Abstract
In this study, interleukin-5 (IL-5) transgenic mice with lifelong eosinophilia were assessed for resistance to primary infections with two tissue-invading nematodes, Nippostrongylus brasiliensis and Toxocara canis. Relative to nontransgenic littermates, three lines of IL-5 transgenic mice with varying degrees of eosinophilia all displayed enhanced resistance to N. brasiliensis. Although the timing of final worm expulsion was similar in transgenic and nontransgenic hosts, intestinal worms in transgenic mice were fewer in number throughout infection, failed to increase in size over the course of the infection, and were much less fecund. In contrast, T. canis larvae were recovered in similar numbers from tissues of transgenic mice with “low” or “high” eosinophilia and from nontransgenic mice. These results and other data suggest that eosinophils can contribute to host resistance to some parasite species. Parasite transit time through the host may correlate with relative sensitivity to eosinophils.
Tissue-invasive helminth species often induce intense tissue and peripheral blood eosinophilia. It has long been argued that eosinophils may kill some species of parasites or at least impede larval migration and development. Early studies suggested that depletion of eosinophils with polyclonal antieosinophil antibodies impaired immunity to Schistosoma mansoni (13) and Trichinella spiralis (7). More recent studies have shown that while monoclonal anti-interleukin-5 (IL-5) antibodies can decrease eosinophilia induced by infections with these parasite species, in all cases the parasite burden remained unchanged (8, 18, 19). Our own data suggest that in IL-5 transgenic mice, eosinophilia may even be counterproductive in primary infections with both S. mansoni and T. spiralis (2, 3). Nevertheless, depletion studies with anti-IL-5 antibody suggest that IL-5 and/or eosinophilia are protective against some helminth species, including Strongyloides venezuelensis and Angiostrongylus cantonensis (11, 17).
This study addresses the importance of eosinophils in resistance to two tissue-invasive intestinal helminths. Nippostrongylus brasiliensis was chosen because it has a very short transit time through the host, and Toxocara canis was selected because it remains in the host for extended periods of time. We used two lines of IL-5 transgenic mice (Tg5C1 and Tg5C2) which have been described elsewhere (2–4, 20) and another previously unreported but related IL-5 transgenic line, Tg5C3 (44 transgene copies). These IL-5 transgenic lines can be divided into “low-” (Tg5C1) and “high-level” (Tg5C2 and Tg5C3) eosinophilia categories, having approximately two (Tg5C1) to eight times (Tg5C2 and Tg5C3) more peripheral blood eosinophils than nontransgenic mice infected with the helminth Mesocestoides corti, a known inducer of eosinophilia (4). Since these lines of IL-5 transgenic mice have proven difficult to establish as homozygotes, all transgenic animals described were heterozygotes, and the nontransgenic control animals were generated from the same litters. N. brasiliensis and T. canis were passaged, cultured, and enumerated using conventional techniques (9, 16). In all experiments mice either were injected subcutaneously (s.c.) at the base of the neck with approximately 500 N. brasiliensis L3 infective larvae or were given 500 infective T. canis eggs by gavage. Statistical significance of the data was assessed by two-tailed Student’s t test using Excel for Macintosh 4.0 (Microsoft), where P < 0.05 was considered significant.
N. brasiliensis egg production.
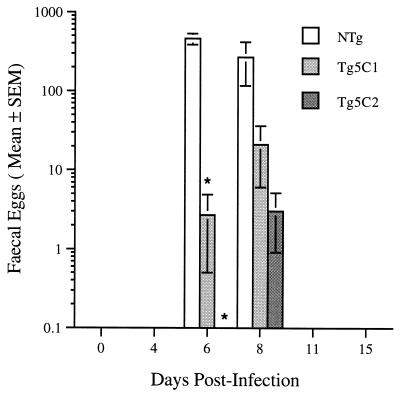
Eggs produced by N. brasiliensis infecting nontransgenic CBA/Ca mice were released in large numbers in the feces from six to eight days postinfection (PI) but were never detected outside of this period (Fig. 1). In contrast, worms infecting IL-5 transgenic CBA/Ca mice produced few eggs and when detected, peak production tended to be delayed by one to two days (Fig. 1). Counts of eggs in feces remained very low throughout the course of infection in Tg5C1 and Tg5C2 transgenic mice (Fig. 1) and in experiments with Tg5C3 mice whose results are not presented. While egg production in low-eosinophilia Tg5C1 mice was greater than in high-eosinophilia Tg5C2 animals, it failed to reach statistical significance. However, a clear difference between the lines was the absence of eggs in any of the Tg5C2 mice on Day 6 PI.
