Abstract
Use of tobacco products contributes to hundreds of thousands of premature deaths and untold millions of dollars in health care costs in this country each year. Nicotine is the principal neuroactive component in tobacco, but, despite ongoing research efforts, the cellular basis of its effects on behavior remains unclear. Efforts to resolve this conundrum have focused on the mesoaccumbens dopamine system, which contributes to the rewarding effects of many addictive drugs, including nicotine. The goal of this review is to outline recent advances and highlight some of the important unanswered questions regarding nicotine’s effects on neuronal excitability and synaptic plasticity within the brain reward pathways.
Keywords: Nicotinic receptor, Dopamine, Ventral tegmental area, LTP
Introduction
The complex behavioral phenomenon of drug addiction is ultimately a biological process, where repeated exposure to a drug alters the activity and metabolism of neurons that are sensitive to that drug. Over time, this alters the properties of individual neurons and the circuits to which they contribute, leading to complex behaviors such as dependence, tolerance, sensitization, and craving (Koob 2000; Nestler and Aghajanian 1997; Vezina 2004). Considerable research effort is now focused on identifying the cellular mechanisms underlying each of these behaviors. This review will focus on the mechanisms underlying the induction of sensitization to nicotine. Sensitization occurs with repeated or prolonged drug treatment and results in enhanced responsiveness to subsequent drug exposure, even after long withdrawal times. The phenomenon can be assayed as an increase in locomotor response to drug administration (Benwell and Balfour 1992; Clarke and Kumar 1983; Kalivas and Stewart 1991; Shim et al. 2001; Stolerman et al. 1973) or as enhanced extracellular dopamine (DA) levels at the projection areas of the midbrain reward pathway, as measured by microdialysis (Benwell and Balfour 1992; Cadoni and Di Chiara 2000; Rahman et al. 2003; Shim et al. 2001; Shoaib et al. 1994). Behavioral sensitization has been implicated in the development of drug addiction (Robinson and Berridge 1993) with its potential relevance to continuous self-administration in animals and drug craving and abuse in human addicts. It may also reflect a drug-induced change in motivation (Robinson and Berridge 2003; Vezina 2004). Although it is important to note that sensitization is not equivalent to drug dependence, several behavioral and neurochemical consequences of repeated noncontingent drug exposure are also associated with drug addiction.
We know that nicotine influences neuronal activity, and ultimately behavior, through its effects on nicotinic acetylcholine receptors (nAChRs). These receptors are pentameric membrane proteins that include two or more agonist binding sites and a central aqueous pore that opens to allow ion flux following agonist binding. Three properties of these receptors that contribute to their physiological effects include activation, desensitization, and upregulation following nicotine exposure. Each of these phenomena is likely to contribute to the behavioral sensitization to nicotine, but the relative importance of each is not known. We previously demonstrated key roles for nAChR activation (Mansvelder and McGehee 2000) and desensitization (Mansvelder et al. 2002) in acute nicotine-induced changes in reward circuitry. The functional importance of nAChR upregulation following prolonged nicotine exposure remains to be explored experimentally.
Circuitry of the Mesoaccumbens Dopamine System
A common feature of addictive drugs, including nicotine, is that they increase DA release in the nucleus accumbens (NAc) at the same concentrations achieved in serum during self-administration (Dani and Heinemann 1996; Picciotto et al. 1998; Stolerman and Jarvis 1995). The principal dopaminergic projections to NAc arise from neurons in the ventral tegmental area (VTA; Fig. 1a). Evidence that enhanced DA release is important in reward has come from VTA lesion studies and microperfusion of the NAc with DA receptor antagonists, both of which result in reduced self-administration of many addictive drugs, including nicotine (Corrigall and Coen 1991; Corrigall et al. 1994, 1992; Louis and Clarke 1998; Museo and Wise 1995; O’Neill et al. 1991; Vezina 2004). Recent behavioral studies suggest that DA encodes information about incentive salience or reward expectation, rather than reward per se (Berridge and Robinson 1998; Schultz 2002). Although DA release may not directly encode reward, it remains a key element in the reward circuitry and the focus of numerous studies on drug addiction.
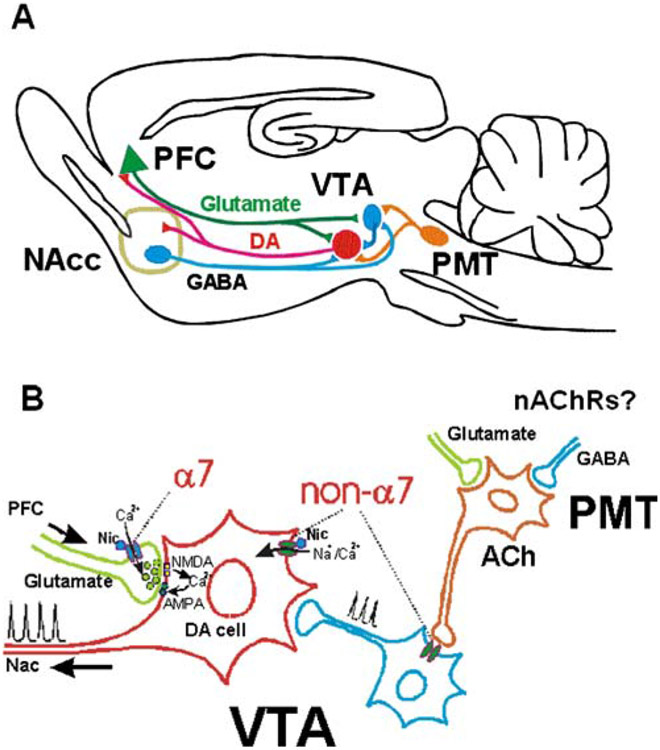
Figure 1.
A simplified diagram of brain reward circuitry. a A simplified schematic of the glutamatergic and GABAergic projections to the VTA (Charara et al. 1996; Kalivas et al. 1993; Omelchenko and Sesack 2006; Sesack et al. 2003; Sesack and Pickel 1992). b Some sites of nAChR expression within the mesoaccumbens dopaminergic circuitry. The cellular localization and functional role of nAChRs within PMT are currently unknown
Principal Excitatory Inputs to the VTA
The principal excitatory inputs to the VTA are glutamatergic projections from prefrontal cortex (PFC), bed nucleus of the stria terminalis, amygdala, and the pontomesencephalic tegmental (PMT) nuclei (Fig. 1a). The Sesack laboratory has demonstrated an interesting segregation of PFC projections to VTA, where VTA neurons that project to cortex receive glutamatergic inputs from PFC, while NAc-projecting VTA neurons do not (Carr and Sesack 2000). The NAc-projecting DA neurons may be primarily controlled by excitatory inputs from the PMT (Sesack et al. 2003), which are apparently under control of PFC inputs (Semba and Fibiger 1992). This polysynaptic arrangement may allow for more modulatory sites within the circuit controlling excitability of the NAc-projecting DA neurons (Fig. 1a).
