Abstract
Background:
Lung cancer is a common cancer in people living with HIV (PLWH). We investigated trends in relative and absolute risk of lung cancer among PLWH within age groups in the United States.
Methods:
We used data from the HIV/AIDS Cancer Match study during 2001–2016. Average annual percentage changes (AAPCs) in lung cancer rates were estimated with multivariable Poisson regression, and standardized incidence ratios (SIRs), and excess absolute risks (EARs) were estimated comparing PLWH to the general US population. We used non-parametric cumulative incidence curves to estimate the 5-year cumulative incidence of lung cancer, and non-Hodgkin lymphoma and Kaposi sarcoma (two AIDS-defining cancers).
Findings:
There were 3,426 lung cancers in 4·3 million person-years of follow-up for PLWH in the U.S. in 2001–2016. Lung cancer rates among PLWH declined 6% per year (95% confidence interval [CI]=−7% to − 5%) during 2001–2016, with greater declines among younger age groups. During 2013–2016, risk of lung cancer among PLWH was 2.0 times higher among 40–49-year-olds (95%CI=1·52–2·61) and 1·3 times higher among 60–69-year-olds (95%CI=1·12–1·52) compared to the general population, while the EARs among PLWH ages 40–49 and 60–69-years-old were 12 (95%CI=4·0–21·9) and 48 (95%CI=6·9–95·5) per 100,000 person-years, respectively. Beginning in 2011, the 5-year cumulative incidence for lung cancer (1·36%) surpassed that of Kaposi sarcoma (0·12%) and non-Hodgkin lymphoma (0·45%) for PLWH aged 60–69 years.
Interpretation:
The risk of lung cancer has decreased for younger PLWH but remains significantly elevated compared to the general population, likely due to a combination of smoking and immunosuppression. For PLWH aged ≥50 years, the risk of lung cancer exceeds that of the most common AIDS-defining cancers, highlighting the importance of lung cancer among the growing older population of PLWH.
Funding:
Intramural Research Program of the National Cancer Institute
Introduction
Rates of lung cancer among people living with HIV (PLWH) are elevated 1·5 to 3-fold compared to the general population (1,2). Previous work has shown increasing lung cancer risk with lower CD4 counts, greater viral loads, and among those with a prior AIDS diagnosis (1,3). In addition, markers of lung inflammation have been shown to be elevated among those with uncontrolled HIV viremia (4). While smoking is more prevalent among PLWH than in the general population, some evidence has suggested that HIV is an independent risk factor for lung cancer and possibly interacts with smoking to enhance lung cancer risk among PLWH who smoke (2,5–8).
The current antiretroviral therapy (ART) era has resulted in a shift in the cancer burden among PLWH from predominantly AIDS-defining cancers (i.e., Kaposi sarcoma [KS], certain non-Hodgkin lymphomas [NHLs], and cervical cancer) (9) to predominantly non-AIDS-defining cancers. By 2030, the proportion of PLWH who are older than 65 years of age is expected to double (9), introducing greater risks for diseases associated with an aging population. Lung cancer is the third most common cancer type in PLWH, following NHL and KS, and the second largest contributor to death among all cancers (10). While lung cancer rates have been declining over time in the United States (11), such trends have not been investigated among PLWH for recent years.
As lung cancer is an important cause of morbidity and mortality among PLWH, updated estimates of both absolute and relative risk are needed overall and across age groups to inform efforts to improve health outcomes in PLWH. In this work, we leveraged a recently updated population-based study of HIV and cancer registries to describe lung cancer risk in PLWH relative to the general population and cumulative incidence of lung cancer among PLWH in the United States (U.S.).
Methods
Study design and participants
We conducted this research using the HIV/AIDS Cancer Match (HACM) Study, an HIV and cancer registry linkage that includes cancer diagnoses from 2001 through 2016 from 13 regions in the United States, including 11 states, Washington D.C. and Puerto Rico. We included individuals older than 20 years of age and restricted to younger than 90 years. HIV registry data capture demographic characteristics and dates of reported HIV, AIDS, and cause of death. Risk groups for HIV acquisition were classified based on gender and recorded mode of transmission (e.g., persons who inject drugs [PWID], men who have sex with men [MSM] excluding PWID, and other/unknown including MSM who inject drugs). We included non-Hispanic White (i.e., White), non-Hispanic Black (i.e., Black), and Hispanic race and ethnicity in analyses due to limited sample sizes of PLWH of other or unknown race and ethnicity, who represented ~1·5% of the total observed person-time in PLWH in our study population and among whom zero cases of lung cancer were observed in some years. We estimated time since HIV diagnosis and prior AIDS diagnosis using HIV surveillance data for all regions excluding Massachusetts, where those data were unavailable. Years of observation were grouped into four time periods: 2001–2004, 2005–2008, 2009–2012, and 2013–2016.
Linked cancer registry data include cancer diagnosis dates, tumor site, morphology, and stage. Using ICD-O3 site codes C340–C349, excluding ICD-O-3 morphology codes 9050–9055, 9140, 9590–9992, we classified lung cancer histologic subtypes into squamous cell carcinoma, adenocarcinoma, small cell carcinoma, large cell carcinoma, and other histologies (SEER Lung Solid Tumor Rules).
The study was approved by institutional review boards at participating registries and was exempt from review at the USA National Institutes of Health. Consent of participants was not required for the use of data collected through public health surveillance. The data used in this article cannot be shared publicly due to the terms of the NCI’s data use agreements with the cancer and HIV surveillance systems.
Statistical analysis
We calculated age standardized incidence rates (IRs) using the 2010 population of PLWH as the standard population and 95% confidence intervals (CIs) using Fay & Feuer method (12). We used Joinpoint to test for any significant changes in trajectory of the age-standardized incidence rates and we did not identify any significant Joinpoints, which suggested that the yearly change was constant and that multivariable Poisson regression would be appropriate. To estimate the average annual percentage change (AAPC) in lung cancer in PLWH, we used a multivariable Poisson regression model to estimate the coefficient for calendar year, adjusted for attained age, sex and HIV risk group, and race and ethnicity with the log person-time as an offset. We used a generalized boosted model to inform the forward selection of covariates for a minimally adjusted model after including the effects of year as a linear term (13). We also tested for a minimum of 10% change in the year coefficient to consider additional confounders, including region, time since HIV diagnosis, and prior AIDS diagnosis. We tested for interactions between year and other variables and then estimated stratified AAPCs when the interaction term was significant.
