Abstract
Objective: To explore the role of endothelial progenitor cell (EPC)-derived exosomal microRNA-382-3p (miR-382-3p) in septic injury in mice. Methods: A murine model of sepsis was introduced by cecal ligation and puncture (CLP). The model mice were treated with EPC-derived exosomes (Exos). The lung, kidney and liver tissues of mice were collected and stained with hematoxylin and eosin. The lymphocytes in murine spleen tissues, and the proportion and phenotype of the T helper cells (Ths) were examined by flow cytometry. The exosomal miRNAs were screened using a microarray analysis. The expressions of miR-382-3p and beta-transducin repeat containing E3 ubiquitin protein ligase (BTRC) were measured to explore possible mechanism of Exos in septic injury in mice. Results: EPC-derived Exos alleviated CLP-induced tissue damage in the lung, kidney and liver tissues in septic mice. They also restored the number of lymphocytes and the concentration of Ths, and reduced the imbalance in Th1 and Th2 cells in mice. The Exos mainly contained miR-382-3p, and miR-382-3p directly targeted BTRC mRNA. Either downregulation of miR-382-3p or upregulation of BTRC blocked the protective roles of Exos in septic injury and immune suppression. Overexpression of BTRC increased the phosphorylation of nuclear factor kappa B (NF-κB) inhibitor α (IκBα) and NF-κB. Conclusion: EPC-derived exosomal miR-382-3p alleviates sepsis-induced organ damage and immune suppression in septic mice through regulating BTRC and the IκBα/NF-κB axis.
Keywords: Endothelial progenitor cells, exosomes, microRNA-382-3p, BTRC, IκBα/NF-κB, sepsis
Introduction
Sepsis is a costly condition and a leading cause of in-hospital death [1]. It is estimated that sepsis affects 31 million people and leads to over 5 million deaths annually worldwide, and this number might be an underestimate due to data from many low-income countries are not included [2]. Despite great efforts in protocol-based care, septic shock mortality remains high at approximately 35% to 40% [3]. Infections reflect frequent self-limiting events, and sepsis usually leads to an imbalance in the body’s reaction to infection and results in organ dysfunction syndrome [4]. Multiple organ dysfunction, importantly, represents the main cause of sepsis-associated death [5]. Despite an increasing understanding of the sepsis pathogenesis, the immune-modulating drugs which target sepsis-associated proinflammatory responses show little survival benefit [6,7]. It is also not surprising that reduced functions in innate immune cells in septic patients correlate with dismal outcome [8]. Reducing immune repression and alleviating organ injury are important issues for survival benefit in septic patients.
Exosomes are a class of extracellular vesicles (Evs) fulfilling key functions in cell-to-cell communication by conveying their contents such as microRNAs (miRNAs), mRNAs and proteins into recipient cells [9]. Exosomes have shown functional significance in bacterial inflammation and sepsis as well [10]. Endothelial progenitor cells (EPCs) and their derived exosomes are capable of differentiating into endothelial cells for tissue repair and angiogenesis [11]. Interestingly, synergistic administration of EPCs and a stromal cell-derived factor-1α analog have been suggested to improve survival in sepsis patients [12]. In addition, an increased proportion of circulating EPCs in patients with acute pancreatitis has been reported to be associated with reduced organ failure [13]. Recruitment of bone marrow-derived EPCs has also been suggested to alleviate multiple organ injury [14]. However, the function of EPC-derived exosomes in sepsis-caused immune imbalance and organ injury remains uncertain. miRNAs represent a major class of exosomal ‘cargoes’ that are closely associated with the development of a multitude of human diseases including sepsis [15]. miRNAs exert their versatile functions through potent regulation of target mRNA transcripts [16]. In the present study, a miRNA microarray analysis suggests that miR-382-3p was abundant in the EPC-derived exosomes, and bioinformatic analyses suggested miR-382-3p targeted beta-transducin repeat containing E3 ubiquitin protein ligase (BTRC) mRNA. Interestingly, miR-382-3p has been reported to alleviate the interleukin-1β-induced inflammatory response of chondrocytes through regulating the nuclear factor kappa B (NF-κB) signaling pathway [17], while BTRC has been suggested to promote phosphorylation and degradation of NF-κB inhibitor alpha (IκBα) and tsubsequent NF-κB activation [18]. IκBα can inhibit translocation of NF-κB into the nucleus, which is a frequent and critical event involving inflammatory responses in human diseases including sepsis [19,20]. We therefore surmised that the exosomal miR-382-3p possibly regulates inflammatory responses and tissue injury in sepsis through regulation of a BTRC/IκBα/NF-κB axis.
Materials and methods
Cell culture and transfection
Mouse bone marrow-sourced EPCs (Cat. No. CP-M140) were purchased from Procell Life Science & Technology Co., Ltd. (Wuhan, Hubei, China). Cells were cultured in EPC-specific complete medium (Cat. No. CM-M140) at 37°C in 5% CO2. The miR-382-3p mimic (5’-UCAUUCACGGACAACACUUUUU-3’) and inhibitor (5’-GAAGUUGUUCGUGGUGGAUUCG-3’) used for cell transfection were procured from Procell as well. All transfections were performed using a Lipofectamine 2000 kit (Invitrogen, Thermo Fisher Scientific Inc., Waltham, MA, USA).
Extraction and identification of exosomes
Exosomes were extracted from EPCs by differential centrifugation. First, the culture medium was centrifuged at 100,000×g at 4°C overnight to exclude the serum-sourced exosomes in the medium. Next, the EPCs were cultured in exosome-exhausted medium for 48 h. The supernatant was centrifuged at 2,000×g at 4°C for 20 min to discard the large cell debris. Then, the cell supernatant was further centrifuged at 12,000×g at 4°C for 45 min to discard the small cell debris. After that, the supernatant was loaded into new tubes and ultra-centrifuged at 120,000×g at 4°C for 2 h to precipitate the exosomes. The supernatant was then removed, and the sediment was resuspended in phosphate-buffered saline (PBS). Thereafter, the samples were filtered using a 0.22-μm filter to exclude the possible contaminants. Purified exosomes were obtained after another 120 min of ultra-centrifugation at 120,000×g at 4°C. The exosomes were resuspended in PBS, and the protein concentration was examined by a BCA kit (Pierce, Waltham, MA, USA). The exosomes extracted from un-transfected EPCs were defined as Exo, while those from EPCs transfected with NC inhibitor/miR-382-3p inhibitor were defined as Exo-NC/Exo-KD, respectively.