FIG. 1.
Mean egg production by N. brasiliensis worms in two lines of IL-5 transgenic mice. No eggs were detected in any Tg5C2 mouse on day 6 PI. Asterisks denote statistically significant comparisons with the corresponding nontransgenic (NTg) control group (P < 0.02). There were three mice/group.
Intestinal N. brasiliensis worms.
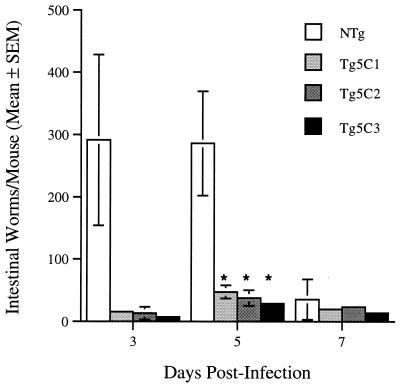
Large numbers of worms were found in the small intestines of nontransgenic mice as early as 3 days PI, and this level of infection was maintained for at least another 2 days (Fig. 2). In contrast, worm numbers were much lower in both low- and high-eosinophilia IL-5 transgenic lines. Intestinal worm numbers in IL-5 transgenic mice in this and other experiments peaked later than in nontransgenic controls.
FIG. 2.
N. brasiliensis intestinal worm burden in IL-5 transgenic and control nontransgenic mice. Asterisks denote statistically significant comparisons with the nontransgenic control group (P ≤ 0.05). There were three to four mice/group.
Low egg counts in transgenic mice were not simply due to a paucity of worms. In a separate experiment, a mean of 27.7 (standard error of the mean, 5.9) worms in nontransgenic mice produced 14.3 eggs/fecal sample, whereas a similar number of worms (28.3 ± 2.3) in Tg5C2 mice did not produce eggs at a level detectable with the same techniques. Thus, while it is likely that few parasites survived migration from the s.c. site of inoculation in IL-5 transgenic mice, those that did reach the gut also appeared to be less fecund. Neither was this result due to an absence of female worms in transgenic mice. In a typical experiment, worm sex ratios of 213 females to 108 males (i.e., 66% female) and 30 females to 14 males (i.e., 69% female) for nontransgenic and transgenic mice, respectively, were determined.
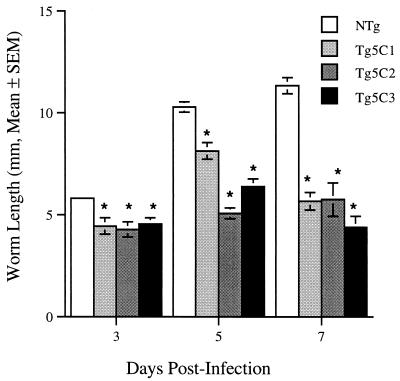
Worms were detected in the gut 3 days after s.c. injection of L3 larvae into nontransgenic mice and typically, they could be detected for 7 to 9 days PI. During this period of residence in the gut of nontransgenic hosts, worms mature (9) and increase in length (Fig. 3). The mean lengths of worms recovered from the lower gastrointestinal tracts of each of the three IL-5 transgenic lines were significantly less (P < 0.003) than those of worms found in nontransgenic mice at all three time points (Fig. 3). Male worms are smaller than female worms (9), but since worm sex ratios were approximately the same in transgenic and nontransgenic hosts, this does not explain the observed differences in average worm size. Taken together, these results suggest that the average growth rates of worms recovered from transgenic animals during the course of infections were lower than those seen in nontransgenic hosts. Worms recovered from transgenic mice were often pale in appearance, suggesting that they were malnourished. Worms also failed to localize in the preferred anterior third of the gut in each of the IL-5 transgenic mouse lines tested (data not shown), and this may adversely affect worm nutrition. The reduced fecundity routinely observed in infections in IL-5 transgenic mice may be directly related to poor growth and development of intestinal worms, but this is reversible. When transferred surgically to naive hosts, intestinal worms recovered from transgenic mice developed sufficiently to produce eggs (unpublished results).
