Abstract
Induced pluripotent stem cells (iPSCs) are a powerful modeling system for medical discovery and translational research. To date, most studies have focused on the potential for iPSCs for regenerative medicine, drug discovery, and disease modeling. However, iPSCs are also a powerful modeling system to investigate the effects of environmental exposure on the cardiovascular system. With the emergence of e‐cigarettes, air pollution, marijuana use, opioids, and microplastics as novel cardiovascular risk factors, iPSCs have the potential for elucidating the effects of these toxins on the body using conventional two‐dimensional (2D) arrays and more advanced tissue engineering approaches with organoid and other three‐dimensional (3D) models. The effects of these environmental factors may be enhanced by genetic polymorphisms that make some individuals more susceptible to the effects of toxins. iPSC disease modeling may reveal important gene–environment interactions that exacerbate cardiovascular disease and predispose some individuals to adverse outcomes. Thus, iPSCs and gene‐editing techniques could play a pivotal role in elucidating the mechanisms of gene–environment interactions and understanding individual variability in susceptibility to environmental effects.
Keywords: cannabis, e‐cigarettes, environment, iPSC, opioids
Subject Categories: Evolution & Ecology, Methods & Resources, Stem Cells & Regenerative Medicine
In this review, J Wu and colleagues discuss the potential of human iPSC as a modeling system to investigate the toxic effects of environmental exposure on human health.

Glossary
- AHA
American Heart Association
- BDNF
Brain‐derived neurotrophic factor
- CB1
Cannabinoid receptor 1
- CB2
Cannabinoid receptor 2
- CRISPR/Cas9
Clustered Regulatory Interspaced Short Palindromic Repeats/Cas9 system
- CRISPRi
Clustered Regulatory Interspaced Short Palindromic Repeats Interference
- CRISPRa
Clustered Regulatory Interspaced Short Palindromic Repeats Activation
- CV
Cardiovascular
- DCM
Dilated cardiomyopathy
- EGFR
Epithelial growth factor receptor
- EHT
Engineered heart tissue
- ENDS
Electronic nicotine delivery systems
- EVALI
e‐cigarette or vaping use associated lung injury
- FDA
Food and Drug Administration
- GABA
γ‐aminobutyric‐acid
- GDNF
Glial cell‐derived neurotrophic factor
- GPCR
G‐protein coupled receptor
- HCM
Hypertrophic cardiomyopathy
- hERG
Human Ether‐à‐go‐go‐Related Gene
- iKR
Inward‐rectifier potassium channels
- iPSC
Induced pluripotent stem cell
- iPSC‐CM
iPSC‐derived cardiomyocyte
- iPSC‐EC
iPSC‐derived endothelial cell
- iPSC‐NC
iPSC‐derived neuronal cell
- iPSC‐SN
iPSC‐derived sensory neuron
- HTS
High‐throughput screening
- KLF4
Krüppel‐like factor 4
- MEA
Multi‐electrode array
- c‐MYC
Myc proto‐oncogene protein
- OSKM
Oct2/4, Sox2, Klf4, c‐Myc
- PBMC
Peripheral blood mononuclear cells
- PDCD4
Programmed cell death protein 4
- PGC‐1
Peroxisome proliferator‐activated receptor γ‐coactivator‐1
- SCD
Sudden cardiac death
- scRNA‐seq
Single‐cell RNA sequencing
- SRY
sex determining region Y
- SOX2 TALEN
sex determining region Y box 2
- TRP
Transcription Activator‐Like Effector Nucleases
- Δ9‐THC
Transient receptor potential channel delta‐9‐tetrahydrocannabinol
Introduction
The discovery of induced pluripotent stem cells (iPSCs) has transformed the field of stem cell biology and regenerative medicine (Yu et al, 2007). Induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs) are both pluripotent stem cells (PSCs) capable of self‐renewal and differentiation into any tissue lineage. Tissues derived from ESCs and iPSCs are largely similar from molecular and functional perspectives (Zhao et al, 2017). However, the use of iPSCs avoids the ethical problems associated with using ESCs, and iPSCs can be obtained readily from a blood sample to isolate peripheral blood mononuclear cells (PBMCs) or tissue samples with fibroblasts. Adult human somatic cells are reprogramed into stem cells via transferring somatic nuclear material into oocytes, followed by cell fusion, and genetic integration of somatic cell chromatin. The development of viral transduction and overexpression of the Yamanaka factors (octamer‐binding protein 3/4 (OCT 3/4; also known as POU5F1), SRY (sex‐determining region Y)‐box 2 (SOX2), Krüppel‐like factor 4 (KLF4), and Myc proto‐oncogene protein (c‐MYC)) was a revolutionary innovation (Takahashi & Yamanaka, 2006; Takahashi et al, 2007; Yu et al, 2007). iPSCs can be differentiated into any tissue cell type using a cocktail of recombinant protein factors and small‐molecule inhibitors, allowing the use of cardiovascular tissue for regenerative medicine, disease modeling, and drug discovery (Obal & Wu, 2020; Paik et al, 2020). While the financial cost and time required for reprogramming, generating, biobanking, and differentiation are high, iPSCs are a more accurate model of human disease than traditional mammalian cell culture and animal models. Leveraging individual genetic information and recent advances in gene editing (Nishiga et al, 2022), iPSCs are proving critical to understanding the molecular mechanisms of human disease and advancing the goals of precision medicine. In addition, with the diverse genetic backgrounds of individuals, a large cohort of iPSC lines has the potential to capture the heterogeneity of disease and drug treatments, which can help us discover the toxic effects of drugs and environmental exposures (Fig 1).
Figure 1. Applications for induced pluripotent stem cells (iPSCs) include disease modeling, drug screening, regenerative medicine, and toxicity profiling.
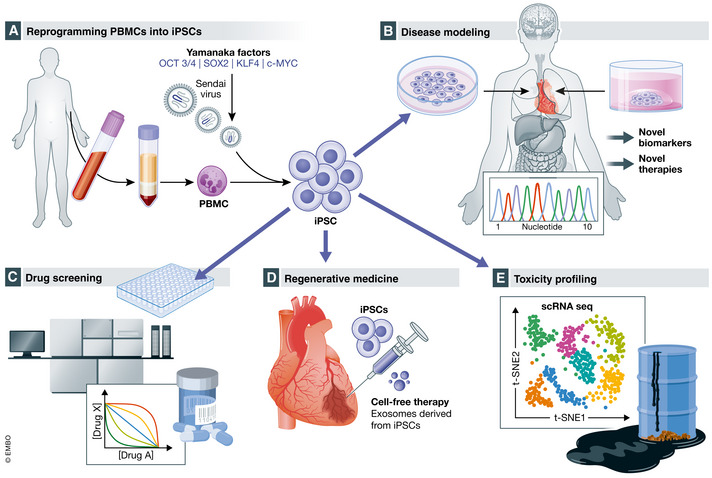
(A) From a single blood draw, peripheral blood mononuclear cells (PBMCs) are isolated and reprogrammed into iPSCs via viral transduction and overexpression of the Yamanaka factors (octamer‐binding protein 3/4 (OCT 3/4; also known as POU5F1), SRY (sex determining region Y)‐box 2 (SOX2), Krüppel‐like factor 4 (KLF4), and Myc proto‐oncogene protein (c‐MYC); Takahashi & Yamanaka, 2006; Takahashi et al, 2007; Yu et al, 2007). (B) iPSCs contain an individual's genetic information, and disease modeling offers the unique opportunity to understand patient‐specific disease mechanisms that might lead to novel biomarkers and therapies. (C) Drug screening traditionally has difficulty translating from small animal and cell culture models to the clinic. iPSCs contain the genetic code of individuals and can be differentiated into any cell type allowing the determination of safety, efficacy, and possibly patient‐specific responses in a dish. (D) Regenerative medicine involves iPSCs and cell‐free therapy with exosomes derived from iPSCs. (E) iPSCs are also ideally suited to testing for the effects of toxins on the different tissue beds and identifying patient‐specific factors that predispose to toxicity. [Colour figure can be viewed at wileyonlinelibrary.com]
Regenerative medicine
Despite great advances in percutaneous coronary intervention and medical management, cardiovascular disease remains the world's leading cause of death, with associated long‐term complications that include malignant arrhythmias and pump failure. iPSCs are a limitless source of tissue that are autologous, and their use helps avoid the need for immunosuppression (Lu et al, 2013). Despite their initial promise and great enthusiasm, preclinical studies have shown varying and sometimes disparate results for improving cardiac function, vasculogenesis, and reducing apoptosis (Nelson et al, 2009; Kawamura et al, 2012; Templin et al, 2012). Although clinical results from adult stem cells have been mixed, there are now several trials focusing on using human ESC‐ or iPSC‐based cardiovascular therapies (Neofytou et al, 2015).
