogenesis therapy in NPC [12]. In addition, recombinant human endostatin injection (Endostar) has been independently developed based on the anti-angiogenesis activity of endostatin by Chinese researchers. Endostar has an additional nine-amino acid sequence at the N-terminus of endostatin that forms his-tag structure. A multicenter phase III clinical study showed that Endostar combined with standard chemotherapy had significant survival benefit in patients with advanced non-small cell lung cancer [13]. Encouraged by the promising results of Endostar in lung carcinoma, Endostar has been approved by China’s State Food and Drug Administration for treating non-small cell lung cancer in 2005. However, there are few clinical studies on the use of radiotherapy and chemotherapy combined with Endostar in the treatment of locally advanced NPC.
Therefore, we conducted a multicenter, randomized controlled, phase II clinical trial to evaluate the survival benefit and safety of Endostar combined with chemotherapy and sequential intensity-modulated radiotherapy (IMRT) for locally advanced NPC. This study provides new insights into the application of Endostar as a novel therapy strategy for locally advanced NPC.
Materials and methods
Study design and patients
This randomized, open, multicenter, phase II clinical trial was approved by the Medical Ethics Committee of Yichang Central People’s Hospital and conducted according to the tenets of the Declaration of Helsinki. The study was registered at ClinicalTrials.gov (NCT02444949). Written informed consent was obtained from all patients. The study design was shown in Figure 1.
Figure 1.

Trial flow diagram.
The study was conducted at four medical centers in Hubei Province from May 2015 to June 2016. Patients were deemed eligible if they met the following criteria: (1) newly diagnosed and pathologically confirmed NPC (stages III-IVa except T3N0; 8th American Joint Commission on Cancer staging system) and with indications for chemoradiotherapy; (2) age ranging from 18 to 70 years; (3) Eastern Cooperative Oncology Group (ECOG) score 0-1; (4) at least one measurable lesion (lesion diameter ≥20 mm) on magnetic resonance imaging (MRI) scan according to the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1; (5) normal routine blood test result and normal liver, kidney, and heart functions; (6) at least 3 months of expected survival. The exclusion criteria were: (1) previous allergic reaction to biological agents; (2) receiving other anticancer treatments; and (3) other conditions, including any of the following: uncontrolled symptoms of central nervous system metastases, significant organ dysfunction, and severe heart disease (including congestive heart failure, uncontrolled arrhythmia, angina requiring long-term medical therapy, valvular heart disease, and myocardial infarction), resistant hypertension, pregnant or lactating women, non-healing infectious wounds, or a history of uncontrolled mental illness.
Treatment protocol
The patients in the experimental group received IC administered as one cycle of PF regimen (cisplatin 25 mg/m2 on days 1-3 and 5-fluorouracil 600 mg/m2 on days 1-5) with concurrent Endostar infusion. Endostar was administered at 30 mg daily via a continuous intravenous pump for 5 days (150 mg Endostar was added to 200 mL of normal saline and infused using a 250 mL pump). IMRT started on day 6 and was administered 5 times per week. After a 4-week break at the end of radiotherapy, Endostar plus PF regimen was administered as AC for up to 3 cycles (a duration of 3 weeks was considered one cycle) unless progressive disease or unacceptable toxic effects had occurred earlier in the study. The patients were subsequently followed up. The patients in the control group underwent the same procedures but without Endostar infusion, and then patients were followed up.
Radiotherapy was performed with image-guided radiotherapy using whole-target IMRT. The gross target volume (GTV) was delineated to include the entire dimension of the neoplastic lesion on imaging, endoscopy, and physical examination. GTVnx and GTVnd were defined as the GTV of the primary tumor and that of the metastatic lymph nodes in the neck, respectively. Clinical target volumes 1 and 2 were high-risk and low-risk clinical target regions, respectively. The planning target volume (PTV) was calculated as the clinical target volume expanded 3-5 mm externally and corrected appropriately in vital organs.
The target-region prescription doses were determined as follows: for T1-2: PGTVnx = 70.4 GY/2.2 GY/F × 32 F, PGTVnd = 70.4 GY/2.2 GY/F × 32 F, PTV1 = 60.16 GY/1.88 GY/F × 32 F, and PTV2 = 55.04 GY/1.72 GY/F × 32 F; for T3-4: PGTVnx = 73.6 GY/2.3 GY/F × 32 F, PGTVnd = 70.4 GY/2.2 GY/F × 32 F, PTV1 = 60.16 GY/1.88 GY/F × 32 F, and PTV2 = 55.04 GY/1.72 GY/F × 32 F. The colony-stimulating factor was used to treat grade ≥2 hematological toxicities, and treatment interruption was allowed for any intolerable severe chemoradiotherapy reactions.
Patient evaluation and follow-up
Patients were considered evaluable for the assessment of treatment response if they received at least two cycles of AC after radiotherapy. The short-term efficacy evaluation was based on the imaging results after the completion of the last AC. The results were divided into complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) according to the RECIST version 1.1. The objective response rate was calculated as the sum of the CR and PR rates.
The patients were followed up once every 3 months for 2 years and once every 6 months thereafter. Follow-up comprised a routine blood test, plain and enhanced MRI scan of the nasopharynx and neck, and computed tomography of the lung and upper abdomen. Brain MRI and bone emission computed tomography were performed as needed.
The primary endpoint was PFS, which was calculated from the registration date to the first observation of progressive disease or death due to any cause, whichever occurred first. Secondary endpoints included OS, which was defined as the date from registration to death due to any cause, and treatment toxicities (adverse reactions to radiotherapy and chemotherapy graded according to the Common Terminology Criteria for Adverse Events version 4.0).
