Abstract
Pneumolysin is a 471-amino-acid toxin produced by Streptococcus pneumoniae which has both cytolytic and complement activation properties. We have constructed a derivative of the type 2 S. pneumoniae strain D39 in which the portion of the pneumolysin gene encoding amino acids 55 to 437 has been deleted in-frame. The virulence of this strain (ΔPly) was compared with those of wild-type D39, a pneumolysin insertion-duplication mutant (PLN-A), and a derivative (PdT) carrying a toxin gene with three point mutations known to abolish both cytolytic activity and complement activation. PdT was intermediate in virulence between D39 and either PLN-A or ΔPly in a mouse intraperitoneal challenge model. This provides unequivocal evidence that pneumolysin has an additional property that is not abolished by point mutations which reduce cytotoxicity and complement activation to virtually undetectable levels.
Pneumolysin, a potent 53-kDa thiol-activated cytolysin produced by virtually all clinical isolates, is a proven virulence factor of Streptococcus pneumoniae (12, 13). Its cytolytic activity involves interaction with cholesterol in target cell membranes and insertion into the lipid bilayer, followed by oligomerization to form transmembrane pores, which bring about cell lysis. Erythrocytes are particularly susceptible to pneumolysin-induced lysis, but theoretically, the toxin can interact with any cell that has cholesterol in its plasma membrane. Indeed, in vitro studies with purified toxin have demonstrated detrimental effects on a wide range of cells and tissues, even at sublytic concentrations (12, 13). However, pneumolysin is a bifunctional toxin, and in addition to its cytotoxic properties, it is capable of directly activating the classical complement pathway in the absence of specific antibody, with a concomitant reduction in serum opsonic activity (14). This is mediated by its capacity to bind directly to the Fc region of human immunoglobulin G (IgG) (10).
Structure-function analysis of pneumolysin has demonstrated that a domain towards the C terminus of the toxin which includes the unique cysteine residue (amino acids [aa] 427 to 437) is critical for cytotoxicity. This cysteine motif is highly conserved among other members of the thiol-activated cytolysin family. Several single amino acid substitutions within this region reduce the cytotoxicity of pneumolysin by up to 99.9% (8, 17). A separate region, which has a degree of amino acid homology with human complement (C)-reactive protein, is responsible for IgG binding and complement activation, and a mutation in this domain (Asp385→Asn) inhibits IgG binding and abolishes complement activation (10).
Inactivation of the pneumolysin gene (ply) by insertion-duplication mutagenesis in either a type 2 or a type 3 strain reduced virulence of S. pneumoniae for mice challenged by either the intranasal (i.n.) or intraperitoneal (i.p.) route (6, 7). Subsequent studies demonstrated that i.n. or intratracheal (i.t.) challenge with ply-negative pneumococci resulted in a less-severe inflammatory response, a reduced rate of multiplication within the lung, a reduced capacity to injure the alveolar-capillary barrier, and a delayed onset of bacteremia compared with those of the wild-type strain (9, 16). Intravenously (i.v.) administered ply-negative pneumococci also exhibited a reduced rate of multiplication in the blood relative to that of the wild-type strain (7). Benton et al. (3) subsequently reported that i.v. challenge of mice with the ply-negative mutant resulted in a chronic becteremia, with numbers of pneumococci in the blood remaining at, or below, 107 CFU per ml for a week in some mice. i.v. administration of an identical dose of the wild-type pneumococcus, however, resulted in fulminant infection; mice invariably died within 28 h, at which time there were approximately 109 to 1010 CFU per ml of blood. Benton et al. concluded that during the first few hours of bacteremia pneumolysin plays a critical role by preventing the generation of inflammation-based immunity, thereby permitting continued exponential net growth of pneumococci (3).
