Abstract
Objective: Revision total hip arthroplasty (THA) imposes physical and financial burdens on patients and depletes limited medical resources. Causes for revision THAs can change as technology changes. Therefore, understanding contemporary causes is essential for clinical decision-making. We analyzed causes and trends associated with revision THA in the 2010s. Methods: We retrospectively identified 803 revision cases after primary THAs were performed at our center from January 2011 to December 2020. Causes for revision were reviewed and compared among patients who were grouped by the date of revision and interval between primary and revision THA. Results: The most common causes were aseptic loosening (66.6%), infection (11.0%), osteolysis (6.0%), periprosthetic fracture (5.5%), and instability (3.5%). The values for incidence of infection, fracture, and instability were higher in the early revision group than in the late revision group (threshold, 2 years after primary surgery, all P<0.05). The proportion of revision THAs increased by 25.6% from 2011-2015 to 2016-2020, and the time between primary and revision THAs increased from 8.8 ± 7.0 years to 10.2 ± 6.8 years (P=0.003). In the last 5 years of the study period, the incidence of aseptic loosening decreased and the rates of osteolysis, acetabular wear by hemiarthroplasty, and instability increased, compared to 2011-2015 (all P<0.05). Conclusions: Aseptic loosening was the most common cause of revision THA. Revisions due to infection, fracture, and instability occurred more frequently during the early post-THA period after primary THA. Revisions due to osteolysis, instability, and acetabular wear have increased in recent years.
Keywords: Total hip arthroplasty, revision THA, aseptic loosening, infection, periprosthetic fracture, instability
Introduction
Total hip arthroplasty (THA) has been performed for >100 years and stands among the most successful orthopedic surgeries during these decades [1]. With economic development and an aging population, THAs continue to be increasingly performed in developing countries [2]. Primary THAs yield increasingly good results, and the absolute number of THAs performed has increased. Nevertheless, the number of failures and revisions have also increased [3,4]. THA failure imposes physical and financial burdens on patients and depletes limited medical resources [5,6]. Obtaining information regarding causes of revision THA can aid surgeons to better understand reasons for failure of primary THA, and help them prepare for revision surgery during the preoperative period [7,8]. Investigation of major causes of primary THA failure can assist doctors with the development of more appropriate prosthesis, improve surgical techniques, and avoid complications and failures.
Studies that examined causes of revision THA have been performed in some countries [6,9-17]. Evaluation of causes of revision is complicated, and some previous studies used large databases that included cases from multiple institutions. Therefore, diagnoses of causes of revision were often not standardized, which could lead to study bias. In addition to the inherent limitations of studies using large databases, some studies have relatively small sample sizes, early research dates, lack of detail in analyses, and unclear classification. Studies from different periods and studies using different data sources have reported varying results. Over the last years, the volume of primary THAs continues to increase. Surgical approaches, prosthesis designs, understanding of diseases, and perioperative management continue to change. These changes contribute to changes in modes of failure, and causes of revision may be different than in the past. Contemporary distributions and trends in causes of revisions remain to be further investigated to enhance primary THA efficacy. The present study aimed (i) to update and analyze contemporary causes of primary revision THA at a large center in a developing country with a relatively large surgical volume over a 10-year period, (ii) to analyze causes of revision THA during the early and late post-THA period, and (iii) to analyze changes and trends in causes of revision.
Materials and methods
Ethical approval
This study was performed in accordance with the principles embodied in the Declaration of Helsinki and its amendments. The study protocol was approved by the Beijing Jishuitan Hospital institutional review board (approval number, 202008-02). The informed consent requirement was waived due to the retrospective nature of the study.
Study design
We retrospectively reviewed records of 803 revisions for primary THA (779 patients, 803 hips). According to the institution’s arthroplasty registration center data, the revisions were performed at our center between January 2011 and December 2020. Each patient’s demographic data (e.g., age, sex, height, weight, and body mass index), dates of primary and revision THA, and failure mechanisms were documented. The time between primary and revision THA was calculated, and revision procedures were divided into two groups according to time; early revisions were performed ≤2 years, and late revisions were performed >2 years, after the primary THA. We also classified the causes of revision THA into the categories of infection, periprosthetic fracture, aseptic loosening, osteolysis (no loosening), instability, adverse local tissue reactions/adverse reaction to metal debris (ALTR/ARMD), acetabular wear by hemiarthroplasty, fracture of ceramic components, or other (e.g., implant fracture, leg length discrepancy, prosthesis impingement, unexplained pain, and soft tissue stimulation). Two experienced surgeons identified the main cause of the revision procedures based on clinical documents, laboratory and imaging examination results, and condition of the retrieved prosthesis. If there were multiple reasons for hip revision, by priority, they would be categorized as infection, periprosthetic fracture/ceramic fracture, aseptic loosening, instability, osteolysis, ALTR/ARMD, or acetabular wear by hemiarthroplasty. The revision procedures were stratified into two groups based on revision date: 2011-2015 or 2016-2020.