Statistical analysis
Pearson’s chi-squared test or Fisher’s exact test and Student’s t-test were performed in SPSS to evaluate the differences in variables and treatment toxicities between the two groups. Kaplan-Meier survival curves were generated using the survminer R package (version 0.4.9) and compared using the log-rank test between the two groups. The Cox proportional hazards model was used in univariate and multivariate analyses to evaluate independent prognostic factors associated with OS and PFS and to calculate the corresponding 95% confidence intervals (CIs). The Cox PH assumption was verified using statistical tests based on the Schoenfeld residuals. Subsequently, subgroup analysis was performed to evaluate the survival benefit in each subgroup. All statistical testing was two-sided, with the significance level set at P<0.05. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 26.0 (IBM Corp.; Armonk, NY, USA) or R software version 4.1.1 (R Foundation; Vienna, Austria).
Results
Patient characteristics
From May 2015 to June 2016, 86 patients diagnosed with locally advanced NPC were enrolled in four medical centers in Hubei Province. Three patients were excluded because two did not meet the inclusion criteria, and one refused to participate. Therefore, 83 patients were randomly divided into two groups: the experimental group (41) and the control group (42), received the assigned treatment, and were evaluable for treatment response, outcomes, and toxicities. The median patient age was 52 years (range: 22-70), and there were 61 men and 22 women. In total, 33 patients (39.8%) were classified as T1/T2, while 50 patients (60.2%) were classified as T3/T4. Most patients were diagnosed as N2 (47%) and N3 (47%), while 32 (38.6%) and 51 (61.4%) patients were diagnosed with stage III and stage IVa NPC, respectively. The control group had 17 stage III (40.5%) and 25 stage IVa (59.5%) NPC cases. The experimental group had 15 patients (36.6%) with stage III and 26 (63.4%) with stage IVa NPC. Some patients in the experimental (n = 3) and control (n = 2) groups completed only two cycles of AC after IMRT due to treatment-related toxicities; the remaining patients completed the treatment plan. All patients were restaged according to the 8th edition of the UICC/AJCC staging system, and the clinical characteristics were well balanced between these two groups (all P>0.05, Table 1).
Table 1.
Clinical characteristics of 83 patients with locally advanced nasopharyngeal carcinoma (NPC)
| Characteristic | Experimental group (n = 41) | Control group (n = 42) | P | |
|---|---|---|---|---|
| Gender | Male | 30 | 31 | 0.947 |
| Female | 11 | 11 | ||
| Age (Years) | Range | 23-70 | 22-70 | 0.297 |
| ECOG score | 0 | 11 | 8 | 0.399 |
| 1 | 30 | 34 | ||
| Pathological types | Keratinized carcinoma | 0 | 1 | 0.676 |
| Non-keratinizing carcinoma | 38 | 37 | ||
| Undifferentiated type | 2 | 4 | ||
| Adenoid cystic carcinoma | 1 | 0 | ||
| Clinical stagea | III | 15 | 17 | 0.716 |
| IVa | 26 | 25 | ||
| T staging | T1 | 3 | 4 | 0.910 |
| T2 | 14 | 12 | ||
| T3 | 15 | 15 | ||
| T4 | 9 | 11 | ||
| N staging | N1 | 3 | 2 | 0.830 |
| N2 | 20 | 19 | ||
| N3 | 18 | 21 |
Staging is based on the American Joint Commission on Cancer (AJCC) eighth edition of the TNM staging system in 2018.
Short-term efficacy
The short-term treatment efficacy after completing the last AC was evaluated in these two groups. Based on the results for the cervical metastatic lymph nodes, CR and PR were confirmed in 31 (75.6%) and 10 (24.4%) cases, respectively, in the experimental group, while 17 (40.5%) and 25 (59.5%) cases, respectively, in the control group, indicating a significantly improved CR rate for lymph nodes in the experimental group (P = 0.001). In addition, the CR rate of cervical N3 lymph nodes in the experimental group was significantly higher than in the control group (55.6% vs. 9.5%, P = 0.004). However, there was no significant difference for curative efficacy on nasopharyngeal lesions between the two groups (P = 0.335), as the cases for CR and PR were 33 (80.5%) and 8 (19.5%), respectively, in the experimental group, while 30 (71.4%) and 12 (28.6%), respectively, in the control group (Table 2).
Table 2.
Short-term efficacy after completion of treatment
| Nasopharyngeal lesions | Cervical metastatic lymph nodes | Cervical N3 lymph nodes | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||||
| Experimental group n (%) | Control group n (%) | χ2 | P | Experimental group n (%) | Control group n (%) | χ2 | P | Experimental group n (%) | Control group n (%) | χ2 | P | |||
| CR | 33 (80.5) | 30 (71.4) | 0.931 | 0.335 | CR | 31 (75.6) | 17 (40.5) | 10.501 | 0.001 | CR | 10 (55.6) | 2 (9.5) | - | 0.004 |
| PR | 8 (19.5) | 12 (28.6) | PR | 10 (24.4) | 25 (59.5) | PR | 8 (44.4) | 19 (90.5) | ||||||
CR, complete response; PR, partial response.
Based on MRI results, the local lesions in both the experimental and the control groups were significantly relieved after treatment compared with pre-treatment images (Figure 2). However, in patients with cervical N3 lymph nodes, the therapeutic efficacy in the experimental group was significantly better than that in the control group (Figure 3).
Figure 2.