We have also constructed a series of derivatives of the type 2 S. pneumoniae strain D39 in which the wild-type ply gene has been replaced by mutated genes encoding toxins with defined point mutations affecting either or both of the cytotoxic and complement activation properties (5). In an i.p. challenge model, pneumococci carrying two ply point mutations (Cys428→Gly and Trp433→Phe) resulting in production of a toxin with only 0.001% residual cytolytic activity was much less virulent than wild-type D39. A D39 derivative producing pneumolysin with just the Trp433→Phe mutation (with 0.1% residual cytolytic activity) was only marginally (albeit statistically significantly) less virulent than the wild-type strain. Interestingly, in this model system, pneumococci carrying the Asp385→Asn ply mutation, which abolishes complement activation, was as virulent as the wild-type strain. However, by using the same pneumococcal mutants, distinct roles have been demonstrated for both toxin properties in the pathogenesis of bronchopneumonia and lobar pneumonia with mouse i.n. and i.t. challenge models. In both models, strains producing pneumolysin lacking either property were less virulent than the wild type (1, 15). In the i.t. challenge model, cytotoxic activity was required for damage to the alveolar-capillary barrier and for optimal bacterial multiplication in the alveoli and lung tissue during the first 6 h of infection. However, the complement activation property was associated with increased numbers of pneumococci in lung tissue and the blood 24 h after infection (15). In the i.n. challenge model, the complement activation property was important for growth of pneumococci in both the lungs and the blood 6 to 24 h after challenge, while the cytotoxic property was associated with increased numbers of pneumococci in the lungs after 24 h (1).
An S. pneumoniae D39 derivative carrying three ply point mutations (Asp385→Asn, Cys428→Gly and Trp433→Phe) abrogating both complement activation and cytolytic properties of the toxin has been shown to be less virulent than strains carrying either Asp385→Asn or Cys428→Gly and Trp433→Phe substitutions in both i.n. and i.v. challenge models (1, 4). However, in both cases, the triple point mutant was significantly more virulent than a D39 derivative in which ply was disrupted by insertion-duplication mutagenesis, leading to the suggestion that pneumolysin has an additional property which contributes to pathogenesis.
The above observations could, of course, be explained by polar effects caused by integration of the insertion-duplication mutagenesis vector pVA891 into ply, although there are no open reading frames immediately downstream of ply in the S. pneumoniae genome. Moreover, construction of the various D39 ply point mutants also involved insertion of pVA891 immediately downstream of the intact ply open reading frame; these constructs included a control strain in which insertion of pVA891 reconstituted a wild-type ply gene, and this strain was fully virulent (1, 5). An alternative explanation for the lower virulence of the ply insertion-duplication mutant could be the production of a truncated gene product, or a ply-vector encoded fusion protein, which is toxic for the pneumococcus. The insertion-duplication mutant was constructed with a derivative of pVA891 containing a 695-bp Sau3A fragment from the middle of ply cloned into the BamHI site of the vector. This was transformed into S. pneumoniae and integrated into the chromosome by a single recombination event (7). Translation of the interrupted gene would be expected to result in a fusion protein containing the first 322 aa of pneumolysin plus an indeterminate number of vector-encoded amino acids. Theoretically, fusions containing vector-encoded amino acids plus the C-terminal 380 aa of pneumolysin could also result from readthrough from vector sequences into the 3′ portion of ply. Furthermore, smaller truncated pneumolysin C-terminal fragments could result from initiation at internal ATG codons in the distal portion of ply.
In the present study we have eliminated these complications by constructing a D39 derivative with an in-frame ply deletion, and we compared its virulence to that of the wild-type strain and derivatives carrying insertion-duplication and point mutations in ply.
Bacterial strains.
The virulent type 2 S. pneumoniae strain D39 (NCTC 7466) has been described previously (2). D39 derivatives in which ply has been disrupted by insertion-duplication mutagenesis (designated PLN-A) or in which the wild-type ply has been replaced by a mutated gene encoding pneumolysin with three amino acid substitutions (Asp385→Asn, Cys428→Gly and Trp433→Phe) abrogating both complement activation and cytolytic properties of the toxin (designated PdT) have also been described previously (5, 7).
