This case series assesses data from 55 US hospitals about neurologic involvement in children and adolescents with acute COVID-19 or multisystem inflammatory syndrome in children.
Key Points
Question
What was the spectrum of SARS-CoV-2–related pediatric severe neurologic involvement in 2021?
Findings
In this case series of 2168 US patients younger than 21 years hospitalized for acute COVID-19 (34%) or multisystem inflammatory syndrome in children (66%), 476 (22%) had neurologic involvement. Of these, 42 (9%) had life-threatening conditions, with 23 (55%) having acute central nervous system (CNS) infections/demyelination; 18 of 42 (43%) died or had new neurologic deficits; and most vaccine-eligible patients were unvaccinated.
Meaning
In 2021, SARS-CoV-2–related severe neurologic involvement in US hospitalized children and adolescents showed a potential increase in diagnoses of acute CNS infections/demyelination.
Abstract
Importance
In 2020 during the COVID-19 pandemic, neurologic involvement was common in children and adolescents hospitalized in the United States for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–related complications.
Objective
To provide an update on the spectrum of SARS-CoV-2–related neurologic involvement among children and adolescents in 2021.
Design, Setting, and Participants
Case series investigation of patients reported to public health surveillance hospitalized with SARS-CoV-2–related illness between December 15, 2020, and December 31, 2021, in 55 US hospitals in 31 states with follow-up at hospital discharge. A total of 2253 patients were enrolled during the investigation period. Patients suspected of having multisystem inflammatory syndrome in children (MIS-C) who did not meet criteria (n = 85) were excluded. Patients (<21 years) with positive SARS-CoV-2 test results (reverse transcriptase–polymerase chain reaction and/or antibody) meeting criteria for MIS-C or acute COVID-19 were included in the analysis.
Exposure
SARS-CoV-2 infection.
Main Outcomes and Measures
Patients with neurologic involvement had acute neurologic signs, symptoms, or diseases on presentation or during hospitalization. Life-threatening neurologic involvement was adjudicated by experts based on clinical and/or neuroradiological features. Type and severity of neurologic involvement, laboratory and imaging data, vaccination status, and hospital discharge outcomes (death or survival with new neurologic deficits).
Results
Of 2168 patients included (58% male; median age, 10.3 years), 1435 (66%) met criteria for MIS-C, and 476 (22%) had documented neurologic involvement. Patients with neurologic involvement vs without were older (median age, 12 vs 10 years) and more frequently had underlying neurologic disorders (107 of 476 [22%] vs 240 of 1692 [14%]). Among those with neurologic involvement, 42 (9%) developed acute SARS-CoV-2–related life-threatening conditions, including central nervous system infection/demyelination (n = 23; 15 with possible/confirmed encephalitis, 6 meningitis, 1 transverse myelitis, 1 nonhemorrhagic leukoencephalopathy), stroke (n = 11), severe encephalopathy (n = 5), acute fulminant cerebral edema (n = 2), and Guillain-Barré syndrome (n = 1). Ten of 42 (24%) survived with new neurologic deficits at discharge and 8 (19%) died. Among patients with life-threatening neurologic conditions, 15 of 16 vaccine-eligible patients (94%) were unvaccinated.
Conclusions and Relevance
SARS-CoV-2–related neurologic involvement persisted in US children and adolescents hospitalized for COVID-19 or MIS-C in 2021 and was again mostly transient. Central nervous system infection/demyelination accounted for a higher proportion of life-threatening conditions, and most vaccine-eligible patients were unvaccinated. COVID-19 vaccination may prevent some SARS-CoV-2–related neurologic complications and merits further study.
Introduction
In 2020, neurologic involvement in severe acute COVID-19 or multisystem inflammatory syndrome in children (MIS-C) was identified in 22% of pediatric patients (365 of 1695) hospitalized at 52 US sites from March to mid-December, and 12% had severe complications.1 In June 2021, the B.1.617.2 (Delta) variant of SARS-CoV-2 became predominant, causing another surge of US pediatric hospitalizations.2 In 2021, children became eligible for COVID-19 vaccination (May 12, 2021, for adolescents,3 November 2, 2021, for children aged 5-11 years4). This update on the extent of SARS-CoV-2–related neurologic involvement and documented hospital outcomes in US children and adolescents evaluates patients hospitalized during 2021 including their COVID-19 vaccination status. Data for the update came from active surveillance performed at hospitals participating in the Overcoming COVID-19 public health surveillance network (eAppendix in Supplement 1).