To compare risks between PLWH and the general population, we used standardized incidence ratios (SIRs) for the included registry areas. SIRs were calculated by dividing the observed number of lung cancers in PLWH by the expected number, estimated by applying general population cancer incidence rates to person-time in the HIV population based on sex, attained age, race and ethnic group, calendar year, and registry. To assess calendar trends of SIRs over the time period, we used Poisson regression adjusting for age group, sex and HIV risk group, and race and ethnicity with the log of the expected number of lung cancers based on the general population as an offset. We estimated the excess absolute risk (EAR) of lung cancer by calculating the difference between the observed and the expected cancer rates as estimated from the general population.
As information on cigarette smoking was not available in the HACM Study, we conducted bias factor analyses to estimate the relative risk (RR) between smoking and lung cancer that would be needed for the true SIR to be null after accounting for smoking (i.e., SIRTrue=1) under varied prevalence of smoking in the general population (A; 20%, 25%, and 30%) and PLWH (B; 40%, 60%, and 80%) using the following bias factor equation(14):
We calculated nonparametric cumulative incidence curves for lung cancer by treating death from causes other than lung cancer as a competing event. Pointwise estimates at 5 years of follow-up in three separate models using time origins of 2001, 2006, and 2011 were calculated within age groups. We included weights in our models to account for left truncation and right censoring due to registries entering and leaving the study at various points. To contextualize the risk of lung cancer in PLWH we estimated cumulative incidence of AIDS-defining cancers (i.e., KS and NHL) using the same approach. All analyses were conducted using R version 4.1.0 (15).
Role of the Funding Source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report, but did review a final version of the manuscript prior to submission.
Results
We observed a total of 4,310,304 years of follow-up among PLWH in the U.S. between January 1, 2001 through December 31, 2016 (IR=79.4/100,000 person-years). The proportion of the population aged 50 and older nearly doubled, from ~22% of the total person-time observed in 2001–2004 to over 40% in the period of 2013–2016 (Table 1). Over time there was a decrease in the proportion of PWID of both genders, and an increase in the proportion of MSM. By 2013–2016, approximately half of the population of PLWH had been living with HIV for at least 10 years, and there was a ~10% decrease in the proportion with a prior AIDS diagnosis compared to 2001–2004.
Table 1.
Descriptive characteristics of person-years contributed in the study population of people living with HIV in the United States over time.
| Characteristic | 2001–2004 | 2005–2008 | 2009–2012 | 2013–2016 |
|---|---|---|---|---|
| Age group | ||||
| 20–39 | 284,608 (38·3%) | 356,772 (30·4%) | 425,480 (26·9%) | 235,996 (29·1%) |
| 40–49 | 299,321 (40·3%) | 474,044 (40·5%) | 567,403 (35·8%) | 237,153 (29·2%) |
| 50–59 | 126,515 (17·0%) | 263,486 (22·5%) | 433,146 (27·3%) | 238,148 (29·3%) |
| 60–69 | 27,181 (3·7%) | 64,188 (5·5%) | 131,154 (8·3%) | 82,998 (10·2%) |
| 70+ | 5,596 (0·8%) | 13,284 (1·1%) | 26,699 (1·7%) | 17,133 (2·1%) |
| HIV risk group | ||||
| Male other / unknown | 170,790 (23·0%) | 278,085 (23·7%) | 383,217 (24·2%) | 182,494 (22·5%) |
| Female other / unknown | 144,548 (19·4%) | 251,947 (21·5%) | 353,794 (22·3%) | 172,893 (21·3%) |
| Male PWID | 125,451 (16·9%) | 161,431 (13·8%) | 174,802 (11·0%) | 73,602 (9·1%) |
| Female PWID | 63,902 (8·6%) | 81,726 (7·0%) | 90,845 (5·7%) | 37,853 (4·7%) |
| MSM | 238,530 (32·1%) | 398,584 (34·0%) | 581,224 (36·7%) | 344,585 (42·5%) |
| Race and ethnicity | ||||
| Hispanic | 187,475 (25·2%) | 310,383 (26·5%) | 399,103 (25·2%) | 208,350 (25·7%) |
| Non-Hispanic Black | 350,350 (47·1%) | 556,115 (47·5%) | 781,135 (49·3%) | 377,419 (46·5%) |
| Non-Hispanic White | 205,394 (27·6%) | 305,275 (26·1%) | 403,644 (25·5%) | 225,660 (27·8%) |
| Time since HIV diagnosis* | ||||
| <=5 | 260,425 (35·0%) | 316,903 (27·5%) | 337,306 (22·1%) | 155,886 (21·3%) |
| 5.01–10 | 226,406 (30·5%) | 335,074 (29·1%) | 416,349 (27·3%) | 187,354 (25·6%) |
| 10.01–15 | 108,513 (14·6%) | 226,730 (19·7%) | 315,264 (20·7%) | 166,042 (22·7%) |
| >=15.01 years | 17,332 (2·3%) | 101,486 (8·8%) | 272,829 (17·9%) | 194,167 (26·5%) |
| Missing | 130,544 (17·6%) | 172,382 (15·0%) | 183,578 (12·0%) | 28,914 (3·9%) |
| Prior AIDS diagnosis* | ||||
| No | 262,460 (35·3%) | 446,698 (38·8%) | 632,278 (41·5%) | 330,237 (45·1%) |
| Yes | 480,759 (64·7%) | 705,877 (61·2%) | 893,049 (58·5%) | 402,126 (54·9%) |
PWID = persons who inject drugs
MSM = men who have sex with men
Numbers for HIV-related factors excluded Massachusetts
Over the course of follow-up there were 3,426 lung cancers observed in PLWH. The most common histologic subtype was adenocarcinoma, accounting for 40% of all lung cancers, followed by squamous cell carcinoma at ~25%; 9% of cases were small cell carcinoma, and 3% were large cell carcinomas. Based on the total expected counts for each histologic group, these proportions were similar to what would be expected in the general population where 43% of lung cancers would be adenocarcinoma and 21% would be squamous cell carcinoma given our population distribution. There was a marked decrease in lung cancer over time, with an age standardized IR of 124·4 per 100,000 person-years in 2001–2004 and 58·3 per 100,000 person-years in 2013–2016 (Table 2), corresponding to a 6% per year (95% CI=−7% to −5%) decline from 2001 to 2016. Similar declines were observed across all histologic subtypes (Table 3). Compared to the men living with HIV in the other/unknown risk group, female PWID (RR=1·62; 95% CI=1·44–1·83) and male PWID (RR=1·26; 95% CI=1·15–1·39) had significantly elevated lung cancer risk, and MSM (RR=0·63; 95% CI=0·57–0·70) and females in the other/unknown risk groups (RR=0·89; 95% CI=0·80–0·99) had significantly lower lung cancer risk. Lung cancer rates increased with age and were similar among Black PLWH (RR=1·07; 95% CI=0·99–1·17) and lower among Hispanic PLWH (RR=0·46; 95% CI=0·41–0·52) compared to White PLWH (Table 3). Findings were similar when looking at histologic subtypes, with some evidence that relative risks associated with age and risk group vary by histologic subtype (Table 3). Prior AIDS diagnosis was associated with a 40% increased risk of lung cancer (RR=1·40; 95% CI=1·29–1·52).
