Abstract
Vaccination of mice with Escherichia coli expressing Brucella Cu/Zn superoxide dismutase (SOD) [E. coli(pBSSOD)] induced a significant level of protection against virulent Brucella abortus challenge, although this level was not as high as the one reached with B. abortus vaccine strain RB51. In addition, vaccination with E. coli(pBSSOD) induced antibodies to Cu/Zn SOD and a strong proliferative response of splenocytes when stimulated in vitro with a thioredoxin-Cu/Zn SOD fusion protein.
Mice and cattle can be protected against Brucella abortus infection by vaccination with viable B. abortus 19 or RB51 (2, 5, 8). At present it is not clear which of the many Brucella antigens in these vaccine strains are responsible for the induction of the protective immunity. The induction of protection with strain RB51, which does not evoke a detectable immune response to the O chain (2, 8), clearly indicates that antigens unrelated to the O chain play a crucial role. Protection induced by strain RB51 is cell mediated, since passive transfer of antibodies does not confer protection while transfer of lymphocytes does (3). In the past, ribosomal protein L7/L12 and certain epitopes of the Cu/Zn superoxide dismutase (SOD) of B. abortus have been implicated in the induction of a protective cell-mediated immune response (6, 12). Our objective in this study was to evaluate the protective capacity of Brucella Cu/Zn SOD in mice immunized with a recombinant Escherichia coli strain expressing this Brucella antigen.
Animals, bacterial strains, and antigens used.
Two-month-old female BALB/c mice were used in this study, as were the following bacterial strains: B. abortus 2308 and RB51 (8), E. coli DH5α (Gibco BRL) with plasmid pBluescript SK(−) (E. coli pBS), and E. coli DH5α expressing Brucella Cu/Zn SOD [E. coli(pBSSOD)]. E. coli(pBSSOD) was constructed as follows. A clone, pBAII-3, containing the gene for Brucella Cu/Zn SOD (sodC) along with its own promoter was initially obtained from a pUC9 genomic library of B. abortus 2308 (unpublished data). A 1.1-kb fragment containing the sodC gene and its promoter sequence was excised from the insert of pBAII-3 with ClaI and subcloned into pBluescript SK(−) (Stratagene); the resulting plasmid is referred to as pBSSOD. In order to purify the recombinant Cu/Zn SOD protein of B. abortus, it was overexpressed in E. coli by using the pThioHis vector of the His-Patch ThioFusion expression system (Invitrogen Corp., San Diego, Calif.). In this vector, the product of the cloned foreign gene is expressed as a fusion protein with a modified version of the E. coli thioredoxin protein. These modifications allow the purification of the recombinant fusion protein by metal affinity chromatography. The sodC gene was PCR amplified from the genomic DNA of strain 2308 by using a primer pair designed based on the nucleotide sequence. (The forward primer was 5′ GAAGTGATGGAATTCTTATTTATTGC 3′; the reverse primer was 5′ AGCGCTGCAGGCTTATCGGAAT 3′.) A restriction site was engineered into each primer by point mutation. The amplified gene fragment was digested with an appropriate restriction enzyme and cloned into pThioHis.
The recombinant plasmid was electroporated into E. coli DH5α, and a single recombinant colony was selected. Expression and purification of the recombinant fusion protein (Thio-SOD) and the thioredoxin alone were performed according to the manufacturer’s suggested procedure. Both preparations were tested for purity by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analysis and were found to be devoid of other E. coli proteins.