Methods
Design and Participants
We performed active surveillance at 55 hospitals in 31 states to identify US patients (<21 years) with severe acute COVID-19 (admitted to an intensive care or step-down unit at a participating site) or who met Centers for Disease Control and Prevention (CDC) criteria for MIS-C hospitalized between December 15, 2020, and December 31, 2021 (eTable 1 in Supplement 1). Patients with acute COVID-19 had a positive result on a SARS-CoV-2 respiratory test (reverse transcriptase–polymerase chain reaction [RT-PCR] or antigen) and symptoms related to COVID-19. Patients with MIS-C had a positive SARS-CoV-2 respiratory or antibody test result. Reporting guidelines for uncontrolled case series were followed.5 The investigation was approved by the central institutional review board at Boston Children’s Hospital and determined to meet the requirement of public health surveillance as defined in 45 CFR 46.102(I)(2) by the CDC with waiver of consent.
Classification of Neurologic Involvement and Outcomes
Data were abstracted from medical records by trained staff.1 Patients with life-threatening neurologic conditions and neurologic deficits (gross impairment in motor, cognitive, or speech and language functions) identified from medical records were adjudicated by neurology, neuroradiology, and critical care experts (K.L.L., T.Y.P., A.G.R.). Cases of encephalitis were adjudicated using standardized case report forms including the International Encephalitis Consortium criteria.6 Race and ethnicity were extracted from medical records, and social vulnerability index7 was calculated from home addresses. Vaccination eligibility and status were confirmed as previously reported (eMethods in Supplement 1).
Statistical Analyses
Descriptive statistics were used to report frequencies. Continuous variables included median and IQR, and categorical variables included counts and percentages. We used a χ2 test, Fisher exact test, or Kruskal-Wallis test to evaluate between-group differences using R (version 4.0.2, R Project for Statistical Computing) with P < .05 considered statistically significant. Missing data were not imputed.
Results
Of 2168 patients (58% male; median age, 10.3 years) with acute COVID-19 (34%) or MIS-C (66%), 476 (22%) had neurologic involvement (Table 1 and eFigure 1 in Supplement 1). Patients with neurologic involvement were older and had more underlying neurologic disorders than those without. Seizures were more common in younger children, and loss of taste and smell was more common in adolescents (eTable 2 and eFigure 2 in Supplement 1).
Table 1. Characteristics and Outcomes of 2168 Patients (<21 Years) Hospitalized for SARS-CoV-2–Related Illness by Reported Neurologic Involvement During the Second Year of the Pandemic (December 15, 2020, to December 31, 2021).
| Characteristic | No. (%) | P value | ||
|---|---|---|---|---|
| All patients | With neurological involvement | Without neurological involvement | ||
| No. | 2168 | 476 | 1692 | |
| Sex | .93 | |||
| Male | 1260 (58) | 278 (58) | 982 (58) | |
| Female | 908 (42) | 198 (42) | 710 (42) | |
| Age, y | <.001 | |||
| Median (IQR) | 10.3 (5.9-14.7) | 11.7 (7.1-15.8) | 10.0 (5.5-14.3) | |
| <1 | 120 (6) | 32 (7) | 88 (5) | |
| 1-<5 | 342 (16) | 44 (9) | 298 (18) | |
| 5-<12 | 832 (38) | 172 (36) | 660 (39) | |
| 12-<18 | 783 (36) | 202 (42) | 581 (34) | |
| 18-<21 | 91 (4) | 26 (5) | 65 (4) | |
| Race and ethnic groupa | .19 | |||
| Asian | 58 (3) | 12 (3) | 46 (3) | |
| Hispanic or Latino | 467 (22) | 93 (20) | 374 (22) | |
| Non-Hispanic Black | 621 (29) | 150 (32) | 471 (28) | |
| Non-Hispanic White | 778 (36) | 180 (38) | 598 (35) | |
