Abstract
Objective
There is a paucity of validated diagnostic interviews for avoidant/restrictive food intake disorder (ARFID) to aid identification and classification of cases for both clinical and research purposes. To evaluate the factor structure, construct validity, and criterion validity of the Pica ARFID and Rumination Disorder Interview (PARDI; ARFID module), we administered the PARDI to 129 children and adolescents ages 9–23 years (M = 16.1) with ARFID (n = 84), subclinical ARFID (n = 11), and healthy controls (n = 34).
Method
We used exploratory factor analysis to examine the factor structure of the PARDI in children, adolescents, and young adults with an ARFID diagnosis, the Kruskal‐Wallis analysis of variance and Spearman correlations to test the construct validity of the measure, and non‐parametric receiver operating characteristic curves to evaluate the criterion validity of the PARDI.
Results
Exploratory factor analysis yielded a 3‐factor structure: (1) concern about aversive consequences of eating, (2) low appetite/low interest in food, and (3) sensory sensitivity. Participants with ARFID demonstrated significantly higher levels of sensory sensitivity, low appetite/low‐food interest, and concern about aversive consequences of eating symptoms relative to control participants. The construct validity for each PARDI subscale was supported and clinical cutoffs for the low appetite/low interest in food (1.1) and sensory sensitivity subscales (0.6) were established.
Discussion
These data present evidence for the factor structure and validity of the PARDI diagnostic interview for diagnosing ARFID in children, adolescents, and young adults, supporting the use of this tool to facilitate ARFID clinical assessment and research.
Public Significance
Due to the paucity of validated diagnostic interviews for avoidant/restrictive food intake disorder (ARFID), we evaluated the factor structure and validity of the Pica ARFID and Rumination Disorder Interview (ARFID module). Findings suggest that the interview assesses 3 components of ARFID: concern about aversive consequences of eating, low‐appetite, and sensory sensitivity, and that clinical threshold scores on the latter two subscales can be used to advance ARFID assessment.
Keywords: assessment, avoidant/restrictive food intake disorder, diagnosis, factor analysis, feeding and eating disorders, Pica, rumination disorder interview, receiver operating characteristic, youth
1. INTRODUCTION
Avoidant/restrictive food intake disorder (ARFID) is a heterogenous psychological disorder characterized by a limitation in the type or quantity of food consumed, not motivated by body shape or weight concerns, leading to significant physical or psychosocial impairment (APA, 2013; WHO, 2018). Individuals affected by ARFID most commonly present with aversion due to the sensory qualities of foods, low appetite/low‐food interest, or concern about aversive consequences of eating (e.g., choking, vomiting), or a combination of the three (Norris et al., 2014; Strand, von Hausswolff‐Juhlin, & Welch, Strand et al., 2019; Thomas et al., 2017; Zickgraf, Lane‐Loney, Essayli, & Ornstein, Zickgraf et al., 2019). While these feeding/eating concerns were previously classified under multiple diagnoses in earlier iterations of the Diagnostic and Statistical Manual of Mental Disorders (DSM), including feeding disorder of infancy and early childhood and eating disorder not otherwise specified, the DSM‐5 ARFID criteria acknowledges the presence of these symptoms across the lifespan. While the true prevalence of ARFID in the general population remains unknown, current estimates suggest that <5% of children and <1% of adults may be affected by ARFID, though data on developmental differences in symptom presentation is lacking (Bertrand et al., 2021; Chen, Chen, Lin, Shen, & Gau, Chen et al., 2019; Hay et al., 2017; Herle et al., 2020). Up to 14% of patients seeking treatment for eating disorders and 32% of patients seeking treatment for feeding problems may have ARFID (Fisher et al., 2014; Forman et al., 2014; Nakai, Nin, Noma, Teramukai, & Wonderlich, Nakai et al., 2016; Nicely, Lane‐Loney, Masciulli, Hollenbeak, & Ornstein, Nicely et al., 2014; Norris et al., 2014; Ornstein et al., 2013; Williams et al., 2015). Given the profound impairments associated with ARFID, including nutritional deficiencies, poor growth/development, need for supplemental feeds, or significant psychosocial dysfunction, the development of tools to reliably identify cases is essential to enhance allocation to needed interventions and derive accurate population level estimates (Hay et al., 2017; Norris et al., 2014).
Since the introduction of ARFID, four diagnostic interviews have been developed or adapted for the assessment of ARFID: the Eating Disorders Assessment for DSM‐5 (EDA‐5)(Sysko et al., 2015), the Eating Disorders Examination (EDE)/Eating Disorders Examination for Children (ChEDE) (Schmidt, Kirsten, Hiemisch, Kiess, & Hilbert, Schmidt et al., 2019), the Pica, ARFID and Rumination Disorder Interview (PARDI) (Bryant‐Waugh et al., 2019), and the Structured Clinical Interview for DSM‐5 (SCID‐5) (First, Williams, Karg, & Spitzer, First et al., 2015). Among these, the PARDI interview can be used across the developmental span, with versions adapted for caregivers of young children (2–3 years), caregivers of children 4 years and older, children, and young people/adults. The EDA‐5 and EDE/ChEDE can be used with children as young as 8 years old, and the SCID can currently be used only among adults, though child and adolescent versions are in preparation. All interviews assess ARFID symptoms across the three most common symptom dimensions (i.e., sensory sensitivity, low appetite/food interest, and concern about aversive consequences of eating), which the leading theory posits are three biologically‐based dimensions of ARFID symptoms that can co‐occur within an individual (Thomas et al., 2017). Understanding the degree to which symptoms from each dimension are present for a patient is essential to identify the most useful behavioral interventions, such as systemic desensitization for sensory sensitivity, meal scheduling for low appetite, and in‐vivo exposure for concerns about aversive consequences of eating. Beyond assessing symptoms within these three domains, the EDE also includes questions relevant to emotionally driven avoidant/restrictive eating (Schmidt et al., 2019). However, the PARDI is the only one of these interviews that also includes a severity rating scale to assess ARFID‐related impairment. The inclusion of an ARFID severity score is essential to enhance the clinical utility of an interview for diagnostic accuracy, treatment planning and tracking of symptom change.
Currently, there are only limited data available regarding the reliability and validity of these diagnostic interviews for ARFID. Preliminary evidence for the inter‐rater reliability and construct validity of the ChEDE ARFID module has been established in a small non‐clinical sample of 8–13 year‐old German children (Schmidt et al., 2019). Further, an investigation of the PARDI demonstrated preliminary evidence for reliability and validity of the PARDI in a sample of 10–22 year‐olds with clinically significant avoidant/restrictive eating (Bryant‐Waugh et al., 2019). However, there remains an urgent need to validate these diagnostic interviews for ARFID in larger samples of individuals with clinically significant symptoms, to enhance diagnostic accuracy of ARFID assessment and enable more precise treatment planning and tracking symptom change for affected individuals.
The aim of the present investigation was to explore the factor structure of the ARFID dimensional and severity items of the PARDI in a sample of children, adolescents, and young adults with ARFID, to enhance the clinical assessment across its major symptom dimensions. As the only semi‐structured clinical interview for ARFID with versions for use across the lifespan by multiple informants, and one that provides both a measure of ARFID symptoms and overall severity, the PARDI stands to fill the existing gap as a comprehensive ARFID assessment tool.
2. METHODS
2.1. Participants
Participants for this study included 129 children and young adults 9–23 years old, 50.4% male, who were recruited as part of an ongoing multidisciplinary study of the neurobiology of ARFID (National Institute of Mental Health R01MH108595). Participants with full (n = 84) and subthreshold (n = 11) ARFID were recruited along with heathy controls (n = 34). Participants were recruited through flyers, social media, the hospital's research recruitment website, local pediatric and adolescent medicine practices, and eating disorder clinics (ARFID cases only). Forty‐three participants with ARFID were receiving treatment for ARFID at an affiliated hospital clinic, and the treating clinician's ratings of ARFID symptoms were obtained from clinic records. The demographic characteristics of the sample are detailed in Table 1. The clinical characteristics of a subset of this sample (N = 49) have been previously reported by Bryant‐Waugh et al. (2019).
TABLE 1.