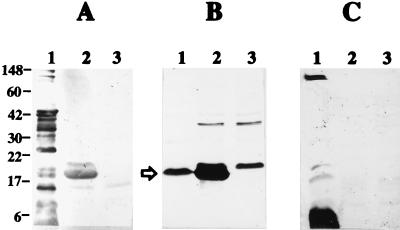
The expression of the Brucella Cu/Zn SOD by the transformed E. coli strain was confirmed by Western blot analysis with goat anti-RB51 (Fig. 1A) and goat anti-Cu/Zn SOD serum (Fig. 1B). The anti-Cu/Zn SOD serum was produced by immunizing a goat with recombinant Brucella Cu/Zn SOD expressed in Salmonella (unpublished data). Figure 1A and B show that E. coli(pBSSOD) indeed expresses the Brucella Cu/Zn SOD. Figure 1A, lane 2, shows that serum from goat immunized three times with sonicates of RB51 cells recognizes the recombinant antigen in E. coli(pBSSOD) but not in E. coli(pBS). Figure 1B, lane 1, shows that the goat anti-Brucella Cu/Zn SOD recognizes only SOD in the Brucella extract, which contains a large number of antigens, as indicated in Fig. 1A, lane 1. Therefore, this serum will recognize the recombinant Cu/Zn SOD expressed by E. coli(pBSSOD). The goat anti-Brucella Cu/Zn SOD serum also contains antibodies which react with two other E. coli proteins not related to Brucella Cu/Zn SOD. These two antigens appear in both E. coli(pBSSOD) and E. coli(pBS) (Fig. 1B, lanes 2 and 3). This reaction is most probably due to the presence of anti-E. coli antibodies in the goat serum due to environmental exposure or Salmonella antigens, which cross-react with these two E. coli proteins.
FIG. 1.
Western blot showing the expression of B. abortus Cu/Zn SOD protein by recombinant E. coli. The antigens separated by sodium dodecyl sulfate–15% polyacrylamide gel electrophoresis were transferred to a nitrocellulose membrane and reacted with different sera. The membranes were developed with appropriate secondary antibodies conjugated with horseradish peroxidase. Lanes 1 to 3 contain the antigens of B. abortus RB51, E. coli(pBSSOD), and E. coli(pBS), respectively. Panels A to C were reacted with hyperimmunized goat serum to sonicates of B. abortus RB51, goat antiserum to B. abortus Cu/Zn SOD protein, and sera from mice vaccinated with strain RB51, respectively. The arrow at the left of panel B indicates the Cu/Zn SOD band. Numbers at the left of panel A indicate the positions of molecular mass markers, in kilodaltons.
Protection experiments.
To test the effects of different immunogens, groups of 10 mice each were vaccinated intraperitoneally (i.p.) with either 2 × 108 CFU of live B. abortus RB51, 1 × 106 CFU of E. coli(pBS), or 1 × 106 CFU of E. coli(pBSSOD). Unvaccinated control mice were injected with 10 mM phosphate-buffered saline (pH 7.2). Five weeks after vaccination, five mice from each group were challenged by i.p. injection of 104 CFU of B. abortus 2308. Experimentally infected mice were killed 2 weeks later by cervical dislocation, spleens were disrupted, and dilutions were plated to determine the number of Brucella CFU per spleen (3). The remaining five mice in each group were used for determinations of the antibody and lymphoproliferative responses to the immunizing antigens. The degrees of protection in vaccinated animals and controls were expressed as mean CFU for spleens in each group obtained after challenge following log10 conversion (5). This experiment was repeated three times, and results from the pooled data are shown. Statistical analyses were performed with Student’s paired t test. Log10 units of protection were obtained by subtracting the mean log10 CFU for the experimental group from the mean log10 CFU of the corresponding control group (3, 9).
Analysis of antibody and lymphoproliferative responses.
To evaluate the production of antibody against Cu/Zn SOD, mice were bled retro-orbitally 5 weeks postimmunization and sera were used for Western blot analysis (14). The lymphocyte proliferation assays were performed as described previously (7). Briefly, 5 weeks postvaccination, mice were killed and single cell suspensions were prepared from the spleens of both the control and vaccinated groups. After elimination of erythrocytes, the splenocytes were cultured in 96-well plates at a concentration of 4 × 105 cells/well in RPMI 1640 medium alone or in the presence of 0.4, 0.08, or 0.016 μg of Thio-SOD antigen or similar quantities of thioredoxin alone. Concentrations of antigen were determined by the Bradford protein assay (Bio-Rad). The cells were cultured for 3 days, pulsed with 0.4 μCi of thymidine (50 Ci/mmol; Amersham, London, United Kingdom) per well for 8 h, and harvested, and the radioactivity was measured in a liquid scintillation counter. Cell proliferation was expressed as mean counts per minute obtained from triplicate cultures obtained from a cell pool of each group. In addition, a stimulation index (SI) was obtained for each experimental group by dividing the counts per minute of cells with antigen by the counts per minute of cells without antigen.
