Abstract
Objectives:
There are limited data on the effect and evolution of risk factors for hepatocellular cancer (HCC) in patients with virologically cured hepatitis C virus infection (HCV).
Methods:
We conducted a retrospective cohort study of HCV patients who achieved sustained virological response (SVR) with direct acting antivirals from 130 Veterans Administration hospitals during 2014–2018, followed through 2021. Cox proportional hazard models were constructed at 3 landmark times (baseline, 12- and 24-months after SVR) to examine associations between demographic, clinical, and behavioral factors and HCC risk, stratified by cirrhosis status.
Results:
Among 92,567 patients (32% cirrhosis), 3,247 cases of HCC were diagnosed during mean follow-up of 2.5 years. In patients with cirrhosis, male sex (hazard ratio (HRs): 1.89, 1.93, 1.99), cirrhosis duration ≥5 years (HRs: 1.71, 1.79, 1.34), varices (HRs: 1.73, 1.60, 1.56), baseline albumin (HRs: 0.48, 0.47, 0.49) and change in albumin (HRs: 0.82, 0.90) predicted HCC risk at each landmark time. HCV genotype 3, prior treatment, bilirubin, smoking, and race influenced HCC risk at baseline, but their effects attenuated over time. In patients without cirrhosis, diabetes (HRs: 1.54, 1.42, 1.47) and hypertension (HRs: 1.59, 1.65, 1.74) were associated with HCC risk at all landmark times. Changes in FIB-4 scores over time were associated with HCC risk both in patients with and without cirrhosis.
Conclusions:
Risk factors for HCC were different in patients with and without cirrhosis and some also evolved during follow-up. These factors can help with risk stratification and HCC surveillance decisions in patients with cured HCV.
Keywords: hepatitis C virus, hepatocellular carcinoma, risk factors, sustained viral response
BACKGROUND
In patients with chronic infection with hepatitis C virus (HCV), successful treatment with direct acting antivirals (DAA) reduces HCC risk. However, data from our group and others show that subsequent risk of HCC persists in many patients after sustained virological response (SVR).1 Over the next two decades, most HCV-related HCC cases are projected to occur in individuals with SVR.2 Yet, decisions on how best to surveil this growing population are based on limited data.
Cirrhosis is the most important risk factor for HCC.3 However, even among patients with cirrhosis, the risk of HCC is not uniform. Some of this may come from the variation in the extent to which the underlying histological lesion (fibrosis) regresses or progresses over time. Several demographic (e.g., age), clinical (e.g., diabetes, obesity), and behavioral factors (e.g., alcohol use, smoking) may also influence HCC risk. There are limited data on the evolution of these risk factors and their effects on HCC risk at different time points after SVR. These factors may also vary in patients with and without cirrhosis. This information is important to clinicians, as identifying patients without cirrhosis but who still may be at risk for HCC can alter surveillance decisions, with more aggressive surveillance considered in those more likely to have a high risk.
Few studies have evaluated HCC risk longitudinally. We examined which risk factors (demographics, comorbidities, and liver/HCV-related) were associated with HCC at the time of SVR, 12-months and 24-months following SVR. We also examined associations of changes in these factors from baseline to different timepoints in subgroups defined based on presence or absence of baseline cirrhosis.
METHODS
DATA SOURCES
We used national data in the US Department of Veterans Affairs (VA) Corporate Data Warehouse (CDW) and Central Cancer Registry (CCR). CDW includes all laboratory test results, pharmacy, diagnosis (International Classification of Diseases [ICD]-9 and ICD-10) codes for all encounters in the VA. CDW also contains information from annual Alcohol Use Disorders Identification Test-concise (AUDIT-C) screen4 and date of death. CCR is a centralized repository for VA patients with cancer and includes information on date of diagnosis, primary site, and histology.
STUDY COHORT
We included patients ≥18 years who achieved SVR with DAA in any of the 130 VA hospitals. We defined DAA treatment as ≥1 filled prescription of DAA (appendix 1) between 1/1/2014 and 12/31/2018. We used 3 months after the date of DAA completion as the index date for start of follow up to consider time needed to determine SVR. We classified patients as SVR if all HCV RNA tests were negative after the end of DAA treatment, with ≥1 HCV RNA test recorded at least 12 weeks after completion. If no test was available after 12-weeks we used a negative test from 4–12 weeks as SVR (6.9% of cohort). We excluded patients who failed to achieve SVR, who had HCC or liver transplant before or within 3 months of DAA treatment, had prior evidence of cancers with a high likelihood of liver metastases (esophageal, stomach, pancreatic, lung, kidney, breast cancer, and melanoma), and patients did not have evidence of ≥1 visit in the VA following end of treatment.
OUTCOME
The outcome was HCC defined based on any instance of HCC recorded in the CCR. We also included patients with ≥2 ICD-9 or ICD-10 codes or HCC in CDW.3, 5–7 The ICD-based definition had a positive predictive value of 84–94% in our and others’8 work. In addition, previously, we reviewed electronic medical records (EMR) of a random sample of 50 HCC cases that had ≥2 ICD codes but not in CCR; all had evidence of HCC in EMR.1 We followed patients to the development of HCC, liver transplant, death, or last VA visit before December 31, 2021, whichever was earlier.
PREDICTOR VARIABLES
The demographic variables included age, sex, race/ethnicity, marital status, and rural status defined at the index date. Other time invariant factors included HCV genotype and previous HCV treatment status was defined based on filled prescriptions for interferon, ribavirin, or receipt of any DAA prior to baseline.
Time varying factors included cirrhosis diagnosis, duration since cirrhosis diagnosis, fibrosis-4 score (FIB-4; and individual components of FIB-4), serum albumin, sodium, bilirubin, international normalized ratio (INR), hemoglobin, HBV surface antigen, diabetes, body mass index (BMI), hypertension, dyslipidemia, active alcohol use disorder, smoking status, and medical and mental health comorbidity. We defined cirrhosis based on ICD codes for cirrhosis or its complications recorded any time prior to baseline or the presence of FIB-4 >3.25 (as defined below).9 We used the date of first instance of cirrhosis code/s as cirrhosis diagnosis date and once present they were presumed to have cirrhosis for the remaining follow-up time. We also used FIB-4 to identify patients with cirrhosis/fibrosis at baseline (FIB-4 >3.25) and used serial changes in FIB-4 to examine the decline or increase in fibrosis. We used the values of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and platelet tests performed within 24-months and nearest to index date to derive the baseline value. We found that 90% of patients had their FIB-4 measured within 4 months prior to their index date. We also calculated FIB-4 at 12-month intervals longitudinally using ALT as the anchor test and selecting the AST and platelet tests performed within 6-months of the anchor ALT. We analyzed FIB-4 as a categorical variable defined based on published cutoffs (>3.25 and <1.45). A previous study demonstrated in patients with HCV that FIB-4 of less than 1.45 has a negative predictive value of 94.7% to exclude advanced fibrosis (F3-F4), and a value over 3.25 has a positive predictive value of 82.1% for advanced fibrosis.10 See Appendix 2 for the specification for other predictors.
STATISTICAL ANALYSES
We used cause-specific Cox proportional hazard models for competing risks12 constructed at three landmark times13, 14 (baseline, and 12- and 24-months after index) to examine the associations between risk factors measured at these landmark times and subsequent HCC risk, stratified by cirrhosis status at baseline.