The exosomes were loaded on copper grids, air-dried, and then stained with phosphotungstic acid at 25°C for 5 min. The shape of the particles was observed under a transmission electron microscope (TEM) (JEM-1400Plus, JEOL, Ltd., Tokyo, Japan). The exosomes were resuspended in PBS and processed using nanoparticle tracking analysis (NTA) particle Metrix equipped with ZetaView PMX 120 (Particle Metrix GmbH, Microtrac, Meerbusch, Germany). The particle size distribution was analyzed using the NTA software (ZetaView 8.04.02). The exosome-specific marker proteins were examined by Western blot using anti-CD9 (ab92726, Abcam Inc., Cambridge, MA, USA) and anti-CD81 (ab109201, Abcam). The secondary antibody was goat anti-rabbit IgG H&L (HRP) (ab205718, Abcam). The exosome proteins were quantified using a BCA kit.
Animal experiments
Male C57BL/6 mice (8-12 weeks old) were procured from Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). The mice were fed in a 12-h light/dark cycle with free access to feed and water. This study was ratified by the Animal Ethical Committee of Affiliated Hospital of Guizhou Medical University. All procedures were conducted according to the Guideline for the Care and Use of Laboratory Animals published by the National Institutes of Health (Bethesda, Maryland, USA).
A murine model of sepsis was induced using cecal ligation and puncture (CLP) as previously reported [21]. In brief, the mice were anesthetized via inhalation of 2% isoflurane. The mice were fixed in a supine position. The abdomen was shaved and cleaned using 70% isopropyl alcohol and 10% povidone-iodine. The peritoneal cavity was exposed by midline laparotomy. The appendix was ligated at 1 cm from the distal end using a 4-0 silk suture, and the blind pouch of the appendix was punctured using a 22-gauge needle. The feces at the perforated site was excluded, and the incision was sewed up using a 4-0 silk suture in two layers. The sham-operated mice underwent similar procedures except for the ligation and puncture. All animals were subcutaneously injected with 0.9% NaCl for resuscitation.
To examine the influence of the Exo treatment on the survival of septic mice, the mice were treated with 50 μg Exo through tail vein injection at 0, 6 and 12 h post CLP procedures, respectively. Ten mice were injected at each time point. To evaluate the function of miR-382-3p on the efficacy of Exo, the Exo-NC/Exo-KD were injected into mice at 0 h after CLP surgery. There were 10 successfully modeled mice in each group (survived over 72 h after surgery). The function of BTRC on Exo efficacy was examined through additional administration of 100 μL lentiviral vector (LV-BTRC) and LV-NC in mice after CLP and Exo treatment. Ten successfully modeled mice were maintained in each group.
Examination of colony-forming units (CFUs)
At the 72 h after surgery, the mice were sacrificed via an overdose of pentobarbital sodium (150 mg/kg, intraperitoneal injection). Then, 5 mL cold PBS was injected into the peritoneal cavity of the dead animals in a sterile condition. The abdomen of mice was shaken for 1 min to evenly distribute the PBS in the cavity. Then, the peritoneal lavage fluid was collected using a pipette, and the samples were cultured on a blood agar plate at 37°C for 24 h to calculate the number of CFUs.
Hematoxylin and eosin (H&E) staining
The lung, liver, and kidney tissues of mice were collected, fixed in 4% formaldehyde, embedded in paraffin, and cut into 4-μm sections. The sections were stained with hematoxylin and eosin (Sigma-Aldrich Chemical Company, St Louis, MO, USA). The staining was observed under a microscope.
The organ injury was evaluated according to the H&E staining, which was scored by three group-blinded pathologists as previously reported [22]. For the lung tissues, the staining was scored according to the tissue congestion and inflammatory infiltration (1-5 points); for the kidney tissues, the staining was scored according to the kidney tubular injury and glomerular shrinkage (0-5 points); for the liver tissues, the staining was scored according to necrosis (0-3 points), bleeding (0-3 points) and infiltration (0-3 points) (total points: 9).
Isolation of spleen lymphocytes and identification of T helper cells (Ths)
The murine spleen tissues were isolated and made into homogenate with a syringe plunger. The homogenate was then filtered using a nylon mesh and then injected into lymphocyte separation medium (Dakewe Biotech Co., Ltd., Shenzhen, China). The medium with spleen cell suspension was further loaded with 200 μL Roswell Park Memorial Institute (RPMI)-1640 and centrifuged at 800 g for 30 min to separate the lymphocyte layer. Then, the layer was further added with 10 mL RPMI and centrifuged at 250 g for 10 min. The supernatant was discarded, and the cells were resuspended in culture medium.
The isolated lymphocytes were blocked by Magic Fc-receptor blocker for 15 min (Abace Biotechnology, Beijing, China) and then incubated in allophycocyanin-labeled anti-CD3 (#100311, BioLegend, San Diego, CA, USA), anti-CD4, anti-interferon-γ (IFNγ, ab210390, Abcam) and anti-interleukin-4 (IL-4, ab95715, Abcam) at 4°C for 30 min. Subsequently, the cells were washed and analyzed using a FACScan cytometer (BD Biosciences, Mountain View, CA).
Exo-based miRNA microarray analysis
Total RNA from the Exo was extracted using the TRIzol reagent (Thermo Fisher Scientific) and a miRNeasy mini kit (Qiagen, Hilden, Germany). Since the total concentration of the exosomal RNA used for microarray analysis was relatively low, the total miRNA was first amplified using a miRNA amplification kit (System Biosciences (SBI), Mountain View, CA, USA). Next, the amplified miRNA samples were labeled using a miRCURY™ Hy3/Hy5 Power kit (Exiqon, Vedbaek, Denmark) and hybridized using a miRCURY™ LNA Array kit (version 18.0, Exiqon). Then, the glass slides were scanned using an Axon GenePix 4000B microarray scanner (Axon Instruments, Foster City, CA, USA). The images were imported in the Genepix Pro (Version 6.0, Axon Instruments) for data extraction. Three independent experiments were performed. miRNAs with foreground signal intensity stronger than the background signal by two times were screened, and those with foreground signal intensity stronger than the background signal by 15 times were considered as highly-enriched miRNAs.
Reverse transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNA was collected using the TRIzol reagent (Thermo Fisher Scientific). To examine the expression of BTRC, total RNA was reverse-transcribed into a PrimeScript RT kit (Takara Biotechnology Ltd., Dalian, China). Real-time qPCR was conducted using TB Green Premix Ex Taq II (Takara) on a QUANT5 PCR system (Applied Biosystems, Inc., Carlsbad, CA, USA), and GAPDH was used as the internal loading for mRNA quantification. The miR-382-3p expression was examined using an all-in-one miRNA RT-qPCR (Genecopoeia, Rockville, MD, USA) with 5S used as the internal control. Relative gene expression was examined by the 2-ΔΔCt method. All primers are shown in Table 1.
Table 1.