Statistical analysis
The Kolmogorov-Smirnov test was used to assess continuous data for a normal distribution. For normally distributed data, mean and standard deviation values were used, and Student’s t-tests were applied. For non-normally distributed data, median and inter-quartile range values were used, and Mann-Whitney U tests were performed. For categorical variables, frequencies and percentages were used; chi-square tests and Fisher’s exact tests were performed as needed. The times between primary and revision THAs were calculated and analyzed using Kaplan-Meier survival curves. SPSS version 23.0 (IBM Corp., Armonk, NY, USA) was used for all analyses. Statistical significance was set at P<0.05.
Results
A total of 803 revision THA procedures were performed at our institution from January 2011 to December 2020 (mean age: 61.1 ± 12.8 years [range, 19-89 years]). Patients’ mean height was 164.8 ± 7.8 cm (range, 143-184 cm), and mean weight was 66.0 ± 12.0 kg (range, 37-100 kg). The mean body mass index was 24.2 ± 3.6 kg/m2 (range, 14.8-35.0 kg/m2). Out of 803 hips, 420 (52.3%) were in women and 383 (47.7%) were in men; there were 415 (51.7%) left hips and 388 (48.3%) right hips (Table 1). The mean time between primary and revision THAs was 9.6 ± 7.0 years (range, 0.02-36.6 years). Less than one-fifth of the patients (141, 17.6%) underwent revision ≤2 years after primary THA (i.e., early revision). In the early revision group, the mean time was 1.1 ± 0.7 years (range, 0.02-2.0 years). In 662 patients (82.4%) who underwent revisions >2 years after primary THA (i.e., late revision), the mean time was 11.4 ± 6.3 years (range, 2.3-36.6 years).
Table 1.
Patient demographics
| All revisions [n=803] | Early revisions [n=141] | Late revisions [n=662] | P | |
|---|---|---|---|---|
| Age (years)* | 61.1 ± 12.8 | 60.4 ± 13.5 | 61.2 ± 12.7 | 0.515 |
| Body mass index (kg/m2)* | 24.2 ± 3.6 | 24.2 ± 3.5 | 24.2 ± 3.6 | 0.996 |
| Sex** | 0.057 | |||
| Men | 383 (47.7%) | 57 (40.4%) | 326 (49.2%) | |
| Women | 420 (52.3%) | 84 (59.6%) | 336 (50.8%) | |
| Side** | 0.872 | |||
| Left | 415 (51.7%) | 72 (51.1%) | 343 (51.8%) | |
| Right | 388 (48.3%) | 69 (48.9%) | 319 (48.2%) | |
| Date of revision** | 0.050 | |||
| 2011-2015 | 356 (44.3%) | 73 (51.8%) | 283 (42.7%) | |
| 2016-2020 | 447 (55.7%) | 68 (48.2%) | 379 (57.3%) |
Mean ± SD, Student’s t-test;
n (%), Chi-square test.
Two experienced surgeons identified the main causes for revision, according to the categories presented in the Study Design section (Figure 1). The causes for revision were aseptic loosening in 535 (66.6%) patients, infection in 88 (11.0%) patients, periprosthetic fracture in 44 (5.5%) patients, osteolysis in 48 (6.0%) patients, acetabular wear by hemiarthroplasty in 24 (3.0%) patients, instability in 28 (3.5%) patients, ALTR/ARMD in 9 (1.1%) patients, ceramic fracture in 8 (1.0%) patients, and others in 19 (2.4%) patients (Figure 2). Aseptic loosening and infection were the leading causes of revision in both the early and late revision groups (Table 2).
Figure 1.

In one case with multiple causes for revision, aseptic loosening was identified as the main cause: (A) preoperative anteroposterior radiograph, polyethylene wear, osteolysis, and prosthesis loosening are present, (B) postoperative anteroposterior radiograph, (C) intraoperative conditions, adverse local tissue reactions (ALTR), and prosthesis loosening are present, and (D) retrieved prosthesis, tribocorrosion of head-neck junction is present, ALTR was an additional failure mode.
Figure 2.
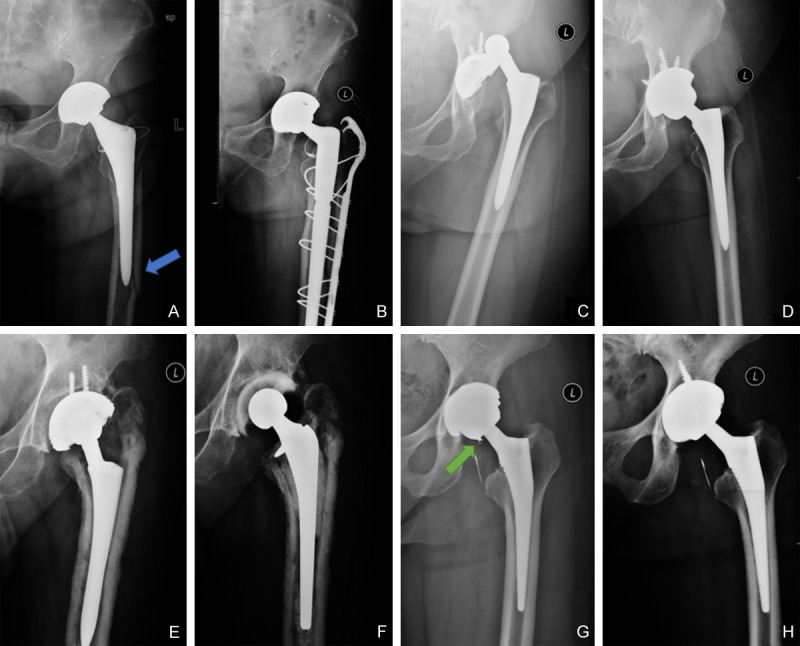
Anteroposterior (AP) radiographs of some causes. (A) pre- and (B) postoperative AP radiographs of periprosthetic fracture (blue arrow), (C) pre- and (D) postoperative AP radiographs of instability, (E) pre- and (F) postoperative AP radiographs of periprosthetic infection, (G) pre- and (H) postoperative AP radiographs of ceramic fracture (green arrow).