We constructed six separate models (at three separate landmark times for patients with and without cirrhosis). The outcome was the time from the landmark to HCC. At each landmark, the data only included the at-risk patients at that time (e.g., those who are alive without HCC).
The predictor variables included time-independent and time-dependent defined at each landmark time. For continuous measures, we assessed both categorical and continuous values to determine the best performing measure. The predictors also incorporated longitudinal information of a patient up to the landmark time, including change in FIB-4 [defined as remained stable at low risk (≤3.25), increased from low (≤3.25) to high risk (>3.25), declined from high (>3.25) to low risk (≤3.25), or remained stable at high risk (>3.25)] as well as changes in serum albumin, sodium, bilirubin, INR, creatinine, and hemoglobin between baseline and each landmark time. Log-transformation was used for variables with a skewed distribution. For model interpretability, we only considered two-way interactions that are frequently observed in the cohort or suggested by literature.
There was a small amount of missing data. We used multiple imputation generated from the fully conditional specification method of SAS PROC MI to create five imputed datasets and then combined the results using multiple imputation. Each imputed dataset went through a model building process with stepwise selection (entry p-value ≤0.10; retaining p-value ≤0.05). The final model included all predictors selected from at least one dataset. For each landmark time, we forced baseline variable/s in the model if the final model/s included the corresponding change variable. Model fit with imputed datasets was checked using usual model checking procedures and no assumptions were violated.
We conducted 3 sensitivity analyses. We refined our cirrhosis definition by including only patients with a diagnosis of cirrhosis or its complications and excluding those with a FIB-4<1.45; we tested the robustness of our HCC definition by including patients with only 1 ICD code for HCC; and we examined the effect of BMI after excluding HCCs diagnosed in the first 12-months.
RESULTS
In total, 113,563 patients received DAA treatment between January 1, 2014, and December 31, 2018. We excluded 9112 without known SVR status, 1919 with prior HCC and 5034 with prior other cancers, 912 who received liver transplant, and 4019 who did not achieve SVR. Hence, our study cohort included 92,567 patients with SVR without prevalent HCC.
The mean age of the overall cohort at baseline was 61.5 years (SD=7.55 year), 96.4% were men, 51.0% were white, and 38.7% were African American (Supplemental Table 1). A total of 26.5% had cirrhosis diagnosed at baseline and an additional 5.5% had a FIB-4 value greater than 3.25, 36.7% had diabetes, 83.1% had hypertension, 51.2% dyslipidemia, and 14.1% had an active alcohol use disorder.
Table 1 displays the characteristics of patients with cirrhosis and their evolution over time. At baseline, 43.5% of patients with cirrhosis had diabetes; it increased to 46.4% at 24-month landmark time. Similarly, the proportion of patients with hypertension increased from 86.6% to 89.7% and dyslipidemia increased from 52.1% to 55.6% between baseline and 24-month landmark time. We found similar trends in patients without cirrhosis (Table 2).
Table 1:
Demographic and clinical characteristics of patients with virologically cured HCV and cirrhosis. Table also shows evolution of these characteristics over time.
| Baseline (N=29,398) | 12-month landmark (N=26,648) | 24-month landmark (N=21,600) | |
|---|---|---|---|
| Demographics | |||
| Age in years, mean (SD) | 62.78 (5.95) | 63.67 (5.91) | 64.48 (5.8) |
| Sex (n, percent) | |||
| Male | 28567 (97.17) | 25884 (97.13) | 20952 (97.0) |
| Female | 831 (2.83) | 764 (2.87) | 648 (3.0) |
| Race/ethnicity | |||
| White not Hispanic | 15107 (51.39) | 13618 (51.1) | 11084 (51.31) |
| African American | 10803 (36.75) | 9873 (37.05) | 7966 (36.88) |
| White Hispanic | 1383 (4.7) | 1264 (4.74) | 1040 (4.81) |
| Other | 727 (2.47) | 660 (2.48) | 534 (2.47) |
| Missing | 1378 (4.69) | 1233 (4.63) | 976 (4.52) |
| Clinical characteristics | |||
| HCV genotype | |||
| 1a | 16198 (55.1) | 14738 (55.31) | 11948 (55.31) |
| 1a/1b | 1043 (3.55) | 950 (3.56) | 773 (3.58) |
| 1b | 6393 (21.75) | 5800 (21.77) | 4815 (22.29) |
| 1-unknown subtype | 841 (2.86) | 781 (2.93) | 678 (3.14) |
| 2 | 2356 (8.01) | 2116 (7.94) | 1634 (7.56) |
| 3 | 1806 (6.14) | 1577 (5.92) | 1191 (5.51) |
| 4, 5, or 6 | 255 (0.87) | 235 (0.88) | 191 (0.88) |
| Missing | 506 (1.72) | 451 (1.69) | 370 (1.71) |
| Prior HCV treatment | |||
| No | 18241 (62.05) | 16447 (61.72) | 13060 (60.46) |
| Yes | 11157 (37.95) | 10201 (38.28) | 8540 (39.54) |
| FIB-4 | |||
| <1.45 | 5859 (19.93) | 6160 (23.12) | 5430 (25.14) |
| 1.45–3.25 | 12756 (43.39) | 13300 (49.91) | 10569 (48.93) |
| >3.25 | 10667 (36.28) | 6571 (24.66) | 4568 (21.15) |
| Missing | 116 (0.39) | 617(2.31) | 1033 (4.78) |
| Cirrhosis duration, months, | 76.92 (57.64) | 88.45 (57.17) | 100.25 (56.17) |
| Cirrhosis duration, categorical | |||
| < 1 year | 4874 (16.58) | -- | -- |
| 1–<2 years | 2424 (8.25) | 4380 (16.44) | -- |
| 2–<5 years | 5873 (19.98) | 5800 (21.77) | 6669 (30.88) |
| ≥ 5 years | 16227 (55.2) | 16468 (61.8) | 14931 (69.13) |
| Varices | |||
| No | 25800 (87.76) | 24644 (92.48) | 19522 (90.38) |
| Yes | 3598 (12.24) | 2004 (7.52) | 2078 (9.62) |
| Ascites | |||
| No | 27187 (92.48) | 25735 (96.57) | 20747 (96.05) |
| Yes | 2211 (7.52) | 913 (3.43) | 853 (3.95) |
| Diabetes | |||
| No | 16618 (56.53) | 14667 (55.04) | 11575 (53.59) |
| Yes | 12780 (43.47) | 11981 (44.96) | 10025 (46.41) |
