Abstract
Recent studies have shown that lycorine, a natural alkaloid compound, plays its anti-cancer role in several human malignancies including bladder cancer. However, the molecular mechanism of lycorine-induced antitumor activity has not been sufficiently investigated. The E3 ubiquitin ligase neural precursor cell expressed developmentally downregulated protein 4 (NEDD4, also known as NEDD4-1) plays a crucial role in tumorigenesis and progression of human cancer. Therefore, depletion of NEDD4 could be a prospective therapeutic strategy for the treatment of cancer. In this study, we investigated whether lycorine restrains tumor by inhibiting the expression of NEDD4 in bladder cancer. We observed that lycorine blocked bladder cancer cell proliferation, colony formation, metastasis and invasion. Moreover, we found that overexpression of NEDD4 in bladder cancer cells significantly promoted cell proliferation and motility, whereas downregulating of the NEDD4 gene expression by lycorine or siRNA suppressed cell growth and movement. Notably, lycorine increased gemcitabine sensitivity in bladder cancer cells. Importantly, lycorine significantly reduced tumor growth, whereas overexpression of NEDD4 accelerated tumor growth and rescued lycorine-triggered tumor inhibition in xenograft mouse model. In conclusion, our study demonstrated that lycorine could exert its antineoplastic activity via suppressing NEDD4 pathway in vitro and in vivo. Therefore, inhibition of NEDD4 expression by lycorine might be a potential efficient strategy for bladder cancer.
Keywords: NEDD4, lycorine, gemcitabine, bladder cancer, growth, migration, invasion
Introduction
Bladder cancer is one of the most aggressive and lethal forms of human malignancies worldwide, ranking the sixth among the most prevalent cancers in men and rating the fifteenth in the common cancers in women [1]. Approximately 90% of all newly diagnosed cases are urothelial cell carcinomas [2]. Multiple most prominent risk factors may induce bladder cancer incidence, including smoking and exposure to occupational environments containing aromatic PAHs, amines and other chemicals [3]. In the recent years, even though the introduction of neoadjuvant chemotherapy (NAC), antibodies, and immune checkpoint inhibitors have achieved certain curative effects and the future looks prospective, it cannot significantly improve the prognosis of advanced patients [4]. Thus, it is urgent to search clinically novel therapeutic targets to benefit the patients with bladder cancer.
Lycorine hydrochloride (LH) is a natural alkaloid isolated from Amaryllidaceae family plant Lycoris radiate, which was initially characterized by Nagakawa et al. in 1956 [5]. In recent years, lycorine was shown to exert various pharmacological effects, including anti-inflammation [6], anti-malarial [7], anti-bacterial [8-10], anti-viral [11] and anti-tumor activities [12-15]. The anti-tumor mechanism of lycorine in a variety of tumors is due to inhibition of cell growth [16], cell cycle [17,18], cell mobility [19,20], and induction of cell apoptosis [21,22] and necrotic cell death [23]. Moreover, lycorine was discovered to induce the proteasomal degradation of HMGB1 and inhibit the dissociation of Bcl-2 from Beclin-1, eventually resulting in autophagy inhibition [24]. Although multiple studies identified the role of lycorine in carcinogenesis, further investigations are necessary to determine the biological roles of lycorine and its relevant underlying mechanisms.
As one of E3 HECT ubiquitin ligases, NEDD4 is frequently highly expressed in multiple cancers including non-small cell lung carcinomas [25], colorectal cancer [26], breast cancer [27] and prostate cancer [28]. Furthermore, NEDD4 mRNA was significantly increased in invasive bladder cancer samples [28]. Moreover, Sun et al. found that inhibition of the expression of NEDD4 suppressed the movement capacity of tumor cells and NEDD4 was an exceptional biomarker for prognosis of gastric cancer [29]. Wang and colleagues found that NEDD4 directly binds to PTEN, resulting in ubiquitination and degradation of PTEN, which first elaborated the carcinogenic and tumor promotion role of NEDD4 [28]. In addition, NEDD4 gene has been thought to be an oncogene principally by regulating various downstream signal transduction pathways [30,31]. For instance, one study described that NEDD4 interacts with and stimulates ubiquitin-mediated protein degradation of LATS1 and, thus, negatively regulated the Hippo tumor suppressor pathway [32]. Recently, Wu et al. demonstrated that reduction of NEDD4 decreases proliferation and invasion of bladder cancer cells, and promotes cell apoptosis [33]. These data suggest that inactivation of NEDD4 could be e a promising approach to benefit human cancer patients.
Here, we attempted to explore the cytotoxic effects of lycorine on proliferation and motility in bladder cancer cells. Moreover, we determined whether lycorine could inactivate NEDD4 expression in bladder cancer cells. Furthermore, we dissected the mechanism of NEDD4 in lycorine-induced inhibition of bladder cancer cell growth and motility. Our results demonstrated that lycorine inhibited bladder cancer cell proliferation and metastasis partly through inhibition of NEDD4 expression, indicating that reduction of NEDD4 by lycorine could be a safe and effective approach for the treatment of bladder cancer.
Materials and methods
Cell culture and reagents
Human bladder cancer cell lines (5637 and T24) were provided by Shanghai Genechem Co., LTD (Shanghai, China). T24 cells were cultured in DMEM medium (Corning) supplemented with 10% FBS, and 5637 cells were maintained in RPMI medium 1640 (Corning) supplemented with 10% FBS, 1% penicillin and streptomycin at 37°C in a humidified atmosphere of 5% CO2. Lycorine (98% purity) was obtained from Solarbio Life Sciences company (Beijing, China). The anti-LATS1, anti-NEDD4 and anti-PTEN antibodies were purchased from Cell Signaling. Anti-tubulin (T9028) primary antibody was obtained from Sigma.
Cell viability analysis
For cell viability analysis, 5000 exponentially growing bladder cancer cells (100 μl/well) were seeded into 96-well plates, and maintained in complete medium containing different concentrations of lycorine. When incubation for an appropriate period of time, 10 μL of CCK-8 solution was added to 96-well and incubated for 1-4 h in 37°C, 5% CO2 incubator. Then, the absorbance at 450 nm was measured by microplate reader.
Colony formation test
In brief, 1000 bladder cancer cells in 2 ml medium were plated in 6-well plates overnight. After treated with different concentrations of lycorine, cells were cultured in the incubator for 2-3 weeks to form macroscopic clones. Cells were fixed with 4% paraformaldehyde and stained with crystal violet. After washed and air-dried, the colonies were photographed and the numbers were counted.
Wound healing assay
T24 and 5637 cells (1 × 106 cells for T24, 1.5 × 106 cells for 5637) were seeded into 6-well plates and cultured for 24 h. After 90-100% confluency, cells were wounded by a 200 μl plastic pipette tips. After washed with PBS, the cells were then treated with various concentrations of lycorine and cultured with medium containing 1% FBS for 20 h. The scratched area was observed under microscopy, and photos were taken with Digital inverted microscope (EVOS1).