Participant characteristic and group comparisons
| ARFID (n = 84) M (SD) or % | Subthreshold ARFID (n = 11) M, (SD) or % | Control (n = 34) M, (SD)or % | Test of group differences | |
|---|---|---|---|---|
| Sex (% female) | 47.6% | 63.6% | 50.0% | Χ2(2) = 1.001, p = .606 |
| Age (M, SD) | 15.9 (4.0) | 16.5 (3.6) | 16.7 (4.4) | F(128) = 0.51, p = .604 |
| Race (%) | Χ2(8) = 23.10, p = .003 | |||
| Asian | 1.2% | 0.0% | 8.8% | |
| Black | 2.4% | 0.0% | 2.9% | |
| White | 94.0% | 72.7% | 76.5% | |
| Multiple | 2.4% | 27.3% | 5.9% | |
| Unknown | 0.0% | 0.0% | 5.9% | |
| Ethnicity | Χ2(4) = 5.67, p = .224 | |||
| Non‐Hispanic/Latino | 90.5% | 90.9% | 85.3% | |
| Hispanic/Latino | 9.5% | 9.0% | 8.8% | |
| Unknown | 0.0% | 0.0% | 5.9% |
| ARFID Symptoms | Rank mean M(SD) | Rank mean M(SD) | Rank mean M(SD) | |
|---|---|---|---|---|
| Concern about aversive consequence | 71.1 0.5(1.0) | 71.1 0.4(0.7) | 48.0 0(0) | Χ2(2) = 15.90, p = .0004 ARFID > C ST > C ARFID = ST, d = 0.1 |
| Low appetite/low interest | 78.8 2.1(1.7) | 75.4 1.8(1.4) | 27.6 0.2(0.3) | Χ2(2) = 47.17, p = .0001 ARFID > C, d = 1.3 ST > C, d = 2.2 ARFID = ST, d = 0.2 |
| Sensory Sensitivity | 79.9 1.7 (1.3) | 81.1 1.6 (1.3) | 23.1 0.03(0.1) | Χ2(2) = 59.89, p = .0001 ARFID > C, d = 1.5 ST > C, d = 2.5 ARFID = ST, d = 0.1 |
Abbreviations: C, control; d, Cohen's d for comparison of mean differences between the groups; ST, subthreshold ARFID.
Participants were eligible for the study if they met diagnostic criteria for ARFID on the Eating Disorder Assessment for DSM‐5 (EDA‐5; ARFID case) (Sysko et al., 2015) or demonstrated significant symptoms of ARFID on the KSADS, defined as clinically significant restriction in food volume or variety, as determined by clinical consensus during weekly assessment supervision meetings with study PI (JTT). Participants were excluded from participating if they experienced: (a) any current feeding or eating disorder (determined by EDA‐5 and a modified KSADS interview) that precludes a DSM‐5 diagnosis of ARFID; (b) disordered eating or purging behaviors due to weight/shape concerns in the past 28 days reported on the Eating Disorder Examination‐Questionnaire (EDE‐Q; global score >4.0) or KSADS interview (Fairburn & Beglin, 2008; Kaufman et al., 1997); (c) suicidal ideation; (d) substance or alcohol use disorders (determined by KSADS‐PL); (e) current or lifetime psychosis reported on the KSADS‐PL; and (f) medical history of intellectual disability (IQ < 70). See Kambanis et al. (2020) for additional exclusion criteria associated with functional magnetic resonance imaging (fMRI) and neuroendocrine assessments not analyzed here. Control participants were 9–23 years old with BMI in the 15th‐85th percentile without a history of a psychological disorders (via EDA‐5 and KSADS) or disordered eating (via EDE‐Q global >4.0). Controls were matched to the ARFID group (full and subthreshold) for sex, age, and pubertal development (Tanner stage). Written informed consent/assent was obtained from all participants (and their caregivers if under 18 years). All procedures were reviewed and approved by the Institutional Review Board of the institution.
2.2. Measures
2.2.1. ARFID diagnosis
ARFID diagnosis was assessed using the Eating Disorders Assessment for DSM‐5 (EDA‐5). The EDA‐5 is a semi‐structured interview designed to assess the presence of DSM‐5 diagnoses of eating and feeding disorders that has been validated for use in adolescents and adults with eating and feeding disorders (Sysko et al., 2015).
2.2.2. ARFID symptoms
ARFID symptoms were assessed using the Pica ARFID and Rumination Disorder Interview (PARDI)‐child version (ages 9–13 years) or young person/adult version (14+ years). The PARDI is a semi‐structured diagnostic interview for ARFID that includes screening questions for weight and shape concerns, to screen for eating disorders that preclude an ARFID diagnosis. Preliminary evidence for the feasibility, reliability, validity of the PARDI has been established in a sample (N = 49) of youth with avoidant/restrictive eating and healthy controls (Bryant‐Waugh et al., 2019). For the present study, we explored the factor structure of data obtained through the participant individual interview.
2.2.3. Anxiety
Anxiety was assessed via self‐report on the Beck Anxiety Inventory (BAI), for participants 18 and older (α = .94, present sample), and the Beck Anxiety Inventory‐Youth version (BAI‐Y), for participants under 18 (α = .89, present sample) (A. T. Beck, Epstein, Brown, & Steer, Beck et al., 1988; J. S. Beck, Beck, & Jolly, Beck et al., 2005), which have been validated for use in adult and pediatric populations, respectively (J. S. Beck et al., 2005; Fydrich, Dowdall, & Chambless, Fydrich et al., 1992; Steer, Kumar, Beck, & Beck, Steer et al., 2001).
2.2.4. Sensory sensitivity
Sensory sensitivity was assessed using an abbreviated version of the Adolescent/ Adult Sensory Profile (AASP). The AASP questionnaire evaluates behavioral responses to sensory experiences and is validated for use in adolescents and adults (Pearson, 2019). A sensory threshold score was derived from the mean of the 8 taste/smell processing items after reverse scoring items from the empirically derived high neurological threshold quadrants so that higher mean scores reflect detection of sensory input at lower thresholds (α = .42, present sample).
2.2.5. Subjective appetite and food interest
Subjective appetite and food interest were assessed using the Adult Eating Behaviors Questionnaire (AEBQ)(Hunot et al., 2016), which has been previously used in adolescent samples that have demonstrated strong support for the factor structure in 14–17 year‐olds (Hunot‐Alexander et al., 2019). Of the eight AEBQ subscales, satiety responsiveness (SR, α = .77, present sample), enjoyment of food (EF, α = .89, present sample), and slowness in eating (SE, α = .86, present sample) scales were examined in this study, with higher scores indicating quicker satiety, greater enjoyment of food, and slower eating.
2.2.6. Clinician rating of ARFID symptom profile
Clinician rating of ARFID symptom profile was obtained for a subsample (N = 43) of participants who were also receiving treatment for ARFID through an affiliated hospital clinic. These ratings were used as the criterion variable in receiver operating characteristic curve analysis of the PARDI. Through standard intake procedures in the clinic, the treating clinician dichotomously rated the presence of clinically significant ARFID symptoms in the domains of concern about aversive consequences, low appetite/low interest, and sensory sensitivity at the clinical intake assessment using their clinical judgment. Treating clinicians had master's or doctoral level training and worked under the supervision of a licensed clinical psychologist or psychiatrist.
2.3. Analytic plan
2.3.1. Factor structure
Factor structure of the PARDI dimensional symptom items and severity items were evaluated using principal factor analysis. Prior to conducting factor analyses, the Kaiser‐Meyer‐Olkin (KMO) measure of sampling adequacy and Bartlett's test of sphericity were used to confirm appropriateness of data for factor analysis. In the principal factor method, the factor loadings are computed using the squared multiple correlations as estimates of the communality, which allows for the assumption of the presence of latent variables. An oblique (promax) rotation was then applied to enhance interpretability of factor loadings, given the likelihood of intercorrelation between factors. A first exploratory factor analysis was conducted using the 31 PARDI items designed to elicit continuous ratings of ARFID symptoms associated with the three most common symptom dimensions, concern about aversive consequences, low appetite/low interest, and sensory sensitivity. Eigen values (>1) and the scree plot slope were examined in combination with the conceptual fit of items to determine the number of factors to be retained. Items with factor loadings of .35 or higher were retained (Hair, Black, Black, Babin, & Anderson, Hair et al., 2010). Cross‐loading items were retained on the factor with the higher factor loading, if it was at least .20 higher than the cross‐loading, otherwise the item was dropped (Costello & Osborne, 2005). A second factor analysis was conducted on the 17 PARDI items intended to assess severity of functional impairments associated with PARDI symptoms using the same procedures. The PARDI item which assesses growth impairment was excluded from this factor analysis, because in this iteration of the PARDI it was only administered to participants under 20 years of age. Based on the factors derived from the exploratory factor analysis, subscales of the PARDI were created by obtaining the mean value of item ratings within each factor. These subscales were then used for subsequent validity analyses.
