Abstract
The toxin-coregulated pilus (TCP) of Vibrio cholerae is essential for colonization. It was recently reported that rfb mutations in V. cholerae 569B cause the translocation arrest of the structural subunit of TCP, raising the possibility that the colonization defects of lipopolysaccharide mutants are due to effects on TCP biogenesis. However, an rfbB gene disruption in either V. cholerae O395 or 569B has no apparent effect on surface TCP production as assessed by immunoelectron microscopy and CTX phage transduction, and an rfbD::Tn5lac mutant of O395 also shows no defect in TCP expression. We conclude that the colonization defect associated with rfb mutations is unrelated to defects in TCP assembly.
The gram-negative bacterium Vibrio cholerae is the causative agent of the diarrheal disease cholera. The profuse secretory diarrhea characteristic of cholera is induced by cholera toxin (18), whose expression is regulated by the ToxR and ToxT transcriptional activators (24). ToxR and ToxT also coordinately regulate the biogenesis of the toxin-coregulated pilus (TCP) (19), a type IV pilus that is absolutely required for intestinal colonization in infant mice and human volunteers (3, 15, 33, 34). TCP also function as receptors for the CTX phage (37). Although TCP are currently thought to play a traditional role in colonization, functioning as adhesin-carrying organelles for host receptors, there are data suggesting that TCP may also alter other surface properties of V. cholerae, rendering cells more hydrophobic (33) and more resistant to the bacteriocidal action of antibodies and complement (12). Bacterial cells expressing high levels of TCP also display autoagglutination, a macroscopic clumping phenomenon that may reflect an ability of V. cholerae to form microcolonies on the intestinal epithelium. A similar autoaggregative phenotype is associated with type IV pilus expression in enteropathogenic Escherichia coli and has been shown to be important for the virulence of that organism in humans (7).
The rfb genes of V. cholerae encode enzymes necessary for lipopolysaccharide (LPS) biosynthesis (13, 32, 39). Previous studies correlated LPS mutations with colonization defects in V. cholerae (11, 29, 36) and other organisms (8, 9, 20, 28, 40), but the mechanism by which LPS mutations decrease colonization remained obscure. However, it was recently reported that an rfbD::Tn5 mutation in V. cholerae 569B prevents translocation of the TcpA pilin, the structural subunit of TCP (17). If true, this finding suggests two possibilities. First, since TCP are critical for colonization, the colonization defects of rfb mutants could be due to interference with proper TCP expression. Second, since TcpA mutations result in serum sensitivity, a phenotype commonly associated with LPS mutations, it is possible that improper TCP assembly affects LPS structure or function.
While searching for genes required for colonization, we identified rfbB and rfbL mutants as colonization-defective strains (11). We accordingly constructed rfbB mutations in V. cholerae O395 and 569B, and in contrast to a previous report (17), we found no defect in the TCP production of these rfb mutants, nor did we detect any reduction in the TCP expression in an rfbD::Tn5lac mutant of O395.
To construct a suicide vector for disrupting rfbB, an internal fragment of rfbB was obtained from the chromosome of V. cholerae C6709 (El Tor, Inaba) by PCR amplification and cloned into pGP704 (23). The resulting plasmid, pSC95, was introduced into O395 (classical, Ogawa) and 569B (classical, Inaba) by plate mating from the E. coli donor SM10 λpir (33), and Apr Smr transconjugants were selected for further analysis. Integration of pSC95 at the rfbB locus was verified by Southern blot analysis (data not shown) for strains SC512 (O395; rfbB::pSC95) and SC539 (569B; rfbB::pSC95).
