Abstract
IMPORTANCE
Cerebral palsy describes the most common physical disability in childhood and occurs in 1 in 500 live births. Historically, the diagnosis has been made between age 12 and 24 months but now can be made before 6 months’ corrected age.
OBJECTIVES
To systematically review best available evidence for early, accurate diagnosis of cerebral palsy and to summarize best available evidence about cerebral palsy-specific early intervention that should follow early diagnosis to optimize neuroplasticity and function.
EVIDENCE REVIEW
This study systematically searched the literature about early diagnosis of cerebral palsy in MEDLINE (1956–2016), EMBASE (1980–2016), CINAHL (1983–2016), and the Cochrane Library (1988–2016) and by hand searching. Search terms included cerebral palsy, diagnosis, detection, prediction, identification, predictive validity, accuracy, sensitivity, and specificity. The study included systematic reviews with or without meta-analyses, criteria of diagnostic accuracy, and evidence-based clinical guidelines. Findings are reported according to the PRISMA statement, and recommendations are reported according to the Appraisal of Guidelines, Research and Evaluation (AGREE) II instrument.
FINDINGS
Six systematic reviews and 2 evidence-based clinical guidelines met inclusion criteria. All included articles had high methodological Quality Assessment of Diagnostic Accuracy Studies (QUADAS) ratings. In infants, clinical signs and symptoms of cerebral palsy emerge and evolve before age 2 years; therefore, a combination of standardized tools should be used to predict risk in conjunction with clinical history. Before 5 months’ corrected age, the most predictive tools for detecting risk are term-age magnetic resonance imaging (86%−89% sensitivity), the Prechtl Qualitative Assessment of General Movements (98% sensitivity), and the Hammersmith Infant Neurological Examination (90% sensitivity). After 5 months’ corrected age, the most predictive tools for detecting risk are magnetic resonance imaging (86%−89% sensitivity) (where safe and feasible), the Hammersmith Infant Neurological Examination (90% sensitivity), and the Developmental Assessment of Young Children (83% C index). Topography and severity of cerebral palsy are more difficult to ascertain in infancy, and magnetic resonance imaging and the Hammersmith Infant Neurological Examination may be helpful in assisting clinical decisions. In high-income countries, 2 in 3 individuals with cerebral palsy will walk, 3 in 4 will talk, and 1 in 2 will have normal intelligence.
CONCLUSIONS AND RELEVANCE
Early diagnosis begins with a medical history and involves using neuroimaging, standardized neurological, and standardized motor assessments that indicate congruent abnormal findings indicative of cerebral palsy. Clinicians should understand the importance of prompt referral to diagnostic-specific early intervention to optimize infant motor and cognitive plasticity, prevent secondary complications, and enhance caregiver well-being.
According to a 2007 report, “Cerebral palsy is a group of permanent disorders of the development of movement and posture, causing activity limitation, that are attributed to non-progressive disturbances that occurred in the developing fetal or infant brain.”1(p9) Cerebral palsy is a clinical diagnosis based on a combination of clinical and neurological signs. Diagnosis typically occurs between age 12 and 24 months.2–4 The following 4 motor types exist but may emerge and change during the first 2 years of life: (1) spasticity (85%−91%); (2) dyskinesia (4%−7%), including dystonia and athetosis; (3) ataxia (4%−6%); and (4) hypotonia (2%), which is not classified in all countries.2 Dyskinesia, ataxia, and hypotonia usually affect all 4 limbs, whereas spasticity is categorized topographically as (1) unilateral (hemiplegia) (38%) and (2) bilateral, including diplegia (lower limbs affected more than upper limbs) (37%) and quadriplegia (all 4 limbs and trunk affected) (24%).2 Comorbidities and functional limitations are common and disabling, including chronic pain (75%), epilepsy (35%), intellectual disability (49%), musculoskeletal problems (eg, hip displacement) (28%), behavioral disorders (26%), sleep disorders (23%), functional blindness (11%), and hearing impairment (4%).5
Cerebral palsy is the most common physical disability in childhood, with a prevalence of 2.1 cases per 1000 in high-income countries.6 The prevalence is declining in Australia and Europe.7,8 Exact rates in countries of low to middle income are less certain9 but appear to be higher, with worse physical disability, because of greater infectious disease burden and prenatal and perinatal care differences.10 The complete causal path to cerebral palsy is unclear in approximately 80% of cases, but risk factors are often identifiable from history taking about conception, pregnancy, birth, and the postneonatal period.11 The full causal path is a complex interplay between several risk factors across multiple epochs,11 including new evidence suggesting that 14% of cases have a genetic component.12–14 Early diagnosis does not preclude further specific etiological investigation, and identifying a specific etiology does not then preclude individuals from also having cerebral palsy. Genetic advances are likely to soon amend the diagnostic process.
Our primary objective was to systematically review best available evidence for early, accurate diagnosis of cerebral palsy. Our secondary objective was to summarize best available evidence about cerebral palsy-specific early intervention that should follow early diagnosis to optimize neuroplasticity and function.
Methods
We conducted a systematic review to develop an international clinical practice guideline in accord with the World Health Organization’s Handbook for Guideline Development15 and the Institute of Medicine’s standards.16 We followed the Equator Network reporting recommendations outlined in the Appraisal of Guidelines, Research and Evaluation (AGREE) II instrument17 and the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement.18 We systematically searched MEDLINE (1956–2016), EMBASE (1980–2016), CINAHL (1983–2016), and the Cochrane Library (1988–2016) and hand searched using the following terms: cerebral palsy, diagnosis, detection, prediction, identification, predictive validity, accuracy, sensitivity, and specificity. We included systematic reviews with or without meta-analyses, criteria of diagnostic accuracy, and evidence-based clinical guidelines. Quality was appraised using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) methodological rating checklist for systematic reviews of diagnostic accuracy.19
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework was used to assess quality and formulate recommendations along a 4-part continuum, including strong for, conditional for, conditional against, and strong against.20 As per the GRADE method, we weighed (1) the balance between desirable and undesirable consequences of different management strategies or not acting; (2) family preferences, including benefits vs risks and inconvenience; and (3) cost. Recommendations were discussed face-to-face among all authors, and the manuscript was reviewed, edited, and agreed on by all coauthors. Authors were clinicians involved in the diagnosis of cerebral palsy, including neurologists, pediatricians, neonatologists, rehabilitation specialists, general practitioners, neuroradiologists, psychiatrists, physical therapists, psychologists, occupational therapists, speech pathologists, nurses, and early educators. Individuals with cerebral palsy and parents also contributed as equal authors, ensuring that recommendations addressed their views and preferences.
Results
Six systematic reviews21–26 and 2 evidence-based clinical guidelines27,28 met inclusion criteria. The methodological quality of the evidence was very high (eTable in the Supplement), enabling strong GRADE recommendations.20 Many standardized tools exist that predict risk of cerebral palsy early. Best available evidence was summarized (eTable in the Supplement), and a PRISMA diagram summarized study flow (eFigure in the Supplement).
Advances in Diagnosis: Early Clinical Diagnosis Is Now Possible
Before age 12 to 24 months was historically regarded as the latent or silent period where cerebral palsy could not be identified accurately. Experts now consider the silent period as outdated because cerebral palsy or “high risk of cerebral palsy” can be accurately predicted before age 6 months’ corrected age.
