Highlights
-
•
Sensitive serum thyroglobulin measurement by LC-MS/MS.
-
•
Detailed standard operating procedure using a commercially available antibody.
-
•
Dimethylsulfoxide-enhanced ionization of a proteotypic thyroglobulin peptide.
-
•
Beads for peptide immunoaffinity purification can be reused at least 5 times.
-
•
Distributable standard reference material (Husky Ref) to facilitate assay harmonization.
Keywords: Thyroglobulin, Differentiated thyroid cancer, Autoantibody, Mass spectrometry
Abbreviations: CPTAC, Clinical Proteomic Tumor Analysis Consortium; DMSO, dimethylsulfoxide; DTC, differentiated thyroid cancer; IAE, immunoaffinity enrichment; LC-MS/MS, liquid chromatography-tandem mass spectrometry; Tg, thyroglobulin; TLCK, tosyllysine chloromethyl ketone hydrochloride
Abstract
Background
Despite its clear advantages over immunoassay-based testing, the measurement of serum thyroglobulin by mass spectrometry remains limited to a handful of institutions. Slow adoption by clinical laboratories could reflect limited accessibility to existing methods that have sensitivity comparable to modern immunoassays, as well as a lack of tools for calibration and assay harmonization.
Methods
We developed and validated a liquid chromatography-tandem mass spectrometry-based assay for the quantification of serum thyroglobulin. The protocol combined peptide immunoaffinity purification using a commercially available, well-characterized monoclonal antibody and mobile phase modification with dimethylsulfoxide (DMSO) for enhanced sensitivity. To facilitate harmonization with other laboratories, we developed a novel, serum-based 5-point distributable reference material (Husky Ref).
Results
The assay demonstrated a lower limit of quantification of 0.15 ng/mL (<20 %CV). Mobile phase DMSO increased signal intensity of the target peptide at least 3-fold, improving quantification at low concentrations. Calibration traceable to Husky Ref enabled harmonization between laboratories in an interlaboratory study.
Conclusions
Sensitive mass spectrometry-based thyroglobulin measurement can be achieved using a monoclonal antibody during peptide immunoaffinity purification and the addition of mobile phase DMSO. Laboratories interested in deploying this assay can utilize the provided standard operating procedure and freely-available Husky Ref reference material.
Introduction
Thyroglobulin (Tg), the substrate of thyroid hormone production, is a tumor marker widely used in the surveillance of differentiated thyroid cancer (DTC) [1]. The measurement of Tg poses challenges related to the size and heterogeneity of the analyte, interferents, and other factors [2], [3]. Immunoassays, in particular, show poor comparability among manufacturers and false negative results in the presence of thyroglobulin autoantibodies (TgAb) [4], [5]. Liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based assays address these shortcomings, demonstrating better harmonization across laboratories while using protein digestion to eliminate interference from antibodies [6], [7]. However, as with the application of LC-MS/MS to protein quantification more generally, Tg testing by LC-MS/MS is still limited to only a handful of institutions [8]. To facilitate broader use, there is a need for more accessibility to existing protocols, including for those LC-MS/MS methods with sensitivity equivalent to modern immunoassays. In addition, long-term solutions are needed to assist in calibration and assay harmonization [3], [8], [9], [10].
Here we describe a distributable LC-MS/MS method for the measurement of serum Tg, with an accompanying standard operating procedure (SOP) to expedite adoption by other laboratories. The method achieves higher sensitivity compared to a previously reported method [11] by replacing polyclonal antibodies used in peptide immunoaffinity enrichment (IAE) with a well-characterized monoclonal antibody developed through the National Cancer Institute-sponsored Clinical Proteomic Tumor Analysis Consortium (CPTAC [12]). We also demonstrate the impact of small changes to the mobile phase (i.e., addition of dimethylsulfoxide) in improving ion intensity of the target surrogate peptide. Given the potential cost associated with IAE (e.g., beads, antibodies), the procedure includes steps for bead regeneration, enabling reuse of beads up to 5 times. Finally, alongside the assay, we present a freely available, 5-point reference material (Husky Ref) to facilitate harmonization between laboratories.
Materials and methods
A complete list of equipment, supplies, and chemicals are provided in the SOP located in the Supplemental Material. The SOP also defines calibration and control materials, sample preparation and bead regeneration procedures, and LC-MS/MS parameters.
