Abstract
Contemporary diets in Western countries are largely acid-inducing and deficient in potassium alkali salts, resulting in low-grade metabolic acidosis. The chronic consumption of acidogenic diets abundant in animal-based foods (meats, dairy, cheese and eggs) poses a substantial challenge to the human body's buffering capacities and chronic retention of acid wherein the progressive loss of bicarbonate stores can cause cellular and tissue damage. An elevated dietary acid load (DAL) has been associated with systemic inflammation and other adverse metabolic conditions. In this narrative review, we examine DAL quantification methods and index observational and clinical evidence on the role of plant-based diets, chiefly vegetarian and vegan, in reducing DAL. Quantitation of protein and amino acid composition and of intake of alkalising organic potassium salts and magnesium show that plant-based diets are most effective at reducing DAL. Results from clinical studies and recommendations in the form of expert committee opinions suggest that for a number of common illnesses, wherein metabolic acidosis is a contributing factor, the regular inclusion of plant-based foods offers measurable benefits for disease prevention and management. Based on available evidence, dietary shifts toward plant-based nutrition effectively reduces dietary-induced, low-grade metabolic acidosis.
Key words: Dietary acid load, Net endogenous acid production, Plant-based diet, Potential renal acid load, Vegan diet, Vegetarian diet
Introduction
Contemporary diets in Western countries are largely acid-inducing and deficient in potassium alkali salts(1,2). This results in a chronic condition known as low-grade metabolic acidosis, subsequent to an increased dietary acid load (DAL) that leads to small net increases in acid (H+) and a reduction in base (HCOO3−). While diet-induced low-grade metabolic acidosis results in only a slight decrease in blood pH, investigations that followed the initial seminal findings of Kurtz et al. have shown that its impact on metabolism can contribute to the worsening of a variety of disorders(3). DiNicolantonio and O'Keefe have classified low-grade metabolic acidosis as a driver of chronic disease(4).
In general, foods of animal origin contain precursors that increase DAL (main precursors of acid include proteins rich in sulphur-containing amino acids, lysine, and arginine), whereas the vast majority of plant-based foods are precursors of base (potassium alkali salts and magnesium). Thus, low-grade metabolic acidosis is frequently found in individuals adhering to contemporary omnivorous Western diets(4). Although there are no clinically apparent or noticeable harms, the chronic retention of acid and the progressive loss of bicarbonate stores can cause cellular and tissue damage. The long-term intake of supraphysiological loads of acid in contemporary net acid-producing diets has been associated with systemic inflammation and other adverse metabolic conditions(2,5,6).
The human body is naturally equipped with multiple systems to buffer and titrate acid in order to prevent the inexorable accumulation of acid(7). However, the body's capacities are limited and may be insufficient under certain circumstances (e.g. in age-related decline in renal functional)(2).
Contemporary Western diets typically produce a total acid load of about 60–100 mEq/d(8,9). However, even in healthy adults, the kidneys can only excrete 40–70 mEq of acid per day before acid is retained in the body(4). When acid production exceeds its excretion, compensatory mechanisms (such as muscle and connective tissue breakdown to eliminate protons along with ammonium(4)) are elicited to minimise systemic acidosis. This chronic acid-related stress is increasingly understood as a continuum, which has chronic metabolic acidosis at its most extreme end, and acidifying diets at its least extreme, yet also detrimental, end(10). Chronic acid-stress has been associated with numerous health repercussions (Fig. 1)(5,6,11).
Fig. 1.
Potential adverse effects of a high DAL: an overview. Based on(5,6,11). DAL, dietary acid load.
Dietary modifications are an effective means to reduce the burden of alimentary acid load(12). A frequent consumption of acid-inducing foods (processed meats, cheese and certain acidifying grains) combined with a low intake of base-inducing foods (fruits, legumes and vegetables) increases DAL(13–15). Plant-based diets (PBDs) that are naturally low in (or exclude) animal products have been shown to reliably reduce DAL(14). Results from clinical studies and recommendations in the form of expert committee opinions suggest that for a number of common illnesses – wherein metabolic acidosis is a contributing factor – the regular inclusion of plant-based foods offers measurable benefits for disease prevention and management(16).
This review examines the contribution of plant-based dietary patterns, chiefly vegetarian and vegan diets, which drastically reduce or exclude animal products, to DAL and summarises growing evidence that dietary shifts toward plant-based nutrition are effective at diminishing dietary-induced low-grade metabolic acidosis.
DAL assessment and quantification
Epidemiological studies and clinical trials regularly rely on estimates of DAL to investigate potential relationships to human health and disease(17). The majority of studies on DAL used at least two common formulas to estimate acid load from diet: the potential renal acid load (PRAL) score by Remer and Manz(18) and the net endogenous acid production (NEAP) score by Frassetto et al.(19).
The PRAL score may be calculated as follows(18):
 |
The PRAL score (hereafter called PRALR) includes intestinal absorption rates for the following micronutrients: potassium, phosphate, magnesium, calcium and also considers protein intake. Previous studies in healthy individuals validated a strong correlation between the PRAL score and urinary pH(18).
NEAP (hereafter called NEAPF) may be estimated based on the formula by Frasetto et al.(19), which considers daily total protein intake and potassium intake.
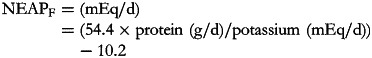 |
Alternatively, there is an additional formula to estimate NEAP proposed by Remer et al. (hereafter termed NEAPR)(20).
Remer et al. estimated NEAP from average intestinal absorption rates of ingested protein and additional minerals (PRALR score) as well as anthropometry-based estimates for organic acid excretion (OAest). Hereby, OAest (mEq/d) was calculated as follows:
The individual body surface area may be calculated with the formula of Du Bois and Du Bois:
 |
NEAP is the net combination of non-carbonic (i.e. fixed) acids from acids ingested in the diet and produced from endogenous metabolic processes, minus the acids that are neutralised or buffered by non-carbonic dietary and endogenously generated base supplies(21,22). Thus, NEAP considers PRAL (e.g. the sum of bases and acids released from diet-derived compounds of cations and anions) in addition to the total non-carbonic organic acids. The aforementioned NEAP scores (NEAPF, NEAPR) have both been validated against net acid excretion (NAE) with satisfying results, reliably estimating NAE).
The three aforementioned scores (NEAPF, NEAPR and PRALR) are the most commonly used scores in the majority of studies. Notably, each score has its own advantages and drawbacks(23). In clinical practice, all scores performed differently(21). Calculation of all three scores is thus recommended, preferably supplemented with estimations of urinary DAL indices (PRAL and NEAP) values, as recently summarised by Parmenter et al.(22).
Western diets typically produce a total DAL ranging from approximately 50 to 75 mEq/d(8,9). In other parts of the world, DAL is substantially lower. One is rural Ghana, where Goldberg et al. reported NAE values of 29⋅2 ± 12⋅2 mEq/d(24). When glancing solely at PRAL values, there are notable differences between common dietary patterns(10). Wesson reported calculated PRAL sums of selected diets and demonstrated that the average dietary intake in the United States results in PRAL sums of approximately 27 mEq/d. Other diets, such as the DASH diet (Dietary Approaches to Stop Hypertension) resulted in substantially lower PRAL sums (about 11 mEq/d). PBDs are characterised by even lower PRAL sums(12). The potential DAL-lowering mechanism of PBDs is discussed in detail hereafter.
Dietary components affecting DAL
The ratio of plant v. animal-based food intake determines DAL(25). When protein containing foods are metabolised, most release acid in the form of hydrogen ions. In contrast, potassium-rich plant foods (mainly fruits and vegetables) produce alkali(25,26).
Protein content and amino acid composition of diet
Unlike carbohydrates or lipids, which do not generate unmetabolisable acidity during their complete oxidation, proteins contain various amino acids whose catabolism is liable to affect the acid–base equilibrium(1). When protein containing foods are metabolised, most release acid in the form of protons(25). The amount, however, depends on the amino acid composition. Some amino acids are neutral, some are acidic and some are alkaline(25,27).
Lysine, arginine and histidine are acidifying because their metabolisation in the liver generates hydrochloric acid (plus glucose and urea)(1,25):
Lysine and arginine intake are substantially higher on a meat-based diet as compared to plant-based (vegan) diet(28,29). While this might be beneficial with regard to DAL, it has also been argued that an insufficient lysine intake could also have adverse effects on human health. Yet, if a diet has at least a modest amount of variability (which is usually the case in economically developed countries), there are no issues regarding sufficient intakes of lysine(30).
