Abstract
Metabolic syndrome is a cluster of biological irregularities that is a known risk factor for cardiovascular disease, stroke, and diabetes. In a case-control study of 555 West African women, we observed that metabolic syndrome was strongly associated with breast cancer and the aggressive triple-negative molecular subtype, highlighting a need for clinical and lifestyle interventions targeting metabolic syndrome to reduce breast cancer risk in this population.
Background:
Metabolic syndrome (MetS) is characterized by a cluster of biological irregularities. The purpose of this analysis was to examine the association of MetS with BC among Nigerian women, and for the first time evaluate this association by molecular subtype.
Materials and Methods:
MetS was defined as having at least 3 out of 5 of: high blood pressure (≥ 130/85 mm Hg), reduced HDL (< 50 mg/dL), elevated triglyceride (> 150 mg/dL), high waist circumference (≥ 80 cm), and prior diagnosis of diabetes or elevated fasting glucose level (≥ 100 mg/dL). Among 296 newly diagnosed BC cases and 259 healthy controls, multivariable logistic regression models were utilized to estimate adjusted odds ratios (aOR) and 95% confidence intervals (95% CI) for the association between MetS and BC overall. Multinomial logistic regression models were used to evaluate each molecular subtype (Luminal A, Luminal B, HER2-enriched and triple-negative or TNBC).
Results:
After adjusting for age, socio-demographic and reproductive risk factors, there was a positive association between MetS and BC (aOR: 1.84, 95% CI: 1.07, 3.16). In stratified analyses, MetS was associated with BC regardless of BMI status; however, the estimate was significant only among normal weight women (aOR: 3.85; 95% CI: 1.25, 11.90). MetS was significantly associated with TNBC subtype (aOR: 4.37, 95% CI: 1.67, 11.44); associations for other molecular subtypes were not statistically significant.
Conclusion:
MetS appears to be a robust risk factor for BC, particularly for TNBC. Public health and clinical interventions can provide substantial benefits in reducing the burden of MetS and preventing BC among Nigerian women.
Keywords: Cholesterol, Hypertension, Diabetes, Nigeria, Triple-negative breast cancer
Introduction
Nigeria accounts for one-sixth of the population on the African continent, making it the most populous country in Africa. Over the last 50 years, Nigeria has experienced dramatically increasing breast cancer (BC) incidence rates, with estimates suggesting a 3-fold increase from 15 to 52 cases per 100,000 from 1973 to 2012.1,2 This is particularly concerning because BC in Nigeria has several striking epidemiologic features reflecting aggressive disease, many of which parallel BC among African American women in the United States (US). First, over 70% of BC cases in Nigeria are diagnosed in the premenopausal years, between ages 20 and 50 years.1,3 This pattern has been observed among Black women in the US and UK, but contrasts with BC observed among White women, which is largely postmenopausal.4-6 Although risk factors for postmenopausal BC such as parity, breastfeeding and mammographic density are well understood,7-9 those for premenopausal BC are not as well characterized and deserve further study. Second, 60% to 80% of BC cases in Nigeria are diagnosed at late-stages (III-IV) with high-grade disease.10-13 Similar aggressive phenotypes have also been documented among Black women in the US.12,14-16 Third, BC tumors in Nigerian women are disproportionately classified as triple-negative BC (TNBC), meaning they are receptor-negative for estrogen, progesterone, and human epidermal growth factor.13,17 BC in Nigeria has also been reported to have mutations in several genes well known to be associated with tumorigenesis and DNA repair, specifically, BRCA1 and BRCA2,18-20 P53 and cyclin D1.12,21 Compared to less aggressive BC subtypes, TNBCs are less responsive to treatment due to the lack of drug-targetable receptors and associated with poorer clinical outcomes.22 Despite this unique and aggressive BC phenotype and exceptionally high mortality rates among women of African descent,23 few studies have focused on understanding differentially patterned risk factors associated with increasing BC incidence and aggressive molecular subtypes among Nigerian women.
Metabolic syndrome (MetS) refers to a cluster of conditions that include central obesity, dyslipidemia, insulin resistance, and hypertension. MetS is an established risk factor for cardiovascular disease, stroke and Type 2 diabetes,24-27 and the individual components that comprise MetS, including obesity,28,29 diabetes,30 and hypertension,31,32 are well known to be associated with increased BC risk. However, MetS is increasingly being evaluated in epidemiologic studies as a significant predictor of BC incidence,33-35 distant metastasis,36 TNBC subtype,37 and aggressive tumor biology.38,39 Strikingly, the associations between MetS and BC are consistently larger than associations with individual conditions comprising MetS, suggesting that there are complex biological processes underlying this association. There is recent evidence of rapidly increasing MetS prevalence among Nigerian women, ranging from 12% in rural areas,40 to 35% to 43% in urban areas,41,42 and 65% to 85% among adults with type 2 diabetes.43,44 However, no study to our knowledge has examined the association of MetS with breast cancer or aggressive subtypes in this population.
The purpose of this study is to evaluate the association between MetS and BC risk and molecular subtypes among Nigerian women. This information may help explain increasing BC incidence and pointedly higher prevalence of aggressive TNBC subtypes in this population, leading to enhanced cancer prevention strategies that may be relevant for Black women who share similar BC features.
Materials and Methods
Study Design
We have previously described the methodology for the Mechanisms for Established and Novel Risk Factors for Breast Cancer in Women of African Descent (MEND) study in detail.45,46 MEND recruited patients who were newly diagnosed with BC between 2015 and 2019 at 4 hospitals in southwest Nigeria. Study requirements were explained to BC patients during their clinical visits by a trained nurse, and individuals who expressed interest were assessed for eligibility. Participants were excluded if they were unable to communicate in English to complete the required baseline survey, and/or had other medical conditions that could interfere with their participation in the study. After providing written and verbal informed consent, participants completed a questionnaire to gather information on sociodemographic characteristics, reproductive history, and past personal and family history of cancer. Subsequently, anthropometric measurements were taken, and blood and tumor biopsy samples were collected and stored in −80°C freezers until shipment to the US for additional analysis. Participants were provided with the supplies necessary for their biopsy in addition to an N500 telephone recharge card (valued at US $1.50) for their participation. We obtained data on healthy controls without BC from the Human Heredity and Health (H3) Africa Chronic Kidney Disease (CKD) Case-Control study.47 The H3 Africa study recruited healthy, community-based adult women from Ghana and Nigeria between 2015 and 2017, overlapping with case recruitment. Like BC cases, controls provided extensive socio-demographic, clinical, family history and behavioral risk factor data, and blood samples. Blood samples for cases and controls were assayed in the same laboratory at the same time, and the laboratory technician was blinded to case status. All recruitment and data collection procedures were approved by the Institutional Review Boards at Duke University and the participating hospitals.
