Abstract
Clinical pharmacist-driven antimicrobial stewardship programs (ASPs) have been successfully implemented. Although relevant guidance and several studies suggest that clinical pharmacists be integrated into the current ASP team model, barriers still exist in Asia, primarily due to lack of dedicated personnel and lack of career advancement. We review the effectiveness and the ideal role of clinical pharmacist among ASPs in Asia. Several studies conducted in Asia have shown the effectiveness of pharmacist-led ASP interventions in hospitals and other healthcare settings. However, opportunities to expand the role of clinical pharmacists in ASPs in Asia exist in the implementation of rapid diagnostic test and drug allergies.
Infections caused by multidrug-resistant (MDR) pathogens can lead to high morbidity and mortality among hospitalized patients. In 2019, the global mortality associated with MDR pathogens was estimated to be ∼4.95 million deaths and the most common drug-resistant pathogens that led to death were Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Streptococcus pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa. 1 Carbapenem-resistant gram-negative bacteria, such as A. baumannii and Enterobacterales, are of grave concern in the United States as well as the European continent and the Asia-Pacific region. 2–4 In the era of MDR pathogens, the availability of active antimicrobial agents has become limited. Therefore, to combat these MDR pathogens, several strategies must be incorporated such as improved infection prevention and control, implementation of antimicrobial stewardship (ASP), active surveillance, and development of new antimicrobial agents. In well-developed countries like the United States, it is well recognized that the ASP team should consist of multidisciplinary healthcare personnel (eg, infectious diseases physician, clinical pharmacist, infection control nurse, and clinical microbiologist) who can influence appropriate antimicrobial prescriptions. Integration of the pharmacist into the ASP team has been shown to increase appropriateness of antimicrobial prescriptions and reduction in antimicrobial consumption, hospital antimicrobial expenditure, and in hospital length of stay. 5–7
Guidelines from the Infectious Diseases Society of America (IDSA), the American Society of Health-System Pharmacist (ASHP), and a consensus statement on ASP from Asia recommended that a pharmacist should be a coleader of the ASP. 8–10 The clinical pharmacist member of the ASP team should have some training in infectious disease. Clinical pharmacists are core members of the ASP team and have an important role in ensuring appropriate antimicrobial prescriptions. According to an ASHP statement, a pharmacist involved in the ASP has responsibilities to promote optimal antimicrobial use, to reduce the transmission of infections, and to educated other healthcare professionals, patients, and the public. 8 The role of the ASP clinical pharmacist includes developing local guidance and formulary choices with the physician coleader, recommending switching from intravenous to oral formulations, identifying de-escalation opportunities, optimizing antimicrobial dose, monitoring drug–drug and drug–food interactions, and monitoring the outcomes of antimicrobial usage (eg, clinical outcomes and antimicrobial consumption). In addition, clinical pharmacists can help educate healthcare personnel on the appropriate use of antimicrobials as part of the processes of patient care. 11 A study in the United States showed that an infectious-diseases–trained pharmacist on the ASP team is associated with reduced antimicrobial consumption, reduced mortality associated with sepsis and respiratory tract infections, and reduced antimicrobial costs. 12 However, barriers to the integration of the clinical pharmacist into the ASP team in general, include the lack of financial support for dedicated personnel, lack of time, lack of career promotion for the pharmacist, and lack of knowledge and experience. Therefore, continued recommendations for clinical pharmacist engagement in the ASP team is essential. We reviewed the literature on the role and effectiveness of the clinical pharmacist in ASP in Asia, with a primary focus on the adult inpatient setting. The relevant studies were identified by searching the PubMed, ScienceDirect, and EMBASE databases from their inception date through August 2022. In this review, included studies were limited to English-language publications. The search strings “pharmacist,” “asia OR bangladesh OR brunei OR bhutan OR darussalam OR cambodia OR china OR guam OR hong kong OR india OR indonesia OR japan OR korea OR lao people’s democratic republic OR macao OR malaysia OR mongolia OR myanmar OR nepal OR northern mariana islands OR palau OR papua new guinea OR philippines OR singapore OR taiwan OR thailand OR timor-leste OR vietnam,” and “antimicrobial stewardship OR antibiotic stewardship” were used to identify papers that met the inclusion criteria. Our outcomes of interest were clinical outcome, proportion of appropriate antibiotic prescription, antibiotic consumption, antibiotic resistance rates, and pharmacist’s rate of recommendation acceptance. We only included papers that mainly emphasized pharmacist-led antimicrobial stewardship interventions. Letters to the editor, editorials, commentaries, review articles, and conference abstracts were excluded. The title and abstracts were screened for eligibility, and data extraction was conduted by K.J. and A.A.
Role of the clinical pharmacist in the ASP team
Several studies from around the world have reported that clinical-pharmacist–led interventions have been successfully implemented. These interventions included performing a prospective audit and feedback, educating healthcare professionals, developing guidance for treatment a specific infection (eg, urinary tract infection and respiratory tract infection) and a specific pathogen (eg, Staphylococcus aureus and Clostridioides difficile), encouraging penicillin allergy delabeling, and facilitating real-time pathogen identification and feedback to the treating physician. 13–20 The infectious diseases (ID) pharmacist is the ideal role model for pharmacist-led ASP interventions. Various studies have supported the effectiveness of ID pharmacist-led ASP interventions. 12,13 However, most successful clinical-pharmacist–led interventions have been performed in high-income countries such as the United States, Canada, Australia, and Japan, as well as the European continent.