Another group that belongs to the acidifying amino acids is sulphur-containing amino acids (methionine, homocysteine and cysteine)(31). Catabolism of these amino acids leads to sulphuric acid generation – a non-metabolizable anion which is a major constituent of DAL(25,27).
The obtained sulphate anions constitute unmetabolizable acidity(1) and are a major contributor to DAL(32). Plant-based proteins tend to be much lower in methionine than animal proteins(33,34). As summarised by McCarty, the methionine fraction in representative plant proteins ranges from 0⋅85 % in lentils to 2⋅26 % in brown rice, whereas that of animal proteins falls into a much higher range (from approximately 2⋅35 to 3⋅11 %)(33). Eggs are often high in methionine(35), whereas the fraction of methionine in legume protein and nut protein is especially low(36). Table 1 summarises the methionine content of selected common foods per kcal (based on(33)). For additional information on amino acid composition in selected foods across foods groups, we refer the interested reader to the work of Gardner et al.(37).
Table 1.
Content of the amino acid methionine in commonly consumed foods of plant and animal origin
 |
Orange colouring: animal-based foods, green colouring: plant-based foods.
Source data adapted from(33).
In this context, mentioning of the amino acid glycine is also warranted. Glycine can act as a functional Methionine antagonist(33), since it can fulfil the role of a methyl group acceptor in a biochemical reaction catalysed by glycine n-methlytransferase – a key enzyme in methyl group metabolism(38). Plant proteins are higher in glycine than most animal proteins(39) and it is not surprising that vegans had the highest plasma concentration of this amino acid in the Epic Oxford cohort study(28).
Glutamate and glutamine content of diet
Glutamine (C5H10N2O3) and glutamate (C5H9NO4) are important for the neutralisation of acid via α-Ketoglutaric acid (C5H6O5). Glutamate is a non-essential neutralising anionic amino acid whose metabolism consumes hydrogen ions to become neutral(25,27,40):
Diet is the major source of glutamine and glutamate(41), and unprocessed plant proteins are usually richer than animal proteins in glutamate(27). In cross-sectional studies, meat eaters thus had a lower glutamine intake than vegetarians and vegans(28). Another prominent example with comparable findings is the INTERMAP study, demonstrating that individuals on a high plant protein/low animal protein diet consumed greater amounts of glutamic acid as compared with their high animal protein/low plant protein counterparts(42). A reservation must be made that this section refers to unprocessed plant foods and not to processed vegan foods enriched with artificial flavours containing monosodium glutamate.
Phosphorus content of diet
Phosphorus and preservative phosphates (phosphoric acid, polyphosphates, etc.) are other important contributors to DAL(14). Phosphate salts are frequently added to bacon, sausages and other processed meats for their antibacterial properties and to condition the colour and flavour of products(43,44). In addition to that, phosphate additives are frequently found in cheese manufacture and milk products(45,46).
Notably, their acidity does not depend on the phosphate anion itself(25). Instead, it depends on the cation to which the phosphate anion is attached and the pH of the food. Phosphoric acid (H3PO4), commonly found in many sodas and cola drinks, is acidic as H+ is released upon metabolisation(25).
Moreover, some of the widely used preservative phosphates and additives are acidic and some are alkaline(25). A frequently encountered acidic phosphate-based additive is calcium pyrophosphate (CaH2P2O7)(25), which is frequently found in quick breads and sweet bakery products(47). Trisodium phosphate (Na3PO4), on the other hand, is alkaline and consumes 2 H+ ions upon metabolisation.
The extra burden from phosphorus coming from processed products alone might reach up to 737 mg/d(48). Glancing at the PRALR formula shows that phosphorus has the highest weighting factor of all micronutrients (0⋅037)(18). An extra intake of 250 mg of phosphorus per day will increase PRAL by more than 9 mEq/d.
It is important to understand the extra ‘DAL burden’ subsequent to a high phosphorus intake. Milk and dairy products account for more than 24 % of phosphorus intake in human diets(49), and phosphorus intake might increase substantially when other foods abundant in phosphate (e.g. soft drinks and canned fish) are consumed(50–52). Table 2 shows the phosphorus content of selected foods(53). In this context, a reservation must be made, that the intestinal absorption of phosphorus from additives used in food manufacturing is substantially higher compared with phosphorus derived from unprocessed animal-based foods. Relativisation is thus necessary when evaluating different phosphorus sources.
Table 2.
Phosphorus and protein content of commonly consumed foods of plant and animal origin
 |
Phosphorous and protein content are expressed per 100 g of uncooked food, as typically provided in nutritional content labelling.
Orange colouring: animal-based foods, green colouring: plant-based foods.
Source data adapted from(53).
Plant foods (vegetables, legumes and seeds), on the other hand, contain phosphorus in the form of phytate, which has a significantly lower bioavailability and neglectable acidising effects(14,54,55). Instead, most plant-based foods have alkalising effects due to their high availability of potassium salts of organic anions(27).
Potassium/organic anion content of diet
As a general rule, almost all fruits and vegetables display negative PRAL values, and the amount of potassium present in those foods reflects their alkalising ability(25,56,57). Organic anions may be considered as virtual precursors of KHCO3 and can be metabolised to bicarbonate(1,58).
Prominent examples include citric acid, malate and potassium citrate (C6H5K3O7)(25). Organic salts such as potassium citrate contain base ions but no hydrogen ions. They are thus capable of binding hydrogen ions during their metabolism to carbon dioxide and water.
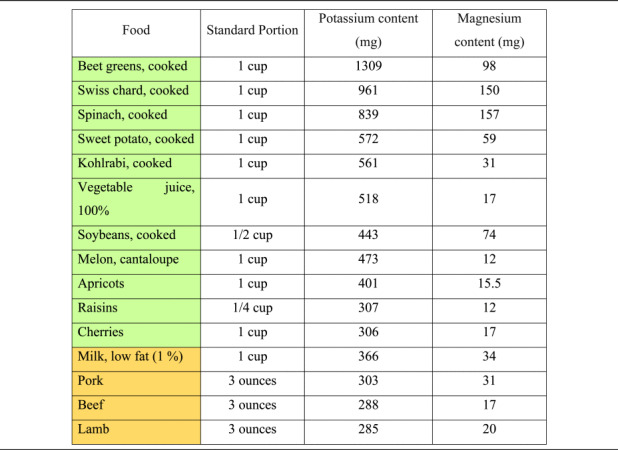
The consumption of hydrogen ions upon metabolisation has alkalising effects(11,27). Except for ripened and processed grains, most plant foods contain substantial quantities of organic anions, whereas they are scarce in animal-based foods(1). Daily food supply of organic anions strongly depends on dietary patterns and ranges from 1 g/d (in low plant consumers) to 3–4 g/d in a diversified omnivorous diet. Vegetarians and vegan usually consume more than 5 g/d of organic anions(1). Potassium content of selected foods is presented in Table 3, based on current data from the dietary guidelines for Americans and the US Department of Agriculture(59,60).
Table 3.
Potassium and magnesium content of selected foods per standard portion
 |
Another important source of organic anions is their production in the colon, mainly short-chain fatty acids (SCFA, including butyrate, acetate and propionate)(1). SCFA are the end-products of microbial fermentation in the distal part of the digestive tract, using specific substrates such as fibre and carbohydrates. SCFA production is closely dependent on nutritional factors and faecal levels of those metabolites correlate positively with the consumption of vegetables, fruits and legumes(61). Significant increases in SCFA production have been observed when omnivores consume a diet rich in fruits and vegetables(62), and it is now widely accepted that a plant-based vegan diet may increase SCFA production by modulation of the gut microbiota(63,64).
Magnesium content of diet
Magnesium is a key micronutrient in the PRAL-formula by Remer and Manz, with a relatively high weighting factor of 0⋅026(18). PBDs are much more abundant in magnesium than omnivorous diets(65,66), and thus have a higher PRAL-lowering capacity. A Danish study revealed that vegan men consume – on average – more than 230 mg of magnesium more than the general population(67), potentially translating into a PRAL-lowering capacity of more than 8 mEq/d. Magnesium content of selected foods is presented in Table 3, based on current data from the US Department of Agriculture(59).
Contribution of increased DAL to chronic illnesses
A number of studies have identified elevated DAL as a factor contributing to various chronic illnesses, such as type 2 diabetes, hyperlipidaemia, cardiometabolic disorders, renal disease, cancer and even pathologies where the metabolic component is less clear, such as mental and musculoskeletal disorders. The underlying pathomechanisms are complex and the subject of ongoing research.