Breast Cancer Cases and Subtyping
We confirmed BC diagnosis in 1 of 2 ways: (1) pathology reports of clinical biopsy samples evaluated by a pathologist from the diagnosing hospital in Nigeria, or (2) pathologic review of samples that were shipped to the US. If either report indicated a cancer diagnosis, we considered the sample to be a confirmed BC case. Immunohistochemistry was performed on confirmed cancer samples either in Nigeria as part of regular standard of care procedures, or at the Duke University BioRepository and Precision Pathology Center. If results from both countries were available, we used US typing because most of the available immunohistochemistry information on cases was from the US. Estrogen receptor (ER) and progesterone receptor (PR) status was scored using the Allred method.48,49 The intensity of staining was categorized as 0 (none), 1 (mild), 2 (moderate), or 3 (strong), and the proportion of nuclear positivity was scored into 0 (0%), 1 (< 1%), 2 (1%-10%), 3 (11%-33%), 4 (33%-66%) or 5 (67%-100%). The numbers from these 2 scores were summed to positive (3-8) or negative (0-2). HER2 status was categorized as negative (scores = 0-1) or positive (score = 3).50 There were no equivocal (score = 2) results in our sample. Based on these categorizations, cancer subtype was determined: Luminal A (ER+ and/or PR+ / HER2−), Luminal B (ER+ and/or PR+ / HER2+) , TN (ER− / PR− / HER2−), or HER2 (ER− / PR− / HER2+). In total, 296 cases and 259 controls were included in this analysis, and there were 124 cases with available data on ER/PR/HER2 status for classification into a molecular subtype.
Metabolic Syndrome and Study Covariates
At enrollment, systolic and diastolic blood pressure measurements were taken 3 times and an average value was recorded. Further, waist circumference, height and weight were collected by the trained research staff. Biospecimen for confirmed breast cancer cases with completed surveys were submitted to the Duke Molecular Physiology Institute Immunoassay laboratory for analysis and tested for HDL and triglycerides using a Beckman DxC600 clinical analyzer, and standard reagents from Beckman (Brea, CA). MetS was defined based on the joint harmonized criteria as having any 3 of: high blood pressure (≥ 130/85 mm Hg), reduced HDL (< 50 mg/d), elevated triglycerides (> 150 mg/d), high waist circumference (≥ 80 cm), and prior diagnosis of diabetes or elevated fasting glucose level (≥ 100 mg/dL). Reproductive and clinical characteristics, including age at menarche, number of pregnancies and births, menopausal status, prior diabetes, and hypertension diagnosis were self-reported by participants. Participants who self-reported a history of cancer or were missing personal cancer history were excluded from analysis.
Statistical Analysis
Differences in demographic, clinical and reproductive characteristics were compared between cases and controls as well as by status of MetS (yes vs. no) using Wilcoxon rank sum tests for continuous variables and χ2 (Chi-squared) tests for categorical variables. Univariable and multivariable logistic regression models were used to test the association between MetS and BC diagnosis. Multivariable models were adjusted for (1) age at enrollment only, (2) age at enrollment, age at menarche, number of pregnancies (categorized as < 4 vs. ≥ 4), number of births (categorized as < 4 vs. ≥ 4), menopausal status and prior hypertension and diabetes status and (3) additionally adjusted for BMI. Further, the association between MetS and BC was stratified by categories of BMI (normal weight, overweight or obese) in univariable and multivariable models (BMI not included in multivariable models). Among a subset of BC cases with cancer subtyping data available, multinomial logistic regression models were used to assess the odds of Luminal A, Luminal B, TN, or HER2 cancer subtypes compared to controls. We evaluated the prevalence of MetS and its individual components among TN cancer subtypes and non-TN subtypes. Additionally, we compared the distribution of MetS between Ghanaian and Nigerian controls, and in sensitivity analyses separately evaluated the association of MetS with BC using the 2 sets of controls. There was no statistically significant difference in MetS prevalence between controls recruited in Nigeria and Ghana (P = .245), and results from the overall analysis was similar to those obtained in models evaluating control groups separately (data not shown), therefore we present overall results. All statistical significance tests were 2-sided with P< .05 defined as significant. Statistical analyses were conducted using SAS Version 9.4 software (SAS institute, Cary, NC, USA).