Drug and toxicity screening
Because they contain an individual's unique genetic makeup, iPSCs are proving to be a powerful platform to discern the beneficial and adverse effects of drugs. Historically, animal models and cell culture systems have provided preclinical safety data for new drugs, and treatments and clinical trials are used to determine safety and efficacy in large heterogeneous populations. While sufficient for most individuals, this approach does not capture the precise cost‐to‐benefit ratio for each person. Currently, iPSCs are expensive and require months to generate. However, once created, iPSCs can be differentiated into any tissue type and provide a limitless supply of tissue for drug and toxicity testing. For example, iPSC‐cardiomyocytes (iPSC‐CMs) can be used to investigate the effects of new antiarrhythmic drugs or caffeine (Luo et al, 2021). iPSC‐derived tissue can advance personalized medicine because they are derived from a single individual, making it feasible to predict which treatments are safe and effective for the individual with an enormous potential for achieving the goals of personalized medicine (Lau et al, 2019).
Disease modeling
Traditional methods of studying human diseases using animal models or patient‐derived tissue samples have been valuable, but many findings nevertheless do not translate to the clinic. Animal models do not always faithfully recapitulate the physiology of human disease, and because of interspecies variation, discoveries based on animal findings may not reflect the human pathophysiology (Matsa & Denning, 2012). Patient‐derived tissue is difficult to acquire and limited in quantity, and lacks longevity in cell culture (Beqqali et al, 2009). Immortalized cell culture models are complicated by genes that facilitate long‐term culture, and may also disrupt the transcriptome and cell function without reflecting patient‐specific genetic information. Derived from terminally differentiated cells, iPSCs provide an exciting new model that has the potential to transform basic science and precision medicine.
iPSCs can be differentiated into any tissue type, including cardiomyocytes, smooth muscle cells, endothelial cells, and fibroblasts. They can be used to identify genes responsible for a disease or be modified by environmental factors in a dish. Gene‐editing tools, such as TALENS, CRISPR‐Cas9, CRISPR‐I, and CRISPR‐A, can facilitate the discovery of molecular mechanisms of a disease (Hsu et al, 2014; Karakikes et al, 2017; Ma et al, 2018; Nishiga et al, 2022). By uncovering novel disease mechanisms, iPSC‐derived tissue is expected to identify novel disease‐specific biomarkers and druggable targets for therapies which may eventually translate into new clinical tools for diagnosis and treatment. Novel disease‐specific biomarkers have the potential to expedite the diagnosis of diseases and facilitate disease management by monitoring response to therapy. iPSCs are also a powerful tool for mechanistic studies that can identify novel drug targets, which are needed to change the scope and dimension of cardiovascular care (Fig 1).
Patient‐specific iPSCs are a limitless source of cardiovascular tissue that have been instrumental in breakthrough studies on the mechanisms of cardiovascular disease such as dilated cardiomyopathy (DCM; Sun et al, 2012), hypertrophic cardiomyopathy (HCM; Lan et al, 2013), arrhythmogenic right ventricular cardiomyopathy (ARVC; Kim et al, 2013; Asimaki et al, 2014), left ventricular non‐compaction (LVNC; Kodo et al, 2016), and LEOPARD syndrome (Carvajal‐Vergara et al, 2010). Imbued with an individual's genetic information, iPSCs are ideally suited for precision medicine (Grskovic et al, 2011) and will usher in a new era of biomarkers and therapies for cardiovascular disease. Simultaneously, iPSC‐derived tissues are an excellent platform to evaluate the effects of drugs, toxins, and environmental exposures (Sayed et al, 2016; Lee et al, 2019). Advances in tissue engineering have allowed for the use of more complex iPSC models such as organoids or engineered heart tissue, which are expected to elucidate interactions between different tissues and cells that contribute to disease pathophysiology (Kim et al, 2022).
Besides these applications, a central question is whether iPSCs can be used to study the impact of the environment on cardiovascular disease in this model. Murine embryonic stem cells have been used to study the effects of environmental toxins (Czyz et al, 2004a; Czyz et al, 2004b; Nikolova et al, 2005). The present review examines the advantages and challenges associated with the use of IPSCs in environmental cardiology.
Environmental cardiology
The environment is emerging as a significant risk factor for cardiovascular disease (Bhatnagar, 2017). However, the effects of environmental toxins from natural or man‐made sources remain unclear especially in the long term. Despite attempts to curb global warming, industrial pollution, and encroachment into natural habitats, humans are continuously exposed to environmental toxins such as particulate matter less than 2.5 micrometers in size (PM2.5; Rajagopalan et al, 2020). After years of declining tobacco use, the rise of e‐cigarettes threatens to renew cardiopulmonary disease. The legalization of marijuana has made cannabinoids more accessible around the world, but the long‐term effects of marijuana on the cardiovascular system remain unclear. A recent study revealed that the psychoactive component of marijuana delta9‐tetrahydrocannabinol (Δ9‐THC) causes vascular inflammation, oxidative stress in iPSC‐derived endothelial cells, and atherosclerosis in mouse models (Wei et al, 2022). Once mislabeled as being non‐addictive, the consequences of chronic marijuana and opioid consumption are now manifesting in adverse cardiovascular outcomes (Jalali et al, 2021; Rohani et al, 2021). The haphazard use of opioids revealed that a class of medications thought to be powerful tools in preventing pain rapidly became a new environmental hazard affecting some 4% of the U.S. population (Skolnick, 2018). Human iPSCs are a powerful model system to study the toxic effects of these different compounds on the human body (Fig 2).
Figure 2. iPSCs offer a unique patient‐specific model to study the effect of the environment on the human body.
