Abstract
Background:
Intracranial subdural empyema (SDE) is a seemingly uncommon life-threatening ailment with varying presentations and outcomes. A variety of risk factors have been associated with predisposition to intracranial SDEs; however, they may be cryptogenic. There is an increased predilection for intracranial SDE in children and teenagers with paranasal sinusitis or middle ear infections. The clinical presentation is non-specific and thus a high index of suspicion is required. Neuroimaging is an invaluable diagnostic tool for early diagnosis and surgical intervention. There have been multiple proponents for either burr hole or craniotomy to treat intracranial SDEs; however, despite surgical intervention, adverse neurologic sequelae and even mortality still occur. Extended antibiotic therapy is mandatory and impacts significantly on patients’ outcomes.
Objectives:
This study describes the demographics, clinical presentation, and surgical outcomes in patients with intracranial SDEs over the study period.
Design:
This is a retrospective single-centre case series.
Setting:
This study takes place in a tertiary referral centre, university hospital.
Materials and Methods:
This was a retrospective review of patients presenting with intracranial SDEs over a 10-year period in a tertiary neurosurgical unit serving south-western Nigeria. Demographic, clinical, and radiologic data were retrieved from patient records.
Results:
Forty-nine patients presented with intracranial SDEs during the review period. These patients aged between 16 months and 75 years, most of whom were 20 years of age and below. The mean age was 21.37 ± 19.29 years with a median age of 15 years. There were 35 males and 14 females giving a male-to-female ratio of 2.5:1. The most common presentations were headache (73.5%), altered sensorium (55.1%), and seizures (38.8%). Forty patients (81.6%) had evacuation of SDE by burr hole and subdural washout. There was a significant post-op residual SDE in six patients. There were eight (16.3%) mortalities in this series. Forty-seven (95.9%) patients had sterile cultures of the subdural pus collection.
Conclusion:
Intracranial SDE affects mostly children and teenagers. Early diagnosis, emergent surgery, extended antibiotic therapy, and concurrent source control employing a multidisciplinary approach are essential in managing this condition. Burr hole and subdural washout help control the disease process, reduce operation time, and may yield outcomes similar to craniotomies, which are more invasive.
Keywords: Antibiotics, burr hole, craniotomy, interhemispheric empyema, Nigeria, sinusitis, subdural empyema (SDE)
Introduction
Subdural empyema (SDE) is a loculated collection of pus in the potential space between the dura and the arachnoid mater. It could occur in the cranial or spinal subdural space, the latter occurring less commonly.[1,2]
Intracranial SDE is a life-threatening ailment requiring early diagnosis and emergent intervention. Despite treatment, adverse neurologic sequelae and even mortality still occur.[1,3] With the advent of the antibiotic era, the present-day improvement in management strategies, and access to investigative modalities, the once-grave prognosis of intracranial SDEs has given way to better outcomes.[2]
SDEs constitute 15–25% of pyogenic intracranial infections.[4] It affects all age groups but is commoner in young males.[4,5,6,7]
The risk factors for intracranial SDEs include meningitis, suppurative otitis media, paranasal sinusitis, mastoiditis, direct infection of the subdural space from traumatic wounds, or neurosurgical procedures. Poorly treated meningitis is the commonest cause in infants.[1,3]
The clinical presentation of intracranial SDE is often non-specific and frequently mimics other disease entities. Common features include headache, altered mental status, focal neurologic deficits, and seizures.[5,7] Intracranial SDEs may occur alongside other extra-axial or intra-axial suppurations and calvarial osteomyelitis.[8]
Broad spectra antibiotic therapy and prompt surgical evacuation are essential for a satisfactory management outcome.
There is a high tendency of incomplete evacuation or recurrence.[9] Antibiotic therapy is recommended for 6–8 weeks.
The objective of this study was to provide demographic information of patients with intracranial SDE, their predisposing factors, clinical features, microbiologic profile, and compare burr hole vs. craniotomy outcomes over a 10-year period. The study centre is a tertiary neurosurgical facility serving the south-western part of Nigeria.
Materials and Methods
We conducted a review of patients who had presented with intracranial SDE between August 2011 and July 2021 at a tertiary hospital in Nigeria.