Central to most chronic lifestyle-related diseases, a high DAL enhances cortisol production(68), which, in turn, may promote insulin resistance(69). Apart from increasing glucocorticoid secretion, a high DAL also reduces catabolic degradation of potentially bioactive glucocorticoids(68). Both mechanisms ensure a steady glucocorticoid supply, which is apparently necessary to increase catabolism of skeletal muscle protein (ensuring an augmented renal glutamine supply) and the subsequent increase in renal ammoniagenesis as discussed below.
Latent metabolic acidosis subsequent to an acidifying diet may also stimulate ammoniagenesis, which allows for a simultaneous elimination of hydrogen ions and anions(27). Ammoniagenesis, however, comes at its price, and has been associated with renal tubulointerstitial injury and subsequent impaired kidney function(58,70). Additional adverse mechanisms include decreased uric acid excretion (potentially resulting in hyperuricaemia)(71,72), increased renal excretion of calcium and magnesium(73), higher insulin-like growth factor (IGF) levels(74) and decreased circulating adiponectin levels through acidosis-induced inhibition of adiponectin gene transcription in adipocytes(74,75).
We summarise main findings on the contribution of DAL to these diverse groups of disorders with the fundamental understanding that the causes and mechanisms of such complex illnesses are of multifactorial nature, implying that it is very likely that more than one of the aforementioned mechanisms is involved.
Type 2 diabetes
A high DAL has been associated with insulin resistance and an increased risk for type 2 diabetes (T2DM) in various large epidemiological cohort studies, including the Teheran Lipid and Glucose Study(76), the Nurses’ Health Study I and II and the Health Professionals’ Follow-up Study(77). Such associations have been found in both children/adolescents(78) and adults(79). A high DAL is not only associated with higher fasting blood glucose levels(80) but also with impaired insulin sensitivity(14,81). Notably, a high DAL may also adversely affect other clinical outcomes in individuals with T2DM. One example is a 2020 study, that demonstrated associations between higher DAL scores and impaired sleep quality and mental health disorders in said individuals(82).
On the other hand, a more alkaline diet has been shown to exert protective effects(13). The particular mechanisms underlying the association between metabolic acidosis and insulin resistance are yet to be elucidated. Apart from DAL-induced increased hepatic gluconeogenesis and disrupted binding of insulin to the insulin receptor, inhibition of insulin signalling pathways may play a crucial role(13). These factors may play an important role when glancing at other adverse clinical outcomes related to a high DAL, including hyperlipidaemia and the increased risk for cardiometabolic disorders.
Hyperlipidaemia and cardiometabolic disorders
In 2008, Murakami et al. reported the findings of a Japanese cross-sectional study comprising 1136 female Japanese students aged 18–22 years(83). The authors reported positive associations of a high DAL with higher systolic and diastolic blood pressure as well as with total and LDL-cholesterol. Associations with hypertriglyceridaemia have been reported in a cross-sectional study including 357 Iranian elderly men(84). Increasing cortisol production caused by mild metabolic acidosis could be the underlying mechanism(85), but additional research is warranted in this poorly understood field(83). Other research suggesting potential associations between a high DAL and obesity(86–88), and hypertension(89,90) – where elevated cortisol levels also play an important role – support this hypothesis. Notably, a high DAL may not only increase the risk for cardiovascular disease(91–93) but may also affect other organs, such as the liver (in the form of non-alcoholic fatty liver disease(94,95)) and the kidneys.
Renal disorders
Numerous clinical and epidemiological studies associated elevated DAL scores with incident chronic kidney disease(96,97) and end-stage renal failure risk(58). A high DAL may contribute to a faster decline in glomerular filtration rate (GFR)(98,99), whereas dietary alkali treatment of metabolic disease in chronic kidney disease preserves GFR and reduce kidney angiotensin-II-activity(100). Renal hyperfiltration subsequent to a high DAL(101) plays a crucial role in the pathogenesis of glomerular disorders and its attenuation is considered a novel therapeutic target in diabetes and obesity-induced kidney disorders(102). This again demonstrates that the effects of a high DAL are not confined to a single organ but may involve the body as a whole.
Studies on the contribution of DAL to kidney disease have gained recognition. Fruit and vegetable treatment of chronic kidney disease-related metabolic acidosis is as effective as oral NaHCO3 when it comes to GFR preservation but reduce cardiovascular risk better than sodium bicarbonate alone(103,104). A committee of experts representing the workgroup of the Kidney Disease Outcomes Quality Initiative (KDOQI) from the National Kidney Foundation, USA, has recently published recommendations for the dietary management of DAL. These are as follows:
‘Statements on Acid Load: Dietary Management of Net Acid Production (NEAP)
In adults with CKD 1–4, we suggest reducing net acid production (NEAP) through increased dietary intake of fruits and vegetables (2C) in order to reduce the rate of decline of residual kidney function.’(105)
Musculoskeletal health and body composition
An elevated acid-load burden from dietary intakes has been associated with poor musculoskeletal health(106,107) and impaired bone health(108). Data from a Japanese study also suggest associations of increased DAL with frailty (particularly weakness and slowness) in older women(109). Faure et al. reported an inverse association between PRAL and the percentage of total lean body mass among senior women in a Swiss-based population, suggesting potentially beneficial effects of a more alkaline diet in said women(110). Their cross-sectional study essentially confirmed the findings by Welch et al., who reported a positive association of a more alkaline PRAL with fat-free mass (%) among women between 18 and 79 years, independent of physical activity and smoking(111). Notably, much additional research is warranted in this field as a recent study associated higher acid diet measures with higher muscle strength – contrary to the common acid hypothesis(112).
Mental health
With regard to mental health, positive associations were found for depression and anxiety(113–115) as well as with emotional problems and hyperactivity in children(116). Systemic inflammation subsequent to a high DAL could play an important aetiological role here, yet the reservation must be made that the involved pathological mechanisms are subject to a controversial debate.
Cancer
Elevated DAL scores have been linked to low-grade inflammation (as indicated by elevated lipid accumulation product levels)(84). It is now widely accepted that low-grade metabolic acidosis may induce peroxidation of biological structures(1). An altered acid–base equilibrium may also modulate molecular activity including adrenal glucocorticoid, IGF-1 and adipocyte cytokine signalling, which contribute to dysregulated cellular metabolism and may play a role in cancer development(74).
DAL-induced low-grade mild metabolic acidosis promote tissue damage and inflammation(11,13,117,118), which may initiate genomic instability on normal cells through the activation of cytokines, which may stimulate tumour invasion and metastases(119,120). Positive associations between a high DAL and various cancers have been reported, including breast cancer(121,122), prostate cancer(123), lung cancer(124), colorectal cancer(125), pancreatic cancer(126), gastric cancer(127), oesophageal cancer(128) as well as head and neck cancers(129). Two meta-analyses confirmed these associations: Keramati et al. and Bahrami et al. independently found higher odds for cancer in individuals with elevated DAL scores(130,131).
PBDs to reduce DAL
Dietary components affecting acid load have been discussed in detail in the previous section. PBDs, including vegetarian and vegan diets, are abundant in potassium salts of organic anions(1,27), while they are at the same time low in phosphorus and preservative phosphates(14,132). Although diversified PBDs contain sufficient amounts of protein, their overall content is usually lower than in omnivorous diets(30). In addition to that, their content of sulphur-containing amino acids is also substantially lower as compared with meat-based diets(33,34).
The combination of these factors qualifies plant-based nutrition as an ideal tool to reduce DAL(132). This section summarises supporting evidence for this glancing at both observational (Table 4) and clinical intervention studies (Table 5).
Table 4.
Observational studies investigating DAL scores in plant-based cohorts
| Study (year) | Location | Participants | Results | Comments |
|---|---|---|---|---|
| Deriemaeker et al.(133) | Belgium | n 60 participants, thereof n 30 vegetarians and n 30 non-vegetarians matched for age, sex and BMI |
|
|
| Ströhle et al.(23) | Germany | n 154 participants, thereof n 56 moderate vegans and n 98 strict vegans. All participants were non-obese, non-smoking adults aged 19–50 years |
|
|
| Knurick et al.(134) | United States of America | n 82 participants, thereof n 27 meat eaters, n 27 lacto-ovo-vegetarians and n 28 vegans. All participants were non-obese, non-smoking adults aged 19–50 years with at least 1 year of dietary adherence |
|
|
| Storz et al.(12) | United States of America | n 191 self-perceived lacto-ovo-vegetarians aged 18 years or older |
|
|
DAL, dietary acid load; NEAP, net endogenous acid production; PRAL, potential renal acid load.