| Other race, non-Hispanic | 52 (2) | 8 (2) | 44 (3) | |
| Unknown | 192 (9) | 33 (7) | 159 (9) | |
| Socioeconomic status | .08 | |||
| Government/public insurance | 1240 (57) | 290 (61) | 950 (56) | |
| Private/self-pay | 869 (40) | 169 (36) | 700 (41) | |
| Unknown insurance | 59 (3) | 15 (3) | 44 (3) | |
| SVI, median (IQR) | 0.59 (0.32-0.82) | 0.65 (0.37-0.81) | 0.58 (0.30-0.82) | .08 |
| Lowest third (least vulnerable) | 534 (25) | 99 (21) | 453 (26) | .05 |
| Middle third | 659 (30) | 140 (29) | 519 (31) | |
| Highest third (most vulnerable) | 876 (40) | 211 (44) | 665 (39) | |
| Missing SVI | 99 (5) | 26 (6) | 73 (4) | |
| SARS-CoV-2 testing | ||||
| RT-PCR performed | 2017 (93) | 458 (96) | 1559 (92) | .003 |
| RT-PCR positive | 1153/2017 (57) | 304/458 (66) | 849/1559 (54) | <.001 |
| Antibody test performed | 1539 (71) | 303 (64) | 1236 (73) | <.001 |
| Antibody test result positive | 1442/1539 (94) | 275/303 (91) | 1167/1236 (94) | .03 |
| Date of admission | ||||
| December 2020-May 2021 (pre-Delta) | 1096 (51) | 217 (46) | 879 (52) | .02 |
| June 2021-December 2021 (Delta) | 1072 (49) | 259 (54) | 813 (48) | |
| Underlying conditionsb | ||||
| Previously healthyc | 1128 (52) | 204 (43) | 924 (55) | <.001 |
| ≥1 Comorbidity, excluding obesity | 788 (36) | 216 (45) | 572 (34) | <.001 |
| Neurological, any condition | 347 (16) | 107 (22) | 240 (14) | <.001 |
| Neurodevelopmental/psychiatricd | 274 (13) | 79 (17) | 195 (12) | .004 |
| Seizure disorder | 114 (5) | 51 (11) | 63 (4) | <.001 |
| Neuromuscular disorderse | 104 (5) | 40 (8) | 64 (4) | <.001 |
| Congenital neurologic disordersf | 30 (1) | 18 (4) | 12 (0.7) | <.001 |
| Static encephalopathy | 26 (1) | 10 (2) | 16 (0.9) | .07 |
| Otherg | 11 (0.5) | 3 (0.6) | 8 (0.5) | .72 |
| Respiratory | 428 (20) | 99 (21) | 329 (19) | .56 |
| Gastrointestinal | 167 (8) | 52 (11) | 115 (7) | .004 |
| Endocrine | 129 (6) | 55 (12) | 74 (4) | <.001 |
| Cardiac | 104 (5) | 30 (6) | 74 (4) | .11 |
| Genetic or metabolic (not obesity) | 81 (4) | 27 (6) | 54 (3) | .02 |
| Hematological | 71 (3) | 16 (3) | 55 (3) | >.99 |
| Oncologic or immune compromised | 64 (3) | 14 (3) | 50 (3) | >.99 |
| Renal | 58 (3) | 22 (5) | 36 (2) | .01 |
| BMI-based obesityh | 708 (36) | 168 (39) | 540 (35) | .12 |
| Organ system involvement | ||||
| Met MIS-C criteria | 1435 (66) | 269 (57) | 1166 (69) | <.001 |
| Organ systems involved, median (IQR) | 4 (3-5) | 5 (3-6) | 3 (2-4) | <.001 |
| Vaccination statusi | ||||
| Vaccine eligible | 664 (31) | 185 (39) | 479 (28) | <.001 |
| Fully | 10 (2) | 4 (2) | 6 (1) | .53 |
| Partially | 17 (3) | 4 (2) | 13 (3) | |
| Unvaccinated | 511 (77) | 147 (79) | 364 (76) | |
| Unknown vaccination status | 126 (19) | 30 (16) | 96 (20) | |
| Outcomes | ||||
| ICU admission | 1560 (72) | 400 (84) | 1160 (69) | <.001 |
| ECMO | 113 (5) | 46 (10) | 67 (4) | <.001 |
| Mechanical ventilation | 336 (16) | 146 (31) | 190 (11) | <.001 |
| Vasopressors | 768 (35) | 226 (48) | 542 (32) | <.001 |
| Length of stay, median (IQR), d | ||||
| ICU | 3 (2-6) | 5 (2-10) | 3 (2-6) | <.001 |
| Hospital | 6 (4-9) | 7 (5-14) | 5 (4-8) | <.001 |
| Died | 56 (3) | 31 (7) | 26 (2) | <.001 |
| Survived, new neurological deficit | 39 (2) | 29 (6) | 9 (0.5) | <.001 |
| Discharged to rehabilitation/other acute care facility | 51 (2) | 23 (5) | 28 (2) | <.001 |
Abbreviations: BMI, body mass index; CNS, central nervous system; ECMO, extracorporeal membrane oxygenation; HIE, hypoxic ischemic encephalopathy; ICU, intensive care unit; MIS-C, multisystem inflammatory syndrome in children; RT-PCR, reverse transcriptase–polymerase chain reaction; SVI, social vulnerability index5 (higher scores indicate higher vulnerability).