Data were obtained from operating theatre, neurocritical care unit, ward, emergency room records, and case files retrieved from the hospital medical records department. The records of all patients with clinical and radiological diagnoses of intracranial SDE during the review period were reviewed and relevant data extracted. Patients with clinical diagnosis but no confirmatory radiology diagnosis were excluded.
Data were collated into a Microsoft Excel spreadsheet and these included patient demographics, clinical features, radiology findings, surgical approach to treatment, microbiology and culture results of the SDE, and treatment outcomes.
Treatment outcomes assessed were in-hospital mortality, residual SDE post-surgery, and need for revision surgery. These outcomes were compared between burr hole-treated and craniotomy-treated patients. Statistical analysis was done using the Microsoft Excel software. Categorized data were presented as frequencies and percentages using tables and graphs. Continuous variables were presented as means, median, and standard deviation. Unadjusted odds ratio (COR) was calculated for the outcomes measured with 95% confidence interval (CI).
Approval was obtained from the Institutional Human Research and Ethics Committee (ref.: ADM/DSCST/HREC/APP/4593).
Resuscitation
All the patients presented through the emergency room and were resuscitated according to the institutional protocol. Patients with Glasgow Coma Scores of 8 and below were intubated and admitted into the intensive care unit for neurocritical care. Patients presenting with seizures were administered anticonvulsants. Meningitic dose of antibiotics was commenced empirically for patients with features of meningism at presentation. All patients with suspected intracranial SDE had contrasted computed tomography (CT) scan or magnetic resonance imaging (MRI) of the brain. Upon radiology diagnosis of intracranial SDE, triple antibiotic regimen was commenced.
Informed consent
Informed consent was acquired for all the patients. The risks of anaesthesia and surgery were outlined. Emphasis was made on the possibility of post-op residual empyema requiring repeat surgery, iatrogenic brain injury, meningitis, and possibility of conversion to a craniotomy in those for whom burr hole treatment was prescribed.
Surgical approach
All the surgeries were done under general anaesthesia, with local anaesthetic infiltration of the site marked for incision and the exit point for the subdural drain.
Burr hole surgery for subdural empyema
Burr hole was made through a 5 cm length scalp incision. Single or two burr holes were employed. Single burr hole, tube aspiration, and saline washout were employed for small volume or localized SDE. The single burr holes were sited at the centre of the SDE collection and widened with a Kerrison rongeur. A French size 8 or 10 feeding tube was tunnelled 5 cm from the incision’s edge in the subgaleal plane to exit the surgical incision. Durotomy was done in a cruciate fashion and the feeding tube advanced into the SDE. SDE specimen was aspirated through the tube with a 5 mL syringe and sent for microscopy, culture, and sensitivity. The remaining SDE was then aspirated with a 5 mL syringe till no effluent returned. The subdural space was subsequently irrigated with normal saline and aspirated till effluent was clear. Subdural tube was left in situ as a drain, secured, and the scalp wound closed in two layers.
Two burr holes (a frontal and a parietal burr hole) were employed for widespread SDE and feeding tubes tunnelled as above and guided into the SDE collection via each burr hole. Culture specimen was taken and the SDE subsequently aspirated via the most-dependent feeding tube, manipulating and re-directing the tubes as necessary. The subdural space was irrigated with normal saline via one subdural tube at a time, with egress of effluent from the second tube and burr hole site till effluent is clear. Subdural washout was routinely done using normal saline to which gentamycin injection had been added (160 mg in 500 ml saline at 0.32 mg/mL).
For the interhemispheric SDEs, burr hole was sited close to the sagittal suture over the collection and widened with a Kerrison rongeur. Cruciate durotomy was done, taking care not to violate the superior sagittal sinus. The feeding tube was guided into the SDE collection, often aided by gentle active brain retraction, and pus aspirated. Saline washout was done, and the interhemispheric drain left in situ.
The subdural tubes were removed bedside after 48 h or when the tubes no longer drained, whichever occurred first.
Antibiotic therapy
Parenteral antibiotic therapy was administered for a minimum of 4 weeks and comprised a third-generation cephalosporin, metronidazole, and gentamicin (or vancomycin). This was subsequently converted to oral antibiotics to complete an 8-week antibiotic course. Oral antibiotics administered included cefuroxime, cefpodoxime, cefepime, cotrimoxazole (based on a sensitivity report for methicillin-resistant Staphylococcus aureus), and metronidazole.