Table 2.
Incidence rates per 100,00 person-years for lung cancer in people living with HIV during 2001–2016.
| 2001–2004 | 2005–2008 | 2009–2012 | 2013–2016 | |
|---|---|---|---|---|
| Age group | ||||
|
| ||||
| 20–39 | 13·0 (10·9–15·1) | 7·9 (6·4–9·3) | 5·2 (4·1–6·3) | 2·1 (1·2–3·1) |
| 40–49 | 73·2 (68·2–78·1) | 56·8 (53·3–60·2) | 42·0 (39·2–44·7) | 23·6 (20·5–26·8) |
| 50–59 | 210·3 (197·4–223·1) | 162·1 (154·2–169·9) | 126·1 (120·7–131·4) | 103·3 (96·7–109·9) |
| 60–69 | 401·0 (362·6–439·4) | 325·6 (303·1–348·1) | 261·5 (247·4–275·6) | 206·0 (190·3–221·8) |
| 70+ | 464·7 (373·5–555·8) | 429·1 (372·3–485·9) | 344·6 (308·7–380·5) | 350·2 (305·0–395·4) |
|
| ||||
| *Overall | 124·4 (114·5–135·0) | 97·9 (91·8–104·3) | 76·1 (71·9–80·4) | 58·3 (53·4–63·5) |
Age-standardized using the age distribution of people living with HIV in 2010
95% confidence interval in paratheses
Table 3.
Relative risks from multivariable Poisson regression of lung cancer and histologic subtypes among people living with HIV in the United States in 2001–2016.
| Factor | Lung | SCC | Adeno | Small | Large |
|---|---|---|---|---|---|
| Calendar year, per year | 0·94 (0·94, 0·95) | 0·96 (0·94, 0·97) | 0·97 (0·96, 0·98) | 0·94 (0·91, 0·97) | 0·86 (0·81, 0·90) |
| Age group | |||||
| 20–39 | 0·05 (0·04, 0·07) | 0·02 (0·01, 0·03) | 0·04 (0·03, 0·06) | 0·04 (0·02, 0·08) | 0·05 (0·01, 0·15) |
| 40–49 | 0·35 (0·32, 0·38) | 0·27 (0·22, 0·33) | 0·36 (0·31, 0·41) | 0·24 (0·17, 0·33) | 0·31 (0·18, 0·53) |
| 50–59 | Ref | Ref | Ref | Ref | Ref |
| 60–69 | 2·09 (1·92, 2·27) | 2·41 (2·05, 2·84) | 1·88 (1·64, 2·16) | 2·53 (1·93, 3·29) | 2·03 (1·16, 3·44) |
| 70+ | 3·04 (2·64, 3·49) | 4·39 (3·44, 5·53) | 2·35 (1·84, 2·97) | 3·10 (1·92, 4·77) | 2·00 (0·6, 5·00) |
| HIV risk group | |||||
| Male other / unknown | Ref | Ref | Ref | Ref | Ref |
| Female other / unknown | 0·89 (0·80, 0·99) | 0·68 (0·55, 0·84) | 1·08 (0·92, 1·27) | 1·33 (0·94, 1·89) | 1·23 (0·68, 2·23) |
| Male PWID | 1·26 (1·15, 1·39) | 1·17 (0·97, 1·41) | 1·43 (1·22, 1·67) | 1·41 (0·99, 2·01) | 1·01 (0·52, 1·90) |
| Female PWID | 1·62 (1·44, 1·83) | 1·10 (0·84, 1·43) | 1·92 (1·59, 2·31) | 2·57 (1·73, 3·80) | 1·54 (0·70, 3·14) |
| MSM | 0·63 (0·57, 0·70) | 0·48 (0·39, 0·59) | 0·79 (0·67, 0·93) | 0·97 (0·69, 1·35) | 0·52 (0·26, 0·99) |
| Race and ethnicity | |||||
| Hispanic | 0·46 (0·41, 0·52) | 0·43 (0·34, 0·54) | 0·55 (0·46, 0·66) | 0·43 (0·30, 0·62) | 0·22 (0·08, 0·51) |
| Non-Hispanic Black | 1·07 (0·99, 1·17) | 0·90 (0·76, 1·06) | 1·23 (1·07, 1·40) | 0·83 (0·64, 1·09) | 1·02 (0·62, 1·73) |
| Non-Hispanic White | Ref | Ref | Ref | Ref | Ref |
SCC (squamous cell carcinoma): ICD-O3 code 8051–2,8070–6, 8078, 8083–4, 8090, 8094, 8120, 8123
Adeno (adenocarcinoma): ICD-O3 code 8015,8050, 8140–1, 8143–5, 8147, 8190, 8201, 8211, 8250–5,8260, 8290, 8310, 8320, 8323, 8333, 8401, 8440, 8470–1, 8480–1, 8490, 8503, 8507, 8550, 8570–2, 8574, 8576
Small (small cell carcinoma): ICD-O3 code 8002, 8041–5
Large (large cell carcinoma): ICD-O3 code 8012–4, 8021, 8034, 8082
Other histologies included in lung cancers: carcinoma not otherwise specified (NOS) (8010); bronchioloalveolar carcinoma (8253), adenosquamous carcinoma (8560); giant cell and spindle cell carcinoma (8031); carcinoid tumor NOS (8240); sarcoma NOS (8800/3); solid carcinoma NOS (8230); spindle cell sarcoma (8801)
Among those 20–39 years of age, the AAPC was −11% (95% CI=−16% to −6%) compared to an AAPC of −6% (95% CI=−7% to −4%) for those 50–59 years old. We noted that the AAPC did not reach statistical significance for those older than 70 years of age (AAPC=−3%; 95% CI=−6% to 1%) (Appendix page 1). In our adjusted model, we found a greater AAPC among Hispanic PLWH (AAPC=−9%; 95% CI=−11% to −7%) compared to non-White (AAPC=−4%; 95% CI=−6% to −3%) and Black PLWH (AAPC=−6%; 95% CI=−7% to − 5%).
