Abstract
Sphingosine 1-phosphate (S1P), which acts via G protein-coupled S1P receptors (S1PRs), is a bioactive lipid essential for vascular integrity and lymphocyte trafficking. The S1P–S1PR signalling axis is a key component of the inflammatory response in autoimmune rheumatic diseases. Several drugs that target S1PRs have been approved for the treatment of multiple sclerosis and inflammatory bowel disease and are under clinical testing for patients with systemic lupus erythematosus (SLE). Preclinical studies support the hypothesis that targeting the S1P–S1PR axis would be beneficial to patients with SLE, rheumatoid arthritis (RA) and systemic sclerosis (SSc) by reducing pathological inflammation. Whereas most preclinical research and development efforts are focused on reducing lymphocyte trafficking, protective effects of circulating S1P on endothelial S1PRs, which maintain the vascular barrier and enable blood circulation while dampening leukocyte extravasation, have been largely overlooked. In this Review, we take a holistic view of S1P–S1PR signalling in lymphocyte and vascular pathobiology. We focus on the potential of S1PR modulators for the treatment of SLE, RA and SSc and summarize the rationale, pathobiology and evidence from preclinical models and clinical studies. Improved understanding of S1P pathobiology in autoimmune rheumatic diseases and S1PR therapeutic modulation is anticipated to lead to efficacious and safer management of these diseases.
Sphingosine 1-phosphate (S1P) is a bioactive lipid molecule secreted into the circulation mainly by erythrocytes and endothelial cells. It binds to five high affinity G protein coupled receptors (GPCRs), termed sphingosine 1-phosphate receptors (S1PRs), which are widely expressed and affect cell proliferation, survival and migration both positively and negatively. S1P receptor 1 (S1PR1, also known as S1P1, encoded by S1PR1, originally cloned as endothelial differentiation gene 1 (EDG1)), is highly expressed on endothelial cells (ECs) and increases vascular barrier function after coupling to guanine nucleotide-binding protein (G protein) Gαi by facilitating the translocation of vascular endothelial (VE)-cadherin (also known as cadherin 5), an EC-specific adhesion molecule, to inter-cellular borders and the assembly of adherens junctions1. EC S1PR1 signalling also has anti-inflammatory effects by curbing the expression of pro-inflammatory adhesion molecules required for leukocyte transmigration2-4 and by limiting the production of the cytokines that contribute to the ‘cytokine storm’ in response to viral infection5. S1PR1 is also expressed on lymphocytes and is a crucial mediator of lymphocyte trafficking. Lymphocytes migrate out of lymph nodes (LNs), in which S1P levels are kept low by degradative enzymes such as phospholipid phosphatase 3 (LPP3) (also known as lipid phosphate phosphohydrolase) and S1P lyase 1 (SGPL1)6,7 towards high concentrations of S1P in lymph and blood, using S1PR1 as an egress receptor8,9.
Whereas S1PR1 functions in vascular and immune systems have been studied extensively, other S1PR isoforms also have important pathophysiological functions10,11. For example, S1PR2 is induced in ECs during pathological conditions and opposes the actions of S1PR1, by enhancing vascular permeability and inducing inflammatory responses12-14. In the immune system, S1PR2 restrains the movement of cells (myeloid cells, germinal centre B cells and γδ T cells) by inhibiting chemotactic receptors15,16. Loss of function of S1PR2 is associated with diffuse large B cell lymphoma severity in humans17. S1PR3 is expressed in pericytes and fibroblasts, and at low levels in ECs, where it can be upregulated in the setting of lung injury18. EC S1PR3 mediates S1P-induced vasoconstriction19, and seems to be crucial for inflammatory and fibrotic responses20,21. S1PR4 polymorphisms are associated with circulating neutrophil numbers in humans22. S1PR5 is the main S1PR facilitating natural killer cell trafficking from bone marrow and secondary lymphoid organs (SLOs)23. Because S1PR1 is essential for lymphocyte trafficking, the major EC S1PR, and the target of newer S1PR modulators used in preclinical studies and clinical trials of rheumatic autoimmune diseases, this Review focuses on S1P–S1PR1 biology.
Four S1PR1 modulators have been FDA approved for the treatment of autoimmune inflammation in multiple sclerosis and one has been FDA approved for ulcerative colitis. Fingolimod, approved in 2010, is a sphingosine analogue that is phosphorylated by sphingosine kinase 2 (SPHK2)24 and binds to S1PR1, S1PR3, S1PR4 and S1PR5 (REF.25). Fingolimod has several known adverse effects, including bradycardia, suppression of pulmonary function, hepatocyte damage and macular oedema. Bradycardia occurs secondary to fingolimod engagement of S1PR1-dependent inward-rectifying potassium channels in human cardiomyocytes26. In most individuals, adverse events are mild or moderate, and bradycardia usually resolves within 4 weeks27. The more recently approved drugs, namely, ozanimod, siponimod and ponesimod, are selective for S1PR1 and S1PR5 (REF.28). As some of the safety issues associated with the use of fingolimod could be related to its engagement of other S1PR isoforms, the newer S1PR modulators with greater specificity for S1PR1 are expected to have fewer adverse effects. The active forms of all four of these drugs bind to S1PR1 on the surface of lymphocytes and induce receptor internalization and degradation, thereby acting as functional antagonists and blocking the ability of autoreactive T cells and B cells to enter tissues (see section S1PR1 modulation of lymphocyte trafficking). However, because S1PR1 is highly expressed on ECs and mediates barrier function of blood vessels, the use of these functional antagonists could lead to vascular leakage29 and downstream inflammatory injury30. Thus, in considering the use of S1PR1 modulators in autoimmune rheumatic disease, one must be cognizant of the potential risks of EC S1PR1 antagonism, particularly in patients with clinical and subclinical pulmonary involvement of their rheumatic disease and inflammatory eye diseases, because S1PR1 is highly expressed in lung and retinal vessels27,31,32. In this Review, we give an overview of S1P–S1PR1 biology, discuss how modulation of the S1P–S1PR1 axis could benefit patients with autoimmune rheumatic diseases and provide a critical assessment of the preclinical and clinical data that support the use of S1PR1 modulators for systemic lupus erythematosus (SLE), rheumatoid arthritis (RA) and systemic sclerosis (SSc).
Basic biology of S1P
S1P production and export
S1P is formed by metabolic breakdown of membrane-derived sphingomyelin (FIG. 1). Hydrolysis of sphingomyelin forms ceramide, which is converted to sphingosine by ceramidase; intracellular sphingosine is phosphorylated into S1P by sphingosine kinase 1 (SPHK1) or SPHK2 and degraded by SGPL1 and S1P phosphatase 1 (Spp1) and Spp211 (FIG. 1). Although the enzymatic properties of SPHKs are similar, differential subcellular localization and tissue specificity of expression suggest some isoform-specific functions. SPHK1 resides predominantly in the cytosol, undergoes translocation to the plasma membrane following phosphorylation and can also be transported outside of the cell33, whereas SPHK2 is localized in the endoplasmic reticulum, nucleus and mitochondria34 (FIG. 1). Sphk1 knockout (KO) mice demonstrate a 65% reduction in circulating S1P levels but have normal tissue S1P levels and do not exhibit dysregulated lymphocyte trafficking or vascular developmental defects35, whereas Sphk2 KO mice have either normal36 or elevated37 levels of circulating S1P, a finding that has been attributed to the ability of Sphk2 to recycle S1P intracellularly38. Double Sphk1–Sphk2 KO embryos show evidence of both vascular and neural defects and die in utero39, whereas mice with postnatally inducible double Sphk1–Sphk2 KO are viable but demonstrate vascular leakage and increased mortality when challenged with inflammatory mediators40.
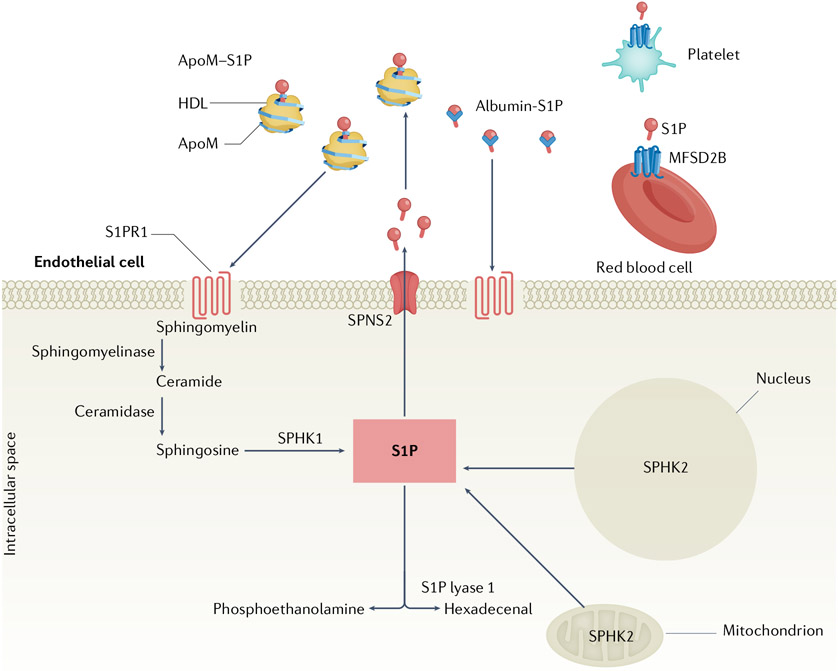
Fig. 1 ∣. Overview of S1P production, export and chaperones.
Sphingosine 1-phosphate (S1P) is produced intracellularly by sphingosine kinases (SPHK1 and SPHK2) and is degraded by S1P lyases and phosphatases. Extracellular transport of S1P is mediated by spinster homologue 2 (SPNS2) on endothelial cells or major facilitator superfamily domain-containing protein 2B (MFSD2B) on red blood cells and platelets. Extracellular S1P binds to apolipoprotein M (ApoM) in HDL or albumin in the circulation and binds S1P receptor 1 (S1PR1), which is expressed on endothelial cells, lymphocytes and many other cell types.
Extracellular S1P is regulated by the LPP3 enzyme; the active site of this key enzyme is located on the extracellular surface and could be involved in sphingolipid uptake41. The polymorphism of PLPP3 (encoding LPP3) is associated with cardiovascular disease in humans, suggesting its importance in vascular function42. LPP3 is highly expressed on ECs and helps to maintain the vascular barrier, as mice with an EC-specific Plpp3 KO have evidence of vascular leakage in vivo43, and silencing of Lpp3 in ECs decreases vascular resistance in vitro44. In the immune system, this enzyme regulates the S1P-dependent trafficking of lymphocytes in the thymus45. As LPP3 is active towards multiple lipid phosphates in the extracellular environment, the role of this protein in autoimmune rheumatic diseases warrants further research.
S1P in lymph and plasma originates from different sources. In lymph, the primary source of S1P is the lymphatic endothelial cell8,46. Lymphatic endothelial cell-specific Sphk1 KO mice have altered lymphatic vascular junctions and decreased egress of lymphocytes to LNs. By contrast, in plasma, erythrocytes and blood vessels ECs are the major sources of S1P47. Erythrocytes use Sphk1 to generate S1P and contribute to circulating levels, and EC-specific double Sphk1–Sphk2 KO mice show a ~30% reduction in plasma S1P levels48.
Although platelets contain high levels of S1P that is produced by Sphk2 (REF.49), they do not contribute to circulating S1P during homeostasis49,50. However, upon activation with agonists, such as thrombin or collagen, platelets release S1P, which, in turn, induces in platelets a change in shape and the release of arachidonic acid and thromboxane49,51, thereby fine tuning thrombotic mechanisms. Platelet-released S1P increases endothelial barrier function52,53, which could limit vascular leakage during thrombosis or inflammatory injury. Moreover, if the level of S1P generated by erythrocytes and ECs becomes insufficient, platelet-derived S1P compensates for essential functions such as embryonic viability52. Platelet-derived S1P has a crucial role in the barrier function of high endothelial venules (HEVs), the vessels that enable trafficking of lymphocytes to LNs. Fibroblastic reticular cells surround HEVs and express podoplanin, which activates platelets, thereby inducing the release of S1P, which in turn maintains HEV barrier integrity54.
Intracellular S1P is transported to the extracellular space by the transporter spinster homologue 2 (SPNS2)55 in ECs and by the major facilitator superfamily domain-containing protein 2B (MFSD2B) in erythrocytes and platelets56 (FIG. 1). Spns2 KO mice (either global gene deletion or endothelial-specific deletion) show reduced plasma S1P level and dramatically reduced T cell and immature B cell egress from the thymus and bone marrow, respectively, suggesting that EC-derived S1P is required for adaptive immune system homeostasis57,58.
Although the cellular and enzymatic contributors to circulating S1P have been defined, the precise mechanisms that maintain physiological S1P levels during homeostasis and inflammation have yet to be elucidated. Inflammatory conditions and ageing lead to reduced plasma S1P levels, suggesting that a stable plasma S1P pool contributes to overall health59-61.
S1P chaperones
Extracellular S1P in the circulation is bound to either albumin, which carries ~30% of plasma S1P, or apolipoprotein M (ApoM), which carries roughly ~65%62 (FIG. 1). ApoM, a lipocalin produced mainly in the liver and kidney, is a constituent of HDL particles. Almost all S1P associated with HDL particles is bound to ApoM62. ApoM has anti-atherogenic properties owing to its ability to regulate triglyceride metabolism, adipocyte biology63,64 and endothelial protection62. ApoM can bind to oxidized phospholipids under pathological conditions, even though ApoM-containing HDL particles represent a minor proportion of the heterogeneous HDL particles65. APOM is located on the MHC class III region of chromosome 6, a region rich in genes involved in innate immunity and inflammation, including TNF (encoding tumour necrosis factor (TNF)), LTA and LTB (encoding lymphotoxin-α and lymphotoxin-β proteins, respectively)66. ApoM KO mice have ~45% reduction in plasma S1P level and are more vulnerable to vascular leakage than wild-type mice but do not show defects in lymphocyte trafficking. However, such mice show increased proliferation of haematopoietic progenitor cells and common lymphoid progenitors in the bone marrow and a heightened adaptive immunity, making them more susceptible to neuroinflammatory insults67. The chaperone (albumin or ApoM) presenting S1P to S1PR1 mediates ‘biased’ signalling, as S1P presented in the context of ApoM in HDL particles shows sustained signalling and enhanced endothelial barrier function compared with albumin-bound S1P62,68-70.