The ply deletion derivative of D39 (designated ΔPly) was constructed as follows. First, S. pneumoniae D39 genomic DNA was digested with ClaI and recircularized, and the region immediately upstream of ply was isolated by inverse PCR (11) with the primers 5′-GGGATCCTGTTCGTAATCTCTCTGTCA-3′ and 5′-GAGGAGCTACCTTGACTCC-3′, which were designed on the basis of the published ply sequence (18). The inverse PCR product was digested with ClaI and SalI, and the resultant 3,050-bp fragment, which contains DNA from the ClaI site 2,883 nucleotides (nt) upstream of the ply initiation codon to the SalI site in codon 55 of ply, was cloned into pBluescript SK (obtained from Stratagene, La Jolla, Calif.). This construct was transformed into Escherichia coli DH5α (Gibco-BRL, Gaithersburg, Md.). The 3′ portion of ply and flanking sequences were isolated by PCR amplification of D39 chromosomal DNA with primers 5′-TGGTGGTCGACGGTTTATGAAAAAACC-3′ and 5′-CCTTTGGCTCGAGCAATCGCTTTATCG-3′. The former primer (plus strand) creates a SalI site (underlined) by changing 3 nt (double underlined); ply codon 438 is shown in boldface. The latter primer (minus strand) anneals to a site approximately 1,100 nt 3′ to the ply termination codon and creates a XhoI site (underlined) by changing 3 nt (double underlined). The resultant PCR product was then digested with SalI and XhoI, and the 1,212-bp fragment was cloned into the similarly restricted pBluescript SK derivative containing the 5′ terminus of ply and flanking sequences. This procedure results in an in-frame fusion of the 5′ region of ply, encoding the 54 N-terminal amino acids, with the 3′ region encoding the 34 C-terminal amino acids (residues 438 to 471). Plasmid DNA was extracted from E. coli DH5α which had been transformed with the above construct, and the complete 4,262-bp pneumococcal DNA insert was then excised by digestion with ClaI and XhoI. This was used to transform S. pneumoniae PLN-A. A derivative in which a double recombination had resulted in replacement of the pVA891-interrupted ply locus with the deleted ply locus (with concomitant loss of the pVA891-encoded erythromycin resistance marker) was isolated after enrichment in the presence of erythromycin and ampicillin, as previously described (7). In the previous study we demonstrated that PLN-A does not contain additional mutations affecting virulence, as back-transformation with an intact copy of ply reconstituted wild-type virulence. To confirm that the transformant isolated in the present study contained the ply deletion mutation, chromosomal DNA was amplified by PCR by using a plus-strand primer annealing 200 nt upstream of the ply initiation codon (5′-TTACAAGACCAACCTTGATTG-3′) and the minus-strand primer annealing downstream of the ply termination codon described above. The PCR product was then sequenced by dye-terminator chemistry on an Applied Biosystems model 373A automated DNA sequencer. The sequence was analyzed using DNASIS and PROSIS Version 7.0 software (Hitachi Software Engineering, South San Francisco, Calif.), and this analysis confirmed that the ply locus of strain ΔPly encodes an in-frame deletion derivative of pneumolysin lacking amino acids 55 to 437.
Virulence studies.
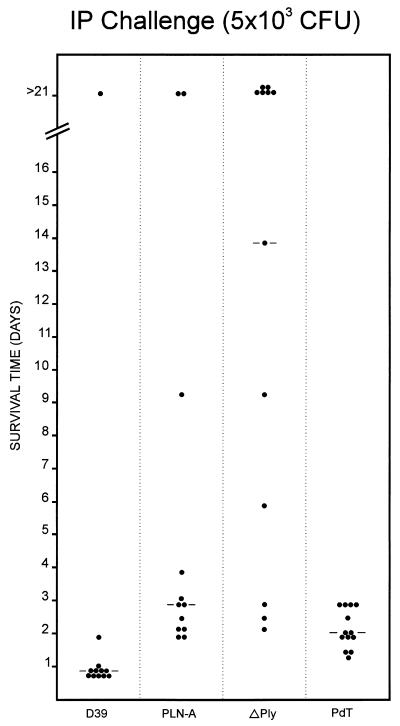
In order to compare the virulence of the various S. pneumoniae strains, D39, PLN-A, PdT, and ΔPly were grown overnight on blood agar (supplemented with 0.2 μg of erythromycin/ml in the case of PLN-A and PdT), inoculated into serum broth (meat extract broth plus 10% horse serum), and incubated at 37°C for 3 h. Production of type 2 capsule was confirmed by quellung reaction by using antisera obtained from Statens Seruminstitut, Copenhagen, Denmark. In vitro growth rates of the three strains were also identical (result not shown). Cultures were then diluted to a density of 5 × 104 CFU/ml, and 0.1-ml volumes were injected i.p. into groups of 12 to 13 BALB/c mice. The survival time of each mouse was recorded (Fig. 1), and differences in median survival time between groups were analyzed using the Mann-Whitney U test (two tailed). Eleven of the 12 mice challenged with the wild-type S. pneumoniae D39 succumbed to the challenge, with a median survival time of 0.9 days. Although all of the mice challenged with PdT succumbed, the median survival time (2.0 days) was significantly greater than that for the D39 group (P < 0.001). The PLN-A and ΔPly groups had median survival times of 2.8 and 13.8 days, respectively, both of which were significantly greater than that for either the D39 group (P < 0.001 in both cases) or the PdT group P < 0.05 and P < 0.002, respectively). Interestingly, the difference in median survival time between the PLN-A and ΔPly groups also reached statistical significance (P < 0.05). However, when the challenge experiment was repeated at a slightly lower dose (5 × 102 CFU) no significant difference in survival times between the PLN-A and ΔPly groups was observed. In this experiment, the median survival times were 8.9 and 7.0 days, respectively; overall survival rates were 6 of 12 and 5 of 12, respectively (result not presented).