The 3 tools with best predictive validity for detecting cerebral palsy before 5 months’ corrected age are (1) neonatal magnetic resonance imaging (MRI) (86%−89% sensitivity),21,27 (2) the Prechtl Qualitative Assessment of General Movements (GMs) (98% sensitivity),21 and (3) the Hammersmith Infant Neurological Examination (HINE) (90% sensitivity)25 (eTable in the Supplement). After 5 months’ corrected age, the most predictive tools for detecting risk are MRI (86%−89% sensitivity) (where safe and feasible), the HINE (90% sensitivity), and the Developmental Assessment of Young Children (83% C index). High-quality evidence also indicates that a trajectory of abnormal GMs or HINE scores, in combination with abnormal MRI, producing congruent findings, is even more accurate than individual clinical assessments in isolation.21,25
To make an early clinical diagnosis before 6 months’ corrected age, a combination of assessments with strong predictive validity coupled with clinical reasoning is recommended. We have made 12 recommendations from best available evidence (Table 1). A highly experienced clinical team should ideally conduct and interpret the standardized assessments and then communicate the news compassionately.
Table 1.
Early Detection and Diagnosis Recommendations From Best Available Evidence
| Recommendations | Strength of Recommendations and Quality of Evidence |
|---|---|
| 1.0 The clinical diagnosis of CP can and should be made as early as possible so that: • The infant can receive diagnostic-specific early intervention and surveillance to optimize neuroplasticity and prevent complications • The parents can receive psychological and financial support (when available) |
Strong recommendation based on moderate-quality evidence for infant and parent outcomes |
| 1.1 When the clinical diagnosis is suspected but cannot be made with certainty, the interim clinical diagnosis of high risk of CP should be given so that: • The infant can receive diagnostic-specific early intervention and surveillance to optimize neuroplasticity and prevent complications • The parents can receive psychological and financial support (when available) • Ongoing diagnostic monitoring can be provided until a diagnosis is reached |
Strong recommendation based on moderate-quality evidence for infant and parent outcomes |
| 2.0 Early standardized assessments and investigations for early detection of CP should always be conducted in populations with newborn-detectable risks (ie, infants born preterm, infants with neonatal encephalopathy, infants with birth defects, and infants admitted to the NICU) | Strong recommendation based on high-quality evidence of test psychometrics |
| Early Detection of CP Before 5 mo CA | |
| 3.0 Option A: The most accurate method for early detection of CP in infants with newborn-detectable risks and younger than 5 mo (CA) is to use a combination of a standardized motor assessment and neuroimaging and history taking about risk factors | Strong recommendation based on high-quality evidence of test psychometrics in newborn-detectable risk populations |
| Standardized motor assessment 3.1 Test: GMs to identify motor dysfunction (95%−98% predictive of CP), combined with neuroimaging |
Strong recommendation based on high-quality evidence of test psychometrics in newborn-detectable risk populations |
| Neuroimaging 3.2 Test: MRI (before sedation is required for neuroimaging) to detect abnormal neuroanatomy in the motor areas of the brain (80%−90% predictive of CP). Note that normal neuroimaging does not automatically preclude the diagnosis of risk of CP |
Strong recommendation based on high-quality evidence of test psychometrics in newborn-detectable risk populations |
| 4.0 Option B: In contexts where the GMs assessment is not available or MRI is not safe or affordable (eg, in countries of low to middle income), early detection of CP in infants with newborn-detectable risks and younger than 5 mo (CA) is still possible and should be carried out to enable access to early intervention | Strong recommendation based on moderate-quality evidence of test psychometrics in newborn-detectable risk populations |
| Standardized neurological assessment 4.1 Test: HINE (scores <57 at 3 mo are 96% predictive of CP) |
Strong recommendation based on moderate-quality evidence of test psychometrics in newborn-detectable risk populations |
| Standardized motor assessment 4.2 Test: TIMP |
Conditional recommendation based on low-quality evidence of test psychometrics in at-risk populations |
| Early Detection of CP After 5 mo CA | |
| Accurate early detection of CP in those with infant-discernible risks and age 5–24 mo can and should still occur as soon as possible, but different diagnostic tools are required | |
| 5.0 Any infant with: (a) Inability to sit independently by age 9 mo, or (b) Hand function asymmetry, or (c) Inability to take weight through the plantar surface (heel and forefoot) of the feet should receive standardized investigations for CP |
Strong recommendation based on high-quality evidence of motor norms |
| 6.0 Option A: The most accurate method for early detection of CP in those with infant detectable risks older than 5 mo (corrected for prematurity) but younger than 2yisto use a combination of a standardized neurological assessment, neuroimaging, and a standardized motor assessment with a history taking about risk factors | Conditional recommendation based on moderate-quality evidence of test psychometrics in newborn-detectable risk populations |
| Standardized neurological assessment 6.1 Test: HINE (90% predictive of CP). Those with HINE scores <73 (at 6, 9, or 12 mo) should be considered at high risk of CP. HINE scores <40 (at 6, 9, or 12 mo) almost always indicate CP, combined with neuroimaging and standardized motor assessments |
Conditional recommendation based on moderate-quality evidence of test psychometrics in newborn-detectable risk populations |
| Neuroimaging 6.2 Test: MRI to detect abnormal neuroanatomy in the motor areas of the brain (sedation may be required from >6 wk up to age 2 y). Well-defined lesions can be seen early, but subtle white matter lesions maybe difficult to detect owing to rapid growth, myelination, and activity-dependent plasticity. Repeated MRI scans are recommended at age 2 y for infants with initially normal findings on MRI (at 12–18 mo) but persistent motor or neurological abnormality, combined with standardized motor assessments |
Conditional recommendation based on moderate-quality evidence of test psychometrics in newborn-detectable risk populations |
| Standardized motor assessment 6.3 Test: DAYC for parents to self-report and quantify motor delay (89% predictive of CP) Additional assessments can improve triangulation of findings 6.4 Tests: AIMS (86% predictive of an abnormal motor outcome) and NSMDA (82% predictive of an abnormal motor outcome) |
Conditional recommendation based on low- to moderate-quality evidence of test psychometrics in newborn-detectable risk populations |
| 7.0 Option B: In contexts where MRI is not safe or affordable, early detection of CP is still possible in those with infant detectable risks between 5 and 24 mo CA and should be carried out to enable access to early intervention | Strong recommendation based on moderate-quality evidence of test psychometrics in newborn-detectable risk populations |
| Standardized neurological assessment 7.1 Test: HINE (90% predictive of CP at age 2–24 mo) HINE scores at 6, 9, or 12 mo: <73 Indicates high risk of CP <40 Indicates abnormal outcome, usually CP |
Strong recommendation based on moderate-quality evidence of test psychometrics in newborn-detectable risk populations |
| Standardized motor assessment 7.2 Test: DAYC to quantify motor delay (89% predictive of CP) 7.3 Test: MAI to quantify motor delay (73% predictive of CP) |
