Abstract
Lysophospholipids, exemplified by lysophosphatidic acid (LPA) and sphingosine 1-phosphate (S1P), are produced by the metabolism and perturbation of biological membranes. Both molecules are established extracellular lipid mediators that signal via specific G protein–coupled receptors in vertebrates. This widespread signaling axis regulates the development, physiological functions, and pathological processes of all organ systems. Indeed, recent research into LPA and S1P has revealed their important roles in cellular stress signaling, inflammation, resolution, and host defense responses. In this review, we focus on how LPA regulates fibrosis, neuropathic pain, abnormal angiogenesis, endometriosis, and disorders of neuroectodermal development such as hydrocephalus and alopecia. In addition, we discuss how S1P controls collective behavior, apoptotic cell clearance, and immunosurveillance of cancers. Advances in lysophospholipid research have led to new therapeutics in autoimmune diseases, with many more in earlier stages of development for a wide variety of diseases, such as fibrotic disorders, vascular diseases, and cancer.
Keywords: lysophospholipids, sphingosine 1-phosphate, lysophosphatidic acid, immunology, vascular biology, fibrosis, cancer
1. INTRODUCTION AND HISTORY OF LYSOPHOSPHOLIPID MEDIATORS
Phospholipids, the main component of biological membranes, usually have two fatty acid chains. Phospholipids with a single fatty acid are called lysophospholipids, even though their concentrations are much lower than those of conventional phospholipids in cells and tissues. Like phospholipids, lysophospholipids are classified by their polar head structure. For example, lysophosphatidylcholine (LPC) has choline in its polar head and lysophosphatidylserine (LysoPS) has l-serine. Lysophospholipids are also classified as glycerolysophospholipids or sphingolysophospholipids depending on whether they have a glycerol or sphingosine backbone, respectively. Lysophospholipids detected in vivo consist of many molecular species that differ in the combinations of their polar groups and acyl groups. Lysophospholipids such as lysophosphatidic acid (LPA), sphingosine 1-phosphate (S1P), lysophosphatidylinositol, and LysoPS have their own synthetic routes; can induce cellular responses such as cellular morphological changes, cell proliferation, cell migration, and cytoskeletal changes; and have various pharmacological actions (1). Thus, these lysophospholipids have been termed bioactive lysophospholipids or lysophospholipid mediators. To elucidate the biological roles of lysophospholipids, researchers must identify their receptors, transporters, and metabolic enzymes. Over the past two decades, many of these key molecules have been identified. In addition, studies of knockout mice have shown that lysophospholipids have a variety of pathophysiological functions (2–6). In this review, we focus on LPA and S1P (Figure 1), two of the bioactive lysophospholipids, and outline their production, mechanism of action, cellular responses, and function in the pathogenesis of disease in multiple organ systems. We also summarize recent findings on S1P and contrast its pathobiology with that of LPA.
Figure 1.
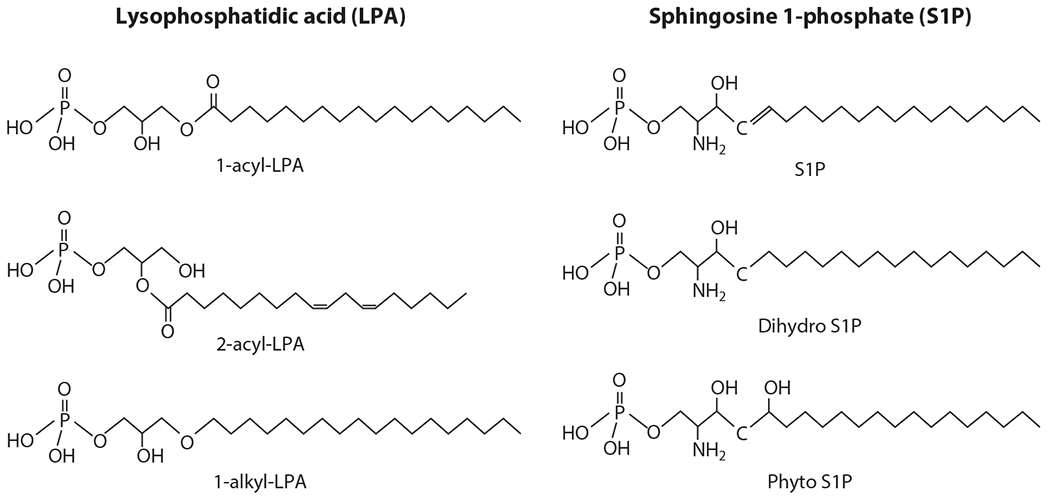
Structures of lysophosphatidic acid (LPA) and sphingosine 1-phosphate (S1P). LPA is classified as 1-acyl-LPA, 2-acyl-LPA, or 1-alkyl-LPA according to the differences in the linkage of a fatty acid to the glycerol backbone. S1P is classified as S1P, dihydro S1P,or phyto S1P on the basis of differences in the sphingosine backbone. The fatty acid carbons of S1P are not diverse, whereas the fatty acids of LPA are extremely diverse in terms of their length and degree of unsaturation, which results in different biological activities (via receptor activation).
Historically, the name lysophosphatide was given to the product formed by the action of snake venom enzymes on phospholipids from egg yolks and brain. Around the turn of the twentieth century, biochemists named such materials lysolecithin and lysocephalin and noticed associated hemolytic and thromboplastic activities, respectively. The famed biochemist Seymour Cohen, along with Erwin Chargaff (7), described the chemical composition of lysophosphatides and suggested the existence of lipid esterases in the formation of substances that contain fatty acids and a phosphoglycerol backbone. Because lysophospholipids were chaotropic for membranes, they have since been considered as lytic compounds involved in cell death. However, LPA regulates arterial ring contraction ex vivo and modulates systemic blood pressure when injected in vivo (8). Moolenaar and colleagues (1, 9) described G protein–coupled mechanisms that are required for the proliferative effects of LPA on fibroblasts, suggesting the existence of specific membrane receptors. This was followed by serendipitous cloning of LPA receptors (LPARs) (10), which ultimately led to the identification and characterization (11) of six receptors for LPA (2). S1P, which was considered an intracellular second messenger involved in cell proliferation induced by growth factors, was demonstrated to be a high-affinity ligand for the G protein–coupled receptor (GPCR) EDG-1, previously cloned as an inducible endothelial gene. It was identified as the first S1P receptor (S1PR1) (12, 13). This also led to the ultimate characterization of four additional S1PRs (S1PR2–5) (Figure 2).
Figure 2.
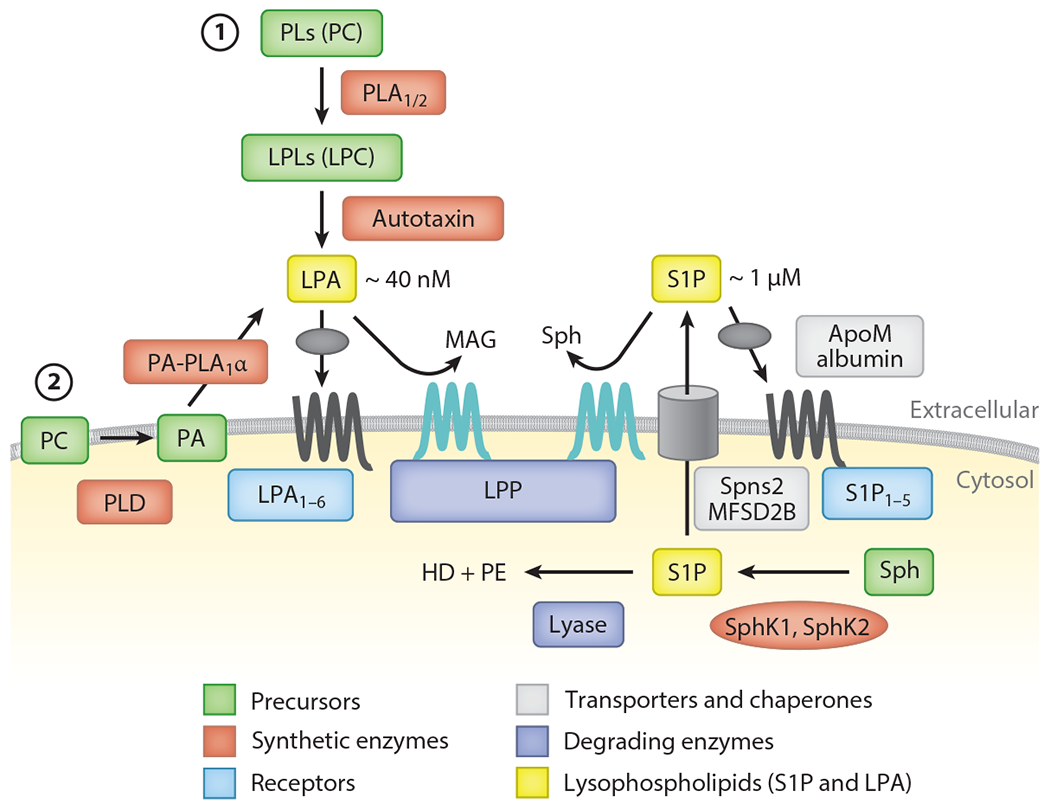
Production, degradation, transport, and action of lysophosphatidic acid (LPA) and sphingosine 1-phosphate (S1P). Whereas LPA is produced extracellularly, S1P is produced intracellularly. At least two pathways are postulated for LPA. (①) In the autotaxin (ATX) pathway, lysophospholipids (LPLs), mainly lysophosphatidylcholine (LPC), are produced by the action of phospholipase A1 or A2 (PLA1/2), and the resulting LPLs are converted to LPA by ATX. (②) In the non-ATX pathway, phosphatidic acid (PA) is generated on the cell membrane, possibly by phospholipase D (PLD), and the resulting PA is converted to LPA by PA-selective phospholipase A1 (PA-PLA1α). LPA targets the six LPA receptors (LPA1–6). LPA is specifically degraded by lipid phosphate phosphatases (LPPs) to monoacylglycerol (MAG) and phosphate. S1P is produced mainly from sphingosine (Sph) by a phosphorylation reaction. Two sphingosine kinases (SphK1 and SphK2) have been identified. S1P produced intracellularly is transported outside by S1P-specific transporters (Spns2 and MFSD2B). Meanwhile, S1P is degraded by lyase to hexadecenal (HD) and phosphoethanolamine (PE). After transport to the extracellular milieu, S1P binds to ApoM on high-density lipoprotein (HDL) and circulates in the bloodstream. LPPs are also responsible for extracellular degradation of S1P. HDL-bound S1P is brought close to the receptors (S1P1–5), which then activates intracellular signaling pathways.
2. PHYSICOCHEMICAL CONSIDERATIONS AND GENERAL PRINCIPLES OF LYSOPHOSPHOLIPID SIGNALING
Membrane phospholipid composition, asymmetry, and turnover are dynamically regulated to maintain the optimal function of cells (14). Cellular perturbations such as biomechanical forces (shear stress, pressure, surface tension), thermal changes, and reactive oxygen species (due to radiation and cellular sources) induce disorder in membrane organization (15, 16). Thus, organisms have developed complex mechanisms to restore homeostatic states of biological membranes. Such mechanisms require the actions of metabolic enzymes, phospholipid transporters/flippases, and binding proteins. Lysophospholipids, which are generated during membrane perturbation and homeostasis, possess unique shapes, solubility, and dynamic properties (17).
In general, the two types of ester bonds found in the structure of phospholipids—phosphodiester and ester bonds—are easily cleaved by esterases. In addition, various types of phospholipases activated in disease states target these chemical bonds, producing lipid metabolites such as eicosanoids and lysophospholipids (14). In contrast, lysophospholipids with ether bonds, lysoplasmalogens, are more stable to esterases. The unique biology of lysoplasmalogens (18, 19) is not covered here.
Because of their single acyl chain content (Figure 1), lysophospholipids are less hydrophobic than diacyl phospholipids. This property enables them to be released from membranes and lipoproteins. Thus, many biophysical and biochemical stimuli induce the formation of lysophospholipids. Highly potent animal venoms and pathogenic microorganisms contain enzymes to rapidly generate lysophospholipids, which mediate part of the toxic effects (20). In general, lysophospholipids are short-lived, as they are rapidly degraded by dephosphorylation, acylation, or deacylation. Cell surface–localized degradative enzymes such as lipid phosphate phosphatases (LPPs) degrade these molecules and thus reduce their activities on cells (Figure 2). Like eicosanoids (prostaglandins and leukotrienes), lysophospholipids are produced by specific pathways and target GPCRs (21) (Figure 2). Through these receptors lysophospholipids exert diverse pathophysiological functions.