| Hypertension | |||
| No | 3943 (13.41) | 3102 (11.64) | 2226 (10.31) |
| Yes | 25455 (86.59) | 23546 (88.36) | 19374 (89.69) |
| Body mass index | |||
| <18.5 | 365 (1.24) | 346 (1.3) | 283 (1.31) |
| 18.5–<25 | 7683 (26.13) | 6513 (24.44) | 4967 (23) |
| 25–<30 | 11144 (37.91) | 9639 (36.17) | 7528 (34.85) |
| ≥30 | 10103 (34.37) | 9501 (35.65) | 7742 (35.84) |
| Missing | 103 (0.35) | 649 (2.44) | 1080 (5.0) |
| Dyslipidemia | |||
| No | 14089 (47.93) | 12808 (48.06) | 9598 (44.44) |
| Yes | 15309 (52.07) | 13840 (51.94) | 12002 (55.56) |
| Chronic kidney disease | |||
| No | 25357 (86.25) | 22725 (85.28) | 18410 (85.23) |
| Yes | 4041 (13.75) | 3923 (14.72) | 3190 (14.77) |
| COPD | |||
| No | 19541 (66.47) | 17477 (65.58) | 14085 (65.21) |
| Yes | 9857 (33.53) | 9171 (34.42) | 7515 (34.79) |
| Heart failure | |||
| No | 26134 (88.9) | 23569 (88.45) | 19127 (88.55) |
| Yes | 3264 (11.1) | 3079 (11.55) | 2473 (11.45) |
| Behavioral factors | |||
| Active alcohol use disorder | |||
| No | 22050 (75.01) | 19339 (72.57) | 15511 (71.81) |
| Yes | 3984 (13.55) | 2675 (10.04) | 2264 (10.48) |
| Missing | 3364 (11.44) | 4634 (17.39) | 3825 (17.71) |
| Smoking | |||
| Current | 13484 (45.87) | 11269 (42.29) | 8601 (39.82) |
| Former | 6721 (22.86) | 6012 (22.56) | 5174 (23.95) |
| Non-Smoker | 3614 (12.29) | 3253 (12.21) | 2898 (13.42) |
| Unknown/Missing | 5579 (18.98) | 6114 (22.94) | 4927 (22.81) |
| Laboratory data (mean, SD) | |||
| Albumin (g/dL) | 3.78 (0.47) | 3.8 (0.46) | 3.78 (0.45) |
| Bilirubin (mg/dL) | 0.93 (0.65) | 0.92 (0.62) | 0.92 (0.61) |
| Sodium (mmol/L) | 138.4 (3.03) | 138.4 (3.01) | 138.4 (2.94) |
| Creatinine (mg/dL) | 1.16 (1.1) | 1.21 (1.18) | 1.2 (1.11) |
| Hemoglobin (g/dL) | 13.4 (1.85) | 13.4 (1.83) | 13.4 (1.8) |
| INR* | 1.13 (0.26) | 1.26 (0.79) | 1.25 (0.73) |
There were 10.5% missing INR at baseline, 38.4% missing at 12-month landmark time, and 54.1% missing at 24-month landmark time.
Table 2:
Demographic and clinical characteristics of patients with virologically cured HCV without cirrhosis. Table also shows evolution of these characteristics over time.
| Baseline (N=63,169) | 12 months (N=58,639) | 24 months (N=46,889) | |
|---|---|---|---|
| Demographics | |||
| Age in years, mean (SD) | 60.83 (8.12) | 61.84 (7.98) | 62.8 (7.66) |
| Sex (n, percent) | |||
| Male | 60670 (96.04) | 56314 (96.04) | 45051 (96.08) |
| Female | 2499 (3.96) | 2325 (3.96) | 1838 (3.92) |
| Race/ethnicity | |||
| White not Hispanic | 32061 (50.75) | 29712 (50.67) | 23727 (50.6) |
| African American | 25038 (39.64) | 23298 (39.73) | 18738 (39.96) |
| White Hispanic | 1886 (2.99) | 1769 (3.02) | 1412 (3.01) |
| Other | 1381 (2.19) | 1275 (2.17) | 990 (2.11) |
| Missing | 2803 (4.44) | 2585 (4.41) | 2022 (4.31) |
| Clinical characteristics | |||
| HCV genotype | |||
| 1a | 34808 (55.1) | 32310 (55.1) | 25791 (55.0) |
| 1a/1b | 1978 (3.13) | 1827 (3.12) | 1464 (3.12) |
| 1b | 13465 (21.32) | 12612 (21.51) | 10372 (22.12) |
| 1-unknown subtype | 1380 (2.18) | 1283 (2.19) | 1059 (2.26) |
| 2 | 6404 (10.14) | 5913 (10.08) | 4606 (9.82) |
| 3 | 3432 (5.43) | 3119 (5.32) | 2318 (4.94) |
| 4, 5, or 6 | 624 (0.99) | 580 (0.99) | 466 (0.99) |
| Missing | 1078 (1.71) | 995 (1.7) | 813 (1.73) |
| Prior HCV treatment | |||
| No | 45222 (71.59) | 41850 (71.37) | 33101 (70.59) |
| Yes | 17947 (28.41) | 16789 (28.63) | 13788 (29.41) |
| FIB-4 | |||
| <1.45 | 32934 (52.14) | 30560 (52.12) | 23972 (51.12) |
| 1.45–3.25 | 29699 (47.02) | 24251 (41.36) | 18338 (39.11) |
| >3.25 | -- | 1174 (2.00) | 959 (2.05) |
| Missing | 536 (0.85) | 2654 (4.52) | 3620 (7.72) |
| Diabetes | |||
| No | 42024 (66.53) | 37839 (64.53) | 29370 (62.64) |
| Yes | 21145 (33.47) | 20800 (35.47) | 17519 (37.36) |
| Hypertension | |||
| No | 11711 (18.54) | 9547 (16.28) | 6820 (14.54) |
| Yes | 51458 (81.46) | 49092 (83.72) | 40069 (85.46) |
| Body mass index | |||
| <18.5 | 868 (1.37) | 854 (1.46) | 676 (1.44) |
| 18.5–<25 | 18501 (29.29) | 16219 (27.66) | 12298 (26.23) |
| 25–<30 | 24539 (38.85) | 21395 (36.49) | 16548 (35.29) |
| ≥30 | 18855 (29.85) | 17523 (29.88) | 13776 (29.38) |
| Missing | 406 (0.64) | 2648 (4.52) | 3591 (7.66) |
| Dyslipidemia | |||
| No | 31059 (49.17) | 27693 (47.23) | 20710 (44.17) |
| Yes | 32110 (50.83) | 30946 (52.77) | 26179 (55.83) |
| Chronic kidney disease | |||
| No | 57317 (90.74) | 52624 (89.74) | 41710 (88.95) |
| Yes | 5852 (9.26) | 6015 (10.26) | 5179 (11.05) |
| COPD | |||
| No | 44974 (71.2) | 40824 (69.62) | 32158 (68.58) |
| Yes | 18195 (28.8) | 17815 (30.38) | 14731 (31.42) |
| Heart failure | |||
| No | 59119 (93.59) | 54536 (93.0) | 43478 (92.73) |
| Yes | 4050 (6.41) | 4103 (7) | 3411 (7.27) |
| Behavioral factors | |||
| Active alcohol use disorder | |||
| No | 46806 (74.1) | 40947 (69.83) | 32179 (68.63) |
| Yes | 9098 (14.4) | 6326 (10.79) | 5448 (11.62) |
| Missing | 7265 (11.5) | 11366 (19.38) | 9262 (19.75) |
| Smoking | |||
| Current | 31978 (50.62) | 26435 (45.08) | 20095 (42.86) |
| Former | 12766 (20.21) | 11860 (20.23) | 10409 (22.2) |
| Non-Smoker | 7132 (11.29) | 6598 (11.25) | 5732 (12.22) |
| Unknown/Missing | 11293 (17.88) | 13746 (23.44) | 10653 (22.72) |
| Laboratory data (mean, SD) | |||
| Albumin (g/dL) | 3.98 (0.37) | 3.98 (0.37) | 3.98 (0.36) |
| Bilirubin (mg/dL) | 0.67 (0.37) | 0.68 (0.37) | 0.67 (0.38) |
| Sodium (mmol/L) | 138.83 (2.84) | 138.43 (2.83) | 138.86 (2.8) |
| Creatinine (mg/dL) | 1.1 (0.83) | 1.14 (0.92) | 1.16 (0.92) |
| Hemoglobin (g/dL) | 14.17 (1.69) | 14.16 (1.68) | 14.11 (1.69) |
| INR* | 1.06 (0.25) | 1.17 (0.64) | 1.21 (0.67) |
There were 21.9% missing INR at baseline, 64.0% missing at 12 month landmark time, and 76.3% missing at 24 month landmark time.