The PMT is made up of two brainstem nuclei known as the pedunculopontine tegmental (PPTg) nucleus and the lateral dorsal tegmental (LDTg) nucleus. The LDTg sends proportionately more projections to VTA than the PPTg (Oakman et al. 1995). It is important to note that PMT projections are approximately 50% cholinergic, 50% glutamatergic, and 25% gamma-aminobutyric acid (GABA)ergic—overlap in neurotransmitter expression in a subset of neurons accounts for a total >100% (Sesack et al. 2003). Although previous anatomical studies suggested that the cholinergic projections to VTA synapse primarily upon non-DA neurons (Garzón et al. 1999), more recent investigations have demonstrated prominent cholinergic projections to both DA and non-DA neurons within the VTA (Omelchenko and Sesack 2006). While functional evidence for nicotinic cholinergic synapses in VTA is rare, we have shown that ACh released onto GABA neurons is important for tonic inhibitory control of DA neurons (Mansvelder et al. 2002).
PMT projections to VTA are of critical importance in the rewarding effects of several different drugs of abuse, including nicotine (Corrigall et al. 2001, 2002; Lanca et al. 2000; Laviolette et al. 2002; Laviolette and van der Kooy 2004). The Corrigall group showed that chemical lesions of PMT cholinergic neurons or administration of either nicotinic antagonists, GABA agonists, or other pharmacological inhibitors of excitation into the PMT reduced nicotine and cocaine self-administration (Corrigall et al. 2001, 2002; Lanca et al. 2000). Schoffelmeer et al. (2002) showed that nicotinic antagonists administered systemically decreased the sensitizing effects of repeated amphetamine injections, implicating cholinergic signaling in the rewarding effects of that drug. The van der Kooy laboratory has presented evidence that PMT lesions eliminate the rewarding effects of nicotine and reveal the aversive effects normally seen with higher doses (Laviolette et al. 2002). Together, these studies illustrate the potentially critical role that these cholinergic centers play in drug reward and highlight the need for further study of drug effects within this area that affects VTA excitability.
Principal Inhibitory Inputs to the VTA
The principal inhibitory inputs to VTA DA neurons are GABAergic, including local interneurons and projections from NAc and the ventral pallidum (Kalivas et al. 1993). We previously showed that nAChR-mediated modulation of GABAergic inputs to VTA DA neurons was pharmacologically similar to the nAChR responses of GABAergic interneurons in VTA (Mansvelder et al. 2002), which supports the idea that these GABA interneurons innervate VTA DA neurons. In addition, lateral habenula (LHb) stimulation inhibits the majority of spontaneously firing DA neurons in the VTA and the substantia nigra pas compacta (SNpc; Christoph et al. 1986; Ji and Shepard 2007). Suppression of the VTA DA neuron activity is likely mediated by the excitatory projections from LHb to the VTA GABA interneurons (Ji and Shepard 2007). A direct projection from the LHb to the midbrain structures including VTA and SNpc has long been established (Lecourtier and Kelly 2007). Interestingly, a recent study by Matsumoto and Hikosaka (2007) has revealed that LHb projections to the VTA mediate the suppression of DA neuron firing in response to aversive environmental stimuli.
Numerous other neurotransmitters and modulators contribute to VTA activity. While this review focuses on ACh, GABA, and glutamate, there are clearly many cellular interactions within the reward pathways that contribute to excitability and the physiological responses to addictive drugs such as nicotine.
Nicotinic Receptors and Nicotine Addiction
Ultimately, nicotine influences neuronal activity, synaptic communication, and behavior through its effects on nAChRs. Some nAChR subtypes and their locations within the brain reward circuitry are schematized in Fig. 1b. nAChR activation results in increased cation flux through the channel, inducing depolarization, increased excitability, and changes in intracellular Ca2+.
Functional Properties of Nicotinic Receptors
Important properties of nicotinic receptors include activation, desensitization, and upregulation following nicotine exposure (Albuquerque et al. 2009). At the cellular and circuit levels, nAChR activation mediates excitatory transmission, primarily in the peripheral nervous system, but some studies have shown nicotinic-receptor-mediated synaptic transmission in the central nervous system (CNS; Finnegan et al. 2004; Frazier et al. 1998). A more prevalent role for these receptors is modification of release of other transmitters (McGehee and Role 1995; Wonnacott 1997). We showed that activation of presynaptic α7 nAChRs on glutamatergic inputs to VTA DA neurons can enhance glutamate release and contribute to long-term potentiation (LTP) induction at that synapse (Mansvelder and McGehee 2000). In the continued presence of agonist, nAChRs show strong desensitization, and the prolonged nicotine exposure during smoking suggests that desensitization may be of key importance for the behavioral effects (Dani and Heinemann 1996). In another study, we found that high-affinity non-α7 nAChRs set the basal GABAergic drive to VTA DA neurons and that physiologically relevant nicotine concentrations desensitize those receptors, leading to disinhibition of the DA neuron (Mansvelder et al. 2002). The same low nicotine concentrations were shown to have minimal desensitizing effects on the presynaptic α7 nAChRs that enhance excitation. The net effect of these modulatory changes is a shift towards greater excitability of the VTA DA neurons. Thus, both activation and desensitization of nAChRs contribute to nicotine’s effects on DA neuron excitability.
The earlier studies outlined above assessed the effects of acute nicotine treatment, but we do not yet know how the circuitry is modified with prolonged nicotine exposure. Long-term inactivation of nAChRs following prolonged nicotine exposure has been reported in studies of the receptors expressed in Xenopus oocytes (Olale et al. 1997). There are some in vivo studies showing depressed nicotinic responses following nicotine treatment (Marks et al. 1993). However, the bulk of the evidence from cellular analyses of receptor function in mammalian expression systems or native receptors in primary cells does not support long-term receptor inactivation as an important functional end point following chronic nicotine exposure. It is possible that homeostatic modifications could occur, as discussed later in this manuscript, but it is unknown whether changes in baseline excitability affect nAChR expression. On the other hand, upregulation of nAChRs following prolonged or repeated agonist exposure has been reported in vitro and in vivo, but it is not known whether this phenomenon contributes to increased excitability of VTA DA neurons. The functional impact of nAChR upregulation within the dopaminergic system is discussed in more detail below.
Nicotinic Receptor Subunit Composition in VTA
Pharmacological and ligand-binding studies have demonstrated considerable diversity in neuronal nAChRs (McGehee and Role 1995; Sargent 1993). To date, 12 nAChR subunit genes have been identified, α2-α10 and β2-β4 (Heinemann et al. 1990). Identifying the subunits that contribute to nAChR responses can be accomplished to a degree with selective agonists and antagonists (McGehee and Role 1995; Sargent 1993). However, one must be cautious when inferring nAChR structure from drug sensitivity, as much of this pharmacological information has come from heterologous expression studies. With this caveat in mind, selective ligands can be used to indicate the contribution of specific subunits to native receptor responses.