Evidence on the efficacy of pharmacist-driven ASPs in Asia
Several studies have reported the effectiveness of ID-pharmacist–driven ASPs in Asian countries such as Japan, Thailand, China, Korea, and India (Table 1). In Japan, ID pharmacist involvement in the ASP of a tertiary-care hospital increased the rate of appropriate blood-culture collection and de-escalation therapy (71% vs 85%; P < .001). 21 A community hospital ASP with clinical pharmacist involvement decreased the duration of antimicrobial treatment in uncomplicated gram-negative bacteremia (8 vs 14 days; P < .001) and resulted in an increase in de-escalation, as reported in the study by Nakamura et al (10.2% vs 30.8%; P < .05). 22,23 A clinical-pharmacist–led ASP intervention among patients with methicillin-resistant Staphylococcus aureus (MRSA) bacteremia led to an increase in targeted antimicrobial administration duration (at least 14 days for uncomplicated bacteremia and 28 days for complicated bacteremia; 44.8% vs 72.1%; P = .027) and early use of anti-MRSA drugs within 24 hours after MRSA was detected (62.3% vs 82.4%; P =.038). 24 A timeout intervention for vancomycin showed a decrease in weekly vancomycin days of therapy (DOT) per 1,000 patient day (coefficient, −0.49; P = .007) as well as a decrease antimicrobial usage in the pharmacist-led arm (coefficient, −0.77; P = .007) compared to an ID physician arm. 25 Finally, a study in Japan reported that clinical pharmacist involvement in an ASP team reduced the volume of antimicrobial prescriptions in a skilled nursing facility (incidence rate ratio [IRR], 0.885; P < .001) (Figure 1). 26
Table 1.
Summary of studies on ASP with clinical pharmacist involvement in Asia classified by countries
| Country | Type of study and population | Intervention | Primary outcomes | Secondary outcomes |
|---|---|---|---|---|
| Japan | ||||
| Uda A, et al. 2022 21 | Study design: Pre and post intervention Study period: 2017-2019 Setting: Tertiary-care hospital Participants: N/A |
ID Pharmacist perform a daily structured review of antibiotic prescriptions, Educating prescribers on antimicrobial therapy, Monthly reporting of department-level rates of blood sampling for culture Comparator: baseline period (May-Dec 2017) vs Intervention period (May-Dec 2018) and post-intervention period (May-Dec 2019 |
- Increased rate of appropriate blood culture collections and de-escalation therapy (71% vs 85%, P < .001) | - Decrease in antipseudomonal agent and carbapenem consumption (P = .016 and P = .004) - Decrease incidence of HA-CDI (P = .031) - Decrease 30-day mortality (P = .005) - Similar length of stay |
| Fukuda T, et al. 2021 22 | Study design: Retrospective cohort Study period: 2013-2015 Setting: uncomplicated gram-negative bacteremia patient, community hospital Participants: 66 |
Pharmacists perform an antimicrobial time-out at 72 hours after blood culture collection, optimized treatment based on the patient’s clinical response and test results Comparator: no pharmacist and no ID physician |
- Decrease duration of antimicrobial treatment (8 vs 14 days, P < .001) | - Higher trend of de-escalation rate (P = .08) - Similar clinical success and failure, recurrence, infectious diseases re-admission, Clostridioides difficile infection, and 30- and 60-day mortality rates |
| Nakamura S, et al. 2021 23 | Study design: Retrospective study Study period: 2018-2019 Setting: Community hospital Participants: 535 |
Pharmacists perform daily follow up, ASP team weekly round Comparator: preintervention period |
- Increase in rate of orders for blood culture (56.3% vs 73.3%, P < .01) - Increased rate of de-escalation (10.2% vs 30.8%, P < .05) - Decrease in piperacillin/tazobactam and carbapenems consumption (P < .01) |
- No different in 30-day mortality - Decrease 30-day recurrence rate (14.7% vs 7.5%, P < .05) - Acceptance rate of pharmacist’s intervention 94.1% |
| Ohashi K, et al. 2018 24 | Study design: Historical-control trial Study period: N/A Setting: MRSA bacteremia patient, Municipal hospital Participants: 94 |
Pharmacists received an alert of blood cultures positive for MRSA and immediate intervention according to the bundle Comparator: preintervention period |
- Increase in compliance rate with the appropriate duration of therapy (44.8% vs 72.1%, P = .027), early use of anti-MRSA drugs (62.3% vs 82.4%, P = .038), higher rate of negative follow-up blood cultures (40% vs 80%, P < .001) | - Decrease in 30-day mortality (41.8% vs 21.6%, P = .044) - Decrease in hospital mortality (58.1% vs 27.5%, P = .003) |
| Hasegawa S, et al. 2021 25 | Study design: Pre and post intervention, crossover trial Study period: 2018-2019 Setting: inpatient tertiary care hospital Participants: 587 patients prescribed IV vancomycin |
Time-out intervention between clinical pharmacist-led time-out arm and an ID physician-led time-out arm Comparator: pre-prescription authorization |
- Decrease in weekly vancomycin DOT per 1000 patient-day in phase 2 (coefficient −0.49, P = .007) - Decrease in antimicrobial usage in the pharmacist-led arm (coefficient −0.77, P = .007) |
- Higher proportion of vancomycin discontinuations within 72 hours in phase 2 - Similar mean vancomycin use, median length of stay, and in-hospital mortality |
| Takito S, et al. 2020 26 | Study design: pre and post intervention Study period: 2013-2017 Setting: Skilled nursing facility Participants: N/A |
Pharmacists provided prescription recommendations to physicians based on Gram stain results Comparator: preintervention period |