The overall SIR for PLWH compared to the general population declined from 2·46 in 2001–2004 (95% CI=2·27–2·65) to 1·48 in 2013–2016 (95% CI=1·36–1·61). SIRs were the largest for younger age groups, among whom the greatest declines in SIRs were also observed. Compared to the general population, lung cancer rates among 20–39-year-old PLWH were 5·2 times higher (95% CI=3·64–7·12) in 2001–2004 and not significantly elevated during 2013–2016 (SIR=1·7; 95% CI=0·55–3·97), and among 40–49-year-olds lung cancer rates were 3·1 times higher (95% CI=2·74–3·59) in 2001–2004and2.0 times higher among 40–49-year-olds (95% CI=1·52–2·67) (Figure 1). In addition, rates of lung cancer among PLWH aged 50–59 and 60–69 years were significantly elevated in 2013–2016 (SIR=1·61 and 1·31, respectively; 95% CI=1·41–1·82 and 1·12–1·52, respectively), but rates were elevated non-significantly among PLWH aged ≥70 years (SIR=1·24; 95% CI=0·94–1·59) (Appendix page 2). By risk group in 2013–2016, lung cancer rates among PLWH were elevated most strongly among female PWID (SIR=3·27; 95% CI=2·45–4·28), with the smallest elevation observed among MSM (SIR=1·13; 95% CI=0·96–1·32), who had a non-significant elevated risk compared to the general population (Appendix page 3).
Figure 1.
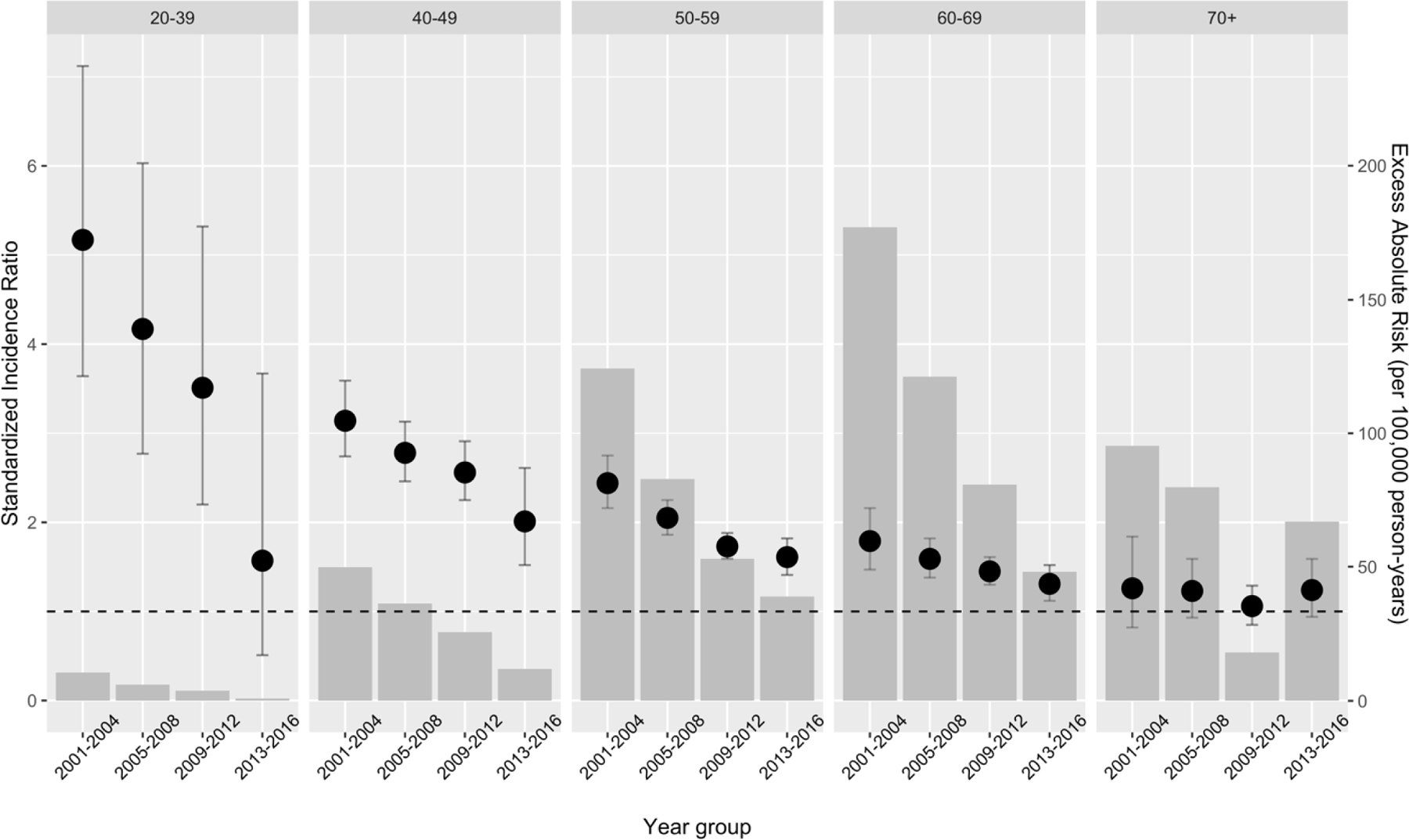
Standardized Incidence Ratio (with 95% Confidence Interval) and Excess Absolute Risk for lung cancer over time for people living with HIV in the United States compared to the general population within age groups.
Standardized Incidence Ratio are shown in black dots and error bars with the scale on the left y-axis with dashed line where SIR=1. Excess Absolute Risk (per 100,00 person-years) are shown in grey bars with the scale on the right y-axis.