When examining changes in FIB-4 values from baseline to 24-months in patients with FIB-4 available at both time periods, 18.6% of patients with cirrhosis remained stable at high risk. In addition, 16.4% of patients had a high-risk FIB-4 at baseline that declined over time. FIB-4 values for few patients (3.5%) were low-risk at baseline but increased to high-risk. Approximately 61% with cirrhosis had low-risk FIB-4 values both at baseline and 24-months. In patients without cirrhosis where everyone had low FIB-4 at baseline, 97.8% remained stable at low-risk and 2.2% increased from low to high risk at 24-months.
RISK OF HCC FOLLOWING DAA TREATMENT
There were 3,247 incident cases of HCC diagnosed during mean 2.51 (SD=1.66) years of follow-up (maximum 7.11 years); 74.0% (n=2,404) were diagnosed in patients with cirrhosis at baseline. In 29,398 patients with cirrhosis, cumulative incidence of HCC was 2.0%, 3.8%, and 5.4% at 1, 2 and 3 years, respectively. In contrast, HCC risk was considerably lower in 63,169 patients without cirrhosis in whom 843 were diagnosed with HCC (0.2%, 0.5%, and 0.8%, respectively) (p-value <0.0001) (Figure 1).
Figure 1.
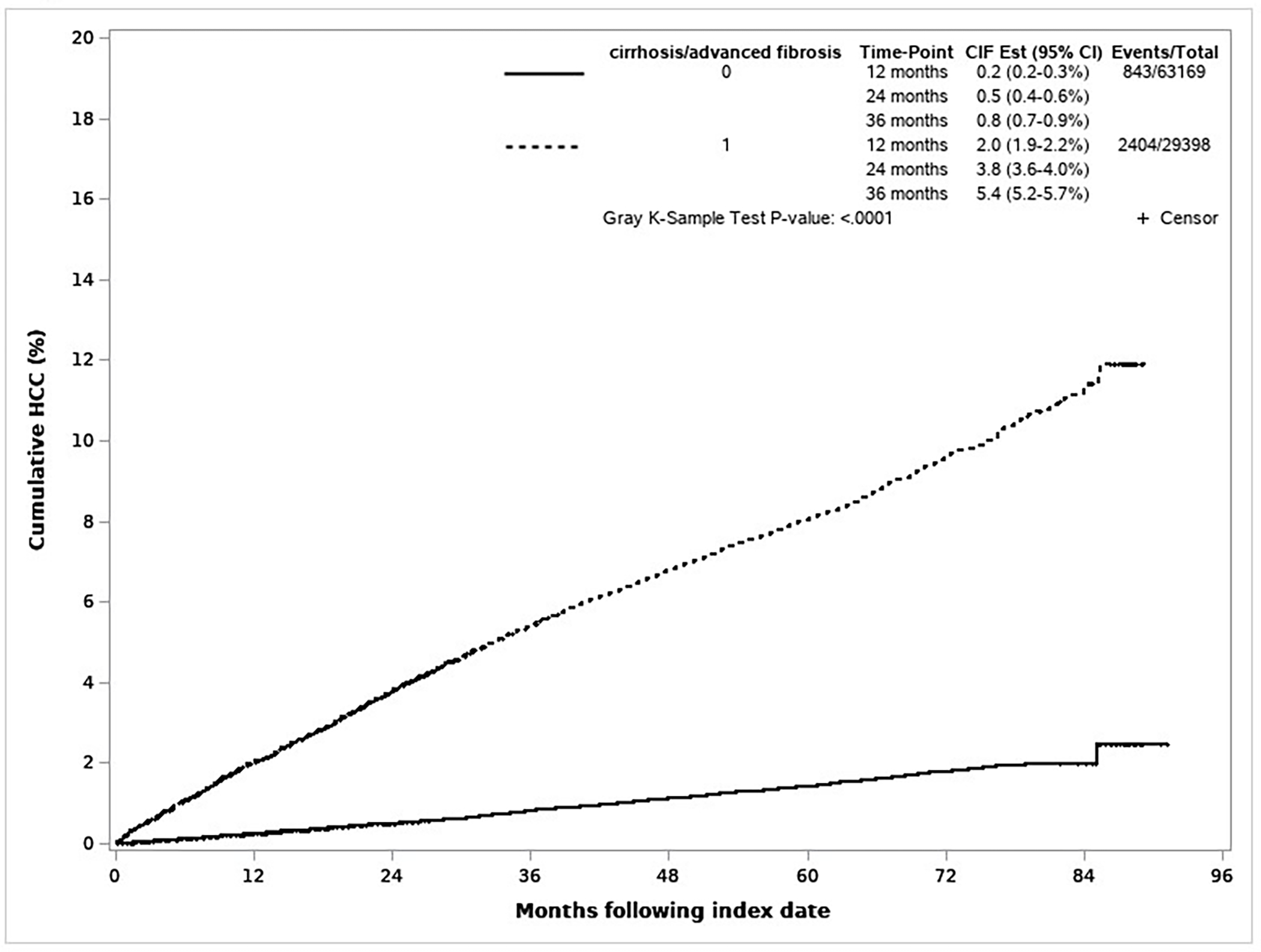
Cumulative incidence of hepatocellular carcinoma in patients with virally cured hepatitis C virus by baseline cirrhosis status
FACTORS ASSOCIATED WITH HCC IN PATIENTS WITH CIRRHOSIS AT BASELINE
In the baseline model (Table 3), in patients with cirrhosis, the risk of HCC was higher in men (adjusted hazard ratio (HR) =1.89, 95% confidence interval (CI)=1.37–2.59) and lower in African American patients (HR=0.78, 95%CI=0.71–0.86). Patients with genotype 3 had a higher risk of HCC (HR=1.47, 95%CI=1.27–1.71) than those with other genotypes. Patients with a longer duration since cirrhosis diagnosis had higher risk (≥5 years vs. <1 year, HR=1.71, 95%CI=1.46–2.00). Serum bilirubin, albumin, presence of varices, and higher FIB-4 were associated with HCC risk. Specifically, HCC risk was 2.5-fold higher (95%CI, 2.11–2.94) in cirrhosis patients who also had FIB-4 >3.25 than those with FIB-4 values <1.45. Patients who were current smokers had an increased risk for HCC than non-smokers (HR=1.32, 95%CI=1.16–1.51). None of the pre-specified interaction terms were significant in the adjusted model at baseline.