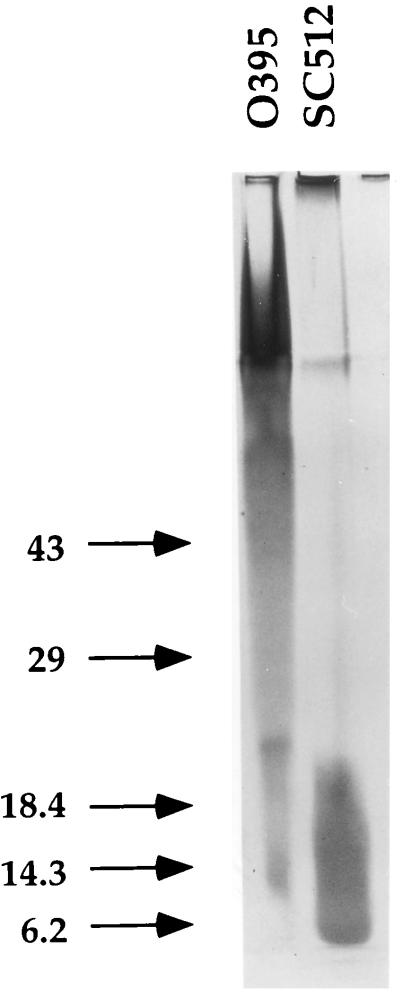
SC512 was analyzed for several phenotypes expected of LPS mutants. As noted with other V. cholerae rfb mutants (39), SC512 exhibited less agglutination with anti-Ogawa typing serum (Difco Laboratories Inc.) in slide agglutination assays than did the parental O395 strain (data not shown). In addition, LPS was purified from SC512 and O395 as described previously (30) and separated on a sodium dodecyl sulfate–12.5% polyacrylamide gel. The patterns observed after silver staining (Fig. 1) were consistent with those previously reported for wild-type and rfb mutant V. cholerae (39). The ability of SC512 to colonize infant mice was tested in competition assays (12) where a competitive index of less than one indicated attenuation relative to a wild-type strain. As expected for an LPS mutant, SC512 demonstrated a severe colonization defect (Table 1).
FIG. 1.
Silver-stained sodium dodecyl sulfate–12% polyacrylamide gel of LPS purified from O395 and SC512. Silver staining was carried out with the Bio-Rad (Hercules, Calif.) silver stain kit in accordance with the manufacturer’s directions. Numbers at the left are molecular weights, in thousands.
TABLE 1.
SC512 phenotypes
| Phenotype | Defining parameter | Value |
|---|---|---|
| Colonization | Competitive indexa | |
| In vivo | <1.8 × 10−4 | |
| In vitro | 2.66 | |
| Serum resistance | SC512/LAC-1 ratio after treatment with: | |
| Untreated serum | <1.6 × 10−4 | |
| Heat-inactivated serum | 1.51 |
The competitive index for colonization is defined as the output ratio of mutant to wild-type bacteria divided by the input ratio of mutant to wild-type bacteria. The in vivo competitive indices shown are averages of the competitive indices obtained for four mice. In one mouse, no mutant bacteria were recovered, and the competitive index was computed assuming that one mutant bacterium had been recovered. This value was then averaged with the competitive indices for the other mice, and the number reported is expressed as less than this average. In vitro competitions were carried out by subculturing 50 μl of the inoculum in 5 ml of LB, incubating the culture at 37°C on a roller overnight, and then plating at appropriate dilutions.
Serum sensitivity is associated with LPS defects in several bacterial species (14, 16, 36), and we tested SC512 in a serum resistance competition assay (12, 36). SC512 and LAC-1 (O395 ΔlacZ [38]) were cultured overnight in Luria broth (LB), subcultured 1:100 into LB, and grown at 37°C to mid-logarithmic phase. Cells were washed once in phosphate-buffered saline, mixed at a 1:1 ratio of SC512 to LAC-1, and diluted 1:10 in phosphate-buffered saline. This mixture was divided into aliquots to which was added either untreated guinea pig serum or heat-inactivated guinea pig serum to a final concentration of 10% serum. These samples were incubated at 37°C for 1 h and subsequently plated on L agar containing X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) to differentiate between Lac+ SC512 and the LAC-1 control. In this assay, SC512 was approximately 10,000-fold more serum sensitive than LAC-1 (Table 1), as determined by comparing the ratio of SC512 to LAC-1 after treatment with untreated guinea pig serum to the ratio of SC512 to LAC-1 after treatment with heat-inactivated serum. The guinea pig serum was obtained from Accurate Chemical and Scientific Corporation (Westbury, N.Y.), and heat inactivation was performed by heating at 55°C for 1 h.
Having established that SC512 displayed phenotypes consistent with an rfb mutation, we examined the strain for phenotypes associated with TCP defects. Autoagglutination, colonization, and serum resistance are all tightly linked to the expression of wild-type TCP (12), but colonization and serum resistance could not in this instance be used to assess TCP production because these phenotypes are also predicted to be affected by LPS mutations. However, TCP also function as CTX phage receptors, and TCP expression can be quantified by a transduction assay employing pCTX-Km, a kanamycin-resistant version of CTX phage (37). With this method, SC512 is as transducible by CTX phage as wild-type V. cholerae (Table 2), indicating that the rfbB::pSC95 mutation does not interfere with TCP expression. LPS mutations do result in a type of autoagglutination, but this autoagglutination is easily distinguishable from TCP-mediated autoagglutination. SC512 showed wild-type, TCP-mediated autoagglutination, further suggesting that TCP expression is unaffected in SC512. Finally, Western analysis and immunoelectron microscopy (IEM) were performed as previously described (12) and showed that SC512 produces wild-type quantities of TcpA (data not shown) and bundled surface TCP (Fig. 2a).