In contrast to findings regarding the SIRs, the largest EARs were among 60–69 and ≥70-year-olds, although all EARs for those ≥70 had estimates that overlapped zero. While EARs have also declined over time for most age groups, EARs for PLWH aged 70 and older increased from an excess of 18 per 100,000 person-years in 2009–2012 to 67 per 100,000 person-years in 2013–2016 (95% CIs = −71 to 138 and −47 to 210, respectively), although neither estimate was statistically different from 0 (Figure 1).
During the time period of our study, the estimated prevalence of smoking in the general population was 26% and 47% among PLWH based on data from NHANES (16). Our bias factor analyses found that in all scenarios the elevated risk observed in PLWH ≥70-years-olds could be plausibly explained by confounding due to smoking prevalence (Appendix page 4). For example, given a prevalence of smoking of 25% in the general population and 40% in PLWH, a relative risk of 2·45 for the effect of smoking on lung cancer could explain the observed SIR of 1·16 in PLWH ≥70-year-olds. For context, estimates for the relative risk of lung cancer for current smoking versus never smoking range between 16–115 for men and 8–121 for women, depending on cancer subtype (17,18). However, no relative risks could be estimated that would explain the elevated risks observed among 20–39 and 40–49-year-olds in scenarios in which smoking prevalence was 40% in PLWH.
We estimated a 5-year cumulative incidence of lung cancer of 0·02% (95% CI=0·01%–0·03%) for PLWH 20–39 years-old using a time of origin of 2011, which increased with each age group to 1·65% (95% CI=1·20%–2·10%) for those 70+ years-old (Figure 2) (Appendix page 5). While the 5-year cumulative incidence of either KS or NHL was greater than that of lung cancer in those younger than 50 years-old, among 50–59-year-old PLWH the cumulative incidence of lung cancer (0·62%; 95% CI=0·56%–0·68%) was equivalent to NHL (0·56%; 95% CI=0·51–0·62%) and greater than KS (0·14%; 95% CI=0·11–0·17%). The 5-year cumulative incidence of lung cancer in 60–69-year-old PLWH at time origin of 2011 was 1·36% (95% CI=1·18%–1·54%); notably greater than NHL (0·45%; 95% CI=0·35–0·56%) and KS (0·12%; 95% CI=0·07%–0·17%). For additional comparisons, we calculated the cumulative incidences using time of origins of 2001 and 2006, separately (Appendix page 5). We note that while the 5-year cumulative incidence of lung cancer for PLWH 60–69-years-old declined across the time period with a cumulative incidence of 1·93% (95% CI=1·03–2·79%) with an origin of 2001, there have also been considerable declines in that of NHL and KS with estimated 5-year cumulative incidences based on the 2001 origin of 1·31% (95% CI=1·01–1·59%) and 0·23% (95% CI=0·11–0·35%), respectively.
Figure 2.
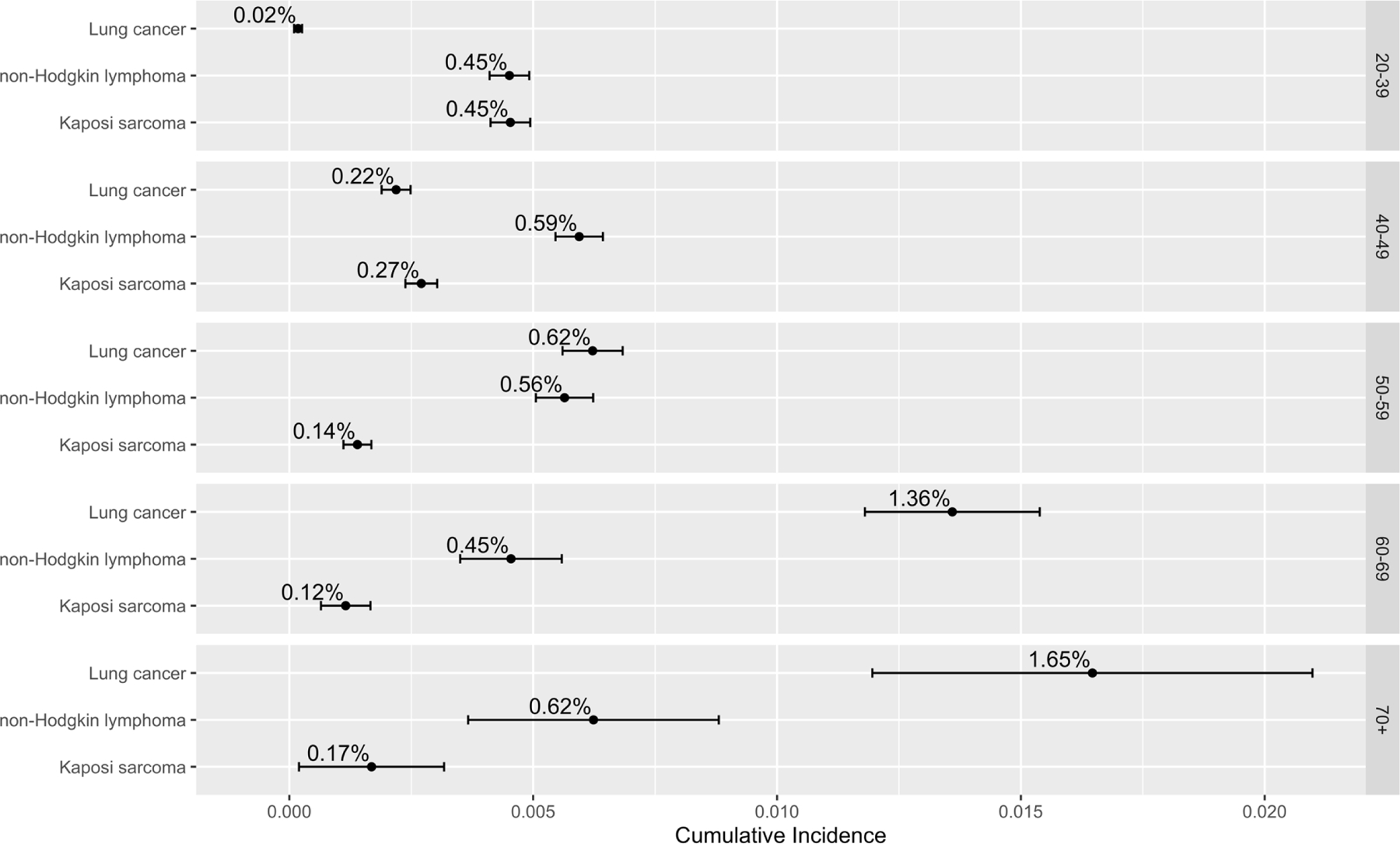
Five-year cumulative incidences of lung cancer in people living with HIV in the United States using time 2011 within age groups.