Cell invasion assay
Briefly, cells were plated in the top insert chambers with 1/8 Matrigel-coated chambers (Corning, 3422) at a density of 2 × 104 to 2 × 105 cells per well in culture medium without 10% FBS. There is 500 μl complete medium including 10% FBS in the lower chamber. After incubated for 24 h, the invaded cells were fixed with 4% paraformaldehyde and washed with PBS and strained with crystal violet for 15 min. After washing with double-distilled water, cells were examined with a microscope.
Transfection
Bladder cancer cells were transfected with NEDD4 siRNA duplexes or cDNA using Lipofectamine 3000 reagent (Invitrogen, USA) according to the manufacturer’s instructions. The sequences of NEDD4 siRNA duplexes are as follows: sense: 5’-GGA GAA UUA UGG GUG UCA ATT-3’; antisense: 5’-UUG ACA CCC AUA AUU CUC CTT-3’. After 48 h, the cells were harvested for subsequent immunoblot analysis.
Western blotting analysis
Cells were harvested and washed with PBS, and then dissolved with RIPA buffer. The cell lysates concentrations were quantified with the BCA protein assay kit (Beyotime, Shanghai, China) according to the product manual. Same amount of each sample was subjected to SDS-PAGE and transferred to a nitrocellulose filter (NC) membrane, and then probed with the appropriate antibody overnight at 4°C with shaking. After being washed for three times with TBST, the membranes were then incubated with second antibody (1:5000) at room temperature for 1 h. Then the expression of protein was visualized using chemiluminescence detection reagents and the GeneGnome XRQ Chemiluminescence Imaging System.
Tumor xenograft experiments
Six-week-old BALB/c nude mice were randomly distributed at three per group, an aliquot of 5 × 106 T24_Control, T24_lycorine, T24_NEDD4, T24_NEDD4 + lycorine cells were subcutaneously injected into the right rear of the mice. When the average tumors achieved approximately 100 mm3, the mice of T24_lycorine and T24_NEDD4 + lycorine groups were constructed by intra-peritoneal injections of lycorine (10 mg/kg/day per mouse) for 15 days and the other two groups mice were injected with PBS. Subcutaneous tumor volumes (mm3) were measured using a vernier caliper every 3 d and calculated by the formula, tumor volume = L × W2 × 0.52 (L: the longest diameter of the tumor, W: the shortest diameter of the tumor).
Statistical analyses
Student’s t test and ANOVA were used to evaluate significance between two groups and multiple groups, respectively. Error bars represent standard deviation. *P<0.05 indicates significance.
Results
Lycorine suppresses cell viability and colony formation
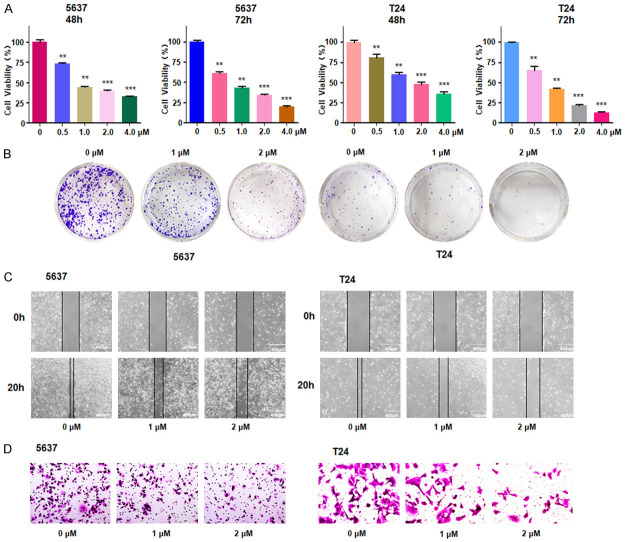
To investigate the potential cytotoxicity of lycorine on T24 and 5637 cell lines, CCK-8 assay was used to determine the cell multiplication in both bladder cancer cells treated with different concentrations of lycorine for 48 hours and 72 hours. The CCK-8 assay showed that lycorine significantly suppressed cell viability in a dose- and time-dependent way in T24 and 5637 cell lines (Figure 1A). Specifically, the 50% inhibitive concentration (IC50) of lycorine at 72 hours for 5637 and T24 cells was found to be around 1 μM (Figure 1A). To further examine the effect of lycorine on cell proliferation, we further conducted the colony formation assays. The colony number was decreased in a dose-dependent manner after treatment with lycorine in both bladder cancer cell lines (Figure 1B).
Figure 1.
Lycorine inhibits cell proliferation and motility. A. CCK-8 results showed that lycorine suppressed cell growth in a dose- and time-dependent way in T24 and 5637 cell lines. **P<0.01 vs. control, ***P<0.001 vs. control. B. The colony formation assay results showed that cell number was obviously decreased after treatment with different concentrations of Lycorine. C. Representative images showed that lycorine attenuated bladder cancer cell migration in 5637 cells and T24 cells. D. Representative images showed that lycorine attenuated bladder cancer cell invasion in 5637 cells and T24 cells.
Lycorine inhibits cell migration and invasion in bladder cancer cells
To determine the toxic effect of lycorine on cell motility, additional experiments were conducted, including wound healing assay and Transwell assay. As shown in Figure 1C, cell migration was significantly inhibited in 5637 and T24 cells treated with lycorine compared with control group. Consistently, Transwell assay results after crystal violet straining revealed that lycorine stimulation decreased cell invasion compared with control group (Figure 1D). Altogether, our findings indicated that lycorine inhibited cell migration and invasion in bladder cancer cells.
Lycorine decreases NEDD4 expression in bladder cancer cells
It has been reported that NEDD4 plays a carcinogenic role in tumorigenesis and cancer development [31]. One study showed that miR-7 inhibited the expression of NEDD4 [34]. We tested whether lycorine could increase the miR-7 expression and subsequently inhibit NEDD4 expression. We found that lycorine increased the miR-7 expression in bladder cancer cells (Figure 2A). Moreover, overexpression of miR-7 suppressed NEDD4 expression in bladder cancer (Figure 2B, 2C). Furthermore, in order to further verify the molecular mechanism of lycorine-mediated anti-tumor effect, we conducted western blotting analysis to investigate the effect of lycorine on the NEDD4 expression in bladder cancer cells. As shown in Figure 2D-F, lycorine treatment markedly suppressed NEDD4 mRNA and protein expressions as compared with the control in both bladder cancer cell lines, while the downstream target proteins of NEDD4 such as PTEN and LATS1 were significantly increased in cells after lycorine treatment.
Figure 2.