- Reduction in the slope of total number of all antimicrobials prescriptions per 100 residents per month (IRR 0.885, P < .001) | - Decrease in number of prescriptions for macrolides and fluoroquinolones per 100 residents per month (P = .154 and .753) - Similar number of prescriptions for cephalosporins per 100 residents per month |
| Thailand | ||||
| Apisarnthanarak A, et al. 2015 5 | Study design: Quasi-experimental, prospective, concurrent groups Study period: Jan 2012-Sep 2012 Setting: Medicine ward, tertiary-care hospital Participants: 574 |
Pharmacists perform PPAF Comparator: standard of care |
- Less likely to use antibiotic inappropriately (P < .001) | - More antibiotic de-escalation (P < .001) - less duration of antibiotic use < 7 days (P < .001) - Shorter hospital length of stay (P < .001) - No difference in mortality |
| Rattanaumpawan P, et al. 2018 6 | Study design: Randomized controlled trial Study period: Feb – Sep 2013 Setting: Medicine ward, tertiary-care hospital Participants: 1632 |
Pharmacists perform PPAF Comparator: ID clinicals fellow perform PPAF |
- Non-inferiority in clinical response rate (44.9% vs 39.7%, P = .20, difference 5.15%, 95% CI 2.69 – 12.98%) | - Similar in microbiological outcomes, antibiotic-associated complications, antibiotic consumption, antibiotic expenditure, and length of stay |
| Jantarathaneewat K, et al. 2021 27 | Study design: Quasi-experimental, prospective, concurrent groups Study period: Aug 2019 – April 2020 Setting: Febrile neutropenic patient, tertiary-care hospital Participants: 90 |
Pharmacist performed daily PPAF and education Comparator: standard ASP |
- Increase appropriateness of prescription (88.9% vs 51.1%, P < .001) | - Trend to lower meropenem, ceftazidime and cefepime similar 30-day ID mortality and length of stay - Acceptance rate of pharmacist’s recommendation 93.8% |
| Jantarathaneewat K, et al. 2022 13 | Study design: Quasi-experimental, prospective, concurrent groups Study period: 2019-2020 Setting: Medicine ward, tertiary-care hospital Participants: 400 |
ID pharmacist Daily prospective audit and feedback Comparator: standard ASP group |
- Guideline adherence was higher in intervention group (79% vs 56.6%, P < .001) | - Trend to decrease 30-day all-cause mortality (15.9% vs 1.5%, P = .344) - Trend towards improved Clinical cure rate (63.6% vs 56.1%, P = .127) - Decrease Carbapenems consumption (P =.042) - Decrease Incidence of multidrug resistant pathogens (P = .049) - Similar Length of stay (P = .085) |
| China | ||||
| Li Z, et al. 2017 30 | Study design: Prospective cohort study Study period: March – April 2014 Setting: Intensive care units, multicenter Participants: 577 |
Pharmacists in 4 ICUs perform daily ward round and communicate with physician when inappropriate are prescribed Control: other 4 ICUs without pharmacists’ involvement |
- Lower all-cause hospital mortality (19.3% vs 29.0%; P = .007) | - Reduce multidrug resistance (31.7% vs 23.8%, P = .037) - Less inappropriate third- and fourth-generation cephalosporin initiation (9.1% vs 15.2%, P = .031) - Shorter duration of mechanical ventilation (P = .184), length of stay in ICU (P = .227), and length of stay in hospital (P = .544) - Acceptance rate of pharmacist’s recommendation 71.9% |
| Du Y, et al. 2020 31 | Study design: Retrospective study Study period: 2016-2018 Setting: Gastroenterology ward, tertiary-care hospital Participants: 1763 |
Pharmacists perform daily ward rounds with physicians, regular review of medical orders, monthly indicator feedback, frequent physician training, and necessary patient education Comparator: pre intervention period |
- Decrease in intensity of antibiotic consumption (coefficient −0.88, P = .01) | - Decrease in proportion of patients receiving combined antibiotics (coefficient −9.91, P = .03) - Decrease in average length of hospital stay (coefficient −1.79, P = .00) - Temporary increase in patients receiving antibiotics (coefficient 4.95, P = .038) |
| Zhou Y, et al. 2015 32 | Study design: prospective study with historical controls Study period: 2010-2013 Setting: Department of urology, tertiary-care hospital Participants: 234 |
Pharmacists led the antibiotic stewardship program at the hospital and on the urological clinical service; performed prospective audits and feedback from 2011 to 2013 Comparator: no pharmacist involvement period (2010) |
- Decrease in antibiotic use density by 58.8% - Average antibiotic cost decreased by US $246.94 |
N/A |
| Xu J, et al. 2022 33 | Study design: Quasi-experimental, concurrent groups, retrospective Study period: 2018-2019 Setting: Department of Vascular and interventional radiology, Tertiary hospital Participants: 1026 |
Pharmacists perform Daily PPAF and guideline development Comparator: ASP team without pharmacist group |
- Average score of inappropriate antimicrobials decreases in intervention group: perioperative antimicrobial prophylaxis (coefficient −0.207, P < .001), Non-surgical antimicrobial prophylaxis (coefficient −0.164, P = .010), Therapeutic use of antibiotics (coefficient −0.0694, P = .003) | - Decreased antimicrobial consumption (P = .017) - Decreased antimicrobial cost (P = .006) - Decreased average cost per defined daily dose (P < .001) - Similar total cost of hospitalization (P = .476) and length of hospital stay (P = .375) - Acceptance rate of pharmacist’s recommendation 52.78% for non-surgical prophylaxis, 76.69% for surgical prophylaxis, 86.18% for treatment |