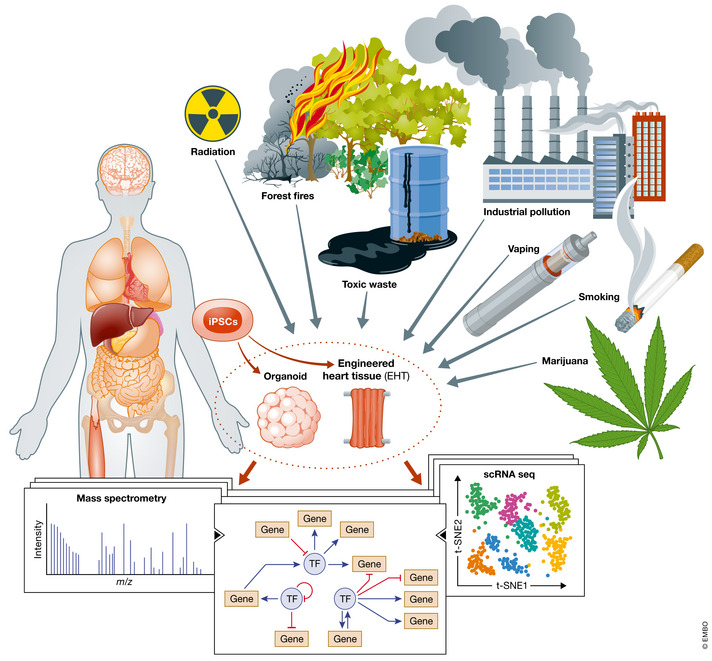
New or unrecognized environmental toxins such as particulate matter 2.5 (PM2.5) from forest fires and industrial pollution, marijuana, e‐cigarette, radiation, or toxic waste exposure might cause adverse effects on the human body. iPSC disease modeling is an opportunity to study the effects of environmental toxins before they result in end‐organ damage or organ failure. iPSCs can be differentiated into organ‐specific cells and assembled into fabricated tissues such as engineered heart tissue or differentiated into organoids comprised of organ‐specific tissue. Using single‐cell RNA sequencing (scRNA‐seq), mass spectrometry, and other ‐omic analysis, iPSC disease modeling could be leveraged to discover harmful effects of environmental factors before epidemiology studies are available and prevent significant morbidity and mortality. The discovery of druggable targets and pathways might lead to the development of new biomarkers and therapies for disease. [Colour figure can be viewed at wileyonlinelibrary.com]
Air pollution
The link between air pollution and cardiovascular disease has been described in epidemiological research (Brook et al, 2004; Kim et al, 2020a; Kim et al, 2020b; Kim et al, 2020c). About 91% of the world's population inhabits areas with poor air quality that exceeds the World Health Organization (WHO) safety limits, and air pollution is estimated to contribute to over 7 million deaths per year, or 1 in 9 deaths worldwide (Seaton et al, 1995; Stieb et al, 2002; Venkatesan, 2016). Fossil fuels are an abundant source of not only particulate matter but also carbon dioxide. Climate change mediated by carbon dioxide can lead to drought and wildfires that also exacerbate human exposure to particulate matter. The mega‐fire event experienced in California culminated in a giga‐fire in the fall of 2020 and led to vast amounts of particulate matter transforming the sky into a Mars‐like landscape, a spectacle that is now repeated worldwide. The long‐term effects of this exposure might not be evident in epidemiological studies for decades.
PM2.5 are capable of traversing through the lungs and entering the circulatory system. Thus, PM2.5 is thought to have adverse effects on the cardiovascular system. Studies in animal models and cell culture suggest that PM2.5 can enter the circulation and has the potential to cause adverse effects on the cardiovascular system (Hoek et al, 2013; Feng et al, 2016). iPSC disease modeling can be used to uncover the toxicities of particulate matter on the body and elucidate the mechanisms of their effects. After exposure to PM2.5, iPSC‐CMs were found to have increased arrhythmias due to upregulation of TRM3 (Cai et al, 2020). This may account for the increased incidence of sudden cardiac death associated with wildfires (Jones et al, 2020). Using iPSC‐derived tissue, investigators may be able to assess the effects of particulate matter on different tissues. The discoveries may inform policymakers and regulatory agencies to craft decisions and laws limiting exposure to PM2.5. The continuing advances in iPSC‐based drug discovery promise to an era of novel therapies for cardiovascular disease.
E‐cigarettes
Smoking has long been associated with lung disease and cancer, and is also a major cause of the cardiovascular disease (CVD), the leading cause of death worldwide (Ambrose & Barua, 2004; Munzel et al, 2020). Despite its deleterious effects, tobacco is consumed by 933 million people worldwide (Collaborators, 2017). While tobacco use in developed nations has dramatically improved over the past 50 years, new tobacco products, such as e‐cigarettes, have created a new generation of tobacco users. Since its introduction in 2007, e‐cigarette use among high school students in the U.S. has proliferated, with a more than 900% increase from 2011 to 2018. The latest National Health Interview Survey (NHIS) estimates that 5.5 million (3.8%) US adults were using e‐cigarettes in 2014. Strikingly, over 26.5% of high school students and 15.2% of middle school students were e‐cigarette users (Wang et al, 2021).
The toxicity and mechanisms of traditional cigarette smoke on the vascular system are well documented, but the effects of e‐cigarettes on the cardiovascular system have not been systematically studied thus far (Ambrose & Barua, 2004; Johnson et al, 2010; Polosa & Caponnetto, 2016; Benowitz & Fraiman, 2017). E‐cigarettes are liquid solutions containing propylene glycol, vegetable glycerin, nicotine, tetrahydrocannabinol (THC), vitamin E acetate, and other components that are vaporized by heating and inhaled as an aerosol. As e‐cigarettes do not involve combustion, they do not produce carbon monoxide or some of the other toxins associated with traditional cigarettes. Initially marketed as a smoking cessation aid, first‐generation e‐cigarettes had a low dose of nicotine and fewer adverse effects, but now e‐cigarette and electronic nicotine delivery systems (ENDS) have evolved to have much higher doses of nicotine. For example, a single pod of Juul™ has the equivalent of 20 cigarettes (one pack) worth of nicotine (Wu et al, 2019). The debate about whether e‐cigarettes will provide long‐term benefit or harm is ongoing, with little data on the cardiac and pulmonary effects (Drummond & Upson, 2014). The emergence of e‐cigarette or vaping use‐associated lung injury (EVALI) has further uncovered the adverse pulmonary effects of e‐cigarettes.
Epidemiology studies will likely take years to fully expose the potential adverse effects of e‐cigarettes on the cardiopulmonary system, and in the meantime, e‐cigarette use has grown dramatically without rigorous toxicology assessment. While studies in small animals and in vitro cell culture suggested adverse effects of e‐cigarettes on the cardiopulmonary system, iPSC disease modeling was the first to identify that e‐cigarette flavorants had deleterious effects on iPSC‐derived endothelial cells in vitro (Lee et al, 2019), helping to steer policymakers in the Food and Drug Administration (FDA) to recommend a ban on flavorings.
The effects of e‐cigarettes on embryonic development remain unclear. iPSC‐derived embryoid bodies contain immature cells and tissues that are an ideal platform to screen for environmental toxicities. When combined with single‐cell sequencing, embryoid bodies revealed potentially toxic effects of nicotine on embryonic development (Guo et al, 2019). A more rigorous and systematic analysis of the effects of e‐cigarettes on the development is needed.