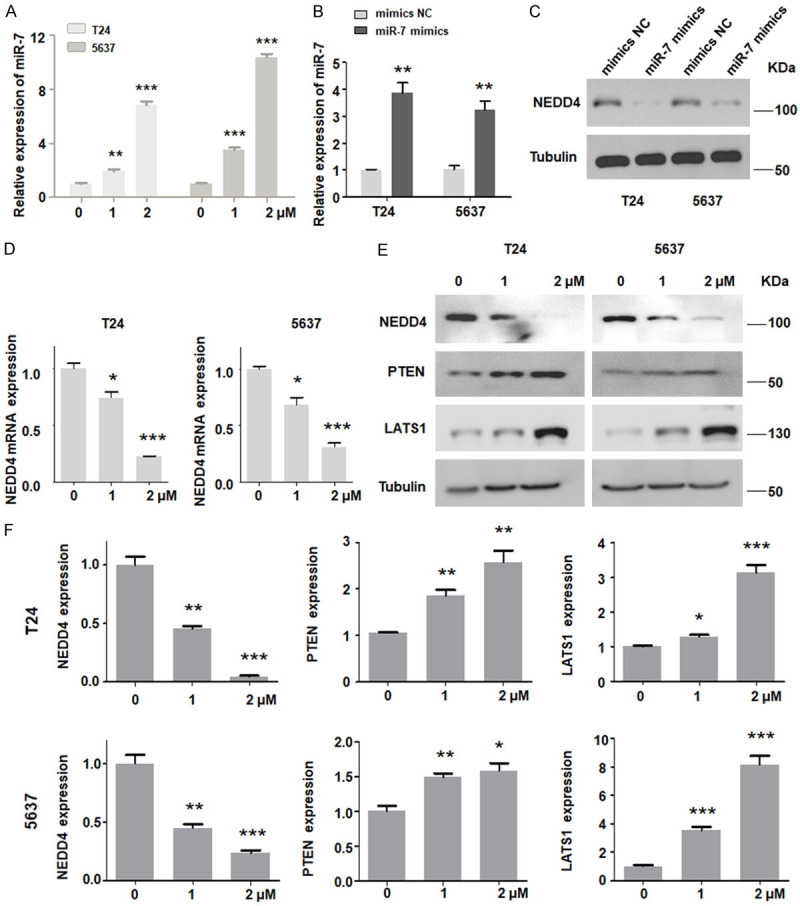
Lycorine inhibits NEDD4 expression. (A) RT-qPCR assay results showed the expression of miR-7 after being treated with lycorine in both T24 and 5637 cells. (B) Both T24 and 5637 cells were transfected with control miRNA or miR-7 mimics, and the expression of miR-7 was determined by RT-qPCR. (C) Over-expression of miR-7 decreased the protein level of NEDD4 in bladder cancer cells. (D, E) Treatment with lycorine reduced both the mRNA and protein levels of NEDD4 in T24 and 5637 cells. (F) Densitometric analyses results are illustrated for (E). *P<0.05, **P<0.01, ***P<0.001 vs. control.
NEDD4 overexpression alleviates lycorine-induced cell growth inhibition
In order to further study whether lycorine plays an anti-tumor role partly via down-regulating NEDD4, bladder cancer cells were transfected with NEDD4 cDNA to overexpress NEDD4 gene, and then were treated with lycorine. Cell proliferative capability was measured by the CCK-8 and colony formation assay. We found that activation of NEDD4 promoted the cell growth of both bladder cancer cell lines (Figure 3A, 3B). Furthermore, overexpression of NEDD4 rescued lycorine-induced cell growth inhibition in 5637 cells and T24 cells (Figure 3A, 3B).
Figure 3.
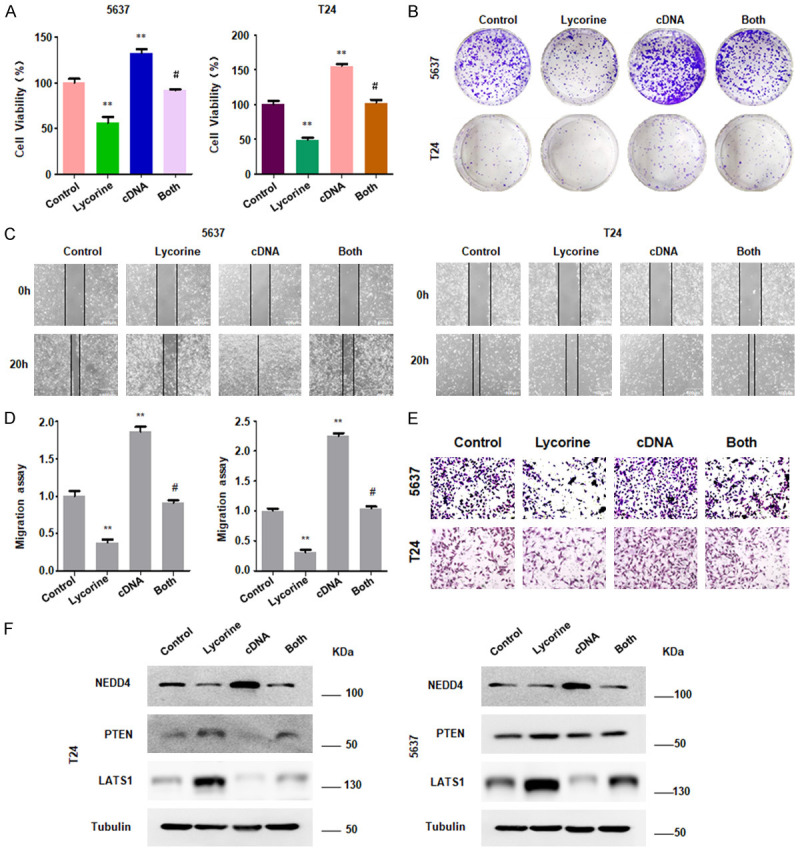
Overexpression of NEDD4 promotes cell growth, migration and invasion. A. CCK-8 assay was performed to determine cell growth in bladder cancer cells after treatment with NEDD4 overexpression and lycorine. Control: pcDNA 3.1 transfection; Lycorine: 1 µM lycorine; cDNA: NEDD4 cDNA transfection; Both: lycorine + NEDD4 cDNA. **P<0.01 vs. Control; #P<0.05 vs. lycorine treatment or NEDD4 cDNA transfection. B. Representative images showed that NEDD4 alleviates lycorine-induced cell colony activity inhibition. C. Wound healing assay was performed to detected cell migration in bladder cancer cells treated with lycorine in combination with NEDD4 cDNA transfection. D. Densitometric analyses results are illustrated for wound healing assay. **P<0.01 vs. Control; #P<0.05 vs. lycorine treatment or NEDD4 cDNA transfection. E. Transwell chambers assay was performed to measure cell invasive ability in bladder cancer cells after treatment with lycorine in combination with NEED4 overexpression. F. Western blotting results showed the expression of NEDD4, PTEN and LATS1 in bladder cancer cells treated with NEDD4 cDNA transfection and lycorine treatment.