Conditional recommendation based on low- to moderate-quality evidence of test psychometrics in newborn-detectable risk populations |
| Early Detection of Motor Severity of CP | |
| Prognosis of long-term motor severity is most accurate in children older than 2 y using the GMFCS | |
| 8.0 In infants younger than 2 y, prognosis of motor severity predictions should be made cautiously and always involve the use of standardized tools because incomplete development of voluntary motor skills or abnormal tone might confound clinical observations. Motor severity of CP in those younger than 2 yis most accurately predicted using the following: | Conditional recommendation based on low-quality evidence |
| Standardized neurological assessment 8.1 Test: HINE. Cutoff scores predict the probable severity HINE scores at 3, 6, 9, or 12 mo: • 50–73 Indicates likely unilateral CP (ie, 95%−99% will walk) • <50 Indicates likely bilateral CP HINE scores at 3–6 mo: • 40–60 Indicates likely GMFCS I-II • <40 Indicates likely GMFCS III-V |
Conditional recommendation based on moderate-quality evidence in newborn-detectable risk populations |
| Neuroimaging 8.2 Test: MRI Nonambulant CP is more likely after: • Bilateral parenchymal hemorrhages (grade IV) • Bilateral cystic periventricular leukomalacia (grade III) • Brain maldevelopment • Basal ganglia injury Ambulant CP is more likely after: • Unilateral lesions (grade IV hemorrhage or perinatal arterial ischemic stroke) • Periventricular leukomalacia (noncystic) • Moderate to severe white matter injury Normal imaging does not preclude CP, and abnormal findings on MRI imaging does not automatically precede CP |
Conditional recommendation based on moderate-quality evidence in newborn-detectable risk populations |
| Early Detection of Motor Subtype and Topography of CP | |
| 9.0 Early detection of motor subtype and topography can be difficult in those younger than 2 y, but wherever possible it is important to identify unilateral vs bilateral CP early because the early interventions (eg, constraint-induced movement therapy) and long-term musculoskeletal outcomes and surveillance needs differ (eg, hip surveillance) | Conditional recommendation based on low- to high-quality evidence |
| Early Intervention | |
| 10.0 The clinical diagnosis of CP or the interim diagnosis of high risk of CP should always be followed by a referral to CP-specific early intervention (eg, constraint-induced movement therapy and hip surveillance). Parent concern is a valid reason to trigger formal diagnostic investigations and referral to early intervention | Strong recommendation based on low- to high-quality evidence |
| Early Detection of Associated Impairments | |
| 11.0 The clinical diagnosis of CP or the interim diagnosis of high risk of CP should always include standard medical investigations for associated impairments and functional limitations (eg, vision impairment, hearing impairment, and epilepsy) | Strong recommendation based on high-quality population register evidence of rates of associated impairments |
| Communicating the Diagnosis Well to Parents | |
| 12.0 Parents experience grief and loss at the time of diagnosis or high-risk notification; therefore, communication with a family should be a series of well-planned and compassionate conversations. Communication should be face-to-face, with both parents or caregivers present (where appropriate), private, honest, jargon free, and with empathic communication tailored to the family, followed by written information, identification of strengths, invitation to ask questions, discussion of feelings, recommendations to use parent-to-parent support, and arrangement of early intervention |
Strong recommendation based on high-quality qualitative parent interviews |
Abbreviations: AIMS, Alberta Infant Motor Scale; CA, corrected age; CP, cerebral palsy; DAYC, Developmental Assessment of Young Children; GMFCS, Gross Motor Function Classification System; GMs, Prechtl Qualitative Assessment of General Movements; HINE, Hammersmith Infant Neurological Examination; MAI, Motor Assessment of Infants; MRI, magnetic resonance imaging; NICU, neonatal intensive care unit; NSMDA, Neuro Sensory Motor Development Assessment; TIMP, Test of Infant Motor Performance.
Interim High Risk of Cerebral Palsy Clinical Diagnosis
When the clinical diagnosis is suspected but cannot be made with certainty, we recommend using the interim clinical diagnosis of high risk of cerebral palsy until a diagnosis is confirmed. We recommend specifying cerebral palsy because infants with cerebral palsy require and benefit from different early interventions than infants “at risk of developmental delay,” “at risk of autism,” “at risk of harm,” or with “social risk.” When the infant is perceived to be at risk of cerebral palsy, he or she should be referred for cerebral palsy-specific early intervention (see the Advances in Treatment section), with regular medical, neurological, and developmental monitoring from the infant’s pediatrician or neurologist to assist with forming a diagnostic picture. To assign the interim clinical diagnosis of high risk of cerebral palsy, the infant must have motor dysfunction (essential criterion) and at least one of the other 2 additional criteria.
Essential Criterion (Required)
Motor Dysfunction
In motor dysfunction, the infant’s quality of movement is reduced (eg, absent fidgety GMs)29 or neurologically abnormal (eg, early observable hand asymmetry or suboptimal HINE scores).30 In addition, the infant’s motor activities may be substantially below those expected for chronological age (eg, abnormal score on a standardized motor assessment or parent and caregiver or clinical observations of head lag, not sitting, inability to grasp, or not reaching for a toy when appropriate).
As a caveat, in milder presentations, especially unilateral cerebral palsy, it is possible for an infant to score within the normal range on a standardized motor assessment, while still displaying abnormal movements. For example, an infant with hemiplegia might obtain a normal fine-motor score but complete the assessment one-handed. Similarly, an infant with diplegia may achieve normal upper limb scores and abnormal lower limb scores, producing a combined total motor score within the normal range Therefore it is essential that assessments be carried out by a professional skilled at determining atypical movement from variation in typical movement.
Additional Criteria (at Least One Required)
Abnormal Neuroimaging
Abnormal MRI21,27 with or without serial cranial ultrasound in preterm infants21,28 may identify neuroanatomical abnormalities predictive of cerebral palsy. The most predictive patterns are (1) white matter injury (cystic periventricular leukomalacia or periventricular hemorrhagic infarctions) (56%), (2) cortical and deep gray matter lesions (basal ganglia or thalamus lesions, watershed injury [para-sagittal injury], multicystic encephalomalacia, or stroke) (18%), and (3) brain maldevelopments (lissencephaly, pachygyria, cortical dysplasia, polymicrogyria, or schizencephaly) (9%).
Clinical History Indicating Risk for Cerebral Palsy
Preconception risks include history of stillbirths, miscarriages, low socioeconomic status, assisted reproduction, and abnormal genetic copy number variations.
Pregnancy risks include genetics, birth defects, multiples, males, maternal thyroid disease or preeclampsia, infection, intrauterine growth restriction, prematurity, and substance abuse.
Perinatal birth risks include acute intrapartum hypoxia-ischemia, seizures, hypoglycemia, jaundice, and infection.
Postneonatal risks include stroke, infection, surgical complications, and accidental and nonaccidental brain injury31 occurring before age 24 months, as per the Surveillance of Cerebral Palsy Europe and Australian Cerebral Palsy Register inclusion criteria.