Table 3:
Factors associated with the development of HCC in patients with virologically cured HCV and cirrhosis.
| Variable | Baseline (N=29,398) | 12 months (N=26,648) | 24 months (N=21,600) | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-Value | HR (95% CI) | P-Value | HR (95% CI) | P-Value | |
| Demographics | ||||||
| Sex (Ref: Female) | ||||||
| Male | 1.89 (1.37–2.59) | <0.0001 | 1.93 (2.79–1.33) | 0.0005 | 1.99 (3.13–1.26) | 0.0031 |
| Race/ethnicity (Ref: White not Hispanic) | ||||||
| African American | 0.78 (0.71–0.86) | <0.0001 | 0.83 (0.75–0.93) | 0.0017 | 0.85 (0.74–0.98) | 0.0206 |
| White Hispanic | 0.89 (0.75–1.07) | 0.21 | 0.89 (0.73–1.10) | 0.2781 | 0.91 (0.71–1.18) | 0.4813 |
| Other | 1.17 (0.93–1.47) | 0.18 | 1.12 (0.86–1.47) | 0.4097 | 1.33 (0.96–1.84) | 0.0865 |
| Clinical Characteristics | ||||||
| HCV genotype (Ref: 1a) | ||||||
| 1a/1b | 0.93 (0.74–1.16) | 0.51 | 0.89 (0.68–1.16) | 0.3915 | 0.96 (0.70–1.32) | 0.7978 |
| 1b | 1.09 (0.98–1.21) | 0.10 | 1.06 (0.94–1.19) | 0.3473 | 0.97 (0.83–1.12) | 0.643 |
| 2 | 0.84 (0.71–0.99) | 0.04 | 0.82 (0.67–1.00) | 0.0515 | 0.78 (0.61–1.00) | 0.0466 |
| 3 | 1.47 (1.27–1.71) | <0.0001 | 1.41 (1.18–1.68) | 0.0002 | 1.35 (1.08–1.69) | 0.0087 |
| 4, 5, or 6 | 1.01 (0.63–1.61) | 0.97 | 1.66 (0.97–2.82) | 0.2066 | 1.31 (0.74–2.34) | 0.3591 |
| Prior HCV treatment | 1.08 (1.00–1.17) | 0.06 | 1.07 (0.97–1.18) | 0.1567 | 1.11 (0.99–1.25) | 0.0847 |
| FIB-4 baseline (Ref: <1.45) | ||||||
| 1.45–3.25 | 2.01 (1.71–2.37) | <0.0001 | N/A | -- | N/A | -- |
| >3.25 | 2.49 (2.11–2.94) | <0.0001 | N/A | -- | N/A | -- |
| Change in FIB-4*
ǂ (Ref: High-High) |
||||||
| (Ref: Low-Low) | ||||||
| High-High | N/A | -- | 1.75 (1.54–1.99) | <.0001 | 1.70 (1.45–2.00) | <0.0001 |
| High-Low | N/A | -- | 1.17 (1.00–1.36) | 0.0456 | 1.40 (1.19–1.65) | <0.0001 |
| Low-High | N/A | -- | 1.86 (1.50–2.30) | <.0001 | 1.81 (1.37–2.38) | <0.0001 |
| Years since cirrhosis diagnosis** | ||||||
| 1–<2 years | 1.41 (1.14–1.74) | 0.001 | Ref | -- | N/A^ | -- |
| 2–<5 years | 1.47 (1.24–1.74) | <0.0001 | 1.58 (1.30–1.93) | <0.0001 | Ref | -- |
| ≥ 5 years | 1.71 (1.46–2.00) | <0.0001 | 1.79 (1.49–2.15) | <0.0001 | 1.34 (1.16–1.55) | <0.0001 |
| Varices | 1.73 (1.57–1.91) | <0.0001 | 1.60 (1.40–1.82) | <0.0001 | 1.56 (1.34–1.82) | <0.0001 |
| Ascites | NS | 1.27 (1.05–1.53) | 0.0135 | 1.33 (1.07–1.66) | 0.0106 | |
| Baseline albumin, g/dl | 0.48 (0.44–0.52) | <0.0001 | 0.47 (0.41–0.52) | <0.0001 | 0.49 (0.42–0.57) | <0.0001 |
| Change in albuminǂ, g/dl | N/A | -- | 0.82 (0.70–0.97) | 0.02 | 0.90 (0.75–1.08) | 0.2627 |
| Baseline bilirubin, (log) mg/dl | 1.24 (1.15–1.34) | <0.0001 | 1.30 (1.17–1.45) | <0.0001 | 1.30 (1.13–1.48) | 0.0002 |
| Change in bilirubinǂ, (log) mg/dl | N/A | -- | 1.20 (1.06–1.36) | 0.0033 | 1.22 (1.06–1.41) | 0.0055 |
| Baseline INR ≥2 (Ref: <2) | 0.83 (0.58–1.18) | 0.30 | N/A | N/A | – | |
| Baseline sodium, mEg/l | NS | -- | 0.99 (0.97–1.01) | 0.3078 | NS | – |
| Change in sodiumǂ, mEq/l | N/A | -- | 1.01 (0.99–1.03) | 0.2883 | NS | – |
| Baseline hemoglobin, g/dl | NS | -- | 1.03 (1.00–1.07) | 0.0745 | 1.05 (1.00–1.09) | 0.0387 |
| Change in hemoglobinǂ, g/dl | N/A | -- | 1.03 (0.99–1.07) | 0.1337 | 1.04 (1.00–1.09) | 0.0715 |
| Behavioral Characteristics | ||||||
| Smoking (Ref: Non-smoker) | ||||||
| Current | 1.32 (1.16–1.51) | <0.0001 | 1.22 (1.05–1.41) | 0.0084 | 1.24 (1.04–1.48) | 0.019 |
| Former | 1.12 (0.97–1.29) | 0.12 | 1.07 (0.91–1.25) | 0.4138 | 1.08 (0.90–1.31) | 0.3891 |
Based on first date of elevated FIB-4; Low-Low indicates FIB-4 was <3.25 at both times, High-High indicates FIB-4 was >3.25 at both times, High-Low indicates FIB-4 was >3.25 at baseline and <3.25 at the landmark times, and Low-High indicates FIB-4 was <3.25 at baseline and increased to >3.25 at the landmark times.
Change variables calculated from baseline to 12 months for the model at 12 month landmark time and from baseline to 24 months for the model at 24 month landmark time.
Reference group for years since cirrhosis diagnosis: Baseline ref: <1 year; 12 month ref: 1-<2 years; 24 month ref: 2-<5 years.
NS: Not selected through model building strategy at given landmark time.
N/A: Not considered for in model building strategy at given landmark time.
: This category is not available at 24 month landmark time.
Abbreviations: HCC-hepatocellular carcinoma; HCV-hepatitis C virus; HR-hazard ratio; CI-confidence interval; Ref-reference group; FIB-4-fibrosis-4 index; INR-international normalized ratio
LONGITUDINAL CHANGES IN FACTORS AND THEIR ASSOCIATIONS WITH HCC IN PATIENTS WITH CIRRHOSIS
Most of the associations observed at baseline persisted in direction and magnitude at 12- and 24-month landmark times, with few notable exceptions. Presence of ascites was associated with an increased risk for HCC at both 12- and 24-months. Changes in serum albumin, bilirubin, and FIB-4 between baseline and landmark times were strongly associated with subsequent risk of HCC. For example, one unit increase in serum bilirubin between baseline and 24-month landmark time was associated with a 22% increase in HCC risk. Compared with patients with persistently low FIB-4 at baseline and 24-months, the risk of HCC was almost 2-fold higher in patients who had persistently high FIB-4 at the time of virological cure and at 24-months (HR=1.70, 95%CI, 1.45–2.00) and 81% higher in patients who were at low risk (FIB-4≤3.25) but increased to high risk (FIB-4>3.25) at 24-months (HR = 1.81, 95% CI = 1.37–2.38). Results were similar in the sensitivity analyses excluding cirrhosis patients with FIB-4<1.45 and including patients with 1 ICD code for HCC (Supplemental Tables 4 and 5).