FIG. 3.
Length of worms recovered from the intestine of IL-5 transgenic and nontransgenic mice. Asterisks denote statistically significant comparisons with the relevant nontransgenic control group for each day of infection (P < 0.003). Values are means (± standard errors of the means) worm length calculated from 6 to 58 worms/group. Worms were recovered from two to four mice/group except for the day 7 nontransgenic group, for which the mean represents measurements for 24 worms found in one mouse only.
Other studies with IL-5-overexpression transgenic mice and with IL-5 receptor α-chain knockout mice show that animals with high levels of IL-5 and eosinophilia also have enhanced resistance to the nematode A. cantonensis (22). Although the IL-5-overexpression transgene construct and background strain employed to generate the mice (24) used by these workers were different from those used in our study, the results support the hypothesis that eosinophilia can impair the migration of some tissue-invasive parasite species and reduce their reproductive success. Treatment of mice with anti-IL-5 antibodies can abolish eosinophilia induced by N. brasiliensis (1), and it has been shown to result in increased parasite load in mice infected with S. venezuelensis (11), A. cantonensis (17), and Onchocerca spp. (6, 12), adding further support to this hypothesis.
Our results and those of another study in which eosinophilia was prevented by treating animals with anti-IL-5 antibodies (10), suggest that IL-5 and eosinophils may not influence greatly the expulsion of adult N. brasiliensis worms. Rather, eosinophils appear to be important in the killing of larvae either at the site of the initial infection or elsewhere during their passage to the gut. Other data (1a) suggest that eosinophils are recruited to the site of inoculation of N. brasiliensis larvae within 6 h and, most significantly, that this is a major phase in which the migration of the parasites is inhibited. However it also seems likely that viable larvae which complete migration to the gut in IL-5 transgenic mice experience further damage or maturational inhibition once they reach this site. The worms do not increase in length from days 3 to 7 PI in IL-5 transgenic hosts, but it has yet to be determined whether egg production is suppressed due to a general effect on nutrition or development or whether it is a more specific effect on mating or fecundity.
Blood and tissue eosinophilia in N. brasiliensis-infected mice.
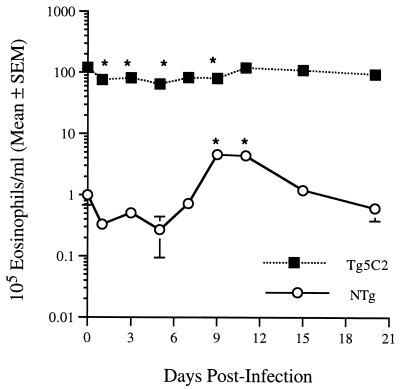
Leukocytes in samples of tail blood were enumerated with a Coulter ZF automated cell counter (Coulter Electronics, Harpenden, England). Differential cell counts were performed on methanol-fixed blood films stained with Giemsa. Mean peripheral blood eosinophil counts in uninfected Tg5C2 mice were approximately 100 times greater than those in nontransgenic mice (Fig. 4). Eosinophils represented 72 and 4% of total peripheral blood leukocytes for Tg5C2 and nontransgenic groups, respectively. Tg5C2 transgenic mice infected with N. brasiliensis showed a 35 to 50% decline in peripheral blood eosinophilia in the early stages of infection (days 1 to 9 PI), but total numbers of eosinophils returned to preinfection levels by 15 days PI, i.e., well after worm expulsion (Fig. 4). A modest decline, followed by a fourfold increase in eosinophil numbers was noted in nontransgenic mice, with counts peaking on days 9 and 11 of infection (maximum of 15% of total leukocytes). This maximum occurred at or soon after the time of worm expulsion (Fig. 4).