| Zhou H, et al. 2021 34 | Study design: pre-and postintervention study Study period: June 2018- March 2019 Setting: Orthopedics department in a tertiary-care hospital Participants: 873 |
Pharmacist-led ASP team participated in ward rounds, reconciled patient’s allergy history, education, and performed standard intradermal skin test with the perioperative antibiotic prophylaxis regimen Comparator: pre-intervention period |
- Decrease in the utilization of intradermal skin tests, from 95.8% to 16.5% (P < .001) - More cephalosporins used as prophylactic antimicrobial (P < .001) - Reduced antimicrobial expenditure by US $150.21 (P < .001) for each patient |
- postintervention population was less likely to undergo an intradermal skin test (OR: 0.008, 95% CI: 0.005–0.014) - Patients in postintervention group had a 5.3-fold higher likelihood (95% CI: 2.95–9.43) of having cephalosporin as prophylactic antimicrobials |
| Wang H, et al. 2019 35 | Study design: Retrospective study Study period: July 2010- Dec 2016 Setting: Tertiary-care hospital Participants: patients with outpatient prescription (17,766,637) and inpatient prescriptions (376,627) |
ASP interventions led by pharmacists such as formulating the activity program and performance management, advising on antibacterial prescriptions and training Comparator: baseline period |
- Decreased in the number of antibiotic prescriptions in the inpatient setting by 59% (P < .05) and the outpatient setting by 33% (P < .05) each month - Decreased number of of antibiotic prophylaxis by 5.71% (P < .001) each month - Decrease DDD from 102.46 to 37.38 DDD/100 bed-days (P < .05) - Significant decrease in resistance rates among E. coli and P. aeruginosa isolates to fluoroquinolones - Significant decrease in the incidence of MRSA - Significant increase in resistance rates of E. coli and K. pneumoniae to carbapenems |
- Increase rational timing of initial dose and rational duration |
| Zhang J, et al. 2020 36 | Study design: prospective, multicenter cohort study Study period: April 2017- Dec 2019 Setting: 17 acute care hospitals across Guizhou Province Participants: 2663 with confirmed infections |
Pharmacist conducted a chart review and provided recommendation to clinician | - More effective clinical response was observed in patients whose provider accepted the ASP recommendation intervention group (81.34% vs 67.16%, P < .001) | - non-ID pharmacist showed similar effective clinical response to ID pharmacist (P = .896) - Acceptance rate of pharmacists ‘recommendation was 5.0% |
| Xu S, et al. 2021 37 | Study design: retrospective, observational pre-and post-intervention study Study period: 2018-2020 Setting: tertiary-care hospital Participants: 524 patients with community-acquired pneumonia eligible for IV to PO conversion |
Prescribers were contacted by pharmacists about patients who were eligible for IV to oral antibiotic switches through computer- generated messages (phase 2) Comparator: prescribers were contacted bypharmacists who verbally informed them of patients who were eligible for an IV to oral conversions (phase 1) | - Increased proportion of patients who were converted to oral therapy on the day they were eligible from 34.8% in phase 1 to 62.7% in phase 2 (P < .05) | - Shorter lengths of IV antibiotic therapy days and hospital stay (P < .05) - Similar total length of antibiotic therapy day (P > .05) |
| Korea | ||||
| Song JY, et al. 2015 38 | Study design: pre and post intervention Study period: 2013 Setting: patient who received double anti-aerobic activity, Tertiary-care hospital Participants: 313 |
Pharmacists perform education and PPAF along with ID physician Comparator: preintervention period |
- Decrease number of patients receiving unnecessary double anti-aerobic activity more than 3 days (26.8 vs 7, P = .005) - Decrease proportion of patients receiving unnecessary double anti-aerobic activity more than 3 days (42.3% vs 13.6%, P < .001) |
- Acceptance rate of pharmacist’s recommendation 93.9% |
| Suh Y, et al. 2021 39 | Study design: retrospective study Study period: Jan-March 2017 Setting: multicenter, Tertiary hospital Participants: 4995 |
ASP with pharmacist involvement who intervene in antimicrobial prescription, perform TDM and monitored antimicrobial-related adverse drug event Comparator: ASP without pharmacist involvement |
- Less incidence proportion of antimicrobial-related adverse event (8.9% vs 14.7%, P < .001) | - multidisciplinary ASPs including clinical pharmacists reduced the risk of antimicrobial-related ADEs by 38% (adjusted odds ratio 0.62; 95% CI 0.50–0.77) |
| India | ||||
| Nampoothiri V, et al. 2021 40 | Study design: Descriptive study Study period: 2016-2017 Setting: Tertiary care hospital Participants: 1326 |
Pharmacists perform daily PPAF Comparator: baseline period |
- Increase appropriateness to 80% in the third year of intervention period - Decrease in antimicrobial consumption |
- Decrease in antimicrobial consumption - Acceptance rate of pharmacist’s recommendation 70% |
DDD, defined daily dose; HA-CDI, hospital-acquired Clostridioides difficile infection; ID, infectious diseases; IRR, incidence rate ratios; IV-PO conversion, intravenous to oral antibiotic conversion ; MRSA, Methicillin-resistant Staphylococcus aureus; TDM, therapeutic drug monitoring; PPAF, prospective audits and feedback.
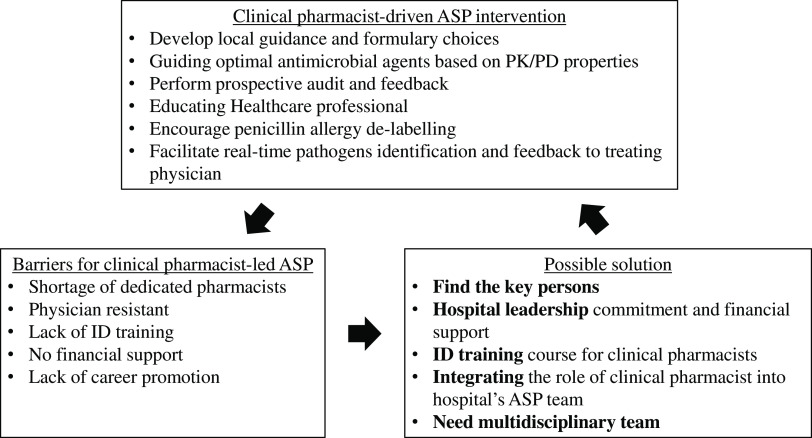
Fig. 1.
Barriers and solutions for clinical pharmacist-driven ASP intervention in Asia.