S1PR1 modulation of lymphocyte trafficking
T cells and B cells lacking S1PR1 are trapped in the thymus and bone marrow, respectively, owing to their inability to sense S1P71-73. During homeostasis, naive lymphocytes egress towards the lymph out of LNs, where S1P levels are kept very low by SGPL1 and LPP3 (REF.6). Lymphocyte cell-surface expression of S1PR1 correlates inversely with extracellular S1P concentration to which the receptor is exposed; thus, it is high in the LNs where lymphocytes are poised for egress8 (FIG. 2). Lymphatic EC production46 and their release of S1P via Spns2 are involved in the egress process46,58,74. In contrast to the lymphocyte expression of S1PR1, the expression CC-chemokine receptor 7 (CCR7), the principal chemokine receptor for CC-chemokine ligand 21 (CCL21) mediating lymphocyte entry and retention in LNs, is downregulated in LNs but high in blood and lymph, facilitating lymphocyte entrance to and transient arrest in SLOs75.
Fig. 2 ∣. Major physiological roles of S1PR1 signalling.
Sphingosine 1-phosphate (S1P) receptor 1 (S1PR1) signalling has roles in lymphocyte trafficking and differentiation and endothelial cell barrier protection. Lymphocytes sense circulating S1P via S1PR1 in lymph nodes where the S1P lyase and phosphatase activities are high, S1PR1 expression is maximal, and lymphocytes are poised to egress. In blood and lymph, high S1P levels induce internalization of S1PR1. Beyond trafficking, S1PR1 signalling restrains CD4+T cell differentiation into regulatory T (Treg) cells and increases their differentiation into T helper 17 (TH17) cells.Thus, functional antagonists of S1P–S1PR1 signalling increase differentiation into Treg cells and decrease differentiation into TH17 cells. On endothelial cells, S1PR1 signalling leads to increased intercellular contacts via adherens junctions mediated, in part, by VE-cadherin and limits vascular leakage and leukocyte transmigration. S1PR1 signalling also limits expression of ICAM1 and VCAM1 in response to inflammatory mediators (such as tumour necrosis factor (TNF)) by blocking NF-κB activation.
Much of the biology of S1P–S1PR1 signalling and its effect on lymphocyte trafficking has been learned from preclinical and clinical experience with fingolimod. Whereas S1P binding to S1PR1 causes internalization of the receptor followed by recycling to the membrane where it can engage extracellular S1P again, fingolimod-P induces S1PR1 internalization, ubiquitination and degradation29, processes that require recruitment of β-arrestin76. This difference occurs because fingolimod-P is metabolically more stable, more poorly dissociated from S1PR1 and more efficient at recruiting β-arrestin than S1P77. The net result is that lymphocytes lose the sensor (that is, S1PR1) required to exit SLOs. Fingolimod can also reduce SphK1 activity and induce its proteasomal degradation, at least in vitro78, which could potentially also contribute to altered lymphocyte trafficking. The lymphocytes affected by fingolimod, namely, naive T cells and central memory T cells, express CCR7 and circulate continuously from the blood to LNs. By contrast, effector memory T cells and CD45RA (also known as receptor-type tyrosine-protein phosphatase C)-expressing effector memory T cells lack expression of CCR7, do not recirculate regularly through LNs and are not affected by fingolimod79. Similar T cell subsets are affected in patients taking ozanimod80 (see section S1PR1 signalling effects on Treg cells). Effector memory T cells present in the circulation remain functionally responsive without impairment of defence against pathogens, consistent with the observation that patients taking fingolimod have a low, albeit increased, risk of infectious complications81,82.
When T cells encounter antigen in the context of dendritic cells (DCs) and become activated, they downregulate S1PR1 transcription and are retained in SLOs via CCR7 retention signals; however, daughter cells begin to recover S1PR1 expression after several divisions and are able to egress in response to S1P75. In the presence of inflammatory signals (such as type I interferon and TNF), lymphocytes express early activation antigen CD69, a negative regulator of S1PR1, which, like CCR7, facilitates lymphocyte arrest in SLOs83. A study published in 2021 has shown that during viral infection or neuroinflammation, S1P levels increase in LNs, thereby hindering T cell egress. Unexpectedly, the source of elevated S1P in LNs was activated CD69+ monocytes, which helped to differentiate T follicular helper cells and T helper 17 (TH17) cells. The precise mechanisms by which monocyte-derived S1P affects T cell differentiation have yet to be delineated84, but T cells with increased retention time in LNs might have enhanced maturation and antigen specificity.
Although fingolimod treatment leads to sequestration of lymphocytes in LNs, FTY720-treated mice do not exhibit hypertrophic SLOs85-87. One possibility is that FTY720 induces lymphocyte apoptosis, as several studies have shown88-91. Another possibility is that FTY720 hinders DC migration to LNs, thereby reducing antigen presentation and T cell and B cell activation, differentiation and proliferation, as has been suggested by some studies92,93. FTY720 may also limit numbers of activated TH cells in the lymphoid tissues, which would result in smaller germinal centers94, impaired affinity maturation, and diminished class-switching recombination, which could also contribute to smaller SLOs.
S1P and adaptive immunity
S1PR1 signalling effects on TH17 cells
Fingolimod is beneficial in multiple sclerosis, at least in part, by keeping auto-aggressive central memory T cells out of the central nervous system (CNS). The central memory T cells subset targeted by fingolimod includes TH17 cells, which produce the inflammatory cytokine IL-17 and are implicated in multiple sclerosis pathogenesis95. Mice with a TH17 cell-specific deletion of S1pr1 (TH17 S1pr1 KO) are completely resistant to the development of experimental autoimmune encephalomyelitis induced by immunization with myelin oligodendrocyte glycoprotein peptide (MOG35–55)96. Congruent with the concept that loss of S1PR1 signalling limits trafficking of auto-aggressive T cells, TH17 S1pr1 KO mice with experimental autoimmune encephalomyelitis had significantly fewer infiltrating TH17 and TH1 cells in the CNS than controls. However, contrary to the expectation that TH17 cells would be trapped in LNs, the percentage of TH17 cells in the LNs was decreased in KO mice, suggesting an additional role for S1PR1 signalling in the generation and/or survival of TH17 cells. These data are supported by earlier studies demonstrating that S1PR1 signalling increases TH17 cell differentiation from CD4+ T cells97 (FIG. 2). Both S1P and SEW-2871, a S1PR1-specific agonist, increased IL-17 production by T cells97. Suppression of TH1 and TH2 cell cytokine production occurred concurrently with the development of TH17 cells97. In another study, mice harbouring a variant of S1pr1 encoding a protein with increased signalling capacity had increased production of IL-17, and splenocytes from mice treated with a SGPL1 inhibitor to increase S1P and S1pr1 signalling also demonstrated enhanced IL-17 production and increased susceptibility to experimental autoimmune encephalomyelitis98. Taken together, these studies provide strong evidence that S1PR1 signalling augments TH17 cell differentiation in appropriately primed CD4+ T cells and that FTY720, by inducing internalization and degradation of S1PR1, attenuates TH17 cell differentiation.
S1PR1 signalling effects on Treg cells
Regulatory T (Treg) cells inhibit the activation and expansion of CD4+ TH cells, suppress differentiation of cytotoxic CD8+ T cells and limit B cell activation. Mice lacking forkhead box protein P3 (Foxp3), the transcription factor required for Treg cell differentiation and maintenance, develop autoimmunity, and inactivating mutations in FOXP3 in humans are associated with severe autoimmunity99-101. Increasing Treg cell numbers and/or function could be beneficial in autoimmune rheumatic disease102. In NZB/NZW lupus-prone mice, adoptive transfer of Treg cells before the onset of clinical disease resulted in decreased progression of kidney injury and mortality103.
S1P–S1PR1 signalling suppresses Treg cell numbers and functions (FIG. 2). CD4+T-specific S1pr1 KO mice have increased numbers of Treg cells that have greater suppressive capacity than those in controls104. Furthermore, mice with CD4+T cells overexpressing S1pr1 have impaired suppressive capacity104. However, a more recent study published in 2017 has added complexity to the paradigm that S1PR1 signalling in T cells negatively affects Treg cells and autoimmunity. When S1pr1 is knocked out in Foxp3+ T cells rather than all CD4+ T cells, Treg cells are trapped in LNs and numbers of tissue-surveilling Treg cells are decreased, resulting in autoimmunity. Moreover, inducible deletion of S1pr1 in Foxp3+ T cells rendered mice more susceptible to experimental autoimmune encephalomyelitis, presumably because of decreased thymic and LN release of Treg cells to the CNS96. In support of the hypothesis that Treg cells use S1PR1 to exit LNs and enter tissues, KO of S1pr1 in CD4+ T cells resulted in Treg cell trapping in LNs, leading to reduced numbers of Treg cells in tumours and restrained tumour growth105. Thus, it is unclear whether the net effect of S1PR1 signalling in lymphocytes is detrimental (by curbing the production of Treg cells from CD4+ T cells) or protective (by enabling Treg cells to egress from LNs to tissues) against autoimmunity. Some insight can be gleaned from mice and patients taking FTY720/fingolimod. FTY720/fingolimod, acting as a functional antagonist on lymphocytes, has been shown to increase the suppressive capacity of Treg cells in both mice (FTY720)106-108 and humans (fingolimod)109-112. Although the numbers of Treg cells are expected to fall during treatment with FTY720/fingolimod secondary to trapping in LNs, they diminish to a lesser extent than those of other T cell populations, resulting in the relative preservation of Treg cells with a memory phenotype110,113,114. Relative preservation of Treg cells compared with other T cell subsets was also observed in patients taking ozanimod80. This finding could be attributed to reduced expression of S1PR1 in Treg cells, the altered chemotaxis to S1P of these cells compared with other T cells, and the ability of Treg cells to use other S1PRs to egress from LNs106.
Because in SLE, RA and SSc there is evidence of an imbalance of TH17 cell–Treg cell populations, S1PR1 modulators, acting as functional antagonists, could help to restore this imbalance and immune tolerance. The ongoing clinical studies using S1PR1 modulators in SLE (NCT03742037) could elucidate whether TH17 cells and/or Treg cells trafficking and/or function are positively affected during treatment.
S1P and vascular homeostasis
S1PR1 signalling effect on vascular barrier
S1PR1 is a crucial mediator of vascular development and function; S1pr1 KO mice are not viable as a result of vascular fragility during embryonic development115. S1PR1 inhibits angiogenic sprouting during development116-118 and stabilizes blood vessels of tumours, which are immature and prone to leakage119. EC S1PR1 is a key mediator of vascular barrier function by facilitating the assembly of VE-cadherin molecules to intercellular borders where they form zipper-like structures that help to maintain the vascular barrier1 (FIG. 2). Mice with an endothelial-specific120 knockdown of S1pr1 or treated with S1PR1 antagonists show evidence of vascular leakage in the lungs67,121 and brain122. Reciprocally, S1PR1 agonists rapidly attenuate vascular leakage, production of inflammatory cytokines and neutrophil infiltration in response to lung injury120,123, and immune complex mediated vasculopathy121. However, significant endocytosis of the endothelial S1PR1 after binding to agonists leads to functional antagonism and cessation of the vascular protective response and could even lead to exacerbation of inflammatory injury and parenchymal fibrosis (see section S1PR1 antagonists in preclinical RA)124.
S1PR1 signalling also induces vasorelaxation by activating endothelial nitric oxide synthase (eNOS)19,125; mice with EC-specific S1pr1 KO have significantly higher blood pressure than littermate controls19. However, in the afferent arterioles of the kidney, S1PR1 agonist SEW-2871 had a vasoconstrictive effect, even at doses as low as 10 nM126. Because afferent arterioles are of major importance in regulating glomerular filtration rate, the vasoconstrictor effect of S1P selectively in afferent but not efferent arterioles strongly implicates S1PR1 signalling in controlling glomerular filtration rate127. How EC S1PR1 signalling affects the pathophysiology of lupus nephritis has yet to be elucidated.
Circulating S1P–ApoM and vascular health
ApoM on HDL particles delivers S1P to S1PR1 and has anti-inflammatory effects by limiting expression of pro-inflammatory adhesion molecules such as ICAM1 and VCAM1, which are required for leukocyte transmigration into tissues2,3 (FIG. 2). As an example of ’biased’ signalling, S1P–ApoM is more effective than S1P–albumin at preventing TNF-induced ICAM1 expression in ECs; this enhanced signalling is secondary to the ability of S1P–ApoM to attenuate NF-κB phosphorylation2, which is essential for TNF-mediated inflammatory responses.
Many lines of evidence suggest that ApoM delivery of S1P to ECs also protects against vascular injury and inflammation, which are important pathogenetic mechanisms in cardiovascular disease. ApoM has been shown to mediate cholesterol efflux and susceptibility to atherosclerosis128. Furthermore, the cardiovascular protective effects of S1P–ApoM were demonstrated in mouse models of myocardial infarction and stroke, in which an engineered S1P chaperone ApoM–S1P Fc attenuated inflammatory damage in response to ischaemic injury69.
ApoM also has protective effects on blood pressure: Apom KO mice showed significantly increased resting blood pressure compared with wild-type mice, and ApoM–S1P Fc increased circulating nitrite levels and protected against angiotensin-induced hypertension, suggesting that ApoM activates the eNOS69.
S1P–S1PR1 modulators in rheumatic disease
SLE, RA and SSc are complex and heterogeneous diseases that share some pathological features, including autoreactive lymphocytes, dysregulated TH17 cells and Treg cells that are not effective enough to quell autoimmune-mediated damage. As detailed above, there is strong evidence that these pathological features could be ameliorated by S1PR1 modulation. Moreover, cytokine-induced vascular permeability in each of these diseases facilitates extravasation of inflammatory mediators129,130, which could be attenuated by EC S1PR1 agonism. The clinical efficacy of fingolimod for the treatment of multiple sclerosis has encouraged the development of several S1PR1-specific agonists and antagonists for the treatment of other autoimmune diseases. In the following sections, we review the evidence that the S1P–S1PR axis is dysfunctional in SLE, RA and SSc and the preclinical and clinical studies supporting the use of S1PR modulators in each of these diseases. We take a holistic view that therapeutic modulation of the S1P signalling axis should consider not only the immune system but also the vascular system and connective tissue cells. Reviews on autoimmune pathophysiology are available131,132.