Results
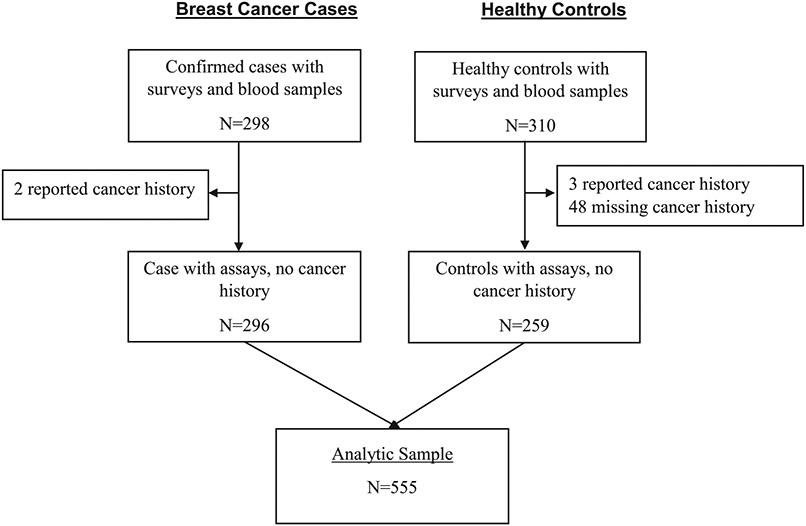
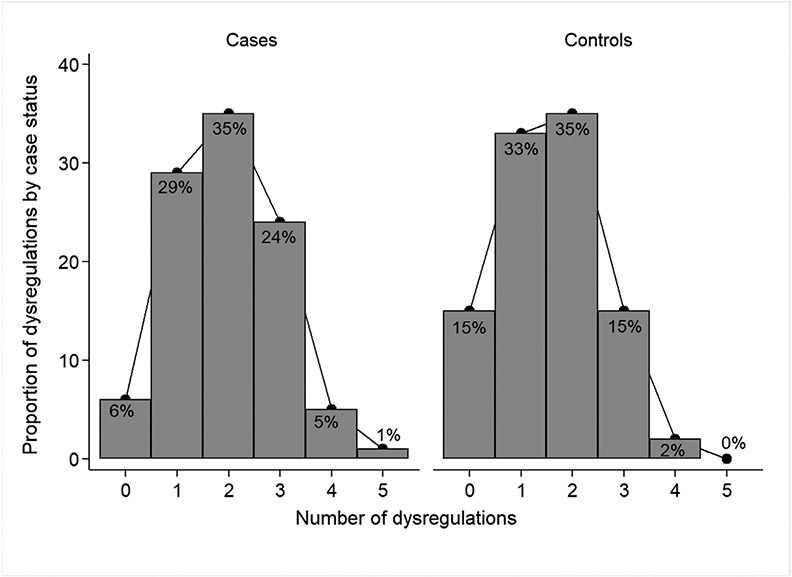
A total of 555 women were included in the study cohort, 296 (53%) were confirmed BC cases, and 259 (47%) were controls (Figure 1). Compared to controls, cases were more likely to have metabolic syndrome (30% vs. 17%, P< .001) (Table 1). They were also less likely to report prior diagnosis of diabetes (1% vs. 15%, P< .001), hypertension (19% vs. 48%, P< .001) and ever having used hormone replacement therapy (0.7% vs. 15% P < .001) compared with controls. No statistically significant differences were found between cases and controls on age at enrollment, age at menarche, number of pregnancies and number of live births (all P value ≥ .507). Compared to women without MetS, women with MetS were older (P < .001) and more likely to be postmenopausal (62% vs. 48%, P = .006) (Table 2). Among cases, 5% had 4 dysregulated MetS components, compared with 2% of controls (Figure 2).
Figure 1.
CONSORT diagram for MetS analysis in MEND.
Table 1.
Study Characteristics for Breast Cancer Cases and Controls.
| Case (N = 296) | Control (N = 259) | Total (N = 555) | P value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (y) a | 48 (23-85) | 49 (18-74) | 49 (18-85) | .632d |
| Clinical characteristics | ||||
| High waist circumference | 220 (75.1%) | 125 (77.2%) | 345 (75.8%) | .621e |
| Elevated triglyceride | 48 (16.2%) | 46 (17.8%) | 94 (16.9%) | .628e |
| Reduced HDL cholesterol | 151 (51.0%) | 122 (47.1%) | 273 (49.2%) | .358e |
| Elevated blood pressure | 80 (27.0%) | 70 (27.5%) | 150 (27.2%) | .911e |
| Elevated fasting glucose/ Diabetes | 77 (26.0%) | 39 (15.1%) | 116 (20.9%) | .002e |
| MetS a | 88 (29.7%) | 44 (17.0%) | 132 (23.8%) | < .001e |
| BMI (Kg/m2) | .086e | |||
| Underweight (< 18.5) | 16 (5.4%) | 6 (2.3%) | 22 (4.0%) | |
| Normal weight (18.5-24.9) | 120 (40.5%) | 93 (35.9%) | 213 (38.4%) | |
| Overweight (25.0-29.9) | 88 (29.7%) | 81 (31.3%) | 167 (30.1%) | |
| Obese (> 29.9) | 65 (22.0%) | 79 (30.5%) | 146 (26.3%) | |
| Height, cm a | 63.1 (56.1-70.1) | 63.0 (51.8-69.5) | 63.0 (51.8-70.1) | .205d |
| Weight, kg a | 143.0 (81.6-255.2) | 149.5 (78.9-289.7) | 145.2 (78.9-289.7) | .019d |
| Systolic BP a | 125.0 (84.0-236.0) | 127.7 (77.7-231.3) | 126.7 (77.7-236.0) | .407d |
| Diastolic BP a | 79.7 (41.0-136.0) | 76.7 (35.3-128.7) | 78.0 (35.3-136.0) | .215d |
| Prior diabetes diagnosis | 3 (1.0%) | 39 (15.1%) | 42 (7.6%) | < .001e |
| Prior hypertension diagnosis | 56 (18.9%) | 125 (48.3%) | 181 (32.6%) | < .001e |
| Reproductive history | ||||
| Age at menarche a | 15.0 (9.0-22.0) | 15.0 (10.0-28.0) | 15.0 (9.0-28.0) | .507d |
| Ever pregnant | 282 (95.3%) | 243 (93.8%) | 525 (94.6%) | .500e |
| Number of pregnancies a,b | 5.0 (1.0-11.0) | 5.0 (1.0-14.0) | 5.0 (1.0-14.0) | .965d |
| Number of births a,b | 4.0 (0.0-10.0) | 4.0 (0.0-16.0) | 4.0 (0.0-16.0) | .523d |
| Menopausal status | .504e | |||
| Pre- or peri-menopause | 143 (48.3%) | 109 (42.1%) | 252 (45.4%) | |
| Postmenopause | 153 (51.7%) | 131 (50.6%) | 284 (51.2%) | |
| Ever used HRT | 2 (0.7%) | 39 (15.1%) | 41 (7.4%) | < .001e |
| Cancer type c | N = 124 | N = 124 | - | |
| Luminal A | 33 (26.6%) | - | 33 (26.6%) | |
| Luminal B | 26 (21.0%) | - | 26 (21.0%) | |
| Triple negative | 37 (29.8%) | - | 37 (29.8%) | |
| HER2 | 28 (22.6%) | - | 28 (22.6%) | |
| Grade c | N = 124 | N = 124 | - | |
| 1 | 2 (1.6%) | - | 2 (1.6%) | |
| 2 | 42 (33.9%) | - | 42 (33.9%) | |
| 3 | 28 (22.5%) | - | 28 (22.5%) | |
| Unknown/Missing | 52 (42.0%) | - | 52 (42.0%) |
MetS defined as any 3 of: blood pressure ≥ 130/85 mm Hg; HDL < 50 mg/d; Triglyceride > 150 mg/d; waist circumference ≥ 80 cm; fasting glucose level ≥ 100 mg/dL or self-reported prior diabetes.