Several clinical-pharmacist–led ASP studies have been conducted in Thailand. A randomized controlled trial in Thailand found that an ASP clinical-pharmacist–led prospective audit and feedback was not inferior to that of an ID fellow in terms of clinical response (difference, 5.15%; 95% CI, 2.69%–12.98%). This finding confirms the concept that a clinical pharmacist can be an alternative to a physician-led ASP implementation strategy. 6 This research was followed by a study conducted in a medicine ward at a tertiary-care hospital that showed that an ID-pharmacist–led ASP intervention, especially in conjunction with an ID physician, can further increase guideline adherence (79% vs 56.6%; P < .001), as well as decrease carbapenem consumption (mean defined daily dose [DDD], 191.94 vs 256.14; P = .042). 5,13 In the specific setting of febrile neutropenic patients, a clinical-pharmacist–led ASP intervention was associated with more appropriate prescriptions (88.9% vs 51.1%; P < .001) without affecting the mortality rate. 27 Finally, clinical pharmacist involvement in the multidisciplinary team support improved adherence to a vancomycin dosing protocol (90.8% vs 55%; P < .001), and the clinical pharmacist can help the ASP team implement antibiotic heterogeneity through periodic antibiotic monitoring and supervision strategy in both medicine and surgical departments in Japan and Thailand. 28,29
In China, a multicenter study conducted in 8 intensive care units (ICUs) at 4 university hospitals resulted in a lower all-cause hospital mortality in 4 ICUs where clinical pharmacists were involved (19.3% vs 29%; P = .007). 30 But this study had limitations including a short study period, potential selection bias of the clinical cohort, and a small sample size. 30 Studies conducted in a gastroenterology ward and a urology ward, respectively, reported reductions in antimicrobial consumption during the respective study periods in which clinical pharmacists were involved (coefficient, −0.88; P = .01). 31,32 A quasi-experimental study on the efficacy of an ID pharmacist with a concurrent control group in the department of vascular and interventional radiology reported a decrease in inappropriate prescriptions, particularly in perioperative antimicrobial prophylaxis (coefficient, −0.207; P < .001), nonsurgical antimicrobial prophylaxis (coefficient, −0.164; P = .010), and therapeutic use of antimicrobials (coefficient, −0.069; P = .003). 33 In an orthopedic department, a pharmacist-led intervention was performed on perioperative antibiotic prophylaxis by standardizing the cephalosporin intradermal skin test. 34 This intervention achieved a reduction the utilization of intradermal skin tests from 95.8% to 16.5% (P < .001) and reduced the cost of antimicrobials by $150.21 (P < .001) for each patient. 34 A pharmacist-led ASP, hospital-wide intervention in China improved antibiotic consumption and achieved a corresponding decrease in the incidence of drug-resistant gram-negative bacteria. 35 A multicenter study also showed that adherence to clinical pharmacist’s recommendations improved clinical response compared with rejection of the clinical pharmacist’s recommendation. 36 Among patients with community-acquired pneumonia, a pharmacist-led intervention converting intravenous to oral administration of antibiotic integrated with computer decision support led to an increase in the proportion of patients who were converted to oral therapy from 34.8% to 62.7% (P < .05) and shortened the lengths of intravenous antibiotic therapy and hospital stay (P < .05). 37
In South Korea, a clinical pharmacist-led intervention that focused on patients receiving antimicrobials with redundant activity. 38 The ID-pharmacist–led intervention achieved a decrease in the number (26.8 vs 7; P = .005) and proportion (42.3% vs 13.6%; P < .001) of patients receiving unnecessary antimicrobials for >3 days. 38 Another retrospective study in South Korea reported that an ASP with clinical pharmacist involvement was associated with a decrease in the incidence of antimicrobial-related adverse events (8.9% vs 14.7%; P < .001). 39 Finally, similar to previous studies, a study on clinical pharmacist involvement in the ASP in India showed an increase in the proportion of appropriate prescriptions during the third year of the intervention period. 40
Similar to those reported in Western developed countries like the United States, the acceptance rates of clinical pharmacist recommendations in Asia range from 70% to 94%. In high-income Asian countries, such as Japan and South Korea, the acceptance rates of pharmacist recommendations tend to be higher (∼90%), whereas in middle-income countries, such as China and India, tend to report lower acceptance rates (70%–90%). The most effective interventions feature daily prospective audit and feedback by the clinical pharmacist along with the ID physician. 41 The relevant outcomes in Asia include adherence to the intervention or bundle, appropriateness of antimicrobial use, antimicrobial consumption, and clinical outcomes such as clinical improvement and mortality. An ASP that includes clinical pharmacists can increase the appropriateness of antimicrobials prescribed and decrease unnecessary antimicrobial use. However, rare reports of the impact of intervention on the development of antimicrobial resistance have emerged, and they may indicate the need to integrate effective infection prevention. The other relevant outcomes, such as cost-effectiveness of clinical pharmacist involvement in the ASP team, should be the subject of future research. Clinical-pharmacist–led ASP interventions in other settings such as ambulatory care, emergency room, high dependency unit, pediatrics, solid-organ transplant unit, patients with cytomegalovirus infection, and patients with invasive candidiasis have been conducted in the United States, Canada, and Europe. 42–48 The addition of delabeling penicillin allergies through penicillin skin testing by the clinical pharmacist to current ASP interventions has effectively reduced antimicrobial-therapy–related costs, clarification of allergies, and optimized antimicrobial therapy. 49,50 Additionally, during the COVID-19 pandemic, ASP interventions were essential to optimizing antimicrobial use when rates of bacterial coinfection were relatively low. 51 In Canada, a clinical trial of a clinical pharmacist-led ASP team on COVID-19 patients is ongoing. 52 Interventions on COVID-19 patients have not been well recognized in Asia; thus, longer follow-up periods with larger sample sizes are needed.
A study in the United States showed that the use of procalcitonin levels to guide interventions by clinical pharmacists led to discontinuation of vancomycin therapy in patients with lower respiratory tract infections and normal procalcitonin levels. 53 Rapid identification of gram-positive bacteria through diagnostic systems like the VITEK-2 automated system, matrix-assisted laser desorption ionization–time-of-flight mass spectrometry (MALDI-TOF MS), and rapid polymerase chain reaction (PCR) with real-time ASP interventions by a clinical pharmacist have been implemented successfully in the United States. 19,20,54,55 Identification of antimicrobial susceptibility using Verigene along with ASP guidance can improve time to initiation of appropriate antimicrobials as well as reduce mortality rates and 30-day readmission rates. 55 Therefore, clinical pharmacist-led ASP interventions should be integrated with rapid diagnostic test.
Barriers and possible solutions
In Asia, some barriers to the implementation of the ID pharmacist-led ASP remain. These include the lack of dedicated personnel, a labor-intensive pharmacist workload, lack of time, lack of knowledge and perception toward ASP by clinicians, shortage of clinical pharmacists with advanced training in infectious diseases, and the lack of integration of the pharmacists’ role with other healthcare professionals when delivering care. 13 A survey in Malaysia revealed that administration support, commitment from leadership, and perseverance are essential to facilitate the role of clinical pharmacists in the ASP. 56 Another survey in South Korea revealed that ASPs were limited only to top-tier general hospitals. The study concluded that a clinical pharmacist is essential to successful implementation of the ASP along with adequate financial reimbursement for the professionals who perform and maintain the ASP. 57 Data from the survey of gaps and opportunities in ASPs in Asia revealed that 80% of respondents had a pharmacist working on ASP activities; however, almost half of the respondents reported no financial support such as salary support, training, or information technology services at their hospitals. 58 Furthermore, a survey conducted in the Asia-Pacific region revealed that 41% of hospitals had no trained ID pharmacists even though most respondents worked in large hospitals. 59 A nationwide survey from the United States revealed that pharmacists with formal ASP responsibilities dedicated 0.6 full-time equivalents (FTE), whereas pharmacists without formal ASP responsibilities spent an average of 0.125 FTE on ASP activities, even though the average program should have 1 FTE allocated for an ASP pharmacist. 60 The lack of data on clinical-pharmacist FTE among ASPs in Asia may be a barrier to implementing effective ASPs in this region.