Two Early Detection Pathways Based on Different Risks
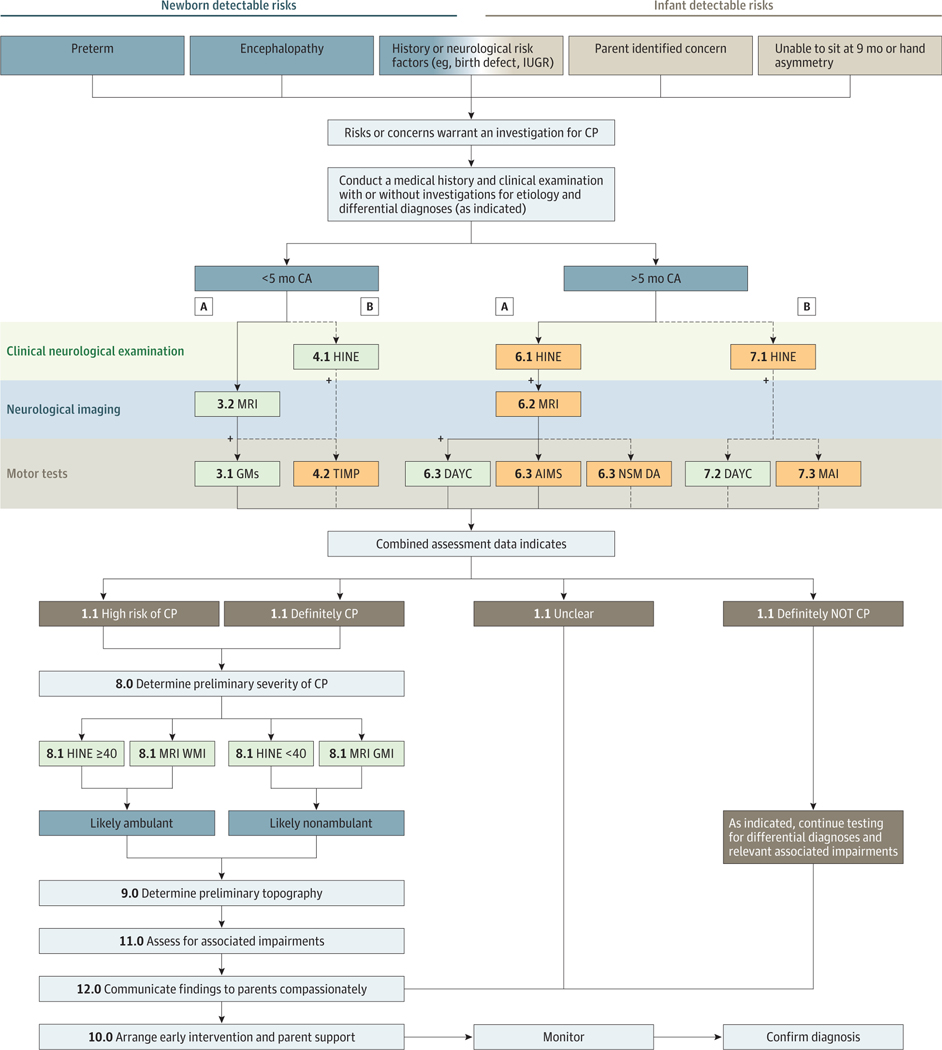
Half of all infants with cerebral palsy have high-risk indicators identifiable in the newborn period, enabling early screening31 (eg, prematurity, atypical intrauterine growth, encephalopathy, genetic abnormalities, and seizures). We have described this population as having “newborn-detectable risks for cerebral palsy,” and this pathway occurs before 5 months’ corrected age. For the other half of all infants with cerebral palsy, the pregnancy and labor may have appeared to be uneventful,31 and parents, caregivers, or community-based professionals first notice delayed motor milestones (eg, not sitting at 9 months or hand asymmetry). This finding may be especially true for infants with unilateral cerebral palsy, who often master early rudimentary motor skills, such as smiling, swallowing, and head control, and it is not until they attempt more complex motor skills, such as grasp, that asymmetries become observable. We have described this population as having “infant detectable risks for cerebral palsy,” and this pathway occurs after 5 months’ corrected age. We developed a conceptual framework for early diagnosis based on these 2 pathways to ensure that the most sensitive and specific tools are used to reduce false-positive and false-negative results. The clinical diagnostic pathway algorithm for these 2 groups varies because the tools have different psychometric properties depending on the infant’s age (Figure).
Figure. Algorithm for Early Diagnosis of Cerebral Palsy or High Risk of Cerebral Palsy.
A indicates the best available evidence pathway. B indicates the next best available evidence pathway when some pathway A tools are not available. The numerals correspond to the numbering in Table 1. AIMS indicates Alberta Infant Motor Scale; CA, corrected age; CP, cerebral palsy; DAYC, Developmental Assessment of Young Children; GMs, Prechtl Qualitative Assessment of General Movements; HINE, Hammersmith Infant Neurological Examination; IUGR, interuterine growth restriction; MAI, Motor Assessment of Infants; MRI, magnetic resonance imaging; NSMDA, Neuro Sensory Motor Development Assessment; TIMP, Test of Infant Motor Performance; and WMI, white matter injury.
Determining Severity
Parents or caregivers will want to learn about the severity of their infant’s physical disability to understand his or her capabilities to plan their future. In infants younger than 2 years, motor severity is difficult to accurately predict for the following reasons: (1) almost half of all infants younger than 2 years have their Gross Motor Function Classification System (GMFCS) reclassified, (2) little natural history data exist about infants with cerebral palsy (eg, the onset of spasticity, dyskinesia, or contractures), (3) motor skills are developing (4) the presence or absence of hypertonia changes and evolves, and (5) there is rapid brain growth and use-dependent reorganization in response to caregiving and therapy. In children 2 years or older severity is reliably classified using the 5-level GMFCS Extended & Revised.32 In infants younger than 2 years, prediction of motor se verity should be made cautiously using standardized tools, including the cutoff scores on the HINE, combined with neuroimaging data.25 Parents or caregivers may mistakenly assume that the diagnosis means their child will need a wheelchair and have an intellectual disability. However, in high-income countries, population data indicate that 2 in 3 individuals with cerebral palsy will walk, 3 in 4 will talk, and 1 in 2 will have normal intelligence.5
Determining Motor Type and Topography
The motor types and topography of cerebral palsy may emerge and change during the first 2 years of life. Cerebral palsy can be difficult to accurately classify early, but clinical signs exist33–37 (Table 2). For example, the onset of spasticity may occur after age 1 year; therefore, the absence of early detectable spasticity does not mean that the infant does not have spastic cerebral palsy. In addition, infants may have more than one motor disorder because spasticity and dystonia often coexist. As the infant’s voluntary activity levels increase, some symptoms may resolve (eg, nonuse of a limb), while other symptoms may worsen (eg, increased involuntary dystonic posturing in response to voluntary movement). Wherever possible, differentiate between unilateral vs bilateral cerebral palsy early because treatments differ.5,38
Table 2.
Clinical Signs Indicating Motor Type and Topography in Infants
| Unilateral Spastic Hemiplegia | Bilateral Spastic Diplegia | Bilateral Spastic Quadriplegia | Dyskinesia | Ataxia |
|---|---|---|---|---|
| GMs34 | ||||
| • Poor repertoire or cramped synchronized GMs, followed by absent fidgety movements plus an asymmetry in segmental movements (eg, wrist or hand). Note that some cases of hemiplegic CP may be missed by GMs | • Cramped synchronized GMs, followed by absent fidgety movements | • Early onset and long duration of cramped synchronized GMs, followed by absent fidgety movements | • Poor repertoire GMs, followed by absent fidgety movements with circular arm movements and finger spreading | • Unknown |
| MRI35,36 | ||||
| • Focal vascular insults (24%) • Malformations (13%) • Unilateral hemorrhage (grade IV) with porencephaly • Lesions in the parietal white matter involving the trigone • Middle cerebral artery stroke with asymmetry of myelination of the PLIC |
• Bilateral white matter injury (31%−60%) • Cystic PVL(grade II-III) with sparse or absent myelination of the PLIC • Moderate to severe white matter injury (also known as PVE) |
• Gray matter injury (34%) • Malformations (16%) • Cystic PVL (grade III) with absent myelination of the PLIC • Severe white matter injury with or without deep nuclear gray matter |
• Gray matter injury (21%) with thalamic and lentiform nuclear injury | • Malformations (18%) • Normal imaging (24%−57%) • Cerebellar injury |
| HINE Scores37 | ||||
| 50–73 | <50 | <50 <40 GMFCS level IV-V |
<50 | Unknown |
| Motor Tests | ||||
| • Asymmetrical hand preference • Stuck in floor sitting (ie, unable to transition out of sitting) • Cruises or steps consistently in one direction or with the same leg always leading • Reduced variation in motor behavior |
• Good hand function compared with lower limb function • Dislike or avoidance of floor sitting • Weight bears on toes • Reduced variation in motor behavior |
• Head lag • Persistent rounded back in supported sitting • Bilateral fisted hands • Slow to reach and grasp with either hand • Reduced variation in motor behavior |
• Twisting arm or neck postures on voluntary movement (may be painful) • Finds midline play difficult, prefers toys positioned at shoulder width • Switches hands during reaching task • Requires a lot of extra time to initiate movement • Voluntary movement and emotion worsens postures • Reduced variation in motor behavior |
• Nonspecific |
Abbreviations: CP, cerebral palsy; GMFCS, Gross Motor Function Classification System; GMs, Prechtl Qualitative Assessment of General Movements; HINE, Hammersmith Infant Neurological Examination; MRI, magnetic resonance imaging; PLIC, posterior limb internal capsule; PVE, periventricular echogenicity; PVL, periventricular leukomalacia.