Discussion
During 2001–2016, lung cancer rates among PLWH declined over time but remained 48% higher than the general population in 2013–2016. Lung cancer rates declined most rapidly among those aged 20–49, and risks were also most elevated in these groups relative to the general population. Though smoking likely contributed to the elevated relative risk, other factors including immunosuppression may also play a role, particularly among younger PLWH. Despite declines in relative risks over time, lung cancer remains an important cancer among older PLWH, as the cumulative incidence of lung cancer among PLWH aged 50 years and older surpasses that of both NHL and KS.
Lung cancer is estimated to have been the second most common cancer among PLWH in 2020 and is expected to increase to represent 15% of all cancers in this population by 2030 (9). We found that the highest risk of lung cancer in PLWH occurs among those 60 years and older, exceeding that of the most common AIDS-defining cancers — NHL and KS. Despite the greater relative risk among younger age groups of PLWH compared to the general population, the greatest excess risks on the absolute scale are among older age groups of PLWH. This finding is particularly important as older individuals comprise an increasing proportion of PLWH and are reaching ages associated with the highest risk of lung cancer. Although the higher prevalence of smoking in PLWH may explain the observed increased risk in these older age groups, we emphasize the public health importance of this additional burden when considering strategies to mitigate the impact of lung cancer in this population.
Cigarette smoking causes 90% of lung cancers in the general population (19) and likely contributes strongly to elevated rates of lung cancer among PLWH, as smoking prevalence in PLWH is known to be higher than in the general population and the primary risk factor for cancer in the modern era, particularly among PWID (6,16,20). The most recent estimates of current smoking status based on the National Health and Nutrition Examination Survey (NHANES) for 1999–2016 reported 47% of PLWH were current smokers compared to 25.5% of survey participants without HIV, with similar declines in smoking prevalence over time (16). While cessation programs tailored to PLWH are merited to bridge the remaining gap in smoking prevalence, more research for the effects of HIV as an independent and synergistic risk factor are also needed (21,22). Our bias analysis showed that differences in smoking prevalence could plausibly explain many of the elevated SIRs observed, though it is likely that improvements in immunosuppression have contributed, particularly among younger PLWH for whom the association between smoking and lung cancer is known to be weaker (17).
In addition to smoking, immunosuppression and pulmonary inflammation likely play a role in the development of lung cancer among PLWH. Lower CD4/CD8 ratio has been associated with higher incidence of lung cancer (23), even independent of the association with episodes of bacterial pneumonia (3). A study that was conducted in France among PLWH also has shown a decline of lung cancer overtime and elevated risk compared to the general population, but a less elevated SIR for PLWH for PLWH who had CD4 cell recovery and were on cART (23). In addition, prior AIDS diagnosis, a marker of prior severe immunosuppression, has been previously shown to be associated with lung cancer and in our study (1). The decrease in SIR for PLWH over time could be due to greater access to more effective antiretroviral treatments, improving the overall health of this population and decreasing progression to immunosuppression and risk of pulmonary infections. Mechanisms that have previously been attributed to both direct oncogenic effects of HIV (24) and indirect result of immunosuppression (14,25), or risk of pulmonary infections and inflammation (3,26,27), may have become less relevant in more recent years as fewer PLWH are progressing to AIDS and have severe immunosuppression. Rates of lung cancer have declined as much as 10% in PLWH aged 20–39 years of age between 2001 and 2016 but these declines have been less dramatic in older age groups and non-significant for those 70 years and older. In combination with declining SIRs over time in younger PLWH, this evidence supports a role of HIV viremia and/or immunocompromised status on lung cancer risk—unless smoking prevalence has disproportionately declined in younger PLWH (we are unaware of data to support that possibility).
As in the general population, most lung cancers in PLWH are not diagnosed until advanced stages (28). These factors have resulted in lung cancer contributing substantially to mortality in PLWH. The US Preventative Services Task Force (USPSTF) has provided some recommendations for the use of low-dose computed tomography (LDCT) for adults aged 50–80 years with a 20 pack-year smoking history who are current smokers or quit within the previous 15 years (29). However, more research specifically on the use of LDCT among PLWH is needed to identify populations at higher risk of death from lung cancer and additional factors that may further be clinically relevant. Previous studies have suggested an interaction between HIV infection and smoking (5,24,25). If true, it may merit more tailored USPSTF guidelines for lung cancer screening for LDCT in PLWH at younger ages or using a less stringent smoking history (30,31). However, recent work on the benefits of LDCT in PLWH found that similar mortality reductions to the general population can be achieved by following the Centers for Medicare & Medicaid Services criteria (i.e. age 55–77, 30-pack-years of smoking, current smoker or quit within 15 years of screening) (32).
While the primary strength of our study is the linkage of two population-based registries representing the largest study for analyzing cancer incidence in PLWH, we were still subject to limitations in the extent to which variables were captured in either of those data sources. As neither HIV surveillance nor cancer registries capture smoking and smoking-related information, we were limited in our ability to adjust for or stratify by smoking history in calculating our estimates. Similarly, information related to socio-economic status which may be relevant to both risk of HIV and lung cancer were unavailable for our analyses. While we emphasize the descriptive nature of our study and the importance of these estimates overall for PLWH, future work to estimate the impacts of smoking and other factors in this population merit investigation.
Using large, national surveillance and registry data in the United States, we have shown an overall decrease in age-standardized incidence rates of lung cancer in PLWH, as well as a decline in the relative excess risk of lung cancer compared to the general population. The reduction in risk for lung cancer in PLWH is most pronounced among those younger than 50 years of age, who show substantial decreases in relative excess risk compared to the general population. However, for the increasing proportion of PLWH who are living beyond 50 years of age, the absolute risk of lung cancer has surpassed that of common AIDS-defining cancers and is a considerable excess risk and public health burden. Strategies for prevention and early detection are necessary to reduce the disparities in lung cancer risk for PLWH.