NEDD4 overexpression alleviates lycorine-induced cell motility inhibition
The results described above demonstrated that NEDD4 plays a protective effect against lycorine-induced cell growth inhibition. To explore the role of NEDD4 on cell migration and invasion, we subsequently performed cell wound healing and cell invasion assay. As shown in Figure 3C-E, overexpression of NEDD4 exhibited significantly increased migratory and invasive cell number. Moreover, NEDD4 overexpression attenuates lycorine-induced cell motility inhibition (Figure 3C-E). Next, we investigated the protein expression of LATS1 and PTEN in bladder cancer cells after NEDD4 overexpression and lycorine treatment. As expected, NEDD4 overexpression suppressed expression of LATS1 and PTEN induced by lycorine, indicating that lycorine exerts anti-tumor effects partly by regulating NEDD4 and its downstream proteins LATS1 and PTEN (Figure 3F).
Knockdown of NEDD4 by siRNA promotes anti-tumor effects of lycorine
To further confirm that lycorine exerts anti-tumor effects through regulating NEDD4, we therefore proceeded to test whether knockdown of NEDD4 affected lycorine-regulate anti-cancer activity. As shown in Figure 4A, 4B, depletion of NEDD4 retarded proliferation of 5637 cells and T24 cells. Moreover, siRNA-induced knockdown of NEDD4 further dramatically promoted cell growth inhibition mediated by lycorine (Figure 4A, 4B). The wound healing assay and invasion assay further showed that siRNA-induced knockdown of NEDD4 suppressed cell migration and invasion in both bladder cancer cell lines (Figure 4C-E). Depletion of NEDD4 by siRNA further promoted lycorine-induced inhibition of cell migration and invasion in bladder cancer cells (Figure 4C-E). To better understand the molecular mechanism, we conducted western blotting analysis to examine the protein levels of LATS1 and PTEN. Knockdown of NEDD4 by siRNA transfection caused an increased protein level of LATS1 and PTEN expression in bladder cancer cells (Figure 4F). Moreover, compared with lycorine treatment alone or siRNA transfection alone, lycorine treatment in combination with NEDD4 siRNA transfection promoted the LATS1 and PTEN expression to more degree (Figure 4F).
Figure 4.
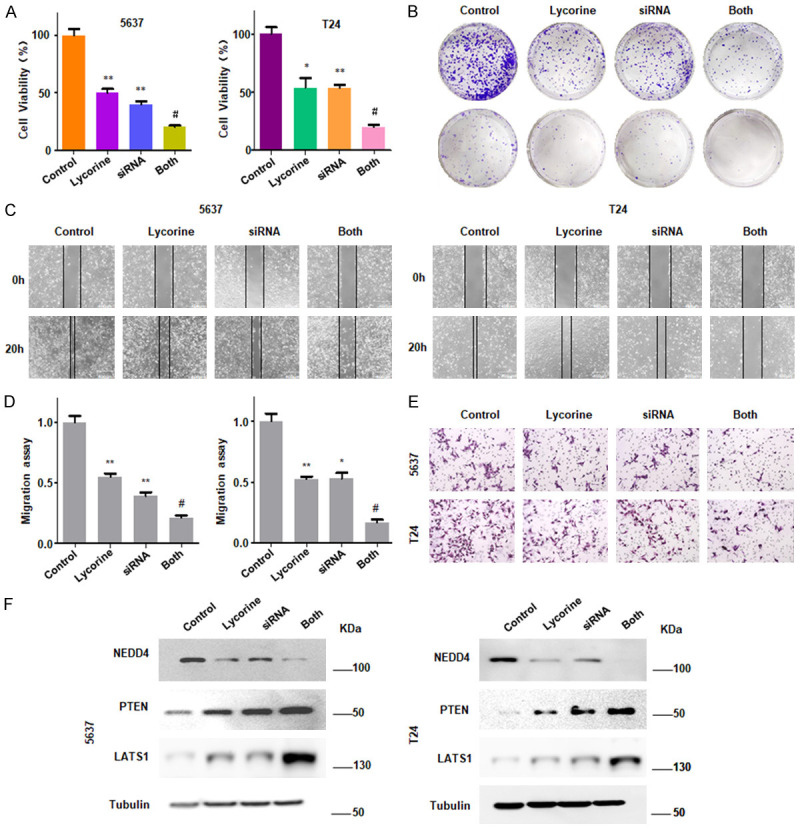
Knockdown of NEDD4 by siRNA reduces cell growth, migration and invasion. A. CCK-8 assay was performed to determine cell growth in bladder cancer cells after treatment with NEDD4 siRNA and lycorine. Control: Negative control siRNA; Lycorine: 1 µM lycorine; siRNA: NEDD4 siRNA; Both: NEDD4 siRNA + lycorine. *P<0.05 vs. Control, **P<0.01 vs. Control; #P<0.05 vs. lycorine treatment or NEDD4 siRNA transfection. B. Representative images showed that NEDD4 siRNA transfection promoted lycorine-induced cell colony activity inhibition. C. Wound healing assay was performed in bladder cancer cells treated with lycorine treatment and NEDD4 siRNA transfection. D. Densitometric analyses results are illustrated for wound healing assay. *P<0.05 vs. Control, **P<0.01 vs. Control; #P<0.05 vs. lycorine treatment or NEDD4 siRNA transfection. E. Transwell chambers assay was performed to measure the effect of knockdown of NEDD4 in combination with lycorine treatment on cell invasion. F. Western blotting results showed the expression of NEDD4, PTEN and LATS1 in bladder cancer cells treated with NEDD4 siRNA transfection and lycorine treatment.