False Positives and False Negatives
Without a laboratory biomarker, an early diagnosis is not always clinically clear-cut because of the possibility of false positives and false negatives.22 Experienced clinicians acknowledge that, because all infants have an expanding and changing voluntary motor repertoire, determining whether their current motor dysfunction is permanent and causing long-term activity limitations, as per the international definition,1 is difficult. False negatives can occur for the following reasons: (1) there is a latency between the initial brain lesion and the later onset of clinical neurological signs (eg, exaggerated spasticity or dystonia from voluntary movement25), (2) approximately 10% have normal neuroimaging,27 (3) half have a seemingly uneventful pregnancy and birth,31 and (4)one-third have the mildest form (GMFCS I)2,32 and may initially achieve all of their motor milestones on time, offering false reassurance about their motor development. False positives can also occur because prematurity, stroke, and encephalopathy do not always result in long-term motor disabilities.25,31 Australian cerebral palsy population register data indicate that less than 5% of registrations are false-positive diagnoses.2 In almost all of these instances, the infant was rediagnosed as having another neurological disability (eg, intellectual disability or autism), not a normal developmental outcome.11
Eighty-six percent of parents of a child with cerebral palsy suspect it before the clinical diagnosis is made.39 Population data indicate that seeking to avoid false-positive results by delaying diagnosis is harmful to parent and caregiver well-being.39 Parents and caregivers dissatisfied with a prolonged diagnostic process are more likely to experience depression39 and lasting anger.40 Parents and caregivers acknowledge that, while receiving the diagnosis is always difficult, they prefer to know earlier rather than later so that they can assist in their infant’sdevelopment.39 Early detection is important for the whole family unit because it helps foster acceptance41 and leads to increased confidence in the infant’s medical team.39 Early detection allows improved access to early intervention and efficient use of resources.
Advances in Treatment: Cerebral Palsy-Specific Early Intervention Improves Outcomes
Neuroscience evidence indicates that brain development and refinement of the motor system continue postnatally, driven by motor cortex activity.42,43 Early active movement and intervention are essential because infants who do not actively use their motor cortex risk losing cortical connections and dedicated function.42,43 Furthermore, there is increasing evidence that the infant’s motor behavior, via discovery and interaction with the environment, controls and generates the growth and development of muscle, ligament, and bone, as well as driving ongoing development of the neuromotor system.44–48
Therefore, the clinical diagnosis of cerebral palsy or high risk of cerebral palsy should always be followed by a referral for the infant to receive cerebral palsy-specific intervention and for the parents or caregivers to receive emotional support. Family concern is a valid reason to trigger formal diagnostic investigations and intervention referrals.
Cerebral palsy-specific early intervention maximizes neuroplasticity42,43 and minimizes deleterious modifications to muscle and bone growth and development.44 Before commencing intervention, unilateral vs bilateral cerebral palsy should be identified because treatments and long-term musculoskeletal outcomes differ.46–48 Randomized clinical trial data are beginning to indicate the following: (1) that infants with hemiplegic cerebral palsy who receive early constraint-induced movement therapy (CIMT) have better hand function than controls in the short term and probably substantially better hand function in the long-term45; (2) that infants with bilateral cerebral palsy who receive regular surveillance and intervention have lower rates of hip displacement, contracture, and scoliosis46–48 (based on population register data); (3) that infants with any type and topography of cerebral palsy who receive Goals-Activity-Motor Enrichment (GAME), which is an early, intense, enriched, task-specific, training-based intervention at home, have better motor and cognitive skills at 1 year than those who receive usual care49; and (4) that improvements are even better when intervention occurs at home50,51 because children learn best in supported natural settings where training is personalized to their enjoyment. Task-specific, motor training-based early intervention (eg, GAME49andCIMT45)are recommended as the new paradigm of care for cerebral palsy because they induce neuroplasticity and produce functional gains.52 Larger replication randomized clinical trials are underway, including the following: (1) Randomised Trial of Rehabilitation Very Early in Congenital Hemiplegia (REACH) (ACTRN12615000180516) (n = 150) CIMT vs bimanual53 and (2) GAME (ACTRN12617000006347) (n = 300) GAME vs usual care.54 In addition, regenerative agents to induce brain repair are being studied, including (1) Preventing Adverse Outcomes of Neonatal Hypoxic Ischaemic Encephalopathy With Erythropoietin: A Randomised Controlled Multicentre Australian Trial (PAEAN) (ACTRN12614000669695) (n = 300) erythropoietin plus hypothermia vs hypothermia alone55 and (2) NCT02612155 (n = 160) umbilical cord blood plus hypothermia vs hypothermia alone.56
The aim of early intervention for children with cerebral palsy should be to (1) optimize motor, cognition, and communication outcomes using interventions that promote learning and neuroplasticity (all have motor impairments, 1 in 2 have intellectual disability, and 1 in 4are nonverbal5); (2) prevent secondary impairments and minimize the influence of complications that worsen function or interfere with learning (3 in 4 have chronic pain, 1 in 3 have hip displacement, 1 in 4 have epilepsy, 1 in 4 have bladder control problems, 1 in 5 have a sleep disorder, 1 in 5 have sialorrhea, 1 in 10 are blind, 1 in 15 require tube feeding, and 1 in 25 are deaf5); and (3) promote parent or caregiver coping and mental health to reduce stress, anxiety, and depression, which are compounded when a behavior disorder is present (1 in 4 have behavior disorders). Recommendations from best available evidence are listed below.
Early Interventions to Optimize Motor, Cognition, and Communication Skills
For motor and cognition, physical and occupational therapy interventions should use child-initiated movement, task-specific practice, and environmental adaptations that stimulate independent task performance.52 These include Learning Games Curriculum (diplegia),57 CIMT or bimanual (hemiplegia),45 and GAME (all subtypes).49
For communication, speech language pathology interventions should foster parent-infant transactions and provide compensation when speech is not possible or is inadequate. Examples include the Hanen It Takes Two to Talk and More Than Words programs, as well as alternative and augmentative communication.58
Interventions to Prevent Secondary Impairments and Minimize Complications
Regarding pain, procedural pain should be avoided where possible because untreated pain elevates the risk for long-term neuropathic pain.59 Recommendations include pharmacological therapy and environmental interventions for ongoing pain and preemptive analgesia for procedural pain.59
Orthopedics
For hips, anteroposterior pelvic radiographs every 6 to 12 months are recommended commencing at age 12 months. This recommendation is in accord with hip surveillance guidelines.60
Neurologic
For epilepsy, standard antiepileptic pharmacological management is recommended.5
Urinary Tract
For the bladder, medical investigations should be conducted because abnormal anatomical findings are common.5 Standard toilet training should be provided over a longer duration because control may take longer.5
Sleep
For sleep, specialist assessments and early treatment are recommended before secondary academic and behavioral problems emerge. Examples include sleep hygiene, parental education, spasticity management, melatonin (2.5–10 mg), and gabapentin (5 mg/kg).5
Oral Care
For sialorrhea, botulinum toxin A, benztropine mesylate, or glycopyrrolate should be considered.61
Ophthalmologic Issues
Vision can be assessed in the first 48 hours of life using the early assessment of visual function in full-term newborns by Ricci et al.62 Any infant with abnormal vision at term-equivalent age should receive vision intervention and be reassessed at 3 months.63 Vision intervention is recommended.