Systemic lupus erythematosus
SLE is the prototypic systemic autoimmune disease, with all components of the immune system contributing to autoimmunity against nucleic acid-containing cellular elements. The innate immune system is activated, in part, by cellular nucleic acid sensors and neutrophil extracellular traps (NETs), which stimulate production of type I interferon, a key contributor to organ damage. Adaptive immune responses are also altered133. T cell dysregulation is evidenced by altered signalling, production of cytokines and proliferation and function of T cell subsets134. In SLE, expansion of follicular TH cells promotes differentiation of autoantigen-specific B cells, whereas depletion of Treg cells is associated with impaired capacity to suppress immune responses and TH17 cells135. B cell function is also disturbed, with increased cytokine production and augmented presentation of antigens to T cells. Consequent expansion of autoreactive B cells and production of autoantibodies leads to intravascular immune complex formation. SLE ECs display an activated, inflamed phenotype, with increased expression of adhesion molecules related to exposure to cytokines (that is, type I interferons), complement activation fragments and products of stimulated innate immune cells136. Immune complexes escape through permeant blood vessels, activate complement, neutrophils and monocytes, and thereby serve as the primary drivers of inflammatory-mediated organ damage137.
All-cause SLE mortality has not improved in the past two decades and remains twofold higher than that in the general population138,139, with infection and cardiovascular disease now the most common causes of mortality140,141. Novel therapies are needed to decrease the use of corticosteroids, induce remission and mitigate end organ damage. S1PR1 modulators could be of particular benefit in SLE because, in addition to their effect on lymphocyte trafficking, they protect the vasculature and reduce the production of type I interferons (see section IFNα production in SLE as target of S1P–S1PR1 modulators).
Evidence that S1P levels are dysregulated in SLE.
S1P levels in SLE have been assessed in small studies142-144, with two showing that patients had elevated S1P that did not correlate with disease activity and another showing that the ceramide:S1P ratios significantly decreased after treatment (n = 22)144, suggesting that a drop in ceramide and/or an increase in S1P was associated with an improvement in SLE. The differing results of these studies could be the result of technical differences in blood drawing and handling, as platelet activation and haemolysis contribute to S1P levels. S1P in serum is higher than in plasma because of the contribution of platelet and erythrocyte S1P; thus, sera levels might not accurately reflect circulating S1P levels. Larger longitudinal studies using plasma rather than serum from patients before and after successful treatment could help to determine whether S1P is a useful biomarker in SLE. It is also unknown whether dysregulated S1P levels contribute to aberrant lymphocyte trafficking and lymphopenia in SLE.
Evidence that ApoM is dysregulated in SLE.
Circulating ApoM levels were reduced in patients with SLE and were inversely correlated with SLE disease activity index (SLEDAI) scores in two studies145,146. In the first study, two groups of patient samples were assessed. In group I (n = 84), bio-banked samples and the corresponding SLEDAI-2K score obtained on the day of collection were evaluated. Group II (n = 140) consisted of patients with SLE participating in studies related to cardiovascular disease over a 2-year period. In group I, ApoM concentrations were significantly lower in patients with active renal disease, skin involvement, leukopenia or anti-dsDNA antibodies than in patients with SLE without these disease manifestations. Endothelial function using a finger plethysmograph (a device measuring arterial blood flow) (EndoPAT, Itamar Medical, Israel) was assessed in patient group II. A low Reactive Hyperemia Index value on EndoPAT indicates endothelial dysfunction147. In support of a vascular protective effect of ApoM, there was an association between Reactive Hyperemia Index and ApoM levels in patients with SLE of 20–45 years of age (n = 60) that remained after adjustment for cardiovascular disease risk factors, medications and SLE disease activity.
In the second study, ApoM levels were compared between individuals newly diagnosed with SLE and age-matched and sex-matched controls (n = 52 per group)145. Because ApoM is produced mainly in the liver and kidney, patients with liver or kidney disease were excluded, as were patients taking steroids or other immunosuppressive agents. ApoM levels were significantly lower in patients with SLE than those in healthy controls and correlated negatively with the levels of SLEDAI scores and the presence of anti-dsDNA antibodies.
Why ApoM levels would drop during periods of increased disease activity is not clear, but data from both humans and mice demonstrate that ApoM expression is downregulated during the acute phase response induced by infection and inflammation148. An interesting and yet unanswered question is whether ApoM undergoes post-translational modifications during chronic inflammation that impair its vascular protective effects. Preclinical studies are needed to determine whether S1P–ApoM delivery attenuates disease activity in mouse models of SLE.
IFNα production in SLE as a target of S1P–S1PR1I modulators.
Dysregulation of type I interferon production is a cornerstone of SLE pathophysiology149-152, and type I interferons, as well as their major producers, plasmacytoid dendritic cells (pDCs), are now targets for therapy in SLE. Understanding how S1P–S1PR1 signalling affects type I interferon production in response to viral infection could reveal mechanistic insights and targets for the treatment of SLE, as viral nucleic acids and human nucleic acids contained in immune complexes and/or NETs in SLE use common molecular pathways to promote the production of type I interferons.
The S1PR1 and S1PR5 agonist CYM-5442 was shown to attenuate IFNα production in pDCs challenged either with influenza virus or CpG-A (a synthetic nucleotide that activates Toll-like receptor 9 (TLR9)). Mechanistically, CYM-5442 induced co-internalization and degradation of S1PR1 and the type I interferon receptor interferon α/β receptor 1 (IFNAR), thereby impeding the ability of IFNα to turn on an autocrine feedback loop and attenuating production of IFNα108. CYM-5442 and other S1PR1 agonists have also been shown to attenuate production of type I interferons and mortality in response to viral infection with influenza A H1N1 in vivo5,153. Interestingly, these studies demonstrated that EC S1PR1 signalling reduces the production of type I interferons and other key mediators of inflammatory injury including CCL2 and CXC-chemokine ligand 10 (CXCL10), implicating ECs as orchestrators of inflammatory injury in this model. Because NETs and immune complexes from patients with SLE have also been demonstrated to induce production of type-I interferons by pDCs154,155 and ECs in a nucleic acid-dependent and endosomal TLR-dependent manner, studies to test whether S1PR1 agonists attenuate NET-driven type I interferon production would help to elucidate whether S1PR1 agonists have the potential to have immunomodulatory roles beyond lymphocyte trafficking in SLE.
Taken together, these studies suggest that S1PR1 modulators could attenuate type I interferon production by pDCs and/or ECs in SLE (FIG. 3). However, preclinical studies investigating a modulatory role of S1PR1 agonists on IFNα production have yielded mixed results. In the NZBWF1 mouse model of SLE, ozanimod attenuated proteinuria and inflammatory renal injury (as assessed by histology) and reduced mortality but did not affect the interfern signature or decrease IFNAR expression on pDCs156. By contrast, cenerimod, another S1PR1 and S1PR5 agonist, reduced type I interferon levels in both plasma and brain of MRL/lpr mice157. It is not known whether distinct signalling properties of these S1PR1 modulators might account for these differences and/or whether one mouse model of SLE is more responsive than others to S1PR1 modulation of IFNα.
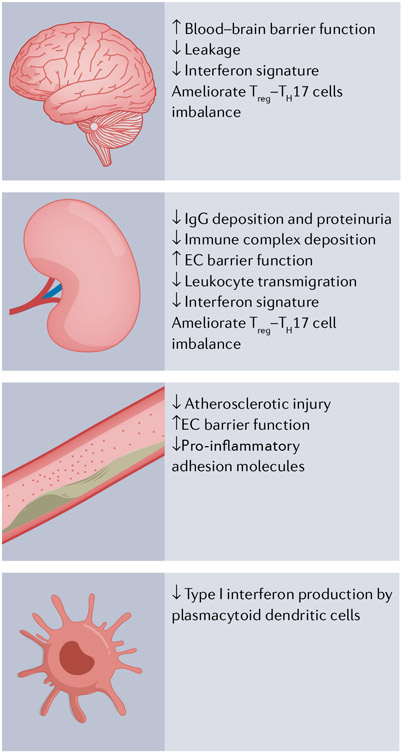
Fig. 3 ∣. Potential mechanisms by which S1PR1 modulators could mitigate disease pathogenesis.
Small-molecule sphingosine 1-phosphate receptor 1 (S1PR1) modulators could ameliorate disease in systemic lupus erythematosus (SLE) by decreasing the trafficking of autoreactive lymphocytes, increasing numbers and functions of regulatory T (Treg) cells, decreasing T helper 17 (TH17) cell differentiation and decreasing autoantibody production. Moreover, S1PR1 modulators could attenuate disease by increasing endothelial cell barrier function, increasing the blood–brain barrier, and decreasing the expression of inflammatory adhesion molecules, which would contribute to decreased leukocyte transmigration. S1PR1 modulators have also been shown to decrease type I interferons in response to viral infection or CpG oligonucleotides, and have the potential to attenuate type I interferons in SLE. These mechanisms could impart protection against renal, neuropsychiatric and atherosclerotic manifestations of SLE. EC, endothelial cell.
Ameliorative effects of FTY720 in preclinical studies of SLE.
The MRL/lpr model of SLE shares many aspects of human disease, including anti-dsDNA antibodies, renal deposition of IgG antibodies and complement, and proteinuria158. MRL/lpr mice have a deletion in Fas causing failure of apoptotic cell death in CD4+ T cells, leading to the persistence of pathogenetic autoreactive TH cells and the subsequent production of autoantibodies159. As a result, these mice develop lymphadenopathy, splenomegaly and spontaneous autoimmune glomerulonephritis. There is evidence that FTY720 treatment markedly attenuates pathogenesis and mortality in the MRL/lpr model of SLE90,160 (TABLE 1). If the major effect of FTY720 was to halt lymphocyte trafficking out of SLOs, one might expect increased lymphadenopathy and splenomegaly in MPL/lpr mice. However, FTY720-treated MRL/lpr mice showed significantly reduced weights of SLOs and decreased numbers of T cells and B cells in the thymus91, suggesting that FTY720 induced lymphocyte apoptosis. In support of this hypothesis, FTY720 did induce lymphocyte apoptosis in vitro, and FTY720-treated mice showed increased numbers of apoptotic cells in SLOs90, implicating lymphocyte apoptosis as a major mechanism contributing to its beneficial effects. FTY720 also significantly suppressed the production of anti-dsDNA antibodies and reduced the deposition of IgG in glomeruli in mice that had already developed renal disease, indicating its therapeutic potential in SLE160.
Table 1 ∣.
Preclinical studies using S1PR1 modulators or SPHK inhibitors in mouse models of SLE
| SLE model |
S1PR modulator |
Target | Outcome | Interferon signature |
Refs |
|---|---|---|---|---|---|
| MRL-lpr | FTY720 | S1PR1, S1PR3, S1PR4 and S1PR5 | ↓Glomerulonephritis and mortality ↓Neuropsychiatric disease, blood–brain barrier permeability, brain tissue cytokines |
NA | 85,90,161 |
| Amiselimod | S1PR1, S1PR4 and S1PR5 | ↓Glomerulonephritis | NA | 87 | |
| Cenerimod | S1PR1 and S1PR5 | ↓Glomerulonephritis, ↓brain tissue cytokines and mortality | Reduced | 157 | |
| KRP-203 | S1PR1 and S1PR4 | ↓Glomerulonephritis and mortality | NA | 86 | |
| NZBWF1 | Ozanimod | S1PR1 and S1PR5 | ↓Glomerulonephritis and mortality | Unchanged | 156 |
| Amiselimod | S1PR1, S1PR4 and S1PR5 | ↓Glomerulonephritis | NA | 87 | |
| BXSB | FTY720 | S1PR1, S1PR3, S1PR4 and S1PR5 | ↓Glomerulonephritis and mortality | NA | 163 |
NA, not assessed; S1P, sphingosine 1-phosphate; S1PR, S1P receptor; SLE, systemic lupus erythematosus.
Neuropsychiatric features in MRL/lpr mice85,161 have some similarities with human disease, and FTY720 has been shown to decrease depression-like behaviour, spatial and recognition memory deficits, and leukocyte infiltration of the choroid plexus85. Mechanistically, FTY720 reduced neuron damage, attenuated the production of inflammatory cytokines in the brain and protected the blood–brain barrier by directly affecting brain microvascular ECs. The brain EC transcriptome showed marked decreases in the expression of genes involved in cellular adhesion and in the interferon response85. As in the aforementioned studies, FTY720 treatment was associated with reduced splenomegaly and lymphadenopathy85. These studies support the need for further work to evaluate the role of FTY720 and/or other S1PR1 modulators as potential therapies for neuropsychiatric SLE.
Finally, in another model of SLE, male mice carry the Yaa mutation inducing overexpression of TLR7, resulting in accelerated autoimmunity and glomerulonephritis162; in this model, FTY720 protected against proteinuria, which occurred in 20% of treated animals compared with 100% of untreated controls, and lowered mortality163.
Taken together, these data support the idea that fingolimod could benefit patients with SLE, particularly those with glomerulonephritis. However, FTY720 has several known adverse effects, including bradycardia, bronchoconstriction and macular oedema. As some of the safety issues related to use of FTY720 are associated with its engagement of S1PR3, newer S1PR modulators with increased specificity for S1PR1 are expected to have fewer adverse effects164.
Newer S1PR modulators in preclinical SLE.
KRP-203 is an S1PR modulator with specificity to S1PR1 and S1PR4. MRL/lpr mice preventively and therapeutically treated with KRP-203 had increased survival86. KRP-203 treatment resulted in a marked decrease in both peripheral lymphocytes and monocytes, attenuated glomerulonephritis, reduced interstitial and perivascular infiltrates, and decreased proteinuria. However, IgG and complement deposition were not decreased. Despite initial adenopathy in MRL/lpr mice treated with KRP-203, there was ultimately a reduction in SLO weights as well as a decrease in the absolute number of lymphocytes in these organs. Again, lymphocyte apoptosis was deemed most likely to account for these findings86.