FACTORS ASSOCIATED WITH HCC IN PATIENTS WITHOUT CIRRHOSIS AT BASELINE
In patients without cirrhosis, patients with hypertension and diabetes had a 1.5-fold higher risk of HCC than their counterparts (Table 4). After controlling for diabetes and hypertension, there was no difference in the risk of HCC between patients with BMI higher than 30 compared with BMI 25–30. Risk of HCC was higher in patients with BMI <25, with the highest risk in patients with BMI <18.5 compared with BMI 25-<30, (HR=1.93, 95%CI=1.20–3.12). The risk of HCC for BMI <18.5 was slightly attenuated after excluding patients with HCC in 12-months after index date (Supplemental Table 6).
Table 4:
Factors associated with the development of HCC in patients with virologically cured HCV without cirrhosis at baseline
| Variable | Baseline (N=63,169) | 12 months (N=58,639 | 24 months (N=46,889) | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-Value | HR (95% CI) | P-Value | HR (95% CI) | P-Value | |
| Clinical Characteristics | ||||||
| HCV genotype (Ref: 1a) |
NS | NS | ||||
| 1a/1b | 1.13 (0.78–1.63) | 0.52 | – | – | – | – |
| 1b | 1.13 (0.96–1.33) | 0.14 | – | – | – | – |
| 2 | 0.81 (0.62–1.06) | 0.13 | – | – | – | – |
| 3 | 1.45 (1.09–1.94) | 0.01 | – | – | – | – |
| 4, 5, or 6 | 1.14 (0.59–2.21) | 0.69 | – | – | – | – |
| Prior HCV treatment | 1.32 (1.14–1.52) | 0.0001 | 1.21 (1.03–1.42) | 0.02 | NS | – |
| Baseline FIB-4 (Ref: <1.45) | ||||||
| 1.45–3.25 | 2.27 (1.96–2.63) | <0.0001 | N/A | – | N/A | – |
| Change in FIB-4 (Ref: Low-low)* | ||||||
| Low-High | N/A | – | 2.58 (1.82–3.66) | <0.0001 | 3.12 (2.12–4.60) | <0.0001 |
| Baseline albumin, g/dl | 0.64 (0.53–0.76) | <0.0001 | 0.63 (0.51–0.78) | <0.0001 | 0.76 (0.58–1.00) | 0.05 |
| Change in albuminǂ, g/dl | 0.92 (0.70–1.22) | 0.57 | 1.12 (0.82–1.52) | 0.47 | ||
| BMI (Ref: 25-<30) | ||||||
| <18.5 | 1.93 (1.20–3.12) | 0.007 | NS | -- | NS | – |
| 18.5-<25 | 1.19 (1.01–1.41) | 0.04 | NS | -- | NS | -- |
| ≥30 | 0.97 (0.82–1.15) | 0.75 | NS | -- | NS | – |
| Hypertension | 1.59 (1.27–1.99) | <0.0001 | 1.65 (1.26–2.14) | 0.0002 | 1.74 (1.24–2.45) | 0.001 |
| Diabetes | 1.54 (1.34–1.78) | <0.0001 | 1.42 (1.22–1.66) | <0.0001 | 1.47 (1.22–1.76) | <0.0001 |
| Behavioral Characteristics | ||||||
| Smoking (Ref: Non-smoker) | ||||||
| Current | 1.46 (1.16–1.84) | 0.002 | 1.22 (0.93–1.61) | 0.14 | 1.24 (0.87–1.76) | 0.22 |
| Former | 1.24 (0.94–1.63) | 0.12 | 1.20 (0.88–1.63) | 0.23 | 1.18 (0.82–1.69) | 0.37 |
Based on first date of elevated FIB-4; Low-Low indicates FIB-4 was <3.25 at both landmark times and Low-High indicates FIB-4 was <3.25 at baseline and increased to >3.25 at the landmark times. For 12 month time period the change was baseline to 12 months and for the 24 month time period the change was baseline to 24 months.
Change variables calculated from baseline to 12 months for the model at 12 month landmark time and from baseline to 24 months for the model at 24 month landmark time.
N/A: Not applicable because not considered for model building strategy at given landmark time.
NS: Not selected by model building strategy at given landmark time.
Abbreviations: HCC-hepatocellular carcinoma; HCV-hepatitis C virus; HR-hazard ratio; CI-confidence interval; Ref-reference group; FIB-4-fibrosis-4 index; BMI-body mass index
Baseline serum albumin and FIB-4 were associated with HCC risk. Patients with baseline FIB-4 between 1.45 and 3.25 had 2.3-fold higher risk of HCC than those with low FIB-4 <1.45 (HR=2.27, 95%CI=1.96–2.63). Prior failure of HCV treatment, HCV genotype 3, and current smoking were also associated with HCC risk in patients without cirrhosis.
LONGITUDINAL CHANGES IN FACTORS AND THEIR ASSOCIATIONS WITH HCC IN PATIENTS WITHOUT CIRRHOSIS
Only metabolic traits (diabetes, hypertension) and change in FIB-4 remained associated with HCC risk at all landmark times. At 24-month landmark time, patients with hypertension and diabetes had 1.7 to 1.5-fold higher risk of HCC than their counterparts. Patients who had an increase from FIB-4 of low risk at baseline to high risk at 24-month landmark time had 3-fold increase in HCC risk (HR=3.12, 95%CI, 2.12–4.60).
CUMULATIVE HCC RISKS IN GROUPS BASED ON CHANGES IN FIB-4
Figure 2 displays the cumulative incidence rates of HCC in patients with cirrhosis stratified by changes in FIB-4 values between baseline and 24-month landmark. Following the 24-month landmark time, the 3-year cumulative incidence of HCC varied from 3.4% (95%CI, 3.1%–3.7%) in patients who had persistently low FIB-4 value to 9.2% (95%CI, 8.3%–10.2%) in patients with persistently high FIB-4. Risk of HCC was low in all subgroups without cirrhosis, with few notable exceptions. In patients who increased from low to high risk, the 2- and 3-year cumulative incidence of HCC was 2.4% (95%CI, 1.6%–3.6%) and 3.0% (95%CI, 2.1%–4.4%), respectively. Cumulative 1, 2- and 3-year risk of HCC in 419 patients without cirrhosis who had hypertension, diabetes and who experienced an increase from low to high risk between baseline and 24-month landmark was 3.1%, 5.7%, and 7.2%, respectively.
Figure 2.