E‐cigarettes include many hazardous and potentially hazardous compounds affecting the cardiopulmonary system. Nicotine has known adverse cardiovascular effects. Even without combustion, these compounds are toxic but need to be evaluated with a high‐throughput model that provides information on the toxicology of these compounds on human tissue. Genetic polymorphisms contribute to the development of vascular disease with traditional cigarette smoking. Investigators from the CARDIoGRAMplusC4D meta‐analysis found that genetic polymorphisms make certain individuals more susceptible to cardiovascular events (van der Harst & Verweij, 2018; Larsson et al, 2020). iPSC disease modeling and transcriptomics can be used to uncover single‐nucleotide polymorphisms (SNPs) that exacerbate cardiovascular disease in the setting of e‐cigarette exposure (Hindy et al, 2018; Levin et al, 2021). In addition to vascular disease, iPSC disease modeling may provide insights into gene and environment interactions with e‐cigarette components that cause arrhythmias. Toxicity studies in iPSC‐CMs have revealed that cinnamaldehyde is associated with cardiotoxicity and altered cardiomyocyte excitability (Nystoriak et al, 2019). More recently, smoking and e‐cigarette exposures were linked to ventricular repolarization and sudden cardiac death (SCD; Ip et al, 2020). The cardiotoxic effects of e‐cigarettes on channels could increase the likelihood of sudden cardiac death in individuals with mutations in ion channels. iPSC disease modeling in conjunction with novel gene‐editing technologies (Nishiga et al, 2022) are being deployed to uncover the possible causal link and identify individuals who may be at a greater risk of SCD after e‐cigarette exposure, as well as possibly identify novel antiarrhythmic agents that might prevent these adverse outcomes.
EVALI underscores the importance of testing e‐cigarette devices before marketing the products to the public. Clinical investigations using bronchoalveolar lavages indicated that nicotine, Δ9‐tetrahydrocannabinol (Δ9‐THC), the psychedelic component of marijuana, and vitamin E acetate, a component used to emulsify Δ9‐THC, were linked to EVALI. Studies in mouse models have found conflicting results, with one study suggesting vitamin E acetate is the causative agent. A more recent study indicates that the vehicle vegetable glycerol and propylene glycol might be sufficient to cause injury (Blount et al, 2019). iPSC‐derived lung tissue may be used to provide a more exhaustive study of the components of e‐cigarettes that cause EVALI.
Marijuana
Associated with EVALI, marijuana is emerging as a new potential and growing threat to cardiopulmonary health. The most popular illicit drug in the world is becoming legalized for medicinal and recreational uses, with rapidly growing rates of marijuana use being reported with legalization (Cerda et al, 2020). The long‐term effects of marijuana remain unclear because of years of restrictions on research, but it is implicated in cardiomyopathy, arrhythmias, and vascular disease from case reports and case series (Pacher et al, 2018). A recent large epidemiological study suggests that the odds ratio of marijuana is greater than traditional cigarettes and can predispose younger patients to premature cardiovascular disease (DeFilippis et al, 2018; Wei et al, 2022).
With legalization, the use of marijuana will increase and expose larger segments of the population to its potentially adverse effects (Pacher & Gao, 2008). Marijuana affects the body via the cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2; Pacher et al, 2018). CB1 activation is associated with endothelial dysfunction and atherosclerosis (El‐Remessy et al, 2011; Rajesh et al, 2012), whereas CB2 activation is related to vascular quiescence (Pacher & Mackie, 2012; Pacher et al, 2018). Marijuana is composed of over a hundred different cannabinoids. The psychedelic component of marijuana, Δ9‐THC, is an agonist of the CB1 receptor and causes inflammation and oxidative stress (El‐Remessy et al, 2011; Pacher et al, 2018). iPSC disease modeling and CRISPR‐Cas9 gene editing were used to elucidate the mechanisms Δ9‐THC‐mediated vascular dysfunction (Wei et al, 2022).
Δ9‐THC promotes inflammation and oxidative stress via the CB1 receptor, and the mechanisms have been previously described for MAP kinase activation and NF‐kb pathways. Briefly, CB1 mediates increased oxidative stress and inflammation implicated in diabetic retinopathy, cardiomyopathy, and endothelial dysfunction (Mukhopadhyay et al, 2010; Rajesh et al, 2010a; El‐Remessy et al, 2011; Rajesh et al, 2012). CB1 activation occurs via the MAP kinase pathway, which causes oxidative stress, inflammation, and cell death in human coronary artery endothelial cells (Liu et al, 2000; Pertwee et al, 2010; Rajesh et al, 2010b). iPSC disease modeling could elucidate the mechanisms accounting for why some individuals are more likely to develop cardiovascular disease or arrhythmias after using cannabinoids. While these studies in primary cells are important for understanding mechanisms of cardiovascular disease, iPSC disease modeling with vascular cells can also be used to reveal novel mechanisms that might serve as biomarkers and lead to novel therapies for the disease. Moreover, patient‐specific polymorphisms may help predict which individuals are more susceptible to adverse effects of marijuana or smoking or vaping. iPSC disease modeling could uncover polymorphisms that make an individual more likely to develop cardiovascular disease with cannabis exposure.
Some recent studies in iPSC‐derived neurons suggest that THC exposure perturbs gene expression profiles and is linked to neuropsychiatric disorders such as schizophrenia (Noh et al, 2017; Guennewig et al, 2018). iPSC‐embryoid body toxicity profiling is likely to reveal more global adverse effects of cannabinoids with the use of transcriptional profiling. Because it is considered an herbal remedy for nausea and vomiting, cannabis is often used by pregnant women, and epidemiological studies on prenatal exposure to cannabis indicated that it is associated with psychiatric disorders of offspring (Roncero et al, 2020). Recent investigations in iPSC‐derived neurons have described neurotoxicity caused by physiologic doses of THC exposure (10 μM) and perturbations in the expression of voltage‐gated calcium channels (Miranda et al, 2020). Indeed, marijuana is associated with cardiac arrhythmias, and iPSC disease modeling can be used to elucidate the molecular mechanisms of how cannabinoids might cause arrhythmias. The long‐term effects of marijuana on the cardiovascular system are not completely understood. However, a recent study showed using UK Biobank data that marijuana was a risk factor for cardiovascular disease (Wei et al, 2022). Here Wei et al (2022) used iPSC disease modeling and small animal models to investigate the mechanisms of Δ9‐THC‐mediated vascular dysfunction via the CB1 receptor. The prevalence of marijuana use is expected to increase with its growing legalization, and is likely to cause additional unanticipated, adverse health effects such as EVALI. Therefore, there is an urgent need to study the impact of marijuana in a pre‐clinical model that is more translatable and relevant to the human body than existing cell culture and animal studies.
Opioids
The current opioid epidemic in the United States has developed in three phases. In the 1990s, most of the opioid‐related deaths were caused by increased prescriptions of natural and semi‐synthetic opioids. A second wave began in 2010 with high usage of heroin (Rudd et al, 2016). The latest wave of opioid‐related deaths is associated with the significant increase in use of illicitly manufactured fentanyl. Overdose‐related deaths are mainly caused by bradycardia and respiratory depression. While recently oxycodone and hydrocodone have been the most commonly used opioids involved in overdose deaths (Mattson et al, 2021), more recently fentanyl and its derivatives are involved in more cardiovascular complications and malignant arrhythmias. Methadone has been well described to inhibit human cardiac ether‐a‐go‐go‐related gene (hERG)‐associated K+ current, the rapid component of the delayed rectifier current (IKr), which determines the duration of the resting QT interval (Katchman et al, 2002). Inhibition of this current results in a prolonged cardiac repolarization phase of the action potential, extending the QT interval and increasing the vulnerability to arrhythmias (Roden, 2004). Nevertheless, the ambulatory data on methadone‐induced cardiac arrests are limited, and perioperative data on QTc prolongation in patients undergoing non‐cardiac surgery suggest that even after administration of more than nine QTc‐prolonging drugs, opioids do not significantly contribute to clinically relevant QTc prolongation (Roden, 2004; Obal et al, 2014).