Protection against challenge by E. coli(pBSSOD).
The results indicate that immunization with viable E. coli(pBSSOD) and strain RB51 induced significant protection (P < 0.004 and P < 0.001, respectively) against challenge with virulent B. abortus 2308 (Table 1). As described previously, viable B. abortus RB51 conferred over 2 log units of protection (3, 8, 11). E. coli expressing the Brucella Cu/Zn SOD also conferred a significant level of protection (1.77 log units), although this level of protection was lower than the level conferred by strain RB51. E. coli not expressing the Brucella antigen did not confer protection (0.09 log unit). There is contradictory information on the role of Cu/Zn SOD as a virulence factor of Brucella, with one study indicating a weak role (4) and another indicating that this protein does not contribute to the virulence of Brucella (13). It is nevertheless possible that E. coli(pBSSOD) may be more virulent than its nonexpressing counterpart and thus may survive longer within mice. Prolonged survival of the recombinant may allow for the presence of activated macrophages at challenge time, and the protection observed in this study may have been nonspecific. To eliminate this possibility we infected mice with E. coli(pBSSOD), E. coli(pBS), and RB51 and cultured their spleens at 1, 2, 3, 4, and 5 weeks postinfection. Neither E. coli strain could be isolated from the mice; RB51 was last isolated in low numbers from some mice at 4 weeks postimmunization, confirming previous studies carried out with the original strain RB51 (8). In a previous study RB51-vaccinated mice did not show substantial nonspecific immunity against Listeria even though the vaccine organism could still be isolated by the fourth week postvaccination (3). The fact that neither E. coli strain could be isolated at 1 week postvaccination indicates that it is highly unlikely that the observed protection was nonspecific. These data indicate that the complete Cu/Zn SOD protein can induce a protective immune response in mice. This contrasts with the results of Tabatabai and Pugh (12), who were unable to induce a protective response in mice using a combination of adjuvants and purified recombinant Brucella Cu/Zn SOD protein as the vaccinal antigen, although some level of protection was induced with synthetic peptides of the protein and adjuvant. It is possible that the protection obtained in our studies with the whole Brucella Cu/Zn SOD protein was due to differences in antigen presentation mechanisms obtained with the use of Cu/Zn SOD expressed in living E. coli compared to its use as a purified protein coadministered with adjuvant.
TABLE 1.
Protection of mice against challenge with B. abortus 2308 after immunization with recombinant E. coli expressing B. abortus Cu/Zn SODa
| Vaccine and dose (CFU) | Log10 CFU of B. abortus 2308 in spleen (mean ± SD) | Log10 units of protection |
|---|---|---|
| Saline control | 7.69 ± 0.33 | 0 |
| RB51 live, 2 × 108 | 5.51 ± 0.39b | 2.18 |
| E. coli(pBSSOD), 1 × 106 | 5.92 ± 0.59c | 1.77 |
| E. coli(pBS), 1 × 106 | 7.60 ± 0.65 | 0.09 |
Mice were challenged i.p. with 104 CFU of strain 2308 5 weeks postvaccination.
P < 0.0001 compared with value for control mice.
P < 0.004 compared with value for control mice.
Stimulation of specific immune responses.