Post-operative brain scan
A repeat brain scan was routine on the 5th day post-surgery. The scan was done earlier in patients who deteriorated clinically before 5 post-operative days elapsed.
Results
Patient demographics
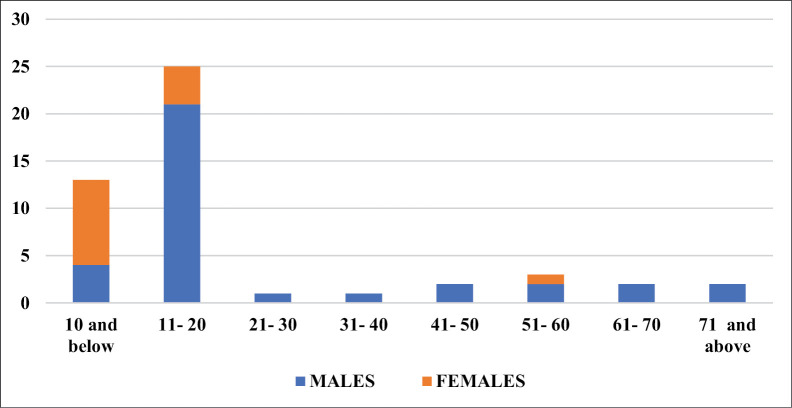
A total of 49 patient records were retrieved for the period under review. The male-to-female ratio was 2.5:1, with age ranging from 16 months to 75 years. The mean age at presentation was 21.37 ± 19.29 years with a median age of 15 years. The age group and sex distribution of patients are as depicted in Figure 1. Most (n = 38; 77.6%) of the patients were aged 20 years and below, with 25 (65.8%) of them being males. There were a total of 14 female patients in this study, with 9 (64.3%) of them aged 10 years and below. The females presented at a younger age, with the male presentations peaking a decade later.
Figure 1.
Age group and sex distribution of patients
Predisposing factors
The predisposing factors to developing intracranial SDE were ascertained in 39 (79.6%) patients. The most frequent (n = 26; 53.1%) predisposing factor was rhinosinusitis, with the maxillary sinusitis (n = 25; 96.2%) being the most frequent. Eleven (44%) patients with maxillary sinusitis had coexisting involvement of other paranasal sinuses.
The commonest surgery complicated by SDE was burr hole drainage of chronic subdural hematoma [Table 1].
Table 1.
Predisposing factors to developing intracranial SDE
| Aetiology | Frequency (N) | Number of | ||
|---|---|---|---|---|
| Sinusitis | 26 (53.1) | |||
| • Frontal | 1 | |||
| • Maxillary | 14 | |||
| • Mixed | Frontal and maxillary | 10 | 11 | |
| Spheno-ethmoidal and maxillary | 1 | |||
| Sepsis | 3 (6.1) | |||
| Post-op | (8.2) | |||
| • Burr hole for chronic subdural haematoma | 2 | |||
| • Burr hole for frontal extradural abscess | 1 | |||
| • Decompressive craniectomy | 1 | |||
| Meningitis | 5 (10.2) | |||
| Otitis | 1 (2.0) | |||
| Cryptogenic | 10 (20.4) | |||
Clinical presentation
The commonest presenting symptoms were headache (73.5%), altered sensorium (55.1%), and seizures (38.8%). The commonest signs were pyrexia (89.8%), long tract signs (49%), and meningismus (26.5%) [Table 2]. The mean duration of symptoms before presentation was 10.06 ± 4.0 days with a range of 5–21 days.
Table 2.