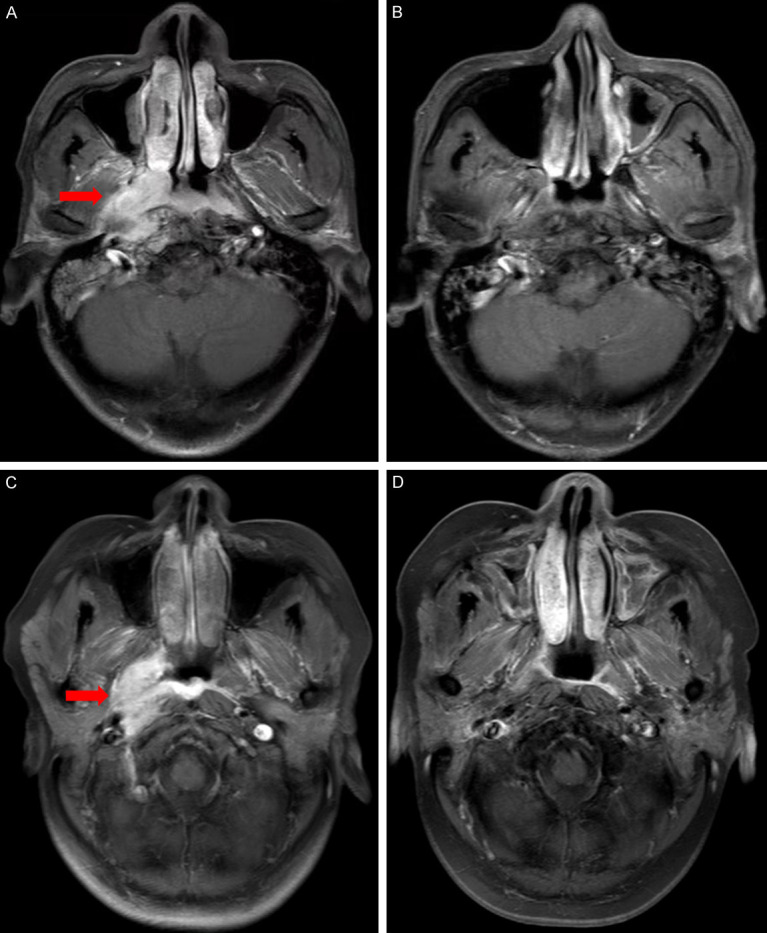
MRI of nasopharyngeal lesions in experimental group and control group before and after treatment. Representative MRI images before and after treatment for experimental and control group. (A) Baseline MRI of nasopharyngeal lesions in a 56-year-old man with nasopharyngeal carcinoma in the experimental group, (B) Reexamination of MRI after treatment showed complete response (CR). (C) Baseline MRI of nasopharyngeal lesions in a 53-year-old woman with nasopharyngeal carcinoma in the control group, (D) Reexamination of MRI after treatment showed complete response (CR).
Figure 3.
MRI of cervical N3 lymph nodes before and after treatment in experimental group and control group. (A) Baseline MRI of cervical N3 lymph nodes in a 61-year-old man with nasopharyngeal carcinoma in the experimental group, (B) Reexamination of MRI after treatment showed complete response (CR) of cervical N3 lymph nodes. (C) Baseline MRI of cervical N3 lymph nodes in a 59-year-old woman with nasopharyngeal carcinoma in the control group, (D) Reexamination of MRI after treatment showed partial response (PR) of cervical N3 lymph nodes.
Long-term efficacy
By the last follow-up date of June 30, 2021, the median patient follow-up time was 69.7 months (range: 15.30-77.50 months), with 1 patient in the experimental group and 2 patients in the control groups loss to follow-up. Our results showed that the patients in the experimental group significantly benefited from the combination therapy with Endostar as the median PFS was 25.6 months in the experimental group, while it was 21.4 months in the control group (HR = 0.64, 95% CI: 0.41-0.99, P = 0.045, Figure 4), There was no significant difference in the median OS between these two groups (50.8 vs. 45.6 months, HR = 0.78, 95% CI: 0.46-1.34, P = 0.374, Figure 5).
Figure 4.
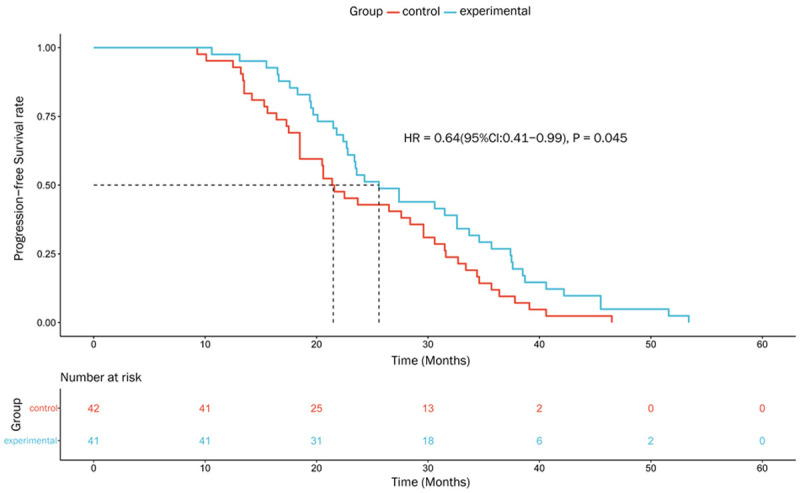
Progression-free survival curves of two groups of patients with locally advanced nasopharyngeal carcinoma.
Figure 5.

Overall survival curves of two groups of patients with locally advanced nasopharyngeal carcinoma.
We also conducted univariable and multivariate analyses to explore the prognostic predictors in our trial and found that clinical stage IVa was an independent negative predictor for PFS (P<0.001, Table 3) and OS (P<0.001, Table 4). In addition, a benefit in PFS was observed for patients in the experimental group from multivariate analyses (HR = 0.53, 95% CI: 0.34-0.84; P = 0.006, Table 3). However, this benefit was not observed in OS (P = 0.374, Table 4).
Table 3.