Median (range).
Among those who were ever pregnant.
Among cancer cases with subtype data available.
Wilcoxon rank sum test.
Chi-Square test.
Table 2.
Study Characteristics of Breast Cancer Cases and Controls by MetS Status.
| Metabolic Syndrome | ||||
|---|---|---|---|---|
| Yes (N = 132) | No (N = 423) | Total (N = 555) | P value | |
| Demographics | ||||
| Age (y) a | 51 (23-85) | 48 (18-82) | 49 (18-85) | < .001d |
| Case status | < .001e | |||
| Control | 44 (33.3%) | 215 (50.8%) | 259 (46.7%) | |
| Case | 88 (66.7%) | 208 (49.2%) | 296 (53.3%) | |
| Clinical characteristics | ||||
| BMI (Kg/m2) | .668e | |||
| Underweight (< 18.5) | 1 (0.8%) | 21 (5.0%) | 22 (4.0%) | |
| Normal weight (18.5-24.9) | 48 (36.4%) | 165 (39.0%) | 213 (38.4%) | |
| Overweight (25.0-29.9) | 44 (33.3%) | 123 (29.1%) | 167 (30.1%) | |
| Obese (> 29.9) | 37 (28.0%) | 109 (25.8%) | 146 (26.3%) | |
| Height, cm a | 63.1 (51.8-69.5) | 63.0 (56.1-70.1) | 63.0 (51.8-70.1) | .708d |
| Weight, kg a | 150.6 (91.5-278.1) | 143.3 (78.9-289.7) | 145.2 (78.9-289.7) | .091d |
| Systolic BP a | 140.7 (87.0-236.0) | 124.0 (77.7-231.3) | 126.7 (77.7-236.0) | < .001d |
| Diastolic BP a | 88.2 (54.0-136.0) | 76.0 (35.3-126.3) | 78.0 (35.3-136.0) | < .001d |
| Prior diabetes diagnosis | 16 (12.1%) | 26 (6.1%) | 42 (7.6%) | .066e |
| Prior hypertension diagnosis | 51 (38.6%) | 130 (30.7%) | 181 (32.6%) | .050e |
| Reproductive history | ||||
| Age at menarche a | 15 (9-19) | 15 (10-28) | 15 (9-28) | .410d |
| Ever pregnant | 130 (98.5%) | 395 (93.4%) | 525 (94.6%) | .025e |
| Number of pregnancies a,b | 5 (1-11) | 4 (1-14) | 5 (1-14) | < .001d |
| Number of births a,b | 4 (0-10) | 3 (0-16) | 4 (0-16) | .001d |
| Menopausal status | .006e | |||
| Pre- or peri-menopause | 47 (35.6%) | 205 (48.5%) | 252 (45.4%) | |
| Postmenopause | 82 (62.1%) | 202 (47.8%) | 284 (51.2%) | |
| Ever used HRT | 8 (6.1%) | 33 (7.8%) | 41 (7.4%) | .421e |
| Cancer type c | N = 43 | N = 81 | N = 124 | .373e |
| Luminal A | 10 (23.3%) | 23 (28.4%) | 33 (26.6%) | |
| Luminal B | 7 (16.3%) | 19 (23.5%) | 26 (20.9%) | |
| Triple negative | 17 (39.5%) | 20 (24.6%) | 37 (29.8%) | |
| HER2 | 9 (20.9%) | 19 (23.5%) | 28 (22%) | |
MetS defined as any 3 of: blood pressure ≥ 130/85 mm Hg; HDL <50 mg/d; Triglyceride > 150 mg/d; waist circumference ≥80 cm; fasting glucose level ≥ 100 mg/dL or self-reported prior diabetes.
Where applicable, missing values were not used in generating p-value.
Median (range).
Among those who were ever pregnant.
Among cancer cases with subtype data available.
Wilcoxon rank sum test.
Chi-Square test.
Figure 2.
Distribution of number of MetS components among cases and controls.
In age-adjusted models (Table 3), MetS was associated with 2-fold increased odds of BC (OR: 2.06, 95% CI: 1.36, 3.11). After adjusting for age, socio-demographic, clinical and reproductive risk factors, the association became slightly attenuated but remained statistically significant (aOR: 1.84, 95% CI: 1.07, 3.16). In models additionally adjusted for BMI, the association was largely consistent (aOR: 1.83, 95% CI: 1.06, 3.15). After stratifying by obesity status, MetS was associated with 4-fold increased odds of BC among women with normal weight in the fully adjusted model (aOR: 3.85, 95% CI: 1.25, 11.90). No statistically significant association was found between MetS and BC among overweight and obese women in fully adjusted models. Increasing numbers of dysregulated MetS components were associated with increasing odds of BC in fully adjusted models, ranging from an almost 3-fold increase (aOR: 2.76, 95% CI: 1.24, 6.17) for 2 dysregulated components, to over 5-fold increased odds (aOR: 5.30, 95% CI: 1.24, 22.75) for 4+ dysregulated components.
Table 3.
Multivariable Adjusted Associations Between MetS and Breast Cancer.
| n/N | Model 1a OR (95% CI) | Model 2b aOR (95% CI) | Model 3c aOR (95% CI) | Model 4d aOR (95% CI) | |
|---|---|---|---|---|---|
| MetS (Overall) | |||||
| No | 208/423 | Ref. | Ref. | Ref. | Ref. |
| Yes | 88/132 | 2.07 (1.37-3.11) | 2.06 (1.36-3.11) | 1.84 (1.07-3.16) | 1.83 (1.06-3.15) |
| MetS (Normal Weight) | |||||
| No | 83/165 | Ref. | Ref. | Ref. | |
| Yes | 37/48 | 3.32 (1.59-6.95) | 3.05 (1.42-6.56) | 3.85 (1.25-11.90) | |
| MetS (Overweight) | |||||
| No | 59/123 | Ref. | Ref. | Ref. | |
| Yes | 29/44 | 2.10 (1.02-4.29) | 2.17 (1.05-4.48) | 1.29 (0.48-3.48) | |
| MetS (Obese) | |||||
| No | 45/109 | Ref. | Ref. | Ref. | |
| Yes | 20/37 | 1.67 (0.79-3.54) | 1.70 (0.80-3.61) | 1.63 (0.64-4.15) | |
| # MetS components | |||||
| 0 | 18/58 | Ref. | Ref. | Ref. | Ref. |
| 1 | 85/170 | 2.22 (1.18-4.18) | 2.22 (1.18-4.19) | 1.87 (0.88-3.98) | 2.07 (0.95-4.52) |
| 2 | 105/195 | 2.59 (1.39-4.84) | 2.60 (1.39-4.87) | 2.46 (1.14-5.33) | 2.76 (1.24-6.17) |
| 3 | 72/111 | 4.10 (2.08-8.09) | 4.13 (2.08-8.19) | 3.69 (1.53-8.88) | 4.09 (1.66-10.07) |
| 4+ | 16/21 | 7.11 (2.26-22.41) | 7.15 (2.26-22.63) | 5.09 (1.21-21.35) | 5.30 (1.24-22.75) |
Logistic regression models predicted odds of breast cancer.