Race and ethnic group were abstracted from the patient’s medical record. Race categories are not mutually exclusive.
Patients may have more than 1 underlying condition.
“Previously healthy” was defined as an absence of reported underlying conditions (including obesity) and taking no prescription medications.
Neurodevelopmental/psychiatric conditions include developmental delay, cognitive delay, gross motor delay, and intellectual disability; psychiatric disorders include attention-deficit/hyperactivity disorder, mood disorder, and autism/autism spectrum disorder.
Neuromuscular disorders include spastic quadriplegia, muscular dystrophy, neuromuscular weakness, and neuromuscular scoliosis.
Congenital neurologic disorders include hydrocephalus, neurogenetic, and neurometabolic disorders.
Other underlying neurological conditions include history of CNS tumor or traumatic brain injury, prior stroke/hypoxic ischemic injury, and history of CNS infection/demyelinating disorder.
The determination of BMI-based obesity was based on the Centers for Disease Control and Prevention national reference standard for age and sex among patients who were at least 2 years of age (n = 426 for patients with neurological involvements and n = 1534 for patients without neurological involvement).
Vaccination eligibility/status was defined as previously reported (eMethods in Supplement 1).
In patients with neurologic involvement, 91% had non–life-threatening neurologic symptoms, most commonly fatigue/weakness, confusion, headache, and loss of taste/smell. Among patients with non–life-threatening neurologic involvement, 90% survived without neurologic deficits, 5% died, and 4% were discharged alive with neurologic deficits related to sequelae of critical illness. A spectrum of life-threatening neurologic conditions and outcomes were identified in 42 of 476 patients (9%) with neurologic involvement, including 23 (55%) with acute central nervous system (CNS) infection/acute disseminated encephalomyelitis (ADEM). Life-threatening neurologic conditions were more frequently reported during the Delta than pre-Delta periods (64% vs 36%). Ten of 42 patients (24%) survived with new neurologic deficits at discharge and 8 (19%) died (Table 2 and eTable 3 in Supplement 1).
Table 2. Life-threatening Neurologic Conditions and Deaths Related to COVID-19 or MIS-C in 42 Hospitalized Patients (<21 Years) During the Second Year of the Pandemic (December 15, 2020, to December 31, 2021).
| Variable | Life-threatening SARS-CoV-2–related neurologic conditions, No. (%) | |||||
|---|---|---|---|---|---|---|
| Any condition | Acute CNS infection or ADEM | Ischemic or hemorrhagic stroke | Severe encephalopathy | Acute fulminant cerebral edema | Guillain-Barré Syndrome | |
| No. | 42 | 23 | 11 | 5 | 2 | 1 |
| Age, median (IQR), ya | 11 (6-16) | 1 Infant | 2 Infants | 1 Infant | 2 Teenagers | 1 School age |
| 1 Toddler | 2 Toddlers | 1 School age | ||||
| 2 Preschoolers | 1 Preschooler | 3 Teenagers | ||||
| 9 School age | 3 School age | |||||
| 9 Teenagers | 3 Teenagers | |||||
| 1 Young adult | ||||||
| Male sex | 29 (67) | 13 (57) | 10 (91) | 4 (80) | 2 (100) | 0 |
| SARS-CoV-2 strain | ||||||
| Pre-Delta (December 15, 2020, to May 2021) | 15 (36) | 9 (39) | 4 (36) | 2 (40) | 0 | 0 |
| Delta (June 2021 to December 31, 2021) | 27 (64) | 14 (61) | 7 (64) | 3 (60) | 2 (100) | 1 (100) |
| RT-PCR or antibody results | ||||||
| RT-PCR positive only | 19 (45) | 9 (39) | 7 (64) | 1 (20) | 1 (50) | 1 (100) |
| Antibody positive only | 13 (31) | 8 (35) | 1 (9) | 3 (60) | 1 (50) | 0 |
| RT-PCR and antibody positive | 10 (24) | 6 (26) | 3 (27) | 1 (20) | 0 | 0 |
| MIS-C diagnosis | 20 (48) | 13 (57) | 3 (27) | 3 (60) | 1 (50) | 0 |
| No major underlying conditions | 16 (38) | 8 (35) | 5 (45) | 2 (40) | 0 | 1 (100) |
| Underlying neurologic disorder | 5 (12) | 1 (4) | 3 (27) | 0 | 1 (50) | 0 |
| Death | 7 (17) | 3 (13) | 3 (27) | 0 | 2 (100) | 0 |
| Discharged alive, new CNS deficit | 11 (26) | 4 (17) | 5 (45) | 0 | 0 | 1 (100) |
| Vaccine eligible | 16 (38) | 11 (48) | 2 (18) | 1 (20) | 2 (100) | 0 |
| Fully or partially vaccinated/eligible | 1/16 (6) | 1/11 (9) | 0 | 0 | 0 | 0 |
Abbreviations: ADEM, acute disseminated encephalomyelitis; CNS, central nervous system; MIS-C, multisystem inflammatory syndrome in children; RT-PCR, reverse transcriptase–polymerase chain reaction.