In brief, sample preparation consisted of denaturation and reduction of samples (400 µL serum) with deoxycholate/tris(2-carboxyethyl)phosphene, alkylation with iodoacetamide, addition of stable-isotope-labeled (SIL) internal standard (IS) peptide, trypsin digestion (30 min), addition of tosyllysine chloromethyl ketone (TLCK) hydrochloride to stop digestion, and peptide IAE. The latter was performed using tosyl activated paramagnetic beads conjugated (via covalent binding) to a commercially-available monoclonal antibody (SISCAPA Assay Technologies, Tg-FSP-31) against the tryptic-peptide sequence FSPDDSAGASALLR (FSP). Eluates were analyzed on an Acquity UPLC system equipped with an HSS T3 VanGuard Pre-column (1.8 µm, 2.1 mm × 5 mm) and HSS T3 C18 analytical column (1.8 µm, 50 × 2.1 mm, both from Waters) and coupled to a Waters Xevo TQS tandem mass spectrometer. Mobile phases (water or methanol with 0.1 % formic acid) included 2 % DMSO for enhanced ionization of the target peptide. The MS instrument was run using multiple reaction monitoring in positive ion mode targeting the doubly charged peptide precursor ion of the FSP peptide and the doubly charged y12 fragment ion. Three synonymous transitions for the y12 fragment were monitored to improve the precision of the assay [13]. Data were analyzed using Waters TargetLynx software. Peak areas of the unlabeled peptide were normalized to the IS peak areas to obtain peak area ratios (PAR). The PAR for the calibration materials were plotted against their concentration set points using linear regression and 1/x weighting [14]. Chicken serum was used as the diluent in preparation of calibrators, controls, as well as samples for assay validation. Its suitability as a substitute matrix (previously reported in [15]) was verified by comparing LC-MS/MS Tg results for a pool of human sera (positive for both Tg and Tg Ab) diluted in two sets, one using chicken serum and the other using Tg-negative, antibody-negative pooled human sera. A bead regeneration procedure (also detailed in the SOP) -- involving multiple phosphate buffered saline (PBS)-based wash steps and treatment of beads with additional (acetic acid-based) elution buffer -- was established and utilized throughout validation studies. Validation studies (detailed in the Supplementary Material) included evaluations of assay linearity, lower limit of quantification, imprecision, interferences, carryover, and agreement with a commercial immunoassay platform. A comparison between assay formats involving sample preparation in microcentrifuge tubes and a 96-well plate was also performed.
A multi-point reference material was developed comprising two sets of sera with approximate concentrations of 0.2, 1, 5, 9, and 12 ng/mL Tg. The material was prepared in the Department of Laboratory Medicine at the University of Washington. One set (standards 1–5) was prepared by serial dilution of Golden West MSG4000 human serum pool (lot#C10003) with chicken serum (Equitech-Bio, Inc., cat # SC30, lot# 190509–0130). A second set (standards A-E) was prepared similarly, with rabbit serum (Gibco, cat# 16120, lot# 2046820) as the diluent. Chicken and rabbit sera lacked detectable signal for FSP. Set points were established through analysis of the material by four clinical laboratories employing LC-MS/MS Tg testing (targeted the FSP peptide), over three days, in duplicate. Additionally, an interlaboratory study was performed to compare evaluation of 12 human-derived samples across the same four laboratories. Two of four institutions used calibrators traceable to the prepared reference material. All laboratories involved in characterization of Husky Ref and interlaboratory study were CLIA-certified, CAP-accredited, or equivalent.
The analysis of de-identified clinical samples for aggregation and publication was approved by the Human Subjects Division at the University of Washington (STUDY00013082). Longitudinal assessment of patients undergoing Tg measurement by LC-MS/MS was approved as a separate study (STUDY00002450) and consent was waived by the IRB for all subjects.
Results
Assay development
Sensitive measurement of serum Tg by LC-MS/MS was implemented using a monoclonal antibody for IAE of the FSP peptide. Addition of small quantities of DMSO to the mobile phase increased ion intensity of the enriched peptide target, as shown for the FSP-IS in Fig. 1. Specifically, an increase from 0 to 0.5 % DMSO resulted in approximately 4-fold increase in peak area when analyzing internal standard peptide (∼4 fmol) contained in a matrix similar to that of a processed serum digest (complete data in Supplemental Table 1 and representative chromatograms in Supplemental Fig. 1). Increases in ion intensity were more modest with further increases in DMSO, with little apparent gain beyond 2 %. In a second experiment, increased ion intensity was replicated by analyzing low and high control sera subjected to LC-MS/MS with post-column DMSO-infusion (mimicking 2 % DMSO in the mobile phase). The peak areas increased at least threefold in the presence of DMSO (Table 1). In a third experiment, examination of full-scan spectra of peptide precursor ions (i.e., no fragmentation) when injecting 5 pmol of synthetic FSP peptide indicated that the gain in signal intensity reflected an absolute increase in ion intensity rather than an effect from charge state consolidation ( Supplemental Table 2 and Supplemental Fig. 2).
Fig. 1.