Table 2.
Causes of early and late revisions, grouped by revision date
| Early revisions [n=141] | Late revisions [n=662] | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| 2011-2015** | 2016-2020** | Overall‡ | 2011-2015## | 2016-2020## | Overall‡ | |
| [n=73] | [n=68] | [n=141] | [n=283] | [n=379] | [n=662] | |
| Aseptic loosening*,#,† | 38 (52.1%) | 24 (35.3%) | 62 (44.0%) | 218 (77%) | 255 (67.3%) | 473 (71.5%) |
| Infection† | 19 (26%) | 17 (25%) | 36 (25.5%) | 24 (8.5%) | 28 (7.4%) | 52 (7.9%) |
| Periprosthetic fracture† | 8 (11%) | 8 (11.8%) | 16 (11.3%) | 15 (5.3%) | 13 (3.4%) | 28 (4.2%) |
| Osteolysis#,† | 0 | 0 | 0 (0.0%) | 11 (3.9%) | 37 (9.8%) | 48 (7.3%) |
| Acetabular wear by hemiarthroplasty# | 1 (1.4%) | 2 (2.9%) | 3 (2.1%) | 3 (1.1%) | 18 (4.7%) | 21 (3.2%) |
| Instability*,† | 4 (5.5%) | 12 (17.6%) | 16 (11.3%) | 3 (1.1%) | 9 (2.4%) | 12 (1.8%) |
| ALTR/ARMD | 0 | 0 | 0 (0.0%) | 4 (1.4%) | 5 (1.3%) | 9 (1.4%) |
| Ceramic fracture | 1 (1.4%) | 2 (2.9%) | 3 (2.1%) | 0 | 5 (1.3%) | 5 (0.8%) |
| Others | 2 (2.7%) | 3 (4.4%) | 5 (3.5%) | 5 (1.8%) | 9 (2.4%) | 14 (2.1%) |
P<0.05 (Comparing 2011-2015 and 2016-2020 in early revisions);
P<0.05 (Comparing 2011-2015 and 2016-2020 in late revisions);
P<0.05 (Comparing all early revisions and all late revisions);
P=0.205 (Fisher’s exact test);
P=0.001 (Fisher’s exact test);
P<0.001 (Fisher’s exact test).
ALTR/ARMD, adverse local tissue reactions/adverse reaction to metal debris.
Since aseptic loosening was a typical cause of late revision, we further analyzed 62 cases of aseptic loosening in the early revision group (44.0%). Sixteen patients (25.8%) presented with femoral component loosening after hemiarthroplasty. In the remaining 46 patients, 29 (63.0%) had acetabular component loosening, 8 (17.4%) had femoral component loosening, and 9 (19.6%) had loosening of both components.
The values for incidence of infection (36, 25.5%), fracture (16, 11.3%), or instability (16, 11.3%) were significantly higher in the early revision group than in the late revision group (all P<0.05). The values for incidence of aseptic loosening (473, 71.5%) or osteolysis (48, 7.3%) were significantly higher in the late revision group (all P<0.05, Figure 3). All cases of revision were performed because of osteolysis and ALTR/ARMD, and most cases of revision due to acetabular wear (87.5%) occurred ≥2 years after primary THA (Table 2).
Figure 3.
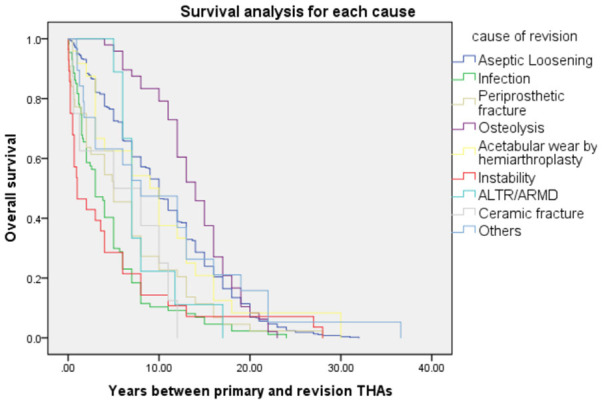
Survival analysis of each cause of revision using the Kaplan-Meier method. Instability (red line, mean time: 4.7 ± 7.3 years) and infection (green line, 4.7 ± 4.9 years) occurred early, whereas osteolysis (purple line, 13.8 ± 4.7 years) occurred late during the postoperative period.