2.3.2. Construct validity
Construct validity was assessed using non‐parametric tests due to non‐normality of PARDI data, resulting from the high frequency of “0” responses among control participants. First, group differences in demographic characteristics were evaluated using the Chi‐square test and analysis of variance. Next, differences in PARDI subscale scores between ARFID, subthreshold ARFID and control groups were evaluated using the Kruskal‐Wallis one‐way analysis of variance with a post hoc Dunn test for multiple comparisons. Within the ARFID group (N = 84), continuous associations between PARDI subscale scores and theoretically associated self‐report questionnaires of anxiety, appetite and food interest, and sensory sensitivity were evaluated using Spearman correlations with a Bonferroni correction for multiple comparisons.
2.3.3. Criterion validity
Criterion validity of the PARDI for identifying clinically significant symptoms of ARFID within each major symptom dimension was evaluated using nonparametric receiver operating characteristic (ROC) curves. Presence of clinically significant symptoms in the domains of sensory sensitivity, low appetite/low interest, and/or concern about aversive consequences, as rated by the treating clinician, was used as the criterion variable (n = 43). Data from control participants (n = 34), coded as symptoms absent in all three dimensions, were also included in the subsample used for ROC analyses, for a total sample of N = 77.
All analyses were conducted using StataSE, version14.2 (StataCorp., 2015).
3. RESULTS
The full sample (N = 129) included children, adolescents, and young adults ages 9–23 years old (M = 16.1, SD = 4.0), 49.2% female, and predominantly White (87.6%) Non‐Hispanic (89.2%). There were no significant group differences in participant age, sex, and ethnicity, whereas the groups varied by racial composition (see Table 1).
3.1. Factor analysis
Among the 31 PARDI interview items intended to measure dimensional symptoms of ARFID (concern about aversive consequences, low appetite/low interest, and sensory sensitivity) the KMO measure of sampling adequacy (KMO = .733) and Bartlett's test of sphericity (p < .001) indicated these data were adequate for factor analysis. The principal factor analysis of these 31 PARDI items first returned a 6‐factor solution, in which 74.0% of the variance was accounted for by the first four factors. Visual inspection of the eigenvalue plot revealed visibly decreased slope after the fourth factor. Upon inspection of rotated factor loadings, among the six items that did not load onto the first four factors, four items were distributed across factors five and six without thematic consistency and two did not load onto any factors. Therefore, we re‐ran the principal factors analysis restricting the model to a 4‐factor solution. In the 4‐factor solution, there was moderate intercorrelation of factors (Table 2) and the slope of the eigenvalue plot visibly decreased after the fourth factor (see Figure 1), however the first three factors accounted for 66.5% of the variance. Upon inspection of rotated factor loadings (Table 3), nearly all items loaded on the first three factors, with only four items loading exclusively on the fourth factor. The nine items that loaded onto Factor 1 pertain to concern about aversive consequences of eating. The eight items that loaded onto Factor 2 pertain to low appetite and low‐food interest. The eight items that loaded onto Factor 3 pertain to sensory sensitivity. Each factor was subsequently scored as a subscale of the PARDI by calculating the mean value of the associated items.
TABLE 2.
Factor intercorrelations for factor analyses
| ARFID dimensional symptoms on the PARDI | ||||
|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | |
| Factor 1 | 1.000 | |||
| Factor 2 | .369 | 1.000 | ||
| Factor 3 | −.012 | .128 | 1.000 | |
| Factor 4 | .280 | .199 | .156 | 1.000 |
| ARFID severity symptoms on the PARDI | ||||
|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 3 | ||
| Factor 1 | 1.000 | |||
| Factor 2 | .324 | 1.000 | ||
| Factor 3 | −.112 | .163 | 1.000 | |
FIGURE 1.
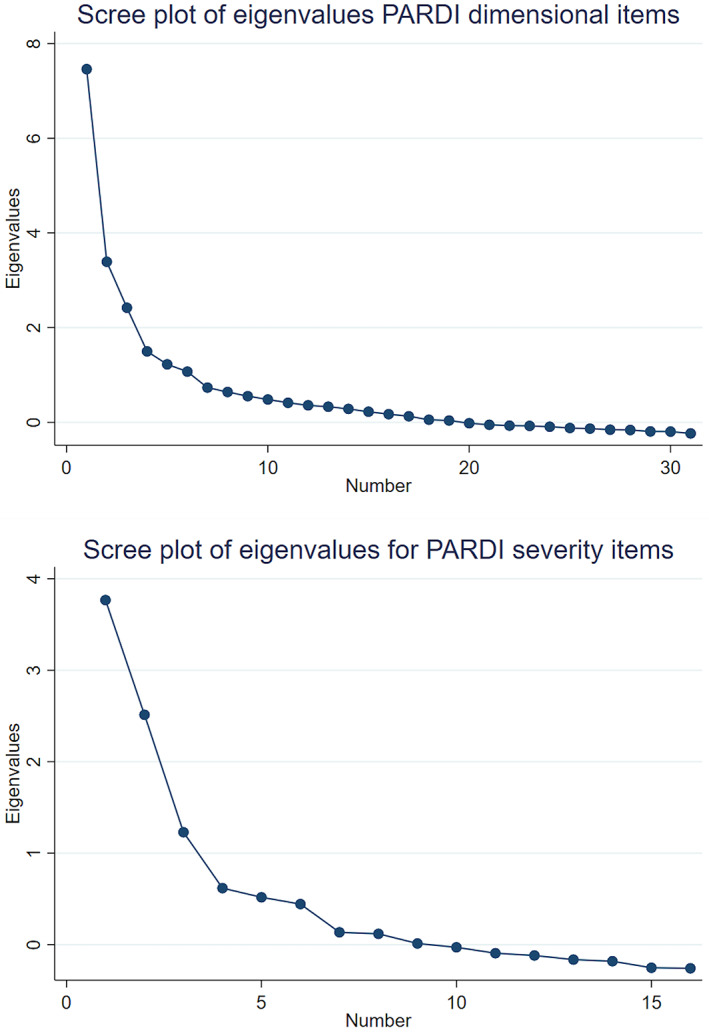
Scree plots of eigen values for Pica ARFID and Rumination Disorder Interview (PARDI) dimensional symptom items and PARDI severity items. ARFID, avoidant/restrictive food intake disorder
TABLE 3.