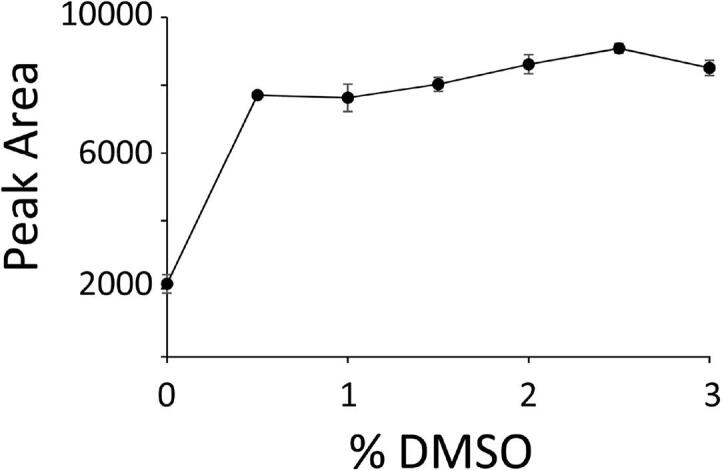
Impact of DMSO on peak area of the FSP-IS peptide. An increase in peak area for the FSP stable-isotope labeled internal standard peptide (injection of 4 femtomoles peptide) was observed with inclusion of DMSO (ranging from 0.5 to 3% v/v) in the mobile phase. The results shown reflect the average peak heights for duplicate injections. The system was fully purged between measurements at the different DMSO mobile phase concentrations.
Table 1.
Impact of DMSO on peak areas for processed quality control samples.
| Controla | Peptide | Peak areab | Fold Increase | |
|---|---|---|---|---|
| No DMSO | 2 % DMSO | |||
| Low | Unlabeled | 416.3 | 3151.3 | 7.6 |
| Labeled IS | 1389.7 | 4334.7 | 3.1 | |
| High | Unlabeled | 1572.3 | 6068.0 | 3.9 |
| Labeled IS | 1224.7 | 4470.7 | 3.7 | |
Digested QC material (estimated thyroglobulin concentrations 0.6 ng/mL and 7.2 ng/mL) were chromatographically resolved and analyzed with either DMSO infused post-column to mimic 2 % DMSO in the mobile phase or water infused post-column.
For unlabeled peptide, peak area reflects the sum of transitions 704.0404 > 586.90, 704.0404 > 586.91, 704.0404 > 586.92; for labeled IS, transitions 707.0300 > 589.89, 707.0300 > 589.90, 707.0300 > 589.91.
Chicken serum was verified as a suitable alternative to human serum as a diluent for the preparation of calibrators, control samples, and assay validation samples based on the data shown in Supplemental Table 3 and Supplemental Fig. 3. Specifically, LC-MS/MS analyses of pooled sera (positive for both Tg and TgAb) produced similar results following dilution of the pool with either chicken serum or Tg-negative human serum (all results within +/- 10 %). The feasibility of re-using antibody-conjugated paramagnetic beads for IAE was explored by examining serial IS recovery from aliquots of a single pooled serum digest. All peak area results from six uses in a 10-day period were within +/- 20 % of the average result (5 of 6 were within 11 %, Supplemental Fig. 4). Based on these results, beads were reused up to five times during the assay validation studies, with each run including simultaneous processing of a chicken serum negative control to demonstrate the absence of integrable signal for unlabeled FSP peptide in samples negative for endogenous Tg.
Assay validation
The assay was linear over the tested range (sample concentrations 0.23 to 12.26 ng/mL, R2 = 0.997, Fig. 2, Supplemental Table 4). The lower limit of quantification (LLOQ) was 0.15 ng/mL based on estimated imprecision < 20 %CV (Supplemental Table 5, Supplemental Fig. 5). Longitudinal measurements of a matrix-matched ultralow control (preparation described in the SOP) resulted in a mean of 0.14 ng/mL and imprecision of 14 %CV (Supplemental Table 6). In a 5x5 design imprecision experiment, intra-day imprecision was 8.6, 3.5, and 3.6 %CV at 0.61 ng/mL, 7.61, and 12.09, respectively (Supplemental Table 7). Inter-day imprecision was 9.3, 3.4, and 6.4 %CV, respectively. Assay recovery was within 100–114 % of that expected for all samples evaluated in the mixing study to assess interference from triglycerides, total protein, or hemoglobin (Supplemental Table 8). Carryover of 0.14 % was observed at Tg concentration of 3,204 ng/mL (Supplemental Table 9). When compared with a commercially available immunoassay (Supplemental Tables 10 and 11, graphed in Supplemental Fig. 6), a consistent bias was not observed. However, correlation was poor in the presence of TgAb. Comparisons between tube and plate formats appear in Supplemental Table 12 and Plate imprecision data are provided in Supplemental Table 13.