Structural, biochemical, pharmacological, and signal transduction properties of GPCRs for LPA and S1P have been extensively discussed in previous reviews (2, 22, 23). In this review, we address the recent findings of lysophospholipid pathophysiology.
3. LYSOPHOSPHOLIPIDS AND PATHOLOGICAL MECHANISMS
3.1. Cellular Stress Signaling
Cell stress due to mechanical trauma, radiation (X-ray, UV), heat, hypoxia, reoxygenation, and microbial insults leads to membrane phospholipid disturbances due to physical forces, presence of phospholipase enzymes, and reactive oxygen species. This leads to the formation of lysophospholipids (24–28). The role of these lipids, which have unique biophysical properties, in membrane function is poorly understood but may well underlie the molecular basis of membrane perturbations, for example, swelling, blebbing, and endocytosis (29). In addition, such molecules, for example, LPA and S1P, are readily solubilized, particularly facilitated by chaperone molecules and lipoprotein particles that can stably bind them. Lysophospholipid molecules released from cell membranes change membrane properties and provide a pool of paracrine mediators that influence neighboring cells that express GPCRs for such mediators. Lysophospholipids that are cell associated can act as autocrine signaling molecules on GPCRs that are expressed in the same cell (4). Such signaling mechanisms have diverse biological effects, as discussed below. GPCRs for lysophospholipids have been found only in vertebrates, whereas the ligands are present throughout all organisms that contain phospholipids in membranes. Thus, it is likely that GPCR signaling of extracellular lysophospholipids coevolved with vertebrates.
As discussed in detail in previous reviews (2, 22), GPCRs for LPA and S1P exhibit diverse signaling mechanisms that depend on differential coupling to multiple cytosolic effectors and coreceptors. Most cells have more than one type of lysophospholipid GPCR, which indicates the widespread nature of this signaling axis in embryo development and physiology. Downstream processes, including cellular contraction, shape change, adhesion (cell–cell and cell–matrix), migration, survival, and death, are all regulated by various lysophospholipid GPCRs. Because these processes are also regulated by multiple signals (i.e., cytokines, growth factors, and hormones), cooperation between multiple receptor-dependent mechanisms may be important for the eventual outcome of cell survival or death during stressful stimuli. The role of lysophospholipid signaling in stress signaling of long-lived cells versus cells that rapidly turn over, for example, neurons and endothelial cells versus neutrophils and epithelial cells, respectively, is currently not known. In addition, their roles in cellular senescence and organismal aging are an understudied area of research.
3.2. Inflammation and Resolution Processes
The five cardinal signs of inflammation, rubor (redness), tumor (swelling), calor (heat), dolor (pain), and functio laesa (loss of function), are induced by multiple mediators. The protective mechanisms of normal inflammation are reversed by resolution processes (30). Lysophospholipids are generated during many of these processes and participate in them (4–6). For example, vasodilation, vasoconstriction, and vascular leak are regulated both positively and negatively by lysophospholipids. Endothelia-expressed Gi-coupled S1PRs mediate vasodilatation, whereas vascular smooth-muscle-expressed Gq- and G12/13-coupled S1PRs and LPARs induce vasoconstriction. The former also inhibit vascular leak by inducing adherens junction assembly in endothelial cells, whereas the latter are either expressed in some endothelial cells constitutively (albeit at lower levels) or induced during inflammatory conditions to promote vascular leak. Thus, lysophospholipids likely influence the function of classical mediators of vascular permeability, for example, histamine, serotonin, and bradykinins. In addition to vascular intrinsic effects, lysophospholipids regulate hematopoietic and immune cells during inflammation. For example, platelet aggregation, red blood cell metabolism, neutrophil phagocytosis, macrophage fate switching, mast cell release of mediators, innate immunity, natural killer and dendritic cell migration, hematopoietic stem/progenitor cell release into circulation, and adaptive (T and B) cell trafficking and tissue residency are all regulated by lysophospholipid signaling via GPCRs (4, 6, 31, 32). Modulation of S1PR1-dependent autoreactive immune cell trafficking by GPCR functional antagonists is currently a first-line pharmacotherapy for multiple sclerosis and is undergoing clinical trials for systemic lupus erythematosus and inflammatory bowel disease. Thus, lysophospholipid GPCRs regulate both innate and adaptive immune responses in a complex manner to affect inflammatory and resolution responses (33–35). In addition, direct action of LPA and S1P on neuronal and neural cells induces pain responses in both central and peripheral nervous tissues (36). Therefore, altered lysophospholipid concentrations due to cell perturbation, vascular leak, immune cell composition changes, or a combination thereof lead to inflammatory and neuropathic pain. In this context, cannabinoid receptors, which are closely related to lysophospholipid receptors, inhibit pain in many systems. In contrast, the role of lysophospholipid mediators in the resolution of inflammatory responses is poorly understood.
3.3. Host Defense and Infectious Disease
Due to their broad roles in cell–cell communication events, lysophospholipids have been implicated in the host defense response against bacterial and viral infections. In systemic bacterial infections (sepsis), reduced level of S1P in plasma is associated with poor outcomes in severe sepsis in humans as well as in preclinical animal models (37). The ability of circulating S1P to maintain vascular tone and maintain blood pressure and organ perfusion appears to be critical for the host to withstand acute circulatory shock and plasma volume depletion (38). Viral infection (influenza and respiratory syncytial virus)–induced cytokine storm, which damages the host and leads to severe disease and death, is attenuated by the activation of vascular endothelial S1PR1 by small-molecule agonists in animal models (39). Suppression of vascular leak and attenuation of the inflammatory responses may be involved in this protective effect. Indeed, in humans with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infections, reduced levels of S1P and chaperone ApoM in plasma are associated with poor outcomes (40, 41). However, because S1PR1 agonists have complex effects on vascular and immune systems, therapeutic modulation of this system awaits additional basic research into pathogenetic mechanisms and the development of novel therapeutics.
4. LYSOPHOSPHATIDIC ACID
4.1. Structure
LPA is the simplest glycerophospholipid, consisting of an acyl group and a phosphate group that is attached covalently to a glycerol backbone (Figure 1). LPA detected in vivo is composed of several molecular species with various types of fatty acids (e.g., oleic acid, arachidonic acid) at either the sn-1 or the sn-2 position of the glycerol backbone. It should be stressed that the structural differences of LPA affect its receptor activation (11, 42) and thus its physiological activity. Specific mechanisms of LPA production as stated below give rise to different LPA molecular species. Liquid chromatography with tandem mass spectrometry (LC-MS/MS) is currently the best method to distinguish and detect LPA species (43). S1P is structurally similar to LPA except that it has a sphingosine backbone (Figure 1). Unlike LPA, S1P is found in only a few molecular species.
4.2. Receptors for Lysophosphatidic Acid
It was recognized early on that LPA and S1P induce similar cellular behaviors, such as cell migration, morphological change, and proliferation (1, 9). LPA1/Edg2/Vzg-1 was proposed by the Chun group (10) as a GPCR for LPA that induced neurite retraction, which was subsequently shown to be correct when the high-affinity ligand binding assays were done. LPA1 belongs to the endothelial differentiation gene (EDG) family (12, 44), which consists of eight members. After the discovery of LPA1/Edg2, the characterization of other EDG family members led to the identification of another two LPARs (LPA2/Edg4 and LPA3/Edg7) (3, 23). EDG-1, originally cloned as an inducible gene from endothelial cells, was identified as an S1PR independently by a novel cell–cell adhesion assay that involved adherens junction assembly (13). The remaining four GPCRs (Edg3, Edg5, Edg6, Edg8) were soon identified as S1PRs. These studies in the late 1990s had revealed that LPA and S1P were recognized by similar but distinct GPCRs. In 2003, Ishii and colleagues (45) identified the fourth LPAR (LPA4/P2Y9). LPA4/P2Y9 belonged to the P2Y receptor family, which in a phylogenetic tree was distant from the EDG family. Subsequently, LPA5/GPR92 and LPA6/P2Y5, both of which are close homologs of LPA4, were reported as the fifth and sixth LPARs, respectively (2). Thus, currently there are six receptors for LPA. These LPARs are divided into two subfamilies: the EDG family LPARs (LPA1, LPA2, and LPA3) and the non-EDG (or P2Y) family LPARs (LPA4, LPA5, and LPA6).
After the discovery of LPARs and S1PRs, several GPCRs for other lysophospholipids were found. These include GPR34, P2Y10, A630033H20, and GPR174 for LysoPS (46) and GPR55 for sugar-containing lysophospholipids such as lysophosphatidylinositol and lysophosphatidylglucose (47). All five of these GPCRs are members of the P2Y family. Generally, P2Y family members recognize nucleotides such as ATP and ADP, which have phosphodiester bonds in their structure. Because the phosphodiester bonds are also present in the structure of lysophospholipids, P2Y family members share a common feature that recognizes the phosphodiester bond in nucleotide or lysophospholipid. The recent elucidation of the crystal structures of P2Y1, P2Y12 (receptors for ADP), and LPA6/P2Y5 also supports this idea (48–50).
4.3. Metabolic Enzymes for Lysophosphatidic Acid
Although LPA and S1P are recognized by similar receptors (i.e., EDG family members), they are produced by different mechanisms. LPA is produced extracellularly (Figure 2), whereas S1P is synthesized intracellularly and is transported outside the cell via specific transporters (51). LPA is also produced inside the cell by the acylation reaction from glycerol-3-phosphate. However, this conserved pathway is thought to be responsible for de novo synthesis of phospholipids and may not be involved in the production of LPA as a signaling molecule.
Extracellular synthesis of LPA can occur via multiple routes. In one route, LPA is converted from lysophospholipids by lysophospholipase D, which cleaves the polar groups of lysophospholipids, including LPC (Figure 2). In 2002, our group (52) and Tokumura et al. (53) purified the responsible enzyme from fetal calf and human sera, respectively, which was shown to be identical to autotaxin (ATX). ATX was originally identified as a motility-stimulating factor in culture media from highly metastatic melanoma cells. This finding led to the idea that ATX promotes the migration of cancer cells via LPAR and that LPA is involved in tumor metastasis and invasion. Indeed, ATX-induced cancer cell migration was effectively blocked when LPA1 receptors on the cancer cells were inactivated (54). Thus, LPARs and the LPA-producing enzyme ATX attracted much attention as targets for cancer treatment.
LPA is also produced by deacylation of phosphatidic acid (PA) (Figure 2). We biochemically identified a secreted enzyme called PA-selective phospholipase A1 [PA-PLA1α, also known as lipase H (LIPH)], which was responsible for deacylating PA on the cell surface (55). However, PA-producing enzymes such as phospholipase D and diacylglycerol kinases are confined to the cytoplasm. Thus, LPA production mediated by PA-PLA1α is thought to be coupled with PA production and transport.
Of the two LPA-producing enzymes, secreted ATX is present in various biological fluids, including plasma, cerebrospinal fluids, urine, and cancer ascites. The ATX substrate, LPC, is also present in these fluids and is especially abundant in plasma and ascites. Thus, LPA appears to be continuously produced and present in various parts of the body that are exposed to these biological fluids. In fact, LPA has been detected in biological fluids such as plasma, cerebrospinal fluids, seminal fluids, and saliva (56–58). Especially in incubated plasma and serum, LPA can be present at the micromolar level, which is sufficient to elicit LPAR activation (59). LPC is also the major component of the cellular phospholipids. Indeed, LPC is present on the outer leaflet of the lipid bilayer of plasma membrane in various cell types. These LPC species also serve as a substrate for ATX. Addition of recombinant ATX protein to the culture can induce cellular migration in serum-free media in a LPAR-dependent manner, suggesting that cellular LPC is the functional substrate for ATX.
LPA and S1P have a unique exposed phosphate group (Figure 1), which is necessary for binding to their receptors and can be a possible target for degradation. Several ecto-phosphatases present on the cell surface (i.e., LPPs) have been identified. LPPs are specific to lipid phosphates such as LPA, S1P, PA, and ceramide 1-phosphate (60) and negatively regulate LPA signaling as well as S1P signaling by dephosphorylating and inactivating LPA and S1P.