Primer sequences for RT-qPCR
| Gene | Primer sequence (5’-3’) |
|---|---|
| miR-382-3p | F: AGTTGTTCGTGGTGGATTC |
| R: GAACATGTCTGCGTATCTC | |
| BTRC | F: GCAGTACGATGAGAGGGTGATC |
| R: CAGAACGGCTTCACAGTGGTGA | |
| GAPDH | F: CATCACTGCCACCCAGAAGACTG |
| R: ATGCCAGTGAGCTTCCCGTTCAG | |
| 5S | F: CTCGCTTCGGCAGCACAT |
| R: TTTGCGTGTCATCCTTGCG |
Note: RT-qPCR, reverse transcription quantitative polymerase chain reaction; miR-382-3p, microRNA-382-3p; BTRC, beta-transducin repeat containing E3 ubiquitin protein ligase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; F: forward; R: reverse.
Western blot analysis
Total protein was collected using the radio-immunoprecipitation assay lysis buffer (Beyotime Biotechnology Co., Ltd., Shanghai, China) containing protease inhibitor. The protein concentration was examined using the BCA kit. Next, an equal volume of protein was run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane (Millipore Corp., Billerica, MA, USA). The membranes were blocked by 5% non-fat milk for 2 h and then hybridized with the primary antibodies against BTRC (1:500, ab137674, Abcam), GAPDH (1:500, ab181603, Abcam), phospho-IκBα (Ser32) (1:1,000, #2859, Cell Signaling Technologies (CST), Beverly, MA, USA) and phospho-NF-κB p65 (Ser536) (1:1,000, #3033, CST) at 4°C overnight and with the secondary antibody goat anti-rabbit IgG (1:1,500, #14708, CST) at 37°C for 2 h. The protein bands were developed using an enhanced chemiluminescence kit (Beyotime) and analyzed using the Image J software (NIH).
Dual-luciferase reporter gene assay
The putative wild type (WT) binding site between miR-382-3p and BTRC mRNA was predicted using the Starbase system (http://starbase.sysu.edu.cn/). The mutant type (MT) binding site was designed as well. The BTRC 3’UTR sequence containing the WT or MT sites was inserted into the pmirGLO luciferase vectors (Promega, Madison, WI, USA) to construct pmirGLO-BTRC-WT and pmirGLO-BTRC-MT luciferase vectors. These vectors were then co-delivered with NC mimic/miR-382-3p mimic into EPCs using the Lipofectamine 2000 kit. After 48 h, relative luciferase activity in cells was examined using a Dual-Luciferase Reporter Assay Kit (Promega) according to the manufacturer’s protocols.
Statistical analysis
GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA) was used for statistical analysis. Data were collected from three independent experiments and shown as mean ± standard deviation (SD). Differences were analyzed using the unpaired t-test (two groups) or one-way or two-way analysis of variance (ANOVA) and Tukey’s multiple comparison (multiple groups). The survival rate of mice was analyzed using the Log-rank test. P < 0.05 was considered a significant difference.
Results
Identification of the EPC-derived exosomes
The EPC-derived exosomes (Exo) were obtained using differential centrifugation. The particles showed typical oval shape under the TEM (Figure 1A). Then, the NTA was performed and suggested that the diameter of the particles was mainly distributed in 30-150 nm (Figure 1B). In addition, western blot analysis confirmed positive expression of the exosome-specific marker proteins CD9 and CD81 in the particles (Figure 1C). These results suggested that the extracted particles were exosomes.
Figure 1.

Identification of the exosomes (Exos). A. Shape of the particles observed under a transmission electron microscope; B. Particle size distribution of the exosomes examined by nanoparticle tracking analysis; C. Expression of the exosome-specific biomarkers CD9 and CD81 examined by western blot analysis. Three independent experiments were performed.
Exo alleviated CLP-induced organ damage in septic mice
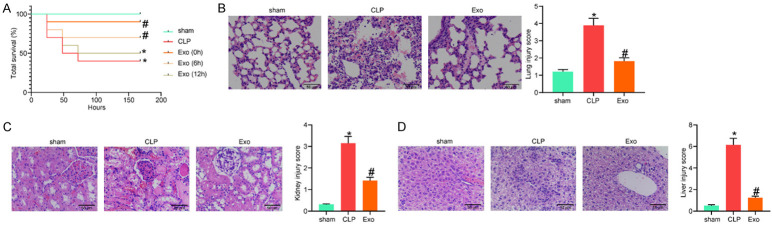
A septic murine model was established by CLP. The model mice were administrated with Exo through tail vein injection at different time points, and then the survival of animals in one week was monitored (Figure 2A). Compared to the sham operation, CLP procedures significantly reduced the survival rate of the mice. At the 0 and 6th h after CLP, Exo treatment enhanced the survival rate in different degrees, while Exo treatment at the 12th h after CLP showed little ameliorating effect on the survival of animals. Therefore, Exo administration at the 0 h after CLP was used as the treating strategy in the subsequent experiments.
Figure 2.
Exos alleviated CLP-induced organ damage in septic mice. (A) Survival rate of mice after cecal ligation and puncture (CLP) procedures and Exo treatment at different time points; (B-D) Pathologic changes in murine lung (B), kidney (C), and liver (D) tissues examined by HE staining. In the survival analysis in (A), n = 10 in each group, while in the subsequent experiments, n = 6 in each group. The survival rate of mice was analyzed by Log-rank test (A). Differences in (B-D) were analyzed by one-way ANOVA followed by Tukey’s multiple comparison. *P < 0.05 vs. the sham group; #P < 0.05 vs. the CLP group.
At the 72nd h after surgery (all mice survived in the initial 72 h were survived in the subsequent one week), the inflammatory injuries in murine lung, kidney and liver tissues were observed by H&E staining (Figure 2B-D). CLP procedures led to significant pathologic changes, such as inflammatory infiltration, alveolar structure damage, hepatic cord disorder and glomerular destruction. Administration of Exo significantly mitigated the CLP-induced symptoms in the organs.
Exo alleviated the immune suppression induced by CLP
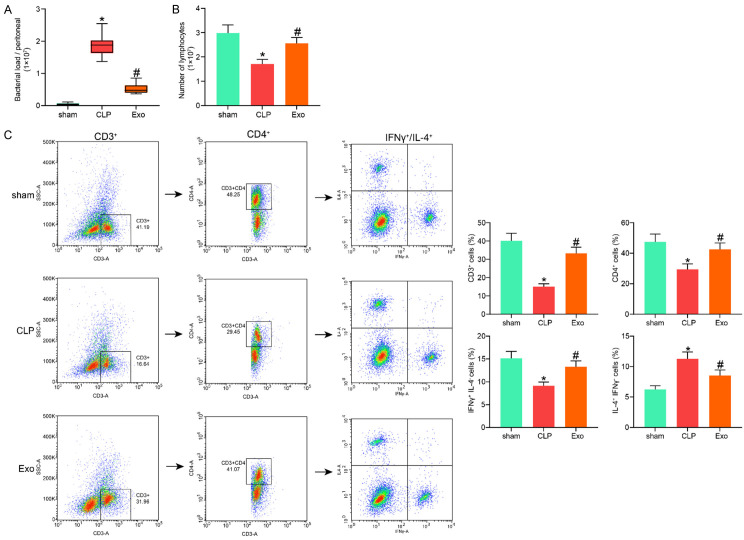
The peritoneal lavage fluid of mice was collected 72 h after CLP surgery and used for 24 h of colony formation (Figure 3A). It was found that the bacterial load (number of formed colonies) in the murine abdominal cavity and the bacterial breeding induced by CLP were notably reduced by Exo treatment.