Feedings
For nonoral feeding, swallowing safety should be comprehensively assessed if concerns or clinical history of pneumonia exists because it is the leading cause of death in individuals with cerebral palsy64 and is mitigated by tube feeding.65 Weight should be measured regularly because severe physical disability elevates the risk for malnutrition.5
Aural
For hearing, standard early hearing accommodations are recommended.5
Interventions to Promote Parent or Caregiver Coping and Mental Health
Parental education in behavior management is recommended. An example is the Positive Parenting Program (Triple P).66
Parent-child attachment interventions are also helpful. Kangaroo Mother Care67 and music therapy68 are examples.
Finally, parent or caregiver mental health interventions69,70 are suggested. One such intervention is Acceptance and Commitment Therapy (ACT).66
Discussion
Clinical Bottom Line
Infants with cerebral palsy require an early diagnosis because motor and cognitive gains are greater from diagnostic-specific early intervention.
An interim diagnosis of high risk of cerebral palsy should be used if a diagnosis of cerebral palsy cannot yet be used with certainty.
Clinical signs emerge and evolve before age 2 years. Therefore, a combination of standardized tools should be used to predict risk.
Before 5 months’ corrected age, MRI, GMs, or the HINE are most predictive of risk for cerebral palsy.
After 5 months’ corrected age, MRI and the HINE are most predictive of risk for cerebral palsy.
In countries of low to middle income where MRI is not available, the HINE is recommended.
Topography and severity of cerebral palsy are important to establish for clinical purposes. Magnetic resonance imaging and the HINE provide guidance.
False positives occur less than 5% of the time with standardized tools.
False negatives resulting in late diagnoses and late intervention are detrimental to parents, caregivers, and infants.
Limitations
This review article has some limitations. First, our literature search revealed that almost all studies focus on identifying cerebral palsy in infants with newborn discernible risks (eg, prematurity and encephalopathy) because these infants are more often in newborn follow-up. Little has been published about early diagnosis in the 50% of all cerebral palsy cases that are discernible later in infancy after a seemingly uneventful pregnancy and birth because these samples are difficult to assemble. Advances in genetics and understanding of congenital anomalies may provide more clues about how to identify these children earlier. Second, no study to date has investigated the combined predictive power of 3 or more of the individual tools identified in this review article and represents a gap in the literature. Third, we have not reviewed or discussed the literature about evidence-based testing for other childhood disabilities on the differential diagnosis list. Fourth, we have not provided a systematic description of the early intervention evidence. More information on assessment tools and early intervention is contained in a related but separate clinical guideline that is being developed from systematic review data.
Conclusions
Cerebral palsy or high risk of cerebral palsy can be diagnosed accurately and early using clinical reasoning and a combination of standardized tools. High-quality evidence indicates that, for infants with newborn-detectable risks before 5 months’ corrected age, the GMs assessment plus neonatal MRI is more than 95% accurate and is thus recommended. For infants with infant detectable risks after 5 months’ corrected age, the HINE plus neonatal MRI is more than 90% accurate and is therefore recommended. The accuracy of these diagnostic methods in infants with later infancy discernible risks for cerebral palsy is not yet known, but they are conditionally recommended. Accurate early diagnosis is possible even when assessments of GMs are not available or MRI is not safe or affordable (eg, in countries of low to middle income) by using the HINE, which detects cerebral palsy with more than 90% accuracy and provides objective information about severity. Early detection of high risk of cerebral palsy, followed by cerebral palsy-specific early intervention, is recommended and should be the standard of care to optimize infant neuroplasticity, prevent complications, and enhance parent and caregiver well-being.
Supplementary Material
Key Points.
Question
What are the most accurate evaluations for diagnosing cerebral palsy early?
Findings
In this systematic review of the literature, we found diagnosis can be accurately made before 6 months’ corrected age. Before 5 months’ corrected age, magnetic resonance imaging plus the General Movements Assessment or the Hammersmith Infant Neurological Examination are recommended; after 5 months’ corrected age, magnetic resonance imaging (where safe and feasible), the Hammersmith Infant Neurological Examination, and the Developmental Assessment of Young Children are recommended.
Meaning
Early diagnosis should be the standard of care because contemporary early interventions optimize neuroplasticity and functional outcomes.
Funding/Support:
The Cerebral Palsy Alliance Research Foundation funded the face-to-face meeting that enabled the authors to jointly make recommendations from evidence. Dr Guzzetta was supported by grant R 15–96 from the Mariani Foundation of Milan.
Role of the Funder/Sponsor:
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: None reported.