Amiselimod, a S1PR modulator with specificity to S1PR1, was shown to decrease anti-dsDNA antibody titres, the number of plasma cells and the development of glomerulonephritis, splenomegaly and lymphadenopathy in the MRL/lpr model when given preventively and therapeutically87. Amiselimod also prevented progression of nephritis in NZBWF1 mice. As expected, the treatment was shown to induce lymphopenia87.
Cenerimod, a S1PR modulator with specificity to S1PR1 and S1PR5, leads to a 500-fold decrease in potency in Ca++ signalling (mediated by Gi–phospholipase C–inositol trisphosphate) compared with S1P, FTY720 and amiselimod, thereby reducing its ability to induce bronchoconstriction ex vivo165. Cenerimod attenuated renal disease and prolonged survival in the MRL/lpr model; treated animals showed decreased circulating blood CD19+ B cells and CD4+ and CD8+ T cells; reduced plasma anti-dsDNA antibodies, proteinuria and brain inflammation; and increased survival. Plasma and tissue levels of IFNα, and other known mediators of SLE, including TNF, IL-6, BAFF (also known as tumour necrosis factor ligand superfamily member 13B) and IL-10, were also reduced157.
Ozanimod was approved by the US FDA for the treatment of multiple sclerosis in 2020 and ulcerative colitis in 2021. It has specificity for S1PR1 and S1PR5, and its active metabolite, RP-101075, decreased proteinuria, renal histological scores and mortality in NZBWF1 mice. RP-101075 treatment suppressed expression of profibrotic and inflammatory genes but did not significantly alter the expression of interferon-inducible genes156. Both ozanimod and RP-101075 significantly reduced peripheral and splenic B cell subsets, but neither treatment was associated with a sustained drop in anti-dsDNA antibodies156.
In summary, multiple S1PR modulators have been shown to attenuate experimental SLE. Cenerimod is particularly promising because of its defined ability to attenuate type I interferon production as well as its unique signalling profile that could mitigate the risk of bronchoconstriction. As discussed in the next section, cenerimod is currently being evaluated in a phase II clinical trial for SLE.
Clinical trials of S1PR1 modulators in human SLE.
Amiselimod has a more favourable cardiac safely profile than fingolimod166. In an open label, non-randomized multicentre phase Ib safety trial, during which immunosuppressive and antimalarial drugs were prohibited, patients were treated with either 0.2 or 0.4 mg of amiselimod for 24 weeks167. In a second part of the trial, low-dose 0.2-mg amiselimod was administered with concomitant submaximal doses of immunosuppressive agents. Individuals with severe active lupus nephritis, CNS lupus, thrombosis, lymphopenia or evidence of cardiovascular disease were excluded. A decrease in peripheral lymphocyte count was observed in all 17 patients treated with active drug without a dose-dependent response, but no severe infections occurred, and there were no serious adverse events. Of the 9 out of 17 individuals (53%) who were positive for anti-dsDNA antibodies at baseline, 8 (89%) had decreased antibody levels by week 24. Although this exploratory study showed that amiselimod was well tolerated, its half-life was determined to be 20–23 days, which could limit its clinical use.
A multicentre, double-blind, placebo-controlled study was conducted to test the safety of cenerimod: patients with SLE were randomized to receive oral cenerimod (0.5, 1, 2 mg or placebo) for 12 weeks157. After an interim safety review, additional individuals were randomized to receive either 4 mg cenerimod or placebo. End points included changes in total lymphocyte count, SLEDAI-2K (modified to exclude lymphopenia) and anti-dsDNA antibodies, pharmacokinetic assessments and adverse events. 1- and 2-mg doses induced a drop in the levels of CD19+ B cells and CD4+ and CD8+ T cells. Cenerimod attenuated plasma IFNα and downstream mediators CXCL9, CXCL10 and galectin 9 in humans with SLE after 12 weeks of treatment157.
An unbiased analysis of circulating B cells revealed two specific cell clusters that were reduced after treatment with cenerimod. Interestingly, these clusters demonstrated elevated expression of CD27 antigen and CD38 antigen (also known as ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1), resembling antibody-secreting cells, which have previously been identified as key mediators of autoimmunity157. Plasma interferon-associated biomarkers were decreased in the majority of patients taking cenerimod. A larger study of cenerimod treatment for up to 12 months is currently ongoing (NCT03742037).
Rheumatoid arthritis
RA is the most prevalent systemic autoimmune rheumatic disease, affecting 1.5 million individuals in the USA, characterized by inflammatory and often erosive arthritis and the presence of autoantibodies against IgG (rheumatoid factor) and anti-citrullinated peptides antibodies168. Despite multiple FDA-approved medications for the treatment of RA, treatment-resistant RA occurs in 6–17% of patients169, and many patients do not have sustained remission. RA-related inflammation increases the risk of cardiovascular disease170,171, with patients having a 2–3-fold higher risk of myocardial infarction and a 50% higher risk of stroke than healthy controls172,173. Thus, there is a real medical need for novel therapies that can be used safely and concomitantly with approved medications to ameliorate the quality of life and lower mortality in patients with RA. The mechanisms by which S1PR1 modulators could ameliorate RA include blockade of auto-aggressive T cells to synovial tissues, restoration of the Treg–TH17 cell imbalance and bolstering of the vascular barrier to attenuate both microvascular121 and atherosclerotic disease174,175.
Evidence that S1P levels are dysregulated in human RA and experimental arthritis.
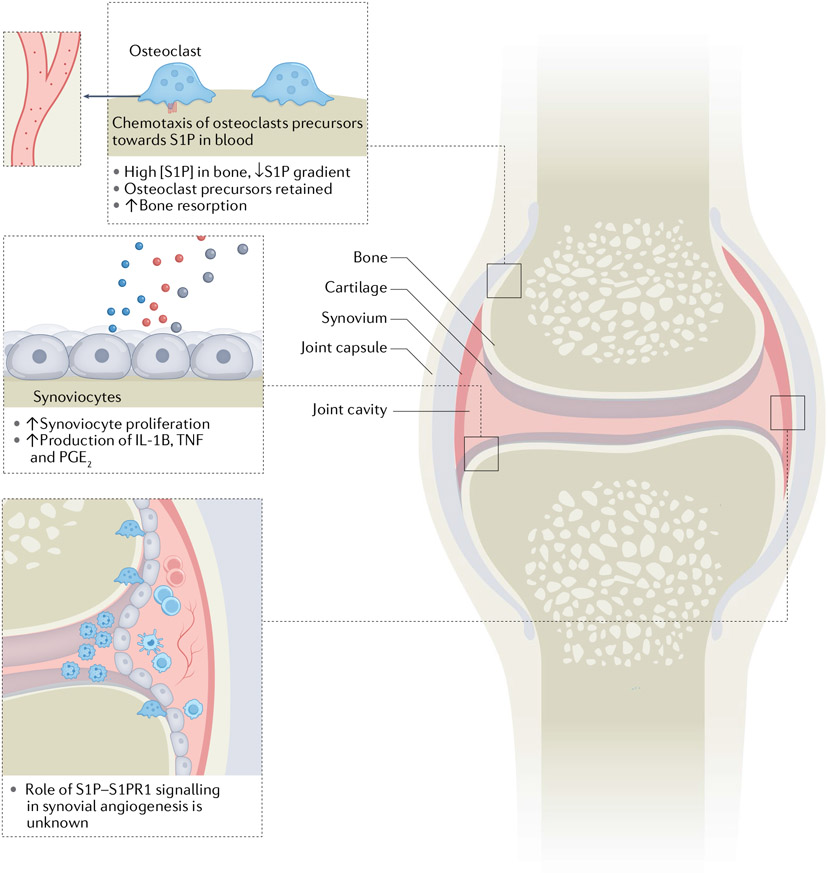
S1P levels have been shown to be elevated in RA synovial fluids compared with osteoarthritis synovial fluids176, which could result from S1P release from activated platelets, fibroblasts and leukocytes176-179 (FIG. 4). S1P has many pro-inflammatory effects in RA, including its ability to induce synoviocyte proliferation180 and synoviocyte production of prostaglandin E2 (PGE2) and cyclooxygenase 2 (COX2, also known as prostaglandin G/H synthase 2) in response to inflammatory cytokines in vitro176. S1P hinders apoptosis of fibroblast-like synoviocytes via S1PR1 (REF.181) and increases receptor activator of nuclear factor κB ligand (RANKL, also known as tumour necrosis factor ligand superfamily member 11) expression on synoviocytes and CD4+ T cells, thereby promoting osteoclastogenesis181, which could contribute to bone erosions. RANKL signalling downregulates the expression of S1PR1 on osteoclast precursors, inhibiting their re-exit into the circulation, and enhances osteoclast maturation and bone resorption182. Because lymphocyte S1PR1 expression and the local S1P level are inversely related, elevated S1P levels in synovial tissues decrease T cell expression of S1PR1, thereby increasing T cell retention time in tissues183,184, potentially exacerbating disease.
Fig. 4 ∣. Contribution of S1P to inflammatory injury and joint destruction in RA.
Elevated sphingosine 1-phosphate (S1P) levels in rheumatoid arthritis (RA) synovium (from multiple sources, including platelets) could contribute to disease pathogenesis by increasing osteoclast precursor retention and bone resorption, and increasing synoviocyte proliferation and production of prostaglandin E2 (PGE2), tumour necrosis factor (TNF) and IL-1β.The effect of increased S1P receptor 1 (S1PR1) signalling on angiogenesis in the RA synovium remains to be elucidated.
Consistent with the findings that S1P contributes to RA pathogenesis, decreasing S1P levels by pharmacological or small interfering RNA knockdown of Sphk1 was shown to reduce the production of key mediators of inflammatory arthritis, including TNF and IL-1β, and attenuate collagen-induced arthritis185,186. Moreover, crossing of TNF transgenic mice, which develop spontaneous arthritis, and Sphk1 KO mice protected offspring from developing the disease187. Mechanistically, the SphK1 KO mice had reduced levels of articular COX2 and fewer synovial TH17 cells than did transgenic TNF–SphK1+/+ littermates. In another study implicating SPHK1 as a contributor to RA pathogenesis, SPHK1-mediated production of S1P inhibited Fas-mediated apoptosis of lymphoblastoid cell lines prepared from peripheral B cells cultured from patients with RA188. However, in other studies, Sphk1 KO mice did not show decreased severity in collagen-induced arthritis189. These apparently discrepant results could be secondary to differences in the genetic strains and the models of arthritis utilized for these studies.
Whereas S1P generation by SPHK1 has been linked to inflammatory signalling via activating S1PR1 signalling, the role of SPHK2 is unclear, with conflicting results regarding SPHK2 roles in inflammation. Although the differences in genetic strains and arthritis models used for these studies could account for these discrepant results, added complexity stems from the fact that Sphk1 activity is increased when Sphk2 is knocked out186,190-193
Evidence that APOM polymorphisms are risk factors for RA.
Polymorphisms in the APOM promoter that result in reduced ApoM levels are associated with an increased risk of RA. Polymorphisms at SNP rs805297 have been shown to decrease APOM transcriptional activity and be significantly associated with RA (OR = 1.56)194. In a study from Korea, this polymorphism was found to be associated with both RA susceptibility and dyslipidaemia, and the A/A genotype was associated with both decreased levels of HDL particles and ApoM but not disease activity195. A study from China of patients with RA (n = 520) showed an association of APOM rs805297 TT genotype with an increased risk of developing RA, with an odds ratio of 4.01 among men. In a sub-analysis of 84 patients, the average plasma concentration of ApoM was significantly higher in patients with RA than in age-matched and sex-matched controls196. Given the protective role of ApoM on the vasculature and the adaptive immune response, the elevated levels in RA are likely to be the consequence of inflammation.
Whether certain APOM polymorphisms predispose to disease or are rather the result of linkage disequilibrium is yet to be elucidated. On the one hand, linkage disequilibrium is supported by the fact that APOM is located in the MHC class III region along with TNF. On the other hand, patients with RA have a more atherogenic lipid profile than healthy controls, even in the preclinical non-inflammatory phase197, raising the possibility that dyslipidaemia contributes to the risk of developing RA. As stated previously, ApoM has been implicated in adipocyte and triglyceride biology, which could affect autoimmune mechanisms. Indeed, anti-inflammatory roles of S1P–ApoM signalling include its ability to decrease ICAM1, VCAM1 and E-selectin expression on ECs in the presence of inflammatory cytokines2,3 and to restrain lymphopoiesis and adaptive immune responses, which could predispose to the development of RA67.
Ameliorative effects of FTY720 in preclinical studies of RA.
As observed in SLE, there is evidence that fingolimod attenuates disease severity in experimental inflammatory arthritis. Supporting the concept that blocking the egress of autoreactive lymphocytes out of SLOs is a major mechanism by which FTY720 ameliorates disease, the ability of FTY720 to attenuate arthritis in both collagen-induced and adjuvant induced arthritis models was associated with reduced numbers of circulating and synovial CD4+ T cells198-202. SKG mice, another RA model, spontaneously develop T cell-mediated autoimmune arthritis owing to a mutation in Zap70, which impairs negative selection of T cells in the thymus203. FTY720 treatment in this model resulted in sequestration of CD4+ T cells in the thymus, reduced numbers of circulating CD4+ T cells and attenuated arthritis204. In addition, FTY720 has been shown to inhibit TNF-induced synoviocyte production of PGE2 (REFS108,204), IL-1β, IL-6 and IL-8 (REF.200). Consistent with the aforementioned studies showing that S1pr1 KO in CD4+ T cells led to increased numbers and function of Treg cells, FTY720, acting as a functional antagonist of S1PR1, attenuated collagen induced arthritis by increasing Treg cell numbers and their ability to induce anergy in collagen-specific T cells108.