Several nAChR classes are functionally expressed in the VTA (Klink et al. 2001; Mansvelder and McGehee 2002; Pidoplichko et al. 1997; Yin and French 2000), and mRNA for many nAChR subunits has been reported (Wada et al. 1989). Specifically, the nAChRs expressed on non-DA, predominantly GABAergic, neurons in the VTA have α4β2 pharmacology (Mansvelder et al. 2002), but α7 nAChR currents can also be elicited (Klink et al. 2001). Pharmacological testing of DA neuron responses to focal application of nicotinic agonists and antagonists in midbrain tissue slices indicates that both α7 and non-α7 nAChRs are expressed by DA neurons, with a lower prevalence of α7 receptors (Pidoplichko et al. 1997). The non-α7 nAChRs on the DA neurons likely include α4β2* and α6β2* (asterisks denote the possibility of other subunits being incorporated in the receptors) with potential contribution of β3 and possibly other subunits (Champtiaux et al. 2003, 2002; Marubio et al. 2003). Ten-nanomolar methyllycaconitine (MLA), which was previously thought to be selective for α7 nAChRs, has also been shown to block a subset of nAChR currents with slow activation kinetics, which may represent the α6-containing nAChRs in DA neurons (Klink et al. 2001). Thus, some caution is necessary in interpreting the effects of MLA in DA neurons, as they are one of the rare cell types that express α6-containing nAChRs. Sensitivity to MLA is still a useful measure as it clearly differentiates the α7- and non-α7 nAChRs. However, use of other more selective antagonists, such as alpha-bungarotoxin (αBGT) and α-conotoxin MII (αCTXMII) would greatly facilitate the pharmacological identification of α7- and α6-containing nAChRs. The cone snail toxin αCTXMII is known to inhibit α3β2 nAChRs (Cartier et al. 1996), and its blockade of nicotine-induced DA release from striatal synaptosomes was previously thought to indicate presence of this receptor subtype (Kulak et al. 1997). However, evidence from mutant mice has shown that αCTXMII likely targets α6 nAChRs in the DA neurons, as the αCTXMII-sensitive component of DA release was absent in α6−/− mice (Champtiaux et al. 2002). Regarding the functional role of the nAChRs expressed in VTA, somatodendritic nAChRs may receive cholinergic input, but this is apparently a meager input, as glutamate antagonists block 100% of the excitatory inputs in VTA recordings in our laboratory (unpublished observation). A predominant role of nAChRs in VTA is synaptic modulation, as seen in many other brain areas (Mansvelder and McGehee 2002; McGehee and Role 1996; Wonnacott 1997).
Localization of nAChRs that Modulate Glutamate Release
There is strong evidence for the expression of α7 nAChRs on presynaptic nerve terminals. Ultrastructural analyses in guinea pig cortex (Fabian-Fine et al. 2001) and rat hippocampus (Lubin et al. 1999) indicate expression of α7 receptor protein on presynaptic structures. Denervation of the interpeduncular nucleus (Clarke et al. 1985) and dorsal spinal cord (Ninkovic and Hunt 1983) corresponds with decreases in αBGT binding. Direct effects of ACh on presynaptic membrane currents were reported in calyx synapses (Coggan et al. 1997), and nAChRs can induce increased [Ca2+]i in presynaptic structures (Gray et al. 1996; McGehee et al. 1995). In sum, these findings show that presynaptic α7 nAChRs modulate glutamate transmission. It should be noted that cultured astrocytes also express functional α7 nAChRs (Sharma and Vijayaraghavan 2001). The activation of these receptors can increase intracellular Ca2+, which could result in vesicular glutamate release (Parpura and Haydon 2000). While this suggests that α7-mediated enhancement of glutamate release in CNS may derive partly from glial sources, neuronal expression of presynaptic nAChRs is clearly an important aspect of this modulation in VTA.
Physiologically Relevant Nicotine Concentrations
There are no studies in which brain levels of nicotine in human smokers were measured. The best approximation has come from assays of nicotine in arterial blood of smokers, which indicate peak levels between 100 and 500 nM with resting levels of 10 to 50 nM (Henningfield et al. 1993). The high-affinity nAChRs including α4β2*, α3β2*, and α6β2* subtypes can be activated, desensitized, and upregulated by these nicotine concentrations (Fenster et al. 1999). It is worth noting that both repeated and continuous nicotine exposure in rat models lead to preferential accumulation of nicotine in the brain, at concentrations several-fold higher than those found in blood (Doura et al. 2008; Ghosheh et al. 2001), suggesting that residual level of nicotine in the heavy smoker’s brain might approach a couple hundred nanomolar while peak brain concentration might approach micromolar levels. Indeed, similar nicotine concentrations activate and desensitize nAChR currents in VTA neurons and modulate synaptic inputs to the DA neurons (Mansvelder et al. 2002; Pidoplichko et al. 1997). Although higher concentrations can have profound effects on neuronal excitability throughout the central and peripheral nervous systems, testing nicotine’s effects within a physiologically relevant range is critically important for correlating cellular or tissue effects with the behavioral effects of the drug.
Nicotinic Receptor Upregulation
Along with differences in pharmacological and biophysical properties, nAChR upregulation following pre-exposure to nicotine varies with subunit composition, cell type, and brain region (Buisson and Bertrand 2001; Flores et al. 1992; Marks et al. 1983; Nguyen et al. 2003; Perry et al. 2007; Schwartz and Kellar 1983, 1985). Prolonged treatment with low, physiologically relevant nicotine concentrations can upregulate α4β2 and α3β2 receptors in heterologous expression systems, as measured by radioligand binding or functional current (Buisson and Bertrand 2001; Wang et al. 1998). The Green laboratory has recently shown upregulation of α6β2 at similar low nicotine concentrations (Walsh et al. 2008). Other receptor subtypes, including α7- and β4-containing nAChRs, appear to be less sensitive to upregulation and require higher nicotine concentrations (>10 μM; Kawai and Berg 2001; Molinari et al. 1998; Rogers and Wonnacott 1997; Schwartz and Kellar 1985). Consistent with the in vitro evidence, in vivo nicotine administration also predominantly upregulates the β2-containing receptors, particularly the α4β2 receptors, with α3β2-like binding increase only in a few discrete areas (Nguyen et al. 2003). However, α6β2* receptors in vivo appear to be distinctively regulated by nicotine with different laboratories reporting increase, decrease, and no change after prolonged exposure to nicotine (Lai et al. 2005; Mugnaini et al. 2006; Parker et al. 2004; Perry et al. 2007). It is possible that nicotine exposure paradigm and presence of accessory subunit such as β3 may affect the outcome of nicotine regulation on the α6β2* receptors (Kuryatov et al. 2008; Perry et al. 2007; Tumkosit et al. 2006).
Nicotinic receptor upregulation has been described as a posttranslational event, in that it is not accompanied by changes in subunit mRNAs (Bencherif et al. 1995; Marks et al. 1992; Peng et al. 1997) or de novo protein synthesis (Peng et al. 1994; Wang et al. 1998). Recently, several studies have converged on the proposal that mechanisms of nicotinic receptor upregulation involve nicotine serving as a molecular chaperon to increase maturation of nascent oligomers of the nicotinic receptors (Corringer et al. 2006; Kuryatov et al. 2005; Sallette et al. 2005). Although a majority of the receptors accumulate inside cell in the heterologous expression systems, a fraction of the upregulated receptors do traffic to the cell surface (Harkness and Millar 2002; Olale et al. 1997; Wang et al. 1998). In addition, using tagged α4 subunits, the Green laboratory reported evidence that upregulation may actually reflect a change in receptor affinity and function rather than a change in receptor number (Vallejo et al. 2005). Additional evidence suggests that alteration in receptor stoichiometry involving more β2 subunits inserted into the channels following nicotine exposure may underlie the change in receptor function and affinity after nicotine exposure (Nelson et al. 2003). Both mechanisms are in agreement with those proposed in previous functional studies demonstrating an upregulation-associated change in receptor channel kinetics (Buisson and Bertrand 2001; Buisson et al. 2000).