Table 5.
Dietary intervention studies investigating DAL scores in plant-based study populations
| Study (year) | Location | Participants | Results | Comments |
|---|---|---|---|---|
| Cosgrove and Johnston(32) | United States of America | n 23 participants, thereof n 7 individuals on a vegan diet for 2 d over 1 week (VEG2), n 8 individuals on a vegan diet for 3 d over 1 week (VEG3) and n 8 individuals on a vegan diet for 7 consecutive days (VEG7) |
|
|
| Müller et al.(132) | Germany | n 45 omnivorous individuals randomly assigned to a vegan diet (n 23) or a meat-rich diet (n 22) for 4 weeks |
|
|
| Kahleova et al.(14) | United States of America | n 244 participants were randomly assigned to an intervention (vegan) (n 122) or control group (n 122) for 16 weeks |
|
|
DAL, dietary acid load; NEAP, net endogenous acid production; PRAL, potential renal acid load.
Observational studies
We identified four observational studies investigating DAL scores in plant-based individuals(12,23,133,134). Three studies investigated lacto-ovo-vegetarians(12,133,134) and two studies also investigated vegans(23,134). The study characteristics may be obtained in a chronological order from Table 4. All studies found negative PRAL values in individuals consuming a plant-based diet, indicating alkalising properties. The lowest PRALR-values were found in a study by Ströhle et al. investigating DAL scores in German vegans (Table 4)(23). Notably, the authors used a modified PRALR formula and omitted calcium in their calculations.
A Belgian study by Deriemaeker et al. also found negative PRALR scores in vegetarians (−10⋅9 ± 19⋅7 mEq/d)(133), however, their diets were less alkalising as compared with the vegans in Ströhle et al.(23). Storz et al. performed a secondary data analysis using data from the National Health and Nutrition Examination Surveys(12). The authors investigated DAL scores in self-identified vegetarians who admitted to occasionally consumed animal products(135). Although median PRALR scores were much higher than in the aforementioned studies, they were still negative (−0⋅44 (−12⋅19 to 11⋅01) mEq/d), also indicating slight alkalising properties.
Generally speaking, vegan diets were associated with lower DAL scores than lacto-ovo-vegetarian diets in all retrieved studies (Table 4). One conceivable explanation is that lacto-ovo-vegetarian diets, which build around eggs, cheese and other dairy products, are usually richer in phosphorus and preservative phosphate (phosphoric acid, polyphosphates) than vegan diets(132,136). Preservative phosphates are characterised by higher gastrointestinal absorption rates and therefore increase the acid load burden from diet(137). We purport that this is one potential factor why vegan diets contribute lower DAL scores than vegetarian diets. An additional difference between these diets is the amino acid composition from protein sources. Protein sources in vegetarian diets include dairy products and/or eggs, which have a greater abundance of sulphur-containing amino acids compared with plant-based protein.
Several large epidemiological investigations suggested that total protein intake is lower in vegan diets as compared with lacto-ovo-vegetarian diets(138). Vegan diets are not deficient in protein but contain significantly higher amounts of plant-based protein(139). One example is the French NutriNet-Santé Study, where vegans consumed on average 12⋅7 g more plant protein per day than vegetarians (46⋅5 g/d v. 33⋅8 g/d)(139). This translates into a substantially higher intake of vegetables, fruits and legumes, which generally have alkalising effects(132). The higher the fruits and vegetable intake, the higher the supply of organic anions(1) and thus the higher the alkalising effect of the diet. A reservation must be made, that the protein intake difference between vegans and vegetarians reported in other studies(140) was not as pronounced, possibly due to geographical and socioeconomic factors known to influence nutrition.
Clinical intervention studies
We also identified several clinical intervention studies that investigated the effects of various PBDs on DAL management(14,32,132) (Table 5). However, in light of the low number of studies in this field, and with regard to the high heterogeneity in diet composition and study designs, we refrained from performing a meta-analysis.
Cosgrove and Johnston examined the impact of adherence to a vegan diet on acid–base balance in health adults(32). In a randomised-controlled trial, they compared three different diets: a vegan diet for 2 d over 1 week (VEG2), a vegan diet for 3 d over 1 week (VEG3), and a vegan diet for 7 consecutive days (VEG7). With regard to the PRAL-lowering effect, the VEG7 diet performed best. After seven consecutive days on a strict vegan diet, mean PRAL values fell substantially from 23⋅7 ± 17⋅7 to −6⋅0 ± 12⋅8 mEq/d. Again, a strict vegan diet yielded alkalising effects. The effect of the other two dietary interventions (VEG2 and VEG3) was less pronounced.
Our group performed a secondary data analysis of a randomised-controlled trial where 45 omnivorous individuals were randomly assigned to either a vegan diet (n 23) or a meat-rich diet (n 22) for 4 weeks(132). After 3 weeks, PRALR scores fell from −5⋅26 ± 4⋅45 to −23⋅57 (23⋅87) mEq/d in vegans. Comparable values were observed in week 4. Notably, the control group comprised individuals on a meat-rich diet, which demonstrated a significant increase in their DAL scores. PRALR scores rose from 3⋅26 ± 17⋅91 to 18⋅78 (21⋅04) mEq/d in individuals on a meat-rich diet. The isocaloric nature of the vegan diet (participants were instructed to avoid weight loss due to a decreased energy intake) deserves special consideration in this context and might have led to underestimations of the PRAL-lowering effect of vegan diets.
Another important study in the field has been conducted by Kahleova et al. in 2021(14). The authors performed a post-hoc analysis of a low-fat vegan dietary intervention that restricted processed foods and reduced fat intake to approximately 10 % of total energy. This diet included grains, legumes, vegetables and fruits and was characterised by a targeted macronutrient distribution of approximately 75 % of energy from carbohydrates, 15 % protein and 10 % fat. After 4 months, median PRALR scores and NEAPF scores dropped significantly in the vegan group (−24⋅3 (−28 to −20⋅5) mEq/d and −25⋅1 (−29⋅1 to −21⋅1) mEq/d, respectively), whereas both scores remained almost identical in the control group (Table 4).
A vegan diet significantly reduced DAL scores in all three studies, however, results from these studies also suggest that dietary adherence is a crucial factor. The simple implementation of one or two ‘vegan days’ per week may be insufficient to achieve an alkalising diet.
Discussion
There is mounting evidence that PBDs (vegetarian or vegan) may be an effective means to reduce DAL. Observational and clinical studies suggest that both can have alkalising effects, although a vegan diet seems most effective. One limitation is that the total amount of studies in this particular field is still limited and that a direct large-scale randomised-controlled study comparing both diets head-to-head is not yet available. Additional research is thus necessary to identify and quantify the factors that appear to make the vegan diet more favourable towards DAL reduction.
The heterogeneity in studies (and dietary interventions) did not allow us to perform a meta-analysis. Although it is desirable to quantify the PRAL-lowering effects of PBDs, our findings strongly suggest that a vegan diet is associated with an alkaline dietary character, whereas vegetarian diets have rather neutral total PRAL values.
Another point of concern is the lack of a defined reference range for PRAL values and the fact that studies comprised heterogeneous study populations across the world. Depending on sex, age and total energy intake, different reference values may be outlined. Although most studies found lower PRAL values in older adults (potentially due to their lower protein and total energy intake(12), this is not univocally the case)(141). We purport that age is an underestimated factor and suggest that future studies should carefully adjust for that. This might be of particular importance with regard to a potentially progressive loss of bicarbonate in older age(142).
Since neither PRAL nor NEAP scores consider protein origin per sé (e.g. animal v. plant-based protein, and the corresponding bioavailability of cations and anions contributing to DAL), it would be interesting to examine whether this factor should be incorporated to delineate diet-specific reference ranges of PRAL and NEAP scores to assess and compare DAL more accurately among individuals adhering to different dietary patterns.
In addition to that, future studies should also investigate whether there are potential adverse effects of an overly alkalising diet. According to Xu et al.(143), excess diet alkalinity and acidity both showed weak associations with higher mortality in Swedish adults. Comparable findings have been reported in an Iranian study by Hejazi et al.(144). Although alkaline diets have been associated with numerous health benefits, we believe that more research is warranted in this area. Quantifying nutrient intake in alkaline diets in comparison with established dietary guidelines would be desirable. A quantification of the effect of colon-produced organic anions and their weighted contribution to DAL would also open a new area of research that has received insufficient attention in the past.