Age categories reported for privacy reasons for subcategories of neurologic conditions are infant (<1 year), toddler (1-2 years), preschooler (3-5 years), school age (6-12 years), teenager (13-17 years), and young adult (18-21 years).
There were 9 possible and 5 confirmed cases of encephalitis (eTable 4 in Supplement 1). Electroencephalography abnormalities included diffuse background slowing (n = 10) and/or focal seizures or epileptic discharges (n = 5). Brain magnetic resonance imaging (MRI) findings were mostly ADEM-like with multifocal, nonenhancing lesions with T2 prolongation and reduced diffusivity mainly in the deep juxtacortical and periventricular white matter, thalami, basal ganglia, brainstem, and posterior fossa and in 1 case cortical involvement in the supratentorium (eTable 4 in Supplement 1). One patient had low titer–positive myelin oligodendrocyte glycoprotein antibody (1:20) with involvement of the left temporal lobe on MRI that resolved on 9-month follow-up brain MRI. Of 23 patients with acute CNS infection/ADEM, outcomes were severe in 7 patients (30%) (Table 2 and eTable 1 in Supplement 1). Representative brain MRI studies from 2 patients with acute encephalitis and 1 with meningitis and cerebral venous sinus thrombosis complication are shown in the Figure.
Figure. Representative Central Nervous System Images From Patients With Life-threatening COVID-19–Related Neurologic Involvement.
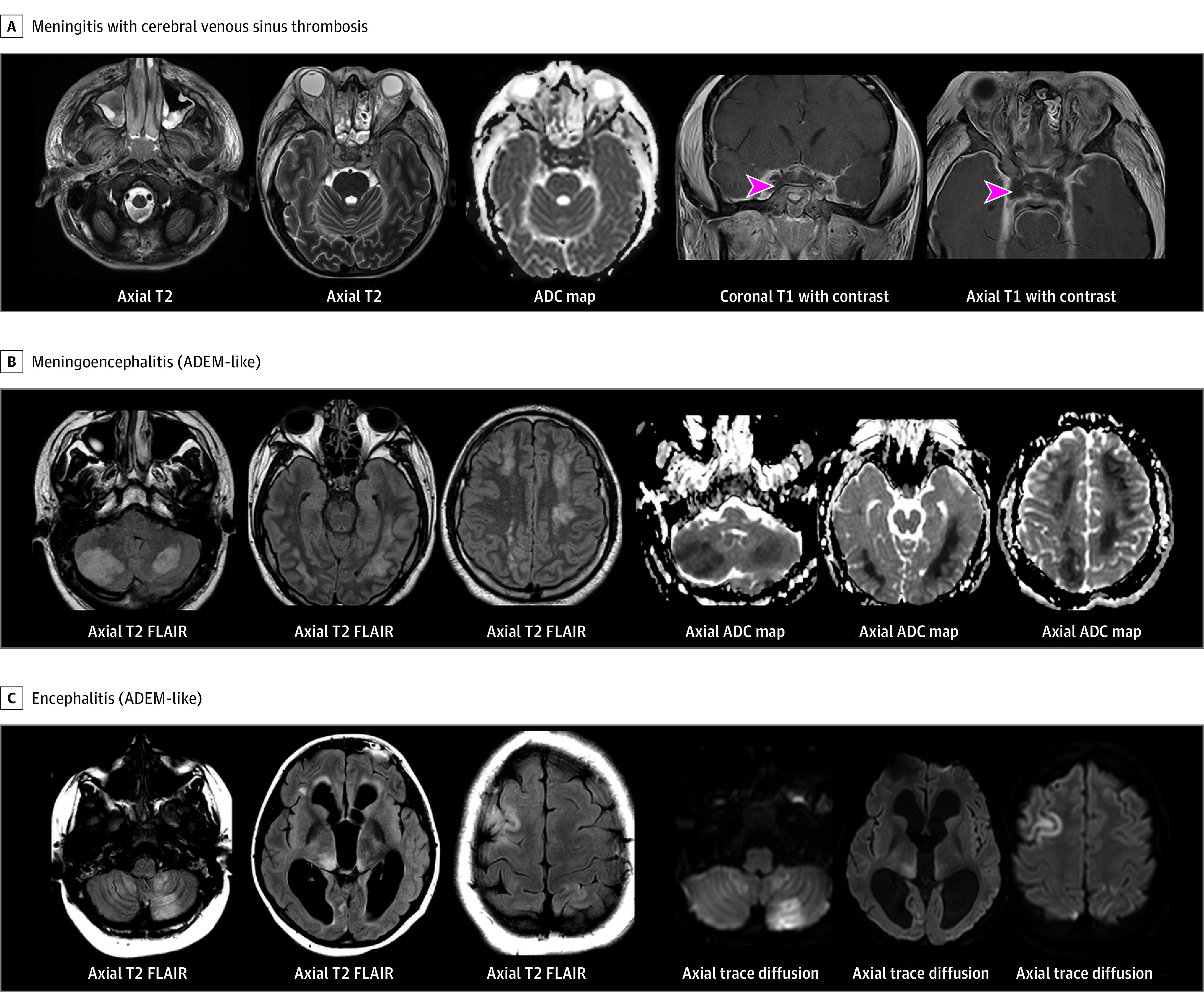
A, Teenager presented with acute respiratory failure, fever, acute onset of altered awareness, confusion, agitation, and difficulty walking. Axial T2 images show myositis of the facial muscles with stranding of subcutaneous fat, sinusitis, and cavernous sinus thrombosis with T2 hypointense signal and reduced diffusivity (apparent diffusion coefficient [ADC] map). Coronal and axial T1 images with contrast demonstrate dural enhancement, filling defects in superior ophthalmic veins and cavernous sinuses consistent with thrombosis (arrowheads). The patient’s brain injury evolved to diagnosis of death by neurologic criteria. B, Teenager with history of obesity, hypertension, and diabetes presented with 3 weeks of