A more subtle effect may be occurring on the cardiovascular system. Epidemiological data suggest that chronic opioid administration is associated with a higher incidence of myocardial infarction and stroke (Chen & Ashburn, 2015). The American Heart Association (AHA) recently advised on the effect of chronic exposure to synthetic opioid analogs on cardiovascular (CV) function underscoring their potential detrimental impact on CV function and health (Chow et al, 2021; Dezfulian et al, 2021). Surprisingly, little is known about how chronic opioids affect endothelial and cardiomyocyte function. Distracted by the apparent short‐term effects of opioids (i.e., respiratory depression and bowel obstruction), long‐term effects have been lurking in the shadows and unrecognized. Recent insights into opioid receptor function and binding of different ligands have provided a broader understanding on the complex regulation of opioid signaling within the cardiovascular system (Gladden et al, 2016; O'Donnell & Jackson, 2017).
Microplastics
The widespread use of plastics and microplastics is a potentially novel hazard (Matthews et al, 2021). A recent study revealed that the abundance of microplastics may be vastly underestimated, with over 5 trillion pieces of plastic in the world's oceans that have a combined mass of 250,000 tons (Eriksen et al, 2014). The United Nations Environment Assembly (UNEP) has estimated that 4.8–12.7 million tons of plastic are introduced into oceans annually (Haward, 2018). The long‐term consequences of such ubiquitous pollution with plastics on the body are unknown but increasingly found in all elements of the aquatic food chain (Lehel & Murphy, 2021).
By studying the effects of microplastics in vitro, iPSCs might provide a window into the effects of plastics. Microplastics are consumed by plankton and are found in all aquatic species (Lehel & Murphy, 2021). The largest source of protein for humans is fish, and the microplastics do not simply transit through the gastrointestinal system but instead accumulate in the circulatory and adipose tissues (Lehel & Murphy, 2021). iPSC disease modeling can be used to understand how microplastics affect the gastrointestinal system, central nervous system, and the cardiovascular system. For example, epigenetic changes and the introduction on mutations in the genetic code may herald the initiation and progression of carcinogenesis. Initial studies with iPSCs have only revealed growth inhibition (Jeong et al, 2018). More recent studies have employed organoid models to understand how microplastics affect the body (Miloradovic et al, 2021; Winkler et al, 2022). Indeed, plastics are likely to modify gene expression and cause disease. More worrisome, plastics may alter epigenetic expression and be transmitted to future generations.
Summary and conclusion
iPSC disease modeling is emerging as a powerful paradigm for understanding the patient‐specific disease mechanisms. The interface with gene‐editing tools and iPSCs allows investigators to elucidate the underlying molecular mechanisms that cause disease. iPSCs, gene‐editing tools, and transcriptomics have the potential to revolutionize toxicology and rapidly advance our understanding of the adverse effects of environmental toxins. Because they contain the genetic code unique to each person, the use of iPSCs promises the discovery of new gene–environment interactions that can decipher why some individuals are more susceptible to environmental factors that exacerbate diseases such as cardiovascular disease.
Author contributions
Joseph C Wu: Conceptualization; resources; supervision; writing – original draft; writing – review and editing. Mark Chandy: Conceptualization; visualization; writing – original draft; writing – review and editing. Detlef Obal: Conceptualization; writing – original draft; writing – review and editing.
In addition to the CRediT author contributions listed above, the contributions in detail are:
MC: manuscript writing and preparing figures; DO: manuscript writing; JCW: manuscript writing and final approval of the manuscript.
Disclosure and competing interests statement
J.C.W. is a co‐founder and SAB of Greenstone Biosciences, and M.C. is a consultant for Greenstone Biosciences, but this manuscript was written independently.
Pending issues.
iPSCs are a powerful model system to investigate the adverse effects of these compounds on the body and avoid animal testing. However, 2D iPSC‐derived tissues are immature and may not fully recapitulate the cellular physiology of somatic cells. With the development of 3D models such as organoids and EHT, and advances in single‐cell RNA sequencing, iPSC disease modeling has improved significantly. 3D models provide a better environment that promotes cellular maturation and development. More importantly, single‐cell RNA sequencing can provide spatial transcriptomics of complex cell–cell interactions. With ongoing advancements in biomaterials, biomedical engineering, and next‐generation sequencing, iPSC disease modeling is continuing to evolve into a high‐throughput screening platform for environmental exposures.
For more information.
For additional information on iPSC Biobanking, cell culture protocols, publications, and contact information to request iPSC lines, please visit the Stanford Cardiovascular Institute (SCVI) Biobank website: https://med.stanford.edu/scvibiobank.html.
Acknowledgements
We thank Blake Wu for his assistance with manuscript preparation. Owing to space limitation, we are unable to include all the important papers relevant to iPSC and environmental exposure research, and we apologize to those investigators who have otherwise contributed substantially to this field. This work was supported by the Stanford Cardiovascular Institute, Tobacco‐Related Disease Research Program (TRDRP) 27IR‐0012, Steven M. Gootter Foundation, P01 HL152953, and American Heart Association 20YVNR3500014 (JCW), Stanford Maternal & Child Health Research Institute (MCHRI) Tip Grant (DO).
EMBO Mol Med (2022) 14: e13260
See the Glossary for abbreviations used in this article.
Contributor Information
Mark Chandy, Email: mchandy2@uwo.ca.
Joseph C Wu, Email: joewu@stanford.edu.