Rotated factor loadings for items assessing ARFID dimensional symptoms on the PARDI
| Items | Factor 1 | Factor 2 | Factor 3 | Factor 4 |
|---|---|---|---|---|
| 50. Over the past month has it bothered you, having food around your mouth or on your hands? | 0.540 | |||
| 51. Over the past month has brushing your teeth bothered you? | 0.438 | |||
| 52. Over the past month have strong food smells bothered you? | 0.652 | |||
| 53. Over the past month have you been sensitive to differences in taste? | 0.705 | |||
| 54. Over the past month have you preferred foods to be a certain temperature? | 0.407 | |||
| 55. Do you cough, choke or gag on certain types or textures/consistencies of food or drinks? | 0.631 | |||
| 56. Over the past month has the smell of food been important to you in deciding whether to eat it? | 0.549 | |||
| 57. Over the past month have you been put off food if it does not look “right”? | 0.551 | |||
| 58. Over the past month have you preferred foods to be a certain color (beige or brown)? | ||||
| 59. Over the past month have you preferred to eat food of a certain brand? | 0.545 | |||
| 60. Some people avoid foods or eat very little because they find it hard to realize when they are hungry. Over the past month, has this happened to you? | 0.776 | |||
| 61. Over the past month, have you forgotten to eat or found it difficult to make time to eat? | 0.459 | 0.339 | ||
| 62. Over the past month, have you looked forward to eating (even if just preferred food) before mealtimes? a | 0.755 | |||
| 63. Over the past month, have you had a good appetite? a | 0.761 | |||
| 64. Over the past month, have you needed to be prompted or reminded to eat? | 0.712 | |||
| 65. Over the past month, have you eaten less when you were upset or worried? | 0.488 | |||
| 66. Over the past month, have you eaten less when you were happy, excited or looking forward to something? | ||||
| 67. Over the past month, how much have you found eating to be a chore? | 0.704 | |||
| 68. Over the past month, have you enjoyed food or eating (even if only a few foods)? a | 0.787 | |||
| 69. Over the past month, have you been full before your meal is finished or sooner than others? | 0.566 | 0.403 | ||
| 70. Over the past month, have you felt uncomfortable when you are full? | 0.567 | |||
| 71b. Over the past 4 weeks, have you been concerned that eating will make you choke? | 0.671 | |||
| 72b. Over the past 4 weeks, have you been concerned that eating will make you be sick (involuntarily) or cause diarrhea? | 0.376 | |||
| 73b. Over the past 4 weeks, have you been worried that food might be bad for you in any way (i.e., may contain an allergen)? | 0.581 | |||
| 74b. Over the past 4 weeks, have you been worried that eating might cause you pain? | 0.513 | |||
| 75. Over the past month, have you felt afraid of eating? | 0.810 | |||
| 76. Over the past month, have you spent much time worrying that something bad might happen if you eat? Has worrying about eating made it hard for you to concentrate? | 0.915 | |||
| 77. Over the past month have you been eating less food because you were afraid that something bad might happen, like being sick, choking, having an allergic reaction, or being in pain? | 0.723 | |||
| 78. Over the past month have you had feelings like a racing heart, sweaty hands, feeling sick when you have seen something that reminded you of: (being sick, choking, etc)? | 0.722 | |||
| 79. Over the past month have you avoided food or mealtimes that you were worried might make you be sick, choke, have an allergic reaction, or be in pain while eating? | 0.911 | |||
| 80. Over the past month have you done anything special before or while you were eating to stop yourself from being sick, choking, having an allergic reaction, or being in pain while eating? | 0.591 | −0.305 | 0.356 |
Note: Bolding indicates item was retained on this factor for subsequent analyses. All items are rated on a 7‐point Likert scale (0–6) with lower scores representing little difficulty and higher scores indicating more severe eating/feeding difficulty.
The rating scale on these PARDI items was reversed, such that higher ratings indicate more problematic eating/feeding behaviors. Items 1–29 and 49 were not included in the factor analysis because they assess medical history, Pica symptoms, and include dichotomous ratings of eating and feeding problems.
The four items that loaded exclusively on to the fourth factor asked about disturbance when brushing teeth, eating less when upset/worried, feeling uncomfortable when full, and fear that food may be harmful. Additionally, two items did not load onto any of the four factors (preferred foods to be a certain color and eating less when happy/excited). Given the lack of conceptual coherence in these items, the fourth factor was eliminated, and these items were dropped from our subsequent validity analyses. Factors 1–3 were then examined for cross‐loading items, and the one cross‐loading item (80) was retained due to having at least a .20 difference between factor loadings. The three subscales of the PARDI, concern about aversive consequences of eating, low appetite/low‐food interest, and sensory sensitivity were then used for the validity analyses.
A second exploratory factor analysis was conducted on a subset of items designed to evaluate the severity of ARFID‐related functional impairments. Among these items the KMO (KMO = .711) and Bartlett's test of sphericity (p < .001) also indicated that the data were adequate for factor analysis. A principal factor method with promax rotation was applied, which yielded a 3‐factor solution that accounted for 91.5% of the variance, with moderate intercorrelation of factors (Table 2). Inspection of eigenvalues also supported a 3‐factor solution (Figure 1). All items were retained and loaded on to three factors that reflected symptom severity associated with restriction in range of foods consumed (factor 1), social impairments due to restricted eating (factor 2) and difficulty meeting nutritional needs (factor 3, Table 4). Two items (29e and 46) were dropped due to cross‐loading. Acceptable internal consistency was also found when all retained severity items were combined to generate overall severity score (α = .77).
TABLE 4.
Rotated factor loadings for items assessing ARFID severity on the PARDI
| Items | 1 | 2 | 3 |
|---|---|---|---|
| 29a. Over the past month, have you been eating a range of fruits? a | 0.495 | ||
| 29b. Over the past month, have you been eating a range of vegetables? a | 0.798 | ||
| 29c. Over the past month, have you been eating a range of protein foods? a | 0.680 | ||
| 29d. Over the past month, have you been eating a range of dairy or calcium‐enriched dairy substitute foods? a | 0.618 | ||
| 29e. Over the past month, have you been eating a range of starchy/carbohydrate foods (e.g., rice, pasta, bread, cereal)? a | 0.454 | 0.361 | |
| 30. Over the past month, have you eaten exactly the same food at mealtimes? | 0.620 | ||
| 32. If someone asked you to try a new food that is not something you have ever eaten before, how likely would you be to try it? How would you feel about trying it? | 0.361 | ||
| 33. Over the past month have your family or doctors been worried that you are finding it difficult to eat enough? | 0.827 | ||
| 34. Over the past 3 months has your doctor or anyone in your family worried that you are not putting on weight or that you are getting thinner? | 0.786 | ||
| 40. Do you think your eating affects your family members/significant others? If so how? Are they worried about your eating? | 0.673 | ||
| 41. Does your eating cause problems for you at home e.g., arguments with parents or brothers and sisters? | 0.522 | ||
| 42. How do you get on at mealtimes? Are they difficult or upsetting? | 0.674 | ||
| 43. When you eat meals with others (e.g., family, friends), do you usually eat something different than they do? | 0.648 | ||
| 46. Do you have to be given a reward, reminded or made to take a bite or to eat? | 0.369 | 0.533 | |
| 47. Does your eating make things hard for you, for example does it make it difficult for you to go to friends' houses or eat at school or stay away from home? | 0.587 | ||
| 48. Does your eating make things hard for you at school? | 0.643 |
Note: Bolding indicates item was retained on this factor. All items are rated on a 7‐point Likert scale (0–6) with lower scores representing little impairment and higher scores indicating more severe eating/feeding impairment.
The rating scale on these PARDI items was reversed, such that higher ratings indicate more restrictive eating behaviors. Items 1–29 and 49 were not included in the factor analysis because they assess medical history, Pica symptoms, and include dichotomous ratings of eating and feeding problems.
3.2. Construct validity
The construct validity of the dimensional symptom subscales of the PARDI was evaluated by comparing scores on these subscales between the participant groups, that is, those with an ARFID diagnosis, subclinical ARFID, and healthy controls via the Kruskal‐Wallis test with a follow‐up Dunn‐test for group differences. The Kruskal‐Wallis test revealed significant differences between the three groups on the sensory sensitivity, low appetite/low interest, and concern about aversive consequences subscales. Follow‐up Dunn test revealed that on the concern about aversive consequences, low appetite, and sensory sensitivity subscale of the PARDI, the mean ranks of participants with ARFID and subthreshold ARFID were significantly higher than control participants (p < .05, Table 1). No differences were found on the PARDI subscales between participants with ARFID and subthreshold ARFID.
Among the participants with an ARFID diagnosis on the PARDI (N = 84), continuous relationships between PARDI subscale scores and self‐reports of anxiety, appetite, food interest, and sensory sensitivity, were tested using Spearman correlations with a Bonferroni correction for multiple comparisons. Due to missing self‐report data, sample sizes were slightly reduced for these analyses. The PARDI concern about aversive consequences subscale significantly correlated with self‐reported anxiety, but the other two subscales of the PARDI did not (Table 5). The low appetite/low‐interest subscale of the PARDI significantly correlated with self‐reported satiety responsiveness and enjoyment of food, whereas the sensory sensitivity subscale of the PARDI did not significantly correlate with these self‐reports. However, the concern about aversive consequences subscale also significantly correlated with self‐reported slowness in eating and satiety responsiveness. Finally, the sensory sensitivity subscale of the PARDI significantly correlated with self‐report of sensory sensitivity, whereas the other two subscales of the PARDI did not.
TABLE 5.