fever, confusion, headache, seizures, orofacial dyskinesias, agitation, slurred speech, difficulty walking, chorea, and left-sided weakness. Axial T2 fluid-attenuated inversion recovery (FLAIR) images demonstrate T2 hyperintense signal in bilateral cerebellar, bilateral temporal, and bilateral centrum semiovale white matter with reduced diffusivity. There was no enhancement, and susceptibility was present in lesions consistent with punctate blood products (not shown). The patient later died by brain death. C, Teenager presented with 3 weeks of fever, headaches, lethargy, confusion, seizure, vomiting, blurry vision, and nystagmus. Axial T2 FLAIR images demonstrate moderate enlargement of lateral and third ventricles, T2 prolongation in bilateral cerebellar hemispheres, bilateral thalami, and bifrontal white matter and cortex with reduced diffusivity on trace diffusion images and no enhancement (not shown). ADEM indicates acute disseminated encephalomyelitis.
Of the 155 vaccine-eligible patients with neurologic involvement and confirmed vaccination status, 147 (95%) were unvaccinated (Table 1 and eTable 5 in Supplement 1), including 15 of 16 patients (94%) with life-threatening neurologic conditions (Table 2).
Discussion
In 2168 US children and adolescents hospitalized with acute COVID-19 or MIS-C during 2021, the frequency, range, and severity of neurologic involvement were similar to the 2020 investigation.1 However, there were 2 major differences between the 2 surveillance periods. First, acute CNS infection/ADEM cases accounted for a higher proportion of life-threatening cases (55% in 2021 vs 19% in 2020). Many of these patients had subacute onset of encephalitis-like symptoms and ADEM-like imaging features and were discharged home, but 30% had severe outcomes. Because the adjudication methods for neurologic involvement were the same in both years, it is possible that the increased number of acute CNS infection/ADEM cases in 2021 were associated with the Delta variant8 or due to more diagnostic investigations in 2021 identifying more cases. Second, most patients with severe COVID-19 or MIS-C associated neurologic involvement who were vaccine eligible were unvaccinated.