DCs express S1PR1 and are also affected by FTY720 and other S1PR1 modulators205. In a study focused on the role of FTY720 in DC trafficking and function in collagen-induced arthritis, FTY720 attenuated production of several cytokines, including TNF, IL-1β and Il-6, as well as the incidence and severity of arthritis92. To determine whether the ability of FTY720 to attenuate arthritis was mediated, at least in part, by an effect on DCs, a DC transfer mouse model of arthritis was used. In this model, murine bone marrow-derived DCs are activated with lipopolysaccharide and collagen in vitro and injected into the paw, causing localized arthritis. Ex vivo treatment of DCs with FTY720 before injection into the paw significantly reduced DC trafficking to draining LNs and attenuated arthritis, demonstrating that DCs are an important target of FTY720 (REF.92).
S1PR1 antagonists in preclinical RA.
Because of their ability to block lymphocyte trafficking, S1PR1 antagonists have been tested for their efficacy in preclinical models of arthritis206. However, although S1PR1 antagonism, induced by either functional or competitive antagonists, mitigates trafficking of central memory T cells and naive T cells to tissues, it also blocks S1PR1 signalling on ECs, leading to vascular leakage. In a study on adjuvant induced arthritis, the S1PR1-specific antagonist NIBR-0213 was effective at preventing arthritis, but it induced diffuse alveolar and interstitial haemorrhage, oedema and inflammation. In mice treated with NIBR-0213 for over 2 weeks, chronic changes were observed, including necrotic and fibrotic foci in both the lung and the heart207. These data suggest that the use of S1PR1 functional antagonists that induce endothelial injury might pose risks for patients with RA, because clinically significant interstitial lung disease occurs in ~10%208-210 of these patients and subclinical disease occurs in up to 60%, as detected by high-resolution CT209.
Clinical trials targeting S1P–S1PR1 axis in RA.
Although fingolimod has not yet been tested for RA treatment in clinical trials, a different strategy to block S1PR1 signalling and induce lymphopenia has been used in phase I and II clinical trials. Inhibition of SGPL1 resulted in >100-fold accumulation of S1P in thymus and SLOs, thereby eliminating the S1P gradient and inducing lymphopenia6. The SGPL1 inhibitor LX2931 (REF.211) was tested in phase II clinical trials on 208 patients with active RA who had incomplete response to methotrexate. There were no safety issues, but analysis of the results suggested that the doses tested were subtherapeutic. No further studies using this agent have been reported, as the study failed to meet its primary end point of ACR20 response at week 12 (REF.212). This approach as a treatment for RA is not supported by preclinical data, as mice with Sgpl1 KO have neutrophilia and increased levels of inflammatory cytokines, particularly IL-17 (REF.213).
Systemic sclerosis
SSc is a rare complex systemic autoimmune rheumatic disease characterized by vasculopathy and fibrosis, mostly of skin, gastrointestinal tract and lungs. Symptomatic interstitial lung disease is present in >50% of patients with diffuse disease and in up to 35% of individuals with limited cutaneous SSc, and subclinical interstitial lung disease is seen on high-resolution CT in up to 90% of patients with SSc214,215. Data from the Scleroderma Lung Study II have shown that the mortality from interstitial lung disease is ~20% with a median follow-up of 3.6 years after the diagnosis of interstitial lung disease216,217. Pulmonary hypertension, either in its primary form or secondary, to severe interstitial lung disease occurs in 8–12% of patients, with 25% mortality at 3 years after pulmonary hypertension diagnosis218. Although there has been improvement in mortality over the past few decades216,219, novel therapies are direly needed to decrease morbidity and mortality in SSc.
The use of S1PR1 modulators could have beneficial effects in SSc. For example, S1PR1 modulators decrease type I interferon production by pDCs220 and thereby have the potential to decrease the upregulated production of type I interferons in SSc221-224. IFNα could contribute to SSc pathogenesis by increasing blood vessel permeability via downregulation of FLI1 (encoding Friend leukaemia integration 1 transcription factor) and CDH5 (encoding VE-cadherin)225 and by contributing to skin fibrosis, as has been shown in mouse studies226. S1PR1 modulators (acting as functional antagonists) have also been shown to diminish differentiation and function of TH17 cells, both of which are increased in patients with SSc227-229 and contribute to fibrosis in animal models of SSc230,231. However, in terms of restoring the TH17–Treg cell imbalance, S1PR1 modulation could be problematic in SSc, as Treg cells have the potential to exacerbate pulmonary fibrosis by increasing TH2 cell immune responses232. Moreover, S1PR1 antagonism, by treatment with either competitive or functional antagonists, can increase the risk of pulmonary vascular leakage.
S1PR1 modulation to target vascular leakage and fibrosis in SSc.
Bolstering EC S1PR1 signalling limits vascular leakage and decreases vascular tone233 and therefore could be of particular benefit in SSc, because vascular leakage is a predominant clinical feature234,235, and almost all patients have Raynaud phenomenon as a result of vascular dysfunction. The vasculopathy of SSc is characterized by increased vascular permeability, decreased VE-cadherin expression and dysregulated angiogenesis236. These features are prominent in mice with an EC-specific KO of Fli1, a transcription factor that is markedly decreased in SSc skin and is a negative regulator of collagen production237. Isolated dermal ECs from these mice show markedly decreased expression of S1pr1 and VE-cadherin. Chromatin immunoprecipitation experiments showed that the promoter of S1pr1 is targeted by Fli1 (REF.237). Taken together, these data support the hypothesis that low levels of Fli1 in SSc ECs lead to decreased transcription of S1PR1 and CDH5 (VE-cadherin), leading to increased vascular instability and leakage.
S1PR1 modulation has also been studied in mouse models of pulmonary fibrosis. Chronic administration of FTY720 or an S1PR1-specific functional antagonist exacerbated vascular leakage and intra-alveolar coagulation in response to bleomycin, leading to increased pulmonary fibrosis and mortality in bleomycin-treated mice124. In in vitro studies, short-term (1-h) EC exposure to S1P or S1PR1 agonists decreased permeability and protected against thrombin-mediated barrier disruption, whereas prolonged (24-h) exposure to S1PR1 agonists exerted the opposite effect124, illustrating that the dose and the frequency of administration of a S1PR1 modulator will determine whether it acts as an agonist or as an antagonist (FIG. 5). In follow-up studies on pulmonary fibrosis induced by bleomycin and FTY720, fibrosis was attenuated by inhibiting thrombin generation, thereby showing that extravascular coagulation induced by vascular leak is a driver of fibrosis. Mechanistically, thrombin binding to protease activated receptor 1 (PAR1) increased activation of transforming growth factor-β1 (TGFβ1) via avβ6 integrin238. Importantly, a study supports the hypothesis that EC S1PR1 protects against pulmonary fibrosis by mitigating vascular leakage, leukocyte infiltration, and intravascular coagulation239. These data support the development of novel EC S1PR1 targeted agonists for the treatment of SSc.
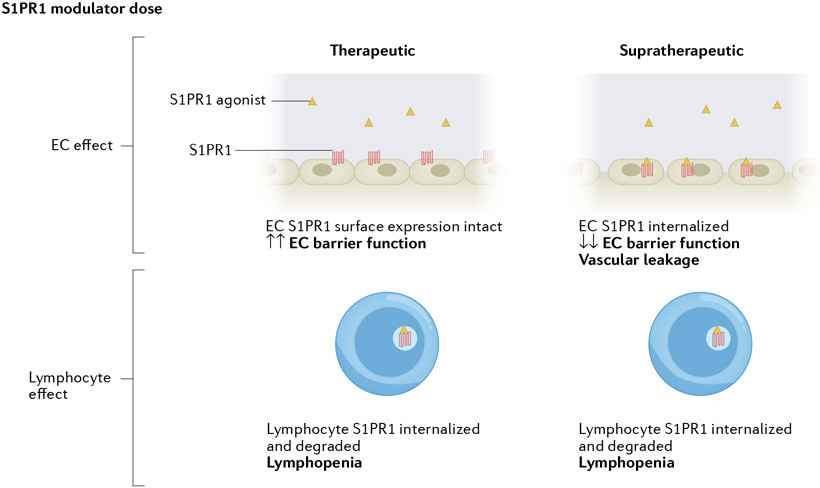
Fig. 5 ∣. S1PR1 modulators act as agonists and/or functional antagonists depending on dose and target cell type.
Sphingosine 1-phosphate (S1P) receptor 1 (S1PR1) modulators have differing effects on endothelial cells (ECs) and lymphocytes. After binding of S1PR modulators to S1PR1, signalling is activated and the S1PR1 modulator–S1PR1 complex can be internalized and degraded in proteasomes and lysosomes. S1PR1-targeted small molecule functional antagonists at therapeutic concentrations induce irreversible internalization and degradation of S1PR1, resulting in lymphopenia. At such doses, endothelial S1PR1 antagonism does not lead to substantial vascular leak, owing to the presence of large receptor reserve. However, at supratherapeutic doses, S1PR1 functional antagonists induce degradation of S1PR1 in both lymphocytes and endothelial cells, leading to lymphopenia as well as vascular leakage.
S1PR modulators in mouse models of SSc.
Cenerimod attenuated the infiltration of immune cells and inflammatory cytokines IL-6, IL-13 and Il-1β in a mouse model of chronic graft-versus-host disease (Scl-cGVHD)240. Consistent with previous data that S1PR1 is a negative regulator of Treg cell differentiation, cenerimod (functioning as an antagonist on CD4+ T cells) increased the percentage of Treg cells both in the spleen and skin compared with control mice240. Moreover, cenerimod decreased IL-6 and collagen deposition in both skin and lung of the bleomycin-induced fibrosis model240. Experiments to assess vascular permeability were not performed in these studies, but the protective effects of cenerimod were possibly related, at least in part, to agonist signalling on ECs240. In addition, because S1PR5 was implicated in the early fibrotic changes in the skin in the bleomycin model of SSc241, one could speculate that cenerimod’s anti-fibrotic effects may have been mediated, at least in part, by modulating S1PR5 signalling. Thus, multiple S1PRs could be involved in the complex pathophysiology of SSc by acting on immune, vascular and connective tissue cells. Such complex biology needs to be understood much better to develop rational therapeutic approaches.
Future directions and challenges
The application of S1PR modulators to inhibit autoreactive lymphocyte trafficking has proven successful in patients with multiple sclerosis and inflammatory bowel disease. However, the complex biology of S1P has hindered the wider application of this therapeutic principle in additional immunological diseases. Further complicating the application of S1PR1 modulators to autoimmune rheumatic diseases, S1PR1 signalling can be enhanced or antagonized by the same drug depending on the concentration of the drug, the duration of treatment and the type of cell targeted (FIG. 5).
It is well established that patients with SLE, RA and SSc show substantial endothelial dysfunction169,234,235,242-244. Although preclinical studies support the use of S1PR1 modulators for SLE, RA and SSc, vascular S1PRs have received scant attention in past therapeutic efforts. The discovery that HDL-bound S1P protects the vascular endothelium during inflammatory insult has provided impetus for therapeutic development. Newer S1PR1 agonists do not induce internalization and degradation of S1PR1 and do not affect lymphocyte percentages in peripheral blood161. We hypothesize that S1PR1 agonists specifically targeting ECs could benefit patients with autoimmune rheumatic disease on standard therapies, because bolstering vascular integrity might synergize with immunosuppressive therapy. A potential benefit of the use of concomitant immunosuppressive and endothelial barrier-targeted drugs is that, although the anti-inflammatory effects of both drugs could be compounded, the immunosuppressive effects are not likely to be, as EC S1PR1-bolstering drugs should attenuate the risk of infection4,5,153. An optimal approach will be to reset the immune cell repertoire while maintaining optimal EC function. We posit that an in-depth understanding of S1P biology is an essential prerequisite in the rational therapeutic development of novel agents to control SLE, RA and SSc.
Key points.
Autoimmune rheumatic diseases are complex and heterogeneous diseases that have fundamental pathophysiological pathways in common.
These dysregulated pathways include lymphocyte autoreactivity, myeloid and endothelial cell activation, and extravasation of inflammatory mediators into tissues.
Chronic inflammatory damage in autoimmune rheumatic disease leads to end organ damage.
Sphingosine 1-phosphate receptor 1 (S1PR1) is expressed on leukocytes and endothelial cells and is an important mediator of lymphocyte trafficking, regulatory T/T helper 17 cell homeostasis and vascular permeability.
Use of S1PR1 modulators in preclinical studies of systemic lupus erythematosus, rheumatoid arthritis and systemic sclerosis has shown promise in attenuating inflammatory injury and end organ damage.
Modulation of S1PR1 signalling in leukocytes and/or endothelial cells warrants further evaluation in clinical studies in autoimmune rheumatic diseases.
Footnotes
Competing interests
T.H. declares the following potential competing interests in 2021–2022: consultancy — Arena Pharmaceuticals, Bristol Myers-Squibb Inc., Janssen Inc; inventor — patents on ApoM-Fc, S1PR2 modulators; speakers fees — Pfizer Inc. The other authors declare no competing interests.