4.4. Physiological Regulation of Lysophosphatidic Acid Levels
As stated above, LPA is present in various body fluids, including blood (serum and plasma), cerebrospinal fluid, seminal plasma, and saliva (57, 61–63). Several clinical studies have reported elevated levels of LPA in plasma in patients with various diseases, including malignant tumors (64, 65), hepatitis (66), and acute coronary syndrome (67, 68), raising the possibility that plasma LPA could be used as a biomarker for these diseases. Although levels of LPA in plasma are clinically significant, reported LPA concentration, even under physiological conditions, can vary by an order of magnitude depending on sample collection and processing techniques. For example, levels of LPA in plasma varied widely in both patients with ovarian cancer (1.0–43.1 μM) and healthy controls (0.1–6.3 μM) (69). Also, LPA concentrations of 200 nM (70), 120 nM (71), and several tens of nanometers (72) in plasma from healthy human subjects have been reported. The analyses have shown a large scattering even in the same test, suggesting that the different ways in which the blood samples were handled and the LPA postsample collection was generated were the reasons for this variation. We recently established an optimized plasma preparation method that precisely reflects the concentration of LPA in the circulating blood (73). When this method was used, LPA levels in human and mouse were much lower than those previously reported, ranging from 40 to 50 nM, the suboptimal concentration for receptor activation. The plasma S1P concentrations reported so far were much higher than those for LPA, ranging from 400 to 1,000 nM (74), the concentrations sufficient to activate S1PRs. Accumulating evidence has suggested that the level of ATX substrates (i.e., LPC) is elevated in various diseases, including neuropathic pain (75) and lung fibrosis (76). Thus, in contrast to S1P, which is available to cells in the blood and lymphatic vascular systems, LPA is thought to be produced locally depending on disease states.
LPA is also detected in cells and tissues. It is an integral intermediate in the de novo synthesis of phospholipids and is present in essentially all cells. However, we know little about whether this intracellular LPA stimulates receptors outside the cell, because unlike S1P, which is synthesized intracellularly and transported outside the cells, transporters for LPA have not been described. In addition, we have been unable to determine whether LPA detected in tissues is present inside or outside the cells. Mice in which LPP3, which regulates the levels of extracellular LPA and S1P, was knocked out had higher levels of LPA and S1P in their tissues (60), clearly showing that a part of tissue-associated LPA and S1P exists outside the cells and is available for both receptors and LPP3.
4.5. Cellular Functions of Lysophosphatidic Acid
As with other GPCR-targeting ligands, LPA induces intracellular signals via G proteins. The G proteins are roughly divided into four subgroups, Gαi, Gαq, Gαs, and Gα12/13, and each LPAR is coupled with a single or multiple G proteins to induce intracellular signals. In various cells and cell lines, the EDG family LPARs LPA1, LPA2 and LPA3 couple with Gαi, Gαq/Gαi, and Gαq, respectively (2). LPA4, LPA5, and LPA6, which do not belong to the EDG family, couple primarily with Gα12/13. The coupling of LPARs with G proteins has been verified only in cells that are easily transfected, such as HEK293 and CHO-K1 cells, so it is uncertain whether the result can be applied to cells that intrinsically express LPARs. For example, although the LPA4 receptor couples with Gαs (77), it is not clear whether LPA4 couples with Gαs in LPA4-expressing cells in vivo.
Before we could understand the holistic pathophysiological functions (i.e., in vivo functions of LPA), we attempted to understand the cellular functions of LPA through each LPAR using reductionist approaches. The functions of LPA at the cellular level have been studied by examining the effect of LPA on various cell types in vitro. For example, LPA promotes cell proliferation (78) and can retract elongating axons of neurons (1, 9). In addition, ATX, one of the LPA-producing enzymes, was originally characterized as a motility factor for cancer cell migration (79).
The development of LPAR gene–deleted mice (knockout) and receptor-selective antagonists (80) has made it possible to verify the cellular functions of LPA in vitro. These analyses revealed that LPA stimulates cell migration of fibroblasts and cancer cells via LPA1 (54) and that LPA repels the growth of nerve cell axons via a Gα12-coupled LPAR (81), possibly LPA6. However, these in vitro functions of LPA have not been demonstrated at the in vivo level. At present, it is unclear whether LPA functions observed in vitro reflect those observed in vivo, as discussed in Section 4.6.
4.6. Pathophysiological Functions of Lysophosphatidic Acid
After the discovery of LPARs and LPA-producing enzymes, most of the pathophysiological functions of LPA have been elucidated by analyzing the knockout mouse phenotypes of LPARs and LPA-producing enzymes. Some pathological functions of LPA have been deduced through studies of human patients that are genetically deficient in LPARs or LPA-producing enzymes. The following subsections describe some examples in both mice and humans.
4.6.1. Fibrosis.
In 2008, Tager et al. (82) first demonstrated that LPA levels are high in bronchoalveolar lavage fluid following lung injury in the bleomycin model of pulmonary fibrosis in mice and in patients with idiopathic pulmonary fibrosis. Furthermore, they showed that mice lacking LPA1 were markedly protected from lung fibrosis and death in the bleomycin model. The absence of LPA1 led to reduced fibroblast recruitment and vascular leak, which are the two hallmarks of injury-induced lung fibrosis. Later, the Natarajan group (83) demonstrated that LPA2 had a protective role in bleomycin-induced lung fibrosis. The involvement of other LPARs in lung fibrosis has not been demonstrated. Among the LPARs, LPA6 is highly expressed in the lung, as are LPA1 and LPA2, implicating a potential role of LPA6 in the development of lung fibrosis. LPA1 has been implicated in the fibrosis of other tissues, such as the kidney and the skin. Using a mouse model of unilateral ureteral obstruction–induced renal fibrosis, the Tager group (84) demonstrated that accumulations of both fibroblasts and myofibroblasts were significantly attenuated in LPA1 knockout mice. The same group also demonstrated that bleomycin-induced skin fibrosis (a model of scleroderma) was significantly attenuated in LPA1 but not in LPA2 knockout mice (85). Thus, LPA1 appears to be responsible for the fibrosis of many organs. Within these tissues, fibroblasts predominantly express LPA1. Indeed, fibroblasts and their relative cells such as chondrocytes, myofibroblasts, and osteoblasts highly express LPA1 receptors. These cells have a critical role in producing extracellular matrices such as collagen and fibronectin. The LPA1 signal appeared to be involved in the development of cartilage and bone in mice (86).
In contrast to the receptors, the LPA-producing enzymes involved in the pathology of fibrosis are less studied. In the bronchoalveolar lavage fluids from both mouse models of lung fibrosis and patients, high levels of ATX proteins were detected with significant levels of LPA and LPC, suggesting the involvement of ATX (Figure 3a). However, this possibility was once denied because levels of LPA in the bronchoalveolar lavage fluids in bleomycin-induced lung fibrosis did not decrease in animals treated with an ATX inhibitor (76). However, the Aidinis group (87) has demonstrated that the ATX-LPA1 axis promoted bleomycin lung fibrosis in mice. They showed increased concentrations of ATX in both murine and human fibrotic lungs. The deletion of the ATX gene specifically in bronchial epithelial cells or macrophages attenuated disease severity. Furthermore, the pharmacological inhibition of ATX dramatically reduced the development of the disease. Another study, performed by Maher et al. (88), showed that treatment with the ATX inhibitor GLPG1690 had positive effects on the progression of lung fibrosis in humans. The observation that ATX inhibitors can stabilize and, in some cases, improve lung function was an encouraging result for patients with idiopathic pulmonary fibrosis, and GLPG1690 is currently in two phase III clinical trials.
Figure 3.
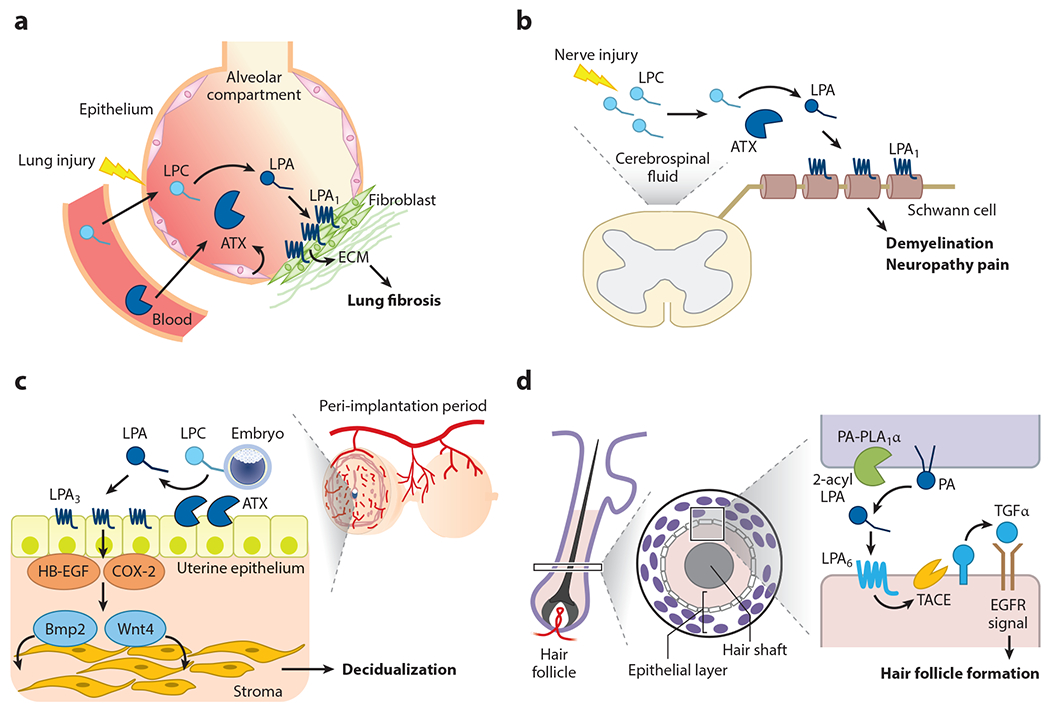
Pathophysiology of lysophosphatidic acid (LPA). LPA signaling via specific LPA-producing enzymes and LPA receptors is involved in pathophysiological conditions, including (a) lung fibrosis, (b) neuropathy pain, (c) uterine decidualization, and (d) hair follicle formation. (a) Upon lung injury, lysophosphatidylcholine (LPC) and autotaxin (ATX) levels increase and activate the LPA1 receptor on fibroblasts in the alveolar compartment, which leads to the progression of fibrosis by depositing extracellular matrix (ECM) components. (b) Upon nerve injury, newly produced LPC is converted to LPA by ATX, which is always present in cerebrospinal fluid. LPA then acts on LPA1 in myelin, inducing demyelination and the subsequent manifestation of pain. (c) When fertilized eggs interact with uteri (implantation), LPC present in eggs is converted to LPA by ATX, which is expressed abundantly on the surface of uteri (uterine epithelium). Accordingly, LPA3 is activated, as the epithelium also expresses a high level of LPA3, which then activates the decidualization factors such as cyclooxygenase-2 (COX-2), heparin-binding epidermal growth factor (HB-EGF), Wnt4, and Bmp2. Ectopic activation of LPA3 also induces endometriosis (not shown). (d) An LPA-producing enzyme, phosphatidic acid (PA)-selective phospholipase A1 (PA-PLA1α), and an LPA receptor, LPA6, are expressed in specific layers of keratinocytes in hair follicles. Activation of LPA6 induces an ectodomain shedding of transforming growth factor α (TGFα), an epidermal growth factor receptor (EGFR) ligand in the skin, which leads to the formation of hair follicles.
4.6.2. Neuropathic pain.
In 2004, Ueda and colleagues (89) found that allodynia and hyperalgesia, induced by neuropathy, were greatly reduced in LPA1 knockout mice. In support of this finding, intraspinal administration of LPA demyelinated the dorsal root nerve, which was also LPA1 dependent. Intraspinal administration of lysolecithin (LPC) has long been used as a model for demyelination and its associated pain (90). Cerebrospinal fluid contains a high concentration of ATX (approximately twice that of plasma). However, unlike plasma, it contains little LPC. Therefore, it was expected that lysolecithin administered into the spinal cord would be converted to LPA by the action of ATX and would induce demyelination and pain via the LPA1 receptor (Figure 3b). The same group demonstrated the plausibility of this hypothesis, showing an important role for the ATX-LPA-LPA1 axis in developing neuropathic pain, at least in rodents (36). These studies have suggested that the production of LPC is induced as a result of nerve injury. Indeed, it is the case both in animal models and in the clinic. The level of various lysophospholipids, including LPC and LPA, can be readily determined by LC-MS/MS. The Yatomi group (91) detected very high levels of LPC and LPA in cerebrospinal fluid of patients with neuropathic pain, including scoliosis, and showed that the levels correlated well with pain. The group also showed that levels of LPC and LPA in cerebrospinal fluid are elevated in a rat model of scoliosis and that ATX inhibitors ameliorate the progression of the condition in this case (75). These studies have demonstrated that LPC and LPA are diagnostic markers and that both ATX and LPA1 are promising targets for neuropathy pain.