TABLE 2.
CTX phage transduction of O395, 569B, and their rfb derivativesa
| Strain | Relevant genotype | Phage used | Transduction frequency |
|---|---|---|---|
| O395 | pCTX-Km | 1.34 × 10−2 ± 2.82 × 10−3 | |
| SC512 | O395; rfbB::pSC95 | pCTX-Km | 1.57 × 10−2 ± 2.43 × 10−3 |
| 569B | pCTX-Km | 1.29 × 10−3 ± 2.13 × 10−4 | |
| SC539 | 569B; rfbB::pSC95 | pCTX-Km | 4.03 × 10−3 ± 5.57 × 10−4 |
| O395 | pCTX704A | 1.72 × 10−2 ± 5.44 × 10−3 | |
| O395-R2 | O395; rfbD::Tn5lac | pCTX704A | 1.71 × 10−2 ± 3.97 × 10−3 |
Cultures were grown overnight as described for the autoagglutination assay (12). A total of 50 μl of each culture was mixed with 50 μl of kanamycin- or ampicillin-marked CTX phage purified as described previously (37); cultures were then incubated at room temperature for 30 min, serially diluted, and plated on L agar containing appropriate antibiotics to determine the transduction frequency. Each assay was conducted in triplicate, and means and standard deviations are shown. pCTX704A is an ampicillin-marked CTX phage (20a).
FIG. 2.
Immunoelectron micrographs of SC512 (O395; rfbB::pSC95) (a), SC539 (569B; rfbB::pSC95) (b), 569B (c), and O395-R2 (O395; rfbD::Tn5lac) (d). Immunogold labelling was performed with polyclonal anti-TCP antiserum and gold-conjugated secondary antibody. Bars = 200 nm.
The results for SC512 clearly demonstrate that an rfbB mutation in O395 does not affect TCP expression. This is in contrast to a previous report asserting that rfb mutations block translocation of the TcpA pilin subunit, a finding that was based primarily on immunofluorescence and IEM analysis of a single rfbD mutant of 569B (17). Multiple studies with the infant mouse model had already demonstrated that 569B is a poorly colonizing strain of low virulence compared to other wild-type strains (1, 2, 4–6), and 569B carries a deletion of the regulatory gene toxS, which is required for full ToxR activation of the toxin and tcp genes in other strains of V. cholerae (22). We therefore initially thought that any effect of rfb mutations on TCP expression might be peculiar to the 569B strain background. The rfbB::pSC95 mutant of 569B (SC539) was therefore analyzed for surface TCP production by IEM (Fig. 2b) and CTX phage transduction (Table 2). SC539 did not display reduced surface expression of TCP relative to 569B by either criterion. It was not possible to determine whether TCP-mediated autoagglutination was affected by the rfbB::pSC95 mutation in 569B, because TCP-mediated autoagglutination is so poor in 569B that it would not be observable against the background autoagglutination caused by the LPS defect.
These data suggested that TCP production might be affected by rfbD mutations but not by rfbB mutations. These two genes act at slightly different points in the O-antigen biosynthetic pathway, and an rfbD mutation could conceivably result in the accumulation of intermediates that somehow interfere with TcpA export. This seemed unlikely because rfbD is immediately downstream of rfbB in the rfb operon (32), and the rfbB::pSC95 mutations are almost certainly polar on rfbD. However, we were able to address the issue definitively by examining TCP expression in an rfbD::Tn5lac mutant of O395 (O395-R2; gift of M. Waldor). O395-R2 showed wild-type, TCP-mediated autoagglutination and wild-type production of TCP when examined by IEM (Fig. 2d) and CTX phage transduction (Table 2). This demonstrates conclusively that TCP expression in O395 is not affected by either rfbB or rfbD mutations.