Despite in vitro evidence, direct assessment of the receptor channel functions after in vivo nicotine pre-exposure has generated conflicting results. Several laboratories have reported increases in nicotinic responses after upregulation (Buisson and Bertrand 2001; Clarke et al. 1988; Nguyen et al. 2004; Rowell and Wonnacott 1990; Yu and Wecker 1994), while others report decreased function (Lapchak et al. 1989; Marks et al. 1993, 1985). More recent in vivo studies have reported functional upregulation of nAChRs following in vivo nicotine exposure. Alkondon and Albuquerque (2005) reported enhanced nAChR-mediated responses in hippocampal slices from P14–15 rats that were pre-exposed to nicotine (two injections of 0.586 mg/kg in less than 24 h). These data suggest that nAChR upregulation can be assayed relatively soon after the last nicotine exposure (<2 h after the last injection). In mice expressing fluorescence-tagged α4 subunits, continuous nicotine infusion through a subcutaneous osmotic mini-pump specifically upregulated α4-containing nAChRs in the midbrain GABA neurons but not the DA neurons; moreover, the cell-type-specific upregulation appeared to be functional, at least in the substantia nigra and hippocampus (Nashmi et al. 2007).
Differences in assays, species, animal age, and treatment paradigms may explain some of the variability in the reported effects of in vivo nicotine on nAChR function, but this emphasizes the importance of using a multidisciplinary approach to assess differences in nAChR function under various treatment conditions. Thus, understanding the cellular basis for the pre-exposure-induced sensitization to nicotine’s biochemical and behavioral effects will also require a convergence of methodologies (Shoaib et al. 1994; Wise 1988).
Long-Term Synaptic Plasticity and Sensitization
The persistent behavioral effects of addictive drugs indicate they can induce nearly permanent changes in CNS function. Ultimately, this long-lasting behavioral plasticity must be explained in terms of its cellular and molecular mechanisms. An attractive and reasonable hypothesis is that long-term synaptic plasticity and other cellular mechanisms of learning and memory contribute to drug addiction (Dani et al. 2001; Kauer 2004; Nestler 2001).
Our laboratory found that nicotine could contribute to LTP induction in the VTA, which was among the first direct demonstrations of long-term synaptic modulation by a drug of abuse (Mansvelder and McGehee 2000). Nicotine-induced glutamate release was shown to contribute to LTP induction. In hippocampal slices, a weak stimulus that induced only short-term potentiation could result in LTP induction when paired with focal ACh application to activate postsynaptic nAChRs (Ji et al. 2001). Bonci and colleagues reported that a single cocaine injection in vivo increased the prevalence of potentiated synapses in the VTA and that the potentiation persisted for up to 5 days following this single drug exposure (Ungless et al. 2001). This was followed by the finding that single injections of several other drugs of abuse, including nicotine, could also potentiate the excitatory inputs to VTA DA neurons (Saal et al. 2003). However, repeated cocaine injections did not enhance the magnitude or prolong the duration of LTP in the VTA (Borgland et al. 2004). Thus, it is not likely that LTP expression in VTA underlies the sensitized phenotype that lasts weeks or months following repeated drug exposure (Shoaib et al. 1994). Given the links between DA release and sensitization, however, it is reasonable to hypothesize that LTP of excitatory inputs to VTA DA neurons is an important step in the induction of sensitization. Interestingly, a recent study by Chen et al. (2008) revealed that only self-administration, but not yoked administration or repeated i.v. infusion, of cocaine lead to persistent LTP in the VTA, lasting over 3 weeks in adult rats. In fact, α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA)/N-methyl-d-aspartate (NMDA) ratios remained elevated for 3 months throughout the abstinence even when the drug-seeking behavior was extinguished. This study emphasizes the importance of contingency in drug-induced LTP in the VTA DA neurons. It remains to be shown whether the observations with cocaine in adolescent rats generalize to nicotine’s effects on adult rats. In addition, the cellular mechanisms underlying LTP induction following nicotine exposure are unknown, beyond a requirement for NMDA receptor activation.
Glutamatergic synapses in the VTA have also been shown to undergo LTD, which can be inhibited by amphetamine exposure (Jones et al. 2000). These observations emphasize the importance of considering all forms of plasticity in testing drug effects. Examination of LTD induction following in vivo nicotine exposure remains to be investigated.
LTP is developmentally regulated, either positively or negatively depending upon the tissue. In the hippocampus CA1 region, LTP is expressed after postnatal day 14 into adulthood (Bolshakov and Siegelbaum 1995). At thalamocortical synapses, LTP and LTD are inducible only until day 14 (Crair and Malenka 1995; Feldman et al. 1999). We have seen LTP expression in VTA between postnatal days 10 and 20 in rats (Mansvelder and McGehee 2000). VTA expression of LTP/LTD was seen by other labs using rats aged 16–28 days (Jones et al. 2000) or 14–42 days (Bonci and Malenka 1999) or mice aged 21–35 days (Saal et al. 2003; Thomas et al. 2000; Ungless et al. 2001). These age ranges suggest that long-term plasticity is expressed in adult VTA. Indeed, cocaine self-administration has been shown to induce LTP in rats aged P80 to P140 in a recent study (Chen et al. 2008). It remains to be demonstrated whether in vivo nicotine can also elicit LTP in adult VTA.
Homeostatic Synaptic Modulation and Drug Sensitization
In addition to LTP and LTD, drug exposure is likely to induce homeostatic changes within reward-associated pathways. This form of plasticity, also referred to as “synaptic scaling,” is a much slower process involving numerous changes in ion channel and neurotransmitter receptor expression that help regulate neuronal firing rates within an optimal range to maintain health and viability (Turrigiano and Nelson 2004). Chronic and repeated exposure to drugs of abuse such as nicotine occurs over extended time periods. Indeed, recent investigations have implicated homeostatic plasticity in the effects of morphine and exogenous cannabinoids on AMPA receptor expression and LTD induction (Glass et al. 2005; Mato et al. 2005). There is extensive evidence supporting the idea the nicotine exposure causes increased excitability within the reward pathways, particularly the VTA. Thus, it is critical to consider possible homeostatic modifications in synaptic strength that may occur in combination with the more immediate activity-dependent plasticity discussed above. It is also important to consider that homeostatic modifications of synaptic strength do not preclude expression of LTP or LTD at these same synaptic inputs, rather, these changes are relevant to the baseline strength from which LTP or LTD would be expressed.
Summary
The acute and persistent effects of nicotine exposure are the focus of ongoing investigations by many different research groups. The extent to which specific receptor functions and cellular consequences contribute to the behavioral effects of the drug remains to be determined. The success of these research efforts may lead to more effective smoking cessation aids. This would decrease smoking-related disease and prolong the lives of the huge numbers of chronic smokers who report a desire to quit but have been unsuccessful with currently available methods.