Research in Context
Evidence before this study
We searched PubMed for peer reviewed articles published during Jan 1, 2000, to Dec 31, 2021, with MeSH terms “lung cancer”, “incidence”, and “HIV”, along with personal collections of study reports and reviews and reference lists of previously identified publications of relevance. Specifically, we examined articles for estimates of the risk of lung cancer among people living with HIV (PLWH) compared to the general population. Incidence of lung cancer has been shown to be 1.5–3 times higher among PLWH compared to either the general population or to a comparison group without HIV. The most recent high-quality work that used a U.S.-based HIV surveillance and cancer registry linkage study with data from 1996 through 2012 found that the relative risk of lung cancer for PLWH in the U.S. compared to the general population has declined over time but remains significantly elevated and is the second most common cancer among PLWH. However, more recent data and age-group specific trends should be investigated. Additionally, most publications have focused on the relative risk rather than the absolute risk of lung cancer.
Added value of this study
The relative risk of lung cancer in PLWH compared to the general population has continued to decline since 2001 and was approximately 1.5 during the most recent time period of 2013–2016. Our findings show that greater declines in the relative risk of lung cancer were observed for the youngest age groups of PLWH. However, the largest absolute excess risks continue to be among the oldest age groups. Importantly, based on our most recent data starting in 2011, we found that the cumulative incidence of lung cancer surpasses that of both non-Hodgkin lymphoma and Kaposi sarcoma for PLWH older than 50 years.
Implications of all the available evidence
Declines in risk of lung cancer for PLWH likely reflects improvements in access to and treatment with combined anti-retroviral therapy, especially for the youngest age group. Prevention and detection of lung cancer for PLWH should be prioritized for the growing proportion of those over 50-years of age.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the support and assistance provided by individuals at the following state HIV/AIDS and cancer registries: Colorado, Connecticut, District of Columbia, Georgia, Louisiana, Maryland, Massachusetts, Michigan, New Jersey, New York, North Carolina, Puerto Rico, and Texas. We also thank Timothy McNeel at Information Management Services for programming support.
The views expressed in this paper are those of the authors and should not be interpreted to reflect the views or official policies of the National Cancer Institute, Centers for Disease Control and Prevention or the Department of Health and Human Services, HIV/AIDS or cancer registries, or their contractors, nor does the mention of trade names, commercial practices, or organizations imply endorsement by the U.S. Government. This research was supported in part by the Intramural Research Program of the National Cancer Institute.
The following cancer registries were supported by the cooperative agreement funded by the Centers for Disease Control and Prevention, National Program of Cancer Registries: Colorado (NU58DP006347–01), Georgia (5U58DP003875–01), Louisiana (NU58DP006332–03-00), Maryland (NU58DP006333), Massachusetts (NU58DP006271–04-00), Michigan (17NU58DP006334), New Jersey (NU58/DP003931–05-00), New York (6NU58/DP006309), North Carolina (1NU58DP006281), Texas (1NU58DP006308). District of Columbia is supported by the Centers for Disease Control and Prevention cooperative agreement DP006302.
The following cancer registries were supported by the SEER Program of the National Cancer Institute: Connecticut (HHSN261201300019I), Louisiana (HHSN261201800007I/ HHSN26100002), Massachusetts (HHSN261201800008l), New Jersey (HHSN261201300021I, N01-PC-2013–00021), and New York (HHSN261201800009I). The New Jersey State Cancer Registry was also supported by the state of New Jersey, the Maryland Cancer Registry was supported by the State of Maryland and the Maryland Cigarette Restitution Fund, the Louisiana Tumor Registry was also supported by the state of Louisiana (0587200015), and the New York State Cancer Registry was also supported by the state of New York.
The following HIV registries were supported by HIV Incidence and Case Surveillance Branch of the Centers for Disease Control and Prevention, National HIV Surveillance Systems: Colorado (NU62PS003960), Connecticut (5U62PS001005–05), Louisiana (NU62PS924522–02-00), Michigan (U62PS004011–02), New Jersey (U62PS004001–2), New York (NU62PS924546–02-00; PS18–1802: Integrated HIV Surveillance and Prevention Programs for Health Departments, National Center for HIV, Viral Hepatitis, STD, and TB Prevention (NCHHSTP)).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
All authors state that they have nothing to disclose.
References
- 1.Chaturvedi AK, Pfeiffer RM, Chang L, Goedert JJ, Biggar RJ, Engels EA. Elevated risk of lung cancer among people with AIDS. AIDS [Internet] 2007. Jan 11;21(2):207–13. Available from: https://journals.lww.com/00002030-200701110-00011 [DOI] [PubMed] [Google Scholar]
- 2.Shiels MS, Cole SR, Mehta SH, Kirk GD. Lung cancer incidence and mortality among HIV-infected and HIV-uninfected injection drug users. J Acquir Immune Defic Syndr [Internet] 2010. Dec;55(4):510–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20838223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sigel K, Wisnivesky J, Crothers K, Gordon K, Brown ST, Rimland D, et al. Immunological and infectious risk factors for lung cancer in US veterans with HIV: a longitudinal cohort study. lancet HIV [Internet] 2017;4(2):e67–73. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27916584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiels MS, Kirk GD, Drummond MB, Dhillon D, Hanash SM, Taguchi A, et al. HIV Infection and Circulating Levels of Prosurfactant Protein B and Surfactant Protein D. J Infect Dis [Internet] 2018. Jan 17;217(3):413–7. Available from: https://academic.oup.com/jid/article/217/3/413/4762465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahale P, Engels EA, Coghill AE, Kahn AR, Shiels MS. Cancer Risk in Older Persons Living With Human Immunodeficiency Virus Infection in the United States. Clin Infect Dis [Internet] 2018;67(1):50–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29325033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altekruse SF, Shiels MS, Modur SP, Land SR, Crothers KA, Kitahata MM, et al. Cancer burden attributable to cigarette smoking among HIV-infected people in North America. AIDS [Internet] 2018. Feb 20;32(4):513–21. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29239891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kirk GD, Merlo C, O’ Driscoll P, Mehta SH, Galai N, Vlahov D, et al. HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin Infect Dis [Internet] 2007. Jul 1;45(1):103–10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17554710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engels EA, Brock MV, Chen J, Hooker CM, Gillison M, Moore RD. Elevated incidence of lung cancer among HIV-infected individuals. J Clin Oncol [Internet] 2006. Mar 20;24(9):1383–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16549832 [DOI] [PubMed] [Google Scholar]
- 9.Shiels MS, Islam JY, Rosenberg PS, Hall HI, Jacobson E, Engels EA. Projected Cancer Incidence Rates and Burden of Incident Cancer Cases in HIV-Infected Adults in the United States Through 2030. Ann Intern Med [Internet] 2018;168(12):866–73. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29801099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horner M-J, Shiels MS, Pfeiffer RM, Engels EA. Deaths Attributable to Cancer in the US Human Immunodeficiency Virus Population During 2001–2015. Clin Infect Dis [Internet] 2021. May 4;72(9):e224–31. Available from: https://academic.oup.com/cid/article/72/9/e224/5876399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Surveillance Research Program; National Cancer Institute;, Division of Cancer Control and Population Sciences (DCCPS) Cancer Stat Facts: Lung and Bronchus Cancer; [Internet]. [cited 2021. Dec 21]. Available from: https://seer.cancer.gov/statfacts/html/lungb.html [Google Scholar]
- 12.Fay MP, Feuer EJ. Confidence intervals for directly standardized rates: a method based on the gamma distribution. Stat Med 1997. Apr;16(7):791–801. [DOI] [PubMed] [Google Scholar]
- 13.Greenwell B, Boehmke B, Cunningham J, Developers G. Generalized Boosted Regression Models 2020.