Spearman correlations of PARDI subscales and self‐reports of anxiety, appetite, and sensory sensitivity
| PARDI‐C | PARDI‐LA | PARDI‐SS | BAI | |||
|---|---|---|---|---|---|---|
| PARDI‐C | 1.000 | |||||
| 84 | ||||||
| PARDI‐LA | .395 | 1.000 | ||||
| 84 | 84 | |||||
| .001 | ||||||
| PARDI‐SS | −.007 | .149 | 1.000 | |||
| 84 | 84 | 84 | ||||
| 1.000 | 1.000 | |||||
| BAI | .328 | .274 | .219 | 1.000 | ||
| 83 | 83 | 83 | 85 | |||
| .015 | .074 | .278 |
| PARDI‐C | PARDI‐LA | PARDI‐SS | AEBQ‐SE | AEBQ‐SR | AEBQ‐EF | |
|---|---|---|---|---|---|---|
| PARDI‐C | 1.000 | |||||
| 84 | ||||||
| PARDI‐LA | .395 | 1.000 | ||||
| 84 | 84 | |||||
| .003 | ||||||
| PARDI‐SS | −.007 | .149 | 1.000 | |||
| 84 | 84 | 84 | ||||
| 1.000 | 1.000 | |||||
| AEBQ‐SE | .335 | .314 | −.061 | 1.000 | ||
| 82 | 82 | 82 | 83 | |||
| .031 | .061 | 1.000 | ||||
| AEBQ‐SR | .345 | .513 | .138 | .489 | 1.000 | |
| 82 | 82 | 82 | 83 | 83 | ||
| .023 | .000 | 1.000 | .000 | |||
| AEBQ‐EF | −.286 | −.735 | −.190 | −.124 | −.365 | 1.000 |
| 82 | 82 | 82 | 83 | 83 | 83 | |
| .140 | .000 | 1.000 | 1.000 | .010 |
| PARDI‐C | PARDI‐LA | PARDI‐SS | AASP‐ST | |||
|---|---|---|---|---|---|---|
| PARDI‐C | 1.000 | |||||
| 84 | ||||||
| PARDI‐LA | .395 | 1.000 | ||||
| 84 | 84 | |||||
| .001 | ||||||
| PARDI‐SS | −.007 | .149 | 1.000 | |||
| 84 | 84 | 84 | ||||
| 1.000 | 1.000 | |||||
| AASP‐ST | .030 | .126 | .478 | 1.000 | ||
| 83 | 83 | 83 | 85 | |||
| 1.000 | 1.000 | .000 |
Note: For each correlation r s , N, and p value corrected for multiple comparisons is reported. Significant correlations are bolded.
Abbreviations: AASP‐ST, adolescent and adult sensory profile sensory threshold; AEBQ‐EF, AEBQ enjoyment of food scale; AEBQ‐SE, ASEBQ slowness in eating scale; AEBQ‐SR, AEBQ satiety responsiveness scale; BAI, Beck Anxiety Inventory; PARDI‐C, PARDI concern about aversive consequences subscale; PARDI‐LA, PARDI low appetite/low‐interest subscale; PARDI‐SS, PARDI sensory sensitivity subscale.
3.3. Criterion validity
Finally, the criterion validity of the three PARDI dimensional symptom subscales for predicting clinical diagnosis of ARFID was evaluated using non‐parametric receiver operating characteristic (ROC) curves. By treating clinician ratings, in this subsample (N = 77), 29.9% of participants presented with low appetite/low interest symptoms of ARFID, 46.8% presented with sensory sensitivity symptoms, and 11.7% presented with concern about aversive consequences symptoms. The ROC curves for both the low appetite/low interest (AUC = .837 [0.734, .939]) and sensory sensitivity subscales (AUC = .884 [.809, .959]) demonstrated excellent criterion validity (Figure 2). On the low appetite/low interest subscale of the PARDI, a clinical cutoff score of 1.1 correctly identified 83.1% of cases (sensitivity, 82.6%; specificity, 83.3%; symptomatic M = 2.4, SD = 1.6; asymptomatic M = 0.6, SD = 1.0). On the sensory sensitivity subscale of the PARDI, a clinical cutoff score of 0.6 correctly identified 84.4% of cases (sensitivity, 83.3%; specificity, 85.4%; symptomatic M = 1.7, SD = 1.3; asymptomatic M = 0.2, SD = 0.5). The concern about aversive consequences subscale of the PARDI ROC curve showed an area under the curve of .802 [0.614, 0.991], with a clinical cutoff of 1.6 correctly classifying 94.8% of cases (Figure 2). However, due to low test sensitivity (55.6%; specificity, 100%; symptomatic M = 1.5, SD = 1.4; asymptomatic M = 0.1, SD = 0.2), these estimates must be interpreted with great caution.
FIGURE 2.
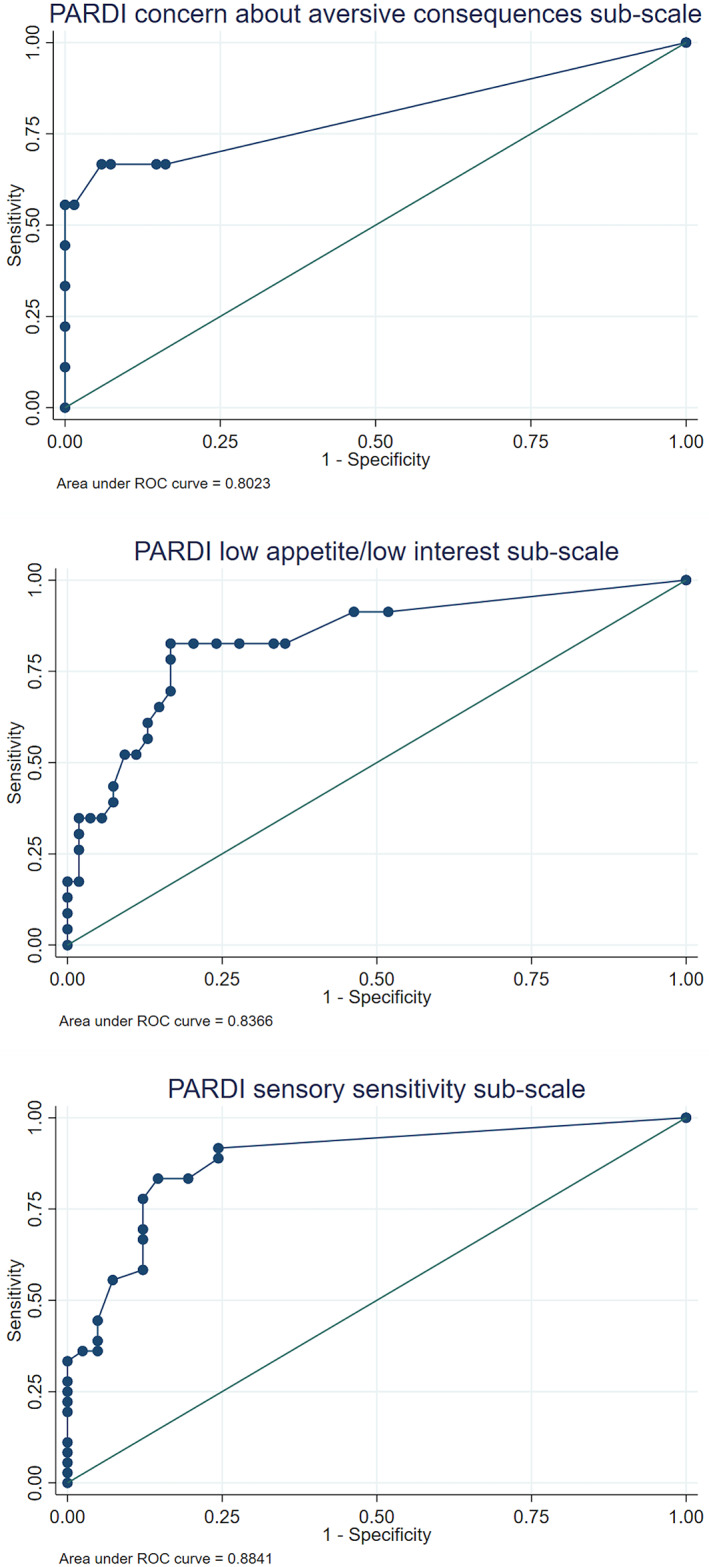
ROC curves for Pica ARFID and Rumination Disorder Interview (PARDI) subscales predicting treating clinician diagnosis of avoidant/restrictive food intake disorder (ARFID).
4. DISCUSSION
These data present evidence for the factor structure and validity of the PARDI diagnostic interview in a sample of children, adolescents, and young adults with ARFID.