Acknowledgements
We thank Robert Mitchum for his helpful suggestions on an earlier draft of this manuscript and W. Green and P. Vezina for encouragement and support of our research efforts. This work was supported by NIH grants DA015918 and DA019695.
Footnotes
Proceedings of the XIII International Symposium on Cholinergic Mechanisms
Contributor Information
Danyan Mao, Department of Anesthesia and Critical Care, University of Chicago, 5841 S Maryland Ave, MC4028, Chicago, IL 60637, USA.
Daniel S. McGehee, Committee on Neurobiology, University of Chicago, 5841 S Maryland Ave, MC4028, Chicago, IL 60637, USA
References
- Albuquerque EX, Pereira EFR, Alkondon M, & Rogers S (2009). Physiological Reviews, 89(1), 73–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkondon M, & Albuquerque EX (2005). Nicotinic receptor subtypes in rat hippocampal slices are differentially sensitive to desensitization and early in vivo functional upregulation by nicotine and to block by bupropion. Journal of Pharmacology and Experimental Therapeutics, 313, 740–750. [DOI] [PubMed] [Google Scholar]
- Bencherif M, Fowler K, Lukas R, & Lippiello P (1995). Mechanisms of up-regulation of neuronal nicotinic acetylcholine receptors in clonal cell lines and primary cultures of fetal rat brain. Journal of Pharmacology and Experimental Therapeutics, 275, 987–994. [PubMed] [Google Scholar]
- Benwell ME, & Balfour DJ (1992). The effects of acute and repeated nicotine treatment on nucleus accumbens dopamine and locomotor activity. British Journal of Pharmacology, 105, 849–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, & Robinson TE (1998). What is the role of dopamine in reward: Hedonic impact, reward learning, or incentive salience? Brain Research. Brain Research Reviews, 28, 309–369. [DOI] [PubMed] [Google Scholar]
- Bolshakov VY, & Siegelbaum SA (1995). Regulation of hippocampal transmitter release during development and long-term potentiation. Science, 269, 1730–1734. [DOI] [PubMed] [Google Scholar]
- Bonci A, & Malenka RC (1999). Properties and plasticity of excitatory synapses on dopaminergic and GABAergic cells in the ventral tegmental area. Journal of Neuroscience, 19, 3723–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, & Bonci A (2004). Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: Electrophysiological and behavioral correlates in individual rats. Journal of Neuroscience, 24, 7482–7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisson B, & Bertrand D (2001). Chronic exposure to nicotine upregulates the human (alpha)4(beta)2 nicotinic acetylcholine receptor function. Journal of Neuroscience, 21, 1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisson B, Vallejo YF, Green WN, & Bertrand D (2000). The unusual nature of epibatidine responses at the alpha4beta2 nicotinic acetylcholine receptor. Neuropharmacology, 39, 2561–2569. [DOI] [PubMed] [Google Scholar]
- Cadoni C, & Di Chiara G (2000). Differential changes in accumbens shell and core dopamine in behavioral sensitization to nicotine. European Journal of Pharmacology, 387, R23–R25. [DOI] [PubMed] [Google Scholar]
- Carr DB, & Sesack SR (2000). Projections from the rat prefrontal cortex to the ventral tegmental area: Target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. Journal of Neuroscience, 20, 3864–3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier GE, Yoshikami D, Gray WR, et al. (1996). A new alpha-conotoxin which targets alpha3beta2 nicotinic acetylcholine receptors. Journal of Biological Chemistry, 271, 7522–7528. [DOI] [PubMed] [Google Scholar]
- Champtiaux N, Han ZY, Bessis A, et al. (2002). Distribution and pharmacology of alpha 6-containing nicotinic acetylcholine receptors analyzed with mutant mice. Journal of Neuroscience, 22, 1208–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M, et al. (2003). Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. Journal of Neuroscience, 23, 7820–7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charara A, Smith Y, & Parent A (1996). Glutamatergic inputs from the pedunculopontine nucleus to midbrain dopaminergic neurons in primates: Phaseolus vulgaris-leucoagglutinin anterograde labeling combined with postembedding glutamate and GABA immunohistochemistry. Journal of Comparative Neurology, 364, 254–266. [DOI] [PubMed] [Google Scholar]
- Chen BT, Bowers MS, Martin M, et al. (2008). Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron, 59, 288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoph G, Leonzio R, & Wilcox K (1986). Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. Journal of Neuroscience, 6, 613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke P, & Kumar R (1983). The effects of nicotine on locomotor activity in non-tolerant and tolerant rats. British Journal of Pharmacology, 78, 329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PB, Schwartz RD, Paul SM, Pert CB, & Pert A (1985). Nicotinic binding in rat brain: Autoradiographic comparison of[3H] acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. Journal of Neuroscience, 5, 1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PB, Fu DS, Jakubovic A, & Fibiger HC (1988). Evidence that mesolimbic dopaminergic activation underlies the locomotor stimulant action of nicotine in rats. Journal of Pharmacology and Experimental Therapeutics, 246, 701–708. [PubMed] [Google Scholar]
- Coggan JS, Paysan J, Conroy WG, & Berg DK (1997). Direct recording of nicotinic responses in presynaptic nerve terminals. Journal of Neuroscience, 17, 5798–5806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, & Coen KM (1991). Selective dopamine antagonists reduce nicotine self-administration. Psychopharmacology (Berl), 104, 171–176. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, & Clarke PB (1992). The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl), 107, 285–289. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, & Adamson KL (1994). Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Research, 653, 278–284. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Zhang J, & Adamson KL (2001). GABA mechanisms in the pedunculopontine tegmental nucleus influence particular aspects of nicotine self-administration selectively in the rat. Psychopharmacology (Berl), 158, 190–197. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Zhang J, & Adamson L (2002). Pharmacological manipulations of the pedunculopontine tegmental nucleus in the rat reduce self-administration of both nicotine and cocaine. Psychopharmacology (Berl), 160, 198–205. [DOI] [PubMed] [Google Scholar]
- Corringer P-J, Sallette J, & Changeux J-P (2006). Nicotine enhances intracellular nicotinic receptor maturation: A novel mechanism of neural plasticity? Journal of Physiology-Paris. The World of the Synapse: Molecular Basis, Pathologies and Drug Discovery, 99, 162–171. [DOI] [PubMed] [Google Scholar]
- Crair MC, & Malenka RC (1995). A critical period for long-term potentiation at thalamocortical synapses. Nature, 375, 325–328. [DOI] [PubMed] [Google Scholar]
- Dani JA, & Heinemann S (1996). Molecular and cellular aspects of nicotine abuse. Neuron, 16, 905–908. [DOI] [PubMed] [Google Scholar]
- Dani JA, Ji D, & Zhou FM (2001). Synaptic plasticity and nicotine addiction. Neuron, 31, 349–352. [DOI] [PubMed] [Google Scholar]