Bolded values indicate significance at P < .05.
Abbreviations: aOR = adjusted odds ratio; CI = confidence interval; MetS = Metabolic syndrome; n = number of breast cancer cases within each category; N = Number of women in each category; OR = odds ratio.
Model 1, unadjusted.
Model 2, adjusted for age.
Model 3, additionally adjusted for reproductive and clinical characteristics: age at menarche, number of pregnancies, number of births, menopausal status, and prior diabetes and hypertension status.
Model 4, additionally adjusted for BMI.
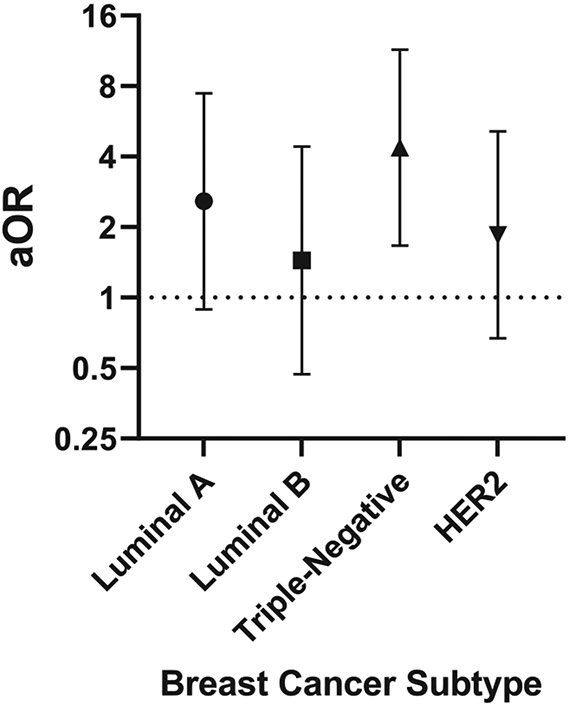
In multivariable multinomial logistic regression models adjusting for reproductive factors, MetS was associated with significantly increased odds of TNBC (aOR: 4.37, 95% CI: 1.67, 11.44), and not statistically significant but higher odds of Luminal A (aOR: 2.58; 95% CI: 0.89, 7.46), Luminal B (aOR: 1.44, 95% CI: 0.47, 4.41) and HER2 (aOR: 1.85; 95% CI: 0.67, 5.13) BC molecular subtypes (Figure 3). Among patients with BC subtypes (Table 4), those with TNBC were more likely to have low HDL cholesterol compared to those with a non-TNBC subtype (76% vs. 53%); however, there was no statistically significant difference in MetS prevalence or in any other individual components of MetS.
Figure 3.
Multivariable adjusted associations between MetS and breast cancer subtype. Logistic regression models predicting odds of breast cancer (MetS yes vs. no) by molecular subtype. Adjusted for reproductive and clinical characteristics: age at menarche, number of pregnancies, number of births, menopausal status and prior diabetes and hypertension status. aOR = adjusted odds ratio.
Table 4.
Distribution of MetS and Components Among TNBC Compared to Non-TNBC Patients.
| TNBC (N = 37) | Non-TNBC (N = 87) | Total (N = 124) | P value | |
|---|---|---|---|---|
| MetS | 17 (46%) | 26 (30%) | 43 (35%) | .101a |
| Waist circumference | 93.4 (12) | 89.5 (13.4) | 90.7 (13.1) | .141b |
| High waist circumference | 31 (86%) | 62 (72%) | 93 (76%) | .109a |
| Triglyceride | 107.8 (65.2) | 98.2 (64.9) | 101.1 (64.8) | .450b |
| Elevated triglyceride | 7 (19%) | 13 (15%) | 20 (16%) | .599a |
| HDL cholesterol | 52.5 (12.4) | 49.5 (17.4) | 47.5 (16.3) | .028b |
| Reduced HDL cholesterol | 28 (76%) | 46 (53%) | 74 (60%) | .027a |
| Elevated blood pressure | 11 (30%) | 25 (28%) | 36 (29%) | 1.000b |
| Elevated fasting glucose/diabetes | 8 (22%) | 21 (24%) | 29 (23%) | .821a |
MetS defined as any 3 of: blood pressure ≥ 130/85 mm Hg; HDL < 50 mg/d; Triglyceride > 150 mg/d; waist circumference ≥ 80 cm; fasting glucose level ≥ 100 mg/dL or self-reported prior diabetes.
Fisher’s exact test.
t test.
Discussion
In this study, we examined the association between MetS and BC among Nigerian women for the first time and evaluated whether this association varies by molecular subtype. Overall, we observed a higher prevalence of MetS among cases compared with controls, and patients with MetS were more likely to be older and obese compared with those without MetS. Further, there was a strong positive association between MetS and BC, a consistent finding among women who were normal weight but not overweight or obese, and after additionally adjusting for BMI. In addition, there was a strong positive association between MetS and TNBC molecular subtype, while associations for other subtypes were positive but not statistically significant.
These robust findings are consistent with findings among other populations. Specifically, a systematic review and meta-analysis of 9 independent cohorts from 5 countries (US, Italy, Switzerland, Uruguay, and Japan) that included over 97,000 females observed that MetS increased BC risk by 47%. Other studies have also reported consistently strong associations between MetS and BC incidence, with odds ratios ranging from 2.5 in Brazil (95% CI: 1.17-5.30),34 to 3.04 in Switzerland (95% CI: 1.75-5.29),35 to 6.28 in Italy (95% CI: 2.79-14.11).33 The consistency of these associations is even more striking given that prior to the publication of the joint harmonized criteria for metabolic syndrome in 2009,51 the definition of MetS varied significantly across studies regarding clinical cut-points and number of components evaluated. Our study provides the first empirical evidence for an association between MetS and BC in Nigerian women.