- 14.Engels EA, Brock MV., Chen J, Hooker CM, Gillison M, Moore RD. Elevated Incidence of Lung Cancer Among HIV-Infected Individuals. J Clin Oncol [Internet] 2006. Mar 20;24(9):1383–8. Available from: 10.1200/JCO.2005.03.4413 [DOI] [PubMed] [Google Scholar]
- 15.R Core Team. R Core Team (2014). R: A language and environment for statistical computing R Found Stat Comput Vienna, Austria URL http//wwwR-project.org/. 2014; [Google Scholar]
- 16.Asfar T, Perez A, Shipman P, Carrico AW, Lee DJ, Alcaide ML, et al. National Estimates of Prevalence, Time-Trend, and Correlates of Smoking in US People Living with HIV (NHANES 1999–2016). Nicotine Tob Res [Internet] 2021. Aug 4;23(8):1308–17. Available from: https://academic.oup.com/ntr/article/23/8/1308/6226483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pesch B, Kendzia B, Gustavsson P, Jöckel K-H, Johnen G, Pohlabeln H, et al. Cigarette smoking and lung cancer--relative risk estimates for the major histological types from a pooled analysis of case-control studies. Int J cancer [Internet] 2012. Sep 1;131(5):1210–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22052329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freedman ND, Abnet CC, Caporaso NE, Fraumeni JF, Murphy G, Hartge P, et al. Impact of changing US cigarette smoking patterns on incident cancer: risks of 20 smoking-related cancers among the women and men of the NIH-AARP cohort. Int J Epidemiol [Internet] 2016. Jun;45(3):846–56. Available from: 10.1093/ije/dyv175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC). What Are the Risk Factors for Lung Cancer? 2021.
- 20.Park LS, Hernández-Ramírez RU, Silverberg MJ, Crothers K, Dubrow R. Prevalence of non-HIV cancer risk factors in persons living with HIV/AIDS: a meta-analysis. AIDS [Internet] 2016. Jan;30(2):273–91. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26691548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ledgerwood DM, Yskes R. Smoking Cessation for People Living With HIV/AIDS: A Literature Review and Synthesis. Nicotine Tob Res [Internet] 2016;18(12):2177–84. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27245237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chew D, Steinberg MB, Thomas P, Swaminathan S, Hodder SL. Evaluation of a Smoking Cessation Program for HIV Infected Individuals in an Urban HIV Clinic: Challenges and Lessons Learned. AIDS Res Treat [Internet] 2014;2014:237834. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25349726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hleyhel M, Hleyhel M, Bouvier AM, Belot A, Tattevin P, Pacanowski J, et al. Risk of non-AIDS-defining cancers among HIV-1-infected individuals in France between 1997 and 2009: results from a French cohort. AIDS [Internet] 2014. Sep 10;28(14):2109–18. Available from: https://journals.lww.com/00002030-201409100-00012 [DOI] [PubMed] [Google Scholar]
- 24.Winstone TA, Man SFP, Hull M, Montaner JS, Sin DD. Epidemic of lung cancer in patients with HIV infection. Chest [Internet] 2013. Feb 1;143(2):305–14. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23381313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sigel K, Makinson A, Thaler J. Lung cancer in persons with HIV. Curr Opin HIV AIDS [Internet] 2017. Jan;12(1):31–8. Available from: https://journals.lww.com/01222929-201701000-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hessol NA, Martínez-Maza O, Levine AM, Morris A, Margolick JB, Cohen MH, et al. Lung cancer incidence and survival among HIV-infected and uninfected women and men. AIDS [Internet] 2015. Jun 19;29(10):1183–93. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25888645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shebl FM, Engels EA, Goedert JJ, Chaturvedi AK. Pulmonary infections and risk of lung cancer among persons with AIDS. J Acquir Immune Defic Syndr [Internet] 2010. Nov;55(3):375–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20736841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coghill AE, Han X, Suneja G, Lin CC, Jemal A, Shiels MS. Advanced stage at diagnosis and elevated mortality among US patients with cancer infected with HIV in the National Cancer Data Base. Cancer [Internet] 2019. May 3;cncr.32158. Available from: 10.1002/cncr.32158 [DOI] [PMC free article] [PubMed]
- 29.Krist AH, Davidson KW, Mangione CM, Barry MJ, Cabana M, Caughey AB, et al. Screening for Lung Cancer. JAMA [Internet] 2021. Mar 9;325(10):962. Available from: https://jamanetwork.com/journals/jama/fullarticle/2777244 [DOI] [PubMed] [Google Scholar]
- 30.Hulbert A, Hooker CM, Keruly JC, Brown T, Horton K, Fishman E, et al. Prospective CT screening for lung cancer in a high-risk population: HIV-positive smokers. J Thorac Oncol [Internet] 2014. Jun;9(6):752–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24828660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sigel K, Wisnivesky J, Shahrir S, Brown ST, Justice A, Kim J, et al. Findings in asymptomatic HIV-infected patients undergoing chest computed tomography testing: implications for lung cancer screening. AIDS [Internet] 2014. Apr 24;28(7):1007–14. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24401647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kong CY, Sigel K, Criss SD, Sheehan DF, Triplette M, Silverberg MJ, et al. Benefits and harms of lung cancer screening in HIV-infected individuals with CD4+cell count at least 500 cells/μl. Aids 2018;32(10):1333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