A total of 356 and 447 revision THAs were performed in the early 5-year group (2011-2015) and recent 5-year group (2016-2020), respectively, which was a 25.6% increase during the 2016-2020 period. The recent 5-year group had a significantly longer prosthesis in situ interval (10.2 ± 6.8 years) than the early 5-year group (8.8 ± 7.0 years) (P=0.003, Figure 4). The mean patient age was also significantly higher (62.5 ± 12.0 years vs. 59.3 ± 13.6 years, respectively, P<0.001). The time to prosthesis revision for aseptic loosening was longer in the recent 5-year group (11.3 ± 6.3 years) than in the early 5-year group (9.7 ± 7.1 years) (P=0.005, Table 3).
Figure 4.

Survival analysis for date of revision, using the Kaplan-Meier method. The mean time between primary and revision THA was 9.6 ± 7.0 years (yellow line) for all revisions. The mean time was longer in 2016-2020 (green line, 10.2 ± 6.8 years) than in 2011-2015 (blue line, 8.8 ± 7.0 years, P=0.003).
Table 3.
Intervals between primary and revision THAs (mean ± SD, years)
| All revisions | 2011-2015 | 2016-2020 | P (Student’s t-test) | |
|---|---|---|---|---|
| Aseptic loosening | 10.5 ± 6.7 | 9.7 ± 7.1 | 11.3 ± 6.3 | 0.005 |
| Infection | 4.7 ± 4.9 | 4.1 ± 4.3 | 5.3 ± 5.4 | 0.281 |
| Periprosthetic fracture | 6.6 ± 6.4 | 7.3 ± 7.1 | 5.8 ± 5.5 | 0.447 |
| Osteolysis | 13.8 ± 4.7 | 11.4 ± 3.6 | 14.5 ± 4.8 | 0.051 |
| Acetabular wear by hemiarthroplasty | 9.9 ± 8.1 | 6.0 ± 4.7 | 10.7 ± 8.5 | 0.302 |
| Instability | 4.7 ± 7.3 | 3.6 ± 4.4 | 5.0 ± 8.2 | 0.653 |
| ALTR/ARMD | 8.3 ± 3.8 | 6.5 ± 1.3 | 9.8 ± 4.6 | 0.220 |
| Ceramic fracture | 6.0 ± 4.8 | 6 (one case) | 6.8 ± 4.6 | 0.651 |
| Others | 10.5 ± 9.5 | 14.0 ± 12.3 | 8.4 ± 7.3 | 0.226 |
THAs, total hip arthroplasties; ALTR/ARMD, adverse local tissue reactions/adverse reaction to metal debris.
The rate of aseptic loosening decreased from 71.9% in the early 5-year group to 62.4% in the recent 5-year group. The rates of osteolysis, acetabular wear by hemiarthroplasty, and instability increased from 3.1% to 8.3%, 1.1% to 4.5%, and 2.0% to 4.7%, respectively, and were higher in the recent 5-year group than in the early 5-year group (all P<0.05). However, the rates of infection, fracture, ALTR, and ceramic fracture did not change significantly (Figure 5).
Figure 5.
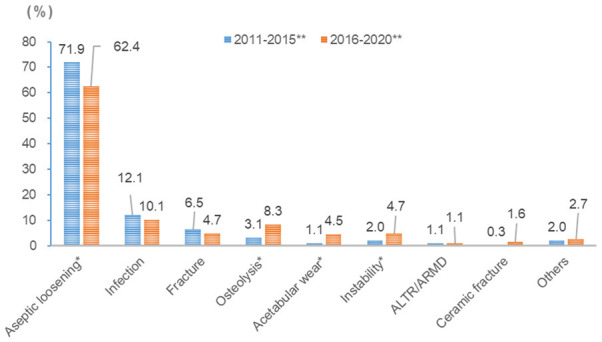
Comparison of proportion of causes of revision total hip arthroplasty (grouped by date of revision) *P<0.05 **P<0.001 (Fisher’s exact test); ALTR/ARMD, adverse local tissue reactions/adverse reaction to metal debris.
For early revisions performed within 2 years after primary THA, the incidence of instability increased from 5.5% in the early 5-year group to 17.6% in the recent 5-year group (P<0.05). The incidence of aseptic loosening decreased from 52.1% in the early 5-year group to 35.3% in the recent 5-year group (P<0.05). As for late revision, the incidence of osteolysis and acetabular wear by hemiarthroplasty increased from 3.9% in the early 5-year group to 9.8% (P<0.05) and from 1.1% to 4.7% (P<0.05), respectively. The incidence of aseptic loosening decreased from 77.0% in the early 5-year group to 67.3% in the recent 5-year group (P<0.05) (Table 2).
Discussion
Although prosthesis and surgical techniques for primary THA have improved over several years, the number of revisions continues to increase [4,17]. This study revealed that the mean prosthesis in situ interval significantly increased from 8.8 to 10.2 years. However, the volume of revision surgeries increased by 25.6% from the 2011-2015 to the 2016-2020 periods. Other studies found that the number of revision THAs increased by >20% over the past 15 years and are expected to continue to increase [4,17]. This change may be primarily attributable to the increased absolute number of THAs [4]. Surgeons will perform more revision THAs in the future, and understanding the contemporary causes and trends of THA failure is vital for clinical decision-making. In this study, we performed a comprehensive analysis of the largest sample ever obtained in a developing country of non-database studies. This study provided and updated valuable results on the causes and trends of revision after primary THA.