Notably, we found a strong positive association between MetS and the TNBC subtype among Nigerian women. Only a handful of studies worldwide, and none in Africa, have examined the association of MetS or individual components with BC molecular subtypes, and existing studies are conflicting. 1 study in the US found a higher prevalence of MetS in TNBC patients relative to non-TNBC patients.37 In contrast, another study in the US found a nonsignificant reduced risk of ER− versus ER+ hormone receptor subtype cancers with MetS.52 Additionally, 1 study in a Chinese population found no association between MetS and TNBC susceptibility.53 Our study contributes to the limited literature on this topic by studying Nigerian patients and highlighting a significant role for MetS in development of TNBC. In the same population, we have previously shown that higher C-reactive protein levels, a measure of inflammation, is also associated with increased odds of TNBC.46 Our results lay important groundwork for future studies that may inform BC prevention strategies among women of African descent, a population disproportionately affected by aggressive TNBCs.
There are several possible mechanisms underlying the association between MetS and breast cancer that may explain our findings.54,55 Higher circulating insulin levels lead to mitogenic, antiapoptotic and angiogenic tumor properties, and insulin may act synergistically with estrogen to promote tumor cell proliferation. There is convincing evidence that hyperinsulinemia and hyperglycemia can lead to decreased availability of insulin-like growth factor (IGF)- binding proteins, or inhibition of sex hormone-binding globulin production, leading to higher circulating levels of testosterone, estrogen and IGF-1, which in turn increase mitogenic activity. There is some evidence that Metformin, often prescribed for patients with type 2 diabetes, inhibits the proliferation of TNBC cells in vitro, however much work remains to better understand mechanisms linking insulin and associated pathways with the TNBC molecular subtype. Obesity has also been shown to increase circulating leptin; leptin regulates metabolism and studies suggest that leptin resistance is a biological mechanism in obesity. Higher leptin has been associated with tumorigenesis via cellular proliferation, angiogenesis, and apoptosis, and in particular, increases the activity of the IGF-1 receptor in TNBC cell lines. Although we did not evaluate insulin or leptin in the present analysis, we hope to explore this further in future analyses. Obesity is also associated with higher levels of circulating estrogen and estradiol, in addition to reduced production of anti-inflammatory proteins, and reduced production of adiponectin, which inhibits tumor growth.56
We observed strong associations for MetS among women who were normal weight, suggesting that obesity is not a sufficient biological mechanism underlying this association. In addition, our results provide evidence that MetS and its components are important risk factors for BC in general, and TNBC, in particular, among Nigerian women. Further studies are needed to better understand the biological mechanisms involved, however risk prevention strategies focused on MetS prevention among Nigerian women may provide significant benefits. Public health interventions including diet and physical activity, and clinical interventions including treatment for diabetes and insulin resistance are actionable strategies that can provide immediate benefits for the prevention of MetS and BC. With these strategies, even modest reductions in MetS prevalence can have significant impact at the population level, and risk prediction models incorporating MetS can help with risk stratification and targeted prevention.
There are several strengths and limitations relevant to the interpretation of this study. To our knowledge, this is the first study examining the association between MetS and BC by molecular subtype in Nigerian women. MetS was defined following the joint harmonized criteria for MetS, enhancing comparability across studies, and measures of MetS components were assessed in a standardized format by trained nurses. BC status was ascertained from pathology reports, and molecular subtyping was done following standard guidelines by a trained pathologist. There are also several potential limitations. The case-control study design limits our ability to rule out reverse causality, however our findings were largely consistent with other case-control studies on this topic, and other prospective studies support our finding of significant positive associations of MetS with BC. In addition, our measure of the diabetes MetS component relied on fasting blood glucose measures for cases (given that only 1% of cases had a previous clinical diagnosis of diabetes) and self-reported diabetes among controls. Despite this, we observed that having 3 or 4 out of 5 MetS components altered was associated with strong and significant associations with BC. Other study covariates were based on self-reports at time of enrollment, increasing the risk of recall bias. Nevertheless, these findings provide unique insights into a highly prevalent and increasing risk factor for Nigerian women that is robustly associated with increased risk of BC. Future studies with prospective cohort designs will be needed to address the limitations outlined here, and molecular studies will be needed to understand the biological mechanisms underlying these associations.
In conclusion, MetS was associated with a strong and significant increase in BC risk and TNBC molecular subtype among Nigerian women. Aggressive public health and clinical interventions targeting MetS, such as diet, physical activity, and treatment for diabetes and dyslipidemia, can provide immediate benefits in reducing the burden of MetS and in reducing the future risk of BC including TNBC.
Clinical Practice Points.
Breast cancer (BC) in Nigeria is disproportionately diagnosed in the premenopausal years and characterized by the aggressive triple-negative (TN) molecular subtype.
Metabolic syndrome (MetS) refers to a cluster of conditions that include central obesity, dyslipidemia, insulin resistance, and hypertension, and is an established risk factor for cardiovascular disease, stroke, and diabetes. MetS is increasingly being identified in clinical and epidemiologic studies as a significant predictor of BC incidence, distant metastasis, tumor subtype, and aggressive tumor biology.
The purpose of this study was to evaluate the association between MetS and BC risk by molecular subtype among Nigerian women. To our knowledge, no prior study has evaluated this association despite increasing MetS prevalence in this population. After adjusting for demographic and reproductive characteristics, we found that MetS was associated with a 2-fold increased odds of BC, and a 4-fold increased odds of the aggressive TNBC molecular subtype.
Our findings suggest that aggressive public health and clinical interventions targeting MetS, such as those addressing diet, physical activity, and treatment for diabetes and dyslipidemia, may provide immediate benefits in reducing the burden of MetS and the future risk of aggressive BC in this population.