Western blot analysis of the humoral immune response indicated that only the sera from mice immunized with recombinant E. coli(pBSSOD) reacted weakly with the Brucella Cu/Zn SOD (data not shown). Mice immunized with viable strain RB51 did not develop antibodies to SOD but did develop antibodies to other Brucella antigens, as described previously (Fig. 1C) (1, 6), a finding also observed in cattle (2, 10). The detection of a weak antibody response to Brucella Cu/Zn SOD in the mice immunized with recombinant E. coli(pBSSOD) is an indication that the mice specifically recognized the antigen and contrasts with the lack of response in the strain RB51-immunized mice. Cu/Zn SOD may be a poor inducer of antibodies due to its relatedness (27% homology) to eukaryotic Cu/Zn SOD (1), and it is possible that the amount of SOD produced by Brucella in vivo is insufficient to induce Cu/Zn SOD-specific antibodies. In contrast, recombinant E. coli(pBSSOD) may express higher levels of Cu/Zn SOD than Brucella and therefore is able to induce a weak but detectable antibody response. Passive transfer of mouse anti-strain RB51 sera does not confer protection on naive mice against challenge (3); however, it is not clear if the anti-SOD antibodies induced by the recombinant E. coli strain played a role in protection, since passive transfer experiments with sera containing the specific antibodies were not carried out in this study. It is unlikely that anti-SOD antibodies would confer protection, since Cu/Zn SOD has not been located on the surfaces of Brucella rough or smooth strains.
The lymphocyte proliferation response results suggest that splenocytes from mice immunized with recombinant E. coli(pBSSOD) and strain RB51 respond in vitro upon stimulation with 0.08 and 0.4 μg of Thio-SOD, while splenocytes from saline- and E. coli-immunized mice did not (Table 2). In vitro stimulation of splenocytes with thioredoxin protein alone never reached an SI above 2, indicating that the response observed with the E. coli(pBSSOD)- and RB51-vaccinated mice was Cu/Zn SOD specific. The ability of Thio-SOD fusion protein to induce an in vitro lymphoproliferative response in the splenocytes from E. coli(pBSSOD)-immunized mice is again an indication that these mice recognized the antigen immunologically. It cannot be concluded from these experiments that the lymphoproliferative response is an indicator of protective immunity, since a variety of other Brucella antigens which are capable of inducing such a response without inducing protection exists (11).
TABLE 2.
Murine splenocyte proliferation and SI in response to Thio-SODa
| Vaccine and dose (CFU) | Proliferative response to Thio-SOD at the following concn (μg/well):
|
|||
|---|---|---|---|---|
| 0 | 0.016 | 0.08 | 0.4 | |
| Saline | 1,200 | 2,060 (1.7) | 2,050 (1.7) | 2,100 (1.8) |
| B. abortus RB51, 2 × 108 | 1,053 | 2,883 (2.7) | 3,848 (3.6) | 8,864 (8.4) |
| E. coli(pBSSOD), 1 × 106 | 2,189 | 4,305 (2.0) | 9,125 (4.2) | 23,029 (10.5) |
| E. coli(pBS), 1 × 106 | 2,300 | 3,598 (1.6) | 3,038 (1.3) | 9,272 (4.0) |
Splenocytes were cultured at 4 × 105 cells per well 5 weeks postimmunization. Each value is the average counts per minute of triplicate cultures of cells obtained from a pool of five mice in each group. Numbers in parentheses are the SIs for each group.
The RB51-vaccinated mice did not demonstrate a stronger anti-Cu/Zn SOD proliferative response than the E. coli(pBSOD)-immunized mice, and as indicated before, these mice did not respond with the production of specific antibodies. It is possible that Brucella does not produce sufficient Cu/Zn SOD or is unable to present the antigen appropriately during its in vivo replication cycle, leading to a lack of a humoral immune response and a lymphocyte proliferative response. This proliferative response is lower than the one that can be expected from the important role in defense that Cu/Zn SOD possesses, as demonstrated in this study, suggesting that Brucella probably evolved a system which down regulates the immune response to Cu/Zn SOD. Since the expression of Brucella Cu/Zn SOD by E. coli was sufficient to induce a detectable immune response to the antigen and significant protection against challenge, it may be possible to construct a Brucella RB51 vaccine which avoids this paucity in the response to Cu/Zn SOD, improving the protective abilities of the vaccine.