Fig. 2.
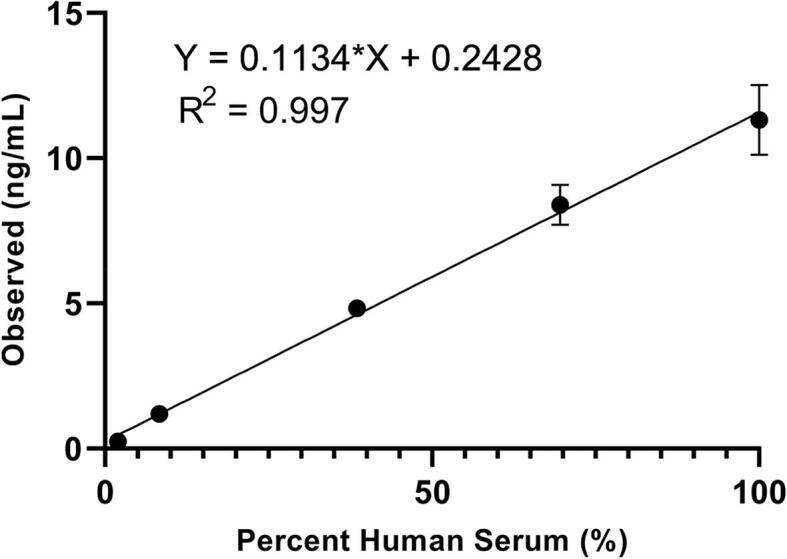
Linearity. Samples were prepared at 5 levels via dilution of pooled human serum (Golden West MSG4000, anti-Tg antibody concentration of 14.60 IU/mL as measured using Beckman Coulter Unicel DxI 800 assay) in Tg-null chicken serum. These 5 sample levels subsequently became the Husky Ref levels 1–5. Percent human serum is based on volumetric dilution of human serum with chicken serum. Set points (0.23, 1.15, 5.14, 8.83, and 12.26 ng/mL) were subsequently established for these five samples via analysis by four different laboratories, over three days, run in duplicate each day.
Reference material evaluation and interlaboratory study
Four laboratories analyzed the reference material produced in our laboratory (Husky Ref, Supplemental Table 14, Supplemental Fig. 8). Each laboratory analyzed each sample in duplicate on each of three days (six measurements per level). Mean concentrations were 0.23, 1.15, 5.14, 8.83, and 12.26 ng/mL for set 1–5 (dilution in chicken serum) and 0.20, 0.97, 5.05, 8.81, and 12.24 for set A-E (dilution in rabbit serum).
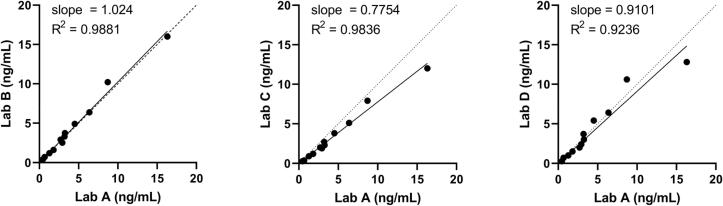
To continually evaluate the performance of our Tg LC-MS/MS assay compared to other laboratories, we established an alternative assessment program, because no appropriate matrix-matched proficiency testing materials are currently available for Tg. Different sets of three de-identified clinical samples (a subset positive for anti-thyroglobulin autoantibodies) were exchanged with three other Clinical Laboratory Improvement Amendments (CLIA)-certified (or equivalent) laboratories three times per year. This interlaboratory study demonstrated good agreement between the laboratories (Fig. 3, Supplemental Table 15). Two laboratories have calibration systems traceable to Husky Ref and had well-harmonized results (Laboratories A and B).
Fig. 3.
Interlaboratory Study (Alternative Assessment). Different sets of three de-identified clinical samples were exchanged with three other Clinical Laboratory Improvement Amendments (CLIA)-certified (or the equivalent) laboratories employing LC-MS/MS Tg measurement. Four sets are shown, comprising 12 samples total. Both Laboratory A (using the assay described here) and Laboratory B utilized calibrators with set points traceable to Husky Ref.
Longitudinal patient measurements
We continued a previously described chart review of patients being monitored for disease recurrence after total thyroidectomy [15]. Disease status was determined based on clinical presentation, physical examination, and biochemical, and imaging results. Disease status was classified as disease-free (e.g., undetectable Tg and no nodules on imaging), stable (stable Tg and no growth of nodules on imaging), or recurrence (up-trending Tg and growth of nodules on imaging). We have followed 34 patients for an average of 3.56 years, all with < 0.15 ng/mL at their original blood draw. All are still clinically disease free, while three have had detectable Tg during their follow up.