Cox regression analysis of PFS
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Age (years) | ||||||
| <52 | 1 | |||||
| ≥52 | 0.86 | 0.56-1.33 | 0.497 | |||
| Gender | ||||||
| Male | 1 | |||||
| Female | 0.66 | 0.4-1.09 | 0.107 | |||
| Pathology | ||||||
| Non-keratinizing | 1 | |||||
| Keratinized | 92.69 | 5.77-1488.4 | 0.001 | 43.05 | 2.67-695.38 | 0.008 |
| Undifferentiated | 2.7 | 1.15-6.33 | 0.022 | 1.73 | 0.68-4.35 | 0.248 |
| Adenoid cystic | 1.97 | 0.27-14.46 | 0.504 | 1.09 | 0.1-11.6 | 0.946 |
| Clinical stage | ||||||
| III | 1 | |||||
| IVa | 5.58 | 3.13-9.96 | <0.001 | 5.54 | 2.29-13.4 | <0.001 |
| T staging | ||||||
| T1-2 | 1 | |||||
| T3-4 | 1.45 | 0.93-2.28 | 0.101 | |||
| N staging | ||||||
| N1 | 1 | |||||
| N2 | 0.78 | 0.3-2.04 | 0.619 | 0.58 | 0.19-1.73 | 0.326 |
| N3 | 2.87 | 1.11-7.4 | 0.029 | 0.69 | 0.19-2.6 | 0.589 |
| ECOG socre | ||||||
| 0 | 1 | |||||
| 1 | 0.8 | 0.47-1.34 | 0.393 | |||
| Group | ||||||
| Control | 1 | |||||
| Experimental | 0.64 | 0.41-0.99 | 0.045 | 0.53 | 0.34-0.84 | 0.006 |
Table 4.
Cox regression analysis of OS
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Age (years) | ||||||
| <52 | 1 | |||||
| ≥52 | 0.88 | 0.52-1.51 | 0.647 | |||
| Gender | ||||||
| Male | 1 | |||||
| Female | 0.85 | 0.46-1.56 | 0.591 | |||
| Pathology | ||||||
| Non-keratinizing | 1 | |||||
| Keratinized | 5.97 | 0.78-45.6 | 0.085 | 3.9 | 0.51-29.91 | 0.19 |
| Undifferentiated | 2.78 | 1.18-6.54 | 0.02 | 2.21 | 0.93-5.23 | 0.072 |
| Adenoid cystic | 1.59 | 0.22-11.59 | 0.649 | 0.91 | 0.12-6.69 | 0.926 |
| Clinical stage | ||||||
| III | 1 | |||||
| IVa | 4.53 | 2.36-8.7 | <0.001 | 4.33 | 2.24-8.36 | <0.001 |
| T staging | ||||||
| T1-2 | 1 | |||||
| T3-4 | 1.71 | 0.97-3.01 | 0.064 | |||
| N staging | ||||||
| N1 | 1 | |||||
| N2 | 1.51 | 0.35-6.46 | 0.576 | |||
| N3 | 3.96 | 0.94-16.6 | 0.06 | |||
| ECOG socre | ||||||
| 0 | 1 | |||||
| 1 | 0.79 | 0.43-1.45 | 0.45 | |||
| Group | ||||||
| Control | 1 | |||||
| Experimental | 0.78 | 0.46-1.34 | 0.374 | |||
Further subgroup analyses were performed for OS in patients stratified by the following characteristics (covariates): gender (male, female), age (<52, ≥52 years), T stage (T1-2, T3-4), N stage (N1, N2, N3), clinical stage (III, IVa), and ECOG score (0, 1). In patients with cervical N3 metastatic lymph nodes in the experimental group, the risk of death was reduced compared to that in the control group, with marginally statistical significance (HR = 0.48, 95% CI: 0.23-0.99, P = 0.046; Figures 6 and 7). No significant interactions between these covariates and the treatment groups were noted (all P>0.05; Figure 6).
Figure 6.

Subgroup analyses of OS.
Figure 7.
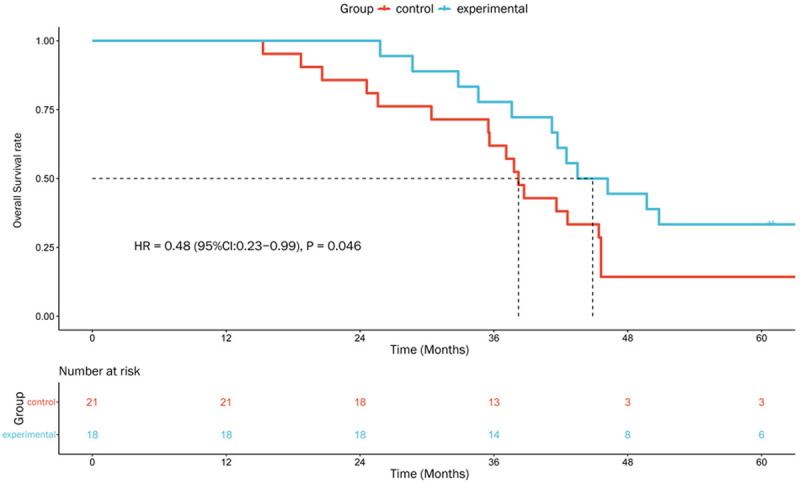
Kaplan-Meier plots of OS in patients with Cervical N3 lymph nodes.
Treatment toxicity
A total of 5 patients did not complete the planned AC cycles due to severe gastrointestinal reactions and hematological toxicity. The most common acute adverse reactions were leukopenia, neutropenia, hemoglobin reduction, nausea and vomiting, liver function damage, and radioactive dermatitis; however, there were no significant differences in the distribution of adverse events between the two groups (P>0.05, Table 5). Furthermore, the incidence of grade 3-4 radiation-induced oral mucositis was significantly lower in the experimental group than in the control group (29.3% vs. 54.8%, P = 0.019).
Table 5.