Animal model data for SARS-CoV-2–induced encephalomyelitis support the possibility that it may contribute to the immunopathogenesis of encephalitis in humans.9 Brain biopsies in 9 adults with fatal COVID-19 revealed extensive inflammation of both white and gray matter without detectable SARS-CoV-2.10 Whether COVID-19 vaccination can prevent SARS-CoV-2–associated neurologic complications merits further study, weighing the immune-mediated vaccine-specific adverse neurologic events.11,12
Limitations
This investigation has multiple limitations. As a public health surveillance investigation, we were unable to study the immunobiology underlying severe complications, including the Delta variant, or assess reasons for low vaccine uptake. It is probable that we did not capture all patients. Fatigue, weakness, and headache are nonspecific symptoms that could lead to overreporting of milder neurologic involvement. Although data collection was standardized, potential misclassification of patients with neurologic involvement, including acute CNS infection/ADEM, may have occurred because of nonstandardized diagnostic investigations at each site, conducted using clinical discretion. ADEM was categorized based on the presence of encephalopathy and acute imaging features only and therefore is unconfirmed based on the 2013 International Pediatric Multiple Sclerosis Society Group criteria.13 Myelin oligodendrocyte glycoprotein has been reported in pediatric patients with COVID-19,14 but we cannot determine in the 1 patient if it represents a primary or autoimmune response to SARS-CoV-2 or is unrelated.
Conclusions
SARS-CoV-2–related neurologic involvement in US children and adolescents hospitalized for COVID-19 or MIS-C persisted in 2021, and acute CNS infection/ADEM accounted for more of the reported life-threatening cases than in 2020. COVID-19 vaccination became available for adolescents and children during 2021, but most vaccine-eligible patients were unvaccinated. COVID-19 vaccination is effective at preventing hospitalization for acute COVID-1915 and MIS-C16 and may decrease associated neurologic complications.
eAppendix. Overcoming COVID-19 Investigators and CDC COVID-19 Response Team
eMethods
eFigure 1. Eligibility flowchart of hospitalized patients with SARS-CoV-2-related neurologic involvement, December 15, 2020, – December 31, 2022
eFigure 2. Presenting neurologic symptoms and most abnormal laboratory values in patients (<21 years) hospitalized for COVID-19 or MIS-C
eTable 1. Case definition used in this investigation for multisystem inflammatory syndrome in children (MIS-C)
eTable 2. Number of patients with presenting neurologic symptoms (2A) and number of patients with most abnormal laboratory values by severity of neurologic involvement (2B)
eTable 3. Life-threatening SARS-CoV-2-related neurologic disorders and outcomes in 42 children and adolescents (<21 years) hospitalized for COVID-19 or MIS-C
eTable 4. Detailed clinical descriptions, treatment and outcomes for 14 patients (<21 years) with clinically adjudicated possible/confirmed acute encephalitis
eTable 5. Vaccination status by SARS-CoV-2-related neurologic involvement and MIS-C versus acute COVID-19 diagnosis
eReferences
Nonauthor collaborators
References
- 1.LaRovere KL, Riggs BJ, Poussaint TY, et al. ; Overcoming COVID-19 Investigators . Neurologic involvement in children and adolescents hospitalized in the United States for COVID-19 or multisystem inflammatory syndrome. JAMA Neurol. 2021;78(5):536-547. doi: 10.1001/jamaneurol.2021.0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wanga V, Gerdes ME, Shi DS, et al. Characteristics and clinical outcomes of children and adolescents aged <18 years hospitalized with COVID-19: six hospitals, United States, July-August 2021. MMWR Morb Mortal Wkly Rep. 2021;70(5152):1766-1772. doi: 10.15585/mmwr.mm705152a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallace M, Woodworth KR, Gargano JW, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine in adolescents aged 12-15 years: United States, May 2021. MMWR Morb Mortal Wkly Rep. 2021;70(20):749-752. doi: 10.15585/mmwr.mm7020e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woodworth KR, Moulia D, Collins JP, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine in children aged 5-11 years: United States, November 2021. MMWR Morb Mortal Wkly Rep. 2021;70(45):1579-1583. doi: 10.15585/mmwr.mm7045e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kempen JH. Appropriate use and reporting of uncontrolled case series in the medical literature. Am J Ophthalmol. 