Symptoms and signs at presentation with intracranial SDE
| Symptoms | Frequency (%) | Signs | Frequency (%) |
|---|---|---|---|
| Headache | 36 (73.5) | Fever | 44 (89.8) |
| Altered sensorium | 27 (55.1) | Hemiplegia | 17 (34.7) |
| Seizure | 19 (38.8) | Meningismus | 13 (26.5) |
| Vomiting | 11 (22.5) | Hemiparesis | 5 (10.2) |
| Ocular swelling | 3 (6.1) | Aphasia | 3 (6.1) |
| Frontal swelling | 4 (8.2) | Monoplegia | 2 (4.1) |
| Discharge from surgical wound | 2 (4.1) | Photophobia | 1 (2.0) |
| Irritability | 1 (2.0) |
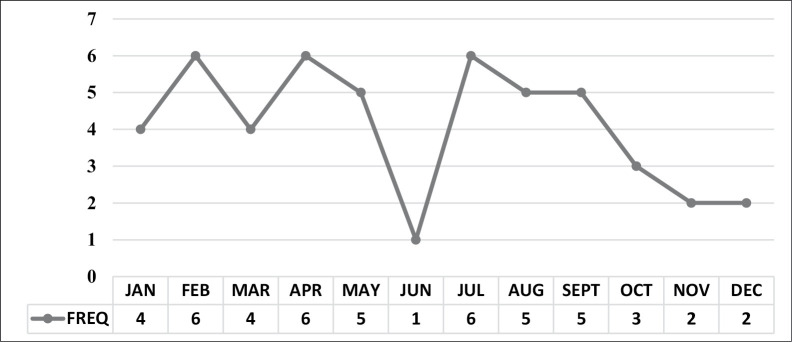
Although there were fewer presentations in the last quarter of each year, there was no seasonal pattern to presentation with intracranial SDE [Figure 2].
Figure 2.
Intracranial SDE presentations by month of the year
All the patients were administered antibiotic therapy prior to neuroimaging and neurosurgical referral.
Neuroradiology findings
The SDE was supratentorial in 48 (98%) patients, with 40 (83.3%) being convexity SDEs. It was bilateral in three (6.1%) patients and seven (14.3%) had co-existing extracranial pus [Table 3]. Figure 3 shows a typical bilateral subdural empyema.
Table 3.
Extent of intracranial SDE on neuroimaging
| Neuroimaging finding | Number of patients (%) |
|---|---|
| Isolated intracranial SDE | 39 (79.6) |
| Intracranial SDE + subperiosteal extension | 6 (12.3) |
| Intracranial SDE + subperiosteal + intra-orbital | 1 (2.0) |
| Intracranial SDE + intra-orbital | 2 (4.1) |
| Intracranial SDE + intracerebral abscesses | 1 (2.0) |
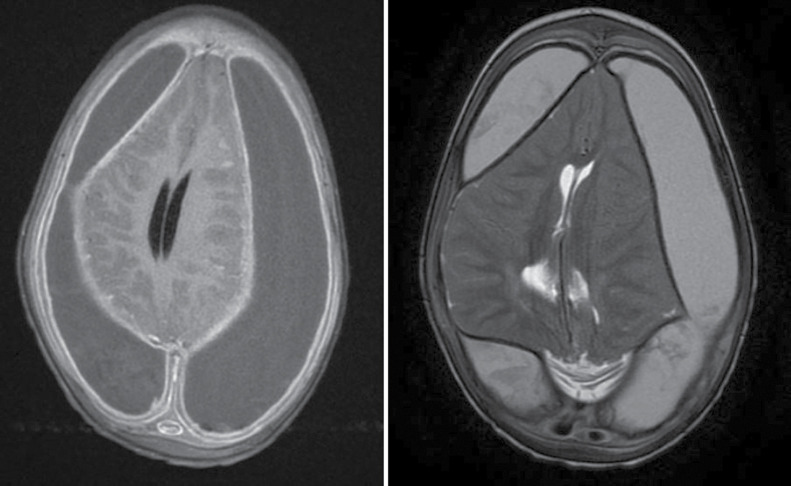
Figure 3.
Brain MRI in a 2-year-old with subdural empyema. Post-gadolinium T1 sequence (left image) shows bilateral subdural collections with mass effect and meningeal enhancement. The collections are hyperintense on the T2-weighted sequence (right image)
Surgical approach
Table 4 shows the surgical approaches employed for patients in this study. Burr hole tube aspiration and saline washout of the SDE were done for 40 (81.6%) patients. An external ventricular drain was inserted for one patient as an adjunct to craniectomy for posterior fossa SDE.
Table 4.
Surgical treatment of patients with SDE
| Surgery | Number of patients (%) |
|---|---|
| Burr hole drainage and washout | 40 (81.6) |
| Debridement of surgical wound | 1 (2.0) |
| Craniotomy | 4 (8.2) |
| Craniectomy | 3 (6.1) |
| External ventricular drain (EVD) insertion (as adjunct treatment) | 1 (2.0) |
| Nil | 1 (2.0) |
Eleven patients had maxillary sinus washouts by the otorhinolaryngologists at the same sitting as the time of surgery for intracranial SDE. One patient had interval mastoidectomy for complicated chronic suppurative otitis media.