Figure 3.
Exos alleviated the immune suppression induced by cecal ligation and puncture (CLP). A. Bacterial load in murine abdominal cavity examined by the colony-forming units; B. Number of the lymphocytes extracted from the murine spleen tissues; C. Phenotype of the lymphocytes analyzed by flow cytometry. In each group, n = 6. Differences in all panels were analyzed by one-way ANOVA followed by Tukey’s multiple comparison. *P < 0.05 vs. the sham group; #P < 0.05 vs. the CLP group.
The lymphocytes were extracted from the murine spleen tissues and counted (Figure 3B). It was found that the CLP significantly reduced the lymphocyte count, but the lymphocyte count was recovered after Exo treatment. We then analyzed the phonotype of the lymphocytes (Figure 3C). The concentration of T cells (CD3+) in the lymphocytes was examined using flow cytometry. It was found that the concentration of T cells was first reduced by CLP but then restored by the Exo. Among the CD3+ cells, the proportion of CD4+ cells (Ths) was significantly reduced in the CLP group. Likewise, the proportion of Ths was re-elevated by Exo treatment. A lower Th2/Th1 (T helper 2/T helper 1) ratio in Ths may lead to better survival in septic patients [23]. Then, CD3+ and CD4+ cells were further screened using flow cytometry. It was found that the proportion of Th1 cells (IFNγ+IL-4-) in the Ths was notably declined, while the proportion of Th2 cells (IL-4+IFNγ-) was increased by CLP, and this trend was reversed by Exo treatment. These results indicated that CLP may reduce the antibacterial reaction against pathogen and consequently increase the risk of secondary infection, while the Exo can alleviate the accumulation of Th2 in Ths.
miR-382-3p was present stably and abundantly in the Exo
Since miRNAs are a major class of cargoes conveyed by exosomes, we then examined the miRNA profiles in the Exo. A total of 12 miRNAs were suggested to be highly enriched in the Exo. Among them, only miR-382-3p showed high enrichment in three independent experiments (Figure 4A), indicating that this miRNA was present stably and abundantly in the Exo. Therefore, miR-382-3p was selected as the subject for the subsequent experiments.
Figure 4.
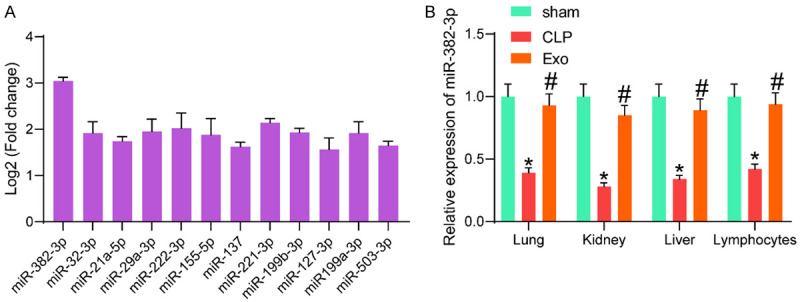
Exos mainly contained miR-382-3p. (A) miRNAs enriched in Exos screened by microarray analysis; (B) Expression of miR-382-3p in murine lung, kidney and liver tissues and in lymphocytes in mice after cecal ligation and puncture (CLP) and Exo treatment examined by RT-qPCR. In each group, n = 6. Differences in (B) were analyzed by one-way ANOVA followed by Tukey’s multiple comparison. *P < 0.05 vs. the sham group; #P < 0.05 vs. the CLP group.
Then, we examined the expression of miR-382-3p in lung, kidney, and liver tissues and in lymphocytes of mice in the sham, CLP and Exo groups. As shown in Figure 4B, the expression of miR-382-3p was significantly reduced after CLP, while the expression of miR-382-3p in these organs was enhanced after Exo treatment. Also, the expression of miR-382-3p in the lymphocytes was examined by RT-qPCR. Compared to that in the lymphocytes from the sham-operated mice, the miR-382-3p expression in the lymphocytes from the CLP-treated mice was significantly reduced. Still, the miR-382-3p expression in the lymphocytes was restored after Exo treatment (Figure 4B).
Knockdown of miR-382-3p blocked the protective effects of Exo on the impaired organs
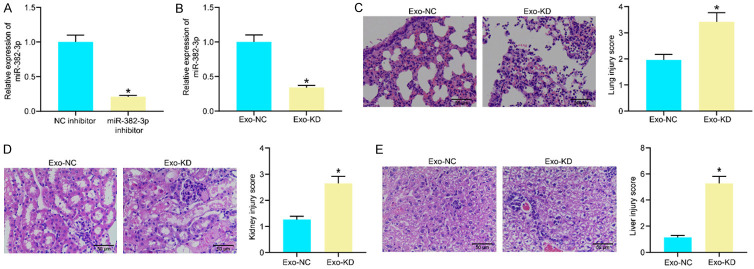
To examine whether miR-382-3p plays a key role in the protective events against sepsis-induced organ injury, downregulation of miR-382-3p was induced in Exo through administration of NC inhibitor/miR-382-3p inhibitor in the EPCs. The successful transfection was confirmed by RT-qPCR (Figure 5A). The corresponding exosomes were collected and named Exo-NC and Exo-KD, respectively. The miR-382-3p expression in the exosomes was examined using RT-qPCR, and successful downregulation of miR-382-3p was confirmed in Exo-KD (Figure 5B). The Exo-NC and Exo-KD were administrated into the CLP-treated mice for further experiments.
Figure 5.
Knockdown of miR-382-3p blocked the protective effects of Exos on the impaired organs. (A) Transfection efficacy of miR-382-3p inhibitor in endothelial progenitor cells examined by RT-qPCR; (B) Expression of Exo-NC and Exo-KD determined by RT-qPCR; (C-E) Pathological changes in murine lung (C), kidney (D) and liver (E) tissues after Exo-NC or Exo-KD treatment examined by H&E staining. In each group, n = 6. Differences in (A-E) were analyzed by the unpaired t test. *P < 0.05 vs. the Exo-NC group.
The pathologic changes in murine organs were observed by H&E staining. Compared to Exo-NC, Exo-KD treatment significantly increased the impairment scores in mice and aggravated inflammatory cell infiltration and tissue injuries in murine lung, kidney, and liver tissues (Figure 5C-E).