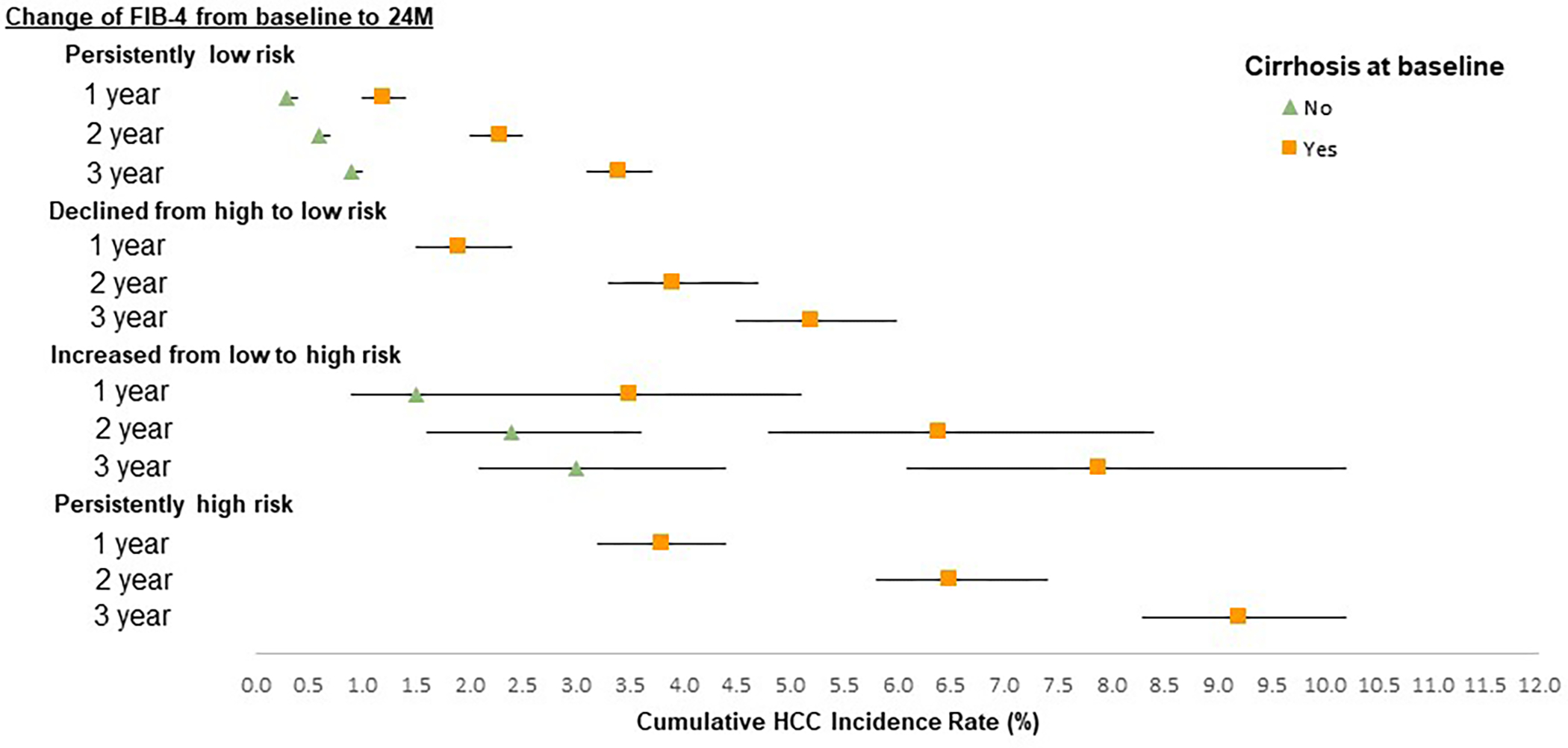
Cumulative incidence rates of hepatocellular carcinoma by various patterns of change of FIB-4 from baseline to 24-months stratified by cirrhosis status
DISCUSSION
Our study has three key findings.
First, HCC risk factors differed in patients with and without cirrhosis. In patients with cirrhosis, several markers of liver disease severity (cirrhosis duration, albumin, bilirubin, varices, and hemoglobin) were associated with increased risk of HCC. In contrast, metabolic traits predicted HCC risk in patients without cirrhosis, underscoring the importance of metabolic traits as modifiable targets for risk reduction after virological cure.
Second, changes in several risk factors over time, especially markers of liver fibrosis, could inform risk prediction in patients with virologically cured HCV. Change in FIB-4 scores was associated with incident HCC in patients with and without cirrhosis. These findings were similar to that of Ioannou et al.15 In our cohort, patients without cirrhosis experienced an increase in FIB-4 over 2 years; 2/3rds of these also had diabetes and hypertension suggesting metabolic traits could have played a role in fibrosis progression. Subsequent risk of HCC in this subgroup approached the HCC incidence in patients with cirrhosis. These data are important because in patients with chronic liver diseases, evaluation of the risk of disease progression currently relies only on the most recent risk factor assessment. Our results show that repeat data at 2-year interval might allow more accurate HCC risk stratification.
Third, our data confirm previous reports that showed that the risk of HCC remain elevated in some groups of patients after SVR.3, 15–17 The trends reported here provide support to the current recommendations about continuing HCC surveillance in virologically cured patients with cirrhosis.
In our cohort, smoking was associated with a higher risk of HCC in patients with cirrhosis. Several constituents of tobacco smoke can promote hepatocarcinogenesis.18–21 We also noted an association between low BMI and high risk of HCC. In a recent study, sarcopenia was associated with an increased risk of developing HCC.22 The exact mechanistic links cannot be explored in this retrospective study but could be related to sarcopenia-associated chronic inflammation, physical inactivity, insulin resistance, and/or vitamin D or Zinc deficiency.
Our study has limitations. It was limited to veterans with HCV, however this cohort represents the largest known HCV-infected cohort in the world with almost complete capture of outcomes. We might have misclassified patients with cirrhosis or HCC. However, we used previously validated algorithms. Fewer than 19.6% of patients with cirrhosis diagnosis at baseline had FIB-4 <1.45 and excluding these patients in a sensitivity analysis did not impact the results. FIB-4 was missing in 0.7–6.8% of patients at different landmark times; there were some differences in these patients, but they were small and clinically inconsequential, rendering selection bias less likely (Supplementary Table 3). We might have missed HCCs in early stages or HCC diagnosed outside of the VA healthcare system. However, this is unlikely to bias our results because our outcome of interest was any incident HCC (and not early HCC per se) and most early HCCs progress to advanced stages within a relatively short course of time. Expanding HCC definition to include those with just 1 ICD code did not change study findings.
In conclusion, our study provides evidence from a well characterized cohort of virologically cured patients with HCV but free of cirrhosis that monitoring changes in FIB-4 might have clinical utility, especially in patients with co-existing diabetes and hypertension. In patients with cirrhosis, evolution of FIB-4 and other select markers of liver disease severity could refine risk stratification. We believe risk assessment based on repeat measurements at 2 years is practical and can improve shared decision-making between patients and their physicians by providing a quantifiable personalized HCC risk assessment, including how it changes over time.
Supplementary Material
STUDY HIGHLIGHTS:
What is known
Hepatitis C virus (HCV) infection is the leading risk factor for hepatocellular carcinoma (HCC).
Risk for HCC remains in patients with cured chronic HCV infection.
What is new here
Risk factors for HCC differed in patients with and without cirrhosis.
Longitudinal changes of risk factors over time, especially markers of liver fibrosis, were important predictors of HCC risk.
These factors can help with risk stratification and HCC surveillance decisions in patients with cured HCV.