REFERENCES
- 1.Rosenbaum P, Paneth N, Leviton A, et al. A report: the definition and classification of cerebral palsy April 2006 [published correction appears in Dev Med Child Neurol. 2007;49(6):480]. Dev Med Child Neurol Suppl. 2007;109:8–14. [PubMed] [Google Scholar]
- 2.Report of the Australian Cerebral Palsy Register, Birth Years 1993–2009, September 2016. https://www.cpregister.com/pubs/pdf/ACPR-Report_Web_2016.pdf. Accessed 2016. [DOI] [PubMed]
- 3.Granild-Jensen JB, Rackauskaite G, Flachs EM, Uldall P. Predictors for early diagnosis of cerebral palsy from national registry data. Dev Med Child Neurol. 2015;57(10):931–935. [DOI] [PubMed] [Google Scholar]
- 4.Hubermann L, Boychuck Z, Shevell M, Majnemer A. Age at referral of children for initial diagnosis of cerebral palsy and rehabilitation: current practices. J Child Neurol. 2016;31(3):364–369. [DOI] [PubMed] [Google Scholar]
- 5.Novak I, Hines M, Goldsmith S, Barclay R. Clinical prognostic messages from a systematic review on cerebral palsy. Pediatrics. 2012;130(5):e1285–e1312. [DOI] [PubMed] [Google Scholar]
- 6.Oskoui M, Coutinho F, Dykeman J, Jetté N, Pringsheim T. An update on the prevalence of cerebral palsy: a systematic review and meta-analysis. Dev Med Child Neurol. 2013;55(6):509–519. [DOI] [PubMed] [Google Scholar]
- 7.Reid SM, Meehan E, McIntyre S, Goldsmith S, Badawi N, Reddihough DS; Australian Cerebral Palsy Register Group. Temporal trends in cerebral palsy by impairment severity and birth gestation. Dev Med Child Neurol. 2016;58(suppl 2):25–35. [DOI] [PubMed] [Google Scholar]
- 8.Sellier E, Platt MJ, Andersen GL, Krägeloh-Mann I, De La Cruz J, Cans C; Surveillance of Cerebral Palsy Network. Decreasing prevalence in cerebral palsy: a multi-site European population-based study, 1980 to 2003. Dev Med Child Neurol. 2016;58(1):85–92. [DOI] [PubMed] [Google Scholar]
- 9.Blair E, Watson L. Epidemiology of cerebral palsy. Semin Fetal Neonatal Med. 2006;11(2):117–125. [DOI] [PubMed] [Google Scholar]
- 10.Khandaker G, Smithers-Sheedy H, Islam J, et al. Bangladesh Cerebral Palsy Register (BCPR): a pilot study to develop a national cerebral palsy (CP) register with surveillance of children for CP. BMC Neurol. 2015;15:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson KB. Causative factors in cerebral palsy. Clin Obstet Gynecol. 2008;51(4):749–762. [DOI] [PubMed] [Google Scholar]
- 12.McMichael G, Bainbridge MN, Haan E, et al. Whole-exome sequencing points to considerable genetic heterogeneity of cerebral palsy. Mol Psychiatry. 2015;20(2):176–182. [DOI] [PubMed] [Google Scholar]
- 13.Oskoui M, Gazzellone MJ, Thiruvahindrapuram B, et al. Clinically relevant copy number variations detected in cerebral palsy. Nat Commun. 2015;6:7949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaefer GB. Genetics considerations in cerebral palsy. Semin Pediatr Neurol. 2008;15(1):21–26. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. WHO Handbook for Guideline Development. Geneva, Switzerland: World Health Organization; 2012. http://apps.who.int/iris/bitstream/10665/75146/1/9789241548441_eng.pdf. Accessed July 2015. [Google Scholar]
- 16.Graham R, Mancher M, Wolman DM, Greenfield S, Steinberg E, eds. Clinical Practice Guidelines We Can Trust. Washington, DC: National Academies Press; July 16, 2011. [PubMed] [Google Scholar]
- 17.Brouwers MC, Kho ME, Browman GP, et al. ; AGREE Next Steps Consortium. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839–E842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrews J, Guyatt G, Oxman AD, et al. GRADE guidelines, 14: going from evidence to recommendations: the significance and presentation of recommendations. J Clin Epidemiol. 2013;66(7):719–725. [DOI] [PubMed] [Google Scholar]
- 21.Bosanquet M, Copeland L, Ware R, Boyd R. A systematic review of tests to predict cerebral palsy in young children. Dev Med Child Neurol. 2013;55(5):418–426. [DOI] [PubMed] [Google Scholar]
- 22.Burger M, Louw QA. The predictive validity of general movements: a systematic review. Eur J Paediatr Neurol. 2009;13(5):408–420. [DOI] [PubMed] [Google Scholar]
- 23.Darsaklis V, Snider LM, Majnemer A, Mazer B. Predictive validity of Prechtl’s method on the qualitative assessment of general movements: a systematic review of the evidence. Dev Med Child Neurol. 2011;53(10):896–906. [DOI] [PubMed] [Google Scholar]
- 24.Heineman KR, Hadders-Algra M. Evaluation of neuromotor function in infancy: a systematic review of available methods. J Dev Behav Pediatr. 2008;29(4):315–323. [DOI] [PubMed] [Google Scholar]
- 25.Romeo DM, Ricci D, Brogna C, Mecuri E. Use of the Hammersmith Infant Neurological Examination in infants with cerebral palsy: a critical review of the literature. Dev Med Child Neurol. 2016;58(3):240–245. [DOI] [PubMed] [Google Scholar]
- 26.Spittle AJ, Doyle LW, Boyd RN. A systematic review of the clinimetric properties of neuromotor assessments for preterm infants during the first year of life. Dev Med Child Neurol. 2008;50(4):254–266. [DOI] [PubMed] [Google Scholar]
- 27.Ashwal S, Russman BS, Blasco PA, et al. ; Quality Standards Subcommittee of the American Academy of Neurology; Practice Committee of the Child Neurology Society. Practice parameter: diagnostic assessment of the child with cerebral palsy: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2004;62(6):851–863. [DOI] [PubMed] [Google Scholar]
- 28.Ment LR, Bada HS, Barnes P, et al. Practice parameter: neuroimaging of the neonate: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2002;58(12):1726–1738. [DOI] [PubMed] [Google Scholar]
- 29.Einspieler C, Prechtl HF. Prechtl’s assessment of general movements: a diagnostic tool for the functional assessment of the young nervous system. Ment Retard Dev Disabil Res Rev. 2005;11 (1):61–67. [DOI] [PubMed] [Google Scholar]
- 30.Haataja L, Mercuri E, Regev R, et al. Optimality score for the neurologic examination of the infant at 12 and 18 months of age. J Pediatr. 1999;135(2, pt 1):153–161. [DOI] [PubMed] [Google Scholar]
- 31.McIntyre S, Morgan C, Walker K, Novak I. Cerebral palsy: don’t delay. Dev Disabil Res Rev. 2011;17(2):114–129. [DOI] [PubMed] [Google Scholar]
- 32.Palisano R, Rosenbaum P, Walter S, et al. Development and validation of a Gross Motor Function Classification System for children with cerebral palsy. Dev Med Child Neurol. 1997;39:214–223. [DOI] [PubMed] [Google Scholar]
- 33.Maitre NL, Slaughter JC, Aschner JL. Early prediction of cerebral palsy after neonatal intensive care using motor development trajectories in infancy. Early Hum Dev. 2013;89(10):781–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Einspieler C, Marschik PB, Bos AF, Ferrari F, Cioni G, Prechtl HF. Early markers for cerebral palsy: insights from the assessment of general movements. Future Neurol. 2012;7:709–717. [Google Scholar]
- 35.de Vries LS, van Haastert IC, Benders MJ, Groenendaal F. Myth: cerebral palsy cannot be predicted by neonatal brain imaging. Semin Fetal Neonatal Med. 2011;16(5):279–287. [DOI] [PubMed] [Google Scholar]
- 36.Reid SM, Dagia CD, Ditchfield MR, Carlin JB, Reddihough DS. Population-based studies of brain imaging patterns in cerebral palsy. Dev Med Child Neurol. 2014;56(3):222–232. [DOI] [PubMed] [Google Scholar]
- 37.Romeo DM, Cioni M, Scoto M, Mazzone L, Palermo F, Romeo MG. Neuromotor development in infants with cerebral palsy investigated by the Hammersmith Infant Neurological Examination during the first year of age. Eur J Paediatr Neurol. 2008;12(1):24–31. [DOI] [PubMed] [Google Scholar]
- 38.Hägglund G, Alriksson-Schmidt A, Lauge-Pedersen H, Rodby-Bousquet E, Wagner P, Westbom L. Prevention of dislocation of the hip in children with cerebral palsy: 20-year results of a population-based prevention programme. Bone Joint J. 2014;96-B(11):1546–1552. [DOI] [PubMed] [Google Scholar]