Knockdown of miR-382-3p blocked the effects of Exo on immune regulation
The peritoneal lavage fluid samples from mice in the Exo-NC and Exo-KD groups were collected and cultured for 24 h. Compared to Exo-NC treatment, Exo-KD led to a notable increase in the number of CFUs in the peritoneal lavage fluid of mice (Figure 6A). The lymphocytes from murine spleen tissues were collected and analyzed. Importantly, Exo-KD treatment also significantly reduced the lymphocyte count in the murine spleens (Figure 6B). The phenotypes of the lymphocytes were identified using flow cytometry (Figure 6C). In the lymphocytes from mice in the Exo-KD group, the proportion of T cells (CD3+) was significantly reduced. In addition, the proportion of Ths (CD3+CD4+) in the T cells was reduced as well. In the Exo-KD group, the Th2 (IL4+) phenotype showed a predominance in the Ths.
Figure 6.
Knockdown of miR-382-3p blocked the effects of Exo on immune regulation. (A) Bacteria load in the peritoneal lavage fluid from mice examined by colony-forming units; (B) Number of the isolated lymphocytes in the spleen tissues; (C) Concentration of Ths in the T cells and the phenotype of the Ths examined by flow cytometry. In each group, n = 6. Differences in (A-C) were analyzed by unpaired t test. *P < 0.05 vs. the Exo-NC group.
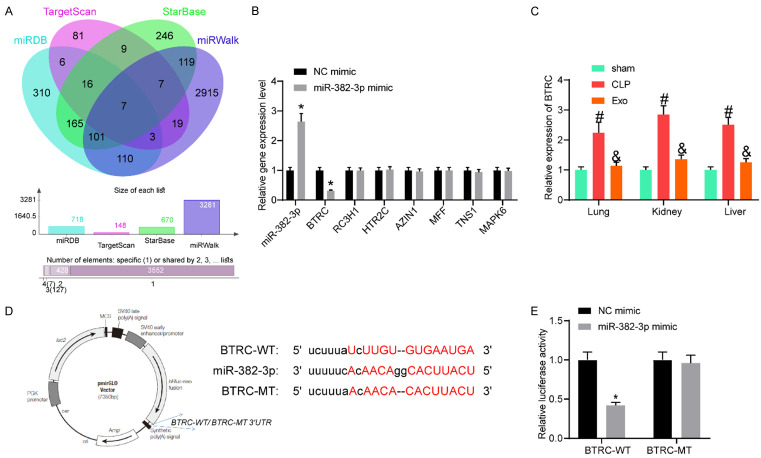
miR-382-3p targeted BTRC mRNA
To further examine the downstream molecules involved, we predicted the potential target mRNAs of mmu-miR-382-3p in several bioinformatic systems including miRDB (http://mirdb.org/), TargetScan (http://www.targetscan.org/vert_72/), Starbase (http://starbase.sysu.edu.cn/) and miRwalk (http://mirwalk.umm.uni-heidelberg.de/). Consequently, a total of 7 mRNAs were predicted to be intersected (Figure 7A). Then, miR-382-3p mimic and the control were transfected into EPCs, and then the expressions of these candidate mRNAs were examined by RT-qPCR (Figure 7B). It was found that only the expression of BTRC in cells was suppressed by miR-382-3p mimic, while the expressions of other candidate transcripts were not significantly changed. Next, the BTRC expression in the lung, kidney and liver tissues of mice in the sham, CLP and Exo groups were examined using RT-qPCR (Figure 7C). Importantly, the CLP procedure increased the expression of BTRC in the lung, kidney, and liver tissues in the mice, while further Exo treatment blocked BTRC expression. The putative binding site between miR-382-3p and BTRC 3’UTR was obtained from StarBase, and the MT sequence was designed (Figure 7D). The dual-luciferase reporter gene assay suggested that the miR-382-3p mimic significantly suppressed the activity of the BTRC-WT luciferase reporter vector, while the luciferase activity of the BTRC-MT vector was not changed (Figure 7E).
Figure 7.
miR-382-3p targeted BTRC mRNA. (A) Possible target mRNAs of miR-382-3p predicted using several bioinformatic systems; (B) Expressions of the candidate mRNAs in endothelial progenitor cells after miR-382-3p mimic transfection examined by RT-qPCR; (C) mRNA expression of BTRC in the murine lung, kidney and liver tissues examined by RT-qPCR; (D) Putative binding site between BTRC and miR-382-3p and the WT sequence prepared for luciferase assay; (E) Luciferase activity of the BTRC-WT and the BTRC-MT luciferase reporter vectors examined by the dual luciferase reporter gene assay. In each group in (C), n = 6. Differences in (B, C and E) were analyzed by two-way ANOVA and Tukey’s multiple comparison. *P < 0.05 vs. the sham group, #P < 0.05 vs. the CLP group, &P < 0.05 vs. the CLP group. BTRC, beta-transducin repeat containing E3 ubiquitin protein ligase; WT, wild type; MT, mutant type.
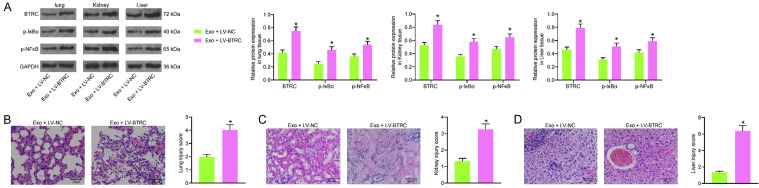
Overexpression of BTRC regulated the IκBα/NFκB signaling pathway and blocked the protective effects of Exo against CLP-induced organ injury
To validate whether BTRC is a target of the exosomal miR-382-3p, the Exo-treated model mice were further injected with LV-BTRC through tail vein, and LV-NC was injected for control. It was previously reported that BTRC (β-TrCP) may promote phosphorylation and degradation of IκBα and trigger NF-κB activation [18]. Therefore, we examined the expression of BTRC and phosphorylation of IκBα/NF-κB in the murine tissues using western blot analysis. Importantly, LV-BTRC significantly increased the protein level of BTRC and the phosphorylation of IκBα and NF-κB in lung, kidney, and liver tissues (Figure 8A). In addition, the tissue injury in these organs was examined using HE staining again. Compared to LV-NC, LV-BTRC transfection significantly increased the tissue impairment scores in mice. The damages in lung, kidney and liver tissues, which were initially alleviated by Exo, were aggravated again upon further BTRC upregulation (Figure 8B-D).
Figure 8.