4.1. Factor structure
The exploratory factor analysis of the dimensional symptom items revealed a 3‐factor structure, with the factors representing concern about aversive consequences, low appetite/low‐interest and sensory sensitivity symptoms. These three factors closely align with the primary ARFID symptom profiles described in the DSM‐5 and observed in clinical populations (Strand et al., 2019). Further, validity analyses partially supported both the construct and criterion validity of the PARDI dimensional symptom subscales for assessing these three major symptom profiles observed among ARFID patients. These data, in combination with a previous preliminary study (Bryant‐Waugh et al., 2019) provide growing evidence for the reliability and validity of the PARDI diagnostic interview among American children, adolescents and young adults with ARFID.
As a result of the factor analysis, six items were not retained for our validity analyses. These items assessed oral sensitivity when brushing teeth, color‐based food preferences, eating less when upset/worried, eating less when happy/excited, feeling uncomfortable when full, and worry about food being harmful. Except for the items assessing reduced eating when upset and uncomfortable feelings of fullness, these items were infrequently endorsed at moderate or higher intensity, less than six times in the present sample. Infrequent endorsement could in part explain why these items did not load onto the three factors derived. Due to their potential clinical utility these items should therefore be further evaluated in larger samples from other countries and across the lifespan before being removed from the interview. The cross‐loading item (80) was retained on the factor on which it loaded most highly (at least .20 greater than the cross‐loadings), which also resulted in the best conceptual fit.
Beyond the factor structure of the dimensional symptom items, an exploratory factor analysis of the severity items also revealed a 3‐factor structure, representing restriction in range of foods consumed (factor 1), social impairments due to restricted eating (factor 2) and difficulty meeting nutritional needs (factor 3). Two items were dropped (29e and 46) due to high cross‐loadings. These three factors provide useful subscales to explore in future clinical investigations of ARFID etiology and intervention, and we suggest that a total mean severity score also be calculated to quantify overall ARFID severity, given its clinical utility and good internal consistency of all severity items (α = .77). Given the importance of dimensional psychiatric ratings for elaborating on existing categorical diagnoses to enhance diagnostic accuracy and track change in treatment (Helzer, Kraemer, & Krueger, Helzer et al., 2006), the inclusion of continuous severity rating on the PARDI is a great advance for ARFID assessment.
4.2. Construct validity
Evidence for the construct and criterion validity of the dimensional symptom subscales was also partially supported. Specifically, participants with a diagnosis of ARFID and subthreshold ARFID experienced higher scores all three subscales (i.e., concern about aversive consequences, low appetite/low‐interest and sensory sensitivity), relative to control participants. These findings suggest that these subscales are measuring clinically relevant symptoms of ARFID and may be sensitive to subthreshold as well as full presentations, though further research is needed in a larger sample. The construct validity of each subscale was partially supported by correlations with self‐report measures of theoretically associated constructs of interest. Specifically, a self‐report of anxiety significantly correlated with the concern about aversive consequences subscale, but not the low‐appetite/low‐interest or sensory sensitivity subscale. Similarly, self‐reports of sensory threshold correlated with only the sensory sensitivity subscale of the PARDI, and not the other two subscales. Further, self‐reports of satiety responsiveness and enjoyment of food significantly correlated in the expected direction with the low‐appetite/low‐interest subscale of the PARDI. However, self‐reports of satiety responsiveness and eating speed also significantly correlated with the concern about aversive consequences subscale of the PARDI to a lesser degree. It is possible that the concern about aversive consequences subscale of the PARDI may be sensitive to increased monitoring of bodily responses and slower intake during eating. These findings partially support the convergent and divergent validity of the three PARDI symptom subscales and suggest that further work is needed in a larger sample to further clarify the constructs assessed via the concern about aversive consequences subscale.
4.3. Criterion validity
Finally, ROC curves were used to evaluate the criterion validity of the PARDI for detecting a clinical diagnosis of ARFID. The extent to which each PARDI symptom subscale could predict the treating clinician's ARFID diagnosis and symptom profile rating was evaluated. Both the low‐appetite/low‐interest and sensory sensitivity subscales showed excellent ability to discriminate between participants with an ARFID diagnosis and those without. For the low‐appetite/low‐interest subscale, a clinical cutoff score of 1.1 classified 83.1% of cases correctly. For the sensory sensitivity scale, a clinical cutoff score of 0.6 correctly identified 84.4% of cases. The lower clinical cutoff for the sensory sensitivity subscale is likely due to high prevalence of sensory sensitivity symptoms in this sample and the observed phenomena that a single aspect of sensory aversion could cause high impairment while other sensory items were rated as non‐problematic, resulting in an overall lower mean score. The high sensitivity and specificity observed at these cutoff scores (>80%), indicates that these PARDI subscale cutoffs can be used to detect clinically meaningful symptoms of ARFID in their respective domains among children, adolescents, and young adults.
The ROC curve analysis for the concern about aversive consequences subscale yielded less useful estimates. Although the area under the curve was consistent with excellent discrimination, the clinical cutoff of 1.6 was also associated with only 56% sensitivity. Alternative clinical cutoffs that would enhance sensitivity were not clinically meaningful, likely due to the low number of ARFID cases presenting with symptoms in this dimension (n = 9). Therefore, we recommend that the clinical cutoff for the concern about aversive consequences subscale be evaluated in a larger sample of youth with ARFID symptoms in this dimension before applying a subscale clinical cutoff in research or clinical practice.
The evidence for 3‐factor factor structure and validity of the PARDI diagnostic interview in a sample of children, adolescents, and young adults with ARFID represents a significant advance in the field of ARFID diagnostic assessment. The PARDI is the first semi‐structured clinical interview for ARFID that directly assesses the core DSM‐5 ARFID symptom dimensions, yields a severity score, and is adapted for use with multiple informants across the lifespan. These aspects of the PARDI offer a significant advantage over other diagnostic interviews for ARFID that address a narrower developmental span, and do not include a severity score that is essential for treatment planning and monitoring outcomes (Cooke, 2020; First et al., 2015; Sysko et al., 2015).
4.4. Limitations
The findings must be interpreted in light of the limitations of this study. Foremost, the sample included 129 children, adolescents, and young adults ages 9–23 years old. Therefore, it was not possible to evaluate relative item functioning by age within this sample, and it is unclear if the findings would generalize to young children, among whom some selectivity is normative (Dovey, Staples, Gibson, & Halford, Dovey et al., 2008; Mascola, Bryson, & Agras, Mascola et al., 2010), or older adult samples. Second, although multi‐informant reports are available for the PARDI, only the self‐interview was used for this investigation. As participants' level of insight may vary due to treatment engagement or other factors, the inclusion of caregiver reports could yield different response patterns by informant type and question type, such as higher severity ratings on observed psychosocial disturbance but lower ratings of fear (van der Ende, Verhulst, & Tiemeier, van der Ende et al., 2012; van der Meer, Dixon, & Rose, van der Meer et al., 2008). However, all participants completed their PARDI interview prior to receiving care through the affiliated hospital clinic, limiting the potential effect of treatment‐related symptom insight on our findings. Further, as not all self‐report measures, such as the AEBQ and abbreviated AASP, were previously validated for use with children as young as 9 years old it is possible that this could influence the reported findings of the PARDI construct validity among younger participants. Given the low‐internal consistency of the abbreviated AASP, the construct validity of the sensory sensitivity scale of the PARDI should be examined relative to more comprehensive sensory assessments. Additionally, the sample was comprised of predominantly Caucasian children, adolescents, and young adults recruited in a northeastern American city, limiting the generalizability of these findings to other regions and cultures. Given that the feeding behaviors often associated with ARFID, such as pressure to eat and family accommodation, are also influenced by culture (Gu, Warkentin, Mais, & Carnell, Gu et al., 2017), it is essential that the factor structure and the validity of the PARDI be evaluated across the lifespan and cultures. Finally, given the sample size and small number of subthreshold ARFID cases examined, future work should evaluate the factor structure in a larger sample and explore the utility of the PARDI to detect differences in sub and full‐threshold symptom presentations.