Discussion
Progress toward accessible, sensitive LC-MS/MS-based Tg measurement
The ability to measure small quantities of serum Tg by “second-generation” immunoassays (functional sensitivity = 0.1 ng/mL) has helped facilitate the transition from standardized therapies for DTC to more personalized care based on risk [16]. However, up to a third of patients with DTC are positive for TgAb, potentially excluding them from evaluation for recurrent disease using the Tg biochemical marker. LC-MS/MS-based quantification allows for continued Tg monitoring in TgAb-positive patients, but utility of the measurement is contingent on attaining comparable sensitivity to immunoassay [5], [16], [17]. Low abundance serum proteins can be difficult to measure by LC-MS/MS due to ion suppression when operating under the flow conditions typical of clinical laboratories (200 to 1000 µL/min). Previously described LC-MS/MS Tg assays reported limits of detection and quantification higher than 0.1 ng/mL (Table 2), primarily utilizing polyclonal antibodies during IAE for enrichment a different target peptide sequence, VIFDANAPVAVR (VIF) [11], [18], [19]. Subsequently, the NCI-sponsored Clinical Proteomic Tumor Analysis Consortium (CPTAC) identified the FSP peptide as a possible target for quantitative Tg measurements by LC-MS/MS and developed an antibody for this purpose [20]. Preference has since shifted toward the FSP peptide, as evident in a more recent method comparison study reported by Netzel et al. in which three of four methods targeted FSP [6]. The peptide can be quickly and reproducibly liberated from Tg using trypsin in a half hour, thus reducing the overall time burden. Furthermore, a monoclonal antibody may be more preferable to polyclonal antibodies over the longer-term, since polyclonal antibodies are often subject to increased batch-to-batch variability. Finally, as shown here, harnessing the monoclonal anti-FSP antibody enables assay sensitivity similar to immunoassay. Recently, Shuford et al. [15] (also utilizing the FSP surrogate peptide) described use of micro-flow chromatography to improve LC-MS/MS sensitivity beyond current immunoassay limits, reporting a lower limit of quantitation (LLOQ) of 0.02 ng/mL. However, this approach requires more specialized chromatography instrumentation and expertise, and thus may not be a workable option for many users.
Table 2.
Assays for Tg Measurement by LC-MS/MS Peptide Immunoaffinity Purification.
| Ref. | Year | Antibody Peptidea | Internal Standardb | Reported Sensitivity | Additional Method Aspectsc |
|---|---|---|---|---|---|
| [11] | 2008 | Polyclonal (rabbit) anti-VIF | tSIL Peptide | LOD: 2.6 ng/mL | |
| [18] | 2012 | Polyclonal (chicken) anti-VIF | tSIL Peptide | LOD: 0.3 ng/mL, LLOQ: 0.4 ng/mL | |
| [19] | 2013 | Polyclonal anti-VIF | cSIL Peptide | LLOQ: 0.5 ng/mL | Protein Precipitation |
| [15] | 2020 | Monoclonal anti-FSP d | cSIL Peptide | LOD: 0.0057 ng/mL LLOQ: 0.02 ng/mL |
Micro-flow chromatography |
| Current method | Monoclonal anti-FSP d | tSIL Peptide | LLOQ: 0.15 ng/mL | Mobile Phase DMSO | |
Tg tryptic peptides: VIF, VIFDANAPVAVR; FSP, FSPDDSAGASALLR.
Reported assays all employ multi-point external calibration, but may differ by internal standard approach, utilizing either tryptic stabile isotope labeled (tSIL) or cleavable/“winged” stable isotope labeled (cSIL) peptide.
All methods use protein digestion and peptide IAE. The method in Ref. 19 also uses an initial protein precipitation step.
Clinical Proteomic Tumor Analysis Consortium (CPTAC) antibody against FSPDDSAGASALLR sequence peptide.
For the presently described method, the addition of DMSO directly increased ionization of the FSP peptide. Use of DMSO as a mobile phase additive in LC-MS assays to enhance protein and peptide detection and improve sensitivity has been demonstrated by multiple laboratories [21], [22], [23], [24]. Here, for the low QC in particular, the increase in peak area for the endogenous peptide was more than twice that for the labeled internal standard (see Table 1), highlighting that a gain in signal intensity does impact actual quantification results at lower concentrations. This finding is consistent with other reports showing that identification of low-abundance peptides benefit from DMSO-enhancement more than high-abundance peptides [22], [23], [24]. A variety of mechanisms for DMSO enhancement of signal intensity have been suggested, including charge state coalescence, improved ionization efficiency, background-ion suppression, and/or improved LC resolution. Our results suggest that DMSO is enhancing Tg FSP peptide signal intensity through improved ionization efficiency and not through improvements in chromatographic resolution (Supplemental Fig. 1) or charge state coalescence (Supplemental Fig. 2).