4.6.3. Normal and pathological angiogenesis.
A study of mice in which ATX, a major LPA-producing enzyme in plasma, was knocked out revealed that LPA signaling had a critical role in embryonic blood vessel formation (92) (Figure 3a). ATX knockout mice were lethal at embryonic day 10.5 due to impaired embryonic vessel formation. The knockout of several key signal molecules downstream of ATX-LPA signaling such as Gα13 and Rho kinases (ROCKs) caused a similar vascular defect phenotype (93). Endothelial cell–specific Gα13 conditional knockout mice also showed this vascular defect (94). Thus, the ATX-LPA-Gα13-ROCK signaling axis in endothelial cells is thought to be involved in regulating embryonic blood vessel formation. LPA4 and LPA6 may be involved in this signaling axis, as both LPARs are coupled mainly with Gα13 and are highly expressed in endothelial cells. This idea is also supported by the finding that impaired vessel formation caused by LPA4 knockout was partially lethal at the neonatal stages (95).
Does LPA signaling regulate blood vessel formation in the adult? Capillary vessels in a solid tumor formed by transplanting cancer cells into mice had abnormalities such as disorderly branching and low blood flow. The abnormal capillaries are thought to impede the delivery of anticancer drugs to tumor tissues and thus to contribute to anticancer drug resistance (96). Recently, Takara et al. (97) reported that after LPA was administered to tumor-bearing mice, the abnormal capillaries were normalized. The LPA effect was observed within 24 h; LPA induced the morphological change (elongation) instead of promoting endothelial cell growth. LPA4 but not LPA6 knockout mice failed to exhibit the normalization action of LPA on tumor blood vessels. These results suggest that LPA4 signaling in endothelial cells contributes to the normalization of tumor vascular function. The authors showed that LPA dramatically enhanced the potency of anticancer drugs in preclinical mouse models of cancer. Thus, LPA4-mediated normalization of tumor blood vessels may contribute to anticancer drug treatment.
4.6.4. Embryo implantation and uterine decidualization.
Analysis of LPA3 receptor knockout mice unexpectedly revealed that LPA signaling via LPA3 in the uterus contributed to embryo implantation and uterine decidualization (98) (Figure 3c). LPA3 knockout female mice showed extremely small litter size compared with wild-type counterparts. Analysis of the uterus of pregnant female LPA3 knockout mice and mice treated with ATX inhibitors revealed that uterine implantation and decidualization rarely occurred in LPA3 knockout mice (99). Decidualization is a series of uterine morphological changes during early pregnancy and is essential for subsequent placenta formation and fetal development. Decidualization occurs only in the vicinity of the embryos. When LPA3, which is highly expressed in epithelial layers of the uterus, is stimulated with an agonist, decidualization was observed throughout the uterus (99). LPA3 signaling in epithelial layers of the uterus leads to the upregulation of two prominent decidual factors, heparin-binding epidermal growth factor (HB-EGF) and cyclooxygenase-2 (COX-2), which then contribute to decidualization by inducing Wnt4 and Bmp2, which are well-characterized executioner molecules for decidualization. Implanted embryos express high levels of LPC, indicating that LPC serves as a substrate for ATX, and the resulting LPC stimulates LPA at the embryo-epithelium boundary, which then induces decidualization via the canonical HB-EGF and COX-2 pathways (Figure 3c).
4.6.5. Endometriosis.
HB-EGF, COX-2, and Wnt4 have been implicated in the progression of endometriosis, the abnormal growth of endometrial tissues outside the uterus (100–102). In the mouse model of endometriosis, endometrial tissues from LPA3 knockout mice were significantly less developed. Thus, the LPA-ATX-LPA3 axis is critical in the development of endometriosis. Furthermore, HB-EGF, COX-2, and Wnt4, as well as Bmp2, are risk factors for sex-hormone-dependent diseases such as prostatic hyperplasia, breast cancer, and ovarian cancer (103–105). Because LPA3 is highly expressed in the prostate, mammary gland, and ovary (11), LPA3 signaling might contribute to the progression of such diseases and is a potential drug target.
4.6.6. Brain development.
Historically, the first LPAR LPA1/Edg2 was identified as a GPCR highly expressed in neuroprogenitor cells in the ventricular zone of the brain. Accordingly, the initial studies performed by the Chun group (106, 107) were focused mainly on the brain. LPA1 knockout mice showed 50% perinatal lethality associated with defects in the olfactory system and other nervous systems. Whole cerebral cortical tissues isolated and cultured ex vivo in the presence of LPA formed thicker cortices through decreased cell death within the ventricular zone and had an increased postmitotic neuronal population (108). The LPA effect was absent in LPA1 and LPA1/LPA2 knockout mice. Pathologically, LPA signaling via LPA1 and LPA2 appeared to be involved in the development of hydrocephalus, as hydrocephalus severity was ameliorated in LPA1 and LPA1/LPA2 knockout mice or by the use of pharmacological LPA1 antagonism. Hydrocephalus is the accumulation of cerebrospinal fluid within the brain and is characterized by macrocephaly. Fetal hydrocephalus, one of the most common neurological diseases of perinatal life, has been linked to overactive LPA signaling in an embryonic mouse model of the disease (109). Several neurological disorders, including hydrocephalus, are strongly correlated with a preceding hemorrhagic event during development. Because LPA is abundant in blood, the hemorrhagic event may result in enhanced LPA signaling through blood exposure in these disorders.
4.6.7. Lymphocyte trafficking.
ATX receptors and LPARs are expressed in specific blood vessels, where they regulate lymphocyte entry into secondary lymphoid organs. Kanda et al. (110) showed that ATX was highly expressed in high endothelial venules (HEVs) of lymphoid organs. Chemokine-activated lymphocytes expressed enhanced receptors for ATX, possibly integrins, and facilitated lymphocyte entry into lymphoid organs by producing LPA. The Miyasaka group also showed that ATX was highly expressed in HEVs (111) and that LPA produced locally in the vicinity of HEVs by ATX facilitated the binding of lymphocytes to HEVs, possibly through LPA4 and LPA6 receptors (112). In addition, same group showed that LPA2 was the receptor involved in lymphocyte migration (113). Thus, like S1P, LPA regulates lymphocyte trafficking, even though its mechanisms of action at secondary lymphoid organs differ significantly.
4.6.8. Hair follicle development and hair growth.
In 2006, Kazantseva et al. (114) reported that LIPH, which encodes the LPA-producing enzyme PA-PLA1α (LIPH), is the causative gene of congenital alopecia, which was found in a gene analysis of a Russian family who suffered from the disease. Subsequent analysis showed that LIPH is the major causative gene of congenital alopecia all over the world (115, 116). In 2008, independent studies showed that a similar type of congenital alopecia was caused by the recessive mutation of an LPA6 receptor–encoding gene (LPAR6) (117, 118). Mutations in Liph and Lpar6 genes cause phenotypes of curly hair in mice and rabbits (119, 120). Because both PA-PLA1α/LIPH and LPA6 are highly expressed in hair follicles, it was assumed that an LPA6 signal evoked by LPA that is produced by PA-PLA1α in the hair follicles has an important function in hair follicle formation (Figure 3d). Furthermore, analyses of hair follicles in PA-PLA1α knockout mice, together with the analyses of mutant mice that showed phenotypes similar to those of PA-PLA1α/LIPH knockout mice, suggested that a PA-PLA1α–LPA–LPA6 axis regulates epidermal growth factor (EGF) signaling in hair follicles (120). In this scheme (Figure 3d), LPA produced by PA-PLA1α stimulates the LPA6 receptor, which is expressed in a specific epithelial layer in the hair follicle. Then, a membrane-bound protease (ADAM17) is activated downstream of LPA6 and TGFα is shed from the membrane. TGFα is an EGF ligand that has a role in hair follicle formation. This model is also supported by the observations that a deficiency in ADAM17 or TGFα resulted in phenotypes similar to those observed in LPA6 and PA-PLA1α deficiencies: curly hair in mice and congenital alopecia in humans (121). Because LPA6 is intact in PA-PLA1α/LIPH-deficient individuals, LPA6 agonists have been expected to be ideal drugs for treatment of congenital alopecia in the clinic. Recent elucidation of the LPA6 X-ray crystal structure will accelerate the development of LPA6 agonists.
5. SPHINGOSINE 1-PHOSPHATE
5.1. Introduction and Background
Although structurally similar, S1P differs from LPA in several aspects. The presence of the amine group on the sphingosine backbone provides a unique solubility and a zwitterionic nature in physiological situations. The poor solubility of S1P required the presence of chaperones, which are binding proteins that transport this lipid to receptors for signaling as well as allow unique extracellular spatial gradients in biological compartments (4). In addition, the sphingosine backbone, in contrast to some fatty acids on LPA that are prone to oxidation, is resistant to oxidative stress. Furthermore, lack of a labile lipid ester linkage makes S1P much more stable as a lysophospholipid. These remaining subsections focus on recent advances in S1P research and on topics not addressed in previous recent reviews on this subject (2, 4–6, 31, 33).
5.2. Signal Transduction and Transcriptional Output
S1PR isotypes have been characterized extensively with respect to signal transduction properties that use G proteins. Downstream of G proteins, small GTPases, cellular ionic fluxes, and protein kinases are activated (2, 22). It is generally accepted that cytosolic signaling mechanisms lead to nuclear transcriptional changes that change cellular phenotypes. Recent studies have begun to elucidate how S1PR signals lead to downstream alterations to the transcription factor and changes to the cellular phenotype (122, 123). During postnatal angiogenesis and retinal vascular maturation events, S1PRs expressed in endothelial cells are needed for suppressing excessive vascular endothelial growth factor (VEGF) signaling, which causes vascular sprouting (124). In addition, cell–cell and cell–matrix adhesive events downstream of S1PRs regulate blood vessel morphogenesis, which is needed for proper blood flow and tissue oxygenation (4). By conducting RNA sequencing analysis and chromatin profiling techniques, we showed that S1PRs downregulate levels of activator protein 1 (AP-1) transcription factor (JunB) (123). Accurate expression levels of this transcription factor are needed for proper neurovascular guidance and for organotypic specialization of the central nervous system (CNS) vasculature, which involves expression of the proper repertoire of transporters and adhesion molecules. In the absence of S1PR signaling, high levels of JunB drive hypersprouting, defective morphogenesis, and differentiation into CNS vasculature by suppressing Norrin/Wnt signaling and enabling excessive VEGF signaling.
In adult animals, vascular S1PR signaling is not needed for host survival or vascular stability. However, animals that lack S1PRs in the endothelial cells exhibit increased vascular leak and mild inflammatory phenotypes. Such animals exhibit exaggerated inflammatory response to stressful stimuli, poor regenerative response of the liver, increased fibrotic responses, poor recovery after traumatic injury, and ischemic insults (4, 6). These studies suggest that normal vascular function requires S1PR signaling in endothelial cells. Some of these phenotypes were recapitulated in mice that lack high-density lipoprotein (HDL)-bound S1P (i.e., Apom knockout mice), albeit to a lesser degree. Thus, plasma HDL-S1P-/endothelial S1PR-dependent vascular function is needed for optimal recovery from stressful stimuli. Unbiased transcriptome analyses and chromatin interrogations suggest that endothelial S1PR signaling restrains extracellular stimuli-activated stress pathways such as AP-1 and nuclear factor κB (NF-κB) transcription factors (122). The specific intracellular mechanisms by which S1PRs restrain these transcription factors are not known.
5.3. Collective Cell Behavior and Apoptotic Cell Clearance
Most biological processes depend on the synchronized behavior of a collection of cells, also known as collective cell behavior. Processes such as cell migration, differentiation, wound healing, maintenance of barriers, and morphogenesis require synchronized behavior of adherent cells, in which each cell can sense the behavior of its neighbors. Localized signal transduction at cell–cell adhesion sites is critical for this phenomenon (125). Recent work has revealed the critical role lysophospholipids play in these processes.
Epithelial monolayers are characterized by high cell turnover while proper barrier function is maintained. Mature epithelial cells migrate from basal to apical regions and die by apoptosis. Dead cells must be removed rapidly while the barrier function of important organs lined by epithelial sheets, for example, the intestine, skin, mucosal surfaces, and airways, is maintained. Defects in apoptotic epithelial cell removal are thought to lead to inflammation and oncogenesis. Recent studies have revealed that apoptotic cells send cell–cell adhesion–dependent signals to neighbors to induce a specific contractile ring, which pushes the apoptotic cell to the apical space while maintaining the barrier. This required cadherin-dependent Rho activation at the contractile ring. S1P signaling via S1PR2 and the G12/13 pathway is essential for this process to occur (126). This coincidental detection system requires the coordinated action of tension-dependent mechanosignaling from the apoptotic cell and lysophospholipid-dependent signals in neighboring cells, which allows the epithelial barrier integrity to be maintained collectively (127). This type of multicellular coordinated behavior may require several GPCRs for lysophospholipids that are ubiquitously present in multiple cellular compartments. Whether processes such as morphogenesis, collective migration, and convergent extension require lysophospholipids is not known, but many of these processes are regulated by both LPARs and S1PRs (128, 129).