References
- Ambrose JA, Barua RS (2004) The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol 43: 1731–1737 [DOI] [PubMed] [Google Scholar]
- Asimaki A, Kapoor S, Plovie E, Karin Arndt A, Adams E, Liu Z, James CA, Judge DP, Calkins H, Churko J et al (2014) Identification of a new modulator of the intercalated disc in a zebrafish model of arrhythmogenic cardiomyopathy. Sci Transl Med 6: 240ra274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Fraiman JB (2017) Cardiovascular effects of electronic cigarettes. Nat Rev Cardiol 14: 447–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beqqali A, van Eldik W, Mummery C, Passier R (2009) Human stem cells as a model for cardiac differentiation and disease. Cell Mol Life Sci 66: 800–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar A (2017) Environmental determinants of cardiovascular disease. Circ Res 121: 162–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount BC, Karwowski MP, Shields PG, Morel‐Espinosa M, Valentin‐Blasini L, Gardner M, Braselton M, Brosius CR, Caron KT, Chambers D et al (2019) Vitamin E acetate in bronchoalveolar‐lavage fluid associated with EVALI. N Engl J Med 382: 697–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC Jr et al (2004) Air pollution and cardiovascular disease: A statement for healthcare professionals from the expert panel on population and prevention science of the American Heart Association. Circulation 109: 2655–2671 [DOI] [PubMed] [Google Scholar]
- Cai B, Xia T, Qian Y, Lu H, Cai R, Wang C (2020) Association between fine particulate matter and fatal hemorrhagic stroke incidence: a time stratified case‐crossover study in Shanghai, China. J Occup Environ Med 62: 916–921 [DOI] [PubMed] [Google Scholar]
- Carvajal‐Vergara X, Sevilla A, D'Souza SL, Ang YS, Schaniel C, Lee DF, Yang L, Kaplan AD, Adler ED, Rozov R et al (2010) Patient‐specific induced pluripotent stem‐cell‐derived models of LEOPARD syndrome. Nature 465: 808–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerda M, Mauro C, Hamilton A, Levy NS, Santaella‐Tenorio J, Hasin D, Wall MM, Keyes KM, Martins SS (2020) Association between recreational marijuana legalization in the United States and changes in marijuana use and cannabis use disorder from 2008 to 2016. JAMA Psychiat 77: 165–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Ashburn MA (2015) Cardiac effects of opioid therapy. Pain Med 16(Suppl 1): S27‐31 [DOI] [PubMed] [Google Scholar]
- Chow SL, Sasson C, Benjamin IJ, Califf RM, Compton WM, Oliva EM, Robson C, Sanchez EJ (2021) Opioid use and its relationship to cardiovascular disease and brain health: a presidential advisory from the American Heart Association. Circulation 144: e218–e232 [DOI] [PubMed] [Google Scholar]
- Collaborators GBDT (2017) Smoking prevalence and attributable disease burden in 195 countries and territories, 1990‐2015: a systematic analysis from the global burden of disease study 2015. Lancet 389: 1885–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czyz J, Guan K, Zeng Q, Nikolova T, Meister A, Schonborn F, Schuderer J, Kuster N, Wobus AM (2004a) High frequency electromagnetic fields (GSM signals) affect gene expression levels in tumor suppressor p53‐deficient embryonic stem cells. Bioelectromagnetics 25: 296–307 [DOI] [PubMed] [Google Scholar]
- Czyz J, Nikolova T, Schuderer J, Kuster N, Wobus AM (2004b) Non‐thermal effects of power‐line magnetic fields (50 Hz) on gene expression levels of pluripotent embryonic stem cells‐the role of tumour suppressor p53. Mutat Res 557: 63–74 [DOI] [PubMed] [Google Scholar]
- DeFilippis EM, Singh A, Divakaran S, Gupta A, Collins BL, Biery D, Qamar A, Fatima A, Ramsis M, Pipilas D et al (2018) Cocaine and marijuana use among young adults with myocardial infarction. J Am Coll Cardiol 71: 2540–2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezfulian C, Orkin AM, Maron BA, Elmer J, Girotra S, Gladwin MT, Merchant RM, Panchal AR, Perman SM, Starks MA et al (2021) Opioid-associated out-of-hospital cardiac arrest: distinctive clinical features and implications for health care and public responses: a scientific statement from the American Heart Association. Circulation 143: e836–e870 [DOI] [PubMed] [Google Scholar]
- Drummond MB, Upson D (2014) Electronic cigarettes. Potential harms and benefits. Ann Am Thorac Soc 11: 236–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Remessy AB, Rajesh M, Mukhopadhyay P, Horvath B, Patel V, Al‐Gayyar MM, Pillai BA, Pacher P (2011) Cannabinoid 1 receptor activation contributes to vascular inflammation and cell death in a mouse model of diabetic retinopathy and a human retinal cell line. Diabetologia 54: 1567–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen M, Lebreton LC, Carson HS, Thiel M, Moore CJ, Borerro JC, Galgani F, Ryan PG, Reisser J (2014) Plastic pollution in the World's oceans: more than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS One 9: e111913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C, Li J, Sun W, Zhang Y, Wang Q (2016) Impact of ambient fine particulate matter (PM2.5) exposure on the risk of influenza‐like‐illness: a time‐series analysis in Beijing, China. Environ Health 15: 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladden RM, Martinez P, Seth P (2016) Fentanyl law enforcement submissions and increases in synthetic opioid‐involved overdose deaths ‐ 27 states, 2013‐2014. MMWR Morb Mortal Wkly Rep 65: 837–843 [DOI] [PubMed] [Google Scholar]
- Grskovic M, Javaherian A, Strulovici B, Daley GQ (2011) Induced pluripotent stem cells: opportunities for disease modelling and drug discovery. Nat Rev Drug Discov 10: 915–929 [DOI] [PubMed] [Google Scholar]
- Guennewig B, Bitar M, Obiorah I, Hanks J, O'Brien EA, Kaczorowski DC, Hurd YL, Roussos P, Brennand KJ, Barry G (2018) THC exposure of human iPSC neurons impacts genes associated with neuropsychiatric disorders. Transl Psychiatry 8: 89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Tian L, Zhang JZ, Kitani T, Paik DT, Lee WH, Wu JC (2019) Single‐cell RNA sequencing of human embryonic stem cell differentiation delineates adverse effects of nicotine on embryonic development. Stem Cell Reports 12: 772–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Harst P, Verweij N (2018) Identification of 64 novel genetic loci provides an expanded view on the genetic architecture of coronary artery disease. Circ Res 122: 433–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haward M (2018) Plastic pollution of the world's seas and oceans as a contemporary challenge in ocean governance. Nat Commun 9: 667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindy G, Wiberg F, Almgren P, Melander O, Orho‐Melander M (2018) Polygenic risk score for coronary heart disease modifies the elevated risk by cigarette smoking for disease incidence. Circ Genom Precis Med 11: e001856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek G, Krishnan RM, Beelen R, Peters A, Ostro B, Brunekreef B, Kaufman JD (2013) Long‐term air pollution exposure and cardio‐ respiratory mortality: a review. Environ Health 12: 43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PD, Lander ES, Zhang F (2014) Development and applications of CRISPR‐Cas9 for genome engineering. Cell 157: 1262–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip M, Diamantakos E, Haptonstall K, Choroomi Y, Moheimani RS, Nguyen KH, Tran E, Gornbein J, Middlekauff HR (2020) Tobacco and electronic cigarettes adversely impact ECG indexes of ventricular repolarization: Implication for sudden death risk. Am J Physiol Heart Circ Physiol 318: H1176–H1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalali Z, Bahrampour S, Khalili P, Khademalhosseini M, Esmaeili Nadimi A (2021) Cohort‐based analysis of paternal opioid use in relation to offspring's BMI and plasma lipid profile. Sci Rep 11: 9462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong CB, Kang HM, Lee YH, Kim MS, Lee JS, Seo JS, Wang M, Lee JS (2018) Nanoplastic ingestion enhances toxicity of persistent organic pollutants (POPs) in the monogonont rotifer Brachionus koreanus via multixenobiotic resistance (MXR) disruption. Environ Sci Technol 52: 11411–11418 [DOI] [PubMed] [Google Scholar]