FIG. 4.
Mean total peripheral blood eosinophils in IL-5 transgenic (closed box) and nontransgenic (open circle) mice infected with N. brasiliensis (five mice/group). Asterisks represent statistically significant differences from values for the corresponding uninfected (day 0) mice (P < 0.05).
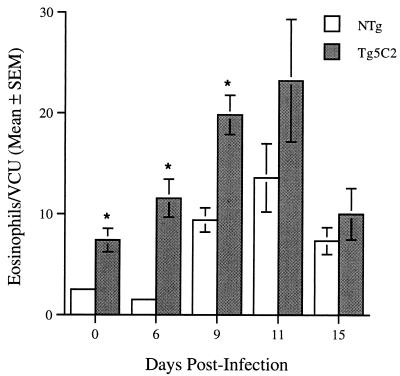
Eosinophils were also enumerated in hematoxylin and eosin-stained histological sections of formalin-fixed small intestine. Samples were taken 3 cm posterior to the pyloric sphincter and eosinophils were enumerated in either 10 or 20 villus crypt units (14) per mouse. Eosinophils were relatively common in the lamina propria and submucosa of the guts of all uninfected mice. The numbers of eosinophils detected in the guts of nontransgenic mice increased dramatically approximately 6 days after worms had arrived in the lumen of the small intestine, i.e., circa day 9 PI (Fig. 5). Eosinophil numbers increased earlier in transgenic than in nontransgenic mice (Fig. 5; day 6 versus day 9 PI, respectively), but peak levels were seen at approximately the same time—at or shortly after worm expulsion (day 11; Fig. 5). Most of the increase in eosinophils in infected transgenic mice occurred within the lamina propria and submucosa. At most times postinfection, intestinal eosinophil numbers were greater in transgenic than in nontransgenic mice. However, the numbers in transgenic mice returned to baseline (uninfected) levels by day 15 PI, while those in the small intestines of nontransgenic mice remained elevated at this time.
FIG. 5.
Tissue eosinophils in histological sections of the small intestines of (IL-5 Tg5C2 transgenic [grey bars] and nontransgenic [NTg] [open bars]) mice infected with N. brasiliensis. Each value is a group mean for 3 or 4 mice and was calculated from means of counts on 10 or 20 villus crypt units (VCU) for each mouse in a group. Asterisks represent a statistically significant difference from values for the corresponding nontransgenic mice on the days indicated (P < 0.05).
While we cannot exclude the possibility that overexpression of IL-5 may mediate resistance through other mechanisms, our results are consistent with the hypothesis that the development and fecundity of adult worms may be affected adversely by the presence of large numbers of eosinophils in the lamina propria of the gut. It is possible that local production of IL-5 enhances activation of eosinophil functions that are deleterious to N. brasiliensis intestinal worms, since expression of IL-5 in gut tissue has been shown to correlate with differential resistance to N. brasiliensis in BALB/c and C57BL/6 mice (25). While IL-5 is also a growth and differentiation factor for B lymphocytes and promotes immunoglobulin A (IgA) production in the mouse, the very short course of a primary infection makes unlikely any contribution from the humoral arm of the adaptive immune response.