5.4. Sphingosine 1-Phosphate and Cancer Immunosurveillance
In situ carcinoma development leads to error-prone DNA replication and introduction of somatic mutations into the proteome. Immune surveillance mechanisms keep such processes in check, as evidenced by the increased development of cancers during chronic immunosuppressive pharmacotherapy. Recent studies suggest that S1P signaling mediates several complex effects on surveillance and antitumor functions of the immune system. For example, S1P secretion from the transporter Spns2 in the lymphatic endothelium of the lymph nodes is critical for efficient T cell egress into lymph and metabolic fitness (130). If this process is blocked by S1PR1 inhibitors, tumor-draining lymph nodes fail to release cells that are essential to mount an antitumor response by precise homing and induction of cytotoxic responses (131). Blockage of sphingosine kinase-1 also leads to an impaired antitumor response due to poor metabolic fitness of immune cells (132). Furthermore, chemokinetic and cytotoxic activities of CD8+ T cells are inhibited if S1P signaling via S1PR1 is inhibited (133). In addition, tissue residency of immune cells is regulated by complex modulatory systems that involve multiple S1PRs (134). However, the role of S1P in the tumor microenvironment is complex and involves interactions with other cell types, for example, T regulatory cells, innate lymphoid cells, myeloid-derived suppressor cells, vascular cells, and tumor-associated fibroblasts.
Given the importance of S1P signaling in lymphocyte trafficking and local immunity, CNS tumors pose an especially daunting problem for successful immunosurveillance and immunotherapy. The blood–brain barrier is a significant barrier for lymphocyte homing to CNS. Further, the presence of CNS tumors downregulates S1PR1 expression in T cells systemically via a process that is incompletely understood (135). Overcoming such tumor-specific processes that inhibit S1P signaling may lead to better immunotherapeutic approaches to combat not only CNS tumors but also other tumors amenable to cytotoxic T cell defenses.
6. CLINICAL AND THERAPEUTIC ISSUES
More than two decades have passed since the receptors for lysophospholipids were discovered. Since then, much has been learned about the pathophysiological roles of LPA and S1P from the studies of receptors, synthetic enzymes, transporters, and chaperones, mainly from the analyses using animal models. Currently, significant challenges in the field are to determine the significance of abnormal lysophospholipid signaling in human diseases and to develop novel therapeutics.
6.1. Measurement of Lysophospholipids in Clinical Samples
Lysophospholipids have been detected in biological fluids such as plasma, serum, urine, saliva, and cerebrospinal fluid. In addition, tools for detecting lysophospholipids precisely and quantitatively, such as LC-MS (43, 56, 70), help us understand the pathophysiological significance of lysophospholipids in the clinic. As stated above, lysophospholipids can be produced as a result of cellular perturbations. Previous studies have indicated that levels of LPA and ATX in blood increased in pathophysiological conditions, including pregnancy (136, 137), liver fibrosis (138), and cancers such as follicular lymphoma (139). Also, patients with acute coronary syndrome (with blocked arteries and a high risk for myocardial infarction) had higher levels of LPA-containing docosahexaenoic acid (DHA) in plasma (68). Cerebrospinal fluid from patients with neuropathy pain showed significantly elevated levels of LPC and LPA. Plasma ATX has been approved as a biomarker for liver cirrhosis since 2018 (63, 140).
6.2. Lysophosphatidic Acid Receptor Modulators
As mentioned above, dysregulation of LPA signaling via LPARs can lead to pathologies such as neuropathy pain, fibrosis, and cancer. Thus, LPARs are promising targets. Various small-molecule ligands for the six LPARs have been developed. These are principally divided into two categories: LPA-like compounds (LPA analogs) and nonlipid ligands. Several groups, including our group, took the former strategy and identified potent and receptor-specific agonists (141–143), which contributed to the elucidation of LPARs. By contrast, nonlipid ligands were developed mainly by pharmaceutical companies, which have been summarized in previous reviews (80, 144).
6.3. Autotaxin Inhibitors
ATX is a major LPA-producing enzyme and thus is involved in many pathological conditions, including neuropathy pain, fibrosis, glaucoma, renal and lung fibrosis, and cancer. Accordingly, researchers in academia and at pharmaceutical and biotechnology companies have tried to develop potent ATX inhibitors. For the current state of development of ATX inhibitors, we refer the reader to two excellent recent reviews (145, 146).
6.4. Sphingosine 1-Phosphate Receptor Modulation by Small-Molecule and Protein Therapeutics
Currently, three small-molecule-based drugs that target the S1PRs have been approved for use in the treatment of multiple sclerosis (both relapsing-remitting and progressive). Several compounds in the same class are being tested for use in the treatment of other autoimmune diseases, including ulcerative colitis and systemic lupus erythematosus. While this manuscript was under review, the US Food and Drug Administration approved an S1PR modulator in the treatment of ulcerative colitis. Such studies target the S1PR1 in autoreactive lymphocytes as a functional antagonist by inducing irreversible GPCR endocytosis (4). However, additional clinical trials for other indications have been initiated that are based on therapeutic targeting of S1PRs on other cell types such as vascular endothelium (https://www.clinicaltrials.gov/ct2/results?recrs=&cond=&term=sphingosine&cntry=&state=&city=&dist=). However, small-molecule-based compounds that exhibit selective GPCR-biased signaling, show tissue selectivity, or both may offer advantages in enhancing efficacy while minimizing adverse effects.
S1P chaperones that bind to the ligand in the extracellular space and present to GPCRs in a specific manner are being explored as potential therapeutics. Due to the larger size of the chaperone-S1P complex, differential effects on immune versus vascular systems have been described, which may offer additional therapeutic opportunities. Moreover, recent studies suggest polarized signaling of S1PRs in apical versus basolateral plasma membranes of adherent cells in tissues. This may also provide an additional level of specificity in therapeutics. For example, blood–brain barrier–penetrating S1PR1 agonists appear to be needed to protect the vasculature in the ischemic brain tissue after stroke (147).
7. EMERGING AREAS, OUTSTANDING QUESTIONS, AND FUTURE PERSPECTIVES
7.1. Non–G Protein–Coupled Receptor Modes of Signaling
As we have discussed, lysophospholipids exert their main effects through GPCRs. However, mechanisms other than GPCRs have also been postulated. The Tominaga group (148) found that LPA activated a subtype of transient receptor potential (TRP) transmembrane calcium channels, TRPV1, and transduced signaling, leading to itch sensing. TRP channels are activated directly or indirectly by a variety of biologically active substances to act as sensors of environmental changes. Thus, one such biologically active substance sensed by TRP channels is LPA. LPA may also activate one of the nuclear receptors, namely the lipid-sensitive peroxisome proliferator-activated receptor γ (PPARγ) (149), and modulate its activity as a transcription factor. Intracellular pools of LPA may be involved in this mode of signaling. The biological significance of the LPA-PPARγ signaling axis is not yet clear.
7.2. Lysophospholipid Reporter Systems
GPCR ligand-sensing systems (reporters) that can monitor the activation of receptors for S1P in living organisms have been developed. Because S1PRs undergo rapid endocytosis in response to ligand binding, transgenic mice expressing fluorescent-protein-tagged S1PRs have been used to provide an indirect measure of extracellular S1P levels in various organs and tissues. This approach was used to define heterogeneous S1P gradients in secondary lymphoid organs such as spleen and lymph nodes (150–152). The Proia group (153, 154) described two mouse models: one that enables detection of S1PR1 activation in real time at the tissue level and another that records receptor activity at cellular resolution in mice. In the former system, upon receptor activation and subsequent β-arrestin2 recruitment, an active luciferase enzyme complex is produced that can be detected by in vivo bioluminescence imaging. In the latter system, β-arrestin2-dependent transcriptional activation induces nuclear GFP, which can be detected by fluorescence microscopy or flow cytometry. This imaging strategy reveals the dynamics and spatial specificity of S1PR1 activation in normal and pathophysiological contexts in vivo (122, 155). Similar approaches may yield novel insights into extracellular lysophospholipid gradients and cellular sites of receptor signaling during normal and pathological conditions both spatially and temporally.
7.3. Direct Measurement of Lysophospholipid Gradients In Vivo
Even though the reporter systems provide the existence of extracellular lysophospholipid gradients, recent advances in MS technology have made it possible to detect LPA and S1P in tissue samples. For example, the increased sensitivity of LC-MS technology allows the detection of 1 fmol of S1P (156), which is sufficient to detect S1P from tiny tissue sections (micron resolution) excised by laser microdissection. MS imaging has emerged as a tool for detecting the spatial localization of various phospholipids. We have recently developed a novel MS imaging method for LPA and S1P based on derivatization on tissue sections (157) that enables the direct visualization of the distribution of LPA and S1P on tissue sections (i.e., lysophospholipid gradients) (Figure 4). This MS imaging method showed marked S1P and LPA accumulation in specific regions of the brain sections from LPP3 or S1P lyase knockout mice. Because lysophospholipid gradients are involved in the physiological homeostatic regulation of organ systems as well as induction of pathological mechanisms, the ability to directly assess them in freshly isolated tissue sections is expected to lead to unprecedented insights.
Figure 4.
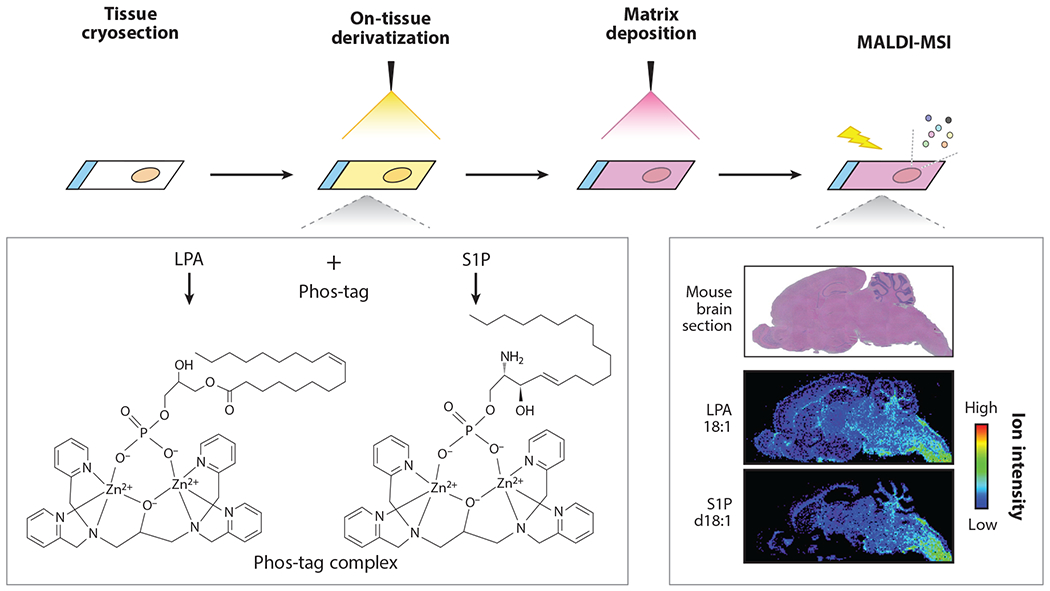
Mass spectrometry imaging of lysophosphatidic acid (LPA) and sphingosine 1-phosphate (S1P). The molecular imaging of LPA and S1P by matrix-assisted laser desorption ionization (MALDI)–mass spectrometry imaging (MSI) is combined with on-tissue derivatization using Phos-tag. LPA and S1P form a complex with Phos-tag, which increases detection sensitivity and selectivity of LPA and S1P in MALDI-MSI analysis. The experimental procedure is as follows: The tissue cryosections are sprayed with Pho-tag. The matrix, organic substances facilitating the ionization, is deposited and then MALDI-MSI analysis is performed. After data processing with spatial information, the distributions of LPA and S1P are visualized as an ion intensity of the Phos-tag complex.