Microbiology
SDE cultures were negative in 47 patients. There were two positive culture results: one yielding methicillin-resistant S. aureus (in a patient with SDE post-burr hole drainage of chronic subdural haematoma) and the second growing Pseudomonas aeruginosa (following a decompressive craniectomy).
Treatment outcomes
There were eight mortalities comprising of seven males and one female with age ranging between 7 and 75 years and a mean age of 41.63 ± 29.05 years. The average duration of symptoms in these patients was 11.5 ± 3.67 days with a range of 7–17 days. One of these patients was treated by craniotomy, whereas the other seven patients had burr hole drainage [Figure 4].
Figure 4.
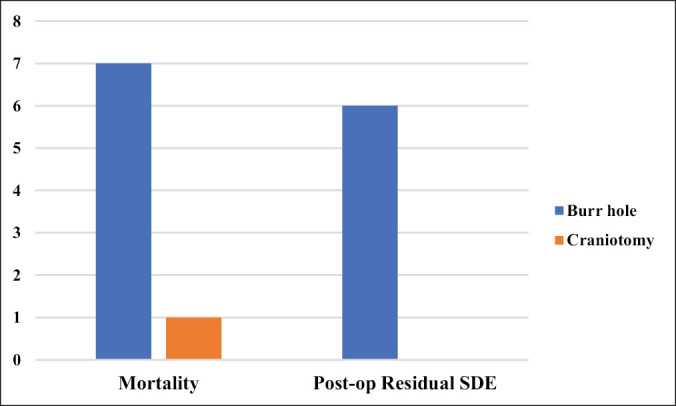
Comparison of outcomes of burr hole and craniotomy treatment for SDE
Six patients had significant residual SDE on post-operative brain scan requiring repeat surgical intervention. All these had primary burr hole drainage and subdural washout of SDE at initial presentation. A repeat subdural washout employing the previously sited burr holes was done.
Discussion
The clinical features of intracranial SDEs are non-specific and misdiagnosis could occur. A high index of suspicion is often required to make a diagnosis.[2] Brain imaging is necessary to confirm the diagnosis and guide localization of the pus pockets intraoperatively.[2]
Intracranial SDEs have a predilection for children and teenagers with paranasal sinusitis or middle ear infections.[10,11,12] In our series, 38 (77.6%) patients were in their first or second decade of life, a finding similar to a South African review of 699 SDE patients by Nathoo et al.,[13] in which 71% were 20 years of age and below, with a mean age of 14.56 ± 12.2 years. This predilection for the younger age group is also documented in other studies, raising the question of what factors could be responsible for the childhood and teenage predisposition to intracranial SDEs.
Pathogenic microorganisms gain entrance via multiple routes into the intracranial space. They could be transported as septic emboli via intracranial vessels, diploic veins, or endolymphatic pathways.[2] Another source is from contiguous infections, most commonly of rhinosinugenic and otogenic origin. Traumatic wounds and cranial neurosurgical procedures have been implicated as possible sources of offending microbes.[2] In our series, the most frequent identified predisposing factor was paranasal sinusitis (53.1%).
Males were 2.5 times more likely than females to present with intracranial SDE in our series. Male predisposition has been alluded to their relatively higher risk of cranial trauma, meningitis, to anatomical differences and higher vascularity of their diploic veins.[14] Other theories that had been proposed to explain the young male predisposition to intracranial SDE include the rapid growth of their frontal sinuses between the age of 7 and 20 years, larger air sinuses, more rigorous nose blowing, and congenital narrowing of paranasal sinus ostia.[13]
The cranial bones develop by membranous ossification from both dura and galea and thus derive their blood supply from both structures with intracranial–extracranial vascular connections. The diploic veins (of Breschet) are valveless and allow bi-directional flow of blood, septic emboli, or pathogenic microbes.[14,15] This mechanism could explain why 20% of our patients and 33.4% in the review of Nathoo et al.[13] had co-existing subperiosteal or subgaleal pus at presentation.