Overexpression of BTRC regulated the IκBα/NFκB signaling pathway and blocked the protective effects of Exo against CLP-induced organ injury. (A) Protein level of BTRC and phosphorylation of IκBα/NF-κB in murine lung, kidney and liver tissues examined by western blot analysis; (B-D) Pathological changes in murine lung (B), kidney (C) and liver (D) tissues after Exo treatment and LV-BTRC transfection determined by H&E staining. In each group, n = 6. Differences were analyzed by two-way ANOVA (A) or the unpaired t test (B and D). *P < 0.05 vs. Exo + LV-NC. BTRC, beta-transducin repeat containing E3 ubiquitin protein ligase; CLP, cecal ligation and puncture.
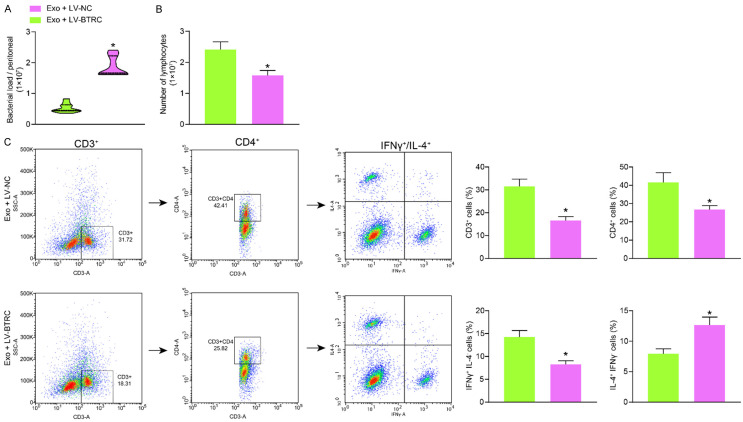
Overexpression of BTRC blocked the effects of Exo on immune regulation
Likewise, the peritoneal lavage fluid samples from Exo-treated mice after LV-NC and LV-BTRC transfection were collected for CFU assay. Importantly, it was found that the bacteria load in the abdominal cavity of the septic mice was increased after BTRC overexpression (Figure 9A). The spleen tissues of these mice were collected. It was found that the number of total lymphocytes (Figure 9B), the concentration of Th cells (CD3+CD4+), as well as the proportion of Th1-phenotype cells (IFNγ+IL-4-), which were increased by Exo, were reduced in the setting of BTRC overexpression (Figure 9C).
Figure 9.
Overexpression of BTRC blocked the effects of Exo on immune regulation. A. Bacteria load in the peritoneal lavage fluid from mice examined by colony-forming units; B. Number of isolated lymphocytes in the spleen tissues; C. Concentration of Th cells (CD3+CD4+) and the phenotype of the lymphocytes examined by flow cytometry. In each group, n = 6. Differences were analyzed by unpaired t test. *P < 0.05 vs. the Exo + LV-NC group. BTRC, beta-transducin repeat containing E3 ubiquitin protein ligase.
Discussion
Septic shock is a fatal organ dysfunction induced by the dysregulated host response to infection [24]. Identifying novel therapeutic tools or biomarkers related to immune regulation and tissue repair is of great significance to improve the outcome of septic patients. EPCs have shown potential relevance to tissue repair in organ systems [14]. Here, we found that the EPC-derived exosomal miR-382-3p alleviated organ damage and immune suppression in a murine model with sepsis, during which the BTRC/IκBα/NF-κB axis was possibly involved.
Acute septic injury in different organs such as lung [25], kidney [26], and liver [27] is a main cause of death of septic patients. Interestingly, EPCs have been found to mitigate paraquat-induced acute lung injury [28] and ischemia-induced kidney injury in murine models [29]. More relevantly, administration of EPCs has also shown beneficiary effects on vascular injury, organ dysfunction, as well as the mortality in preclinical septic models [12,30,31]. Importantly, emerging evidence has suggested that the exosomes play a part in the tissue-repair events by the EPCs [32,33]. This is also applied in sepsis. For instance, EPC-secreted Evs were reported to protect against sepsis-induced kidney injury in mice [34]. The EPC-sourced exosomes were found to prevent microvascular dysfunction and improve the survival and outcomes of septic mice [35]. Here in this study, we validated that the Exo treatment enhanced the survival of septic mice and alleviated the inflammatory infiltration, organ damage, and dysfunction in the murine lung, kidney and liver tissues. Importantly, the protective role of Exo on mouse survival was largely depended on the time of administration, since only a timely treatment of Exo (0 h and 6 h after CLP, rather than 12 h) offered significant survival benefits for the mice.
As for the immune regulation, we first confirmed that the bacterial load in murine abdominal cavity induced by CLP was significantly reduced by Exo treatment. Sepsis can also reduce the concentration of Ths in the lymphocytes [36], and a higher concentration of Ths usually indicates a higher survival rate of septic patients [37]. In concert with this, sepsis can decrease the ratio of Th1 to Th2 cells [38], and a lower Th2/Th1 ratio is correlated with better survival in septic patients [23]. Here, the spleen tissues from mice were collected for analysis. Consequently, the number of lymphocytes in spleen tissues, the concentration of Ths in lymphocytes and the proportion of Th1 cells in the Ths, were significantly reduced by CLP but recovered after Exo treatment. These results, collectively, indicated that the Exo might enhance the immune response and reduce the bacteria load in septic mice.
As aforementioned, miRNAs are a major class of contents of exosomes which play key roles in the exosome-mediated events, and miR-93-5p and miR-126-3p were reported as key factors responsible for the protective functions of EPC-exosomes in the previous reports [34,35]. Here, a microarray analysis suggested that miR-382-3p was highly enriched in the Exo. Although the relevance of miR-382-3p to tissue repair and sepsis has not been concerned before, a previous study showed anti-inflammatory effects of miR-382-3p against inflammation in chondrocytes [17]. Knockdown of miR-382-3p blocked the protective events against organ injury and blocked the immune activation mediated by Exo, which validated that the miR-382-3p was, at least in part, responsible for the functions of the Exo. However, we did not directly inject the miR-382-3p mimic into septic mice to observe its anti-sepsis effects. This would need to be verified in further studies.
The bioinformatic analyses and cellular experiments confirmed BTRC as a direct target of miR-382-3p. Upregulation of BTRC has been reported to enhance phosphorylation and degradation of IκBα and the following phosphorylation and nuclear translocation of NF-κB [18,39]. NF-κB plays a critical role in inflammation and is frequently involved in inflammatory diseases [40]. Activation of NF-κB is also a major contributor to the increase in the chemokines and cytokines, leading to organ injury in sepsis [41]. Importantly, our study found that overexpression of BTRC increased the phosphorylation of IκBα/NF-κB. Also, the tissue repair and immune activity mediated by Exo were blocked in the setting of BTRC upregulation.