NEDD4 overexpression alleviates lycorine-induced tumor inhibition in vivo
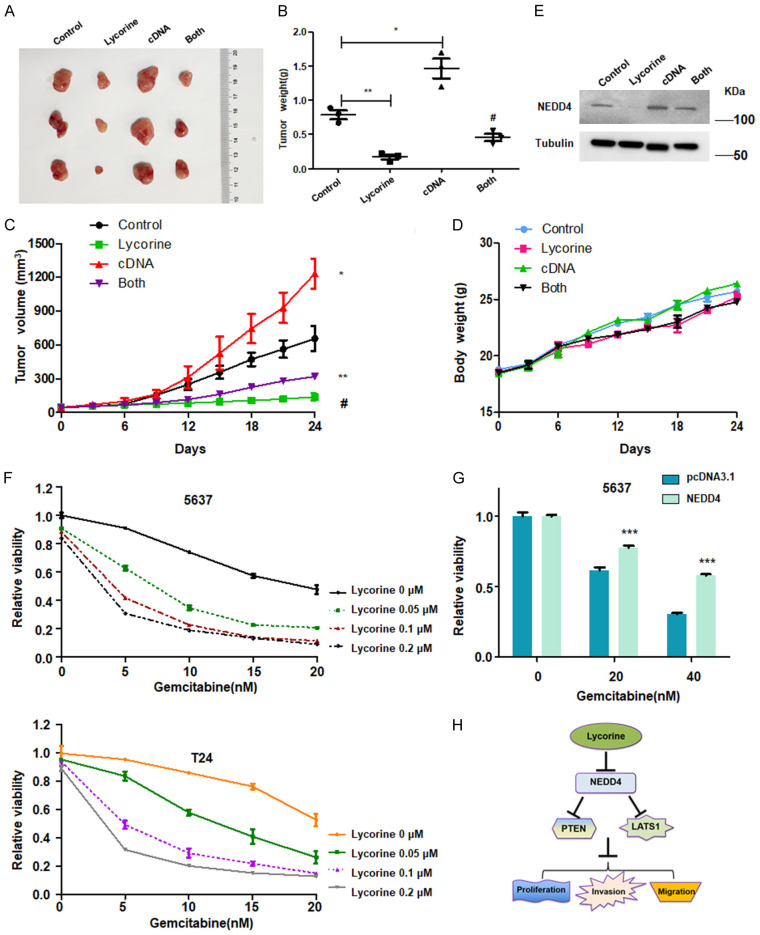
It has been shown that lycorine suppressed bladder cancer tumorigenesis in vivo [35]. To further validate whether overexpression of NEDD4 rescued lycorine-induced bladder tumor inhibition in vivo, we established the bladder cancer subcutaneous tumor xenograft model. We observed that lycorine significantly inhibited tumor growth (Figure 5A-C). On the other hand, upregulation of NEDD4 promoted tumor growth and rescued tumor growth suppression induced by lycorine treatment (Figure 5A-D). The decreased expression of NEDD4 by lycorine exposure was also abrogated by NEDD4 overexpression in tumor tissues (Figure 5E).
Figure 5.
Overexpression of NEDD4 rescues lycorine-induced tumor inhibition in vivo. (A) T24_Control and T24_NEDD4 bladder cancer cells were injected subcutaneously into the nude mice. When the average tumor volume achieved a volume of about 100 mm3, the mice were constructed by intra-peritoneal injections of either PBS or lycorine (10 mg/kg/day per mouse) for 15 days. (B) Weights of the xenograft tumors in (A) were illustrated. (C) In vivo tumor growth was measured by Vernier caliper over the indicated time period. (D) The body weights of the nude mice over the indicated time period. (E) IB analysis of the NEDD4 protein levels in the dissected tumor tissues. (F) CCK-8 assay was performed to determine cell viability in bladder cancer cells after treatment with different doses of gemcitabine and lycorine for 48 h. (G) CCK-8 assay data showed that overexpression of NEDD4 reduced the sensitivity of bladder cancer cells to gemcitabine. (H) A schematic illustration shows how lycorine suppresses cell growth and invasion via down-regulation of NEDD4 pathway in bladder cancer.
Lycorine increases gemcitabine sensitivity in bladder cancer cells
To investigate whether lycorine could increase gemcitabine sensitivity in T24 and 5637 cell lines, CCK-8 assay was performed to determine the cell viability in both bladder cancer cells treated with different concentrations of lycorine in combination with different doses of gemcitabine for 48 hours. Data from the CCK-8 assay revealed that lycorine markedly increased gemcitabine sensitivity in a dose-dependent manner in T24 and 5637 cell lines (Figure 5F). 0.5 μM lycorine treatment increased gemcitabine-induced cell viability suppression about 40-60% in T24 and 5637 cells (Figure 5F), suggesting that lycorine enhanced gemcitabine sensitivity in bladder cancer cells. Moreover, we observed that overexpression of NEDD4 reduced the gemcitabine sensitivity in bladder cancer cells (Figure 5G). Together, lycorine increased gemcitabine sensitivity partly via downregulation of NEDD4 in bladder cancer.
Discussion
Jimenez and colleagues previously found the anti-neoplastic effect of lycorine via inhibition of protein synthesis in 1976 [5]. Subsequently, numerous researches have been conducted to investigate the potential targets and possible mechanism behind lycorine performs antitumor activity on multiple types of cancers. Additionally, Zhang et al. found that lycorine suppressed tumor growth and motility of colorectal cancer cells by activating the ROS/p38 and AKT signaling pathway [16]. Recently, it has been reported that lycorine induced reactive oxygen species-mediated apoptosis by the JNK signaling pathway and mitochondrial apoptotic pathway in the oral squamous cell carcinoma cells [22]. Yuan et al. found that lycorine triggered cell cycle arrest at the G1/S transition and suppressed tumor growth of human osteosarcoma cells via inhibition of Wnt/beta-catenin, ERK1/2/MAPK and PI3K/AKT signaling pathway [36]. However, the effect and mechanism of lycorine on bladder cancer have not yet been fully clarified. In this study, we found that lycorine suppressed cell viability and motility and reduced tumor growth in mice, suggesting that lycorine could a new drug candidate to develop novel bladder cancer therapeutics.
NEDD4 E3 ligase has been known to play an oncogenic role in a variety of cellular processes via ubiquitination and degradation of multiple substrates [31]. Recently, NEDD4 was shown to perform vital function in tumorigenesis and progression, including cell proliferation [37], cell cycle [38], apoptosis [28], metastasis [39,40], and autophagy [41]. Chen et al. reported that NEDD4 directly binds with p21, increases p21 ubiquitylation and targets p21 for degradation [42]. Moreover, one study identified that NEDD4 regulates migration and invasion of glioma cells via interacting with CNrasGEF and promoting its poly-ubiquitination and degradation in vitro [43]. Studies also showed that the tumor suppressor Beclin 1 is ubiquitinated and degraded by NEDD4 via lysine-11-linked polyubiquitination. Similarly, our data suggested that overexpression of NEDD4 enhanced cell growth and invasion, while depletion of NEDD4 by siRNA suppressed cell growth and motility in bladder cancer cells. Taken together, our findings showed that NEDD4 played an oncogenic role in bladder cancer and targeting NEDD4 could be a promising and optimum anticancer therapeutic strategy.