To successfully encourage pharmacist involvement in their respective ASPs, each institution should identify its own gaps and challenges. 9 Prioritizing formal support and approval from hospital leadership and making a case for the return on investment of clinical pharmacist involvement in ASP activities are essential. 9 Illustrating to administrators that the role of the clinical pharmacist in the ASP team is that of an expert in antimicrobial delivery can potentially lead to a modification in their role in routine activities in the hospital pharmacy. A full-time antimicrobial stewardship pharmacist has been well recognized as a viable advanced career path for clinical pharmacists in the United States, Canada, and Europe.
In Asia, especially in developing countries, most ID-trained clinical pharmacists perform their ASP activities along with their routine work without additional renumeration. Without financial support from hospital administration, the ID-trained clinical pharmacist will not be able to complete their tasks on the ASP team. A possible solution is to require administrative support before hospitals are granted accreditation. The pharmacy council should promote the clinical pharmacist’s role in the ASP team and support the continuing education for pharmacist who are interested in or work for the ASP team. Furthermore, the role of the clinical pharmacist on the ASP team should be integrated into the curricula of doctor of pharmacy programs to encourage participation of pharmacists in the multidisciplinary ASP team. A recent study identified a trend toward increased clinical pharmacist involvement in ASP teams in Japan. These researchers also recommended that policy makers and stakeholders promote and support the evidence-based activities of clinical-pharmacist–led ASPs for small to medium-sized hospitals. 61
This review had several limitations. It was difficult to determine which factors were important to the success of the ASP because each study involved a multifaceted intervention and different outcomes were measured. Our review is mostly applicable to the adult inpatient population rather than other settings such as the outpatient setting and pediatric population. Publication bias may exist, especially in the middle- and low-income countries. The standardized definitions for processes and outcome measures in ASP is necessary for future studies. Future studies that stratify interventions based on the income level of the country would provide more insight and would improve the homogeneity of the data. The role of clinical pharmacist in ASPs in Asia is limited to the inpatient setting. Further studies on other settings are needed before any recommendations can be made on pediatric and ambulatory settings.
According to previous studies, clinical pharmacist involvement in the ASP team can be implemented successfully in Asia. However, some barriers to widespread implementation of clinical-pharmacist–driven ASPs remain. The approval of and financial support from hospital leadership are important steps in initiating an ASP strategy. Integrating a clinical pharmacist’s role in an ASP team as a policy and financial support along with career advancement for the clinical pharmacist will encourage the proliferation and acceptance of clinical-pharmacist–driven ASPs. Training in infectious diseases is essential for clinical pharmacists on ASP teams. Moreover, the role of clinical pharmacist can be expanded if ASPs are implemented in other areas such as ambulatory care, the emergency departments, and units housing immunocompromised patients. Integrated rapid diagnostic methods and real-time ASP interventions are attractive strategies that will expand the clinical pharmacy service.
Acknowledgments
Financial support
No financial support was provided relevant to this article.
Conflicts of interest
All authors report no conflicts of interest relevant to this article.
References
- 1.Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 2022;399:629–655. [DOI] [PMC free article] [PubMed]
- 2. US Department of Health and Human Services. Antibiotic resistance threats in the United States 2019. Centers for Disease Control and Prevention website. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. Published 2019. Accessed October 7, 2022.
- 3. Antimicrobial resistance surveillance in Europe 2020–2022. European Centre for Disease Control and Prevention website. https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-surveillance-europe-2022-2020-data. Published January 26, 2022. Accessed October 7, 2022.
- 4. Yam ELY, Hsu LY, Yap EP, et al. Antimicrobial resistance in the Asia Pacific region: a meeting report. Antimicrob Resist Infect Control 2019;8:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Apisarnthanarak A, Lapcharoen P, Vanichkul P, Srisaeng-Ngoen T, Mundy LM. Design and analysis of a pharmacist-enhanced antimicrobial stewardship program in Thailand. Am J Infect Control 2015;43:956–959. [DOI] [PubMed] [Google Scholar]
- 6. Rattanaumpawan P, Upapan P, Thamlikitkul V. A noninferiority cluster-randomized controlled trial on antibiotic postprescription review and authorization by trained general pharmacists and infectious disease clinical fellows. Infect Control Hosp Epidemiol 2018;39:1154–1162. [DOI] [PubMed] [Google Scholar]
- 7. Saha SK, Hawes L, Mazza D. Effectiveness of interventions involving pharmacists on antibiotic prescribing by general practitioners: a systematic review and meta-analysis. J Antimicrob Chemother 2019;74:1173–1181. [DOI] [PubMed] [Google Scholar]
- 8. ASHP statement on the pharmacist’s role in antimicrobial stewardship and infection prevention and control. Am J Health Syst Pharm 2010;67:575–577. [DOI] [PubMed] [Google Scholar]
- 9. Apisarnthanarak A, Kwa AL, Chiu CH, et al. Antimicrobial stewardship for acute-care hospitals: an Asian perspective. Infect Control Hosp Epidemiol 2018;39:1237–1245. [DOI] [PubMed] [Google Scholar]
- 10. Barlam TF, Cosgrove SE, Abbo LM, et al. Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin Infect Dis 2016;62:e51–e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garau J, Bassetti M. Role of pharmacists in antimicrobial stewardship programmes. Int J Clin Pharm 2018;40:948–952. [DOI] [PubMed] [Google Scholar]
- 12. Yu K, Rho J, Morcos M, et al. Evaluation of dedicated infectious diseases pharmacists on antimicrobial stewardship teams. Am J Health Syst Pharm 2014;71:1019–1028. [DOI] [PubMed] [Google Scholar]