The results of this study performed at our center confirmed that aseptic loosening (66.6%) is the most common cause of revision THA. This is true for most hip arthroplasty centers. Aseptic loosening was found by Ulrich et al. [10] and Iamthanaporn et al. [18] as the leading cause of revision; they reported values for incidence of 52% and 58%, respectively. The reasons for the high incidence of aseptic loosening are multifaceted. They include prosthesis design, surgical technique, and long-term use. Conventional polyethylene has been widely used for prosthesis fabrication since its introduction by Charnley in 1962 [19]. This generates wear particles and causes osteolysis that can lead to aseptic loosening [20-22]. At our center, aseptic loosening within 2 years after primary THA was mainly due to acetabular component loosening (38 hips). The main reason for this result was likely inadequate fixation of the acetabular component, although complete information on the polyethylene used could not be obtained. Poor cup press fit and osseointegration of the cementless acetabular component are related to errors in surgical technique, acetabular shape, and cup design [23-25]. Malposition of the acetabular component and center of rotation can increase the hip load, resulting in early acetabular component loosening [24]. Osteolysis can be associated with ALTR/ARMD and elevated serum metal ion concentrations [26,27], which leads to implant loosening. Lehil et al. [28] found that the trends in THA implant usage between 2001 and 2012 favored cementless fixation and highly cross-linked polyethylene liners. Use of conventional polyethylene liners and metal-on-metal bearing surfaces declined to <1%. Use of these improved implants decreased wear and revision rates and prolonged survival times of prosthesis [20,22,29,30]. This change could explain why the rate of aseptic loosening decreased from 71.9% in the early 5-year to 62.4% in the recent 5-year period. The prosthesis in situ interval (from 9.7 ± 7.1 to 11.3 ± 6.3 years) was longer in these patients and the incidence of early aseptic loosening significantly decreased from 52.1% to 35.3% during the recent 5-year period.
Infection (11.0%) was the second leading cause of revision procedures. Results of previous studies indicated that the incidence of infection ranges from 7% to 15.6% [3,9,10,31]. In our study, the rate of infection was not significantly reduced during the recent 5 years. This difference was likely because in most cases, the primary surgery is performed at other centers. Occurrence of infection is closely related to the experience of the surgical team. Bozic et al. [32] found that a higher surgical volume is associated with a lower risk of infection. In a comparison performed by Lachiewicz et al. [33], two cohorts of 100 consecutive revisions performed by a single surgeon 10 years apart revealed a decreased rate of revision due to infection (from 10% to 7%). Education regarding prophylaxis against infection and delicate surgical manipulation will contribute to a decrease in the incidence of infection-related revision.
The incidence of revision performed because of instability was 3.5%, with a significant increase from 2.0% during the early 5 years to 4.7% during the recent 5 years. Instability occurred more frequently in the early revision group (11.3%) than in the late revision group (1.8%). Instability, a typical cause of early failure, is currently the major cause of revision (14.6-22.5%) at some medical centers [10,11,15,17]. The main causes of instability include position of the prosthesis, surgical techniques, surgical approaches, and patient factors [34-37]. The increasing trend will remind surgeons that improved medical practices are needed for the prevention of prosthesis dislocation.
To reduce the dislocation rate, appropriate soft tissue tension is needed using adequate intraoperative leg length and offset reconstruction, and strict postoperative posture protection. The use of elevated or lipped liners, larger femoral head sizes, dual-mobility implants, and high-offset stems may benefit hip stability with an adequate range of motion [38,39]. With respect to early revision, we found increased numbers and proportions of revisions for instability (from 5.5% in the early 5 years to 17.6% in the recent 5 years). Currently, THA stability can be affected by the surgical approach and the surgeon’s experience [37]. Therefore, use of the direct anterior approach and robot-assisted THA have emerged as techniques that offer more stability and provide faster postoperative rehabilitation [40,41].
This study revealed that 17% of revisions were performed at <2 years after the primary THA. The early revision rate decreased from 20.5% in the early 5-year group to 15.2% in the recent 5-year group. The interval between primary and revision THAs was prolonged (8.8 ± 7.0 years vs. 10.2 ± 6.8 years, P=0.003) in the recent 5-year group, indicating a longer prosthesis in situ lifetime. This result was probably due to the reduction in revisions for aseptic loosening in the early failure group (from 38 to 24 hips). However, failures due to infection (25.5% vs. 7.9%), periprosthetic fracture (11.3% vs. 4.2%), or instability (11.3% vs. 1.8%) were more frequent in the early failure group than in the late failure group. As found in other studies [3,14,17,42], infection and instability are significant causes of revision during the early post-THA period, because they are more strongly associated with surgical technique errors [3,10,14]. On the other hand, aseptic loosening and osteolysis are associated with prosthesis design and material science [3,14,42]. Surgeons should be well-trained to avoid early failures, including those due to infection and instability. Similar to this study, some studies found that periprosthetic fractures are more likely to occur early after primary THA [10,17]. Some early cases might be associated with the extension of tiny cracks and injuries that were not detected intraoperatively. Others can be trauma-related when high axial and torsional loads are placed on the cementless implant prior to osseointegration [43-45]. Cemented implants should be considered for patients with risk factors for periprosthetic fractures, such as older age, female sex, and osteoporosis [43,46-48].