- 39.Baird G, McConachie H, Scrutton D. Parents’ perceptions of disclosure of the diagnosis of cerebral palsy. Arch Dis Child. 2000;83(6):475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller J, Colligan J, Colver A. A qualitative study, using focused interviews, of the information needs of families whose children’s names are on a cerebral palsy register. Child Care Health Dev. 2003; 29(6):465–471. [DOI] [PubMed] [Google Scholar]
- 41.Rentinck IC, Ketelaar M, Schuengel C, et al. Short-term changes in parents’ resolution regarding their young child’s diagnosis of cerebral palsy. Child Care Health Dev. 2010;36(5):703–708. [DOI] [PubMed] [Google Scholar]
- 42.Eyre J. Corticospinal tract development and activity dependent plasticity. In: Shepherd R, ed. Cerebral Palsy in Infancy. Oxford, England: Elsevier; 2014:53–66. [Google Scholar]
- 43.Martin JH, Chakrabarty S, Friel KM. Harnessing activity-dependent plasticity to repair the damaged corticospinal tract in an animal model of cerebral palsy. Dev Med Child Neurol. 2011;53(suppl 4):9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shepherd RB, ed. Cerebral Palsy in Infancy: Targeted Activity to Optimize Early Growth and Development. Oxford, England: Elsevier Health Sciences; 2014. [Google Scholar]
- 45.Eliasson AC, Holmefur M. The influence of early modified constraint-induced movement therapy training on the longitudinal development of hand function in children with unilateral cerebral palsy. Dev Med Child Neurol. 2015;57(1):89–94. [DOI] [PubMed] [Google Scholar]
- 46.Elkamil AI, Andersen GL, Hägglund G, Lamvik T, Skranes J, Vik T. Prevalence of hip dislocation among children with cerebral palsy in regions with and without a surveillance programme: a cross sectional study in Sweden and Norway. BMC Musculoskelet Disord. 2011;12:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hägglund G, Andersson S, Düppe H, Lauge-Pedersen H, Nordmark E, Westbom L. Prevention of dislocation of the hip in children with cerebral palsy: the first ten years of a population-based prevention programme. J Bone Joint Surg Br. 2005;87(1):95–101. [PubMed] [Google Scholar]
- 48.Scrutton D, Baird G, Smeeton N. Hip dysplasia in bilateral cerebral palsy: incidence and natural history in children aged 18 months to 5 years. Dev Med Child Neurol. 2001;43(9):586–600. [DOI] [PubMed] [Google Scholar]
- 49.Morgan C, Novak I, Dale RC, Guzzetta A, Badawi N. Single blind randomised controlled trial of GAME (Goals-Activity-Motor Enrichment) in infants at high risk of cerebral palsy. Res Dev Disabil. 2016;55:256–267. [DOI] [PubMed] [Google Scholar]
- 50.Rostami HR, Malamiri RA. Effect of treatment environment on modified constraint-induced movement therapy results in children with spastic hemiplegic cerebral palsy: a randomized controlled trial. Disabil Rehabil. 2012;34(1):40–44. [DOI] [PubMed] [Google Scholar]
- 51.Novak I, Cusick A, Lannin N. Occupational therapy home programs for cerebral palsy: double-blind, randomized, controlled trial. Pediatrics. 2009;124(4):e606–e614. [DOI] [PubMed] [Google Scholar]
- 52.Morgan C, Darrah J, Gordon AM, et al. Effectiveness of motor interventions in infants with cerebral palsy: a systematic review. Dev Med Child Neurol. 2016;58(9):900–909. [DOI] [PubMed] [Google Scholar]
- 53.ANZCTR.org.au. Multisite randomised trial comparing infant-friendly modified constraint induced movement therapy and infant-friendly bimanual therapy to improve development of reach and grasp, fine motor skills and cognition for infants with asymmetric brain injuries. ACTRN12615000180516. http://www.anzctr.org.au/TrialSearch.aspx?searchTxt=12615000180516&isBasic=True. Accessed June 3, 2017
- 54.ANZCTR.org.au. Harnessing Neuroplasticity to Improve Motor Performance in Infants With Cerebral Palsy: a pragmatic randomized controlled trial. ACTRN12617000006347. http://www.anzctr.org.au/TrialSearch.aspx?searchTxt=12617000006347&isBasic=True. Accessed June 3, 2017.
- 55.ANZCTR.org.au. Preventing Adverse Outcomes of Neonatal Hypoxic Ischaemic Encephalopathy With Erythropoietin: a Phase III randomised placebo controlled multicentre clinical trial. ACTRN12614000669695. http://www.anzctr.org.au/TrialSearch.aspx?searchTxt=12614000669695&isBasic=True. Accessed June 3, 2017.
- 56.clinicaltrials.gov. A multi-site study of autologous cord blood cells for hypoxic ischemic encephalopathy. NCT02612155. https://clinicaltrials.gov/ct2/results?term=02612155&Search=Search. Accessed June 3, 2017.
- 57.Palmer FB, Shapiro BK, Wachtel RC, et al. The effects of physical therapy on cerebral palsy: a controlled trial in infants with spastic diplegia. N Engl J Med. 1988;318(13):803–808. [DOI] [PubMed] [Google Scholar]
- 58.Chorna O, Hamm E, Cummings C, Fetters A, Maitre NL. Speech and language interventions for infants aged 0 to 2 years at high risk for cerebral palsy: a systematic review. Dev Med Child Neurol. 2017;59(4):355–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anand KJ; International Evidence-Based Group for Neonatal Pain. Consensus statement for the prevention and management of pain in the newborn. Arch Pediatr Adolesc Med. 2001;155(2):173–180. [DOI] [PubMed] [Google Scholar]
- 60.Wynter M, Gibson N, Willoughby KL, et al. ; National Hip Surveillance Working Group. Australian hip surveillance guidelines for children with cerebral palsy: 5-year review. Dev Med Child Neurol. 2015;57(9):808–820. [DOI] [PubMed] [Google Scholar]
- 61.Walshe M, Smith M, Pennington L. Interventions for drooling in children with cerebral palsy. Cochrane Database Syst Rev. 2012;11: CD008624. [DOI] [PMC free article] [PubMed]
- 62.Ricci D, Cesarini L, Groppo M, et al. Early assessment of visual function in full term newborns. Early Hum Dev. 2008;84(2):107–113. [DOI] [PubMed] [Google Scholar]
- 63.Ricci D, Romeo DM, Gallini F, et al. Early visual assessment in preterm infants with and without brain lesions: correlation with visual and neurodevelopmental outcome at 12 months. Early Hum Dev. 2011;87(3):177–182. [DOI] [PubMed] [Google Scholar]
- 64.Blair E, Watson L, Badawi N, Stanley FJ. Life expectancy among people with cerebral palsy in Western Australia. Dev Med Child Neurol. 2001;43 (8):508–515. [DOI] [PubMed] [Google Scholar]
- 65.Sullivan PB, Juszczak E, Bachlet AM, et al. Gastrostomy tube feeding in children with cerebral palsy: a prospective, longitudinal study. Dev Med Child Neurol. 2005;47(2):77–85. [DOI] [PubMed] [Google Scholar]
- 66.Whittingham K, Sanders MR, McKinlay L, Boyd RN. Parenting intervention combined with Acceptance and Commitment Therapy: a trial with families of children with cerebral palsy. J Pediatr Psychol. 2016;41(5):531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Athanasopoulou E, Fox JR. Effects of Kangaroo Mother Care on maternal mood and interaction patterns between parents and their preterm, low birth weight infants: a systematic review. Infant Ment Health J. 2014;35(3):245–262. [DOI] [PubMed] [Google Scholar]
- 68.Bieleninik Ł, Ghetti C, Gold C. Music therapy for preterm infants and their parents: a meta-analysis. Pediatrics. 2016;138(3)pii:e20160971. [DOI] [PubMed] [Google Scholar]
- 69.Brecht C, Shaw RJ, Horwitz SM, John NH. Effectiveness of therapeutic behavioral interventions for parents of low birth weight premature infants: a review. Infant Ment Health J. 2012;33(6):651–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kraljevic M, Warnock FF. Early educational and behavioral RCT interventions to reduce maternal symptoms of psychological trauma following preterm birth: a systematic review. J Perinat Neonatal Nurs. 2013;27(4):311–327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.