8. OUTLOOK
More than two decades have passed since the lysophospholipid receptors were cloned and first described. Much has been learned from cellular and pharmacological studies of receptor isoforms and the studies using mice in which genes for LPARs and S1PRs and metabolic enzymes have been knocked out. We now understand that lysophospholipid signaling is widespread and essential in vertebrate embryogenesis, postnatal homeostasis, and various disease processes. However, many key questions remain about fundamental logic in lysophospholipid signaling. For example, we have little insight into why multiple lysophospholipids exist to activate a multitude of receptors in all organ systems examined so far. Given that membrane perturbation would lead to changes in the levels of lysophospholipids, the generality of this signaling system is perhaps not surprising. However, this multiorgan ubiquity also poses a major challenge in fully understanding the physiological and pathological impact. New technologies that address such challenges are warranted. Furthermore, therapeutic intervention strategies become challenging due to unwanted side effects from targeting a single receptor or metabolic enzyme that is involved in the regulation of multiple processes. Nevertheless, drugs that target the S1PRs have become established as therapeutics that are currently benefiting hundreds of thousands of patients worldwide. Clinical trials are underway for additional targets in this signaling axis, namely ATX inhibitors, LPAR modulators, and sphingosine kinase inhibitors. Recent progress in basic lysophospholipid research as well as new technologies such as MS imaging, receptor reporters, photoactivatable lysophospholipids, and ligand sensors should be of great help during this exciting time in the field of lysophospholipid research.
ACKNOWLEDGMENTS
This work was supported by the Leading Advanced Projects for medical innovation (LEAP) (grant no. JP18gm0010004h0002 to J.A. and K.K.) from the Japan Agency for Medical Research and Development (AMED) and KAKENHI (grant no.JP15H05899 to J.A.). T.H. acknowledges grant support from the National Institutes of Health (R35HL135821, R01EY031715, and R56AG069825).
Footnotes
DISCLOSURE STATEMENT
T.H. is an inventor named on patents and patent applications related to ApoM-Fc, ApoM+-HDL, and S1PR modulators and has consulted for Pfizer Inc., Sandoz Inc., Novartis Inc., SPARC Inc., Bristol Myers Squibb Inc., and Arena Pharmaceuticals Inc. The other authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Moolenaar WH, van Meeteren LA, Giepmans BN. 2004. The ins and outs of lysophosphatidic acid signaling. Bioessays 26:870–81 [DOI] [PubMed] [Google Scholar]
- 2.Kihara Y, Maceyka M, Spiegel S, Chun J. 2014. Lysophospholipid receptor nomenclature review: IUPHAR Review 8. Br. J. Pharmacol 171:3575–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aikawa S, Hashimoto T, Kano K, Aoki J. 2015. Lysophosphatidic acid as a lipid mediator with multiple biological actions. J. Biochem 157:81–89 [DOI] [PubMed] [Google Scholar]
- 4.Cartier A, Hla T. 2019. Sphingosine 1-phosphate: lipid signaling in pathology and therapy. Science 366:eaar5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engelbrecht E, MacRae CA, Hla T. 2021. Lysolipids in vascular development, biology, and disease. Arterioscler. Thromb. Vasc. Biol 41(2):564–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Proia RL, Hla T. 2015. Emerging biology of sphingosine-1-phosphate: its role in pathogenesis and therapy. J. Clin. Investig 125:1379–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chargaff E, Cohen SS. 1939. On lysophosphatides. J. Biol. Chem 129:619–28 [Google Scholar]
- 8.Tokumura A, Fukuzawa K, Tsukatani H. 1978. Effects of synthetic and natural lysophosphatidic acids on the arterial blood pressure of different animal species. Lipids 13:572–74 [DOI] [PubMed] [Google Scholar]
- 9.van Corven EJ, Groenink A, Jalink K, Eichholtz T, Moolenaar WH. 1989. Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell 59:45–54 [DOI] [PubMed] [Google Scholar]
- 10.Hecht JH, Weiner JA, Post SR, Chun J. 1996. Ventricular zone gene-1 (vzg-1) encodes a lysophosphatidic acid receptor expressed in neurogenic regions of the developing cerebral cortex. J. Cell Biol 135:1071–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bandoh K, Aoki J, Hosono H, Kobayashi S, Kobayashi T, et al. 1999. Molecular cloning and characterization of a novel human G-protein-coupled receptor, EDG7, for lysophosphatidic acid. J. Biol. Chem 274:27776–85 [DOI] [PubMed] [Google Scholar]
- 12.Hla T, Maciag T. 1990. An abundant transcript induced in differentiating human endothelial cells encodes a polypeptide with structural similarities to G-protein-coupled receptors. J. Biol. Chem 265:9308–13 [PubMed] [Google Scholar]
- 13.Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, et al. 1998. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science 279:1552–55 [DOI] [PubMed] [Google Scholar]
- 14.Hishikawa D, Hashidate T, Shimizu T, Shindou H. 2014. Diversity and function of membrane glycerophospholipids generated by the remodeling pathway in mammalian cells. J. Lipid Res 55:799–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holthuis JC, Menon AK. 2014. Lipid landscapes and pipelines in membrane homeostasis. Nature 510:48–57 [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi T, Menon AK. 2018. Transbilayer lipid asymmetry. Curr. Biol 28:R386–91 [DOI] [PubMed] [Google Scholar]
- 17.Harayama T, Shimizu T. 2020. Roles of polyunsaturated fatty acids, from mediators to membranes. J. Lipid Res 61:1150–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hossain MS, Mawatari S, Fujino T. 2020. Biological functions of plasmalogens. Adv. Exp. Med. Biol 1299:171–93 [DOI] [PubMed] [Google Scholar]
- 19.Farooqui AA, Horrocks LA. 2001. Plasmalogens, phospholipase A2,and docosahexaenoic acid turnover in brain tissue. J. Mol. Neurosci 16:263–72 [DOI] [PubMed] [Google Scholar]
- 20.Zambelli VO, Picolo G, Fernandes CAH, Fontes MRM, Cury Y. 2017. Secreted phospholipases A2 from animal venoms in pain and analgesia. Toxins 9:406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishii S, Nagase T, Shimizu T. 2002. Platelet-activating factor receptor. Prostaglandins Other Lipid Mediat. 68–69:599–609 [DOI] [PubMed] [Google Scholar]
- 22.Rosen H, Gonzalez-Cabrera PJ, Sanna MG, Brown S. 2009. Sphingosine 1-phosphate receptor signaling. Annu. Rev. Biochem 78:743–68 [DOI] [PubMed] [Google Scholar]
- 23.Makide K, Uwamizu A, Shinjo Y, Ishiguro J, Okutani M, et al. 2014. Novel lysophospholipid receptors: their structure and function. J. Lipid Res 55:1986–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pena LA, Fuks Z, Kolesnick R. 1997. Stress-induced apoptosis and the sphingomyelin pathway. Biochem. Pharmacol 53:615–21 [DOI] [PubMed] [Google Scholar]
- 25.Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, et al. 2008. Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ. Res 102:669–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee H, Liao JJ, Graeler M, Huang MC, Goetzl EJ. 2002. Lysophospholipid regulation of mononuclear phagocytes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1582:175–77 [DOI] [PubMed] [Google Scholar]
- 27.Karliner JS. 2002. Lysophospholipids and the cardiovascular system. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1582:216–21 [DOI] [PubMed] [Google Scholar]
- 28.Zager RA, Iwata M, Conrad DS, Burkhart KM, Igarashi Y. 1997. Altered ceramide and sphingosine expression during the induction phase of ischemic acute renal failure. Kidney Int. 52:60–70 [DOI] [PubMed] [Google Scholar]
- 29.Troyer DA, Kreisberg JI, Venkatachalam MA. 1986. Lipid alterations in LLC-PK1 cells exposed to mercuric chloride. Kidney Int. 29:530–38 [DOI] [PubMed] [Google Scholar]
- 30.Serhan CN, Gupta SK, Perretti M, Godson C, Brennan E, et al. 2020. The atlas of inflammation resolution (AIR). Mol. Aspects Med 74:100894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baeyens A, Fang V, Chen C, Schwab SR. 2015. Exit strategies: S1P signaling and T cell migration. Trends Immunol. 36:778–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dixit D, Okuniewska M, Schwab SR. 2019. Secrets and lyase: control of sphingosine 1-phosphate distribution. Immunol. Rev 289:173–85 [DOI] [PubMed] [Google Scholar]
- 33.Maceyka M, Spiegel S. 2014. Sphingolipid metabolites in inflammatory disease. Nature 510:58–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee SC, Dacheux MA, Norman DD, Balazs L, Torres RM, et al. 2020. Regulation of tumor immunity by lysophosphatidic acid. Cancers 12:1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suryadevara V, Ramchandran R, Kamp DW, Natarajan V. 2020. Lipid mediators regulate pulmonary fibrosis: potential mechanisms and signaling pathways. Int. J. Mol. Sci 21:4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueda H 2021. Pathogenic mechanisms of lipid mediator lysophosphatidic acid in chronic pain. Prog. Lipid Res 81:101079. [DOI] [PubMed] [Google Scholar]
- 37.Frej C, Linder A, Happonen KE, Taylor FB, Lupu F, Dahlbäck B. 2016. Sphingosine 1-phosphate and its carrier apolipoprotein M in human sepsis and in Escherichia coli sepsis in baboons. J. Cell Mol. Med 20:1170–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olivera A, Eisner C, Kitamura Y, Dillahunt S, Allende L, et al. 2010. Sphingosine kinase 1 and sphingosine-1-phosphate receptor 2 are vital to recovery from anaphylactic shock in mice. J. Clin. Investig 120:1429–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosen H, Oldstone MBA. 2021. The riddle of the Sphinx: why sphingosine-1-phosphate may help define molecular mechanisms underlying risk stratification for serious COVID-19 infections. EMBO Mol. Med 13:e13533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marfia G, Navone S, Guarnaccia L, Campanella R, Mondoni M, et al. 2021. Decreased serum level of sphingosine-1-phosphate: a novel predictor of clinical severity in COVID-19. EMBO Mol. Med 13:e13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song JW, Lam SM, Fan X, Cao WJ, Wang SY, et al. 2020. Omics-driven systems interrogation of metabolic dysregulation in COVID-19 pathogenesis. Cell Metab. 32:188–202.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yanagida K, Masago K, Nakanishi H, Kihara Y, Hamano F, et al. 2009. Identification and characterization of a novel lysophosphatidic acid receptor, p2y5/LPA6. J. Biol. Chem 284:17731–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okudaira M, Inoue A, Shuto A, Nakanaga K, Kano K, et al. 2014. Separation and quantification of 2-acyl-1-lysophospholipids and 1-acyl-2-lysophospholipids in biological samples by LC-MS/MS. J. Lipid Res 55:2178–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee MJ, Evans M, Hla T. 1996. The inducible G protein-coupled receptor edg-1 signals via the Gi/mitogen-activated protein kinase pathway. J. Biol. Chem 271:11272–79 [DOI] [PubMed] [Google Scholar]
- 45.Noguchi K, Ishii S, Shimizu T. 2003. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J. Biol. Chem 278:25600–6 [DOI] [PubMed] [Google Scholar]
- 46.Inoue A, Ishiguro J, Kitamura H, Arima N, Okutani M, et al. 2012. TGFα shedding assay: an accurate and versatile method for detecting GPCR activation. Nat. Methods 9:1021–29 [DOI] [PubMed] [Google Scholar]
- 47.Guy AT, Nagatsuka Y, Ooashi N, Inoue M, Nakata A, et al. 2015. Glycerophospholipid regulation of modality-specific sensory axon guidance in the spinal cord. Science 349:974–77 [DOI] [PubMed] [Google Scholar]
- 48.Taniguchi R, Inoue A, Sayama M, Uwamizu A, Yamashita K, et al. 2017. Structural insights into ligand recognition by the lysophosphatidic acid receptor LPA6. Nature 548:356–60 [DOI] [PubMed] [Google Scholar]
- 49.Zhang D, Gao ZG, Zhang K, Kiselev E, Crane S, et al. 2015. Two disparate ligand-binding sites in the human P2Y1 receptor. Nature 520:317–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang K, Zhang J, Gao ZG, Zhang D, Zhu L, et al. 2014. Structure of the human P2Y12 receptor in complex with an antithrombotic drug. Nature 509:115–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishi T, Kobayashi N, Hisano Y, Kawahara A, Yamaguchi A. 2014. Molecular and physiological functions of sphingosine 1-phosphate transporters. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1841:759–65 [DOI] [PubMed] [Google Scholar]