For clinical laboratories already employing LC-MS/MS, the slow adoption of these platforms for protein quantification could reflect the need to address method aspects not typically applicable to small-molecule targets (e.g., protein digestion, analyte enrichment, and selection of proteotypic signature peptides). Provision of an SOP when reporting these methods in the literature may thus be particularly important in facilitating use for other users. Considerations to reduce cost may also improve accessibility. For this purpose, we specifically explored reuse of antibody-conjugated tosyl-activated paramagnetic beads. As described, similar recovery of FSP IS was maintained over 5 rounds of Ab-bead reuse. Reuse of the beads was then carried throughout method validation. Care must be taken through the bead washing and regeneration process to avoid bead loss; to mitigate this, bead reuse is limited to five cycles in the current protocol. Appropriate controls are required to ensure beads have been adequately regenerated following each use. Here, a chicken serum negative control was utilized to ensure previous Tg was cleared from the beads. Passing criteria consisted of absent signal for unlabeled Tg when the negative control was processed alongside human-derived samples. Meanwhile, use of the ultralow control (approximate [Tg] = 0.15 ng/mL) ensures sufficient beads have been recovered to allow detection of small Tg quantities. Of note, Shuford et al. also utilized chicken serum as a blank surrogate matrix. Although potential interference for the FSP peptide was described, this was only apparent at Tg concentrations<0.02 ng/mL and is not relevant to our assay (with LLOQ of 0.15 ng/mL).
Community-driven solutions for calibration and harmonization
Calibration is a major potential hurdle when deploying an LC-MS/MS-based assay for protein quantification. As with most protein targets, Tg is, in reality, a family of analytes, comprising various post-translationally modified states with different conformations. Furthermore, modifications, such as glycosylation, and phosphorylation, are often altered in malignancy, which can impact epitope recognition by reagent antibodies or enzymatic digestion [3]. Notably, immunoassays have experienced a continual failure to harmonize despite widespread traceability to an international standard reference material derived from human tissue, BCR 457 [9], [10]. While the use of proprietary antibodies and other reagents may permanently exclude the possibility of harmonization for immunoassays, LC-MS/MS methods have already shown promising results [6]. BCR 457 has notably been depleted, calling for the production of new reference material. The material described here (freely available from the authors) is also a community resource developed via NIH-funding and represents a path to improve Tg method harmonization moving forward. The material is serum-based from a commercial product derived from a large number of donors. We are able to provide the material to laboratories, as they develop or characterize their own calibrators.
Potential method limitations
The method has several potential limitations that should be considered. First, use of DMSO in the mobile phase may not appeal to all chromatography users due to concerns related to part degradation (e.g., swelling of PEEK tubing). We have not observed these effects in the five to six years we have used the described protocol, but other systems could, at least in theory, be affected. Second, the described assay does not employ a qualifier transition for the FSP peptide, due the lack of a second transition of sufficient intensity for monitoring. Measuring multiple transitions offers the possibility to check for contaminants or interferences but is difficult to implement for the FSP peptide at current sensitivity limitations. Shuford et al., on the other hand, identified additional useful transitions, benefitting from the additional sensitivity gained via micro-flow technology. Of note, the present method does employ so-called “synonymous” transitions, as described above. Importantly, the reason we use this technique is based on our own observations of improved ion count precision. The theoretical foundation for the benefit is unclear, and this approach may not be available or advantageous on all mass spectrometers.
Conclusions
LC-MS/MS-based Tg measurement enables continued testing in patients positive for TgAb, but is still limited to a few laboratories. Accessible examples with transparent protocols are needed, particularly for those assays meeting or exceeding the sensitivity of the modern Tg immunoassay. Hopefully, the described method, along with its standard operating procedure and associated reference material, will address the needs of laboratories interested in transitioning to mass spectrometry-based thyroglobulin measurement.
CRediT authorship contribution statement
Junyan Shi: Investigation, Formal analysis, Writing – original draft. William S. Phipps: Formal analysis, Writing – original draft, Visualization, Writing – review & editing. Benjamin Y. Owusu: Writing – original draft. Clark M. Henderson: Conceptualization, Investigation, Visualization. Thomas J. Laha: Investigation, Writing – review & editing. Jessica O. Becker: Investigation, Writing – original draft, Writing – review & editing. Morteza Razavi: Methodology, Writing – review & editing. Michelle A. Emrick: Project administration, Investigation, Writing – review & editing. Andrew N. Hoofnagle: Conceptualization, Methodology, Supervision, Funding acquisition, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was partially supported by NIH grant U24CA160034 as well as the National Cancer Institute’s Office of Cancer Clinical Proteomics Research, Clinical Proteomic Tumor Analysis Consortium (CPTAC) Antibody Characterization Program. The authors acknowledge early method development completed by the employees of SISCAPA Assay Technologies. The authors thank Dr. Chris Shuford from LabCorp, Laura Regner and Dr. Ravinder Singh from Mayo Clinic, and Grace van der Gugten and Dr. Dan Holmes from St. Paul’s Hospital for coordinating specimen exchange and testing in the alternative assessment of the Tg LC-MS/MS assays.