2011;151(1):7-10.e1. doi: 10.1016/j.ajo.2010.08.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Venkatesan A, Tunkel AR, Bloch KC, et al. ; International Encephalitis Consortium . Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the International Encephalitis Consortium. Clin Infect Dis. 2013;57(8):1114-1128. doi: 10.1093/cid/cit458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flanagan BE, Hallisey EJ, Adams E, Lavery A. Measuring community vulnerability to natural and anthropogenic hazards: the Centers for Disease Control and Prevention’s Social Vulnerability Index. J Environ Health. 2018;80(10):34-36. [PMC free article] [PubMed] [Google Scholar]
- 8.Twohig KA, Nyberg T, Zaidi A, et al. ; COVID-19 Genomics UK (COG-UK) consortium . Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: a cohort study. Lancet Infect Dis. 2022;22(1):35-42. doi: 10.1016/S1473-3099(21)00475-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray RS, Cai GY, Hoel K, Zhang JY, Soike KF, Cabirac GF. Coronavirus infects and causes demyelination in primate central nervous system. Virology. 1992;188(1):274-284. doi: 10.1016/0042-6822(92)90757-G [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schurink B, Roos E, Radonic T, et al. Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe. 2020;1(7):e290-e299. doi: 10.1016/S2666-5247(20)30144-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flannery P, Yang I, Keyvani M, Sakoulas G. Acute psychosis due to anti-N-methyl D-aspartate receptor encephalitis following COVID-19 vaccination: a case report. Front Neurol. 2021;12:764197. doi: 10.3389/fneur.2021.764197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zlotnik Y, Gadoth A, Abu-Salameh I, Horev A, Novoa R, Ifergane G. Case report: anti-LGI1 encephalitis following COVID-19 vaccination. Front Immunol. 2022;12:813487. doi: 10.3389/fimmu.2021.813487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krupp LB, Tardieu M, Amato MP, et al. ; International Pediatric Multiple Sclerosis Study Group . International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler. 2013;19(10):1261-1267. doi: 10.1177/1352458513484547 [DOI] [PubMed] [Google Scholar]
- 14.Ray STJ, Abdel-Mannan O, Sa M, et al. ; CoroNerve study group . Neurological manifestations of SARS-CoV-2 infection in hospitalised children and adolescents in the UK: a prospective national cohort study. Lancet Child Adolesc Health. 2021;5(9):631-641. doi: 10.1016/S2352-4642(21)00193-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olson SM, Newhams MM, Halasa NB, et al. ; Overcoming Covid-19 Investigators . Effectiveness of BNT162b2 vaccine against critical Covid-19 in adolescents. N Engl J Med. 2022;386(8):713-723. doi: 10.1056/NEJMoa2117995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zambrano LD, Newhams MM, Olson SM, et al. ; Overcoming COVID-19 Investigators . BNT162b2 mRNA vaccination against COVID-19 is associated with decreased likelihood of multisystem inflammatory syndrome in U.S. children ages 5-18 years. Clin Infect Dis. 2022;ciac637. doi: 10.1093/cid/ciac637 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Overcoming COVID-19 Investigators and CDC COVID-19 Response Team
eMethods
eFigure 1. Eligibility flowchart of hospitalized patients with SARS-CoV-2-related neurologic involvement, December 15, 2020, – December 31, 2022
eFigure 2. Presenting neurologic symptoms and most abnormal laboratory values in patients (<21 years) hospitalized for COVID-19 or MIS-C
eTable 1. Case definition used in this investigation for multisystem inflammatory syndrome in children (MIS-C)
eTable 2. Number of patients with presenting neurologic symptoms (2A) and number of patients with most abnormal laboratory values by severity of neurologic involvement (2B)
eTable 3. Life-threatening SARS-CoV-2-related neurologic disorders and outcomes in 42 children and adolescents (<21 years) hospitalized for COVID-19 or MIS-C
eTable 4. Detailed clinical descriptions, treatment and outcomes for 14 patients (<21 years) with clinically adjudicated possible/confirmed acute encephalitis
eTable 5. Vaccination status by SARS-CoV-2-related neurologic involvement and MIS-C versus acute COVID-19 diagnosis
eReferences
Nonauthor collaborators