- Johnson HM, Gossett LK, Piper ME, Aeschlimann SE, Korcarz CE, Baker TB, Fiore MC, Stein JH (2010) Effects of smoking and smoking cessation on endothelial function: 1‐year outcomes from a randomized clinical trial. J Am Coll Cardiol 55: 1988–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CG, Rappold AG, Vargo J, Cascio WE, Kharrazi M, McNally B, Hoshiko S, with the CARES Surveillance Group (2020) Out‐of‐hospital cardiac arrests and wildfire‐related particulate matter during 2015‐2017 California wildfires. J Am Heart Assoc 9: e014125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakikes I, Termglinchan V, Cepeda DA, Lee J, Diecke S, Hendel A, Itzhaki I, Ameen M, Shrestha R, Wu H et al (2017) A comprehensive TALEN‐based knockout library for generating human‐induced pluripotent stem cell‐based models for cardiovascular diseases. Circ Res 120: 1561–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katchman AN, McGroary KA, Kilborn MJ, Kornick CA, Manfredi PL, Woosley RL, Ebert SN (2002) Influence of opioid agonists on cardiac human ether‐a‐go‐go‐related gene K(+) currents. J Pharmacol Exp Ther 303: 688–694 [DOI] [PubMed] [Google Scholar]
- Kawamura M, Miyagawa S, Miki K, Saito A, Fukushima S, Higuchi T, Kawamura T, Kuratani T, Daimon T, Shimizu T et al (2012) Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell‐derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation 126: S29–S37 [DOI] [PubMed] [Google Scholar]
- Kim C, Wong J, Wen J, Wang S, Wang C, Spiering S, Kan NG, Forcales S, Puri PL, Leone TC et al (2013) Studying arrhythmogenic right ventricular dysplasia with patient‐specific iPSCs. Nature 494: 105–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Kim WH, Kim YY, Park HY (2020a) Air pollution and central nervous system disease: A review of the impact of fine particulate matter on neurological disorders. Front Public Health 8: 575330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IS, Yang PS, Lee J, Yu HT, Kim TH, Uhm JS, Kim JY, Pak HN, Lee MH, Joung B (2020b) Long‐term fine particulate matter exposure and cardiovascular mortality in the general population: a nationwide cohort study. J Cardiol 75: 549–558 [DOI] [PubMed] [Google Scholar]
- Kim OJ, Lee SH, Kang SH, Kim SY (2020c) Incident cardiovascular disease and particulate matter air pollution in South Korea using a population‐based and nationwide cohort of 0.2 million adults. Environ Health 19: 113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Kamm RD, Vunjak‐Novakovic G, Wu JC (2022) Progress in multicellular human cardiac organoids for clinical applications. Cell Stem Cell 29: 503–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodo K, Ong SG, Jahanbani F, Termglinchan V, Hirono K, InanlooRahatloo K, Ebert AD, Shukla P, Abilez OJ, Churko JM et al (2016) iPSC‐derived cardiomyocytes reveal abnormal TGF‐beta signalling in left ventricular non‐compaction cardiomyopathy. Nat Cell Biol 18: 1031–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan F, Lee AS, Liang P, Sanchez‐Freire V, Nguyen PK, Wang L, Han L, Yen M, Wang Y, Sun N et al (2013) Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient‐specific induced pluripotent stem cells. Cell Stem Cell 12: 101–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson SC, Mason AM, Back M, Klarin D, Damrauer SM, Million Veteran P, Michaelsson K, Burgess S (2020) Genetic predisposition to smoking in relation to 14 cardiovascular diseases. Eur Heart J 41: 3304–3310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau E, Paik DT, Wu JC (2019) Systems‐wide approaches in induced pluripotent stem cell models. Annu Rev Pathol 14: 395–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WH, Ong S‐G, Zhou Y, Tian L, Bae HR, Baker N, Whitlatch A, Mohammadi L, Guo H, Nadeau KC et al (2019) Modeling cardiovascular risks of E‐cigarettes with human‐induced pluripotent stem cell–derived endothelial cells. J Am Coll Cardiol 73: 2722–2737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehel J, Murphy S (2021) Microplastics in the food chain: Food safety and environmental aspects. Rev Environ Contam Toxicol 259: 1–49 [DOI] [PubMed] [Google Scholar]
- Levin MG, Klarin D, Assimes TL, Freiberg MS, Ingelsson E, Lynch J, Natarajan P, O'Donnell C, Rader DJ, Tsao PS et al (2021) Genetics of smoking and risk of atherosclerotic cardiovascular diseases: a mendelian randomization study. JAMA Netw Open 4: e2034461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Gao B, Mirshahi F, Sanyal AJ, Khanolkar AD, Makriyannis A, Kunos G (2000) Functional CB1 cannabinoid receptors in human vascular endothelial cells. Biochem J 346: 835–840 [PMC free article] [PubMed] [Google Scholar]
- Lu TY, Lin B, Kim J, Sullivan M, Tobita K, Salama G, Yang L (2013) Repopulation of decellularized mouse heart with human induced pluripotent stem cell‐derived cardiovascular progenitor cells. Nat Commun 4: 2307 [DOI] [PubMed] [Google Scholar]
- Luo YS, Chen Z, Blanchette AD, Zhou YH, Wright FA, Baker ES, Chiu WA, Rusyn I (2021) Relationships between constituents of energy drinks and beating parameters in human induced pluripotent stem cell (iPSC)‐derived cardiomyocytes. Food Chem Toxicol 149: 111979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Zhang JZ, Itzhaki I, Zhang SL, Chen H, Haddad F, Kitani T, Wilson KD, Tian L, Shrestha R et al (2018) Determining the pathogenicity of a genomic variant of uncertain significance using CRISPR/Cas9 and human‐induced pluripotent stem cells. Circulation 138: 2666–2681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsa E, Denning C (2012) In vitro uses of human pluripotent stem cell‐derived cardiomyocytes. J Cardiovasc Transl Res 5: 581–592 [DOI] [PubMed] [Google Scholar]
- Matthews S, Mai L, Jeong CB, Lee JS, Zeng EY, Xu EG (2021) Key mechanisms of micro‐ and nanoplastic (MNP) toxicity across taxonomic groups. Comp Biochem Physiol C Toxicol Pharmacol 247: 109056 [DOI] [PubMed] [Google Scholar]
- Mattson CL, Tanz LJ, Quinn K, Kariisa M, Patel P, Davis NL (2021) Trends and geographic patterns in drug and synthetic opioid overdose deaths ‐ United States, 2013‐2019. MMWR Morb Mortal Wkly Rep 70: 202–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miloradovic D, Pavlovic D, Jankovic MG, Nikolic S, Papic M, Milivojevic N, Stojkovic M, Ljujic B (2021) Human embryos, induced pluripotent stem cells, and organoids: models to assess the effects of environmental plastic pollution. Front Cell Dev Biol 9: 709183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda CC, Barata T, Vaz SH, Ferreira C, Quintas A, Bekman EP (2020) hiPSC‐based model of prenatal exposure to cannabinoids: effect on neuronal differentiation. Front Mol Neurosci 13: 119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay P, Rajesh M, Batkai S, Patel V, Kashiwaya Y, Liaudet L, Evgenov OV, Mackie K, Hasko G, Pacher P (2010) CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin‐induced cardiomyopathy and in human cardiomyocytes. Cardiovasc Res 85: 773–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzel T, Hahad O, Kuntic M, Keaney JF, Deanfield JE, Daiber A (2020) Effects of tobacco cigarettes, e‐cigarettes, and waterpipe smoking on endothelial function and clinical outcomes. Eur Heart J 41: 4057–4070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson TJ, Martinez‐Fernandez A, Yamada S, Perez‐Terzic C, Ikeda Y, Terzic A (2009) Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation 120: 408–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neofytou E, O'Brien CG, Couture LA, Wu JC (2015) Hurdles to clinical translation of human induced pluripotent stem cells. J Clin Invest 125: 2551–2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova T, Czyz J, Rolletschek A, Blyszczuk P, Fuchs J, Jovtchev G, Schuderer J, Kuster N, Wobus AM (2005) Electromagnetic fields affect transcript levels of apoptosis‐related genes in embryonic stem cell‐derived neural progenitor cells. FASEB J 19: 1686–1688 [DOI] [PubMed] [Google Scholar]