It may be that because TCP expression is much weaker in 569B than in O395, an rfb-related defect in TcpA translocation could under certain circumstances be observed in 569B. Subtle differences in culture conditions have long been known to have profound effects on TCP production (23), and such effects might account for the detection of a relationship between rfb mutations and TCP in a strain where TCP expression is already low. Nevertheless, the results presented here clearly indicate that, even in 569B, an inability to produce O antigen does not cause a further defect in surface TCP expression.
It remains possible that rfb mutations reduce colonization via a more subtle effect on TCP function. Type IV pilins in Neisseria meningitidis, Neisseria gonorrhoeae, and Pseudomonas aeruginosa are glycosylated (10, 25, 31), and glycosylation has been proposed to affect certain aspects of meningococcal pilus function (21, 35). Although LPS biosynthesis genes do not appear to be involved in modification of neisserial pilins, it may be that rfb mutations in V. cholerae affect TCP modification and, therefore, colonization without grossly affecting TCP biogenesis. However, since there is currently no evidence that TCP are glycosylated, we suggest that LPS itself is important for colonization. This conclusion is not unprecedented, given that LPS mutations have been found to affect colonization in several enteric bacteria, including Yersinia enterocolitica (40), E. coli (8, 9), Salmonella typhimurium (20), and Shigella flexneri (28). Although the exact role of LPS in colonization is unknown, LPS is known to be involved in resistance to antibiotics and complement-mediated killing (26, 27). One possibility is that LPS defects might render bacteria more susceptible to gut-associated bacteriocidal substances. Additional studies with V. cholerae could focus on differences between TCP and LPS mutants (e.g., sensitivity to bile salts, proteases, lactoferrin, and intestinal defensins) that might provide an explanation for the intestinal colonization defect exhibited by LPS mutants.
Acknowledgments
We thank M. Ericsson for assistance with the electron microscopy.
This work was supported by National Institutes of Health grant AI26289 (to J.J.M.). S.L.C. is an Illick Fellow of the Ryan Foundation.
REFERENCES
- 1.Attridge S R, Rowley D. The role of the flagellum in the adherence of Vibrio cholerae. J Infect Dis. 1983;147:864–872. doi: 10.1093/infdis/147.5.864. [DOI] [PubMed] [Google Scholar]
- 2.Attridge S R, Rowley D. The specificity of Vibrio cholerae adherence and the significance of the slime agglutinin as a second mediator of in vitro attachment. J Infect Dis. 1983;147:873–881. doi: 10.1093/infdis/147.5.873. [DOI] [PubMed] [Google Scholar]
- 3.Attridge S R, Voss E, Manning P A. The role of toxin-coregulated pili in the pathogenesis of Vibrio cholerae O1 El Tor. Microb Pathog. 1993;15:421–431. doi: 10.1006/mpat.1993.1091. [DOI] [PubMed] [Google Scholar]
- 4.Baselski V, Briggs R, Parker C. Intestinal fluid accumulation induced by oral challenge with Vibrio cholerae or cholera toxin in infant mice. Infect Immun. 1977;15:704–712. doi: 10.1128/iai.15.3.704-712.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baselski V S, Medina R A, Parker C D. Survival and multiplication of Vibrio cholerae in the upper bowel of infant mice. Infect Immun. 1978;22:435–440. doi: 10.1128/iai.22.2.435-440.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baselski V S, Parker C D. Intestinal distribution of Vibrio cholerae in orally infected infant mice: kinetics of recovery of radiolabel and viable cells. Infect Immun. 1978;21:518–525. doi: 10.1128/iai.21.2.518-525.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bieber D, Ramer S W, Wu C-Y, Murray W J, Tobe T, Fernandez R, Schoolnik G K. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science. 1998;280:2114–2118. doi: 10.1126/science.280.5372.2114. [DOI] [PubMed] [Google Scholar]