Acute adverse reactions in the two groups
| Toxicity | Experimental group n (%) | Control group n (%) | χ2 | P* | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Any grade | Grade 3/4 | Any grade | Grade 3/4 | |||
| Leukopenia | 38 (92.7) | 9 (22.0) | 37 (88.1) | 9 (21.4) | 0.003 | 0.954 |
| Neutropenia | 35 (85.4) | 14 (34.1) | 33 (78.6) | 9 (21.4) | 1.675 | 0.196 |
| Hemoglobin decrease | 33 (80.5) | 0 | 34 (81.0) | 1 (2.4) | - | 1.0 |
| Thrombocytopenia | 2 (4.9) | 0 | 4 (9.5) | 0 | - | - |
| Nausea/vomiting | 40 (97.6) | 7 (17.1) | 38 (90.5) | 8 (19.0) | 0.055 | 1.0 |
| Diarrhoea | 6 (14.6) | 0 | 5 (11.9) | 0 | - | - |
| Liver dysfunction | 25 (61.0) | 0 | 21 (50%) | 0 | - | - |
| Renal dysfunction | 3 (7.3) | 1 (2.4) | 5 (11.9) | 0 | - | 0.494 |
| Oral mucositis | 39 (95.1) | 12 (29.3) | 40 (95.2) | 23 (54.8) | 5.529 | 0.019 |
| Skin reaction | 33 (80.5) | 1 (2.4) | 32 (76.2) | 2 (4.8) | 0 | 1.0 |
| Hypertension | 2 (4.9) | 0 | 0 | 0 | - | - |
| Cardiac arrhythmia | 1 (2.4) | 0 | 0 | 0 | - | - |
P values showed the results of grade 3/4 acute toxicity in two groups.
The overall adverse reaction to the Endostar combinational therapy was tolerable as no grade 3 or 4 arrhythmia, hypertension, liver/kidney function damage, thrombocytopenia, or anemia were observed in the experimental group throughout the treatment period. In addition, bleeding or coagulation dysfunction were not observed in either group. The most common late toxicities were dry mouth, hearing loss, subcutaneous fibrosis, and temporal lobe injury. There was no occurrence of radioactive cerebral nerve palsy, osteonecrosis, pituitary injury, mouth opening limitation, or vision loss. Importantly, there was no significant difference in the late adverse reactions between the two groups (P>0.05).
Discussion
Various strategies combining radiotherapy and chemotherapy, including IC plus radiotherapy, CCRT, and AC after radiotherapy, have been implemented to improve the outcomes of patients with locally advanced NPC. Nevertheless, no breakthrough in efficacy has been achieved, and 40-60% of patients with locally advanced NPC eventually develop locoregional recurrences or distant metastases [14]. The results of our trial demonstrated that Endostar combined with PF regimen and sequential IMRT significantly improved PFS in locally advanced NPC and had tolerable toxicities, suggesting that adding anti-angiogenic drugs to systemic therapy is a feasible therapeutic strategy.
Previous reports have shown that treatment using anti-angiogenic drugs (e.g., bevacizumab) can considerably improve the prognoses and significantly reduce the risk of death in patients with solid tumors, including non-small cell lung cancer, colorectal cancer, and liver cancer [15-17]. This encouraging result inspired us to assess the effect of anti-angiogenic drugs in combination therapy. We chose to use anti-angiogenesis agent Endostar because, unlike the single-target VEGF-A inhibitor bevacizumab, Endostar can act on multiple targets, including VEGF, VEGF receptor-2, and platelet-derived growth factor receptor, to directly inhibit vascular endothelial cell proliferation, thereby suppressing tumor growth [18]. Yang et al. [19] have demonstrated that Endostar significantly inhibits the formation and growth of the vascular endothelial CNE-2 cells-derived xenograft tumor in nude mice. Similar results have also been obtained in the study where Endostar, combined with IMRT, significantly inhibits the growth of NPC xenografts. A phase II clinical trial has confirmed that adding Endostar to gemcitabine and cisplatin chemotherapy leads to significant tumor regression, controls disease progression, and improves the prognosis in metastatic NPC patients [20]. However, the clinical studies on Endostar are limited to patients with distal metastasis or patients experiencing drug resistant to initial treatment. There are few studies on the efficacy and safety of Endostar combined with chemotherapy and sequential IMRT for the treatment of locally advanced NPC, which was the goal of our current study.
CCRT with a platinum-based regimen has been the standard treatment for locoregionally advanced NPC [3,21]. Nevertheless, several clinical trials found that IC plus CCRT did not improve local/regional control, disease-free survival, and OS compared to IC plus IMRT alone [22-24]. One reason for this finding could be that the Intergroup 0099 trial was conducted in the era of traditional radiotherapy, while IMRT may provide greater local control and lower toxicities than two-dimensional general radiotherapy [25]. Additionally, IC can increase treatment-related toxicities, leading to treatment interruption in some patients [26,27]. Consistent with this, Yang et al. [28] demonstrated that IC plus IMRT alone had similar favorable treatment outcomes as CCRT but resulted in fewer incidences of leukopenia and anemia. Considering the treatment-related toxicities and patient compliance with combination therapy, we designed the Endostar with IC-IMRT-AC strategy for the experimental group and IC-IMRT-AC strategy without Endostar for the control group. The PF regimen has been proven to be an optimal IC which can improve PFS and OS [29].