Acknowledgments
The authors acknowledge the role of the H3Africa Consortium in making this research possible through the sharing of data. The National Institutes of Health (USA) and Wellcome Trust (UK) have provided the core funding for the H3Africa Consortium. The authors thank the many MEND investigators who contributed substantially to the inception and design of the study, and the patients and families who participated in the MEND study for their vital contribution in advancing the science of cancer in Nigeria and globally. The authors acknowledge the important contribution of the MEND research nurses: Cordelia Ibeneme, Peju Olabanji, Rebecca Israel, Esther Akinwale, Deborah Awodeyi, and Shukurat Oduola. This work was supported by grants from the National Institutes of Health, National Cancer Institute, Fogarty International Center: K01TW010271, T.A. and NIH 1P30DK124723-01. The views expressed in this paper do not represent the views of the National Institutes of Health, H3Africa Consortium, or their funders.
Footnotes
Disclosure
The authors declare no potential conflicts of interest.
Ethics Statement
This study was approved by Duke University and the participating hospitals’ Institutional Review Boards (Pro00102004). All participants included provided informed consent.
Data Availability Statement
The data that support the findings of the study are available from the corresponding author upon reasonable request.
References
- 1.Ihekwaba FN. Breast cancer in Nigerian women. Br J Surg. 1992;79:771–775. [DOI] [PubMed] [Google Scholar]
- 2.Jedy-Agba E, Curado MP, Ogunbiyi O, et al. Cancer incidence in Nigeria: a report from population-based cancer registries. Cancer Epidemiol. 2012;36:e271–e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adesunkanmi AR, Lawal OO, Adelusola KA, Durosimi MA. The severity, outcome and challenges of breast cancer in Nigeria. Breast. 2006;15:399–409. [DOI] [PubMed] [Google Scholar]
- 4.Jack RH, Davies EA, Moller H. Breast cancer and age in Black and White women in South East England. Int J Cancer. 2012;130:1227–1229. [DOI] [PubMed] [Google Scholar]
- 5.Brinton LA, Sherman ME, Carreon JD, Anderson WF. Recent trends in breast cancer among younger women in the United States. J Natl Cancer Inst. 2008;100:1643–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joslyn SA, Foote ML, Nasseri K, Coughlin SS, Howe HL. Racial and ethnic disparities in breast cancer rates by age: NAACCR Breast Cancer Project. Breast Cancer Res Treat. 2005;92:97–105. [DOI] [PubMed] [Google Scholar]
- 7.Trentham-Dietz A, Sprague BL, Hampton JM, et al. Modification of breast cancer risk according to age and menopausal status: a combined analysis of five population-based case-control studies. Breast Cancer Res Treat. 2014;145:165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woolcott CG, Koga K, Conroy SM, et al. Mammographic density, parity and age at first birth, and risk of breast cancer: an analysis of four case-control studies. Breast Cancer Res Treat. 2012;132:1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao YT, Shu XO, Dai Q, et al. Association of menstrual and reproductive factors with breast cancer risk: results from the Shanghai Breast Cancer Study. Int J Cancer. 2000;87:295–300. [DOI] [PubMed] [Google Scholar]
- 10.Anyanwu SN. Temporal trends in breast cancer presentation in the third world. J Exp Clin Cancer Res. 2008;27:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adisa CA, Eleweke N, Alfred AA, et al. Biology of breast cancer in Nigerian women: a pilot study. Ann Afr Med. 2012;11:169–175. [DOI] [PubMed] [Google Scholar]
- 12.Gukas ID, Girling AC, Mandong BM, Prime W, Jennings BA, Leinster SJ. A comparison of clinicopathological features and molecular markers in British and Nigerian women with breast cancer. Clin Med Oncol. 2008;2:347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gukas ID, Jennings BA, Mandong BM, et al. Clinicopathological features and molecular markers of breast cancer in Jos, Nigeria. West Afr J Med. 2005;24:209–213. [DOI] [PubMed] [Google Scholar]
- 14.Bowen RL, Duffy SW, Ryan DA, Hart IR, Jones JL. Early onset of breast cancer in a group of British black women. Br J Cancer. 2008;98:277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lannin DR, Mathews HF, Mitchell J, Swanson MS, Swanson FH, Edwards MS. Influence of socioeconomic and cultural factors on racial differences in late-stage presentation of breast cancer. JAMA. 1998;279:1801–1807. [DOI] [PubMed] [Google Scholar]
- 16.Elledge RM, Clark GM, Chamness GC, Osborne CK. Tumor biologic factors and breast cancer prognosis among white, Hispanic, and black women in the United States. J Natl Cancer Inst. 1994;86:705–712. [DOI] [PubMed] [Google Scholar]
- 17.Ihemelandu CU, Leffall LD Jr, Dewitty RL, et al. Molecular breast cancer subtypes in premenopausal and postmenopausal African-American women: age-specific prevalence and survival. J Surg Res. 2007;143:109–118. [DOI] [PubMed] [Google Scholar]
- 18.Agboola AJ, Musa AA, Wanangwa N, et al. Molecular characteristics and prognostic features of breast cancer in Nigerian compared with UK women. Breast Cancer Res Treat. 2012;135:555–569. [DOI] [PubMed] [Google Scholar]
- 19.Fackenthal JD, Zhang J, Zhang B, et al. High prevalence of BRCA1 and BRCA2 mutations in unselected Nigerian breast cancer patients. Int J Cancer. 2012;131:1114–1123. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Fackenthal JD, Zheng Y, et al. Recurrent BRCA1 and BRCA2 mutations in breast cancer patients of African ancestry. Breast Cancer Res Treat. 2012;134:889–894. [DOI] [PubMed] [Google Scholar]
- 21.Agboola AO, Banjo AA, Anunobi CC, et al. Molecular profiling of breast cancer in Nigerian women identifies an altered p53 pathway as a major mechanism underlying its poor prognosis compared with British counterpart. Malays J Pathol. 2014;36:3–17. [PubMed] [Google Scholar]
- 22.Kumar P, Aggarwal R. An overview of triple-negative breast cancer. Arch Gynecol Obstet. 2016;293:247–269. [DOI] [PubMed] [Google Scholar]
- 23.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. [DOI] [PubMed] [Google Scholar]
- 24.Nilsson PM, Engstrom G, Hedblad B. The metabolic syndrome and incidence of cardiovascular disease in non-diabetic subjects–a population-based study comparing three different definitions. Diabet Med. 2007;24:464–472. [DOI] [PubMed] [Google Scholar]