Finally, it is noteworthy that with the ongoing international promotion of plant-based nutrition and the strong growth of food manufacturing of plant-based products, there is a greater consumption of (non-dairy) plant-based cheese alternatives and meat substitutes is also increasing(145). Numerous plant-based cheese alternatives based on nuts, oils, grains, soy and other plant products have been developed – yet their effect on DAL is basically unexplored. The traditional PRAL tables usually date back to over to decades(20), and do not index these new products. A first attempt in this context has been made by Deriemaeker et al. who quantified the PRAL values of typical products consumed by vegetarians (in mEq/100 g)(133) (Table 6). Additional research in this area is warranted to better understand the impact of those ‘relatively new’ foods on DAL.
Table 6.
Selected PRAL values of typical products consumed by vegetarians v. non-vegetarians (in mEq/100 g): an overview
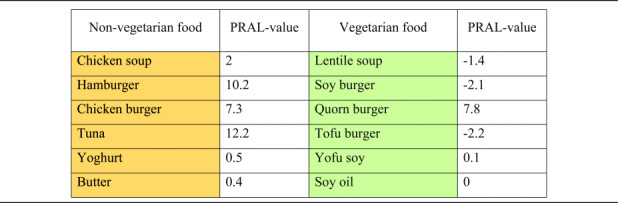 |
PRAL, potential renal acid load.
Orange colouring: animal-based foods, green colouring: plant-based foods.
PRAL values modified from(133).
Conclusion
Multiple observational and clinical studies suggest that vegetarian and vegan diets are an effective means to reduce DAL. The vegan diet in particular appears to have alkalising effects and might be more effective than a vegetarian diet to lower PRAL-scores. The lower content of phosphorus, total protein and sulphur-containing amino acids and the abundance of potassium salts from organic anions makes this dietary pattern particularly effective. Additional trials are warranted to understand the impact of the various plant-based dietary patterns on DAL. In this context, it is also of paramount importance to better understand the impact of plant-based cheese and meat alternatives, which are based on nuts, oils, grains, soy and other plant products.
Acknowledgements
In memory of Stefan Skaper.
The present study received no specific grant from any funding agency, commercial or not-for-profit sectors.
M. A. S., A. L. R. and L. H. have made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data. M. A. S. conceptualised the review. M. A. S. visualised the article. M. A. S. drafted the first version of the manuscript. M. A. S., L. H. and A. L. R. revised it critically for important intellectual content. M. A. S., L. H. and A. L. R. gave final approval of the version to be published. The corresponding author agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
The author declares no conflict of interest.
The specific dataset associated with this study will be made available by the corresponding author upon reasonable request.
This is a review article without human participants.
References
- 1.Demigné C, Sabboh H, Puel C, et al. (2004) Organic anions and potassium salts in nutrition and metabolism. Nutr Res Rev 17, 249–258. [DOI] [PubMed] [Google Scholar]
- 2.Frassetto L, Morris RC, Sellmeyer DE, et al. (2001) Diet, evolution and aging – the pathophysiologic effects of the post-agricultural inversion of the potassium-to-sodium and base-to-chloride ratios in the human diet. Eur J Nutr 40, 200–213. [DOI] [PubMed] [Google Scholar]
- 3.Kurtz I, Maher T, Hulter HN, et al. (1983) Effect of diet on plasma acid-base composition in normal humans. Kidney Int 24, 670–680. [DOI] [PubMed] [Google Scholar]
- 4.DiNicolantonio JJ & O'Keefe J (2021) Low-grade metabolic acidosis as a driver of chronic disease: a 21st century public health crisis. Open Heart 8, e001730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carnauba RA, Baptistella AB, Paschoal V, et al. (2017) Diet-induced low-grade metabolic acidosis and clinical outcomes: a review. Nutrients 9, E538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostrowska J, Janiszewska J & Szostak-Węgierek D (2020) Dietary acid load and cardiometabolic risk factors – a narrative review. Nutrients 12, E3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frassetto L, Banerjee T, Powe N, et al. (2018) Acid balance, dietary acid load, and bone effects—a controversial subject. Nutrients 10, 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemann J (1999) Relationship between urinary calcium and net acid excretion as determined by dietary protein and potassium: a review. Nephron 81, 18–25. [DOI] [PubMed] [Google Scholar]
- 9.Gannon RHT, Millward DJ, Brown JE, et al. (2008) Estimates of daily net endogenous acid production in the elderly UK population: analysis of the National Diet and Nutrition Survey (NDNS) of British adults aged 65 years and over. Br J Nutr 100, 615–623. [DOI] [PubMed] [Google Scholar]
- 10.Wesson DE (2021) The continuum of acid stress. Clin J Am Soc Nephrol 16, 1292–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osuna-Padilla IA, Leal-Escobar G, Garza-García CA, et al. (2019) Dietary acid load: mechanisms and evidence of its health repercussions. Nefrologia (Engl Ed) 39, 343–354. [DOI] [PubMed] [Google Scholar]
- 12.Storz MA & Ronco AL (2022) Reduced dietary acid load in U.S. vegetarian adults: results from the National Health and Nutrition Examination Survey. Food Sci Nutr 10, 2091–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams RS, Kozan P & Samocha-Bonet D (2016) The role of dietary acid load and mild metabolic acidosis in insulin resistance in humans. Biochimie 124, 171–177. [DOI] [PubMed] [Google Scholar]
- 14.Kahleova H, McCann J, Alwarith J, et al. (2021) A plant-based diet in overweight adults in a 16-week randomized clinical trial: the role of dietary acid load. Clin Nutr ESPEN 44, 150–158. [DOI] [PubMed] [Google Scholar]
- 15.Storz MA, Ronco AL & Lombardo M (2022) Dietary acid load in gluten-free diets: results from a cross-sectional study. Nutrients 14, 3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adair KE & Bowden RG (2020) Ameliorating chronic kidney disease using a whole food plant-based diet. Nutrients 12, 1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zwart SR, Rice BL, Dlouhy H, et al. (2018) Dietary acid load and bone turnover during long-duration spaceflight and bed rest. Am J Clin Nutr 107, 834–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Remer T & Manz F (1994) Estimation of the renal net acid excretion by adults consuming diets containing variable amounts of protein. Am J Clin Nutr 59, 1356–1361. [DOI] [PubMed] [Google Scholar]
- 19.Frassetto LA, Todd KM, Morris RC, et al. (1998) Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. Am J Clin Nutr 68, 576–583. [DOI] [PubMed] [Google Scholar]
- 20.Remer T & Manz F (1995) Potential renal acid load of foods and its influence on urine pH. J Am Diet Assoc 95, 791–797. [DOI] [PubMed] [Google Scholar]
- 21.Parmenter BH, Slater GJ & Frassetto LA (2017) Accuracy and precision of estimation equations to predict net endogenous acid excretion using the Australian food database. Nutr Diet 74, 308–312. [DOI] [PubMed] [Google Scholar]
- 22.Parmenter BH, Dymock M, Banerjee T, et al. (2020) Performance of predictive equations and biochemical measures quantifying net endogenous acid production and the potential renal acid load. Kidney Int Rep 5, 1738–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ströhle A, Waldmann A, Koschizke J, et al. (2011) Diet-dependent net endogenous acid load of vegan diets in relation to food groups and bone health-related nutrients: results from the German vegan study. Ann Nutr Metab 59, 117–126. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg GR, Dalzell SE, Jarjou LMA, et al. (2017) Dietary potential renal acid load and net acid excretion in rural and urban pre-menopausal Gambian women. Proc Nutr Soc 76. [Google Scholar]