Our study didn’t find statistical difference in the CR rates of nasopharyngeal lesions between the experimental and control groups. However, Endostar in the combination treatment was an independent prognostic factor affecting the PFS of the patients, and the CR rate of cervical metastatic lymph nodes was significantly higher, especially the CR rate of cervical N3 lymph nodes. Furthermore, subgroup analyses identified that Endostar combined chemoradiotherapy reduced the risk of death in patients with cervical N3 metastatic lymph nodes by 52%. Although no significant interaction was observed, these findings were consistent with the results reported by Kang et al. [30] and Li et al. [31]. Moreover, our results suggested that Endostar combined with chemoradiotherapy has synergistic effects in treating NPC. It is well known that VEGF expression correlates with the microvessel density within a tumor, and metastatic NPC has higher level of circulating VEGF [31]. Since VEGF plays an important role in lymph node metastasis through the induction of angiogenesis in NPC, its inhibition by Endostar will suppress the development of metastatic tumor. In addition, Endostar also promotes the apoptosis of tumor and vascular endothelial cells and improves hypoxia in tumor tissues, which may enhance radiotherapy sensitivity [32]. The use of Endostar significantly prolonged PFS by more than four months, and the 2- and 5-year OS rates were also improved. Nevertheless, our study didn’t show a statistically significant difference in OS outcomes, which might be related to the limited sample size and the short follow-up time.
With respect to treatment-related toxicities, the most common acute adverse reactions we observed in both treatment groups were leukopenia, neutropenia, hemoglobin reduction, nausea and vomiting, liver function damage, and radioactive dermatitis. Importantly, the patients in the experimental group didn’t suffer worse adverse reactions than the patients in the control group, suggesting that the inclusion of Endostar did not result in any unusual or severe-grade events; particularly, no grade 3 or 4 hypertension, cardiac arrhythmia, hemorrhage, and coagulation dysfunction were observed, and the compliance with the protocol therapy remained ideal. Notably, there was a statistically significant decrease in the incidence of radiation-induced grade 3-4 oral mucositis in the experimental group. It is well known that radiation-induced oral mucositis is one of the most common complications after chemoradiotherapy in patients with head and neck cancer. The accompanying side effects of radiation-induced oral mucositis, including severe pain, increased risk of systemic and local infections, and mouth dysfunction, severely affect the patients’ quality of life. Furthermore, these side effects may cause treatment interruption and extended treatment course. Hence, it is clinically significant that Endostar can reduce the incidence of radiation-induced oral mucositis. As for the molecular mechanisms underlying the effect of this Endostar, it has been reported that Endostar can reduce the incidence of radiation-induced lung injury by normalizing the structure and function of vasoganglion and downregulating the inflammatory mediator TGF-β1 [33]. Consistently, the expression of VEGF in NPC is related directly to tumor microvessel density [34]. Endostar can upregulate the endogenous anti-angiogenesis activity and downregulate VEGF activity, thereby leadings to the reduced number of microvessel and lower oxygen consumption by immature blood vessels, the reduced inflammatory exudation, and the improved recovery of necrotic tissues [18,35]. Data from our current study supported these findings and demonstrated that Endostar combined with chemoradiotherapy not only improved the tolerance of patients with locally advanced NPC but also effectively reduced the incidence of adverse reactions to radiation-related oral mucositis. Taken together, Endostar combined with chemoradiotherapy may provide a new strategy for the prevention and treatment of radiation injury.
This study had some limitations. First, as a phase II clinical trial, this study included a small sample size. Second, the follow-up time should be longer, and the long-term survival endpoint has yet to be observed in some patients. Multicenter, large-scale, and longer-term follow-up studies are needed to verify the findings from this study.
In conclusion, Endostar in combination with PF chemotherapy and sequential IMRT significantly improves the PFS of patients with locally advanced NPC, has tolerable treatment-related toxicities, increases the CR rate of cervical N3 lymph nodes, and reduces the incidence of radiation-related oral mucositis. These findings provide the rationale for conducting further studies to confirm the effects of this novel combination therapy.
Acknowledgements
This study was funded by the Major Project of Hubei Provincial Health and Family Planning Commission (No: WJ2017Z026), and the Project of Natural Science Foundation of Hubei Province (No: 2014CFB312).
Disclosure of conflict of interest
None.
Abbreviations
- NPC
nasopharyngeal carcinoma
- CCRT
Platinum-based concurrent chemoradiotherapy
- IC
induction chemotherapy
- AC
adjuvant chemotherapy
- CR
complete response
- IMRT
intensity-modulated radiotherapy
- OS
overall survival
- PFS
progression-free survival