FIG. 1.
Survival time of mice after i.p. challenge. Groups of 12 to 13 BALB/c mice were injected i.p. with approximately 5 × 103 CFU of the indicated strains. The survival time of each mouse is indicated. The broken lines denote the median survival time for each group.
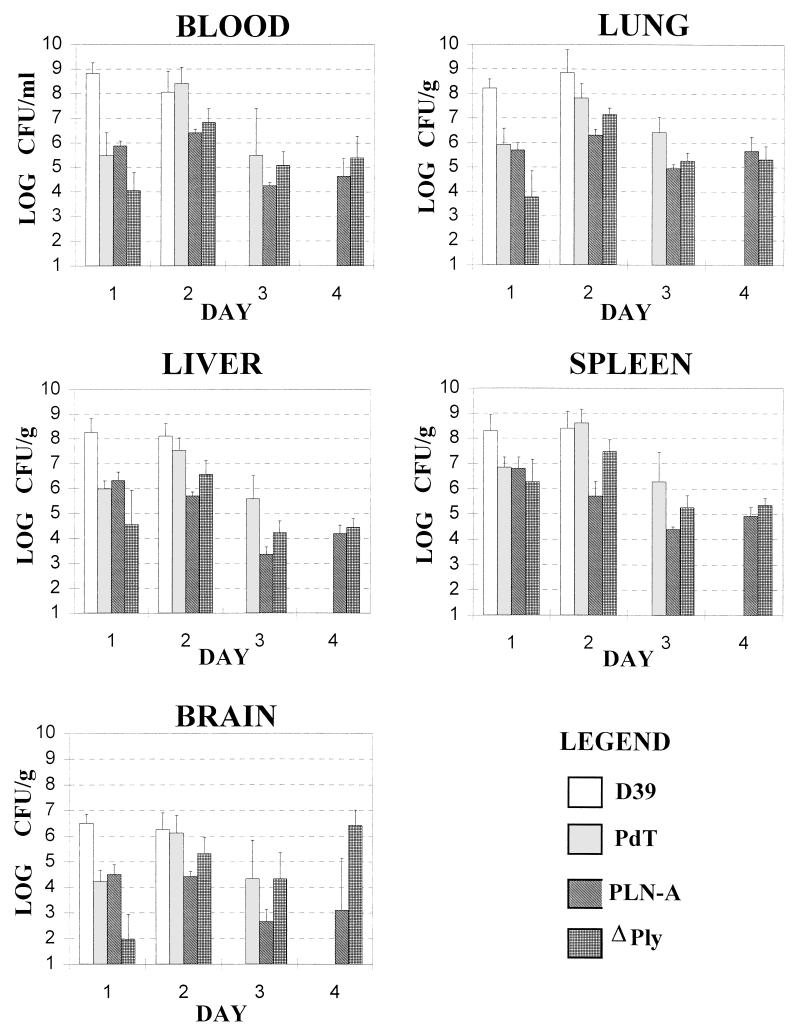
To further examine the contribution of pneumolysin to pathogenesis, the numbers of the various D39 derivatives in blood, lungs, liver, spleen, and brain were monitored after challenge of mice. Groups of 16 BALB/c mice were challenged i.p. with 5 × 102 CFU of D39, PLN-A, PdT, or ΔPly. Four mice chosen at random from each group were sacrificed 1, 2, 3, or 4 days after challenge (where there were sufficient survivors). For each mouse, heart blood was collected into a heparinized tube, and the lungs, liver, spleen, and brain were removed and rinsed extensively, weighed, and homogenized in sterile saline. Serial dilutions of each sample were plated on blood agar to determine the viable count (Fig. 2). The significance of differences in viable counts between various groups was determined by Student’s t test after logarithmic transformation. On day 1 after challenge, the numbers of D39 were significantly greater than those of any of the pneumolysin-deficient strains for all tissues examined (0.001 < P < 0.05). On day 2 after challenge, differences between the numbers of D39 and PdT were no longer apparent for any of the tissues. In contrast, significantly higher numbers of D39 than PLN-A were still observed for all tissues (0.002 < P < 0.05). Also, the numbers of D39 still exceeded those of ΔPly in all tissues, although this only reached statistical significance for the liver samples (P < 0.05). All mice challenged with D39 had died by day 3, but the numbers of PdT in the liver and lungs were significantly greater than those of PLN-A (P < 0.05 and P < 0.01, respectively). On day 3 the numbers of PdT in the lungs were also significantly greater than those of ΔPly (P < 0.05). However, there was no significant difference between the numbers of PLN-A and ΔPly in any of the tissues. On day 4 after challenge, only mice challenged with PLN-A or ΔPly were still alive, and again there was no significant difference between the numbers of these strains for any of the tissues sampled.