Acknowledgments
This work was supported by FONDECYT grant 1961146, grant 96.036.002-1.1D from the Dirección de Investigación, Universidad de Concepción, and grant S-95-46 from the Dirección de Investigación, Universidad Austral de Chile.
REFERENCES
- 1.Beck B L, Tabatabai L B, Mayfield J E. A protein isolated from Brucella abortus is a Cu-Zn superoxide dismutase. Biochemistry. 1991;29:372–376. doi: 10.1021/bi00454a010. [DOI] [PubMed] [Google Scholar]
- 2.Cheville N F, Stevens M G, Jensen A E, Tatum F M, Halling S M. Immune response and protection against infection and abortion in cattle experimentally vaccinated with mutant strains of Brucella abortus. Am J Vet Res. 1993;54:1591–1597. [PubMed] [Google Scholar]
- 3.Jiménez de Bagüés M P, Elzer P H, Jones S M, Blasco J M, Enright F M, Schurig G G, Winter A J. Vaccination with Brucella abortus rough mutant RB51 protects BALB/c mice against virulent strains of Brucella abortus, Brucella melitensis, and Brucella ovis. Infect Immun. 1994;62:4990–4996. doi: 10.1128/iai.62.11.4990-4996.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Latimer E, Simmers J, Sriranganathan N, Roop M, Schurig G G, Boyle S M. Brucella abortus deficient in copper/zinc superoxide dismutase is virulent in Balb/C mice. Microb Pathog. 1992;12:105–113. doi: 10.1016/0882-4010(92)90113-3. [DOI] [PubMed] [Google Scholar]
- 5.Montaraz J A, Winter A J. Comparison of living and nonliving vaccines for Brucella abortus in BALB/c mice. Infect Immun. 1986;53:245–251. doi: 10.1128/iai.53.2.245-251.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliveira S C, Splitter G A. Immunization of mice with recombinant L7/L12 ribosomal protein confers protection against Brucella abortus infection. Vaccine. 1996;14:959–962. doi: 10.1016/0264-410x(96)00018-7. [DOI] [PubMed] [Google Scholar]
- 7.Oñate A, Folch H. 18.5 Kda protein: an interesting antigen of Brucella. Arch Med Vet. 1995;27:93–102. [Google Scholar]
- 8.Schurig G G, Roop R M, Bagchi T, Boyle S, Buhrman D, Sriranganathan N. Biological properties of RB51; a stable rough strain of Brucella abortus. Vet Microbiol. 1991;28:171–188. doi: 10.1016/0378-1135(91)90091-s. [DOI] [PubMed] [Google Scholar]
- 9.Snedecor G W, Cochran W G. Statistical methods. 8th ed. Ames: Iowa State University; 1989. [Google Scholar]
- 10.Stevens M G, Tabatabai L B, Olsen C S, Cheville N F. Immune response to superoxide dismutase and synthetic peptides of superoxide dismutase in cattle vaccinated with Brucella abortus strain 19 or RB51. Vet Immunol. 1994;41:383–389. doi: 10.1016/0378-1135(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 11.Stevens M G, Olsen S C, Pugh G W, Jr, Brees D. Comparison of immune responses and resistance to brucellosis in mice vaccinated with Brucella abortus 19 or RB51. Infect Immun. 1995;63:264–270. doi: 10.1128/iai.63.1.264-270.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tabatabai L B, Pugh G W., Jr Modulation of immune response in BALB/c mice vaccinated with Brucella abortus Cu-Zn superoxide dismutase synthetic peptide vaccine. Vaccine. 1994;12:919–924. doi: 10.1016/0264-410x(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 13.Tatum F M, Detilleux P G, Sacks J M, Halling S M. Construction of Cu-Zn superoxide dismutase deletion mutants of Brucella abortus: analysis of survival in vitro in epithelial and phagocytic cells and in vivo in mice. Infect Immun. 1992;60:2863–2869. doi: 10.1128/iai.60.7.2863-2869.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]