- 52.Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, et al. 2002. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J. Cell Biol 158:227–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tokumura A, Majima E, Kariya Y, Tominaga K, Kogure K, et al. 2002. Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J. Biol. Chem 277:39436–42 [DOI] [PubMed] [Google Scholar]
- 54.Hama K, Aoki J, Fukaya M, Kishi Y, Sakai T, et al. 2004. Lysophosphatidic acid and autotaxin stimulate cell motility of neoplastic and non-neoplastic cells through LPA1. J. Biol. Chem 279:17634–39 [DOI] [PubMed] [Google Scholar]
- 55.Sonoda H, Aoki J, Hiramatsu T, Ishida M, Bandoh K, et al. 2002. A novel phosphatidic acid-selective phospholipase A1 that produces lysophosphatidic acid. J. Biol. Chem 277:34254–63 [DOI] [PubMed] [Google Scholar]
- 56.Bathena SP, Huang J, Nunn ME, Miyamoto T, Parrish LC, et al. 2011. Quantitative determination of lysophosphatidic acids (LPAs) in human saliva and gingival crevicular fluid (GCF) by LC-MS/MS. J. Pharm. Biomed. Anal 56:402–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuwajima K, Sumitani M, Kurano M, Kano K, Nishikawa M, et al. 2018. Lysophosphatidic acid is associated with neuropathic pain intensity in humans: an exploratory study. PLOS ONE 13:e0207310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanaka M, Kishi Y, Takanezawa Y, Kakehi Y, Aoki J, Arai H. 2004. Prostatic acid phosphatase degrades lysophosphatidic acid in seminal plasma. FEBS Lett. 571:197–204 [DOI] [PubMed] [Google Scholar]
- 59.Aoki J, Taira A, Takanezawa Y, Kishi Y, Hama K, et al. 2002. Serum lysophosphatidic acid is produced through diverse phospholipase pathways. J. Biol. Chem 277:48737–44 [DOI] [PubMed] [Google Scholar]
- 60.Brindley DN, Pilquil C. 2009. Lipid phosphate phosphatases and signaling. J. Lipid Res 50(Suppl.):S225–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hama K, Bandoh K, Kakehi Y, Aoki J, Arai H. 2002. Lysophosphatidic acid (LPA) receptors are activated differentially by biological fluids: possible role of LPA-binding proteins in activation of LPA receptors. FEBS Lett. 523:187–92 [DOI] [PubMed] [Google Scholar]
- 62.Sugiura T, Nakane S, Kishimoto S, Waku K, Yoshioka Y, Tokumura A. 2002. Lysophosphatidic acid, a growth factor-like lipid, in the saliva. J. Lipid Res 43:2049–55 [DOI] [PubMed] [Google Scholar]
- 63.Yatomi Y, Kurano M, Ikeda H, Igarashi K, Kano K, Aoki J. 2018. Lysophospholipids in laboratory medicine. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci 94:373–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abdul Rahman M, Mohamad Haron DE, Hollows RJ, Abdul Ghani ZDF, Ali Mohd M, et al. 2020. Profiling lysophosphatidic acid levels in plasma from head and neck cancer patients. PeerJ 8:e9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bese T, Barbaros M, Baykara E, Guralp O, Cengiz S, et al. 2010. Comparison of total plasma lysophosphatidic acid and serum CA-125 as a tumor marker in the diagnosis and follow-up of patients with epithelial ovarian cancer. J. Gynecol. Oncol 21:248–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watanabe N, Ikeda H, Nakamura K, Ohkawa R, Kume Y, et al. 2007. Plasma lysophosphatidic acid level and serum autotaxin activity are increased in liver injury in rats in relation to its severity. Life Sci. 81:1009–15 [DOI] [PubMed] [Google Scholar]
- 67.Dohi T, Miyauchi K, Ohkawa R, Nakamura K, Kishimoto T, et al. 2012. Increased circulating plasma lysophosphatidic acid in patients with acute coronary syndrome. Clin. Chim. Acta 413:207–12 [DOI] [PubMed] [Google Scholar]
- 68.Kurano M, Suzuki A, Inoue A, Tokuhara Y, Kano K, et al.2015. Possible involvement of minor lysophospholipids in the increase in plasma lysophosphatidic acid in acute coronary syndrome. Arterioscler. Thromb. Vasc. Biol 35:463–70 [DOI] [PubMed] [Google Scholar]
- 69.Xu Y, Shen Z, Wiper DW, Wu M, Morton RE, et al. 1998. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA 280:719–23 [DOI] [PubMed] [Google Scholar]
- 70.Onorato JM, Shipkova P, Minnich A, Aubry AF, Easter J, Tymiak A. 2014. Challenges in accurate quantitation of lysophosphatidic acids in human biofluids. J. Lipid Res 55:1784–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nakamura K, Kishimoto T, Ohkawa R, Okubo S, Tozuka M, et al. 2007. Suppression of lysophosphatidic acid and lysophosphatidylcholine formation in the plasma in vitro: proposal of a plasma sample preparation method for laboratory testing of these lipids. Anal. Biochem 367:20–27 [DOI] [PubMed] [Google Scholar]
- 72.Yagi T, Kuschner CE, Shoaib M, Choudhary RC, Becker LB, et al. 2019. Relative ratios enhance the diagnostic power of phospholipids in distinguishing benign and cancerous ovarian masses. Cancers 12:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kano K, Matsumoto H, Kono N, Kurano M, Yatomi Y, Aoki J. 2021. Suppressing postcollection lysophosphatidic acid metabolism improves the precision of plasma LPA quantification. J. Lipid Res 62:100029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hammad SM, Pierce JS, Soodavar F, Smith KJ, Al Gadban MM, et al. 2010. Blood sphingolipidomics in healthy humans: impact of sample collection methodology. J. Lipid Res 51:3074–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Uranbileg B, Ito N, Kurano M, Saigusa D, Saito R, et al. 2019. Alteration of the lysophosphatidic acid and its precursor lysophosphatidylcholine levels in spinal cord stenosis: a study using a rat cauda equina compression model. Sci. Rep 9:16578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Black KE, Berdyshev E, Bain G, Castelino FV, Shea BS, et al. 2016. Autotaxin activity increases locally following lung injury, but is not required for pulmonary lysophosphatidic acid production or fibrosis. FASEB J. 30:2435–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee C-W, Rivera R, Gardell S, Dubin AE, Chun J. 2006. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J. Biol. Chem 281:23589–97 [DOI] [PubMed] [Google Scholar]
- 78.Jones SM, Kazlauskas A. 2001. Growth-factor-dependent mitogenesis requires two distinct phases of signalling. Nat. Cell Biol 3:165–72 [DOI] [PubMed] [Google Scholar]
- 79.Stracke ML, Krutzsch HC, Unsworth EJ, Årestad A, Cioce V, et al. 1992. Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J. Biol. Chem 267:2524–29 [PubMed] [Google Scholar]
- 80.Llona-Minguez S, Ghassemian A, Helleday T. 2015. Lysophosphatidic acid receptor (LPAR) modulators: the current pharmacological toolbox. Prog. Lipid Res 58:51–75 [DOI] [PubMed] [Google Scholar]
- 81.Fukushima N, Furuta D, Tsujiuchi T. 2011. Coordinated interactions between actin and microtubules through crosslinkers in neurite retraction induced by lysophosphatidic acid. Neurochem. Int 59:109–13 [DOI] [PubMed] [Google Scholar]
- 82.Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, et al. 2008. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat. Med 14:45–54 [DOI] [PubMed] [Google Scholar]
- 83.Huang LS, Fu P, Patel P, Harijith A, Sun T, et al. 2013. Lysophosphatidic acid receptor-2 deficiency confers protection against bleomycin-induced lung injury and fibrosis in mice. Am. J. Respir. Cell Mol. Biol 49:912–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sakai N, Chun J, Duffield JS, Lagares D, Wada T, et al. 2017. Lysophosphatidic acid signaling through its receptor initiates profibrotic epithelial cell fibroblast communication mediated by epithelial cell derived connective tissue growth factor. Kidney Int. 91:628–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Castelino FV, Seiders J, Bain G, Brooks SF, King CD, et al. 2011. Amelioration of dermal fibrosis by genetic deletion or pharmacologic antagonism of lysophosphatidic acid receptor 1 in a mouse model of scleroderma. Arthritis Rheum. 63:1405–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nishioka T, Arima N, Kano K, Hama K, Itai E, et al. 2016. ATX-LPA1 axis contributes to proliferation of chondrocytes by regulating fibronectin assembly leading to proper cartilage formation. Sci. Rep 6:23433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ninou I, Kaffe E, Müller S, Budd DC, Stevenson CS, et al. 2018. Pharmacologic targeting of the ATX/LPA axis attenuates bleomycin-induced pulmonary fibrosis. Pulm. Pharmacol. Ther 52:32–40 [DOI] [PubMed] [Google Scholar]
- 88.Maher TM, van der Aar EM, Van de Steen O, Allamassey L, Desrivot J, et al. 2018. Safety, tolerability, pharmacokinetics, and pharmacodynamics of GLPG1690, a novel autotaxin inhibitor, to treat idiopathic pulmonary fibrosis (FLORA): a phase 2a randomised placebo-controlled trial. Lancet Respir. Med 6:627–35 [DOI] [PubMed] [Google Scholar]
- 89.Inoue M, Rashid MH, Fujita R, Contos JJ, Chun J, Ueda H. 2004. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat. Med 10:712–18 [DOI] [PubMed] [Google Scholar]
- 90.Hall SM. 1972. The effect of injections of lysophosphatidyl choline into white matter of the adult mouse spinal cord. J. Cell Sci 10:535–46 [DOI] [PubMed] [Google Scholar]
- 91.Hayakawa K, Kurano M, Ohya J, Oichi T, Kano K, et al. 2019. Lysophosphatidic acids and their substrate lysophospholipids in cerebrospinal fluid as objective biomarkers for evaluating the severity of lumbar spinal stenosis. Sci. Rep 9:9144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tanaka M, Okudaira S, Kishi Y, Ohkawa R, Iseki S, et al. 2006. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J. Biol. Chem 281:25822–30 [DOI] [PubMed] [Google Scholar]
- 93.Offermanns S, Mancino V, Revel JP, Simon MI. 1997. Vascular system defects and impaired cell chemokinesis as a result of Gα13 deficiency. Science 275:533–36 [DOI] [PubMed] [Google Scholar]
- 94.Ruppel KM, Willison D, Kataoka H, Wang A, Zheng Y-W, et al. 2005. Essential role for Gα13 in endothelial cells during embryonic development. PNAS 102:8281–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sumida H, Noguchi K, Kihara Y, Abe M, Yanagida K, et al. 2010. LPA4 regulates blood and lymphatic vessel formation during mouse embryogenesis. Blood 116:5060–70 [DOI] [PubMed] [Google Scholar]
- 96.Jain RK. 2001. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat. Med 7:987–89 [DOI] [PubMed] [Google Scholar]
- 97.Takara K, Eino D, Ando K, Yasuda D, Naito H, et al. 2017. Lysophosphatidic acid receptor 4 activation augments drug delivery in tumors by tightening endothelial cell-cell contact. Cell Rep. 20:2072–86 [DOI] [PubMed] [Google Scholar]
- 98.Ye X, Hama K, Contos JJ, Anliker B, Inoue A, et al. 2005. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature 435:104–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aikawa S, Kano K, Inoue A, Wang J, Saigusa D, et al. 2017. Autotaxin–lysophosphatidic acid–LPA3 signaling at the embryo-epithelial boundary controls decidualization pathways. EMBO J. 36:2146–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arosh JA, Lee J, Balasubbramanian D, Stanley JA, Long CR, et al. 2015. Molecular and preclinical basis to inhibit PGE2 receptors EP2 and EP4 as a novel nonsteroidal therapy for endometriosis. PNAS 112:9716–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ferrandina G, Ranelletti FO, Lauriola L, Fanfani F, Legge F, et al. 2002. Cyclooxygenase-2 (COX-2), epidermal growth factor receptor (EGFR), and Her-2/neu expression in ovarian cancer. Gynecol. Oncol 85:305–10 [DOI] [PubMed] [Google Scholar]
- 102.Levin ER. 2003. Bidirectional signaling between the estrogen receptor and the epidermal growth factor receptor. Mol. Endocrinol 17:309–17 [DOI] [PubMed] [Google Scholar]
- 103.Miller MA, Moss ML, Powell G, Petrovich R, Edwards L, et al. 2015. Targeting autocrine HB-EGF signaling with specific ADAM12 inhibition using recombinant ADAM12 prodomain. Sci. Rep 5:15150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ota H, Igarashi S, Sasaki M, Tanaka T. 2001. Distribution of cyclooxygenase-2 in eutopic and ectopic endometrium in endometriosis and adenomyosis. Hum. Reprod 16:561–66 [DOI] [PubMed] [Google Scholar]