- 8.Bilge S S, Vary J C, Jr, Dowell S F, Tarr P I. Role of the Escherichia coli O157:H7 O side chain in adherence and analysis of an rfb locus. Infect Immun. 1996;64:4795–4801. doi: 10.1128/iai.64.11.4795-4801.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown P K, Curtiss R I. Unique chromosomal regions associated with virulence of an avian pathogenic Escherichia coli strain. Proc Natl Acad Sci USA. 1996;93:11149–11154. doi: 10.1073/pnas.93.20.11149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castric P. pilO, a gene required for glycosylation of Pseudomonas aeruginosa 1244 pilin. Microbiology. 1995;141:1247–1254. doi: 10.1099/13500872-141-5-1247. [DOI] [PubMed] [Google Scholar]
- 11.Chiang S L, Mekalanos J J. Use of signature-tagged transposon mutagenesis to identify Vibrio cholerae genes critical for colonization. Mol Microbiol. 1998;27:797–806. doi: 10.1046/j.1365-2958.1998.00726.x. [DOI] [PubMed] [Google Scholar]
- 12.Chiang S L, Taylor R K, Koomey M, Mekalanos J J. Single amino acid substitutions in the N-terminus of Vibrio cholerae TcpA affect colonization, autoagglutination, and serum resistance. Mol Microbiol. 1995;17:1133–1142. doi: 10.1111/j.1365-2958.1995.mmi_17061133.x. [DOI] [PubMed] [Google Scholar]
- 13.Fallarino A, Mavrangelos C, Stroeher U H, Manning P A. Identification of additional genes required for O-antigen biosynthesis in Vibrio cholerae O1. J Bacteriol. 1997;179:2147–2153. doi: 10.1128/jb.179.7.2147-2153.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammerschmidt S, Birkholz C, Zähringer U, Robertson B D, van Putten J, Ebeling O, Frosch M. Contribution of genes from the capsule gene complex (cps) to lipooligosaccharide biosynthesis and serum resistance in Neisseria meningitidis. Mol Microbiol. 1994;11:885–896. doi: 10.1111/j.1365-2958.1994.tb00367.x. [DOI] [PubMed] [Google Scholar]
- 15.Herrington D A, Hall R H, Losonsky G, Mekalanos J J, Taylor R K, Levine M M. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J Exp Med. 1988;168:1487–1492. doi: 10.1084/jem.168.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong M, Payne S M. Effect of mutations in Shigella flexneri chromosomal and plasmid-encoded lipopolysaccharide genes on invasion and serum resistance. Mol Microbiol. 1997;24:779–791. doi: 10.1046/j.1365-2958.1997.3731744.x. [DOI] [PubMed] [Google Scholar]
- 17.Iredell J R, Manning P A. Outer membrane translocation arrest of the TcpA pilin subunit in rfb mutants of Vibrio cholerae O1 strain 569B. J Bacteriol. 1997;179:2038–2046. doi: 10.1128/jb.179.6.2038-2046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaper J B, Fasano A, Trucksis M. Toxins of Vibrio cholerae. In: Wachsmuth I K, Blake P A, Olsvik Ø, editors. Vibrio cholerae and cholera: molecular to global perspectives. Washington, D.C: ASM Press; 1994. pp. 145–176. [Google Scholar]
- 19.Kaufman M R, Taylor R K. The toxin-coregulated pilus: biogenesis and function. In: Wachsmuth I K, Blake P A, Olsvik Ø, editors. Vibrio cholerae and cholera: molecular to global perspectives. Washington, D.C: ASM Press; 1994. pp. 187–202. [Google Scholar]
- 20.Licht T R, Krogfelt K A, Cohen P S, Poulsen L K, Urbance J, Molin S. Role of lipopolysaccharide in colonization of the mouse intestine by Salmonella typhimurium studied by in situ hybridization. Infect Immun. 1996;64:3811–3817. doi: 10.1128/iai.64.9.3811-3817.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a.Lin, W. Unpublished data.