- 25.Passey C (2017) Reducing the dietary acid load: how a more alkaline diet benefits patients with chronic kidney disease. J Ren Nutr 27, 151–160. [DOI] [PubMed] [Google Scholar]
- 26.Storz MA, Müller A & Ronco AL (2022) Nutrient intake and dietary acid load of special diets in the NHANES: a descriptive analysis (2009–2018). Int J Environ Res Public Health 19, 5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adeva MM & Souto G (2011) Diet-induced metabolic acidosis. Clin Nutr 30, 416–421. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt JA, Rinaldi S, Scalbert A, et al. (2016) Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: a cross-sectional analysis in the EPIC-Oxford cohort. Eur J Clin Nutr 70, 306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dietrich S, Trefflich I, Ueland PM, et al. (2022) Amino acid intake and plasma concentrations and their interplay with gut microbiota in vegans and omnivores in Germany. Eur J Nutr 61, 2103–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mariotti F & Gardner CD (2019) Dietary protein and amino acids in vegetarian diets—a review. Nutrients 11, 2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsen T, Øvrebø B, Turner C, et al. (2018) Combining dietary sulfur amino acid restriction with polyunsaturated fatty acid intake in humans: a randomized controlled pilot trial. Nutrients 10, 1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cosgrove K & Johnston CS (2017) Examining the impact of adherence to a vegan diet on acid-base balance in healthy adults. Plant Foods Hum Nutr 72, 308–313. [DOI] [PubMed] [Google Scholar]
- 33.McCarty MF, Barroso-Aranda J & Contreras F (2009) The low-methionine content of vegan diets may make methionine restriction feasible as a life extension strategy. Med Hypotheses 72, 125–128. [DOI] [PubMed] [Google Scholar]
- 34.Dong Z, Gao X, Chinchilli VM, et al. (2020) Association of sulfur amino acid consumption with cardiometabolic risk factors: cross-sectional findings from NHANES III. EClinicalMedicine 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Attia YA, Al-Harthi MA, Korish MA, et al. (2020) Protein and amino acid content in four brands of commercial table eggs in retail markets in relation to human requirements. Animals (Basel) 10, 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahrens S, Venkatachalam M, Mistry AM, et al. (2005) Almond (Prunus dulcis L.) protein quality. Plant Foods Hum Nutr 60, 123–128. [DOI] [PubMed] [Google Scholar]
- 37.Gardner CD, Hartle JC, Garrett RD, et al. (2019) Maximizing the intersection of human health and the health of the environment with regard to the amount and type of protein produced and consumed in the United States. Nutr Rev 77, 197–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luka Z, Pakhomova S, Loukachevitch LV, et al. (2012) Differences in folate–protein interactions result in differing inhibition of native rat liver and recombinant glycine N-methyltransferase by 5-methyltetrahydrofolate. Biochim Biophys Acta 1824, 286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krajcovicova-Kudlackova M, Babinska K & Valachovicova M (2005) Health benefits and risks of plant proteins. Bratisl Lek Listy 106, 231–234. [PubMed] [Google Scholar]
- 40.Xiao D, Zeng L, Yao K, et al. (2016) The glutamine-alpha-ketoglutarate (AKG) metabolism and its nutritional implications. Amino Acids 48, 2067–2080. [DOI] [PubMed] [Google Scholar]
- 41.Ma W, Heianza Y, Huang T, et al. (2018) Dietary glutamine, glutamate and mortality: two large prospective studies in US men and women. Int J Epidemiol 47, 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elliott P, Stamler J, Dyer AR, et al. (2006) Association between protein intake and blood pressure: the INTERMAP study. Arch Intern Med 166, 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Delgado-Pando G, Ekonomou SI, Stratakos AC, et al. (2021) Clean label alternatives in meat products. Foods 10, 1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.European Food Safety Authority. Re-evaluation of the safety of phosphates (E 338–341, E 343, E 450–452). https://www.efsa.europa.eu/en/efsajournal/pub/5674 (accessed June 2022).
- 45.Lucey JA & Fox PF (1993) Importance of calcium and phosphate in cheese manufacture: a review. J Dairy Sci 76, 1714–1724. [Google Scholar]
- 46.Seth K & Bajwa U (2015) Effect of acidulants on the recovery of milk constituents and quality of Mozzarella processed cheese. J Food Sci Technol 52, 1561–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calvo MS, Moshfegh AJ & Tucker KL (2014) Assessing the health impact of phosphorus in the food supply: issues and considerations. Adv Nutr 5, 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.León JB, Sullivan CM & Sehgal AR (2013) The prevalence of phosphorus-containing food additives in top-selling foods in grocery stores. J Ren Nutr 23, 265–270.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Górska-Warsewicz H, Rejman K, Laskowski W, et al. (2019) Milk and dairy products and their nutritional contribution to the average Polish diet. Nutrients 11, 1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ritz E, Hahn K, Ketteler M, et al. (2012) Phosphate additives in food—a health risk. Dtsch Arztebl Int 109, 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guerrero-Romero F, Rodríguez-Moran M & Reyes E (1999) Consumption of soft drinks with phosphoric acid as a risk factor for the development of hypocalcemia in postmenopausal women. J Clin Epidemiol 52, 1007–1010. [DOI] [PubMed] [Google Scholar]
- 52.Barzel US & Massey LK (1998) Excess dietary protein can adversely affect bone. J Nutr 128, 1051–1053. [DOI] [PubMed] [Google Scholar]
- 53.Barril-Cuadrado G, Puchulu MB & Sánchez-Tomero JA (2013) Table showing dietary phosphorus/protein ratio for the Spanish population. Usefulness in chronic kidney disease. Nefrologia 33, 362–371. [DOI] [PubMed] [Google Scholar]
- 54.Ravindran V, Ravindran G & Sivalogan S (1994) Total and phytate phosphorus contents of various foods and feedstuffs of plant origin. Food Chem 50, 133–136. [Google Scholar]
- 55.Lott JNA, Ockenden I, Raboy V, et al. (2000) Phytic acid and phosphorus in crop seeds and fruits: a global estimate. Seed Sci Res 10, 11–33. [Google Scholar]
- 56.Remer T (2000) Influence of diet on acid-base balance. Semin Dial 13, 221–226. [DOI] [PubMed] [Google Scholar]
- 57.Remer T (2001) Influence of nutrition on acid-base balance – metabolic aspects. Eur J Nutr 40, 214–220. [DOI] [PubMed] [Google Scholar]
- 58.Van den Berg E, Hospers FAP, Navis G, et al. (2011) Dietary acid load and rapid progression to end-stage renal disease of diabetic nephropathy in westernized south Asian people. J Nephrol 24, 11–17. [DOI] [PubMed] [Google Scholar]
- 59.Dietary Guidelines for Americans. Food sources of potassium. https://www.dietaryguidelines.gov/food-sources-potassium (accessed June 2022).
- 60.FoodData Central. https://fdc.nal.usda.gov/index.html (accessed September 2022).