- 105.Zong Y, Huang J, Sankarasharma D,Morikawa T, Fukayama M, et al. 2012. Stromal epigenetic dysregulation is sufficient to initiate mouse prostate cancer via paracrine Wnt signaling. PNAS 109:E3395–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Contos JJ, Fukushima N, Weiner JA, Kaushal D, Chun J. 2000. Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. PNAS 97:13384–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Harrison SM, Reavill C, Brown G, Brown JT, Cluderay JE, et al. 2003. LPA1 receptor-deficient mice have phenotypic changes observed in psychiatric disease. Mol. Cell Neurosci 24:1170–79 [DOI] [PubMed] [Google Scholar]
- 108.Kingsbury MA, Rehen SK, Contos JJ, Higgins CM, Chun J. 2003. Non-proliferative effects of lysophosphatidic acid enhance cortical growth and folding. Nat. Neurosci 6:1292–99 [DOI] [PubMed] [Google Scholar]
- 109.Yung YC, Mutoh T, Lin ME, Noguchi K, Rivera RR, et al. 2011. Lysophosphatidic acid signaling may initiate fetal hydrocephalus. Sci. Transl. Med 3:99ra87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kanda H, Newton R, Klein R, Morita Y, Gunn MD, Rosen SD. 2008. Autotaxin, an ectoenzyme that produces lysophosphatidic acid, promotes the entry of lymphocytes into secondary lymphoid organs. Nat. Immunol 9:415–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nakasaki T, Tanaka T, Okudaira S, Hirosawa M, Umemoto E, et al. 2008. Involvement of the lysophosphatidic acid-generating enzyme autotaxin in lymphocyte-endothelial cell interactions. Am. J. Pathol 173:1566–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hata E, Sasaki N, Takeda A, Tohya K, Umemoto E, et al. 2016. Lysophosphatidic acid receptors LPA4 and LPA6 differentially promote lymphocyte transmigration across high endothelial venules in lymph nodes. Int. Immunol 28:283–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Takeda A, Kobayashi D, Aoi K, Sasaki N, Sugiura Y, et al. 2016. Fibroblastic reticular cell-derived lysophosphatidic acid regulates confined intranodal T-cell motility. eLife 5:e10561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kazantseva A, Goltsov A, Zinchenko R, Grigorenko AP, Abrukova AV, et al. 2006. Human hair growth deficiency is linked to a genetic defect in the phospholipase gene LIPH. Science 314:982–85 [DOI] [PubMed] [Google Scholar]
- 115.Mehmood S, Jan A, Raza SI, Ahmad F, Younus M, et al. 2016. Disease causing homozygous variants in the human hairless gene. Int. J. Dermatol 55:977–81 [DOI] [PubMed] [Google Scholar]
- 116.Shimomura Y, Ito M, Christiano AM. 2009.Mutations in the LIPH gene in three Japanese families with autosomal recessive woolly hair/hypotrichosis. J. Dermatol. Sci 56:205–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pasternack SM, von Kugelgen I, Al Aboud K, Lee YA, Ruschendorf F, et al. 2008. G protein-coupled receptor P2Y5 and its ligand LPA are involved in maintenance of human hair growth. Nat. Genet 40:329–34 [DOI] [PubMed] [Google Scholar]
- 118.Shimomura Y, Wajid M, Ishii Y, Shapiro L, Petukhova L, et al. 2008. Disruption of P2RY5, an orphan G protein-coupled receptor, underlies autosomal recessive woolly hair. Nat. Genet 40:335–39 [DOI] [PubMed] [Google Scholar]
- 119.Diribarne M, Mata X, Chantry-Darmon C, Vaiman A, Auvinet G, et al. 2011. A deletion in exon 9 of the LIPH gene is responsible for the rex hair coat phenotype in rabbits (Oryctolagus cuniculus). PLOS ONE 6:e19281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Inoue A, Arima N, Ishiguro J, Prestwich GD, Arai H, Aoki J. 2011. LPA-producing enzyme PA-PLA1α regulates hair follicle development by modulating EGFR signalling. EMBO J. 30:4248–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Blaydon DC, Biancheri P, Di W-L, Plagnol V, Cabral RM, et al. 2011. Inflammatory skin and bowel disease linked to ADAM17 deletion. N. Engl. J. Med 365:1502–8 [DOI] [PubMed] [Google Scholar]
- 122.Engelbrecht E, Levesque MV, He L, Vanlandewijck M, Nitzsche A, et al. 2020. Sphingosine 1-phosphate-regulated transcriptomes in heterogenous arterial and lymphatic endothelium of the aorta. eLife 9:e52690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yanagida K, Engelbrecht E, Niaudet C, Jung B, Gaengel K, et al. 2020. Sphingosine 1-phosphate receptor signaling establishes AP-1 gradients to allow for retinal endothelial cell specialization. Dev. Cell 52:779–93.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jung B, Obinata H, Galvani S, Mendelson K, Ding BS, et al. 2012. Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Dev. Cell 23:600–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schumacher L 2019. Collective cell migration in development. Adv. Exp. Med. Biol 1146:105–16 [DOI] [PubMed] [Google Scholar]
- 126.Gudipaty SA, Rosenblatt J. 2017. Epithelial cell extrusion: pathways and pathologies. Semin. Cell Dev. Biol 67:132–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Duszyc K, Gomez GA, Lagendijk AK, Yau M-K, Nanavati BN, et al. 2021. Mechanotransduction activates RhoA in the neighbors of apoptotic epithelial cells to engage apical extrusion. Curr. Biol 31:1326–36.e5 [DOI] [PubMed] [Google Scholar]
- 128.Mendelson K, Pandey S, Hisano Y, Carellini F, Das BC, et al. 2017. The ceramide synthase 2b gene mediates genomic sensing and regulation of sphingosine levels during zebrafish embryogenesis. eLife 6:e21992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fukui H, Fukuhara S, Mochizuki N. 2016. S1P-S1p2 signaling in cardiac precursor cells migration. In Etiology and Morphogenesis of Congenital Heart Disease: From Gene Function and Cellular Interaction to Morphology, ed. Nakanishi T, Markwald RR, Baldwin HS, Keller BB, Srivastava D, Yamagishi H, pp. 125–26. Tokyo: Springer Japan; [PubMed] [Google Scholar]
- 130.Mendoza A, Fang V, Chen C, Serasinghe M, Verma A, et al. 2017. Lymphatic endothelial S1P promotes mitochondrial function and survival in naive T cells. Nature 546:158–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fransen MF, Schoonderwoerd M, Knopf P, Camps MG, Hawinkels LJ, et al. 2018. Tumor-draining lymph nodes are pivotal in PD-1/PD-L1 checkpoint therapy. JCI Insight 3:e124507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chakraborty P, Vaena SG, Thyagarajan K, Chatterjee S, Al-Khami A, et al. 2019. Pro-survival lipid sphingosine-1-phosphate metabolically programs T cells to limit anti-tumor activity. Cell Rep. 28:1879–93.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Onishi H, Kiyota A, Koya N, Tanaka H, Umebayashi M, et al. 2014. Random migration contributes to cytotoxicity of activated CD8+ T-lymphocytes but not NK cells. Anticancer Res. 34:3947–56 [PubMed] [Google Scholar]
- 134.Rathinasamy A, Domschke C, Ge Y, Bohm HH, Dettling S, et al. 2017. Tumor specific regulatory T cells in the bone marrow of breast cancer patients selectively upregulate the emigration receptor S1P1. Cancer Immunol. Immunother 66:593–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chongsathidkiet P, Jackson C, Koyama S, Loebel F, Cui X, et al. 2018. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat. Med 24:1459–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Masuda A, Fujii T, Iwasawa Y, Nakamura K, Ohkawa R, et al. 2011. Serum autotaxin measurements in pregnant women: application for the differentiation of normal pregnancy and pregnancy-induced hypertension. Clin. Chim. Acta 412:1944–50 [DOI] [PubMed] [Google Scholar]
- 137.Tokumura A, Kanaya Y, Miyake M, Yamano S, Irahara M, Fukuzawa K. 2002. Increased production of bioactive lysophosphatidic acid by serum lysophospholipase D in human pregnancy. Biol. Reprod 67:1386–92 [DOI] [PubMed] [Google Scholar]
- 138.Ikeda H, Yatomi Y. 2012. Autotaxin in liver fibrosis. Clin. Chim. Acta 413:1817–21 [DOI] [PubMed] [Google Scholar]
- 139.Masuda A, Nakamura K, Izutsu K, Igarashi K, Ohkawa R, et al. 2008. Serum autotaxin measurement in haematological malignancies: a promising marker for follicular lymphoma. Br. J. Haematol 143:60–70 [DOI] [PubMed] [Google Scholar]
- 140.Shao X, Uojima H, Setsu T, Okubo T, Atsukawa M, et al. 2020. Usefulness of autotaxin for the complications of liver cirrhosis. World J. Gastroenterol 26:97–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jiang G, Inoue A, Aoki J, Prestwich GD. 2013. Phosphorothioate analogs of sn-2 radyl lysophosphatidic acid (LPA): metabolically stabilized LPA receptor agonists. Bioorg. Med. Chem. Lett 23:1865–69 [DOI] [PubMed] [Google Scholar]
- 142.Kano K, Arima N, Ohgami M, Aoki J. 2008. LPA and its analogs-attractive tools for elucidation of LPA biology and drug development. Curr. Med. Chem 15:2122–31 [DOI] [PubMed] [Google Scholar]
- 143.Kuo B, Szabó E, Lee SC, Balogh A, Norman D, et al. 2018. The LPA2 receptor agonist Radioprotectin-1 spares Lgr5-positive intestinal stem cells from radiation injury in murine enteroids. Cell Signal. 51:23–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Parrill AL. 2014. Design of anticancer lysophosphatidic acid agonists and antagonists. Future Med. Chem 6:871–83 [DOI] [PubMed] [Google Scholar]
- 145.Matralis AN, Afantitis A, Aidinis V. 2019. Development and therapeutic potential of autotaxin small molecule inhibitors: from bench to advanced clinical trials. Med. Res. Rev 39:976–1013 [DOI] [PubMed] [Google Scholar]
- 146.Salgado-Polo F, Perrakis A. 2019. The structural binding mode of the four autotaxin inhibitor types that differentially affect catalytic and non-catalytic functions. Cancers 11:1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nitzsche A, Poittevin M, Benarab A, Bonnin P, Faraco G, et al. 2021. Endothelial S1P1 signaling counteracts infarct expansion in ischemic stroke. Circ. Res 128(3):363–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kittaka H, Uchida K, Fukuta N, Tominaga M. 2017. Lysophosphatidic acid-induced itch is mediated by signalling of LPA5 receptor, phospholipase D and TRPA1/TRPV1. J. Physiol 595:2681–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.McIntyre TM, Pontsler AV, Silva AR, St Hilaire A, Xu Y, et al. 2003. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARγ agonist. PNAS 100:131–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fang V, Chaluvadi VS, Ramos-Perez WD, Mendoza A, Baeyens A, et al. 2017. Gradients of the signaling lipid S1P in lymph nodes position natural killer cells and regulate their interferon-γ response. Nat. Immunol 18:15–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ramos-Perez WD, Fang V, Escalante-Alcalde D, Cammer M, Schwab SR. 2015. A map of the distribution of sphingosine 1-phosphate in the spleen. Nat. Immunol 16:1245–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sarkisyan G, Gay LJ, Nguyen N, Felding BH, Rosen H. 2014. Host endothelial S1PR1 regulation of vascular permeability modulates tumor growth. Am. J. Physiol. Cell Physiol 307:C14–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kono M, Conlon EG, Lux SY, Yanagida K, Hla T, Proia RL. 2017. Bioluminescence imaging of G protein-coupled receptor activation in living mice. Nat. Commun 8:1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kono M, Tucker AE, Tran J, Bergner JB, Turner EM, Proia RL. 2014. Sphingosine-1-phosphate receptor 1 reporter mice reveal receptor activation sites in vivo. J. Clin. Investig 124:2076–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nitzsche A, Poittevin M, Benarab A, Bonnin P, Faraco G, et al. 2021. Endothelial S1P1 signaling counteracts infarct expansion in ischemic stroke. Circ. Res 128:363–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang J, Kano K, Saigusa D, Aoki J. 2019. Measurement of the spatial distribution of S1P in small quantities of tissues: development and application of a highly sensitive LC-MS/MS method combined with laser microdissection. Mass Spectrom. 8:A0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Iwama T, Kano K, Saigusa D, Ekroos K, van Echten-Deckert G, et al. 2021. Development of an on-tissue derivatization method for MALDI mass spectrometry imaging of bioactive lipids containing phosphate monoester using Phos-tag. Anal. Chem 93:3867–75 [DOI] [PubMed] [Google Scholar]