This study had some limitations. First, we used a retrospective study design. Thus, data available for further investigations were limited. To address this, we tried our best to obtain all potential data, and two experienced surgeons analyzed these data. Second, this study was performed at a single center, which might have led to selection bias. Therefore, to better describe the prevalence of revision THA, a larger standardized database is needed. Third, we could not obtain all information on the patients’ primary THAs, as they underwent the procedures at different hospitals. A nationwide database of arthroplasty registration would help to compensate for this limitation. Studies with more details are needed to address these limitations.
Conclusions
THA failure is a persistent problem. To our knowledge, the sample size used in this study was the largest ever used in studies performed in developing countries. We found that aseptic loosening remained the most common reason for revision THA, particularly during the late post-THA period. Infection, instability, and periprosthetic fractures occurred more frequently in the early failure group. Given the increasing trend in instability, it is necessary to apply appropriate management to decrease the risk of dislocation. Assessing the causes and trends of failure after THA will provide valuable data for surgeons and manufacturers to guide prosthesis design, improve surgical techniques, and change clinical outcomes. This information may even provide a basis for the formulation of relevant national policies.
Disclosure of conflict of interest
None.
References
- 1.Harris WH. The first 50 years of total hip arthroplasty: lessons learned. Clin Orthop Relat Res. 2009;467:28–31. doi: 10.1007/s11999-008-0467-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bian Y, Cheng K, Chang X, Weng X. Reports and analysis of amount of hip and knee arthroplasty in China from 2011 to 2019. Chin J Orthop. 2020:1453–1460. [Google Scholar]
- 3.Haynes JA, Stambough JB, Sassoon AA, Johnson SR, Clohisy JC, Nunley RM. Contemporary surgical indications and referral trends in revision total hip arthroplasty: a 10-year review. J Arthroplasty. 2016;31:622–625. doi: 10.1016/j.arth.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 4.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 5.Lie SA, Havelin LI, Furnes ON, Engesaeter LB, Vollset SE. Failure rates for 4762 revision total hip arthroplasties in the Norwegian Arthroplasty Register. J Bone Joint Surg Br. 2004;86:504–509. [PubMed] [Google Scholar]
- 6.Bozic KJ, Kamath AF, Ong K, Lau E, Kurtz S, Chan V, Vail TP, Rubash H, Berry DJ. Comparative epidemiology of revision arthroplasty: failed THA poses greater clinical and economic burdens than failed TKA. Clin Orthop Relat Res. 2015;473:2131–2138. doi: 10.1007/s11999-014-4078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry DJ, Harmsen WS, Cabanela ME, Morrey BF. Twenty-five-year survivorship of two thousand consecutive primary Charnley total hip replacements: factors affecting survivorship of acetabular and femoral components. J Bone Joint Surg Am. 2002;84:171–177. doi: 10.2106/00004623-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Ong A, Wong KL, Lai M, Garino JP, Steinberg ME. Early failure of precoated femoral components in primary total hip arthroplasty. J Bone Joint Surg Am. 2002;84:786–792. doi: 10.2106/00004623-200205000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Clohisy JC, Calvert G, Tull F, McDonald D, Maloney WJ. Reasons for revision hip surgery: a retrospective review. Clin Orthop Relat Res. 2004:188–192. doi: 10.1097/01.blo.0000150126.73024.42. [DOI] [PubMed] [Google Scholar]
- 10.Ulrich SD, Seyler TM, Bennett D, Delanois RE, Saleh KJ, Thongtrangan I, Kuskowski M, Cheng EY, Sharkey PF, Parvizi J, Stiehl JB, Mont MA. Total hip arthroplasties: what are the reasons for revision? Int Orthop. 2008;32:597–604. doi: 10.1007/s00264-007-0364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J Bone Joint Surg Am. 2009;91:128–133. doi: 10.2106/JBJS.H.00155. [DOI] [PubMed] [Google Scholar]