The clinical presentation of intracranial SDE is often due to the mass effect of the purulent exudates, inflammation of the adjacent brain and vasculature, associated thrombosis, cerebral oedema, and systemic response of the individual to the infective process. The SDE is unhindered in its spread through the subdural space. Thrombosis of cortical vessels contributes to venous stasis, brain swelling, and strokes in these patients.[2]
In our series, the commonest presenting symptoms were headache, altered sensorium, and seizures. The mean duration of symptoms before presentation was 10.06 ± 4.0 days, with a range of 5–21 days. The commonest signs were pyrexia, long tract signs, and meningismus [Table 2]. These findings are similar to those of Nathoo et al.[13] in Durban, South Africa and Dill et al.[16] in Birmingham, USA. Signs and symptoms relating to the primary source of infection may also be present. The absence of meningeal signs does not exclude the possible diagnosis of intracranial SDE as observed in this series.
There is debate as to the surgical approach for optimum evacuation of intracranial SDEs. Decision-making is guided by findings on neuroimaging, the surgeon’s preference, and experience. Many studies document the superiority of craniotomy to burr hole evacuation of SDEs, whereas others document good outcomes with burr hole treatment.[3,15]
During burr hole drainage of SDEs, delaying introduction of the drainage tube into the subdural collection following durotomy, from our experience, often leads to obliteration of the subdural space due to the egress of the empyema and attendant brain swelling. These increase the likelihood of inadvertent trauma to the underlying brain during tube insertion and increase the tendency of residual pockets of pus getting isolated from the reach of the subdural drain. We routinely tunnelled the feeding tube to exit 5 cm from the incision’s edge, and ready for insertion, before making the durotomy to avoid delay in its insertion.
Seven (17.5%) of the 40 patients in whom primary burr hole treatment was employed demised, compared to 1 out of 7 who had primary craniotomies [odds ratio (COR): 1.2727; 95% CI 0.1317–12.3034; P = 0.835]. Post-op residual SDE was present in six of the burr hole-treated patients and none in the craniotomy group [(COR) 2.8261; 95% CI 0.1431–55.8019; P = 0.4948].
In a series of 66 patients by Bannister et al.,[12] they listed four factors that impacted outcome, one of which was the surgical approach employed. In their study, survival was 91% following primary craniotomy and 81% following secondary surgery. Survival was 51% in those who had burr hole drainage. They expressed concerns about brain trauma from subdural tubes and a tendency for burr holes to be occluded by the expanding brain, thus preventing complete empyema drainage.
Nathoo et al.[13] reported a 12.2% mortality in their series and identified factors associated with adverse outcomes. These included ventriculitis, need for intensive care, cerebral infarction, among others.
French et al.[17] treated 28 SDE patients with craniotomy and 4 employing burr holes. They reported a 7.1% re-do craniotomy rate as opposed to 75% of the burr hole-treated patients who had subsequent craniotomy. A similar review by Liu et al. reported that 11.1% and 13.3% of their burr hole- and craniotomy-treated patients, respectively, required subsequent revision surgery.
Miller et al.[18] in their series posited that appropriately sited burr hole, subdural drain, and subdural instillation of antibiotics proved a successful method of intracranial SDE treatment with low perioperative mortality.
The contrasting findings among different authors as regards outcomes following burr hole or craniotomy treatment of intracranial SDEs suggest that surgeon factors, patient factors, or procedural factors could have played a role in the inconsistent outcomes with the treatment methods.
From our experience, burr hole treatment appears a reliable treatment option for intracranial SDEs. It is time-saving, reduces the average anaesthesia time, but requires experience to achieve desired goals of outcome comparable to craniotomies. The surgeon must keep a mental track of the volume of saline introduced into the subdural space during washouts and ensure most of it either egresses or is actively aspirated gently with a low volume syringe (preferably a 5 mL syringe).