- 25.Athyros VG, Ganotakis ES, Elisaf MS, et al. Prevalence of vascular disease in metabolic syndrome using three proposed definitions. Int J Cardiol. 2007;117:204–210. [DOI] [PubMed] [Google Scholar]
- 26.Koren-Morag N, Goldbourt U, Tanne D. Relation between the metabolic syndrome and ischemic stroke or transient ischemic attack: a prospective cohort study in patients with atherosclerotic cardiovascular disease. Stroke. 2005;36:1366–1371. [DOI] [PubMed] [Google Scholar]
- 27.Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. 2008;28:629–636. [DOI] [PubMed] [Google Scholar]
- 28.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. [DOI] [PubMed] [Google Scholar]
- 29.Ogundiran TO, Huo D, Adenipekun A, et al. Body fat distribution and breast cancer risk: findings from the Nigerian breast cancer study. Cancer Causes Control. 2012;23:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicolucci A. Epidemiological aspects of neoplasms in diabetes. Acta Diabetol. 2010;47:87–95. [DOI] [PubMed] [Google Scholar]
- 31.Pereira A, Garmendia ML, Alvarado ME, Albala C. Hypertension and the risk of breast cancer in Chilean women: a case-control study. Asian Pac J Cancer Prev. 2012;13:5829–5834. [DOI] [PubMed] [Google Scholar]
- 32.Soler M, Chatenoud L, Negri E, Parazzini F, Franceschi S, la Vecchia C. Hypertension and hormone-related neoplasms in women. Hypertension. 1999;34:320–325. [DOI] [PubMed] [Google Scholar]
- 33.Capasso I, Esposito E, de Laurentiis M, et al. Metabolic syndrome-breast cancer link varies by intrinsic molecular subtype. Diabetol Metab Syndr. 2014;6:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porto LA, Lora KJ, Soares JC, Costa LO. Metabolic syndrome is an independent risk factor for breast cancer. Arch Gynecol Obstet. 2011;284:1271–1276. [DOI] [PubMed] [Google Scholar]
- 35.Rosato V, Bosetti C, Talamini R, et al. Metabolic syndrome and the risk of breast cancer in postmenopausal women. Ann Oncol. 2011;22:2687–2692. [DOI] [PubMed] [Google Scholar]
- 36.Berrino F, Villarini A, Traina A, et al. Metabolic syndrome and breast cancer prognosis. Breast Cancer Res Treat. 2014;147:159–165. [DOI] [PubMed] [Google Scholar]
- 37.Maiti B, Kundranda MN, Spiro TP, Daw HA. The association of metabolic syndrome with triple-negative breast cancer. Breast Cancer Res Treat. 2010;121:479–483. [DOI] [PubMed] [Google Scholar]
- 38.Calip GS, Malone KE, Gralow JR, Stergachis A, Hubbard RA, Boudreau DM. Metabolic syndrome and outcomes following early-stage breast cancer. Breast Cancer Res Treat. 2014;148:363–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Healy LA, Ryan AM, Carroll P, et al. Metabolic syndrome, central obesity and insulin resistance are associated with adverse pathological features in postmenopausal breast cancer. Clin Oncol. 2010;22:281–288. [DOI] [PubMed] [Google Scholar]
- 40.Adegoke OA, Adedoyin RA, Balogun MO, Adebayo RA, Bisiriyu LA, Salawu AA. Prevalence of metabolic syndrome in a rural community in Nigeria. Metab Syndr Relat Disord.. 2010;8:59–62. [DOI] [PubMed] [Google Scholar]
- 41.Adedoyin RA, Afolabi A, Adegoke OO, Akintomide AO, Awotidebe TO. Relationship between socioeconomic status and metabolic syndrome among Nigerian adults. Diabet Metab Syndr. 2013;7:91–94. [DOI] [PubMed] [Google Scholar]
- 42.Siminialayi IM, Emem-Chioma PC, Odia OJ. Prevalence of metabolic syndrome in urban and suburban Rivers State, Nigeria: international diabetes federation and adult treatment panel III definitions. Niger Postgrad Med J. 2010;17:147–153. [PubMed] [Google Scholar]
- 43.Onesi SO, Ignatius UE. Metabolic syndrome: Performance of five different diagnostic criterias. Indian J Endocrinol Metabol. 2014;18:496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Udenze IC, Azinge EC, Arikawe AP, et al. The prevalence of metabolic syndrome in persons with type 2 diabetes at the Lagos University Tteaching Hospital, Lagos, Nigeria. West Afr J Med. 2013;32:126–132. [PubMed] [Google Scholar]
- 45.Akinyemiju T, Salako O, Daramola A, et al. Collaborative molecular epidemiology study of metabolic dysregulation, DNA methylation, and breast cancer risk among Nigerian women: MEND study objectives and design. J Global Oncol. 2019;5:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gupta A, Oyekunle T, Salako O, et al. Association of high-sensitivity C-reactive protein and odds of breast cancer by molecular subtype: analysis of the MEND study. 2021;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Osafo C, Raji YR, Burke D, et al. Human Heredity and Health (H3) in Africa kidney disease research network: a focus on methods in Sub-Saharan Africa. Clin J Am Soc Nephrol. 2015;10:2279–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mukherjee T. Interpretation of ER and Her2neu hormonal receptor in breast cancer. Med J Armed Forces India. 2016;72:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med. 2010;134:e48–e72. [DOI] [PubMed] [Google Scholar]
- 50.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch Pathol Lab Med. 2007;131:18–43. [DOI] [PubMed] [Google Scholar]
- 51.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. [DOI] [PubMed] [Google Scholar]
- 52.Dibaba DT, Braithwaite D, Akinyemiju T. Metabolic syndrome and the risk of breast cancer and subtypes by race, menopause and BMI. Cancers. 2018;10(9):299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fan Y, Ding X, Wang J, et al. Decreased serum HDL at initial diagnosis correlates with worse outcomes for triple-negative breast cancer but not non-TNBCs. Int J Biol Markers. 2015;30:e200–e207. [DOI] [PubMed] [Google Scholar]
- 54.Davis AA, Kaklamani VG. Metabolic syndrome and triple-negative breast cancer: a new paradigm. Int J Breast Cancer. 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhandari R, Kelley GA, Hartley TA, Rockett IR. Metabolic syndrome is associated with increased breast cancer risk: a systematic review with meta-analysis. Int J Breast Cancer. 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hauner D, Hauner H. Metabolic syndrome and breast cancer: is there a link? Breast Care. 2014;9:277–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of the study are available from the corresponding author upon reasonable request.