Footnotes
Peer review under responsibility of “MSACL”.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jmsacl.2022.09.005.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Momesso D.P., Vaisman F., Yang S.P., Bulzico D.A., Corbo R., Vaisman M., Tuttle R.M. Dynamic Risk Stratification in Patients with Differentiated Thyroid Cancer Treated Without Radioactive Iodine. J. Clin. Endocrinol. Metab. 2016;101:2692–2700. doi: 10.1210/jc.2015-4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giovanella L., Feldt-Rasmussen U., Verburg F.A., Grebe S.K., Plebani M., Clark P.M. Thyroglobulin measurement by highly sensitive assays: focus on laboratory challenges. Clin. Chem. Lab. Med. 2015;53:1301–1314. doi: 10.1515/cclm-2014-0813. [DOI] [PubMed] [Google Scholar]
- 3.Shuford C.M., Walters J.J., Holland P.M., Sreenivasan U., Askari N., Ray K., Grant R.P. Absolute Protein Quantification by Mass Spectrometry: Not as Simple as Advertised. Anal. Chem. 2017;89:7406–7415. doi: 10.1021/acs.analchem.7b00858. [DOI] [PubMed] [Google Scholar]
- 4.Spencer C., Petrovic I., Fatemi S. Current thyroglobulin autoantibody (TgAb) assays often fail to detect interfering TgAb that can result in the reporting of falsely low/undetectable serum Tg IMA values for patients with differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 2011;96:1283–1291. doi: 10.1210/jc.2010-2762. [DOI] [PubMed] [Google Scholar]
- 5.Netzel B.C., Grebe S.K.G., Carranza Leon B.G., Castro M.R., Clark P.M., Hoofnagle A.N., Spencer C.A., Turcu A.F., Algeciras-Schimnich A. Thyroglobulin (Tg) Testing Revisited: Tg Assays, TgAb Assays, and Correlation of Results With Clinical Outcomes. J. Clin. Endocrinol. Metab. 2015;100:E1074–E1083. doi: 10.1210/jc.2015-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Netzel B.C., Grant R.P., Hoofnagle A.N., Rockwood A.L., Shuford C.M., Grebe S.K.G. First Steps toward Harmonization of LC-MS/MS Thyroglobulin Assays. Clin. Chem. 2016;62:297–299. doi: 10.1373/clinchem.2015.245266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoofnagle A.N., Roth M.Y. Clinical review: improving the measurement of serum thyroglobulin with mass spectrometry. J. Clin. Endocrinol. Metab. 2013;98:1343–1352. doi: 10.1210/jc.2012-4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seger C., Salzmann L. After another decade: LC–MS/MS became routine in clinical diagnostics. Clin. Biochem. 2020;82:2–11. doi: 10.1016/j.clinbiochem.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Feldt-Rasmussen U., Profilis C., Colinet E., Black E., Bornet H., Bourdoux P., Carayon P., Ericsson U.B., Koutras D.A., Lamas de Leon L., DeNayer P., Pacini F., Palumbo G., Santos A., Schlumberger M., Seidel C., Van Herle A.J., De Vijlder J.J. Human thyroglobulin reference material (CRM 457). 1st Part: Assessment of homogeneity, stability and immunoreactivity. Ann. Biol. Clin. (Paris). 1996;54:337–342. http://www.ncbi.nlm.nih.gov/pubmed/9092300 [PubMed] [Google Scholar]
- 10.Feldt-Rasmussen U., Profilis C., Colinet E., Black E., Bornet H., Bourdoux P., Carayon P., Ericsson U.B., Koutras D.A., Lamas de Leon L., DeNayer P., Pacini F., Palumbo G., Santos A., Schlumberger M., Seidel C., Van Herle A.J., DeVijlder J.J. Human thyroglobulin reference material (CRM 457). 2nd Part: Physicochemical characterization and certification. Ann. Biol. Clin. (Paris). 1996;54:343–348. http://www.ncbi.nlm.nih.gov/pubmed/9092301 [PubMed] [Google Scholar]