4.5. Application of findings
Despite these limitations, the data presented demonstrate growing evidence for the 3‐factor structure of the dimensional symptom items of the PARDI and indicate that these subscales provide a valid assessment of youth and young adult ARFID symptoms within the dimensions of concern about aversive consequences of eating, low appetite/low interest, and sensory sensitivity. This empirical evidence for the PARDI is a great advance for ARFID diagnostic assessment, as it is the first free‐standing multi‐informant assessment tool that yields continuous ratings for ARFID symptom dimensions and severity. These three subscales can be used in clinical research to advance our understanding of the etiology of ARFID symptoms, including better understanding the link between symptoms and dimension specific biological vulnerabilities posited in the 3‐dimensional neurobiological model of ARFID and clarifying how they interact with psychological and social factors to promote emotionally‐driven food avoidance (Harris et al., 2019; Thomas et al., 2017). Finally, the clinical cutoff scores for two of the three subscales supported by these data, provide useful guidance to clinicians aiming to formulate an ARFID diagnosis and identify clinically significant symptoms within each major ARFID symptom dimension to aid their selection of interventions best suited to their patient's ARFID symptom profile.
AUTHOR CONTRIBUTIONS
Christine E Cooper‐Vince: Conceptualization; data curation; formal analysis; methodology; writing – original draft; writing – review and editing. Chika Nwaka: Conceptualization; writing – original draft; writing – review and editing. Kamryn T. Eddy: Investigation; writing – review and editing. Madhusmita Misra: Investigation; writing – review and editing. Natalia A Hadaway: Data curation; investigation; project administration; writing – review and editing. Kendra Becker: Data curation; investigation; project administration; writing – review and editing. Elizabeth Lawson: Conceptualization; funding acquisition; investigation; writing – review and editing. Lucy Cooke: Conceptualization; writing – review and editing. Rachel Bryant‐Waugh: Conceptualization; investigation; methodology; writing – review and editing. Jennifer J. Thomas: Conceptualization; funding acquisition; investigation; methodology; writing – review and editing. Nadia Micali: Conceptualization; investigation; methodology; supervision; writing – review and editing.
CONFLICT OF INTEREST
Drs. Eddy, Becker, and Thomas receive royalties from Cambridge University Press for the sale of their books on ARFID. Dr. Lawson has a financial interest in OXT Therapeutics, a company developing oxytocin‐based therapeutics for obesity and metabolic disease. Dr. Lawson has also received an investigator‐initiated grant from Tonix Pharmaceuticals. Dr. Lawson's interests were reviewed and are managed by MGH and Mass General Brigham (f/k/a/ Partners Healthcare) in accordance with their conflict of interest policies. Dr. Misra serves as a consultant for Abbvie and Sanofi and has served on the Scientific Advisory Board for Abbvie and Ipsen.
ACKNOWLEDGEMENTS
This project and Jennifer J Thomas's, Elizabeth A. Lawson's, and Nadia Micali's time was supported by NIH R01 MH108595. Elizabeth A. Lawson's time was also supported by K24 MH120568. Christine Cooper‐Vince's time was supported by funding from the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska‐Curie grant agreement No 892437. Open access funding provided by Universite de Geneve.
Cooper‐Vince, C. E. , Nwaka, C. , Eddy, K. T. , Misra, M. , Hadaway, N. A. , Becker, K. R. , Lawson, E. A. , Cooke, L. , Bryant‐Waugh, R. , Thomas, J. J. , & Micali, N. (2022). The factor structure and validity of a diagnostic interview for avoidant/restrictive food intake disorder in a sample of children, adolescents, and young adults. International Journal of Eating Disorders, 55(11), 1575–1588. 10.1002/eat.23792
Action Editor: Ruth Striegel Weissman
Rachel Bryant‐Waugh, Jennifer J Thomas, and Nadia Micali are co‐senior authors.
Funding information European Union Horizon 2020, Grant/Award Number: Marie Skłodowska‐Curie grant No 892437; National Institute of Mental Health, Grant/Award Numbers: K24 MH120568, R01 MH108595
DATA AVAILABILITY STATEMENT
The data analyzed for current study are available from the corresponding author on reasonable request.
REFERENCES
- APA . (2013). Diagnostic and statistical manual of mental disorders DMS V (5th ed.). American Psychiatric Association. [Google Scholar]
- Beck, A. T. , Epstein, N. , Brown, G. , & Steer, R. A. (1988). An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology, 56(6), 893–897. 10.1037//0022-006x.56.6.893 [DOI] [PubMed] [Google Scholar]
- Beck, J. S. , Beck, A. T. , & Jolly, J. B. (2005). Beck youth inventories. In BYI‐II (2nd ed.). Pearson Clinical. [Google Scholar]
- Bertrand, V. , Tiburce, L. , Sabatier, T. , Dufour, D. , Déchelotte, P. , & Tavolacci, M.‐P. (2021). Estimated prevalence and care pathway of feeding and eating disorders in a French pediatric population. Nutrients, 13(6), 2048. 10.3390/nu13062048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant‐Waugh, R. , Micali, N. , Cooke, L. , Lawson, E. A. , Eddy, K. T. , & Thomas, J. J. (2019). Development of the pica, ARFID, and rumination disorder interview, a multi‐informant, semi‐structured interview of feeding disorders across the lifespan: A pilot study for ages 10–22. International Journal of Eating Disorders, 52, 378–387. 10.1002/eat.22958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y.‐L. , Chen, W. J. , Lin, K.‐C. , Shen, L.‐J. , & Gau, S. S.‐F. (2019). Prevalence of DSM‐5 mental disorders in a nationally representative sample of children in Taiwan: Methodology and main findings. Epidemiology and Psychiatric Sciences, 1‐9, e15. 10.1017/S2045796018000793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke, L. (2020). Baseline and outcome measures. In Bryant‐Waugh R. & Higgins C. (Eds.), Avoidant restrictive food intake disorder in childhood and adolescence a clinical guide (pp. 69–86). Routledge. [Google Scholar]
- Costello, A. B. , & Osborne, J. W. (2005). Best practices in exploratory factor analysis: Four recommendations for getting the Most from your analysis. Practical Assessment, Research, and Evaluation, 10, 1–9. 10.7275/jyj1-4868 [DOI] [Google Scholar]
- Dovey, T. M. , Staples, P. A. , Gibson, E. L. , & Halford, J. C. (2008). Food neophobia and 'picky/fussy' eating in children: A review. Appetite, 50(2–3), 181–193. 10.1016/j.appet.2007.09.009 [DOI] [PubMed] [Google Scholar]
- Fairburn, C. , & Beglin, S. (2008). The eating disorder examination questionnaire. In Fairburn C. (Ed.), Cognitive behavior therapy and eating disorders (pp. 309–313). Guilford Press. [Google Scholar]
- First, M. , Williams, J. , Karg, R. , & Spitzer, R. (2015). Structured clinical interview for DSM‐5—Research version. American Psychiatric Association. [Google Scholar]
- Fisher, M. M. , Rosen, D. S. , Ornstein, R. M. , Mammel, K. A. , Katzman, D. K. , Rome, E. S. , Callahan, S. T., Malizio, J., Kearney, S., & Walsh, B. T. (2014). Characteristics of avoidant/restrictive food intake disorder in children and adolescents: A "new disorder" in DSM‐5. Journal of Adolescent Health, 55, 49–52. 10.1016/j.jadohealth.2013.11.013 [DOI] [PubMed] [Google Scholar]
- Forman, S. F. , McKenzie, N. , Hehn, R. , Monge, M. C. , Kapphahn, C. J. , Mammel, K. A. , Callahan, S. T., Sigel, E. J., Bravender, T., Romano, M., Rome, E. S., Robinson, K. A., Fisher, M., Malizio, J. B., Rosen, D. S., Hergenroeder, A. C., Buckelew, S. M., Jay, M. S., Lindenbaum, J., Rickert, V. I., & Woods, E. R. (2014). Predictors of outcome at 1 year in adolescents with DSM‐5 restrictive eating disorders: Report of the national eating disorders quality improvement collaborative. Journal of Adolescent Health, 55, 750–756. 10.1016/j.jadohealth.2014.06.014 [DOI] [PubMed] [Google Scholar]