References
- 1.Lee MJ et al. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell 99, 301–312 (1999). [DOI] [PubMed] [Google Scholar]
- 2.Galvani S et al. HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Sci. Signal 8, ra79 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruiz M et al. High-density lipoprotein-associated apolipoprotein M limits endothelial inflammation by delivering sphingosine-1-phosphate to the sphingosine-1-phosphate receptor 1. Arterioscler. Thromb. Vasc. Biol 37, 118–129 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Jiang H et al. Sphingosine 1-phosphate receptor 1 (S1PR1) agonist CYM5442 inhibits expression of intracellular adhesion molecule 1 (ICAM1) in endothelial cells infected with influenza A viruses. PLoS One 12, e0175188 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teijaro JR et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell 146, 980–991 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwab SR et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 309, 1735–1739 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Ramos-Perez WD et al. A map of the distribution of sphingosine 1-phosphate in the spleen. Nat. Immunol 16, 1245–52. (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pappu R et al. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science 316, 295–298 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Cyster JG & Schwab SR Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu. Rev. Immunol 30, 69–94 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Cartier A & Hla T Sphingosine 1-phosphate: lipid signaling in pathology and therapy. Science 366, eaar5551 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Proia RL & Hla T Emerging biology of sphingosine-1-phosphate: its role in pathogenesis and therapy. J. Clin. Invest 125, 1379–1387 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanchez T et al. PTEN as an effector in the signaling of antimigratory G protein-coupled receptor. Proc. Natl Acad. Sci. USA 102, 4312–4317 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchez T et al. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler. Thromb. Vasc. Biol 27, 1312–1318 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Cruz-Orengo L et al. Enhanced sphingosine-1-phosphate receptor 2 expression underlies female CNS autoimmunity susceptibility. J. Clin. Invest 124, 2571–84. (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michaud J, Im DS & Hla T Inhibitory role of sphingosine 1-phosphate receptor 2 in macrophage recruitment during inflammation. J. Immunol 184, 1475–1483 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laidlaw BJ et al. Sphingosine-1-phosphate receptor 2 restrains egress of γδ T cells from the skin. J. Exp. Med 216, 1487–1496 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muppidi JR et al. Loss of signalling via Gα 13 in germinal centre B-cell-derived lymphoma. Nature 516, 254–258 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun X et al. Sphingosine-1-phosphate receptor-3 is a novel biomarker in acute lung injury. Am. J. Respir. Cell Mol. Biol 47, 628–636 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cantalupo A et al. S1PR1 (sphingosine-1-phosphate receptor 1) signaling regulates blood flow and pressure. Hypertension 70, 426–434 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murakami K et al. Knock out of S1P3 receptor signaling attenuates inflammation and fibrosis in bleomycin-induced lung injury mice model. PLoS One 9, e106792 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takuwa N et al. S1P3-mediated cardiac fibrosis in sphingosine kinase 1 transgenic mice involves reactive oxygen species. Cardiovasc. Res 85, 484–93. (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Group, C. C. H. W. Meta-analysis of rare and common exome chip variants identifies S1PR4 and other loci influencing blood cell traits. Nat. Genet 48, 867–876 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drouillard A et al. S1PR5 is essential for human natural killer cell migration toward sphingosine-1 phosphate. J. Allergy Clin. Immunol 141, 2265–2268.e1 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Kharel Y et al. Sphingosine kinase 2 is required for modulation of lymphocyte traffic by FTY720. J. Biol. Chem 280, 36865–36872 (2005). [DOI] [PubMed] [Google Scholar]
- 25.Chun J & Hartung H Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin. Neuropharmacol 33, 91–101 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fryer RM et al. The clinically-tested S1P receptor agonists, FTY720 and BAF312, demonstrate subtype-specific bradycardia (S1P1) and hypertension (S1P3) in rat. PLoS One 7, e52985 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camm J et al. Cardiac and vascular effects of fingolimod: mechanistic basis and clinical implications. Am. Heart J 168, 632–644 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez-Cabrera PJ et al. S1P signaling: new therapies and opportunities. F1000Prime Rep. 6, 109 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oo ML et al. Engagement of S1P1-degradative mechanisms leads to vascular leak in mice. J. Clin. Invest 121, 2290–2300 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jain N & Bhatti MT Fingolimod-associated macular edema: incidence, detection, and management. Neurology 78, 672–680 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Chae SS, Proia RL & Hla T Constitutive expression of the S1P1 receptor in adult tissues. Prostaglandins Other Lipid Mediat. 73, 141–150 (2004). [DOI] [PubMed] [Google Scholar]
- 32.Yanagida K et al. Sphingosine 1-phosphate receptor signaling establishes AP-1 gradients to allow for retinal endothelial cell specialization. Dev. Cell 52, 779–793.e7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ancellin N et al. Extracellular export of sphingosine kinase-1 enzyme. Sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J. Biol. Chem 277, 6667–6675 (2002). [DOI] [PubMed] [Google Scholar]
- 34.Igarashi N et al. Sphingosine kinase 2 is a nuclear protein and inhibits DNA synthesis. J. Biol. Chem 278, 46832–46839 (2003). [DOI] [PubMed] [Google Scholar]
- 35.Allende ML et al. Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J. Biol. Chem 279, 52487–52492 (2004). [DOI] [PubMed] [Google Scholar]
- 36.Zemann B et al. Sphingosine kinase type 2 is essential for lymphopenia induced by the immunomodulatory drug FTY720. Blood 107, 1454–1458 (2006). [DOI] [PubMed] [Google Scholar]
- 37.Kharel Y et al. Sphingosine kinase type 2 inhibition elevates circulating sphingosine 1-phosphate. Biochem. J 447, 149–157 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kharel Y et al. Sphingosine kinase 2 inhibition and blood sphingosine 1-phosphate levels. J. Pharmacol. Exp. Ther 355, 23–31 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mizugishi K et al. Essential role for sphingosine kinases in neural and vascular development. Mol. Cell Biol 25, 11113–11121 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Camerer E et al. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J. Clin. Invest 119, 1871–1879 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smyth SS et al. Roles for lysophosphatidic acid signaling in vascular development and disease. Biochim. Biophys. Acta 1865, 158734 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Engelbrecht E, MacRae CA & Hla T Lysolipids in vascular development, biology, and disease. Arterioscler. Thromb. Vasc. Biol 41, 564–584 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panchatcharam M et al. Mice with targeted inactivation of ppap2b in endothelial and hematopoietic cells display enhanced vascular inflammation and permeability. Arterioscler. Thromb. Vasc. Biol 34, 837–845 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chatterjee I et al. Endothelial lipid phosphate phosphatase-3 deficiency that disrupts the endothelial barrier function is a modifier of cardiovascular development. Cardiovasc. Res 111, 105–118 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Breart B et al. Lipid phosphate phosphatase 3 enables efficient thymic egress. J. Exp. Med 208, 1267–1278 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pham TH et al. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J. Exp. Med 207, 17–27 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Venkataraman K et al. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ. Res 102, 669–676 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiong Y et al. Erythrocyte-derived sphingosine 1-phosphate is essential for vascular development. J. Clin. Invest 124, 4823–4828 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Urtz N et al. Sphingosine 1-cphosphate produced by sphingosine kinase 2 intrinsically controls platelet aggregation in vitro and in vivo. Circ. Res 117, 376–387 (2015). [DOI] [PubMed] [Google Scholar]
- 50.Chandrakanthan M et al. Deletion of Mfsd2b impairs thrombotic functions of platelets. Nat. Commun 12, 2286 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jonnalagadda D et al. Granule-mediated release of sphingosine-1-phosphate by activated platelets. Biochim. Biophys. Acta 1841, 1581–1589 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gazit SL et al. Platelet and erythrocyte sources of S1P are redundant for vascular development and homeostasis, but both rendered essential after plasma S1P depletion in anaphylactic shock. Circ. Res 119, e110–126 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schaphorst KL et al. Role of sphingosine-1 phosphate in the enhancement of endothelial barrier integrity by platelet-released products. Am. J. Physiol. Lung Cell Mol. Physiol 285, L258–L267 (2003). [DOI] [PubMed] [Google Scholar]
- 54.Herzog BH et al. Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2. Nature 502, 105–109 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawahara A et al. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science 323, 524–527 (2009). [DOI] [PubMed] [Google Scholar]
- 56.Vu TM et al. Mfsd2b is essential for the sphingosine-1-phosphate export in erythrocytes and platelets. Nature 550, 524–528 (2017). [DOI] [PubMed] [Google Scholar]
- 57.Fukuhara S et al. The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J. Clin. Invest 122, 1416–1426 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hisano Y et al. Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLoS One 7, e38941 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ding BS et al. Aging suppresses sphingosine-1-phosphate chaperone ApoM in circulation resulting in maladaptive organ repair. Dev. Cell 53, 677–690.e4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Winkler MS et al. Decreased serum concentrations of sphingosine-1-phosphate in sepsis. Crit. Care 19, 372 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Winkler MS et al. Sphingosine-1-phosphate: a potential biomarker and therapeutic target for endothelial dysfunction and sepsis? Shock 47, 666–672 (2017). [DOI] [PubMed] [Google Scholar]
- 62.Christoffersen C et al. Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc. Natl Acad. Sci. USA 108, 9613–9618 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Christoffersen C et al. The apolipoprotein M/S1P axis controls triglyceride metabolism and brown fat activity. Cell Rep. 22, 175–188 (2018). [DOI] [PubMed] [Google Scholar]
- 64.Christoffersen C et al. Effect of apolipoprotein M on high density lipoprotein metabolism and atherosclerosis in low density lipoprotein receptor knock-out mice. J. Biol. Chem 283, 1839–1847 (2008). [DOI] [PubMed] [Google Scholar]
- 65.Elsoe S et al. Apolipoprotein M binds oxidized phospholipids and increases the antioxidant effect of HDL. Atherosclerosis 221, 91–97 (2012). [DOI] [PubMed] [Google Scholar]
- 66.Dahlback B & Nielsen LB Apolipoprotein M — a novel player in high-density lipoprotein metabolism and atherosclerosis. Curr. Opin. Lipidol 17, 291–295 (2006). [DOI] [PubMed] [Google Scholar]
- 67.Blaho VA et al. HDL-bound sphingosine-1-phosphate restrains lymphopoiesis and neuroinflammation. Nature 523, 342–346 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wilkerson BA et al. Sphingosine 1-phosphate (S1P) carrier-dependent regulation of endothelial barrier: high density lipoprotein (HDL)-S1P prolongs endothelial barrier enhancement as compared with albumin-S1P via effects on levels, trafficking, and signaling of S1P1. J. Biol. Chem 287, 44645–44653 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Swendeman SL et al. An engineered S1P chaperone attenuates hypertension and ischemic injury. Sci. Signal 10, eaal2722 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Christensen PM et al. Impaired endothelial barrier function in apolipoprotein M-deficient mice is dependent on sphingosine-1-phosphate receptor 1. FASEB J. 30, 2351–2359 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matloubian M et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360 (2004). [DOI] [PubMed] [Google Scholar]
- 72.Allende ML et al. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J. Biol. Chem 279, 15396–15401 (2004). [DOI] [PubMed] [Google Scholar]
- 73.Allende ML et al. S1P1 receptor directs the release of immature B cells from bone marrow into blood. J. Exp. Med 207, 1113–1124 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mendoza A et al. The transporter Spns2 is required for secretion of lymph but not plasma sphingosine-1-phosphate. Cell Rep. 2, 1104–1110 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pham TH et al. S1P1 receptor signaling overrides retention mediated by Gαi-coupled receptors to promote T cell egress. Immunity 28, 122–133 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Birker-Robaczewska M et al. S1P1 modulator-induced Gαi signaling and β-arrestin recruitment are both necessary to induce rapid and efficient reduction of blood lymphocyte count in vivo. Mol. Pharmacol 93, 109–118 (2018). [DOI] [PubMed] [Google Scholar]
- 77.Sykes DA et al. Investigating the molecular mechanisms through which FTY720-P causes persistent S1P1 receptor internalization. Br. J. Pharmacol 171, 4797–4807 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lim KG et al. FTY720 analogues as sphingosine kinase 1 inhibitors: enzyme inhibition kinetics, allosterism, proteasomal degradation, and actin rearrangement in MCF-7 breast cancer cells. J. Biol. Chem 286, 18633–18640 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mehling M et al. FTY720 therapy exerts differential effects on T cell subsets in multiple sclerosis. Neurology 71, 1261–1267 (2008). [DOI] [PubMed] [Google Scholar]
- 80.Harris S et al. Effect of the sphingosine-1-phosphate receptor modulator ozanimod on leukocyte subtypes in relapsing MS. Neurol. Neuroimmunol. Neuroinflamm 7, e839 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Luna G et al. Infection risks among patients with multiple sclerosis treated with fingolimod, natalizumab, rituximab, and injectable therapies. JAMA Neurol. 77, 184–191 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao Z et al. Incidence and risk of infection associated with fingolimod in patients with multiple sclerosis: a systematic review and meta-analysis of 8,448 patients from 12 randomized controlled trials. Front. Immunol 12, 611711 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shiow LR et al. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 440, 540–544 (2006). [DOI] [PubMed] [Google Scholar]