- Doura MB, Gold AB, Keller AB, & Perry DC (2008). Adult and periadolescent rats differ in expression of nicotinic cholinergic receptor subtypes and in the response of these subtypes to chronic nicotine exposure. Brain Research, 1215, 40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian-Fine R, Skehel P, Errington ML, et al. (2001). Ultrastructural distribution of the alpha7 nicotinic acetylcholine receptor subunit in rat hippocampus. Journal of Neuroscience, 21, 7993–8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE, Nicoll RA, & Malenka RC (1999). Synaptic plasticity at thalamocortical synapses in developing rat somatosensory cortex: LTP, LTD, and silent synapses. Journal of Neurobiology, 41, 92–101. [PubMed] [Google Scholar]
- Fenster CP, Whitworth TL, Sheffield EB, Quick MW, & Lester RA (1999). Upregulation of surface alpha4beta2 nicotinic receptors is initiated by receptor desensitization after chronic exposure to nicotine. Journal of Neuroscience, 19, 4804–4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan TF, Li DP, Chen SR, & Pan HL (2004). Activation of mu-opioid receptors inhibits synaptic inputs to spinally projecting rostral ventromedial medulla neurons. Journal of Pharmacology and Experimental Therapeutics, 309, 476–483. [DOI] [PubMed] [Google Scholar]
- Flores C, Rogers S, Pabreza L, Wolfe B, & Kellar K (1992). A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Molecular Pharmacology, 41, 31–37. [PubMed] [Google Scholar]
- Frazier CJ, Buhler AV, Weiner JL, & Dunwiddie TV (1998). Synaptic potentials mediated via alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors in rat hippocampal interneurons. Journal of Neuroscience, 18, 8228–8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzón M, Vaughan RA, Uhl GR, Kuhar MJ, & Pickel VM (1999). Cholinergic axon terminals in the ventral tegmental area target a subpopulation of neurons expressing low levels of the dopamine transporter. Journal of Comparative Neurology, 410, 197–210. [DOI] [PubMed] [Google Scholar]
- Ghosheh OA, Dwoskin LP, Miller DK, & Crooks PA (2001). Accumulation of nicotine and its metabolites in rat brain after intermittent or continuous peripheral administration of [2′-14C] nicotine. Drug Metabolism and Disposition, 29, 645–651. [PubMed] [Google Scholar]
- Glass MJ, Kruzich PJ, Colago EE, Kreek MJ, & Pickel VM (2005). Increased AMPA GluR1 receptor subunit labeling on the plasma membrane of dendrites in the basolateral amygdala of rats self-administering morphine. Synapse, 58, 1–12. [DOI] [PubMed] [Google Scholar]
- Gray R, Rajan AS, Radcliffe KA, Yakehiro M, & Dani JA (1996). Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature, 383, 713–716. [DOI] [PubMed] [Google Scholar]
- Harkness PC, & Millar NS (2002). Changes in conformation and subcellular distribution of alpha4beta2 nicotinic acetylcholine receptors revealed by chronic nicotine treatment and expression of subunit chimeras. Journal of Neuroscience, 22, 10172–10181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann S, Boulter J, Deneris E, et al. (1990). The brain nicotinic acetylcholine receptor gene family. Progress in Brain Research, 86, 195–203. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Stapleton JM, Benowitz NL, Grayson RF, & London ED (1993). Higher levels of nicotine in arterial than in venous blood after cigarette smoking. Drug and Alcohol Dependence, 33, 23–29. [DOI] [PubMed] [Google Scholar]
- Ji H, & Shepard PD (2007). Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABAA receptor-mediated mechanism. Journal of Neuroscience, 27, 6923–6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Lape R, & Dani JA (2001). Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron, 31, 131–141. [DOI] [PubMed] [Google Scholar]
- Jones S, Kornblum JL, & Kauer JA (2000). Amphetamine blocks long-term synaptic depression in the ventral tegmental area. Journal of Neuroscience, 20, 5575–5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, & Stewart J (1991). Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Research Reviews, 16, 223–244. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Churchill L, & Klitenick MA (1993). GABA and enkephalin projection from the nucleus accumbens and ventral pallidum to the ventral tegmental area. Neuroscience, 57, 1047–1060. [DOI] [PubMed] [Google Scholar]
- Kauer JA (2004). Learning mechanisms in addiction: Synaptic plasticity in the ventral tegmental area as a result of exposure to drugs of abuse. Annual Review of Physiology, 66, 447–475. [DOI] [PubMed] [Google Scholar]
- Kawai H, & Berg DK (2001). Nicotinic acetylcholine receptors containing alpha 7 subunits on rat cortical neurons do not undergo long-lasting inactivation even when up-regulated by chronic nicotine exposure. Journal of Neurochemistry, 78, 1367–1378. [DOI] [PubMed] [Google Scholar]
- Klink R, de Kerchove d’Exaerde A, Zoli M, & Changeux JP (2001). Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. Journal of Neuroscience, 21, 1452–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2000). Neurobiology of addiction. Toward the development of new therapies. Annals of the New York Academy of Sciences, 909, 170–185. [DOI] [PubMed] [Google Scholar]
- Kulak JM, Nguyen TA, Olivera BM, & McIntosh JM (1997). Alpha-conotoxin MII blocks nicotine-stimulated dopamine release in rat striatal synaptosomes. Journal of Neuroscience, 17, 5263–5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Luo J, Cooper J, & Lindstrom J (2005). Nicotine acts as a pharmacological chaperone to up-regulate human {alpha}4 {beta}2 acetylcholine receptors. Molecular Pharmacology, 68, 1839–1851. [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Onksen J, & Lindstrom J (2008). Roles of accessory subunits in {alpha}4{beta}2* nicotinic receptors. Molecular Pharmacology, 74, 132–143. [DOI] [PubMed] [Google Scholar]
- Lai A, Parameswaran N, Khwaja M, et al. (2005). Long-term nicotine treatment decreases striatal {alpha}6* nicotinic acetylcholine receptor sites and function in mice. Molecular Pharmacology, 67, 1639–1647. [DOI] [PubMed] [Google Scholar]
- Lanca AJ, Adamson KL, Coen KM, Chow BL, & Corrigall WA (2000). The pedunculopontine tegmental nucleus and the role of cholinergic neurons in nicotine self-administration in the rat: A correlative neuroanatomical and behavioral study. Neuroscience, 96, 735–742. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Araujo DM, Quirion R, & Collier B (1989). Effect of chronic nicotine treatment on nicotinic autoreceptor function and N-[3H]methylcarbamylcholine binding sites in the rat brain. Journal of Neurochemistry, 52, 483–491. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, & van der Kooy D (2004). The neurobiology of nicotine addiction: Bridging the gap from molecules to behaviour. Nature Reviews Neuroscience, 5, 55–65. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Alexson TO, & van der Kooy D (2002). Lesions of the tegmental pedunculopontine nucleus block the rewarding effects and reveal the aversive effects of nicotine in the ventral tegmental area. Journal of Neuroscience, 22, 8653–8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecourtier L, & Kelly PH (2007). A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neuroscience & Biobehavioral Reviews, 31, 658–672. [DOI] [PubMed] [Google Scholar]