- 61.Tomova A, Bukovsky I, Rembert E, et al. (2019) The effects of vegetarian and vegan diets on gut microbiota. Frontiers in Nutrition 6, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Filippis F, Pellegrini N, Vannini L, et al. (2016) High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 65, 1812–1821. [DOI] [PubMed] [Google Scholar]
- 63.Sakkas H, Bozidis P, Touzios C, et al. (2020) Nutritional status and the influence of the vegan diet on the gut microbiota and human health. Medicina (Kaunas) 56, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Losno EA, Sieferle K, Perez-Cueto FJA, et al. (2021) Vegan diet and the gut microbiota composition in healthy adults. Nutrients 13, 2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koebnick C, Leitzmann R, García AL, et al. (2005) Long-term effect of a plant-based diet on magnesium status during pregnancy. Eur J Clin Nutr 59, 219–225. [DOI] [PubMed] [Google Scholar]
- 66.Craig WJ (2009) Health effects of vegan diets. Am J Clin Nutr 89, 1627S–1633S. [DOI] [PubMed] [Google Scholar]
- 67.Kristensen NB, Madsen ML, Hansen TH, et al. (2015) Intake of macro- and micronutrients in Danish vegans. Nutr J 14, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Esche J, Shi L, Sánchez-Guijo A, et al. (2016) Higher diet-dependent renal acid load associates with higher glucocorticoid secretion and potentially bioactive free glucocorticoids in healthy children. Kidney Int 90, 325–333. [DOI] [PubMed] [Google Scholar]
- 69.Souto G, Donapetry C, Calviño J, et al. (2011) Metabolic acidosis-induced insulin resistance and cardiovascular risk. Metab Syndr Relat Disord 9, 247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krupp D, Esche J, Mensink GBM, et al. (2018) Dietary acid load and potassium intake associate with blood pressure and hypertension prevalence in a representative sample of the German adult population. Nutrients 10, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shin D & Lee KW (2021) Dietary acid load is positively associated with the incidence of hyperuricemia in middle-aged and older Korean adults: findings from the Korean Genome and epidemiology study. Int J Environ Res Public Health 18, 10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Esche J, Krupp D, Mensink GBM, et al. (2018) Dietary potential renal acid load is positively associated with serum uric acid and odds of hyperuricemia in the German adult population. J Nutr 148, 49–55. [DOI] [PubMed] [Google Scholar]
- 73.Sahın N & Gunsen U (2022) Dietary acid load and cardiovascular diseases. Crit Rev Food Sci Nutr, 1–6. [DOI] [PubMed] [Google Scholar]
- 74.Robey IF (2012) Examining the relationship between diet-induced acidosis and cancer. Nutr Metab (Lond) 9, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Disthabanchong S, Niticharoenpong K, Radinahamed P, et al. (2011) Metabolic acidosis lowers circulating adiponectin through inhibition of adiponectin gene transcription. Nephrol Dial Transplant 26, 592–598. [DOI] [PubMed] [Google Scholar]
- 76.Moghadam SK, Bahadoran Z, Mirmiran P, et al. (2016) Association between dietary acid load and insulin resistance: Tehran lipid and glucose study. Prev Nutr Food Sci 21, 104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kiefte-de Jong JC, Li Y, Chen M, et al. (2017) Diet-dependent acid load and type 2 diabetes: pooled results from three prospective cohort studies. Diabetologia 60, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Caferoglu Z, Erdal B, Hatipoglu N, et al. (2021) The effects of diet quality and dietary acid load on insulin resistance in overweight children and adolescents. Endocrinol Diabetes Nutr 69, 426–432. [DOI] [PubMed] [Google Scholar]
- 79.Akter S, Kurotani K, Kashino I, et al. (2016) High dietary acid load score is associated with increased risk of type 2 diabetes in Japanese men: the Japan Public Health Center-based Prospective Study. J Nutr 146, 1076–1083. [DOI] [PubMed] [Google Scholar]
- 80.Lim SY, Chan YM, Ramachandran V, et al. (2021) Dietary acid load and its interaction with IGF1 (rs35767 and rs7136446) and IL6 (rs1800796) polymorphisms on metabolic traits among postmenopausal women. Nutrients 13, 2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gæde J, Nielsen T, Madsen ML, et al. (2018) Population-based studies of relationships between dietary acidity load, insulin resistance and incident diabetes in Danes. Nutr J 17, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Daneshzad E, Keshavarz S-A, Qorbani M, et al. (2020) Association of dietary acid load and plant-based diet index with sleep, stress, anxiety and depression in diabetic women. Br J Nutr 123, 901–912. [DOI] [PubMed] [Google Scholar]
- 83.Murakami K, Sasaki S, Takahashi Y, et al. (2008) Association between dietary acid-base load and cardiometabolic risk factors in young Japanese women. Br J Nutr 100, 642–651. [DOI] [PubMed] [Google Scholar]
- 84.Jafari A, Ghanbari M, Shahinfar H, et al. (2021) The association between dietary acid load with cardiometabolic risk factors and inflammatory markers amongst elderly men: a cross-sectional study. Int J Clin Pract 75, e14109. [DOI] [PubMed] [Google Scholar]
- 85.Maurer M, Riesen W, Muser J, et al. (2003) Neutralization of western diet inhibits bone resorption independently of K intake and reduces cortisol secretion in humans. Am J Physiol Renal Physiol 284, F32–F40. [DOI] [PubMed] [Google Scholar]
- 86.Arisawa K, Katsuura-Kamano S, Uemura H, et al. (2020) Association of dietary acid load with the prevalence of metabolic syndrome among participants in baseline survey of the Japan Multi-Institutional Collaborative Cohort Study. Nutrients 12, 1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fatahi S, Qorbani M, Surkan PJ, et al. (2021) Associations between dietary acid load and obesity among Iranian women. J Cardiovasc Thorac Res 13, 285–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Iwase H, Tanaka M, Kobayashi Y, et al. (2015) Lower vegetable protein intake and higher dietary acid load associated with lower carbohydrate intake are risk factors for metabolic syndrome in patients with type 2 diabetes: post-hoc analysis of a cross-sectional study. J Diabetes Invest 6, 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Daneshzad E, Haghighatdoost F & Azadbakht L (2019) Dietary acid load and cardiometabolic risk factors: a systematic review and meta-analysis of observational studies. Public Health Nutr 22, 2823–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang L, Curhan GC & Forman JP (2009) Diet-dependent net acid load and risk of incident hypertension in United States women. Hypertension 54, 751–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Han E, Kim G, Hong N, et al. (2016) Association between dietary acid load and the risk of cardiovascular disease: nationwide surveys (KNHANES 2008–2011). Cardiovasc Diabetol 15, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mazidi M, Mikhailidis DP & Banach M (2018) Higher dietary acid load is associated with higher likelihood of peripheral arterial disease among American adults. J Diabetes Complications 32, 565–569. [DOI] [PubMed] [Google Scholar]
- 93.Sanz JM, Sergi D, Colombari S, et al. (2022) Dietary acid load but not Mediterranean diet adherence score is associated with metabolic and cardiovascular health state: a population observational study from northern Italy. Front Nutr 9, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Emamat H, Farhadnejad H, Poustchi H, et al. (2022) The association between dietary acid load and odds of non-alcoholic fatty liver disease: a case-control study. Nutr Health, 2601060221088383. [DOI] [PubMed] [Google Scholar]
- 95.Alferink LJM, Kiefte-de Jong JC, Erler NS, et al. (2019) Diet-dependent acid load—the missing link between an animal protein-rich diet and nonalcoholic fatty liver disease? J Clin Endocrinol Metab 104, 6325–6337. [DOI] [PubMed] [Google Scholar]
- 96.Mirmiran P, Yuzbashian E, Bahadoran Z, et al. (2016) Dietary acid-base load and risk of chronic kidney disease in adults: Tehran lipid and glucose study. Iran J Kidney Dis 10, 119–125. [PubMed] [Google Scholar]
- 97.Rebholz CM, Coresh J, Grams ME, et al. (2015) Dietary acid load and incident chronic kidney disease: results from the ARIC study. Am J Nephrol 42, 427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Scialla JJ, Appel LJ, Astor BC, et al. (2012) Net endogenous acid production is associated with a faster decline in GFR in African Americans. Kidney Int 82, 106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Banerjee T, Crews DC, Wesson DE, et al. (2014) Dietary acid load and chronic kidney disease among adults in the United States. BMC Nephrol 15, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goraya N, Simoni J, Jo C-H, et al. (2014) Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int 86, 1031–1038. [DOI] [PubMed] [Google Scholar]
- 101.So R, Song S, Lee JE, et al. (2016) The association between renal hyperfiltration and the sources of habitual protein intake and dietary acid load in a general population with preserved renal function: the KoGES study. PLoS ONE 11, e0166495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chagnac A, Zingerman B, Rozen-Zvi B, et al. (2019) Consequences of glomerular hyperfiltration: the role of physical forces in the pathogenesis of chronic kidney disease in diabetes and obesity. Nephron 143, 38–42. [DOI] [PubMed] [Google Scholar]
- 103.Goraya N, Munoz-Maldonado Y, Simoni J, et al. (2019) Fruit and vegetable treatment of chronic kidney disease-related metabolic acidosis reduces cardiovascular risk better than sodium bicarbonate. Am J Nephrol 49, 438–448. [DOI] [PubMed] [Google Scholar]