FINANCIAL SUPPORT:
This material is based upon work supported by Department of Defense (DoD W81XWH1910689 and W81XWH1910690). The work is also supported in part by the National Cancer Institute U01 CA230997-01 and R01 CA256977, by the Cancer Prevention & Research Institute of Texas (CPRIT RP200633), the Veterans Administration Center for Innovations in Quality, Effectiveness and Safety (CIN 13–413), Michael E. DeBakey VA Medical Center, Houston, and by the Texas and the Center for Gastrointestinal Development, Infection and Injury (NIDDK P30 DK 56338). The work of Dr. Li was partially funded by NIH R01DK118079 and P30CA016672.
ABBREVIATIONS:
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- SVR
sustained virological response
- HR
hazard ratio
- CI
confidence interval
- DAA
direct acting antivirals
- VA
US Department of Veterans Affairs
- CDW
Corporate Data Warehouse
- CCR
Central Cancer Registry
- ICD
International Classification of Diseases
- AUDIT
C-Alcohol Use Disorders Identification Test-concise
- EMR
electronic medical records
- FIB
4-fibrosis-4 score
- BMI
body mass index
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- INR
international normalized ratio
- Ref
reference group
Appendix 1
Names of direct acting antivirals used to define the cohort include sofosbuvir, simeprevir, ledipasvir, combination of paritaprevir/ritonavir, ombitasvir and dasabuvir, daclatasvir, sofosbuvir/velpatasvir, elbasvir/grazoprevir, sofosbuvir/velpatasvir/voxilaprevir, or glecaprevir/pibrentasvir
Appendix 2
We derived serial values of other laboratory tests (serum albumin, sodium, bilirubin, INR, hemoglobin) using the data within 24-months prior and nearest to index. We defined HBV based on any positive hepatitis B surface antigen before index. We defined active alcohol use disorder based on AUDIT-C of at least 4 (≥3 for women) in the past 12-months. We ascertained smoking status as ever, never and current smokers. We derived BMI based on height and weight within 12-months before and closest to index. Each of these variables were updated annually using the values closest to the 12- and 24-month time points.
We defined medical (diabetes, hypertension, dyslipidemia, congestive heart failure, chronic obstructive pulmonary disease, cancer, and chronic kidney disease) and mental health comorbidity (anxiety, depression) based on two outpatient or one inpatient diagnosis any time before index. (ICD-9 and 10 codes in Supplemental Table 2). We also calculated the Deyo comorbidity score based on diagnoses recorded within 1 year before index.11
Footnotes
POTENTIAL COMPETING INTERESTS: None to report
GUARANTOR OF THE ARTICLE: Dr. Fasiha Kanwal
DISCLAIMER: The opinions and assertions contained herein are the sole views of the authors and are not to be construed as official or as reflecting the views of the Department of Veterans Affairs or the United States.
REFERENCES
- 1.Kanwal F, Kramer JR, Asch SM, et al. Long-Term Risk of Hepatocellular Carcinoma in HCV Patients Treated With Direct Acting Antiviral Agents. Hepatology. 2020;71(1):44–55. [DOI] [PubMed] [Google Scholar]
- 2.Chen Q, Ayer T, Adee MG, et al. Assessment of Incidence of and Surveillance Burden for Hepatocellular Carcinoma Among Patients With Hepatitis C in the Era of Direct-Acting Antiviral Agents. JAMA Netw Open. 2020;3(11):e2021173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanwal F, Kramer J, Asch SM, et al. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology. 2017;153(4):996–1005.e1001. [DOI] [PubMed] [Google Scholar]
- 4.Bradley KA, Williams EC, Achtmeyer CE, et al. Implementation of evidence-based alcohol screening in the Veterans Health Administration. Am J Manag Care. 2006;12(10):597–606. [PubMed] [Google Scholar]
- 5.Davila JA, Weston A, Smalley W, et al. Utilization of screening for hepatocellular carcinoma in the United States. J Clin Gastroenterol. 2007;41(8):777–782. [DOI] [PubMed] [Google Scholar]
- 6.Kanwal F, Hoang T, Kramer JR, et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011;140(4):1182–1188.e1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kramer JR, Giordano TP, Souchek J, et al. The effect of HIV coinfection on the risk of cirrhosis and hepatocellular carcinoma in U.S. veterans with hepatitis C. Am J Gastroenterol. 2005;100(1):56–63. [DOI] [PubMed] [Google Scholar]
- 8.Moon AM, Weiss NS, Beste LA, et al. No Association Between Screening for Hepatocellular Carcinoma and Reduced Cancer-Related Mortality in Patients With Cirrhosis. Gastroenterology. 2018;155(4):1128–1139.e1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yongpisarn T, Thimphitthaya C, Laoveeravat P, et al. Non-invasive tests for predicting liver outcomes in chronic hepatitis C patients: A systematic review and meta-analysis. World J Hepatol. 2021;13(8):949–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32–36. [DOI] [PubMed] [Google Scholar]
- 11.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 12.Wolbers M, Koller MT, Witteman JC, et al. Prognostic models with competing risks: methods and application to coronary risk prediction. Epidemiology. 2009;20(4):555–561. [DOI] [PubMed] [Google Scholar]
- 13.Dafni U Landmark analysis at the 25-year landmark point. Circ Cardiovasc Qual Outcomes. 2011;4(3):363–371. [DOI] [PubMed] [Google Scholar]
- 14.van Houwelingen H, Putter H. Dynamic prediction in clinical survival analysis: CRC Press; 2011. [Google Scholar]
- 15.Ioannou GN, Beste LA, Green PK, et al. Increased Risk for Hepatocellular Carcinoma Persists Up to 10 Years After HCV Eradication in Patients With Baseline Cirrhosis or High FIB-4 Scores. Gastroenterology. 2019;157(5):1264–1278.e1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan PPY, Levy MT, Shackel N, et al. Hepatocellular carcinoma incidence post direct-acting antivirals in hepatitis C-related advanced fibrosis/cirrhosis patients in Australia. Hepatobiliary Pancreat Dis Int. 2020;19(6):541–546. [DOI] [PubMed] [Google Scholar]
- 17.Ioannou GN, Green PK, Beste LA, et al. Development of models estimating the risk of hepatocellular carcinoma after antiviral treatment for hepatitis C. J Hepatol. 2018;69(5):1088–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim NH, Jung YS, Hong HP, et al. Association between cotinine-verified smoking status and risk of nonalcoholic fatty liver disease. Liver Int. 2018;38(8):1487–1494. [DOI] [PubMed] [Google Scholar]
- 19.Lee YC, Cohet C, Yang YC, et al. Meta-analysis of epidemiologic studies on cigarette smoking and liver cancer. Int J Epidemiol. 2009;38(6):1497–1511. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y, Dai M, Bi Y, et al. Active smoking, passive smoking, and risk of nonalcoholic fatty liver disease (NAFLD): a population-based study in China. J Epidemiol. 2013;23(2):115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zein CO, Unalp A, Colvin R, et al. Smoking and severity of hepatic fibrosis in nonalcoholic fatty liver disease. J Hepatol. 2011;54(4):753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng Z, Zhao H, Jiang Y, et al. Sarcopenia associates with increased risk of hepatocellular carcinoma among male patients with cirrhosis. Clin Nutr. 2020;39(10):3132–3139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