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Mao YP, Xie FY, Liu LZ, Sun Y, Li L, Tang LL, Liao XB, Xu HY, Chen L, Lai SZ, Lin AH, Liu MZ, Ma J. Re-evaluation of 6th edition of AJCC staging system for nasopharyngeal carcinoma and proposed improvement based on magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2009;73:1326–1334. doi: 10.1016/j.ijrobp.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Zhao C, Ghimire B, Hong MH, Liu Q, Zhang Y, Guo Y, Huang YJ, Guan ZZ. The role of concurrent chemoradiotherapy in the treatment of locoregionally advanced nasopharyngeal carcinoma among endemic population: a meta-analysis of the phase III randomized trials. BMC Cancer. 2010;10:10. doi: 10.1186/1471-2407-10-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yi J, Huang X, Gao L, Luo J, Zhang S, Wang K, Qu Y, Xiao J, Xu G. Intensity-modulated radiotherapy with simultaneous integrated boost for locoregionally advanced nasopharyngeal carcinoma. Radiat Oncol. 2014;9:56. doi: 10.1186/1748-717X-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen L, Hu CS, Chen XZ, Hu GQ, Cheng ZB, Sun Y, Li WX, Chen YY, Xie FY, Liang SB, Chen Y, Xu TT, Li B, Long GX, Wang SY, Zheng BM, Guo Y, Sun Y, Mao YP, Tang LL, Chen YM, Liu MZ, Ma J. Adjuvant chemotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase 3 multicentre randomised controlled trial. Eur J Cancer. 2017;75:150–158. doi: 10.1016/j.ejca.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Hong RL, Hsiao CF, Ting LL, Ko JY, Wang CW, Chang JTC, Lou PJ, Wang HM, Tsai MH, Lai SC, Liu TW. Final results of a randomized phase III trial of induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in patients with stage IVA and IVB nasopharyngeal carcinoma-Taiwan Cooperative Oncology Group (TCOG) 1303 Study. Ann Oncol. 2018;29:1972–1979. doi: 10.1093/annonc/mdy249. [DOI] [PubMed] [Google Scholar]
- 7.Sun X, Su S, Chen C, Han F, Zhao C, Xiao W, Deng X, Huang S, Lin C, Lu T. Long-term outcomes of intensity-modulated radiotherapy for 868 patients with nasopharyngeal carcinoma: an analysis of survival and treatment toxicities. Radiother Oncol. 2014;110:398–403. doi: 10.1016/j.radonc.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 8.Ou X, Zhou X, Shi Q, Xing X, Yang Y, Xu T, Shen C, Wang X, He X, Kong L, Ying H, Hu C. Treatment outcomes and late toxicities of 869 patients with nasopharyngeal carcinoma treated with definitive intensity modulated radiation therapy: new insight into the value of total dose of cisplatin and radiation boost. Oncotarget. 2015;6:38381–38397. doi: 10.18632/oncotarget.5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzen F, Cassidy J. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J. Clin. Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 10.Tan J, Jiang L, Cheng X, Wang C, Chen J, Huang X, Xie P, Xia D, Wang R, Zhang Y. Association between VEGF-460T/C gene polymorphism and clinical outcomes of nasopharyngeal carcinoma treated with intensity-modulated radiation therapy. Onco Targets Ther. 2017;10:909–918. doi: 10.2147/OTT.S126159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li YH, Hu CF, Shao Q, Huang MY, Hou JH, Xie D, Zeng YX, Shao JY. Elevated expressions of survivin and VEGF protein are strong independent predictors of survival in advanced nasopharyngeal carcinoma. J Transl Med. 2008;6:1. doi: 10.1186/1479-5876-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee NY, Zhang Q, Pfister DG, Kim J, Garden AS, Mechalakos J, Hu K, Le QT, Colevas AD, Glisson BS, Chan AT, Ang KK. Addition of bevacizumab to standard chemoradiation for locoregionally advanced nasopharyngeal carcinoma (RTOG 0615): a phase 2 multi-institutional trial. Lancet Oncol. 2012;13:172–180. doi: 10.1016/S1470-2045(11)70303-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Sun Y, Liu Y, Yu Q, Zhang Y, Li K, Zhu Y, Zhou Q, Hou M, Guan Z, Li W, Zhuang W, Wang D, Liang H, Qin F, Lu H, Liu X, Sun H, Zhang Y, Wang J, Luo S, Yang R, Tu Y, Wang X, Song S, Zhou J, You L, Wang J, Yao C. Results of randomized, multicenter, double-blind phase III trial of rh-endostatin (YH-16) in treatment of advanced non-small cell lung cancer patients. Zhongguo Fei Ai Za Zhi. 2005;8:283–290. doi: 10.3779/j.issn.1009-3419.2005.04.07. [DOI] [PubMed] [Google Scholar]
- 14.Lee AW, Lau WH, Tung SY, Chua DT, Chappell R, Xu L, Siu L, Sze WM, Leung TW, Sham JS, Ngan RK, Law SC, Yau TK, Au JS, O’Sullivan B, Pang ES, O SK, Au GK, Lau JT Hong Kong Nasopharyngeal Cancer Study Group. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 Trial by the Hong Kong Nasopharyngeal Cancer Study Group. J. Clin. Oncol. 2005;23:6966–6975. doi: 10.1200/JCO.2004.00.7542. [DOI] [PubMed] [Google Scholar]
- 15.Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodriguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, Finley G, Lee A, Coleman S, Deng Y, Kowanetz M, Shankar G, Lin W, Socinski MA IMpower150 Study Group. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019;7:387–401. doi: 10.1016/S2213-2600(19)30084-0. [DOI] [PubMed] [Google Scholar]
- 16.Kuboki Y, Nishina T, Shinozaki E, Yamazaki K, Shitara K, Okamoto W, Kajiwara T, Matsumoto T, Tsushima T, Mochizuki N, Nomura S, Doi T, Sato A, Ohtsu A, Yoshino T. TAS-102 plus bevacizumab for patients with metastatic colorectal cancer refractory to standard therapies (C-TASK FORCE): an investigator-initiated, open-label, single-arm, multicentre, phase 1/2 study. Lancet Oncol. 2017;18:1172–1181. doi: 10.1016/S1470-2045(17)30425-4. [DOI] [PubMed] [Google Scholar]
- 17.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL IMbrave150 Investigators. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 18.Ling Y, Yang Y, Lu N, You QD, Wang S, Gao Y, Chen Y, Guo QL. Endostar, a novel recombinant human endostatin, exerts antiangiogenic effect via blocking VEGF-induced tyrosine phosphorylation of KDR/Flk-1 of endothelial cells. Biochem Biophys Res Commun. 2007;361:79–84. doi: 10.1016/j.bbrc.2007.06.155. [DOI] [PubMed] [Google Scholar]