- 104.Goraya N, Simoni J, Jo C-H, et al. (2013) A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin J Am Soc Nephrol 8, 371–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ikizler TA, Burrowes JD, Byham-Gray LD, et al. (2020) KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis 76, S1–S107. [DOI] [PubMed] [Google Scholar]
- 106.Chan R, Leung J & Woo J (2015) Association between estimated net endogenous acid production and subsequent decline in muscle mass over four years in ambulatory older Chinese people in Hong Kong: a prospective cohort study. J Gerontol A Biol Sci Med Sci 70, 905–911. [DOI] [PubMed] [Google Scholar]
- 107.Hayhoe RPG, Abdelhamid A, Luben RN, et al. (2020) Dietary acid-base load and its association with risk of osteoporotic fractures and low estimated skeletal muscle mass. Eur J Clin Nutr 74, 33–42. [DOI] [PubMed] [Google Scholar]
- 108.Alexy U, Remer T, Manz F, et al. (2005) Long-term protein intake and dietary potential renal acid load are associated with bone modeling and remodeling at the proximal radius in healthy children. Am J Clin Nutr 82, 1107–1114. [DOI] [PubMed] [Google Scholar]
- 109.Kataya Y, Murakami K, Kobayashi S, et al. (2018) Higher dietary acid load is associated with a higher prevalence of frailty, particularly slowness/weakness and low physical activity, in elderly Japanese women. Eur J Nutr 57, 1639–1650. [DOI] [PubMed] [Google Scholar]
- 110.Faure AM, Fischer K, Dawson-Hughes B, et al. (2017) Gender-specific association between dietary acid load and total lean body mass and its dependency on protein intake in seniors. Osteoporos Int 28, 3451–3462. [DOI] [PubMed] [Google Scholar]
- 111.Welch AA, MacGregor AJ, Skinner J, et al. (2013) A higher alkaline dietary load is associated with greater indexes of skeletal muscle mass in women. Osteoporos Int 24, 1899–1908. [DOI] [PubMed] [Google Scholar]
- 112.Mohammadpour S, Ghorbaninejad P, Shahavandi M, et al. (2022) Interaction of dietary acid load and general and central obesity with muscle strength and skeletal muscle mass. Clin Nutr ESPEN 48, 361–369. [DOI] [PubMed] [Google Scholar]
- 113.Milajerdi A, Hassanzadeh Keshteli A, Haghighatdoost F, et al. (2020) Dietary acid load in relation to depression and anxiety in adults. J Hum Nutr Diet 33, 48–55. [DOI] [PubMed] [Google Scholar]
- 114.Mozaffari H, Siassi F, Guilani B, et al. (2020) Association of dietary acid-base load and psychological disorders among Iranian women: a cross-sectional study. Complement Ther Med 53, 102503. [DOI] [PubMed] [Google Scholar]
- 115.Tessou KD, Lemus H, Hsu F-C, et al. (2021) Independent and joint impacts of acid-producing diets and depression on physical health among breast cancer survivors. Nutrients 13, 2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bühlmeier J, Harris C, Koletzko S, et al. (2018) Dietary acid load and mental health outcomes in children and adolescents: results from the GINIplus and LISA birth cohort studies. Nutrients 10, 582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wu T, Seaver P, Lemus H, et al. (2019) Associations between dietary acid load and biomarkers of inflammation and hyperglycemia in breast cancer survivors. Nutrients 11, E1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Storz MA & Ronco AL (2022) Quantifying dietary acid load in U.S. cancer survivors: an exploratory study using NHANES data. BMC Nutr 8, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Moellering RE, Black KC, Krishnamurty C, et al. (2008) Acid treatment of melanoma cells selects for invasive phenotypes. Clin Exp Metastasis 25, 411–425. [DOI] [PubMed] [Google Scholar]
- 120.Gillies RJ, Pilot C, Marunaka Y, et al. (2019) Targeting acidity in cancer and diabetes. Biochim Biophys Acta Rev Cancer 1871, 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Park Y-MM, Steck SE, Fung TT, et al. (2019) Higher diet-dependent acid load is associated with risk of breast cancer: findings from the sister study. Int J Cancer 144, 1834–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ronco AL, Martinez-Lopez W, Mendoza BA, et al. (2021) Epidemiologic evidence for association between a high dietary acid load and the breast cancer risk. Sci Med J 3, 166–176. [Google Scholar]
- 123.Ronco AL, Storz MA, Martínez-López W, et al. (2021) High dietary acid load is associated with prostate cancer risk: an epidemiological study. World Cancer Res J 8, e2119. [Google Scholar]
- 124.Ronco AL, Martínez-López W, Calderón JM, et al. (2021) Dietary acid load and lung cancer risk: a case-control study in men. Cancer Treat Res Commun 28, 100382. [DOI] [PubMed] [Google Scholar]
- 125.Jafari Nasab S, Rafiee P, Bahrami A, et al. (2021) Diet-dependent acid load and the risk of colorectal cancer and adenoma: a case-control study. Public Health Nutr 24, 4474–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shi L-W, Wu Y-L, Hu J-J, et al. (2021) Dietary acid load and the risk of pancreatic cancer: a prospective cohort study. Cancer Epidemiol Biomark Prev 30, 1009–1019. [DOI] [PubMed] [Google Scholar]
- 127.Ronco AL, Martínez-López W, Calderón JM, et al. (2022) Dietary acid load and risk of gastric cancer: a case-control study. World Cancer Res J 9, e2403. [Google Scholar]
- 128.Ronco AL, Martínez-López W, Calderón JM, et al. Dietary acid load and esophageal cancer risk: a case-control study. Thoracic Cancer, 1–8. doi: 10.1111/1759-7714.14612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ronco AL, Martínez-López W, Calderón JM, et al. Dietary acid load and risk of head and neck and oral cavity cancers: an epidemiologic study. Oral Science International, 1–10. doi: 10.1002/osi2.1150. [DOI] [Google Scholar]
- 130.Keramati M, Kheirouri S, Musazadeh V, et al. (2022) Association of high dietary acid load with the risk of cancer: a systematic review and meta-analysis of observational studies. Front Nutr 9, 816797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bahrami A, Khalesi S, Ghafouri-Taleghani F, et al. (2022) Dietary acid load and the risk of cancer: a systematic review and dose-response meta-analysis of observational studies. Eur J Cancer Prev 31, 577–584. [DOI] [PubMed] [Google Scholar]
- 132.Müller A, Zimmermann-Klemd AM, Lederer A-K, et al. (2021) A vegan diet is associated with a significant reduction in dietary acid load: post hoc analysis of a randomized controlled trial in healthy individuals. Int J Environ Res Public Health 18, 9998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Deriemaeker P, Aerenhouts D, Hebbelinck M, et al. (2010) Nutrient based estimation of acid-base balance in vegetarians and non-vegetarians. Plant Foods Hum Nutr 65, 77–82. [DOI] [PubMed] [Google Scholar]
- 134.Knurick JR, Johnston CS, Wherry SJ, et al. (2015) Comparison of correlates of bone mineral density in individuals adhering to lacto-ovo, vegan, or omnivore diets: a cross-sectional investigation. Nutrients 7, 3416–3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Juan W, Yamini S & Britten P (2015) Food intake patterns of self-identified vegetarians among the U.S. population, 2007–2010. Procedia Food Sci 4, 86–93. [Google Scholar]
- 136.D'Alessandro C, Piccoli GB & Cupisti A (2015) The ‘phosphorus pyramid’: a visual tool for dietary phosphate management in dialysis and CKD patients. BMC Nephrol 16, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Scialla JJ & Anderson CAM (2013) Dietary acid load: a novel nutritional target in chronic kidney disease? Adv Chronic Kidney Dis 20, 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sobiecki JG, Appleby PN, Bradbury KE, et al. (2016) High compliance with dietary recommendations in a cohort of meat eaters, fish eaters, vegetarians, and vegans: results from the European Prospective Investigation into Cancer and Nutrition-Oxford study. Nutr Res 36, 464–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Allès B, Baudry J, Méjean C, et al. (2017) Comparison of sociodemographic and nutritional characteristics between self-reported vegetarians, vegans, and meat-eaters from the NutriNet-Santé study. Nutrients 9, E1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Orlich MJ & Fraser GE (2014) Vegetarian diets in the Adventist Health Study 2: a review of initial published findings. Am J Clin Nutr 100, 353S–358S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Alam I, Alam I, Paracha PI, et al. (2012) Higher estimates of daily dietary net endogenous acid production (NEAP) in the elderly as compared to the young in a healthy, free-living elderly population of Pakistan. Clin Interv Aging 7, 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Al-Kindi SG, Sarode A, Zullo M, et al. (2020) Serum bicarbonate concentration and cause-specific mortality: the National Health and Nutrition Examination Survey 1999–2010. Mayo Clin Proc 95, 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xu H, Åkesson A, Orsini N, et al. (2016) Modest U-shaped association between dietary acid load and risk of all-cause and cardiovascular mortality in adults. J Nutr 146, 1580–1585. [DOI] [PubMed] [Google Scholar]
- 144.Hejazi E, Emamat H, Sharafkhah M, et al. (2021) Dietary acid load and mortality from all causes, CVD and cancer: results from the Golestan Cohort Study. Br J Nutr, 1–7. [DOI] [PubMed] [Google Scholar]
- 145.Craig WJ, Mangels AR & Brothers CJ (2022) Nutritional profiles of non-dairy plant-based cheese alternatives. Nutrients 14, 1247. [DOI] [PMC free article] [PubMed] [Google Scholar]