- 84.Baeyens A et al. Monocyte-derived S1P in the lymph node regulates immune responses. Nature 592, 290–295 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mike EV et al. Neuropsychiatric systemic lupus erythematosus is dependent on sphingosine-1-phosphate signaling. Front. Immunol 9, 2189 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wenderfer SE, Stepkowski SM & Braun MC Increased survival and reduced renal injury in MRL/lpr mice treated with a novel sphingosine-1-phosphate receptor agonist. Kidney Int. 74, 1319–1326 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sugahara K et al. Amiselimod (MT-1303), a novel sphingosine 1-phosphate receptor-1 modulator, potently inhibits the progression of lupus nephritis in two murine SLE models. J. Immunol. Res 2019, 5821589 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Suzuki S et al. A new immunosuppressant, FTY720, induces bcl-2-associated apoptotic cell death in human lymphocytes. Immunology 89, 518–523 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nagahara Y et al. Evidence that FTY720 induces T cell apoptosis in vivo. Immunopharmacology 48, 75–85 (2000). [DOI] [PubMed] [Google Scholar]
- 90.Suzuki S et al. The in vivo induction of lymphocyte apoptosis in MRL-lpr/lpr mice treated with FTY720. Clin. Exp. Immunol 107, 103–111 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Morris MA et al. Transient T cell accumulation in lymph nodes and sustained lymphopenia in mice treated with FTY720. Eur. J. Immunol 35, 3570–3580 (2005). [DOI] [PubMed] [Google Scholar]
- 92.Han Y et al. FTY720 abrogates collagen-induced arthritis by hindering dendritic cell migration to local lymph nodes. J. Immunol 195, 4126–4135 (2015). [DOI] [PubMed] [Google Scholar]
- 93.Lan YY et al. The sphingosine-1-phosphate receptor agonist FTY720 modulates dendritic cell trafficking in vivo. Am. J. Transpl 5, 2649–2659. (2005). [DOI] [PubMed] [Google Scholar]
- 94.Han S et al. FTY720 suppresses humoral immunity by inhibiting germinal center reaction. Blood 104, 4129–4133 (2004). [DOI] [PubMed] [Google Scholar]
- 95.Moser T et al. The role of TH17 cells in multiple sclerosis: therapeutic implications. Autoimmun. Rev 19, 102647 (2020). [DOI] [PubMed] [Google Scholar]
- 96.Eken A et al. S1P1 deletion differentially affects TH17 and regulatory T cells. Sci. Rep 7, 12905 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liao JJ, Huang MC & Goetzl EJ Cutting edge: alternative signaling of Th17 cell development by sphingosine 1-phosphate. J. Immunol 178, 5425–5428 (2007). [DOI] [PubMed] [Google Scholar]
- 98.Garris CS et al. Defective sphingosine 1-phosphate receptor 1 (S1P1) phosphorylation exacerbates TH17-mediated autoimmune neuroinflammation. Nat. Immunol 14, 1166–1172 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Khattri R et al. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol 4, 337–342 (2003). [DOI] [PubMed] [Google Scholar]
- 100.Fontenot JD, Gavin MA & Rudensky AY Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol 4, 330–336 (2003). [DOI] [PubMed] [Google Scholar]
- 101.Ochs HD, Gambineri E & Torgerson TR IPEX, FOXP3 and regulatory T-cells: a model for autoimmunity. Immunol. Res 38, 112–121 (2007). [DOI] [PubMed] [Google Scholar]
- 102.Ohl K & Tenbrock K Regulatory T cells in systemic lupus erythematosus. Eur. J. Immunol 45, 344–355 (2015). [DOI] [PubMed] [Google Scholar]
- 103.Scalapino KJ et al. Suppression of disease in New Zealand Black/New Zealand White lupus-prone mice by adoptive transfer of ex vivo expanded regulatory T cells. J. Immunol 177, 1451–1459 (2006). [DOI] [PubMed] [Google Scholar]
- 104.Liu G et al. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat. Immunol 10, 769–777 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Priceman SJ et al. S1PR1 is crucial for accumulation of regulatory T cells in tumors via STAT3. Cell Rep. 6, 992–999 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sawicka E et al. The sphingosine 1-phosphate receptor agonist FTY720 differentially affects the sequestration of CD4+/CD25+ T-regulatory cells and enhances their functional activity. J. Immunol 175, 7973–7980 (2005). [DOI] [PubMed] [Google Scholar]
- 107.Daniel C et al. FTY720 ameliorates Th1-mediated colitis in mice by directly affecting the functional activity of CD4+CD25+ regulatory T cells. J. Immunol 178, 2458–2468 (2007). [DOI] [PubMed] [Google Scholar]
- 108.Miller DC et al. The CII-specific autoimmune T-cell response develops in the presence of FTY720 but is regulated by enhanced Treg cells that inhibit the development of autoimmune arthritis. Arthritis Res. Ther 18, 8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Haas J et al. Fingolimod does not impair T-cell release from the thymus and beneficially affects Treg function in patients with multiple sclerosis. Mult. Scler 21, 1521–1532 (2015). [DOI] [PubMed] [Google Scholar]
- 110.Hjorth M, Dandu N & Mellergard J Treatment effects of fingolimod in multiple sclerosis: selective changes in peripheral blood lymphocyte subsets. PLoS One 15, e0228380 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Muls N et al. Fingolimod increases CD39-expressing regulatory T cells in multiple sclerosis patients. PLoS One 9, e113025 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dominguez-Villar M et al. Fingolimod modulates T cell phenotype and regulatory T cell plasticity in vivo. J. Autoimmun 96, 40–49 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Serpero LD et al. Fingolimod modulates peripheral effector and regulatory T cells in MS patients. J. Neuroimmune Pharmacol 8, 1106–1113 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ghadiri M et al. Pre-treatment T-cell subsets associate with fingolimod treatment responsiveness in multiple sclerosis. Sci. Rep 10, 356 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu Y et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Invest 106, 951–961 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ben Shoham A et al. S1P1 inhibits sprouting angiogenesis during vascular development. Development 139, 3859–3869 (2012). [DOI] [PubMed] [Google Scholar]
- 117.Jung B et al. Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Dev. Cell 23, 600–610 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gaengel K et al. The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Dev. Cell 23, 587–599 (2012). [DOI] [PubMed] [Google Scholar]
- 119.Cartier A et al. Endothelial sphingosine 1-phosphate receptors promote vascular normalization and antitumor therapy. Proc. Natl Acad. Sci. USA 117, 3157–3166 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Natarajan V et al. Sphingosine-1-phosphate, FTY720, and sphingosine-1-phosphate receptors in the pathobiology of acute lung injury. Am. J. Respir. Cell Mol. Biol 49, 6–17 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Burg N et al. Sphingosine 1-phosphate receptor 1 signaling maintains endothelial cell barrier function and protects against immune complex-induced vascular injury. Arthritis Rheumatol. 70, 1879–1889 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yanagida K et al. Size-selective opening of the blood-brain barrier by targeting endothelial sphingosine 1-phosphate receptor 1. Proc. Natl Acad. Sci. USA 114, 4531–4536 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Stone ML et al. Sphingosine-1-phosphate receptor 1 agonism attenuates lung ischemia-reperfusion injury. Am. J. Physiol. Lung Cell Mol. Physiol 308, L1245–L1252 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shea BS et al. Prolonged exposure to sphingosine 1-phosphate receptor-1 agonists exacerbates vascular leak, fibrosis, and mortality after lung injury. Am. J. Respir. Cell Mol. Biol 43, 662–673 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Igarashi J, Bernier SG & Michel T Sphingosine 1-phosphate and activation of endothelial nitric-oxide synthase. differential regulation of Akt and MAP kinase pathways by EDG and bradykinin receptors in vascular endothelial cells. J. Biol. Chem 276, 12420–12426 (2001). [DOI] [PubMed] [Google Scholar]
- 126.Guan Z et al. Sphingosine-1-phosphate evokes unique segment-specific vasoconstriction of the renal microvasculature. J. Am. Soc. Nephrol 25, 1774–1785 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guan Z et al. Mechanisms of sphingosine-1-phosphate-mediated vasoconstriction of rat afferent arterioles. Acta Physiol. 10.1111/apha.12913 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wolfrum C, Poy MN & Stoffel M Apolipoprotein M is required for prebeta-HDL formation and cholesterol efflux to HDL and protects against atherosclerosis. Nat. Med 11, 418–422 (2005). [DOI] [PubMed] [Google Scholar]
- 129.Silver RM, Metcalf JF & LeRoy EC Interstitial lung disease in scleroderma. Immune complexes in sera and bronchoalveolar lavage fluid. Arthritis Rheum. 29, 525–531 (1986). [DOI] [PubMed] [Google Scholar]
- 130.Murdaca G et al. Endothelial dysfunction in rheumatic autoimmune diseases. Atherosclerosis 224, 309–317 (2012). [DOI] [PubMed] [Google Scholar]
- 131.McGinley M & Cohen JA Sphingosine 1-phosphate receptor modulators in multiple sclerosis and other conditions. Lancet 398, 1184–1194 (2021). [DOI] [PubMed] [Google Scholar]
- 132.Perez-Jeldres T, Alvarez-Lobos M & Rivera-Nieves J Targeting sphingosine-1-phosphate signaling in immune-mediated diseases: beyond multiple sclerosis. Drugs 81, 985–1002 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Frangou E, Georgakis S & Bertsias G Update on the cellular and molecular aspects of lupus nephritis. Clin. Immunol 216, 108445 (2020). [DOI] [PubMed] [Google Scholar]
- 134.Katsuyama T, Tsokos GC & Moulton VR Aberrant T cell signaling and subsets in systemic lupus erythematosus. Front. Immunol 9, 1088 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hamilton JA, Hsu HC & Mountz JD Autoreactive B cells in SLE, villains or innocent bystanders? Immunol. Rev 292, 120–138 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mancardi D et al. Endothelial dysfunction and cardiovascular risk in lupus nephritis: New roles for old players? Eur. J. Clin. Invest 51, e13441 (2021). [DOI] [PubMed] [Google Scholar]
- 137.Anders HJ et al. Lupus nephritis. Nat. Rev. Dis. Prim 6, 7 (2020). [DOI] [PubMed] [Google Scholar]
- 138.Jorge AM et al. Unchanging premature mortality trends in systemic lupus erythematosus: a general population-based study (1999–2014). Rheumatology 57, 337–344 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Moghaddam B et al. All-cause and cause-specific mortality in systemic lupus erythematosus: a population-based study. Rheumatology 61, 367–376 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ocampo-Piraquive V et al. Mortality in systemic lupus erythematosus: causes, predictors and interventions. Expert. Rev. Clin. Immunol 14, 1043–1053 (2018). [DOI] [PubMed] [Google Scholar]
- 141.Ballocca F et al. Predictors of cardiovascular events in patients with systemic lupus erythematosus (SLE): a systematic review and meta-analysis. Eur. J. Prev. Cardiol 22, 1435–1441 (2015). [DOI] [PubMed] [Google Scholar]
- 142.Watson L et al. Increased serum concentration of sphingosine-1-phosphate in juvenile-onset systemic lupus erythematosus. J. Clin. Immunol 32, 1019–1025 (2012). [DOI] [PubMed] [Google Scholar]
- 143.Patyna S et al. Blood ceramides as novel markers for renal impairment in systemic lupus erythematosus. Prostaglandins Other Lipid Mediat. 144, 106348 (2019). [DOI] [PubMed] [Google Scholar]
- 144.Checa A et al. Dysregulations in circulating sphingolipids associate with disease activity indices in female patients with systemic lupus erythematosus: a cross-sectional study. Lupus 26, 1023–1033 (2017). [DOI] [PubMed] [Google Scholar]
- 145.Du W et al. Low apolipoprotein M serum levels correlate with systemic lupus erythematosus disease activity and apolipoprotein M gene polymorphisms with lupus. Lipids Health Dis. 16, 88 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tyden H et al. Low plasma concentrations of apolipoprotein M are associated with disease activity and endothelial dysfunction in systemic lupus erythematosus. Arthritis Res. Ther 21, 110 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Axtell AL, Gomari FA & Cooke J Assessing endothelial vasodilator function with the Endo-PAT 2000. J. Vis. Exp 10.3791/2167 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Feingold KR et al. Infection and inflammation decrease apolipoprotein M expression. Atherosclerosis 199, 19–26 (2008). [DOI] [PubMed] [Google Scholar]
- 149.Crow MK Interferon-alpha: a therapeutic target in systemic lupus erythematosus. Rheum. Dis. Clin. North. Am 36, 173–186 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Crow MK & Wohlgemuth J Microarray analysis of gene expression in lupus. Arthritis Res. Ther 5, 279–287 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Niewold TB et al. High serum IFN-α activity is a heritable risk factor for systemic lupus erythematosus. Genes. Immun 8, 492–502 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ronnblom L, Alm GV & Eloranta ML The type I interferon system in the development of lupus. Semin. Immunol 23, 113–121 (2011). [DOI] [PubMed] [Google Scholar]
- 153.Zhao J et al. Combination of sphingosine-1-phosphate receptor 1 (S1PR1) agonist and antiviral drug: a potential therapy against pathogenic influenza virus. Sci. Rep 9, 5272 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 154.Garcia-Romo GS et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med 3, 73ra20 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Blanco LP et al. RNA externalized by neutrophil extracellular traps promotes inflammatory pathways in endothelial cells. Arthritis Rheumatol. 73, 2282–2292 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Taylor Meadows KR et al. Ozanimod (RPC1063), a selective S1PR1 and S1PR5 modulator, reduces chronic inflammation and alleviates kidney pathology in murine systemic lupus erythematosus. PLoS One 13, e0193236 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Strasser DS et al. Preclinical to clinical translation of cenerimod, a novel S1P1 receptor modulator, in systemic lupus erythematosus. RMD Open 6, e001261 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu J et al. Genomic view of systemic autoimmunity in MRLlpr mice. Genes Immun. 7, 156–168 (2006). [DOI] [PubMed] [Google Scholar]
- 159.Singer GG et al. Apoptosis, Fas and systemic autoimmunity: the MRL-lpr/lpr model. Curr. Opin. Immunol 6, 913–920 (1994). [DOI] [PubMed] [Google Scholar]
- 160.Okazaki H et al. Effects of FTY720 in MRL-lpr/lpr mice: therapeutic potential in systemic lupus erythematosus. J. Rheumatol 29, 707–716 (2002). [PubMed] [Google Scholar]
- 161.Shi D et al. FTY720 attenuates behavioral deficits in a murine model of systemic lupus erythematosus. Brain Behav. Immun 70, 293–304 (2018). [DOI] [PubMed] [Google Scholar]
- 162.Subramanian S et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc. Natl Acad. Sci. USA 103, 9970–9975 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ando S et al. FTY720 exerts a survival advantage through the prevention of end-stage glomerular inflammation in lupus-prone BXSB mice. Biochem. Biophys. Res. Commun 394, 804–810 (2010). [DOI] [PubMed] [Google Scholar]
- 164.Comi G et al. Benefit-risk profile of sphingosine-1-phosphate receptor modulators in relapsing and secondary progressive multiple sclerosis. Drugs 77, 1755–1768 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Piali L et al. Cenerimod, a novel selective S1P1 receptor modulator with unique signaling properties. Pharmacol. Res. Perspect 5, e00370 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sugahara K et al. Amiselimod, a novel sphingosine 1-phosphate receptor-1 modulator, has potent therapeutic efficacy for autoimmune diseases, with low bradycardia risk. Br. J. Pharmacol 174, 15–27 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tanaka Y et al. Amiselimod, a sphingosine 1-phosphate receptor-1 modulator, for systemic lupus erythematosus: a multicenter, open-label exploratory study. Lupus 29, 1902–1913 (2020). [DOI] [PubMed] [Google Scholar]