- 19.Yang ZP, Du YG, Xie YH, Li JR, Song YW. Inhibition effects of combination of radiotherapy and endostar on rats with nasopharyngeal carcinoma xenografts and its mechanism. Chin J Cancer Prevention Treatment. 2012;19:259–262. [Google Scholar]
- 20.Jin T, Li B, Chen XZ. A phase II trial of Endostar combined with gemcitabine and cisplatin chemotherapy in patients with metastatic nasopharyngeal carcinoma ( NCT01612286) Oncol Res. 2013;21:317–323. doi: 10.3727/096504014X13983417587401. [DOI] [PubMed] [Google Scholar]
- 21.Baujat B, Audry H, Bourhis J, Chan AT, Onat H, Chua DT, Kwong DL, Al-Sarraf M, Chi KH, Hareyama M, Leung SF, Thephamongkhol K, Pignon JP MAC-NPC Collaborative Group. Chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys. 2006;64:47–56. doi: 10.1016/j.ijrobp.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 22.Lin S, Lu JJ, Han L, Chen Q, Pan J. Sequential chemotherapy and intensity-modulated radiation therapy in the management of locoregionally advanced nasopharyngeal carcinoma: experience of 370 consecutive cases. BMC Cancer. 2010;10:39. doi: 10.1186/1471-2407-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Shan GP, Li P, Cheng PJ. The role of concurrent chemotherapy to intensity-modulated radiotherapy (IMRT) after neoadjuvant docetaxel and cisplatin treatment in locoregionally advanced nasopharyngeal carcinoma. Med Oncol. 2015;32:41. doi: 10.1007/s12032-015-0505-2. [DOI] [PubMed] [Google Scholar]
- 24.Wei Z, Zhang Z, Luo J, Li N, Peng X. Induction chemotherapy plus IMRT alone versus induction chemotherapy plus IMRT-based concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: a retrospective cohort study. J Cancer Res Clin Oncol. 2019;145:1857–1864. doi: 10.1007/s00432-019-02925-z. [DOI] [PubMed] [Google Scholar]
- 25.Lee AW, Ng WT, Chan LL, Hung WM, Chan CC, Sze HC, Chan OS, Chang AT, Yeung RM. Evolution of treatment for nasopharyngeal cancer--success and setback in the intensity-modulated radiotherapy era. Radiother Oncol. 2014;110:377–384. doi: 10.1016/j.radonc.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Lee AW, Ng W, Chan OS, Sze HC. If concurrent-adjuvant chemoradiotherapy is beneficial for locoregionally advanced nasopharyngeal carcinoma, would changing the sequence to induction-concurrent achieve better outcome? J Radiat Oncol. 2012;1:107–115. [Google Scholar]
- 27.Huang X, Chen X, Zhao C, Wang J, Wang K, Wang L, Miao J, Cao C, Jin T, Zhang Y, Qu Y, Chen X, Liu Q, Zhang S, Zhang J, Luo J, Xiao J, Xu G, Gao L, Yi J. Adding concurrent chemotherapy to intensity-modulated radiotherapy does not improve treatment outcomes for stage II nasopharyngeal carcinoma: a phase 2 multicenter clinical trial. Front Oncol. 2020;10:1314. doi: 10.3389/fonc.2020.01314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Z, Cai Z, Cai Q, Hong Y, Zhang C, Huang K, Lin Z, Li M. Sequential induction chemotherapy plus intensity-modulated radiotherapy versus concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: the three-year report of a phase II, single center, randomized, non-inferiority trial. Cancer Med. 2021;10:3886–3895. doi: 10.1002/cam4.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Q, Cao SM, Guo L, Hua YJ, Huang PY, Zhang XL, Lin M, You R, Zou X, Liu YP, Xie YL, Wang ZQ, Mai HQ, Chen QY, Tang LQ, Mo HY, Cao KJ, Qian CN, Zhao C, Xiang YQ, Zhang XP, Lin ZX, Li WX, Liu Q, Li JB, Ling L, Guo X, Hong MH, Chen MY. Induction chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase III multicentre randomised controlled trial. Eur J Cancer. 2019;119:87–96. doi: 10.1016/j.ejca.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 30.Kang M, Wang F, Liao X, Zhou P, Wang R. Intensity-modulated radiotherapy combined with endostar has similar efficacy but weaker acute adverse reactions than IMRT combined with chemotherapy in the treatment of locally advanced nasopharyngeal carcinoma. Medicine (Baltimore) 2018;97:e11118. doi: 10.1097/MD.0000000000011118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Tian Y, Jin F, Wu W, Long J, Ouyang J, Zhou Y. A phase II multicenter randomized controlled trial to compare standard chemoradiation with or without recombinant human endostatin injection (Endostar) therapy for the treatment of locally advanced nasopharyngeal carcinoma: long-term outcomes update. Curr Probl Cancer. 2020;44:100492. doi: 10.1016/j.currproblcancer.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Wen QL, Meng MB, Yang B, Tu LL, Jia L, Zhou L, Xu Y, Lu Y. Endostar, a recombined humanized endostatin, enhances the radioresponse for human nasopharyngeal carcinoma and human lung adenocarcinoma xenografts in mice. Cancer Sci. 2009;100:1510–1519. doi: 10.1111/j.1349-7006.2009.01193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang K, Yang S, Zhu Y, Mo A, Zhang D, Liu L. Protection against acute radiation-induced lung injury: a novel role for the anti-angiogenic agent Endostar. Mol Med Rep. 2012;6:309–315. doi: 10.3892/mmr.2012.903. [DOI] [PubMed] [Google Scholar]
- 34.Guang-Wu H, Sunagawa M, Jie-En L, Shimada S, Gang Z, Tokeshi Y, Kosugi T. The relationship between microvessel density, the expression of vascular endothelial growth factor (VEGF), and the extension of nasopharyngeal carcinoma. Laryngoscope. 2000;110:2066–2069. doi: 10.1097/00005537-200012000-00017. [DOI] [PubMed] [Google Scholar]
- 35.Peng F, Xu Z, Wang J, Chen Y, Li Q, Zuo Y, Chen J, Hu X, Zhou Q, Wang Y, Ma H, Bao Y, Chen M. Recombinant human endostatin normalizes tumor vasculature and enhances radiation response in xenografted human nasopharyngeal carcinoma models. PLoS One. 2012;7:e34646. doi: 10.1371/journal.pone.0034646. [DOI] [PMC free article] [PubMed] [Google Scholar]