- Fydrich, T. , Dowdall, D. , & Chambless, D. L. (1992). Reliability and validity of the beck anxiety inventory. Journal of Anxiety Disorders, 6(1), 55–61. 10.1016/0887-6185(92)90026-4 [DOI] [Google Scholar]
- Gu, C. , Warkentin, S. , Mais, L. A. , & Carnell, S. (2017). Ethnic differences in parental feeding behaviors in UKparents of preschoolers. Appetite, 113, 398–404. 10.1016/j.appet.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hair, J. F. , Black, W. C. , Black, B. , Babin, B. J. , & Anderson, R. E. (2010). Multivariate data analysis: Global edition (7th Edition ed.). Person Prentic Hall. [Google Scholar]
- Harris, A. A. , Romer, A. L. , Hanna, E. K. , Keeling, L. A. , LaBar, K. S. , Sinnott‐Armstrong, W. , Strauman, T. J., Wagner, H. R., Marcus, M. D., & Zucker, N. L. (2019). The central role of disgust in disorders of food avoidance. International Journal of Eating Disorders, 52(5), 543–553. 10.1002/eat.23047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay, P. , Mitchison, D. , Collado, A. E. L. , González‐Chica, D. A. , Stocks, N. , & Touyz, S. (2017). Burden and health‐related quality of life of eating disorders, including avoidant/restrictive food intake disorder (ARFID), in the Australian population. Journal of Eating Disorders, 5, 21. 10.1186/s40337-017-0149-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helzer, J. E. , Kraemer, H. C. , & Krueger, R. F. (2006). The feasibility and need for dimensional psychiatric diagnoses. Psychological Medicine, 36(12), 1671–1680. 10.1017/S003329170600821X [DOI] [PubMed] [Google Scholar]
- Herle, M. , Stavola, B. D. , Hübel, C. , Ferreira, D. L. S. , Abdulkadir, M. , Yilmaz, Z. , Loos, R., Bryant‐Waugh, R., Bulik, C. M., & Micali, N. (2020). Eating behavior trajectories in the first 10 years of life and their relationship with BMI. International Journal of Obesity, 44(8), 1766–1775. 10.1038/s41366-020-0581-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunot, C. , Fildes, A. , Croker, H. , Llewellyn, C. H. , Wardle, J. , & Beeken, R. J. (2016). Appetitive traits and relationships with BMI in adults: Development of the adult eating behaviour questionnaire. Appetite, 105, 1095–8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunot‐Alexander, C. , Beeken, R. J. , Goodman, W. , Fildes, A. , Croker, H. , Llewellyn, C. , & Steinsbekk, S. (2019). Confirmation of the factor structure and reliability of the 'Adult eating behavior Questionnaire' in an adolescent sample. Frontiers in Psychology, 10, 1991. 10.3389/fpsyg.2019.01991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambanis, P. E. , Kuhnle, M. C. , Wons, O. B. , Jo, J. H. , Keshishian, A. C. , Hauser, K. , Becker, K. R., Franko, D. L., Misra, M., Micali, N., Lawson, E. A., Eddy, K. T., & Thomas, J. J. (2020). Prevalence and correlates of psychiatric comorbidities in children and adolescents with full and subthreshold avoidant/restrictive food intake disorder. International Journal of Eating Disorders, 53(2), 256–265. 10.1002/eat.23191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman, J. , Birmaher, B. , Brent, D. , Rao, U. , Flynn, C. , Moreci, P. , Williamson, D., & Ryan, N. (1997). Schedule for affective disorders and schizophrenia for school‐age children‐present and lifetime version (K‐SADS‐PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry, 36(7), 980–988. 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- Mascola, A. J. , Bryson, S. W. , & Agras, W. S. (2010). Picky eating during childhood: A longitudinal study to age 11years. Eating Behaviors, 11, 253–257. 10.1016/j.eatbeh.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai, Y. , Nin, K. , Noma, S. I. , Teramukai, S. , & Wonderlich, S. A. (2016). Characteristics of avoidant/restrictive food intake disorder in a cohort of adult patients. European Eating Disorders Review, 1, 2015–2017. 10.1002/erv.2476 [DOI] [PubMed] [Google Scholar]
- Nicely, T. A. , Lane‐Loney, S. , Masciulli, E. , Hollenbeak, C. S. , & Ornstein, R. M. (2014). Prevalence and characteristics of avoidant/restrictive food intake disorder in a cohort of young patients in day treatment for eating disorders. Journal of Eating Disorders, 2, 21. 10.1186/s40337-014-0021-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris, M. L. , Robinson, A. , Obeid, N. , Harrison, M. , Spettigue, W. , & Henderson, K. (2014). Exploring avoidant/restrictive food intake disorder in eating disordered patients: A descriptive study. International Journal of Eating Disorders, 47, 495–499. 10.1002/eat.22217 [DOI] [PubMed] [Google Scholar]
- Ornstein, R. M. , Rosen, D. S. , Mammel, K. A. , Callahan, S. T. , Forman, S. , Jay, M. S. , Fisher, M., Rome, E., & Walsh, B. T. (2013). Distribution of eating disorders in children and adolescents using the proposed DSM‐5 criteria for feeding and eating disorders. Journal of Adolescent Health, 53, 303–305. 10.1016/j.jadohealth.2013.03.025 [DOI] [PubMed] [Google Scholar]
- Pearson . (2019). Adolescent/Adult Sensory Profile: Technical Report . http://images.pearsonclinical.com/Images/pdf/technical_reports/AD_Adult_SP_TR_Web.pdf
- Schmidt, R. , Kirsten, T. , Hiemisch, A. , Kiess, W. , & Hilbert, A. (2019). Interview‐based assessment of avoidant/restrictive food intake disorder (ARFID): A pilot study evaluating an ARFID module for the eating disorder examination. International Journal of Eating Disorders, 52(4), 388–397. 10.1002/eat.23063 [DOI] [PubMed] [Google Scholar]
- StataCorp . (2015). Stata/SE 14.2. StataCorp. [Google Scholar]
- Steer, R. A. , Kumar, G. , Beck, J. S. , & Beck, A. T. (2001). Evidence for the construct validities of the Beck youth inventories with child psychiatric outpatients. Psychological Reports, 89(3), 559–565. 10.2466/pr0.2001.89.3.559 [DOI] [PubMed] [Google Scholar]
- Strand, M. , von Hausswolff‐Juhlin, Y. , & Welch, E. (2019). A systematic scoping review of diagnostic validity in avoidant/restrictive food intake disorder. International Journal of Eating Disorders, 52(4), 331–360. 10.1002/eat.22962 [DOI] [PubMed] [Google Scholar]
- Sysko, R. , Glasofer, D. R. , Hildebrandt, T. , Klimek, P. , Mitchell, J. E. , Berg, K. C. , Peterson, C. B., Wonderlich, S. A., & Walsh, B. T. (2015). The eating disorder assessment for DSM‐5 (EDA‐5): Development and validation of a structured interview for feeding and eating disorders. International Journal of Eating Disorders, 48(5), 452–463. 10.1002/eat.22388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, J. J. , Lawson, E. A. , Micali, N. , Misra, M. , Deckersbach, T. , & Eddy, K. T. (2017). Avoidant/restrictive food intake disorder: A three‐dimensional model of neurobiology with implications for etiology and treatment. Current Psychiatry Reports, 19(8), 54. 10.1007/s11920-017-0795-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ende, J. , Verhulst, F. C. , & Tiemeier, H. (2012). Agreement of informants on emotional and behavioral problems from childhood to adulthood. Psychological Assessment, 24(2), 293–300. 10.1037/a0025500 [DOI] [PubMed] [Google Scholar]
- van der Meer, M. , Dixon, A. , & Rose, D. (2008). Parent and child agreement on reports of problem behaviour obtained from a screening questionnaire, the SDQ. European Child and Adolescent Psychiatry, 17(8), 491–497. 10.1007/s00787-008-0691-y [DOI] [PubMed] [Google Scholar]
- WHO . (2018). International classification of diseases for mortality and morbidity statistics https://icd.who.int/browse11/l-m/en
- Williams, K. E. , Hendy, H. M. , Field, D. G. , Belousov, Y. , Riegel, K. , & Harclerode, W. (2015). Implications of avoidant/restrictive food intake disorder (ARFID) on children with feeding problems. Children's Health Care, 44, 307–321. 10.1080/02739615.2014.921789 [DOI] [Google Scholar]
- Zickgraf, H. F. , Lane‐Loney, S. , Essayli, J. H. , & Ornstein, R. M. (2019). Further support for diagnostically meaningful ARFID symptom presentations in an adolescent medicine partial hospitalization program. International Journal of Eating Disorders, 52, 402–409. 10.1002/eat.23016 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data analyzed for current study are available from the corresponding author on reasonable request.