Emerging evidence has revealed that several natural compounds performed anticancer activities via targeting NEDD4 in various types of cancers. For example, curcumin was reported to repress proliferation and invasion of pancreatic cancer cells via inhibiting NEDD4 expression [44]. Diosgenin displayed antitumor activity via reducing the expression of NEDD4 in prostate cancer cells [45]. Another study showed that nitidine chloride decreased the expression of NEDD4 in lung cancer cells [46]. In the present study, we reported that lycorine might be an inhibitor of NEDD4 in bladder cancer. Several noncoding RNAs have been reported to regulate NEDD4 expression, including LINC01198 and SNHG1 [47,48]. It has been accepted that noncoding RNAs play an essential role in oncogenesis and tumor progression [49-52]. Therefore, lycorine could regulate the noncoding RNAs to govern the expression of NEDD4 in bladder cancer, which is required for further validation.
NEDD4 have been reported to involve in drug resistance [53-55]. One study showed that NEDD4 participated in cisplatin resistance in nasopharyngeal carcinoma cells [54]. Another study revealed that NEDD4 promoted PTEN degradation and induced erlotinib resistance in NSCLC cells and enhanced temozolomide resistance in glioma [47,55,56]. NEDD4 E3 ligase could be a target for bortezomib sensitivity in multiple myeloma [53]. Therefore, targeting NEDD4 might reverse drug resistance in cancer cells. Our findings suggest that lycorine-mediated cell proliferation suppression and invasion inhibition and gemcitabine sensitivity in bladder cancer cells could be partly via reduction of NEDD4 (Figure 5H).
In summary, the inhibitory effect of lycorine and NEDD4 oncogenic function on bladder cancer cells have been reported. Lycorine induced the PTEN expression in bladder cancer cells have also been demonstrated. In our study, the novelty is that lycorine inhibited the expression of NEDD4 in bladder cancer cells, leading to upregulation of PTEN and suppression of bladder tumor promotion. Thus, our data provides that lycorine could be a promising novel agent for the treatment of bladder cancer.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81902579); Natural Science Foundation of Jiangsu Higher Education Institutions (19KJB320021) and the advanced research funds for the Second Affiliated Hospital of Soochow University (SDFEYBS1813, SDFEYGJ1802), CNNC science fund for talented young scholars (51010), and Suzhou Science and Technology Development Project (SYS2020139).
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Fleshner NE, Herr HW, Stewart AK, Murphy GP, Mettlin C, Menck HR. The National Cancer Data Base report on bladder carcinoma. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1996;78:1505–1513. doi: 10.1002/(sici)1097-0142(19961001)78:7<1505::aid-cncr19>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 3.Zhang M, Zhang X, Yu M, Zhang W, Zhang D, Zeng S, Wang X, Hu X. A novel ferroptosis-related gene model for overall survival predictions of bladder urothelial carcinoma patients. Front Oncol. 2021;11:698856. doi: 10.3389/fonc.2021.698856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemke EA, Shah AY. Management of advanced bladder cancer: an update. J Adv Pract Oncol. 2018;9:410–416. [PMC free article] [PubMed] [Google Scholar]
- 5.Jimenez A, Santos A, Alonso G, Vazquez D. Inhibitors of protein synthesis in eukarytic cells. Comparative effects of some amaryllidaceae alkaloids. Biochim Biophys Acta. 1976;425:342–348. doi: 10.1016/0005-2787(76)90261-6. [DOI] [PubMed] [Google Scholar]
- 6.Mikami M, Kitahara M, Kitano M, Ariki Y, Mimaki Y, Sashida Y, Yamazaki M, Yui S. Suppressive activity of lycoricidinol (narciclasine) against cytotoxicity of neutrophil-derived calprotectin, and its suppressive effect on rat adjuvant arthritis model. Biol Pharm Bull. 1999;22:674–678. doi: 10.1248/bpb.22.674. [DOI] [PubMed] [Google Scholar]
- 7.Sener B, Orhan I, Satayavivad J. Antimalarial activity screening of some alkaloids and the plant extracts from Amaryllidaceae. Phytother Res. 2003;17:1220–1223. doi: 10.1002/ptr.1346. [DOI] [PubMed] [Google Scholar]
- 8.Casu L, Cottiglia F, Leonti M, De Logu A, Agus E, Tse-Dinh YC, Lombardo V, Sissi C. Ungeremine effectively targets mammalian as well as bacterial type I and type II topoisomerases. Bioorg Med Chem Lett. 2011;21:7041–7044. doi: 10.1016/j.bmcl.2011.09.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan CX, Schrader KK, Mizuno CS, Rimando AM. Activity of lycorine analogues against the fish bacterial pathogen Flavobacterium columnare. J Agric Food Chem. 2011;59:5977–5985. doi: 10.1021/jf200452z. [DOI] [PubMed] [Google Scholar]
- 10.Bendaif H, Melhaoui A, Ramdani M, Elmsellem H, Douez C, El Ouadi Y. Antibacterial activity and virtual screening by molecular docking of lycorine from Pancratium foetidum Pom (Moroccan endemic Amaryllidaceae) Microb Pathog. 2018;115:138–145. doi: 10.1016/j.micpath.2017.12.037. [DOI] [PubMed] [Google Scholar]
- 11.Hwang YC, Chu JJ, Yang PL, Chen W, Yates MV. Rapid identification of inhibitors that interfere with poliovirus replication using a cell-based assay. Antiviral Res. 2008;77:232–236. doi: 10.1016/j.antiviral.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J, Hu WX, He LF, Ye M, Li Y. Effects of lycorine on HL-60 cells via arresting cell cycle and inducing apoptosis. FEBS Lett. 2004;578:245–250. doi: 10.1016/j.febslet.2004.10.095. [DOI] [PubMed] [Google Scholar]
- 13.Ying X, Huang A, Xing Y, Lan L, Yi Z, He P. Lycorine inhibits breast cancer growth and metastasis via inducing apoptosis and blocking Src/FAK-involved pathway. Sci China Life Sci. 2017;60:417–428. doi: 10.1007/s11427-016-0368-y. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Xu J, Xing G. Lycorine inhibits the growth and metastasis of breast cancer through the blockage of STAT3 signaling pathway. Acta Biochim Biophys Sin (Shanghai) 2017;49:771–779. doi: 10.1093/abbs/gmx076. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Liu J, Tang LJ, Shi YW, Ren W, Hu WX. Apoptosis induced by lycorine in KM3 cells is associated with the G0/G1 cell cycle arrest. Oncol Rep. 2007;17:377–384. [PubMed] [Google Scholar]