- 168.Smolen JS, Aletaha D & McInnes IB Rheumatoid arthritis. Lancet 388, 2023–2038 (2016). [DOI] [PubMed] [Google Scholar]
- 169.Buch MH, Eyre S & McGonagle D Persistent inflammatory and non-inflammatory mechanisms in refractory rheumatoid arthritis. Nat. Rev. Rheumatol 17, 17–33 (2021). [DOI] [PubMed] [Google Scholar]
- 170.Solomon DH et al. Explaining the cardiovascular risk associated with rheumatoid arthritis: traditional risk factors versus markers of rheumatoid arthritis severity. Ann. Rheum. Dis 69, 1920–1925 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Amigues I et al. Myocardial inflammation, measured using 18-fluorodeoxyglucose positron emission tomography with computed tomography, is associated with disease activity in rheumatoid arthritis. Arthritis Rheumatol. 71, 496–506 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Jagpal A & Navarro-Millan I Cardiovascular co-morbidity in patients with rheumatoid arthritis: a narrative review of risk factors, cardiovascular risk assessment and treatment. BMC Rheumatol. 2, 10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Solomon DH et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation 107, 1303–1307 (2003). [DOI] [PubMed] [Google Scholar]
- 174.Sluiter TJ et al. Endothelial barrier function and leukocyte transmigration in atherosclerosis. Biomedicines 9, 328 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nofer JR High-density lipoprotein, sphingosine 1-phosphate, and atherosclerosis. J. Clin. Lipidol 2, 4–11 (2008). [DOI] [PubMed] [Google Scholar]
- 176.Kitano M et al. Sphingosine 1-phosphate/sphingosine 1-phosphate receptor 1 signaling in rheumatoid synovium: regulation of synovial proliferation and inflammatory gene expression. Arthritis Rheum. 54, 742–753 (2006). [DOI] [PubMed] [Google Scholar]
- 177.Endresen GK Evidence for activation of platelets in the synovial fluid from patients with rheumatoid arthritis. Rheumatol. Int 9, 19–24 (1989). [DOI] [PubMed] [Google Scholar]
- 178.Boilard E et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science 327, 580–583 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhao C et al. Chemical hypoxia brings to light altered autocrine sphingosine-1-phosphate signalling in rheumatoid arthritis synovial fibroblasts. Mediators Inflamm. 2015, 436525 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lai WQ, Melendez AJ & Leung B Role of sphingosine kinase and sphingosine-1-phosphate in inflammatory arthritis. World J. Biol. Chem 1, 321–326 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhao C et al. Specific and overlapping sphingosine-1-phosphate receptor functions in human synoviocytes: impact of TNF-alpha. J. Lipid Res 49, 2323–2337 (2008). [DOI] [PubMed] [Google Scholar]
- 182.Ishii M et al. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature 458, 524–528 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jaigirdar SA et al. Sphingosine-1-phosphate promotes the persistence of activated CD4 T cells in inflamed sites. Front. Immunol 8, 1627 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ledgerwood LG et al. The sphingosine 1-phosphate receptor 1 causes tissue retention by inhibiting the entry of peripheral tissue T lymphocytes into afferent lymphatics. Nat. Immunol 9, 42–53 (2008). [DOI] [PubMed] [Google Scholar]
- 185.Lai WQ et al. Anti-inflammatory effects of sphingosine kinase modulation in inflammatory arthritis. J. Immunol 181, 8010–8017 (2008). [DOI] [PubMed] [Google Scholar]
- 186.Lai WQ et al. Distinct roles of sphingosine kinase 1 and 2 in murine collagen-induced arthritis. J. Immunol 183, 2097–2103 (2009). [DOI] [PubMed] [Google Scholar]
- 187.Baker DA et al. Genetic sphingosine kinase 1 deficiency significantly decreases synovial inflammation and joint erosions in murine TNF-alpha-induced arthritis. J. Immunol 185, 2570–2579 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pi X et al. Sphingosine kinase 1-mediated inhibition of Fas death signaling in rheumatoid arthritis B lymphoblastoid cells. Arthritis Rheum. 54, 754–764 (2006). [DOI] [PubMed] [Google Scholar]
- 189.Michaud J et al. Normal acute and chronic inflammatory responses in sphingosine kinase 1 knockout mice. FEBS Lett. 580, 4607–4612 (2006). [DOI] [PubMed] [Google Scholar]
- 190.Baker DA et al. Impact of sphingosine kinase 2 deficiency on the development of TNF-alpha-induced inflammatory arthritis. Rheumatol. Int 33, 2677–2681 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yoshimoto T et al. Positive modulation of IL-12 signaling by sphingosine kinase 2 associating with the IL-12 receptor beta 1 cytoplasmic region. J. Immunol 171, 1352–1359 (2003). [DOI] [PubMed] [Google Scholar]
- 192.Pyne S, Adams DR & Pyne NJ Sphingosine kinases as druggable targets. Handb. Exp. Pharmacol 259, 49–76 (2020). [DOI] [PubMed] [Google Scholar]
- 193.Fitzpatrick LR et al. Attenuation of arthritis in rodents by a novel orally-available inhibitor of sphingosine kinase. Inflammopharmacology 19, 75–87 (2011). [DOI] [PubMed] [Google Scholar]
- 194.Hu HJ et al. Common variants at the promoter region of the APOM confer a risk of rheumatoid arthritis. Exp. Mol. Med 43, 613–621 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Park YJ et al. The APOM polymorphism as a novel risk factor for dyslipidaemia in rheumatoid arthritis: a possible shared link between disease susceptibility and dyslipidaemia. Clin. Exp. Rheumatol 31, 180–188 (2013). [PubMed] [Google Scholar]
- 196.Huang Y et al. Apolipoprotein m (APOM) levels and APOM rs805297 G/T polymorphism are associated with increased risk of rheumatoid arthritis. Joint Bone Spine 81, 32–36 (2014). [DOI] [PubMed] [Google Scholar]
- 197.van Halm VP et al. Lipids and inflammation: serial measurements of the lipid profile of blood donors who later developed rheumatoid arthritis. Ann. Rheum. Dis 66, 184–188 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Matsuura M et al. Effect of FTY720, a novel immunosuppressant, on adjuvant-induced arthritis in rats. Inflamm. Res 49, 404–410 (2000). [DOI] [PubMed] [Google Scholar]
- 199.Matsuura M, Imayoshi T & Okumoto T Effect of FTY720, a novel immunosuppressant, on adjuvant- and collagen-induced arthritis in rats. Int. J. Immunopharmacol 22, 323–331 (2000). [DOI] [PubMed] [Google Scholar]
- 200.Zhu C et al. FTY720 inhibits the development of collagen-induced arthritis in mice by suppressing the recruitment of CD4+ T lymphocytes. Drug Des. Devel. Ther 15, 1981–1992 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wang F et al. Reduction of CD4 positive T cells and improvement of pathological changes of collagen-induced arthritis by FTY720. Eur. J. Pharmacol 573, 230–240 (2007). [DOI] [PubMed] [Google Scholar]
- 202.Jin J et al. Sphingosine-1-phosphate receptor subtype 1 (S1P1) modulator IMMH001 regulates adjuvant- and collagen-induced arthritis. Front. Pharmacol 10, 1085 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sakaguchi N et al. Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature 426, 454–460 (2003). [DOI] [PubMed] [Google Scholar]
- 204.Tsunemi S et al. Effects of the novel immunosuppressant FTY720 in a murine rheumatoid arthritis model. Clin. Immunol 136, 197–204 (2010). [DOI] [PubMed] [Google Scholar]
- 205.Karuppuchamy T et al. Sphingosine-1-phosphate receptor-1 (S1P1) is expressed by lymphocytes, dendritic cells, and endothelium and modulated during inflammatory bowel disease. Mucosal Immunol. 10, 162–171 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Fujii Y et al. Amelioration of collagen-induced arthritis by a novel S1P1 antagonist with immunomodulatory activities. J. Immunol 188, 206–215 (2012). [DOI] [PubMed] [Google Scholar]
- 207.Bigaud M et al. Pathophysiological consequences of a break in S1P1-dependent homeostasis of vascular permeability revealed by S1P1 competitive antagonism. PLoS One 11, e0168252 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bongartz T et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 62, 1583–1591 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kadura S & Raghu G Rheumatoid arthritis-interstitial lung disease: manifestations and current concepts in pathogenesis and management. Eur. Respir. Rev 30, 210011 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Huang S et al. Rheumatoid arthritis-associated interstitial lung disease: current update on prevalence, risk factors, and pharmacologic treatment. Curr. Treatm. Opt. Rheumatol 6, 337–353 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Bagdanoff JT et al. Inhibition of sphingosine 1-phosphate lyase for the treatment of rheumatoid arthritis: discovery of (E)-1-(4-((1R,2S,3R)-1,2,3,4-tetrahydroxybutyl)-1H-imidazol-2-yl)ethanone oxime (LX2931) and (1R,2S,3R)-1-(2-(isoxazol-3-yl)-1H-imidazol-4-yl)butane-1,2,3,4-tetraol (LX2932). J. Med. Chem 53, 8650–8662 (2010). [DOI] [PubMed] [Google Scholar]
- 212.Fleischmann R Novel small-molecular therapeutics for rheumatoid arthritis. Curr. Opin. Rheumatol 24, 335–341 (2012). [DOI] [PubMed] [Google Scholar]
- 213.Allende ML et al. Sphingosine-1-phosphate lyase deficiency produces a pro-inflammatory response while impairing neutrophil trafficking. J. Biol. Chem 286, 7348–7358 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ruaro B et al. The treatment of lung involvement in systemic sclerosis. Pharmaceuticals 14, 154 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Denton CP, Wells AU & Coghlan JG Major lung complications of systemic sclerosis. Nat. Rev. Rheumatol 14, 511–527 (2018). [DOI] [PubMed] [Google Scholar]
- 216.Volkmann ER & Fischer A Update on morbidity and mortality in systemic sclerosis-related interstitial lung disease. J. Scleroderma Relat. Disord 6, 11–20 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Tashkin DP et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir. Med 4, 708–719 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Chung L et al. Survival and predictors of mortality in systemic sclerosis-associated pulmonary arterial hypertension: outcomes from the pulmonary hypertension assessment and recognition of outcomes in scleroderma registry. Arthritis Care Res. 66, 489–495 (2014). [DOI] [PubMed] [Google Scholar]
- 219.Rubio-Rivas M et al. Mortality and survival in systemic sclerosis: systematic review and meta-analysis. Semin. Arthritis Rheum 44, 208–219 (2014). [DOI] [PubMed] [Google Scholar]
- 220.Teijaro JR et al. S1PR1-mediated IFNAR1 degradation modulates plasmacytoid dendritic cell interferon-alpha autoamplification. Proc. Natl Acad. Sci. USA 113, 1351–1356 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Assassi S et al. Systemic sclerosis and lupus: points in an interferon-mediated continuum. Arthritis Rheum. 62, 589–598 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Brkic Z et al. The interferon type I signature is present in systemic sclerosis before overt fibrosis and might contribute to its pathogenesis through high BAFF gene expression and high collagen synthesis. Ann. Rheum. Dis 75, 1567–1573 (2016). [DOI] [PubMed] [Google Scholar]
- 223.Goldberg A et al. Dose-escalation of human anti-interferon-alpha receptor monoclonal antibody MEDI-546 in subjects with systemic sclerosis: a phase 1, multicenter, open label study. Arthritis Res. Ther 16, R57 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ciechomska M & Skalska U Targeting interferons as a strategy for systemic sclerosis treatment. Immunol. Lett 195, 45–54 (2018). [DOI] [PubMed] [Google Scholar]
- 225.Chrobak I et al. Interferon-gamma promotes vascular remodeling in human microvascular endothelial cells by upregulating endothelin (ET)-1 and transforming growth factor (TGF) β2. J. Cell Physiol 228, 1774–1783 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ah Kioon MD et al. Plasmacytoid dendritic cells promote systemic sclerosis with a key role for TLR8. Sci. Transl. Med 10, eaam8458 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Radstake TR et al. The pronounced Th17 profile in systemic sclerosis (SSc) together with intracellular expression of TGFβ and IFNγ distinguishes SSc phenotypes. PLoS One 4, e5903 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Gabsi A et al. TH17 cells expressing CD146 are significantly increased in patients with systemic sclerosis. Sci. Rep 9, 17721 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Goncalves RSG et al. IL-17 and related cytokines involved in systemic sclerosis: perspectives. Autoimmunity 51, 1–9 (2018). [DOI] [PubMed] [Google Scholar]
- 230.Park MJ et al. IL-1-IL-17 signaling axis contributes to fibrosis and inflammation in two different murine models of systemic sclerosis. Front. Immunol 9, 1611 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Moon J et al. Metformin ameliorates scleroderma via inhibiting Th17 cells and reducing mTOR-STAT3 signaling in skin fibroblasts. J. Transl. Med 19, 192 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Frantz C et al. Regulatory T cells in systemic sclerosis. Front. Immunol 9, 2356 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Igarashi J & Michel T Sphingosine-1-phosphate and modulation of vascular tone. Cardiovasc. Res 82, 212–220 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Frech TM et al. Vascular leak is a central feature in the pathogenesis of systemic sclerosis. J. Rheumatol 39, 1385–1391 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Bruni C et al. Vascular leaking, a pivotal and early pathogenetic event in systemic sclerosis: should the door be closed? Front. Immunol 9, 2045 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Fleming JN & Schwartz SM The pathology of scleroderma vascular disease. Rheum. Dis. Clin. North Am 34, 41–55 (2008). [DOI] [PubMed] [Google Scholar]
- 237.Asano Y et al. Endothelial Fli1 deficiency impairs vascular homeostasis: a role in scleroderma vasculopathy. Am. J. Pathol 176, 1983–1998 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Shea BS et al. Uncoupling of the profibrotic and hemostatic effects of thrombin in lung fibrosis. JCI Insight 2, e86608 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Knipe RS et al. Endothelial-specific loss of sphingosine-1-phosphate receptor 1 increases vascular permeability and exacerbates bleomycin-induced pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol 66, 38–52 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Kano M et al. Attenuation of murine sclerodermatous models by the selective S1P1 receptor modulator cenerimod. Sci. Rep 9, 658 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Schmidt KG et al. Sphingosine-1-phosphate receptor 5 modulates early-stage processes during fibrogenesis in a mouse model of systemic sclerosis: a pilot study. Front. Immunol 8, 1242 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Salmon JE & Roman MJ Subclinical atherosclerosis in rheumatoid arthritis and systemic lupus erythematosus. Am. J. Med 121, S3–S8 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Roman MJ et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N. Engl. J. Med 349, 2399–2406 (2003). [DOI] [PubMed] [Google Scholar]
- 244.Villanueva E et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol 187, 538–552 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]