- 21.Marceau M, Forest K, Béretti J-L, Tainer J, Nassif X. Consequences of the loss of O-linked glycosylation of meningococcal type IV pilin on piliation and pilus-mediated adhesion. Mol Microbiol. 1998;27:705–715. doi: 10.1046/j.1365-2958.1998.00706.x. [DOI] [PubMed] [Google Scholar]
- 22.Miller V L, DiRita V J, Mekalanos J J. Identification of toxS, a regulatory gene whose product enhances ToxR-mediated activation of the cholera toxin promoter. J Bacteriol. 1989;171:1288–1293. doi: 10.1128/jb.171.3.1288-1293.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ottemann K M, Mekalanos J J. Regulation of cholera toxin expression. In: Wachsmuth I K, Blake P A, Olsvik Ø, editors. Vibrio cholerae and cholera: molecular to global perspectives. Washington, D.C: ASM Press; 1994. pp. 177–185. [Google Scholar]
- 25.Parge H E, Forest K T, Hickey M J, Christensen D A, Getzoff E D, Tainer J A. Structure of the fibre-forming protein pilin at 2.6 Å resolution. Nature. 1995;378:32–38. doi: 10.1038/378032a0. [DOI] [PubMed] [Google Scholar]
- 26.Raetz C R H. Bacterial lipopolysaccharides: a remarkable family of bioactive macroamphiphiles. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C: ASM Press; 1996. pp. 1035–1063. [Google Scholar]
- 27.Roantree R J. Salmonella O antigen and virulence. Annu Rev Microbiol. 1967;21:443–466. doi: 10.1146/annurev.mi.21.100167.002303. [DOI] [PubMed] [Google Scholar]
- 28.Sandlin R C, Lampel K A, Keasler S P, Goldberg M B, Stolzer A L, Maurelli A T. Avirulence of rough mutants of Shigella flexneri: requirement of O antigen for correct unipolar localization of IcsA in the bacterial outer membrane. Infect Immun. 1995;63:229–237. doi: 10.1128/iai.63.1.229-237.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sigel S P, Lanier S, Baselski V S, Parker C D. In vivo evaluation of pathogenicity of clinical and environmental isolates of Vibrio cholerae. Infect Immun. 1980;28:681–687. doi: 10.1128/iai.28.3.681-687.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slauch J M, Mahan M J, Michetti P, Neutra M R, Mekalanos J J. Acetylation (O-factor 5) affects the structural and immunological properties of Salmonella typhimurium lipopolysaccharide O antigen. Infect Immun. 1995;63:437–441. doi: 10.1128/iai.63.2.437-441.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stimson E, Virji M, Makepeace K, Dell A, Morris H R, Payne G, Saunders J R, Jennings M P, Barker S, Panico M, Blench I, Moxon E R. Meningococcal pilin: a glycoprotein substituted with digalactosyl 2,4-diacetamido-2,4,6-trideoxyhexose. Mol Microbiol. 1995;17:1201–1214. doi: 10.1111/j.1365-2958.1995.mmi_17061201.x. [DOI] [PubMed] [Google Scholar]
- 32.Stroeher U H, Karageorgos L E, Morona R, Manning P A. Serotype conversion in Vibrio cholerae O1. Proc Natl Acad Sci USA. 1992;89:2566–2570. doi: 10.1073/pnas.89.7.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor R K, Miller V L, Furlong D B, Mekalanos J J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci USA. 1987;84:2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thelin K H, Taylor R K. Toxin-coregulated pilus, but not mannose-sensitive hemagglutinin, is required for colonization by Vibrio cholerae O1 El Tor biotype and O139 strains. Infect Immun. 1996;64:2853–2856. doi: 10.1128/iai.64.7.2853-2856.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Virji M, Saunders J R, Sims G, Makepeace K, Maskell D, Ferguson D J P. Pilus-facilitated adherence of Neisseria meningitidis to human epithelial and endothelial cells: modulation of adherence phenotype occurs concurrently with changes in primary amino acid sequence and the glycosylation status of pilin. Mol Microbiol. 1993;10:1013–1028. doi: 10.1111/j.1365-2958.1993.tb00972.x. [DOI] [PubMed] [Google Scholar]
- 36.Waldor M K, Colwell R, Mekalanos J J. The Vibrio cholerae O139 serogroup antigen includes O-antigen capsule and lipopolysaccharide virulence determinants. Proc Natl Acad Sci USA. 1994;91:11388–11392. doi: 10.1073/pnas.91.24.11388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waldor M K, Mekalanos J J. Lysogenic conversion by a filamentous phage encoding cholera toxin. Science. 1996;272:1910–1914. doi: 10.1126/science.272.5270.1910. [DOI] [PubMed] [Google Scholar]
- 38.Waldor M K, Mekalanos J J. ToxR regulates virulence gene expression in non-O1 strains of Vibrio cholerae that cause epidemic cholera. Infect Immun. 1994;62:72–78. doi: 10.1128/iai.62.1.72-78.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward H M, Manning P A. Mapping of chromosomal loci associated with lipopolysaccharide synthesis and serotype specificity in Vibrio cholerae O1 by transposon mutagenesis using Tn5 and Tn2680. Mol Gen Genet. 1989;218:367–370. doi: 10.1007/BF00331294. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Radziejewska-Lebrecht J, Krajewska-Pietrasik D, Toivanen P, Skurnik M. Molecular and chemical characterization of the lipopolysaccharide O-antigen and its role in the virulence of Yersinia enterocolitica serotype O:8. Mol Microbiol. 1997;23:63–76. doi: 10.1046/j.1365-2958.1997.1871558.x. [DOI] [PubMed] [Google Scholar]