FIG. 2.
Numbers of S. pneumoniae in tissues after i.p. challenge. Groups of 16 BALB/c mice were challenged i.p. with 5 × 102 CFU of D39, PLN-A, PdT, or ΔPly. Four mice chosen at random from each group were sacrificed 1, 2, 3, or 4 days after challenge (where there were sufficient survivors). Numbers of each strain in blood (expressed as log10 CFU/ml) or in homogenates of lungs, liver, spleen, or brain (expressed as log10 CFU/g of tissue) were determined as described in the text. Data are the means for each group, with the standard errors indicated by the bars.
Conclusions.
In the present study we have examined the comparative virulence of the type 2 S. pneumoniae strain D39 and derivatives containing insertion-duplication, deletion, or point mutations in the ply gene. Virulence was assessed on the basis of survival time after i.p. challenge and the numbers of pneumococci in blood, lungs, brain, liver, or spleen at various times after challenge. PdT, which produces pneumolysin with three amino acid substitutions (Asp385→Asn, Cys428→Gly, and Trp433→Phe) abrogating both complement activation and cytolytic properties of the toxin, was less virulent than the wild-type D39 but was more virulent than either the ply insertion-duplication mutant PLN-A or the ply deletion mutant ΔPly. A difference between the virulence of PdT and PLN-A has been observed previously with i.n. or i.v. challenge models (1, 4), but this could have been due to polar effects of the pVA891-mediated insertion-duplication event or perhaps to toxicity of truncated pneumolysin polypeptides or fusion proteins resulting from insertion of plasmid sequences into ply. Both these possibilities have been eliminated by the present study as in the i.p. challenge model the virulence of ΔPly is similar to that of PLN-A.
This study provides unequivocal evidence that pneumolysin has an additional property that is not abolished by point mutations which reduce cytotoxicity and complement activation to virtually undetectable levels. Moreover, this property contributes significantly to the pathogenesis of disease. PdT has only 0.001% residual pneumolysin cytolytic activity, as judged by hemolysis assay (5), because of the Cys428→Gly and Trp433→Phe mutations, and it is difficult to imagine this trace level being of pathogenic significance. However, although the Asp385→Asn mutation in PdT reduces the complement activation property of pneumolysin to undetectable levels, the capacity to bind the Fc region of IgG is not completely abolished. Indeed, purified pneumolysin with this point mutation can still bind 27 and 14%, respectively, of the human IgG and Fc bound by the wild-type toxin (10). Failure to prevent the establishment of a nonspecific protective inflammatory response during the early stages of bacteremic infection has been proposed as an explanation for the inability of pneumolysin-negative pneumococci to rapidly overwhelm the host (3, 4). It remains a possibility that binding of IgG and Fc by pneumolysin may contribute to the blockade of such a response independent of classical complement pathway activation.
Acknowledgments
This work was supported by a grant from the National Health and Medical Research Council of Australia.