- Nishiga M, Liu C, Qi LS, Wu JC (2022) The use of new CRISPR tools in cardiovascular research and medicine. Nat Rev Cardiol 19: 505–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh H, Shao Z, Coyle JT, Chung S (2017) Modeling schizophrenia pathogenesis using patient‐derived induced pluripotent stem cells (iPSCs). Biochim Biophys Acta Mol Basis Dis 1863: 2382–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystoriak MA, Kilfoil PJ, Lorkiewicz PK, Ramesh B, Kuehl PJ, McDonald J, Bhatnagar A, Conklin DJ (2019) Comparative effects of parent and heated cinnamaldehyde on the function of human iPSC‐derived cardiac myocytes. Toxicol In Vitro 61: 104648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obal D, Wu JC (2020) Induced pluripotent stem cells as a platform to understand patient‐specific responses to opioids and anaesthetics. Br J Pharmacol 177: 4581–4594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obal D, Yang D, Sessler DI (2014) Perioperative doses of ondansetron or dolasetron do not lengthen the QT interval. Mayo Clin Proc 89: 69–80 [DOI] [PubMed] [Google Scholar]
- O'Donnell FT, Jackson DL (2017) Opioid use disorder and pregnancy. Mo Med 114: 181–186 [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Gao B (2008) Endocannabinoids and liver disease. III. Endocannabinoid effects on immune cells: Implications for inflammatory liver diseases. Am J Physiol Gastrointest Liver Physiol 294: G850–G854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacher P, Mackie K (2012) Interplay of cannabinoid 2 (CB2) receptors with nitric oxide synthases, oxidative and nitrative stress, and cell death during remote neurodegeneration. J Mol Med 90: 347–351 [DOI] [PubMed] [Google Scholar]
- Pacher P, Steffens S, Hasko G, Schindler TH, Kunos G (2018) Cardiovascular effects of marijuana and synthetic cannabinoids: the good, the bad, and the ugly. Nat Rev Cardiol 15: 151–166 [DOI] [PubMed] [Google Scholar]
- Paik DT, Chandy M, Wu JC (2020) Patient and disease‐specific induced pluripotent stem cells for discovery of personalized cardiovascular drugs and therapeutics. Pharmacol Rev 72: 320–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K et al (2010) International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB(1) and CB(2). Pharmacol Rev 62: 588–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosa R, Caponnetto P (2016) The health effects of electronic cigarettes. N Engl J Med 375: 2608–2609 [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Brauer M, Bhatnagar A, Bhatt DL, Brook JR, Huang W, Munzel T, Newby D, Siegel J, Brook RD et al (2020) Personal‐level protective actions against particulate matter air pollution exposure: a scientific statement from the American Heart Association. Circulation 142: e411–e431 [DOI] [PubMed] [Google Scholar]
- Rajesh M, Mukhopadhyay P, Bátkai S, Patel V, Saito K, Matsumoto S, Kashiwaya Y, Horváth B, Mukhopadhyay B, Becker L et al (2010a) Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol 56: 2115–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajesh M, Mukhopadhyay P, Hasko G, Liaudet L, Mackie K, Pacher P (2010b) Cannabinoid‐1 receptor activation induces reactive oxygen species‐dependent and ‐independent mitogen‐activated protein kinase activation and cell death in human coronary artery endothelial cells. Br J Pharmacol 160: 688–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajesh M, Batkai S, Kechrid M, Mukhopadhyay P, Lee WS, Horvath B, Holovac E, Cinar R, Liaudet L, Mackie K et al (2012) Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes 61: 716–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden DM (2004) Antiarrhythmic drugs: Past, present, and future. Heart Rhythm 1: 57C–66C [DOI] [PubMed] [Google Scholar]
- Rohani F, Rezayat AA, Zarifian A, Nour MG, Vakilian F, Sahebkar A, Dadgarmoghaddam M (2021) Opioid dependency and myocardial infarction: a systematic review and meta‐analysis. Curr Rev Clin Exp Pharmacol 16: 330–340 [DOI] [PubMed] [Google Scholar]
- Roncero C, Valriberas‐Herrero I, Mezzatesta‐Gava M, Villegas JL, Aguilar L, Grau‐Lopez L (2020) Cannabis use during pregnancy and its relationship with fetal developmental outcomes and psychiatric disorders. A systematic review. Reprod Health 17: 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd RA, Seth P, David F, Scholl L (2016) Increases in drug and opioid‐involved overdose deaths ‐ United States, 2010‐2015. MMWR Morb Mortal Wkly Rep 65: 1445–1452 [DOI] [PubMed] [Google Scholar]
- Sayed N, Liu C, Wu JC (2016) Translation of human‐induced pluripotent stem cells: from clinical trial in a dish to precision medicine. J Am Coll Cardiol 67: 2161–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaton A, MacNee W, Donaldson K, Godden D (1995) Particulate air pollution and acute health effects. Lancet 345: 176–178 [DOI] [PubMed] [Google Scholar]
- Skolnick P (2018) The opioid epidemic: crisis and solutions. Annu Rev Pharmacol Toxicol 58: 143–159 [DOI] [PubMed] [Google Scholar]
- Stieb DM, Judek S, Burnett RT (2002) Meta‐analysis of time‐series studies of air pollution and mortality: effects of gases and particles and the influence of cause of death, age, and season. J Air Waste Manag Assoc 52: 470–484 [DOI] [PubMed] [Google Scholar]
- Sun N, Yazawa M, Liu J, Han L, Sanchez‐Freire V, Abilez OJ, Navarrete EG, Hu S, Wang L, Lee A et al (2012) Patient‐specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci Transl Med 4: 130ra147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872 [DOI] [PubMed] [Google Scholar]
- Templin C, Zweigerdt R, Schwanke K, Olmer R, Ghadri JR, Emmert MY, Muller E, Kuest SM, Cohrs S, Schibli R et al (2012) Transplantation and tracking of human‐induced pluripotent stem cells in a pig model of myocardial infarction: assessment of cell survival, engraftment, and distribution by hybrid single photon emission computed tomography/computed tomography of sodium iodide symporter transgene expression. Circulation 126: 430–439 [DOI] [PubMed] [Google Scholar]
- Venkatesan P (2016) WHO report: air pollution is a major threat to health. Lancet Respir Med 4: 351 [DOI] [PubMed] [Google Scholar]
- Wang TW, Gentzke AS, Neff LJ, Glidden EV, Jamal A, Park‐Lee E, Ren C, Cullen KA, King BA, Hacker KA (2021) Characteristics of e‐cigarette use behaviors among US youth, 2020. JAMA Netw Open 4: e2111336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei TT, Chandy M, Nishiga M, Zhang A, Kumar KK, Thomas D, Manhas A, Rhee S, Justesen JM, Chen IY et al (2022) Cannabinoid receptor 1 antagonist genistein attenuates marijuana‐induced vascular inflammation. Cell 185: e1623 [DOI] [PubMed] [Google Scholar]
- Winkler AS, Cherubini A, Rusconi F, Santo N, Madaschi L, Pistoni C, Moschetti G, Sarnicola ML, Crosti M, Rosso L et al (2022) Human airway organoids and microplastic fibers: a new exposure model for emerging contaminants. Environ Int 163: 107200 [DOI] [PubMed] [Google Scholar]
- Wu JC, Rhee JW, Sallam K (2019) Electronic cigarettes: where there is smoke there is disease. J Am Coll Cardiol 74: 3121–3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga‐Otto K, Antosiewicz‐Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R et al (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318: 1917–1920 [DOI] [PubMed] [Google Scholar]
- Zhao MT, Chen H, Liu Q, Shao NY, Sayed N, Wo HT, Zhang JZ, Ong SG, Liu C, Kim Y et al (2017) Molecular and functional resemblance of differentiated cells derived from isogenic human iPSCs and SCNT‐derived ESCs. Proc Natl Acad Sci U S A 114: E11111–E11120 [DOI] [PMC free article] [PubMed] [Google Scholar]