- 16.Zhang P, Yuan X, Yu T, Huang H, Yang C, Zhang L, Yang S, Luo X, Luo J. Lycorine inhibits cell proliferation, migration and invasion, and primarily exerts in vitro cytostatic effects in human colorectal cancer via activating the ROS/p38 and AKT signaling pathways. Oncol Rep. 2021;45:19. doi: 10.3892/or.2021.7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li L, Dai HJ, Ye M, Wang SL, Xiao XJ, Zheng J, Chen HY, Luo YH, Liu J. Lycorine induces cell-cycle arrest in the G0/G1 phase in K562 cells via HDAC inhibition. Cancer Cell Int. 2012;12:49. doi: 10.1186/1475-2867-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Hu JL, Shi BW, He Y, Hu WX. Up-regulation of p21 and TNF-alpha is mediated in lycorine-induced death of HL-60 cells. Cancer Cell Int. 2010;10:25. doi: 10.1186/1475-2867-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu M, Peng S, He Y, Qin M, Cong X, Xing Y, Liu M, Yi Z. Lycorine is a novel inhibitor of the growth and metastasis of hormone-refractory prostate cancer. Oncotarget. 2015;6:15348–15361. doi: 10.18632/oncotarget.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao L, Feng Y, Ge C, Xu X, Wang S, Li X, Zhang K, Wang C, Dai F, Xie S. Identification of molecular anti-metastasis mechanisms of lycorine in colorectal cancer by RNA-seq analysis. Phytomedicine. 2021;85:153530. doi: 10.1016/j.phymed.2021.153530. [DOI] [PubMed] [Google Scholar]
- 21.Li C, Deng C, Pan G, Wang X, Zhang K, Dong Z, Zhao G, Tan M, Hu X, Shi S, Du J, Ji H, Wang X, Yang L, Cui H. Lycorine hydrochloride inhibits cell proliferation and induces apoptosis through promoting FBXW7-MCL1 axis in gastric cancer. J Exp Clin Cancer Res. 2020;39:230. doi: 10.1186/s13046-020-01743-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li MH, Liao X, Li C, Wang TT, Sun YS, Yang K, Jiang PW, Shi ST, Zhang WX, Zhang K, Li C, Yang P. Lycorine hydrochloride induces reactive oxygen species-mediated apoptosis via the mitochondrial apoptotic pathway and the JNK signaling pathway in the oral squamous cell carcinoma HSC-3 cell line. Oncol Lett. 2021;21:236. doi: 10.3892/ol.2021.12497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo Y, Roy M, Xiao X, Sun S, Liang L, Chen H, Fu Y, Sun Y, Zhu M, Ye M, Liu J. Lycorine induces programmed necrosis in the multiple myeloma cell line ARH-77. Tumour Biol. 2015;36:2937–2945. doi: 10.1007/s13277-014-2924-7. [DOI] [PubMed] [Google Scholar]
- 24.Roy M, Liang L, Xiao X, Peng Y, Luo Y, Zhou W, Zhang J, Qiu L, Zhang S, Liu F, Ye M, Zhou W, Liu J. Lycorine downregulates HMGB1 to inhibit autophagy and enhances bortezomib activity in multiple myeloma. Theranostics. 2016;6:2209–2224. doi: 10.7150/thno.15584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amodio N, Scrima M, Palaia L, Salman AN, Quintiero A, Franco R, Botti G, Pirozzi P, Rocco G, De Rosa N, Viglietto G. Oncogenic role of the E3 ubiquitin ligase NEDD4-1, a PTEN negative regulator, in non-small-cell lung carcinomas. Am J Pathol. 2010;177:2622–2634. doi: 10.2353/ajpath.2010.091075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong SW, Moon JH, Kim JS, Shin JS, Jung KA, Lee WK, Jeong SY, Hwang JJ, Lee SJ, Suh YA, Kim I, Nam KY, Han S, Kim JE, Kim KP, Hong YS, Lee JL, Lee WJ, Choi EK, Lee JS, Jin DH, Kim TW. p34 is a novel regulator of the oncogenic behavior of NEDD4-1 and PTEN. Cell Death Differ. 2014;21:146–160. doi: 10.1038/cdd.2013.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung S, Li C, Jeong D, Lee S, Ohk J, Park M, Han S, Duan J, Kim C, Yang Y, Kim KI, Lim JS, Kang YS, Lee MS. Oncogenic function of p34SEI-1 via NEDD4-1-mediated PTEN ubiquitination/degradation and activation of the PI3K/AKT pathway. Int J Oncol. 2013;43:1587–1595. doi: 10.3892/ijo.2013.2064. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Trotman LC, Koppie T, Alimonti A, Chen Z, Gao Z, Wang J, Erdjument-Bromage H, Tempst P, Cordon-Cardo C, Pandolfi PP, Jiang X. NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell. 2007;128:129–139. doi: 10.1016/j.cell.2006.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun A, Yu G, Dou X, Yan X, Yang W, Lin Q. Nedd4-1 is an exceptional prognostic biomarker for gastric cardia adenocarcinoma and functionally associated with metastasis. Mol Cancer. 2014;13:248. doi: 10.1186/1476-4598-13-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ye X, Wang L, Shang B, Wang Z, Wei W. NEDD4: a promising target for cancer therapy. Curr Cancer Drug Targets. 2014;14:549–556. doi: 10.2174/1568009614666140725092430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang ZW, Hu X, Ye M, Lin M, Chu M, Shen X. NEDD4 E3 ligase: functions and mechanism in human cancer. Semin Cancer Biol. 2020;67:92–101. doi: 10.1016/j.semcancer.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Salah Z, Cohen S, Itzhaki E, Aqeilan RI. NEDD4 E3 ligase inhibits the activity of the Hippo pathway by targeting LATS1 for degradation. Cell Cycle. 2013;12:3817–3823. doi: 10.4161/cc.26672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wen W, Li J, Wang L, Xing Y, Li X, Ruan H, Xi X, Xiong J, Kuang R. Inhibition of NEDD4 inhibits cell growth and invasion and induces cell apoptosis in bladder cancer cells. Cell Cycle. 2017;16:1509–1514. doi: 10.1080/15384101.2017.1338220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang D, Zhang M, Tan Z. Bone marrow stem cell-exo-derived TSG-6 attenuates 1-methyl-4-phenylpyridinium+-induced neurotoxicity via the STAT3/miR-7/NEDD4/LRRK2 axis. J Neuropathol Exp Neurol. 2022;81:621–634. doi: 10.1093/jnen/nlac049. [DOI] [PubMed] [Google Scholar]
- 35.Wang C, Wang Q, Li X, Jin Z, Xu P, Xu N, Xu A, Xu Y, Zheng S, Zheng J, Liu C, Huang P. Lycorine induces apoptosis of bladder cancer T24 cells by inhibiting phospho-Akt and activating the intrinsic apoptotic cascade. Biochem Biophys Res Commun. 2017;483:197–202. doi: 10.1016/j.bbrc.2016.12.168. [DOI] [PubMed] [Google Scholar]