Collectively, the present study suggests that EPC-derived exosomal miR-382-3p targets BTRC and decreases phosphorylation of IκBα/NF-κB, thereby alleviating CLP-induced organ injury and immune suppression in septic mice (Figure 10). However, in the present study, we mainly focused on the function of Exo on the organ injury and immune activity in vivo. The in vitro experiments concerning septic injury in lung, kidney and liver cells, and the effect of Exo on the injury in vitro were not concerned. Studying the function of Exo in organ protection in a cellular perspective may enhance the comprehensiveness of the study. We would like to study this in the near future. Since exosomes may offer specific advantages thanks to the reduced immunogenicity while increased safety profile, the Exo might serve as a promising tool for the management of organ injury induced by sepsis.
Figure 10.
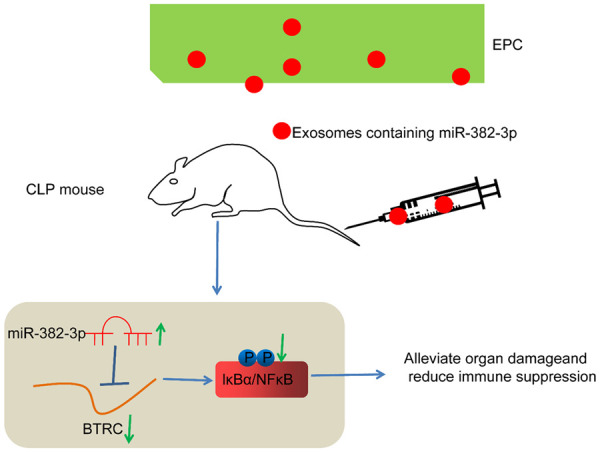
Diagram for the molecular mechanism. Endothelial progenitor cell-derived exosomal miR-382-3p targets BTRC and decreases phosphorylation of IκBα/NF-κB, therefore alleviating CLP-induced organ injury and immune suppression in septic mice. BTRC, beta-transducin repeat containing E3 ubiquitin protein ligase.
Disclosure of conflict of interest
None.
References
- 1.McVeigh SE. Sepsis management in the emergency department. Nurs Clin North Am. 2020;55:71–79. doi: 10.1016/j.cnur.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K International Forum of Acute Care Trialists. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193:259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 3.Vincent JL, Jones G, David S, Olariu E, Cadwell KK. Frequency and mortality of septic shock in Europe and North America: a systematic review and meta-analysis. Crit Care. 2019;23:196. doi: 10.1186/s13054-019-2478-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grondman I, Pirvu A, Riza A, Ioana M, Netea MG. Biomarkers of inflammation and the etiology of sepsis. Biochem Soc Trans. 2020;48:1–14. doi: 10.1042/BST20190029. [DOI] [PubMed] [Google Scholar]
- 5.Weiss SL, Zhang D, Bush J, Graham K, Starr J, Tuluc F, Henrickson S, Kilbaugh T, Deutschman CS, Murdock D, McGowan FX Jr, Becker L, Wallace DC. Persistent mitochondrial dysfunction linked to prolonged organ dysfunction in pediatric sepsis. Crit Care Med. 2019;47:1433–1441. doi: 10.1097/CCM.0000000000003931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angus DC. The search for effective therapy for sepsis: back to the drawing board? JAMA. 2011;306:2614–2615. doi: 10.1001/jama.2011.1853. [DOI] [PubMed] [Google Scholar]
- 7.Marshall JC. Why have clinical trials in sepsis failed? Trends Mol Med. 2014;20:195–203. doi: 10.1016/j.molmed.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis. 2013;13:260–268. doi: 10.1016/S1473-3099(13)70001-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kourembanas S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol. 2015;77:13–27. doi: 10.1146/annurev-physiol-021014-071641. [DOI] [PubMed] [Google Scholar]
- 10.Wu J, Wang Y, Li L. Functional significance of exosomes applied in sepsis: a novel approach to therapy. Biochim Biophys Acta Mol Basis Dis. 2017;1863:292–297. doi: 10.1016/j.bbadis.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 11.Ma C, Wang J, Liu H, Chen Y, Ma X, Chen S, Chen Y, Bihl JI, Yang YI. Moderate exercise enhances endothelial progenitor cell exosomes release and function. Med Sci Sports Exerc. 2018;50:2024–2032. doi: 10.1249/MSS.0000000000001672. [DOI] [PubMed] [Google Scholar]
- 12.Fan H, Goodwin AJ, Chang E, Zingarelli B, Borg K, Guan S, Halushka PV, Cook JA. Endothelial progenitor cells and a stromal cell-derived factor-1alpha analogue synergistically improve survival in sepsis. Am J Respir Crit Care Med. 2014;189:1509–1519. doi: 10.1164/rccm.201312-2163OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J, Zou GJ, Yang L, Rong S, Li BQ, Tong ZH, Li WQ, Li JS. Early prediction of persistent organ failure by circulating endothelial progenitor cells in patients with acute pancreatitis. Shock. 2018;50:265–272. doi: 10.1097/SHK.0000000000001065. [DOI] [PubMed] [Google Scholar]
- 14.Mao SZ, Ye X, Liu G, Song D, Liu SF. An obligatory role of NF-kappaB in mediating bone marrow derived endothelial progenitor cell recruitment and proliferation following endotoxemic multiple organ injury in mice. PLoS One. 2014;9:e111087. doi: 10.1371/journal.pone.0111087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kingsley SMK, Bhat BV. Role of microRNAs in sepsis. Inflamm Res. 2017;66:553–569. doi: 10.1007/s00011-017-1031-9. [DOI] [PubMed] [Google Scholar]
- 16.Iwakawa HO, Tomari Y. The functions of microRNAs: mRNA decay and translational repression. Trends Cell Biol. 2015;25:651–665. doi: 10.1016/j.tcb.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 17.Lei J, Fu Y, Zhuang Y, Zhang K, Lu D. miR-382-3p suppressed IL-1beta induced inflammatory response of chondrocytes via the TLR4/MyD88/NF-kappaB signaling pathway by directly targeting CX43. J Cell Physiol. 2019;234:23160–23168. doi: 10.1002/jcp.28882. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Yuan Y, Xu J, Xiao K, Xu Y, Guo T, Zhang L, Wang J, Zheng H. beta-TrCP restricts lipopolysaccharide (LPS)-induced activation of TRAF6-IKK pathway upstream of IkappaBalpha signaling. Front Immunol. 2018;9:2930. doi: 10.3389/fimmu.2018.02930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi H, Kim Y, Mirzaaghasi A, Heo J, Kim YN, Shin JH, Kim S, Kim NH, Cho ES, In Yook J, Yoo TH, Song E, Kim P, Shin EC, Chung K, Choi K, Choi C. Exosome-based delivery of super-repressor IkappaBalpha relieves sepsis-associated organ damage and mortality. Sci Adv. 2020;6:eaaz6980. doi: 10.1126/sciadv.aaz6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mussbacher M, Salzmann M, Brostjan C, Hoesel B, Schoergenhofer C, Datler H, Hohensinner P, Basilio J, Petzelbauer P, Assinger A, Schmid JA. Cell type-specific roles of NF-kappaB linking inflammation and thrombosis. Front Immunol. 