- 11.Hoofnagle A.N., Becker J.O., Wener M.H., Heinecke J.W. Quantification of thyroglobulin, a low-abundance serum protein, by immunoaffinity peptide enrichment and tandem mass spectrometry. Clin. Chem. 2008;54:1796–1804. doi: 10.1373/clinchem.2008.109652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez H., Pennington S.R. Revolutionizing Precision Oncology through Collaborative Proteogenomics and Data Sharing. Cell. 2018;173:535–539. doi: 10.1016/j.cell.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Best C.M., Riley D.V., Laha T.J., Pflaum H., Zelnick L.R., Hsu S., Thummel K.E., Foster-Schubert K.E., Kuzma J.N., Cromer G., Larson I., Hagman D.K., Heshelman K., Kratz M., de Boer I.H., Hoofnagle A.N. Vitamin D in human serum and adipose tissue after supplementation. Am. J. Clin. Nutr. 2020 doi: 10.1093/ajcn/nqaa295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gu H., Liu G., Wang J., Aubry A.-F., Arnold M.E. Selecting the correct weighting factors for linear and quadratic calibration curves with least-squares regression algorithm in bioanalytical LC-MS/MS assays and impacts of using incorrect weighting factors on curve stability, data quality, and assay perfo. Anal. Chem. 2014;86:8959–8966. doi: 10.1021/ac5018265. [DOI] [PubMed] [Google Scholar]
- 15.Shuford C.M., Johnson J.S., Thompson J.W., Holland P.L., Hoofnagle A.N., Grant R.P. More sensitivity is always better: Measuring sub-clinical levels of serum thyroglobulin on a µLC-MS/MS system. Clin. Mass Spectrom. (Del Mar, Calif.). 2020;15:29–35. doi: 10.1016/j.clinms.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spencer C., LoPresti J., Fatemi S. How sensitive (second-generation) thyroglobulin measurement is changing paradigms for monitoring patients with differentiated thyroid cancer, in the absence or presence of thyroglobulin autoantibodies. Curr. Opin. Endocrinol. Diabetes. Obes. 2014;21:394–404. doi: 10.1097/MED.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azmat U., Porter K., Senter L., Ringel M.D., Nabhan F. Thyroglobulin Liquid Chromatography-Tandem Mass Spectrometry Has a Low Sensitivity for Detecting Structural Disease in Patients with Antithyroglobulin Antibodies. Thyroid. 2017;27:74–80. doi: 10.1089/thy.2016.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke N.J., Zhang Y., Reitz R.E. A novel mass spectrometry-based assay for the accurate measurement of thyroglobulin from patient samples containing antithyroglobulin autoantibodies. J. Investig. Med. 2012;60:1157–1163. doi: 10.2310/JIM.0b013e318276deb4. [DOI] [PubMed] [Google Scholar]
- 19.Kushnir M.M., Rockwood A.L., Roberts W.L., Abraham D., Hoofnagle A.N., Meikle A.W. Measurement of thyroglobulin by liquid chromatography-tandem mass spectrometry in serum and plasma in the presence of antithyroglobulin autoantibodies. Clin. Chem. 2013;59:982–990. doi: 10.1373/clinchem.2012.195594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Razavi M., Pope M.E., Soste M.V., Eyford B.A., Jackson A.M., Anderson N.L., Pearson T.W. MALDI Immunoscreening (MiSCREEN): A method for selection of anti-peptide monoclonal antibodies for use in immunoproteomics. J. Immunol. Methods. 2011;364:50–64. doi: 10.1016/j.jim.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valeja S.G., Tipton J.D., Emmett M.R., Marshall A.G. New reagents for enhanced liquid chromatographic separation and charging of intact protein ions for electrospray ionization mass spectrometry. Anal. Chem. 2010;82:7515–7519. doi: 10.1021/ac1016858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hahne H., Pachl F., Ruprecht B., Maier S.K., Klaeger S., Helm D., Médard G., Wilm M., Lemeer S., Kuster B. DMSO enhances electrospray response, boosting sensitivity of proteomic experiments. Nat. Methods. 2013;10:989–991. doi: 10.1038/nmeth.2610. [DOI] [PubMed] [Google Scholar]
- 23.Strzelecka D., Holman S.W., Eyers C.E. Evaluation of dimethyl sulfoxide (DMSO) as a mobile phase additive during top 3 label-free quantitative proteomics. Int. J. Mass Spectrom. 2015;391:157–160. doi: 10.1016/j.ijms.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Judák P., Grainger J., Goebel C., Van Eenoo P., Deventer K. DMSO Assisted Electrospray Ionization for the Detection of Small Peptide Hormones in Urine by Dilute-and-Shoot-Liquid-Chromatography-High Resolution Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2017;28:1657–1665. doi: 10.1007/s13361-017-1670-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.