- Louis M, & Clarke PB (1998). Effect of ventral tegmental 6-hydroxydopamine lesions on the locomotor stimulant action of nicotine in rats. Neuropharmacology, 37, 1503–1513. [DOI] [PubMed] [Google Scholar]
- Lubin M, Erisir A, & Aoki C (1999). Ultrastructural immunolocalization of the alpha 7 nAChR subunit in guinea pig medial prefrontal cortex. Annals of the New York Academy of Sciences, 868, 628–632. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, & McGehee DS (2000). Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron, 27, 349–357. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, & McGehee DS (2002). Cellular and synaptic mechanisms of nicotine addiction. Journal of Neurobiology, 53, 606–617. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, & McGehee DS (2002). Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron, 33, 905–919. [DOI] [PubMed] [Google Scholar]
- Marks MJ, Stitzel JA, & Collins AC (1985). Time course study of the effects of chronic nicotine infusion on drug response and brain receptors. Journal of Pharmacology and Experimental Therapeutics, 235, 619–628. [PubMed] [Google Scholar]
- Marks M, Burch J, & Collins A (1983). Effects of chronic nicotine infusion on tolerance development and nicotinic receptors. Journal of Pharmacology and Experimental Therapeutics, 226, 817–825. [PubMed] [Google Scholar]
- Marks M, Pauly J, Gross S, et al. (1992). Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. Journal of Neuroscience, 12, 2765–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Grady SR, & Collins AC (1993). Downregulation of nicotinic receptor function after chronic nicotine infusion. Journal of Pharmacology and Experimental Therapeutics, 266, 1268–1276. [PubMed] [Google Scholar]
- Marubio LM, Gardier AM, Durier S, et al. (2003). Effects of nicotine in the dopaminergic system of mice lacking the alpha4 subunit of neuronal nicotinic acetylcholine receptors. European Journal of Neuroscience, 17, 1329–1337. [DOI] [PubMed] [Google Scholar]
- Mato S, Robbe D, Puente N, Grandes P, & Manzoni OJ (2005). Presynaptic homeostatic plasticity rescues long-term depression after chronic delta 9-tetrahydrocannabinol exposure. Journal of Neuroscience, 25, 11619–11627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, & Hikosaka O (2007). Lateral habenula as a source of negative reward signals in dopamine neurons. Nature, 447, 1111–1115. [DOI] [PubMed] [Google Scholar]
- McGehee DS, & Role LW (1995). Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annual Review of Physiology, 57, 521–546. [DOI] [PubMed] [Google Scholar]
- McGehee DS, & Role LW (1996). Presynaptic ionotropic receptors. Current Opinion in Neurobiology, 6, 342–349. [DOI] [PubMed] [Google Scholar]
- McGehee DS, Heath MJS, Gelber S, Devay P, & Role LW (1995). Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science, 269, 1692–1696. [DOI] [PubMed] [Google Scholar]
- Molinari EJ, Delbono O, Messi ML, et al. (1998). Up-regulation of human alpha7 nicotinic receptors by chronic treatment with activator and antagonist ligands. European Journal of Pharmacology, 347, 131–139. [DOI] [PubMed] [Google Scholar]
- Mugnaini M, Garzotti M, Sartori I, et al. (2006). Selective down-regulation of [125I]Y0-[alpha]-conotoxin MII binding in rat mesostriatal dopamine pathway following continuous infusion of nicotine. Neuroscience, 137, 565–572. [DOI] [PubMed] [Google Scholar]
- Museo E, & Wise RA (1995). Cytisine-induced behavioral activation: Delineation of neuroanatomical locus of action. Brain Research, 670, 257–263. [DOI] [PubMed] [Google Scholar]
- Nashmi R, Xiao C, Deshpande P, et al. (2007). Chronic nicotine cell specifically upregulates functional {alpha}4* nicotinic receptors: Basis for both tolerance in midbrain and enhanced long-term potentiation in perforant path. Journal of Neuroscience, 27, 8202–8218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson ME, Kuryatov A, Choi CH, Zhou Y, & Lindstrom J (2003). Alternate stoichiometries of alpha 4beta 2 nicotinic acetylcholine receptors. Molecular Pharmacology, 63, 332–341. [DOI] [PubMed] [Google Scholar]
- Nestler EJ (2001). Molecular basis of long-term plasticity underlying addiction. Nature Reviews. Neuroscience, 2, 119–128. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, & Aghajanian GK (1997). Molecular and cellular basis of addiction. Science, 278, 58–63. [DOI] [PubMed] [Google Scholar]
- Nguyen HN, Rasmussen BA, & Perry DC (2003). Subtype-selective up-regulation by chronic nicotine of high-affinity nicotinic receptors in rat brain demonstrated by receptor autoradiography. Journal of Pharmacology and Experimental Therapeutics, 307, 1090–1097. [DOI] [PubMed] [Google Scholar]
- Nguyen HN, Rasmussen BA, & Perry DC (2004). Binding and functional activity of nicotinic cholinergic receptors in selected rat brain regions are increased following long-term but not short-term nicotine treatment. Journal of Neurochemistry, 90, 40–49. [DOI] [PubMed] [Google Scholar]
- Ninkovic M, & Hunt SP (1983). Alpha-bungarotoxin binding sites on sensory neurones and their axonal transport in sensory afferents. Brain Research, 272, 57–69. [DOI] [PubMed] [Google Scholar]
- Oakman SA, Faris PL, Kerr PE, Cozzari C, & Hartman BK (1995). Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area. Journal of Neuroscience, 15, 5859–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olale F, Gerzanich V, Kuryatov A, Wang F, & Lindstrom J (1997). Chronic nicotine exposure differentially affects the function of human alpha3, alpha4, and alpha7 neuronal nicotinic receptor subtypes. Journal of Pharmacology and Experimental Therapeutics, 283, 675–683. [PubMed] [Google Scholar]
- Omelchenko N, & Sesack SR (2006). Cholinergic axons in the rat ventral tegmental area synapse preferentially onto mesoaccumbens dopamine neurons. Journal of Comparative Neurology, 494, 863–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill MF, Dourish CT, & Iversen SD (1991). Evidence for an involvement of D1 and D2 dopamine receptors in mediating nicotine-induced hyperactivity in rats. Psychopharmacology (Berl), 104, 343–350. [DOI] [PubMed] [Google Scholar]
- Parker SL, Fu Y, McAllen K, et al. (2004). Up-regulation of brain nicotinic acetylcholine receptors in the rat during long-term self-administration of nicotine: Disproportionate increase of the alpha6 subunit. Molecular Pharmacology, 65, 611–622. [DOI] [PubMed] [Google Scholar]
- Parpura V, & Haydon PG (2000). Physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proceedings of the National Academy of Sciences of the United States of America, 97, 8629–8634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, Gerzanich V, Anand R, Whiting P, & Lindstrom J (1994). Nicotine-induced increase in neuronal nicotinic receptors results from a decrease in the rate of receptor turnover. Molecular Pharmacology, 46, 523–530. [PubMed] [Google Scholar]
- Peng X, Gerzanich V, Anand R, Wang F, & Lindstrom J (1997). Chronic nicotine treatment up-regulates alpha3 and alpha7 acetylcholine receptor subtypes expressed by the human neuroblastoma cell line SH-SY5Y. Molecular Pharmacology, 51, 776–784. [DOI] [PubMed] [Google Scholar]