2019;10:85. doi: 10.3389/fimmu.2019.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gurien SD, Aziz M, Jin H, Wang H, He M, Al-Abed Y, Nicastro JM, Coppa GF, Wang P. Extracellular microRNA 130b-3p inhibits eCIRP-induced inflammation. EMBO Rep. 2020;21:e48075. doi: 10.15252/embr.201948075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Wang S, Zhan B, He W, Chu L, Qiu D, Li N, Wan Y, Zhang H, Chen X, Fang Q, Shen J, Yang X. Therapeutic effect of Schistosoma japonicum cystatin on bacterial sepsis in mice. Parasit Vectors. 2017;10:222. doi: 10.1186/s13071-017-2162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xue M, Xie J, Liu L, Huang Y, Guo F, Xu J, Yang Y, Qiu H. Early and dynamic alterations of Th2/Th1 in previously immunocompetent patients with community-acquired severe sepsis: a prospective observational study. J Transl Med. 2019;17:57. doi: 10.1186/s12967-019-1811-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, Angus DC, Rubenfeld GD, Singer M Sepsis Definitions Task Force. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315:775–787. doi: 10.1001/jama.2016.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng Q, Pan B, Alam HB, Liang Y, Wu Z, Liu B, Mor-Vaknin N, Duan X, Williams AM, Tian Y, Zhang J, Li Y. Citrullinated histone H3 as a therapeutic target for endotoxic shock in mice. Front Immunol. 2019;10:2957. doi: 10.3389/fimmu.2019.02957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nusshag C, Rupp C, Schmitt F, Krautkramer E, Speer C, Kalble F, Tamulyte S, Bruckner T, Zeier M, Reiser J, Weigand MA, Uhle F, Merle U, Morath C, Brenner T. Cell cycle biomarkers and soluble urokinase-type plasminogen activator receptor for the prediction of sepsis-induced acute kidney injury requiring renal replacement therapy: a prospective, exploratory study. Crit Care Med. 2019;47:e999–e1007. doi: 10.1097/CCM.0000000000004042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Y, Li Y, Liu X, Pan Y, Sun Z, Xue Y, Wang T, Dou H, Hou Y. SPIONs enhances IL-10-producing macrophages to relieve sepsis via Cav1-Notch1/HES1-mediated autophagy. Int J Nanomedicine. 2019;14:6779–6797. doi: 10.2147/IJN.S215055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin Y, Liu W, Liu X, Ma T, Yang C, Cai Q, Liu Z. Transplantation of endothelial progenitor cells attenuated paraquat-induced acute lung injury via miR-141-3p-Notch-Nrf2 axis. Cell Biosci. 2018;8:21. doi: 10.1186/s13578-018-0219-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen WC, Chou YH, Huang HP, Sheen JF, Hung SC, Chen HF. Induced pluripotent stem cell-derived endothelial progenitor cells attenuate ischemic acute kidney injury and cardiac dysfunction. Stem Cell Res Ther. 2018;9:344. doi: 10.1186/s13287-018-1092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guldner A, Maron-Gutierrez T, Abreu SC, Xisto DG, Senegaglia AC, Barcelos PR, Silva JD, Brofman P, de Abreu MG, Rocco PR. Expanded endothelial progenitor cells mitigate lung injury in septic mice. Stem Cell Res Ther. 2015;6:230. doi: 10.1186/s13287-015-0226-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu X, Yang J, Li N, Wu R, Tian H, Song H, Wang H. Role of endothelial progenitor cell transplantation in rats with sepsis. Transplant Proc. 2015;47:2991–3001. doi: 10.1016/j.transproceed.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Chen C, Wei L, Li Q, Niu X, Xu Y, Wang Y, Zhao J. Exosomes derived from endothelial progenitor cells attenuate vascular repair and accelerate reendothelialization by enhancing endothelial function. Cytotherapy. 2016;18:253–262. doi: 10.1016/j.jcyt.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, Chen C, Hu B, Niu X, Liu X, Zhang G, Zhang C, Li Q, Wang Y. Exosomes derived from human endothelial progenitor cells accelerate cutaneous wound healing by promoting angiogenesis through Erk1/2 signaling. Int J Biol Sci. 2016;12:1472–1487. doi: 10.7150/ijbs.15514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He Z, Wang H, Yue L. Endothelial progenitor cells-secreted extracellular vesicles containing microRNA-93-5p confer protection against sepsis-induced acute kidney injury via the KDM6B/H3K27me3/TNF-alpha axis. Exp Cell Res. 2020;395:112173. doi: 10.1016/j.yexcr.2020.112173. [DOI] [PubMed] [Google Scholar]
- 35.Zhou Y, Li P, Goodwin AJ, Cook JA, Halushka PV, Chang E, Fan H. Exosomes from endothelial progenitor cells improve the outcome of a murine model of sepsis. Mol Ther. 2018;26:1375–1384. doi: 10.1016/j.ymthe.2018.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inoue S, Suzuki-Utsunomiya K, Okada Y, Taira T, Iida Y, Miura N, Tsuji T, Yamagiwa T, Morita S, Chiba T, Sato T, Inokuchi S. Reduction of immunocompetent T cells followed by prolonged lymphopenia in severe sepsis in the elderly. Crit Care Med. 2013;41:810–819. doi: 10.1097/CCM.0b013e318274645f. [DOI] [PubMed] [Google Scholar]
- 37.Chen X, Ye J, Ye J. Analysis of peripheral blood lymphocyte subsets and prognosis in patients with septic shock. Microbiol Immunol. 2011;55:736–742. doi: 10.1111/j.1348-0421.2011.00373.x. [DOI] [PubMed] [Google Scholar]
- 38.Yeh CL, Tanuseputero SA, Wu JM, Tseng YR, Yang PJ, Lee PC, Yeh SL, Lin MT. Intravenous arginine administration benefits CD4(+) T-cell homeostasis and attenuates liver inflammation in mice with polymicrobial sepsis. Nutrients. 2020;12:1047. doi: 10.3390/nu12041047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mu N, Gu J, Huang T, Zhang C, Shu Z, Li M, Hao Q, Li W, Zhang W, Zhao J, Zhang Y, Huang L, Wang S, Jin X, Xue X, Zhang W, Zhang Y. A novel NF-kappaB/YY1/microRNA-10a regulatory circuit in fibroblast-like synoviocytes regulates inflammation in rheumatoid arthritis. Sci Rep. 2016;6:20059. doi: 10.1038/srep20059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haddy FJ. Acute renal failure and sepsis. N Engl J Med. 2004;351:2347–2349. [PubMed] [Google Scholar]