- 12.Havelin LI, Fenstad AM, Salomonsson R, Mehnert F, Furnes O, Overgaard S, Pedersen AB, Herberts P, Kärrholm J, Garellick G. The Nordic Arthroplasty Register Association: a unique collaboration between 3 national hip arthroplasty registries with 280,201 THRs. Acta Orthop. 2009;80:393–401. doi: 10.3109/17453670903039544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jafari SM, Coyle C, Mortazavi SM, Sharkey PF, Parvizi J. Revision hip arthroplasty: infection is the most common cause of failure. Clin Orthop Relat Res. 2010;468:2046–2051. doi: 10.1007/s11999-010-1251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melvin JS, Karthikeyan T, Cope R, Fehring TK. Early failures in total hip arthroplasty -- a changing paradigm. J Arthroplasty. 2014;29:1285–1288. doi: 10.1016/j.arth.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 15.Gwam CU, Mistry JB, Mohamed NS, Thomas M, Bigart KC, Mont MA, Delanois RE. Current epidemiology of revision total hip arthroplasty in the United States: national inpatient sample 2009 to 2013. J Arthroplasty. 2017;32:2088–2092. doi: 10.1016/j.arth.2017.02.046. [DOI] [PubMed] [Google Scholar]
- 16.Goldman AH, Sierra RJ, Trousdale RT, Lewallen DG, Berry DJ, Abdel MP. The Lawrence D. Dorr surgical techniques & technologies award: why are contemporary revision total hip arthroplasties failing? An analysis of 2500 cases. J Arthroplasty. 2019;34:S11–S16. doi: 10.1016/j.arth.2019.01.031. [DOI] [PubMed] [Google Scholar]
- 17.Kelmer G, Stone AH, Turcotte J, King PJ. Reasons for revision: primary total hip arthroplasty mechanisms of failure. J Am Acad Orthop Surg. 2021;29:78–87. doi: 10.5435/JAAOS-D-19-00860. [DOI] [PubMed] [Google Scholar]
- 18.Iamthanaporn K, Chareancholvanich K, Pornrattanamaneewong C. Revision primary total hip replacement: causes and risk factors. J Med Assoc Thai. 2015;98:93–99. [PubMed] [Google Scholar]
- 19.Callaghan JJ, Templeton JE, Liu SS, Pedersen DR, Goetz DD, Sullivan PM, Johnston RC. Results of Charnley total hip arthroplasty at a minimum of thirty years. A concise follow-up of a previous report. J Bone Joint Surg Am. 2004;86:690–695. doi: 10.2106/00004623-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Lachiewicz PF, Kleeman LT, Seyler T. Bearing surfaces for total hip arthroplasty. J Am Acad Orthop Surg. 2018;26:45–57. doi: 10.5435/JAAOS-D-15-00754. [DOI] [PubMed] [Google Scholar]
- 21.Campbell P, Shen FW, McKellop H. Biologic and tribologic considerations of alternative bearing surfaces. Clin Orthop Relat Res. 2004:98–111. doi: 10.1097/00003086-200401000-00017. [DOI] [PubMed] [Google Scholar]
- 22.de Steiger R, Lorimer M, Graves SE. Cross-linked polyethylene for total hip arthroplasty markedly reduces revision surgery at 16 years. J Bone Joint Surg Am. 2018;100:1281–1288. doi: 10.2106/JBJS.17.01221. [DOI] [PubMed] [Google Scholar]
- 23.García-Rey E, García-Cimbrelo E, Cruz-Pardos A. Cup press fit in uncemented THA depends on sex, acetabular shape, and surgical technique. Clin Orthop Relat Res. 2012;470:3014–3023. doi: 10.1007/s11999-012-2381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miettinen SS, Mäkinen TJ, Laaksonen I, Mäkelä K, Huhtala H, Kettunen J, Remes V. Early aseptic loosening of cementless monoblock acetabular components. Int Orthop. 2017;41:715–722. doi: 10.1007/s00264-016-3254-8. [DOI] [PubMed] [Google Scholar]
- 25.Long WJ, Nayyar S, Chen KK, Novikov D, Davidovitch RI, Vigdorchik JM. Early aseptic loosening of the Tritanium primary acetabular component with screw fixation. Arthroplast Today. 2018;4:169–174. doi: 10.1016/j.artd.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osman K, Panagiotidou AP, Khan M, Blunn G, Haddad FS. Corrosion at the head-neck interface of current designs of modular femoral components: essential questions and answers relating to corrosion in modular head-neck junctions. Bone Joint J. 2016;98-b:579–584. doi: 10.1302/0301-620X.98B5.35592. [DOI] [PubMed] [Google Scholar]
- 27.Bauer TW, Campbell PA, Hallerberg G. How have new bearing surfaces altered the local biological reactions to byproducts of wear and modularity? Clin Orthop Relat Res. 2014;472:3687–3698. doi: 10.1007/s11999-014-3817-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehil MS, Bozic KJ. Trends in total hip arthroplasty implant utilization in the United States. J Arthroplasty. 2014;29:1915–1918. doi: 10.1016/j.arth.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Prock-Gibbs H, Pumilia CA, Meckmongkol T, Lovejoy J, Mumith A, Coathup M. Incidence of osteolysis and aseptic loosening following metal-on-highly cross-linked polyethylene hip arthroplasty: a systematic review of studies with up to 15-year follow-up. J Bone Joint Surg Am. 2021;103:728–740. doi: 10.2106/JBJS.20.01086. [DOI] [PubMed] [Google Scholar]
- 30.Tsukamoto M, Mori T, Ohnishi H, Uchida S, Sakai A. Highly cross-linked polyethylene reduces osteolysis incidence and wear-related reoperation rate in cementless total hip arthroplasty compared with conventional polyethylene at a mean 12-year follow-up. J Arthroplasty. 2017;32:3771–3776. doi: 10.1016/j.arth.2017.06.047. [DOI] [PubMed] [Google Scholar]