- 13. Jantarathaneewat K, Montakantikul P, Weber DJ, Nanthapisal S, Rutjanawech S, Apisarnthanarak A. Impact of an infectious diseases pharmacist-led intervention on antimicrobial stewardship program guideline adherence at a Thai medical center. Am J Health Syst Pharm 2022;79:1266–1272. [DOI] [PubMed] [Google Scholar]
- 14. Saleh D, Abu Farha R, Alefishat E. Impact of educational intervention to promote jordanian community pharmacists’ knowledge and perception towards antimicrobial stewardship: pre–post interventional study. Infect Drug Resist 2021;14:3019–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCormick JZ, Cardwell SM, Wheelock C, Wong CM, Vander Weide LA. Impact of ambulatory antimicrobial stewardship on prescribing patterns for urinary tract infections. J Clin Pharm Ther 2020;45:1312–1319. [DOI] [PubMed] [Google Scholar]
- 16. Devchand M, Kirkpatrick CMJ, Stevenson W, et al. Evaluation of a pharmacist-led penicillin allergy de-labelling ward round: a novel antimicrobial stewardship intervention. J Antimicrob Chemother 2019;74:1725–1730. [DOI] [PubMed] [Google Scholar]
- 17. Arensman K, Dela-Pena J, Miller JL, et al. Impact of mandatory infectious diseases consultation and real-time antimicrobial stewardship pharmacist intervention on Staphylococcus aureus bacteremia bundle adherence. Open Forum Infect Dis 2020;7:ofaa184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bishop PA, Isache C, McCarter YS, Smotherman C, Gautam S, Jankowski CA. Clinical impact of a pharmacist-led antimicrobial stewardship initiative evaluating patients with Clostridioides difficile colitis. J Investig Med 2020;68:888–892. [DOI] [PubMed] [Google Scholar]
- 19. Wong JR, Bauer KA, Mangino JE, Goff DA. Antimicrobial stewardship pharmacist interventions for coagulase-negative staphylococci positive blood cultures using rapid polymerase chain reaction. Ann Pharmacother 2012;46:1484–1490. [DOI] [PubMed] [Google Scholar]
- 20. Heyerly A, Jones R, Bokhart G, Shoaff M, Fisher D. Implementation of a pharmacist-directed antimicrobial stewardship protocol utilizing rapid diagnostic testing. Hosp Pharm 2016;51:815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Uda A, Ebisawa K, Sakon H, et al. Sustained improvements in antimicrobial therapy and clinical outcomes following a pharmacist-led antimicrobial stewardship intervention: uncontrolled before–after study. J Clin Med 2022;11:566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fukuda T, Tanuma K, Iio S, Saito J, Komura M, Yamatani A. Impact of a pharmacist-led antimicrobial stewardship program on the number of days of antimicrobial therapy for uncomplicated gram-negative bacteremia in a community hospital. Cureus 2021;13:e14635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nakamura S, Arima T, Tashiro R, et al. Impact of an antimicrobial stewardship in a 126-bed community hospital with close communication between pharmacists working on post-prescription audit, ward pharmacists, and the antimicrobial stewardship team. J Pharm Health Care Sci 2021;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ohashi K, Matsuoka T, Shinoda Y, et al. Evaluation of treatment outcomes of patients with MRSA bacteremia following antimicrobial stewardship programs with pharmacist intervention. Int J Clin Pract 2018;72:e13065. [DOI] [PubMed] [Google Scholar]
- 25. Hasegawa S, Tagashira Y, Murakami S, et al. Antimicrobial time-out for vancomycin by infectious disease physicians versus clinical pharmacists: a before–after crossover trial. Open Forum Infect Dis 2021;8:ofab125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Takito S, Kusama Y, Fukuda H, Kutsuna S. Pharmacist-supported antimicrobial stewardship in a retirement home. J Infect Chemother 2020;26:858–861. [DOI] [PubMed] [Google Scholar]
- 27. Jantarathaneewat K, Apisarnthanarak A, Limvorapitak W, Weber DJ, Montakantikul P. Pharmacist-driven antibiotic stewardship program in febrile neutropenic patients: a single-site prospective study in Thailand. Antibiotics (Basel) 2021;10:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Katawethiwong P, Apisarnthanarak A, Jantarathaneewat K, Weber DJ, Warren DK, Suwantarat N. Effectiveness of a vancomycin dosing protocol guided by area under the concentration-time curve to minimal inhibitory concentration (AUC/MIC) with multidisciplinary team support to improve hospital-wide adherence to a vancomycin dosing protocol: a pilot study. Infect Control Hosp Epidemiol 2022;43:1043–1048. [DOI] [PubMed] [Google Scholar]
- 29. Chaononghin S, Jantarathaneewat K, Weber DJ, Warren DK, Apisarnthanarak A. Impact of antibiotic heterogeneity by periodic antibiotic monitoring and supervision strategy at two units with different prevalences of multidrug-resistant organisms. Infect Control Hosp Epidemiol 2021. doi:10.1017/ice.2021.231. [DOI] [PubMed]
- 30. Li Z, Cheng B, Zhang K, et al. Pharmacist-driven antimicrobial stewardship in intensive care units in East China: a multicenter prospective cohort study. Am J Infect Control 2017;45:983–989. [DOI] [PubMed] [Google Scholar]
- 31. Du Y, Li J, Wang X, et al. Impact of a multifaceted pharmacist-led intervention on antimicrobial stewardship in a gastroenterology ward: a segmented regression analysis. Front Pharmacol 2020;11:442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhou Y, Ma LY, Zhao X, Tian SH, Sun LY, Cui YM. Impact of pharmacist intervention on antibiotic use and prophylactic antibiotic use in urology clean operations. J Clin Pharm Ther 2015;40:404–408. [DOI] [PubMed] [Google Scholar]
- 33. Xu J, Huang J, Yu Y, et al. The impact of a multifaceted pharmacist-led antimicrobial stewardship program on antibiotic use: evidence from a quasi-experimental study in the department of vascular and interventional radiology in a Chinese tertiary hospital. Front Pharmacol 2022;13:832078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou H, Liu L, Sun X, et al. The impact of pharmacist intervention on prophylactic antibiotics use in orthopedic surgery at a hospital in China. Medicine (Baltimore) 2021;100:e28458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Infectious Diseases Society of America (IDSA) position statement: why IDSA did not endorse the Surviving Sepsis campaign guidelines. Clin Infect Dis 2018;66:1631–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang J, Li X, He R, et al. The effectiveness of clinical pharmacist-led consultation in the treatment of infectious diseases: a prospective, multicenter, cohort study. Front Pharmacol 2020;11:575022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu S, Wang X, Song Z, Han F, Zhang C. Impact and barriers of a pharmacist-led practice with computerized reminders on intravenous to oral antibiotic conversion for community-acquired pneumonia inpatients. J Clin Pharm Ther 2021;46:1055–1061. [DOI] [PubMed] [Google Scholar]