- 36.Yuan XH, Zhang P, Yu TT, Huang HK, Zhang LL, Yang CM, Tan T, Yang SD, Luo XJ, Luo JY. Lycorine inhibits tumor growth of human osteosarcoma cells by blocking Wnt/beta-catenin, ERK1/2/MAPK and PI3K/AKT signaling pathway. Am J Transl Res. 2020;12:5381–5398. [PMC free article] [PubMed] [Google Scholar]
- 37.Sun M, Cai J, Anderson RA, Sun Y. Type I gamma phosphatidylinositol phosphate 5-kinase i5 controls the ubiquitination and degradation of the tumor suppressor mitogen-inducible gene 6. J Biol Chem. 2016;291:21461–21473. doi: 10.1074/jbc.M116.736041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al Sorkhy M, Craig R, Market B, Ard R, Porter LA. The cyclin-dependent kinase activator, Spy1A, is targeted for degradation by the ubiquitin ligase NEDD4. J Biol Chem. 2009;284:2617–2627. doi: 10.1074/jbc.M804847200. [DOI] [PubMed] [Google Scholar]
- 39.Edwin F, Anderson K, Patel TB. HECT domain-containing E3 ubiquitin ligase Nedd4 interacts with and ubiquitinates Sprouty2. J Biol Chem. 2010;285:255–264. doi: 10.1074/jbc.M109.030882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawabe H, Neeb A, Dimova K, Young SM Jr, Takeda M, Katsurabayashi S, Mitkovski M, Malakhova OA, Zhang DE, Umikawa M, Kariya K, Goebbels S, Nave KA, Rosenmund C, Jahn O, Rhee J, Brose N. Regulation of Rap2A by the ubiquitin ligase Nedd4-1 controls neurite development. Neuron. 2010;65:358–372. doi: 10.1016/j.neuron.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Platta HW, Abrahamsen H, Thoresen SB, Stenmark H. Nedd4-dependent lysine-11-linked polyubiquitination of the tumour suppressor Beclin 1. Biochem J. 2012;441:399–406. doi: 10.1042/BJ20111424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang S, Yu C, Yang X, Hong H, Lu J, Hu W, Hao X, Li S, Aikemu B, Yang G, He Z, Zhang L, Xue P, Cai Z, Ma J, Zang L, Feng B, Yuan F, Sun J, Zheng M. N-myc downstream-regulated gene 1 inhibits the proliferation of colorectal cancer through emulative antagonizing NEDD4-mediated ubiquitylation of p21. J Exp Clin Cancer Res. 2019;38:490. doi: 10.1186/s13046-019-1476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang H, Nie W, Zhang X, Zhang G, Li Z, Wu H, Shi Q, Chen Y, Ding Z, Zhou X, Yu R. NEDD4-1 regulates migration and invasion of glioma cells through CNrasGEF ubiquitination in vitro. PLoS One. 2013;8:e82789. doi: 10.1371/journal.pone.0082789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Su J, Zhou X, Yin X, Wang L, Zhao Z, Hou Y, Zheng N, Xia J, Wang Z. The effects of curcumin on proliferation, apoptosis, invasion, and NEDD4 expression in pancreatic cancer. Biochem Pharmacol. 2017;140:28–40. doi: 10.1016/j.bcp.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 45.Zhang J, Xie JJ, Zhou SJ, Chen J, Hu Q, Pu JX, Lu JL. Diosgenin inhibits the expression of NEDD4 in prostate cancer cells. Am J Transl Res. 2019;11:3461–3471. [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J, Cao R, Lian C, Cao T, Shi Y, Ma J, Wang P, Xia J. Nitidine chloride suppresses NEDD4 expression in lung cancer cells. Aging (Albany NY) 2020;13:782–793. doi: 10.18632/aging.202185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen WL, Chen HJ, Hou GQ, Zhang XH, Ge JW. LINC01198 promotes proliferation and temozolomide resistance in a NEDD4-1-dependent manner, repressing PTEN expression in glioma. Aging (Albany NY) 2019;11:6053–6068. doi: 10.18632/aging.102162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang Y, Wu W, Jiao G, Chen Y, Liu H. LncRNA SNHG1 modulates p38 MAPK pathway through Nedd4 and thus inhibits osteogenic differentiation of bone marrow mesenchymal stem cells. Life Sci. 2019;228:208–214. doi: 10.1016/j.lfs.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 49.Liu J, Shang G. The roles of noncoding RNAs in the development of osteosarcoma stem cells and potential therapeutic targets. Front Cell Dev Biol. 2022;10:773038. doi: 10.3389/fcell.2022.773038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen X, Liu Y, Liu H, Wang ZW, Zhu X. Unraveling diverse roles of noncoding RNAs in various human papillomavirus negative cancers. Pharmacol Ther. 2022;238:108188. doi: 10.1016/j.pharmthera.2022.108188. [DOI] [PubMed] [Google Scholar]
- 51.Jiang W, Xia J, Xie S, Zou R, Pan S, Wang ZW, Assaraf YG, Zhu X. Long non-coding RNAs as a determinant of cancer drug resistance: towards the overcoming of chemoresistance via modulation of lncRNAs. Drug Resist Updat. 2020;50:100683. doi: 10.1016/j.drup.2020.100683. [DOI] [PubMed] [Google Scholar]
- 52.Jiang W, Pan S, Chen X, Wang ZW, Zhu X. The role of lncRNAs and circRNAs in the PD-1/PD-L1 pathway in cancer immunotherapy. Mol Cancer. 2021;20:116. doi: 10.1186/s12943-021-01406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang X, Gu H, Zhang E, Chen Q, Cao W, Yan H, Chen J, Yang L, Lv N, He J, Yi Q, Cai Z. The NEDD4-1 E3 ubiquitin ligase: a potential molecular target for bortezomib sensitivity in multiple myeloma. Int J Cancer. 2020;146:1963–1978. doi: 10.1002/ijc.32615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feng S, Yang G, Yang H, Liang Z, Zhang R, Fan Y, Zhang G. NEDD4 is involved in acquisition of epithelial-mesenchymal transition in cisplatin-resistant nasopharyngeal carcinoma cells. Cell Cycle. 2017;16:869–878. doi: 10.1080/15384101.2017.1308617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun H, Ma H, Wang J, Xia L, Zhu G, Wang Z, Sun J, Chen Z. Phosphatase and tensin homolog deleted on chromosome 10 degradation induced by NEDD4 promotes acquired erlotinib resistance in non-small-cell lung cancer. Tumour Biol. 2017;39:1010428317709639. doi: 10.1177/1010428317709639. [DOI] [PubMed] [Google Scholar]
- 56.Chuang HY, Hsu LY, Pan CM, Pikatan NW, Yadav VK, Fong IH, Chen CH, Yeh CT, Chiu SC. The E3 ubiquitin ligase NEDD4-1 mediates temozolomide-resistant glioblastoma through PTEN attenuation and redox imbalance in Nrf2-HO-1 axis. Int J Mol Sci. 2021;22:10247. doi: 10.3390/ijms221910247. [DOI] [PMC free article] [PubMed] [Google Scholar]