- 31.Badarudeen S, Shu AC, Ong KL, Baykal D, Lau E, Malkani AL. Complications after revision total hip arthroplasty in the medicare population. J Arthroplasty. 2017;32:1954–1958. doi: 10.1016/j.arth.2017.01.037. [DOI] [PubMed] [Google Scholar]
- 32.Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD. The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am. 2010;92:2643–2652. doi: 10.2106/JBJS.I.01477. [DOI] [PubMed] [Google Scholar]
- 33.Lachiewicz PF, Soileau ES. Changing indications for revision total hip arthroplasty. J Surg Orthop Adv. 2005;14:82–84. [PubMed] [Google Scholar]
- 34.Pivec R, Johnson AJ, Mears SC, Mont MA. Hip arthroplasty. Lancet. 2012;380:1768–1777. doi: 10.1016/S0140-6736(12)60607-2. [DOI] [PubMed] [Google Scholar]
- 35.Houdek MT, Watts CD, Wyles CC, Trousdale RT, Milbrandt TA, Taunton MJ. Total hip arthroplasty in patients with cerebral palsy: a cohort study matched to patients with osteoarthritis. J Bone Joint Surg Am. 2017;99:488–493. doi: 10.2106/JBJS.16.00528. [DOI] [PubMed] [Google Scholar]
- 36.Aziz KT, Best MJ, Skolasky RL, Ponnusamy KE, Sterling RS, Khanuja HS. Lupus and perioperative complications in elective primary total hip or knee arthroplasty. Clin Orthop Surg. 2020;12:37–42. doi: 10.4055/cios.2020.12.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdel MP, von Roth P, Jennings MT, Hanssen AD, Pagnano MW. What safe zone? The vast majority of dislocated THAs are within the Lewinnek safe zone for acetabular component position. Clin Orthop Relat Res. 2016;474:386–391. doi: 10.1007/s11999-015-4432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rowan FE, Benjamin B, Pietrak JR, Haddad FS. Prevention of dislocation after total hip arthroplasty. J Arthroplasty. 2018;33:1316–1324. doi: 10.1016/j.arth.2018.01.047. [DOI] [PubMed] [Google Scholar]
- 39.Wright-Chisem J, Elbuluk AM, Mayman DJ, Jerabek SA, Sculco PK, Vigdorchik JM. The journey to preventing dislocation after total hip arthroplasty: how did we get here? Bone Joint J. 2022;104-b:8–11. doi: 10.1302/0301-620X.104B1.BJJ-2021-0823.R1. [DOI] [PubMed] [Google Scholar]
- 40.Tsukada S, Wakui M. Lower dislocation rate following total hip arthroplasty via direct anterior approach than via posterior approach: five-year-average follow-up results. Open Orthop J. 2015;9:157–162. doi: 10.2174/1874325001509010157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Subramanian P, Wainwright TW, Bahadori S, Middleton RG. A review of the evolution of robotic-assisted total hip arthroplasty. Hip Int. 2019;29:232–238. doi: 10.1177/1120700019828286. [DOI] [PubMed] [Google Scholar]
- 42.Cnudde P, Bülow E, Nemes S, Tyson Y, Mohaddes M, Rolfson O. Association between patient survival following reoperation after total hip replacement and the reason for reoperation: an analysis of 9,926 patients in the Swedish Hip Arthroplasty Register. Acta Orthop. 2019;90:226–230. doi: 10.1080/17453674.2019.1597062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pakarinen OA, Neuvonen PS, Lainiala OS, Reito ARP, Eskelinen AP. Periprosthetic femoral fracture is a leading cause of early revision with taper-slip stems in primary total hip arthroplasty: an analysis of 2765 total hip arthroplasties from a high-volume hospital. J Arthroplasty. 2021;36:3703–3708. e3702. doi: 10.1016/j.arth.2021.06.022. [DOI] [PubMed] [Google Scholar]
- 44.Taunton MJ, Dorr LD, Long WT, Dastane MR, Berry DJ. Early postoperative femur fracture after uncemented collarless primary total hip arthroplasty: characterization and results of treatment. J Arthroplasty. 2015;30:2008–2011. doi: 10.1016/j.arth.2015.05.044. [DOI] [PubMed] [Google Scholar]
- 45.Saemann M, Darowski M, Hennicke NS, Bader R, Sander M, Kluess D. Experimental analysis of early periprosthetic femoral fractures with uncemented straight hip stems. Clin Biomech (Bristol, Avon) 2022;91:105543. doi: 10.1016/j.clinbiomech.2021.105543. [DOI] [PubMed] [Google Scholar]
- 46.Lindberg-Larsen M, Jørgensen CC, Solgaard S, Kjersgaard AG, Kehlet H. Increased risk of intraoperative and early postoperative periprosthetic femoral fracture with uncemented stems. Acta Orthop. 2017;88:390–394. doi: 10.1080/17453674.2017.1302908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sershon RA, McDonald JF 3rd, Ho H, Hamilton WG. Periprosthetic femur fracture risk: influenced by stem choice, not surgical approach. J Arthroplasty. 2021;36:S363–S366. doi: 10.1016/j.arth.2021.02.012. [DOI] [PubMed] [Google Scholar]
- 48.Konow T, Baetz J, Melsheimer O, Grimberg A, Morlock M. Factors influencing periprosthetic femoral fracture risk. Bone Joint J. 2021;103-b:650–658. doi: 10.1302/0301-620X.103B4.BJJ-2020-1046.R2. [DOI] [PubMed] [Google Scholar]