- 38. Song YJ, Kim M, Huh S, et al. Impact of an antimicrobial stewardship program on unnecessary double anaerobic coverage prescription. Infect Chemother 2015;47:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Suh Y, Ah YM, Chun HJ, et al. Potential impact of the involvement of clinical pharmacists in antimicrobial stewardship programs on the incidence of antimicrobial-related adverse events in hospitalized patients: a multicenter retrospective study. Antibiotics (Basel) 2021;10:853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nampoothiri V, Sudhir AS, Joseph MV, et al. Mapping the implementation of a clinical pharmacist-driven antimicrobial stewardship programme at a tertiary-care centre in South India. Antibiotics (Basel) 2021;10(2):220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Molloy L, McGrath E, Thomas R, Kaye KS, Rybak MJ. Acceptance of pharmacist-driven antimicrobial stewardship recommendations with differing levels of physician involvement in a children’s hospital. Clin Pediatr (Phila) 2017;56:744–751. [DOI] [PubMed] [Google Scholar]
- 42. Westerhof LR, Dumkow LE, Hanrahan TL, McPharlin SV, Egwuatu NE. Outcomes of an ambulatory care pharmacist-led antimicrobial stewardship program within a family medicine resident clinic. Infect Control Hosp Epidemiol 2021;42:715–721. [DOI] [PubMed] [Google Scholar]
- 43. Wang N, Athans V, Neuner E, Bollinger J, Spinner M, Brizendine K. A pharmacist-driven antimicrobial stewardship intervention targeting cytomegalovirus viremia in ambulatory solid organ transplant recipients. Transpl Infect Dis 2018;20:e12991. [DOI] [PubMed] [Google Scholar]
- 44. Reed EE, West JE, Keating EA, et al. Improving the management of candidemia through antimicrobial stewardship interventions. Diagn Microbiol Infect Dis 2014;78:157–161. [DOI] [PubMed] [Google Scholar]
- 45. MacMillan KM, MacInnis M, Fitzpatrick E, et al. Evaluation of a pharmacist-led antimicrobial stewardship service in a pediatric emergency department. Int J Clin Pharm 2019;41:1592–1598. [DOI] [PubMed] [Google Scholar]
- 46. Dumkow LE, Beuschel TS, Brandt KL. Expanding antimicrobial stewardship to urgent care centers through a pharmacist-led culture follow-up program. Infect Dis Ther 2017;6:453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Coll A, Kinnear M, Kinnear A. Design of antimicrobial stewardship care bundles on the high-dependency unit. Int J Clin Pharm 2012;34:845–854. [DOI] [PubMed] [Google Scholar]
- 48. Burns KW, Johnson KM, Pham SN, Egwuatu NE, Dumkow LE. Implementing outpatient antimicrobial stewardship in a primary care office through ambulatory care pharmacist–led audit and feedback. J Am Pharm Assoc 2020;60:e246–e251. [DOI] [PubMed] [Google Scholar]
- 49. Kurtz K, Heyerly A, Bokhart G, Simpson W. Impact of a pharmacist-driven penicillin allergy skin testing protocol on antimicrobial stewardship in a tertiary-care hospital. Hosp Pharm 2021;56:136–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Harmon S, Richardson T, Simons H, Monforte S, Fanning S, Harrington K. The clinical and financial impact of a pharmacist-driven penicillin skin testing program on antimicrobial stewardship practices. Hosp Pharm 2020;55:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pierce J, Stevens MP. COVID-19 and antimicrobial stewardship: lessons learned, best practices, and future implications. Int J Infect Dis 2021;113:103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen JZ, Hoang HL, Yaskina M, et al. Efficacy and safety of antimicrobial stewardship prospective audit and feedback in patients hospitalized with COVID-19: a protocol for a pragmatic clinical trial. PLoS One 2022;17:e0265493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Watkins AB, Van Schooneveld TC, Reha CG, Anderson J, McGinnis K, Bergman SJ. Use of a novel clinical decision support tool for pharmacist-led antimicrobial stewardship in patients with normal procalcitonin. Pharmacy (Basel) 2021;9:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Beganovic M, Costello M, Wieczorkiewicz SM. Effect of matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) alone versus MALDI-TOF MS combined with real-time antimicrobial stewardship interventions on time to optimal antimicrobial therapy in patients with positive blood cultures. J Clin Microbiol 2017;55:1437–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mahrous AJ, Thabit AK, Elarabi S, Fleisher J. Clinical impact of pharmacist-directed antimicrobial stewardship guidance following blood culture rapid diagnostic testing. J Hosp Infect 2020;106:436–446. [DOI] [PubMed] [Google Scholar]
- 56. Lai WM, Islahudin FH, Ambaras Khan R, Chong WW. Pharmacists’ perspectives of their roles in antimicrobial stewardship: a qualitative study among hospital pharmacists in Malaysia. Antibiotics (Basel) 2022;11:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Park S, Kang JE, Choi HJ, et al. Antimicrobial stewardship programs in community health systems perceived by physicians and pharmacists: a qualitative study with gap analysis. Antibiotics (Basel) 2019;8:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chang F-Y, Chuang Y-C, Veeraraghavan B, et al. Gaps and opportunities in antimicrobial stewardship programs in Asia: a survey of 10 countries. Open Forum Infect Dis 2021;8 suppl 1:S57–S58. [Google Scholar]
- 59. Lee TH, Lye DC, Chung DR, et al. Antimicrobial stewardship capacity and manpower needs in the Asia Pacific. J Glob Antimicrob Resist 2021;24:387–394. [DOI] [PubMed] [Google Scholar]
- 60. Dionne B, Wagner JL, Chastain DB, Rosenthal M, Mahoney MV, Bland CM. Which pharmacists are performing antimicrobial stewardship: a national survey and a call for collaborative efforts. Antimicrob Steward Healthc Epidemiol 2022;2:e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Maeda M, Miyake T, Inose R, et al. Bibliometric analysis of pharmacist’s research on antimicrobial stewardship in Japan: an interrupted time series analysis on the implementation of the certification system for infection control pharmacists. J Pharm Health Care Sci 2021;7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]