Percutaneous trans-fontanelle subdural aspiration of intracranial subdural and intra-axial pus has been documented; however, this should be employed with great caution.[7,13,15,19]
Patients with multiloculated pus collections are more likely to benefit from craniotomies as burr hole will not aid complete SDE evacuation. This has also been alluded to in other studies.[20]
Specimen of the subdural pus must always be sent for microscopy and culture (both aerobic and anaerobic) and/or fungal studies where suspected. In literature, the positive culture rate for subdural pus is said to be between 54% and 81%,[1] a finding which was in contrast to that in our series with a 4.1% positive culture rate. Other studies had also reported higher culture rates.[13,15,16,17] In a 10-year review of cases of intracranial SDE in an eastern Nigerian facility, the authors reported a 15.4% positive culture rate.[6] Our low positive yields from SDE cultures may be attributable to the large percentage of patients administered antibiotic therapy before presentation to tertiary care, a hypothesis that was also alluded to by Chikani et al.[6]
The commonly reported subdural pus isolates in literature are Streptococcus spp., Staphylococcus spp., Haemophilus influenza, and Proteus mirabilis, Salmonella typhi, and Escherichia coli.[2,18,20] Often, more than one organism is isolated. The causative organisms correlate with the age of the patient and the primary source of infection.[19] In our series, the positive cultures were from patients who had SDE complicating previous neurosurgical procedures. One yielded methicillin-resistant S. aureus and the other, P. aeruginosa.
Expedited surgical treatment and empirical parenteral antibiotic therapy improve the chances of good neurological outcomes. In our centre, empiric triple-antibiotic therapy with vancomycin, metronidazole, and a third-generation cephalosporin was routinely administered once the diagnosis was confirmed. The triple-antibiotic therapy was continued for a minimum of 4 weeks unless microbiology results advised a review. Parenteral therapy was subsequently converted to oral antibiotics for another 2–4 weeks. Patients with minimal SDE residuals on post-operative neuroimaging were managed non-operatively, with extended intravenous antibiotics and follow-up neuroimaging.
Post-operatively, we recommend a repeat brain imaging if there is a drop in level of consciousness, persistent fever, persisting focal neurologic deficits or on the 5th day post-surgery, whichever comes first. We routinely requested a contrast-enhanced CT scan of the brain, due to ease of access and relative affordability compared with MRIs.
Inter-specialty collaboration and multidisciplinary approach to management are essential for good outcomes. Concurrent treatment of the source of infection with surgery and/or appropriate antibiotics not only aid early clearance of infection but also reduce the rate of recurrence.[1] It is important to address predisposing sinus collections and complicated chronic suppurative otitis media. Mastoid bone involvement would necessitate surgical excision of the osteomyelitic bone (tympanomastoidectomy).[21]
Neurorehabilitation is a key component to post-operative care and should be commenced as early as possible. A number of these patients present with focal neurologic deficits at presentation or new deficits from onward progression of the disease process, attributable to possible vascular-related sequela. These often resolve following surgical evacuation, antibiotic therapy, and early involvement of a dedicated neuro-rehabilitation team in the patients care.
Limitations
The retrospective design of this study and a few patients in the craniotomy group could have led to outcome bias.
Conclusion
Intracranial SDE still presents a clinical challenge even in this antibiotic era. Early diagnosis, multidisciplinary consultations, expedited surgical treatment, and early commencement of empirical broad spectrum antibiotic therapy are the goals of treatment. Burr hole drainage and saline washout of the SDE are treatment options in managing these patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Ethical approval
The authors certify that they secured ethical clearance and obtained approval for this study from the Institutional Human Research and Ethics Committee (ref.: ADM/DSCST/HREC/APP/4593).
Acknowledgement
We thank the other colleagues and staff who played several roles in the management of the patients.