REFERENCES
- 1.Alexander J E, Berry A M, Paton J C, Rubins J B, Andrew P W, Mitchell T J. Amino acid changes affecting the activity of pneumolysin alter the behaviour of pneumococci in pneumonia. Microb Pathog. 1998;24:167–174. doi: 10.1006/mpat.1997.0185. [DOI] [PubMed] [Google Scholar]
- 2.Avery O T, MacLeod C M, McCarty M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. Induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III. J Exp Med. 1944;79:137–158. doi: 10.1084/jem.79.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benton K A, Everson M P, Briles D E. A pneumolysin-negative mutant of Streptococcus pneumoniae causes chronic bacteremia rather than acute sepsis in mice. Infect Immun. 1995;63:448–455. doi: 10.1128/iai.63.2.448-455.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benton K A, Paton J C, Briles D E. The hemolytic and complement-activating properties of pneumolysin do not contribute individually to virulence in a pneumococcal bacteremia model. Microb Pathog. 1997;23:201–209. doi: 10.1006/mpat.1997.0150. [DOI] [PubMed] [Google Scholar]
- 5.Berry A M, Alexander J E, Mitchell T J, Andrew P W, Hansman D, Paton J C. Effect of defined point mutations in the pneumolysin gene on the virulence of Streptococcus pneumoniae. Infect Immun. 1995;63:1969–1974. doi: 10.1128/iai.63.5.1969-1974.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berry A M, Paton J C, Hansman D. Effect of insertional inactivation of the genes encoding pneumolysin and autolysin on the virulence of Streptococcus pneumoniae type 3. Microb Pathog. 1992;12:87–93. doi: 10.1016/0882-4010(92)90111-z. [DOI] [PubMed] [Google Scholar]
- 7.Berry A M, Yother J, Briles D E, Hansman D, Paton J C. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect Immun. 1989;57:2037–2042. doi: 10.1128/iai.57.7.2037-2042.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boulnois G J, Paton J C, Mitchell T J, Andrew P W. Structure and function of pneumolysin, the multifunctional, thiol-activated toxin of Streptococcus pneumoniae. Mol Microbiol. 1991;5:2611–2616. doi: 10.1111/j.1365-2958.1991.tb01969.x. [DOI] [PubMed] [Google Scholar]
- 9.Canvin J R, Marvin A P, Sivakumaran M, Paton J C, Boulnois G J, Andrew P W, Mitchell T J. The role of pneumolysin and autolysin in the pathology of pneumonia and septicaemia in mice infected with a type 2 pneumococcus. J Infect Dis. 1995;172:119–123. doi: 10.1093/infdis/172.1.119. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell T J, Andrew P W, Saunders F K, Smith A N, Boulnois G J. Complement activation and antibody binding by pneumolysin via a region of the toxin homologous to a human acute-phase protein. Mol Microbiol. 1991;5:1883–1888. doi: 10.1111/j.1365-2958.1991.tb00812.x. [DOI] [PubMed] [Google Scholar]
- 11.Ochman H, Gerber A S, Hartl D L. Genetic applications of an inverse polymerase chain reaction. Genetics. 1988;120:621–623. doi: 10.1093/genetics/120.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paton J C. The contribution of pneumolysin to the pathogenicity of Streptococcus pneumoniae. Trends Microbiol. 1996;4:103–106. doi: 10.1016/0966-842X(96)81526-5. [DOI] [PubMed] [Google Scholar]
- 13.Paton J C, Andrew P W, Boulnois G J, Mitchell T J. Molecular analysis of the pathogenicity of Streptococcus pneumoniae: the role of pneumococcal proteins. Annu Rev Microbiol. 1993;47:89–115. doi: 10.1146/annurev.mi.47.100193.000513. [DOI] [PubMed] [Google Scholar]
- 14.Paton J C, Rowan-Kelly B, Ferrante A. Activation of human complement by the pneumococcal toxin, pneumolysin. Infect Immun. 1984;43:1085–1087. doi: 10.1128/iai.43.3.1085-1087.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubins J B, Charboneau D, Fasching C, Berry A M, Paton J C, Alexander J E, Andrew P W, Mitchell T J, Janoff E N. Distinct roles for pneumolysin’s cytotoxic and complement activities in the pathogenesis of pneumococcal pneumonia. Am J Respir Crit Care Med. 1996;153:1339–1346. doi: 10.1164/ajrccm.153.4.8616564. [DOI] [PubMed] [Google Scholar]
- 16.Rubins J B, Charboneau D, Paton J C, Mitchell T J, Andrew P W, Janoff E N. Dual function of pneumolysin in the early pathogenesis of murine pneumococcal pneumonia. J Clin Investig. 1995;95:142–150. doi: 10.1172/JCI117631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saunders F K, Mitchell T J, Walker J A, Andrew P W, Boulnois G J. Pneumolysin, the thiol-activated toxin of Streptococcus pneumoniae, does not require a thiol group for in vitro activity. Infect Immun. 1989;57:2547–2552. doi: 10.1128/iai.57.8.2547-2552.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker J A, Allen R L, Falmagne P, Johnson M K, Boulnois G J. Molecular cloning, characterization, and complete nucleotide sequence of the gene for pneumolysin, the sulfhydryl-activated toxin of Streptococcus pneumoniae. Infect Immun. 1987;55:1184–1189. doi: 10.1128/iai.55.5.1184-1189.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]