- Perry DC, Mao D, Gold AB, et al. (2007). Chronic nicotine differentially regulates {alpha}6- and beta3-containing nicotinic cholinergic receptors in rat brain. Journal of Pharmacology and Experimental Therapeutics, 322, 306–315. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, et al. (1998). Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature, 391, 173–177. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, & Dani JA (1997). Nicotine activates and desensitizes midbrain dopamine neurons. Nature, 390, 401–404. [DOI] [PubMed] [Google Scholar]
- Rahman S, Zhang J, & Corrigall WA (2003). Effects of acute and chronic nicotine on somatodendritic dopamine release of the rat ventral tegmental area: In vivo microdialysis study. Neuroscience Letters, 348, 61–64. [DOI] [PubMed] [Google Scholar]
- Robinson TE, & Berridge KC (1993). The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research. Brain Research Reviews, 18, 247–291. [DOI] [PubMed] [Google Scholar]
- Robinson TE, & Berridge KC (2003). Addiction. Annual Review of Psychology, 54, 25–53. [DOI] [PubMed] [Google Scholar]
- Rogers AT, & Wonnacott S (1997). Differential upregulation of alpha 7 and alpha 3 subunit-containing nicotinic acetylcholine receptors in rat hippocampal and PC12 cell cultures. Biochemical Society Transactions, 25, 544S. [DOI] [PubMed] [Google Scholar]
- Rowell PP, & Wonnacott S (1990). Evidence for functional activity of up-regulated nicotine binding sites in rat striatal synaptosomes. Journal of Neurochemistry, 55, 2105–2110. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, & Malenka RC (2003). Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron, 37, 577–582. [DOI] [PubMed] [Google Scholar]
- Sallette J, Pons S, Devillers-Thiery A, et al. (2005). Nicotine upregulates its own receptors through enhanced intracellular maturation. Neuron, 46, 595–607. [DOI] [PubMed] [Google Scholar]
- Sargent PB (1993). The diversity of neuronal nicotinic acetylcholine receptors. Annual Review of Neuroscience, 16, 403–443. [DOI] [PubMed] [Google Scholar]
- Schoffelmeer ANM, De Vries TJ, Wardeh G, van de Ven HWM, & Vanderschuren LJMJ (2002). Psychostimulant-induced behavioral sensitization depends on nicotinic receptor activation. Journal of Neuroscience, 22, 3269–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W (2002). Getting formal with dopamine and reward. Neuron, 36, 241–263. [DOI] [PubMed] [Google Scholar]
- Schwartz R, & Kellar KJ (1983). Nicotinic cholinergic receptor binding sites in the brain: Regulation in vivo. Science, 220, 214–216. [DOI] [PubMed] [Google Scholar]
- Schwartz RD, & Kellar KJ (1985). In vivo regulation of [3H] acetylcholine recognition sites in brain by nicotinic cholinergic drugs. Journal of Neurochemistry, 45, 427–433. [DOI] [PubMed] [Google Scholar]
- Semba K, & Fibiger H (1992). Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: A retro- and anterograde transport and immunohistochemical study. Journal of Comparative Neurology, 323, 387–410. [DOI] [PubMed] [Google Scholar]
- Sesack SR, & Pickel VM (1992). Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. Journal of Comparative Neurology, 320, 145–160. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Carr DB, Omelchenko N, & Pinto A (2003). Anatomical substrates for glutamate-dopamine interactions: Evidence for specificity of connections and extrasynaptic actions. Annals of the New York Academy of Sciences, 1003, 36–52. [DOI] [PubMed] [Google Scholar]
- Sharma G, & Vijayaraghavan S (2001). Nicotinic cholinergic signaling in hippocampal astrocytes involves calcium-induced calcium release from intracellular stores. Proceedings of the National Academy of Sciences of the United States of America, 98, 4148–4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim I, Javaid JI, Wirtshafter D, et al. (2001). Nicotine-induced behavioral sensitization is associated with extracellular dopamine release and expression of c-Fos in the striatum and nucleus accumbens of the rat. Behavioural Brain Research, 121, 137–147. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Benwell ME, Akbar MT, Stolerman IP, & Balfour DJ (1994). Behavioural and neurochemical adaptations to nicotine in rats: Influence of NMDA antagonists. British Journal of Pharmacology, 111, 1073–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, & Jarvis MJ (1995). The scientific case that nicotine is addictive. Psychopharmacology (Berl), 117, 2–10. [DOI] [PubMed] [Google Scholar]
- Stolerman I, Fink R, & Jarvik M (1973). Acute and chronic tolerance to nicotine measured by activity in rats. Psychopharmacologia, 30, 329–342. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Malenka RC, & Bonci A (2000). Modulation of long-term depression by dopamine in the mesolimbic system. Journal of Neuroscience, 20, 5581–5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumkosit P, Kuryatov A, Luo J, & Lindstrom J (2006). beta3 subunits promote expression and nicotine-induced up-regulation of human nicotinic {alpha}6* nicotinic acetylcholine receptors expressed in transfected cell lines. Molecular Pharmacology, 70, 1358–1368. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, & Nelson SB (2004). Homeostatic plasticity in the developing nervous system. Nature Reviews. Neuroscience, 5, 97–107. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, & Bonci A (2001). Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature, 411, 583–587. [DOI] [PubMed] [Google Scholar]
- Vallejo YF, Buisson B, Bertrand D, & Green WN (2005). Chronic nicotine exposure upregulates nicotinic receptors by a novel mechanism. Journal of Neuroscience, 25, 5563–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P (2004). Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neuroscience and Biobehavioral Reviews, 27, 827–839. [DOI] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, et al. (1989). Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: A hybridization histochemical study in the rat. Journal of Comparative Neurology, 284, 314–335. [DOI] [PubMed] [Google Scholar]
- Walsh H, Govind AP, Mastro R, et al. (2008). Up-regulation of nicotinic receptors by nicotine varies with receptor subtype. Journal of Biological Chemistry, 283, 6022–6032. [DOI] [PubMed] [Google Scholar]
- Wang F, Nelson ME, Kuryatov A, et al. (1998). Chronic nicotine treatment up-regulates human alpha3 beta2 but not alpha3 beta4 acetylcholine receptors stably transfected in human embryonic kidney cells. Journal of Biological Chemistry, 273, 28721–28732. [DOI] [PubMed] [Google Scholar]
- Wise RA (1988). Psychomotor stimulant properties of addictive drugs. Annals of the New York Academy of Sciences, 537, 228–234. [DOI] [PubMed] [Google Scholar]
- Wonnacott S (1997). Presynaptic nicotinic ACh receptors. Trends in Neurosciences, 20, 92–98. [DOI] [PubMed] [Google Scholar]
- Yin R, & French ED (2000). A comparison of the effects of nicotine on dopamine and non-dopamine neurons in the rat ventral tegmental area: An in vitro electrophysiological study. Brain Research Bulletin, 51, 507–514. [DOI] [PubMed] [Google Scholar]
- Yu ZJ, & Wecker L (1994). Chronic nicotine administration differentially affects neurotransmitter release from rat striatal slices. Journal of Neurochemistry, 63, 186–194. [DOI] [PubMed] [Google Scholar]