References
- 1.Agrawal A, Timothy J, Pandit L, Shetty L, Shetty JP. A review of subdural empyema and its management. Infect Dis Clin Pract. 2007;15:149–53. [Google Scholar]
- 2.Yoon J, O’Bryan CM, Redmond M. Intracranial subdural empyema—A mini review. J Infectiol. 2020;3:1–5. [Google Scholar]
- 3.Hendaus MA. Subdural empyema in children. Glob J Health Sci. 2013;5:54–9. doi: 10.5539/gjhs.v5n6p54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lefebvre L, Metellus P, Dufour H, Bruder N. Linezolid for treatment of subdural empyema due to Streptococcus: Case reports. Surg Neurol. 2009;71:89–91. doi: 10.1016/j.surneu.2007.06.083. discussion 91. [DOI] [PubMed] [Google Scholar]
- 5.De Bonis P, Anile C, Pompucci A, Labonia M, Lucantoni C, Mangiola A. Cranial and spinal subdural empyema. Br J Neurosurg. 2009;23:335–40. doi: 10.1080/02688690902939902. [DOI] [PubMed] [Google Scholar]
- 6.Chikani MC, Mezue W, Okorie E, Mbachu C, Ndubisi C, Chikani UN. Subdural empyema: Clinical presentations and management options for an uncommon neurosurgical emergency in a developing country. Niger J Clin Pract. 2017;20:1221–5. doi: 10.4103/njcp.njcp_340_16. [DOI] [PubMed] [Google Scholar]
- 7.Kanu OO, Esezobor CI, Ojo OA, Asoegwu CN, Nnoli C, Dawang Y, et al. Infantile supratentorial subdural empyema managed by percutaneous aspiration: An outcome study in a Nigerian city. Sudan J Paediatr. 2019;19:37–43. doi: 10.24911/SJP.106-1520470056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osman Farah J, Kandasamy J, May P, Buxton N, Mallucci C. Subdural empyema secondary to sinus infection in children. Childs Nerv Syst. 2009;25:199–205. doi: 10.1007/s00381-008-0665-x. [DOI] [PubMed] [Google Scholar]
- 9.Yilmaz N, Kiymaz N, Yilmaz C, Bay A, Yuca SA, Mumcu C, et al. Surgical treatment outcome of subdural empyema: A clinical study. Pediatr Neurosurg. 2006;42:293–8. doi: 10.1159/000094065. [DOI] [PubMed] [Google Scholar]
- 10.Anthony D, Hockley BW. Surgical management of subdural empyema. Childs Brain. 1983;10:294–300. doi: 10.1159/000120128. [DOI] [PubMed] [Google Scholar]
- 11.Pattisapu JV, Parent AD. Subdural empyemas in children. Pediatr Neurosci. 1987;13:251–4. doi: 10.1159/000120338. [DOI] [PubMed] [Google Scholar]
- 12.Bannister G, Williams B, Smith S. Treatment of subdural empyema. J Neurosurg. 1981;55:82–8. doi: 10.3171/jns.1981.55.1.0082. [DOI] [PubMed] [Google Scholar]
- 13.Nathoo N, Nadvi SS, van Dellen JR, Gouws E. Intracranial subdural empyemas in the era of computed tomography: A review of 699 cases. Neurosurgery. 1999;44:529–35. doi: 10.1097/00006123-199903000-00055. discussion 535-6. [DOI] [PubMed] [Google Scholar]
- 14.Maniglia AJ, Goodwin WJ, Arnold JE, Ganz E. Intracranial abscesses secondary to nasal, sinus, and orbital infections in adults and children. Arch Otolaryngol Head Neck Surg. 1989;115:1424–9. doi: 10.1001/archotol.1989.01860360026011. [DOI] [PubMed] [Google Scholar]
- 15.Liu ZH, Chen NY, Tu PH, Lee ST, Wu CT. The treatment and outcome of postmeningitic subdural empyema in infants. J Neurosurg Pediatr. 2010;6:38–42. doi: 10.3171/2010.4.PEDS09433. [DOI] [PubMed] [Google Scholar]
- 16.Dill SR, Cobbs CG, McDonald CK. Subdural empyema: Analysis of 32 cases and review. Clin Infect Dis. 1995;20:372–86. doi: 10.1093/clinids/20.2.372. [DOI] [PubMed] [Google Scholar]
- 17.French H, Schaefer N, Keijzers G, Barison D, Olson S. Intracranial subdural empyema: A 10-year case series. Ochsner J. 2014;14:188–94. [PMC free article] [PubMed] [Google Scholar]
- 18.Miller ES, Dias PS, Uttley D. Management of subdural empyema: A series of 24 cases. J Neurol Neurosurg Psychiatry. 1987;50:1415–8. doi: 10.1136/jnnp.50.11.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanu OO, Ojo O, Esezobor C, Bankole O, Olatosi J, Ogunleye E, et al. Pediatric brain abscess—Etiology, management challenges and outcome in Lagos Nigeria. Surg Neurol Int. 2021;12:592. doi: 10.25259/SNI_605_2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muzumdar D, Biyani N, Deopujari C. Subdural empyema in children. Childs Nerv Syst. 2018;34:1881–7. doi: 10.1007/s00381-018-3907-6. [DOI] [PubMed] [Google Scholar]
- 21.Antunes CM, Nogueira JF, Filipe MA. Posterior fossa subdural empyema: A severe and usually late diagnosed complication. Sinapse. 2020;20:60–4. [Google Scholar]