T. canis infection in IL-5 transgenic mice.
T. canis infections induce very substantial blood eosinophilia, and the granulomas which form around larvae are rich in eosinophils. Eosinophilia is suppressed in T. canis-infected pregnant and lactating dogs, and it has been suggested that this may facilitate parasite transmission to newborn offspring. Treatment of infected mice with anti-IL-5 antibodies suppresses both blood and tissue eosinophilia induced by this parasite but does not seem to influence the number of larvae subsequently recovered from the liver (15). Our experiments were designed to assess the significance of preexisting eosinophilia on the survival and migration of T. canis larvae. Twenty-eight days postinfection, the numbers of larvae recovered from the brain, liver, and muscles were similar in Tg5C1 (low eosinophilia) and Tg5C2 (high eosinophilia) IL-5 transgenic mice and in nontransgenic CBA/Ca mice (Table 1). At this time, peripheral blood eosinophil counts in nontransgenic mice had risen approximately 20-fold, while those in infected Tg5C1 and Tg5C2 mice were 10- and 20-fold higher, respectively, than in infected nontransgenic animals. Eosinophils were prominent in liver granulomas found in both transgenic and nontransgenic mice, suggesting that recruitment of these leukocytes to areas of larval deposition was unimpaired in all lines (data not shown).
TABLE 1.
T. canis larvae recovered from tissues of IL-5 transgenic and nontransgenic mice infected with 500 eggs per os 28 days previously
| Mouse group | No. of larvae recovered from
|
Total no. of larvae recovered | ||
|---|---|---|---|---|
| Liver | Brain | Muscle | ||
| Nontransgenic | 25 ± 1a | 84 ± 19 | 52 ± 5 | 160 ± 16 |
| Tg5C1 | 20 ± 3 | 76 ± 7 | 58 ± 1 | 154 ± 10 |
| Tg5C2 | 19 ± 5 | 85 ± 31 | 38 ± 11 | 142 ± 40 |
Values are means ± standard errors of the means for seven mice/group. No statistically significant differences in the number of larvae recovered from transgenic mice compared to those from nontransgenic mice (P < 0.05) were found.
Sugane and colleagues (21) studied T. canis infections in mice expressing a different IL-5 transgene construct (24) and found that overexpression of IL-5 and eosinophilia did not influence the number of larvae recoverable from the lungs within the first 2 weeks of exposure to the parasite. The present study supports and extends both this observation and our earlier studies (15). Even late in infection (i.e., 28 days PI) we could find no effects of eosinophilia on larval numbers recovered from either eosinophil-rich tissues, such as liver and muscle, or from relatively eosinophil-poor brain tissue. Our observations are also consistent with the report that mice unable to mount an eosinophil response due to disruption of IL-5 genes (23) carry numbers of larvae similar to those of wild-type mice. In our experimental model, unlike natural infections in pregnant and lactating dogs, eosinophilia cannot be dramatically down-regulated during T. canis infections. Our findings suggest therefore, that the suppression of eosinophilia seen during pregnancy in dogs may not be essential for T. canis larval migration and transmission to offspring.
This study provides evidence that a preexisting state of eosinophilia enhances resistance to primary infections with N. brasiliensis but does not promote clearance of T. canis larvae. On first appraisal, IL-5 transgenic mice may seem an artificial model from which to draw conclusions about immunity to parasites in natural infections. However, many humans and other animals are exposed to tissue-invasive parasites for much of their lives. Although initially generated as part of a parasite-specific response, a state of constant eosinophilia may contribute nonspecifically to immunity to newly encountered helminth species. The resistance of IL-5 transgenic mice to infection with N. brasiliensis, without preexisting specific immunity, provides evidence to support the hypothesis that eosinophilia alone can confer at least partial resistance. Preexisting eosinophilia, especially in the tissues, might be expected to be most beneficial for resistance to parasites which normally spend only a short period of time in tissues of the host, i.e., where only an early response can be effective in preventing passage of larvae to definitive sites, such as the gut.
It seems likely that although infections with many parasite species induce eosinophilia, not all helminths will be susceptible to these leukocytes. In contrast to organisms such as N. brasiliensis, helminths which parasitize the host for long periods are likely to have evolved strategies which make them resistant to the actions of eosinophils. Treatment with anti-IL-5 antibody does not seem to alter parasite burdens in mice infected with a range of other parasite species, including S. mansoni (18, 19) and T. spiralis (8). Our earlier studies with other parasite species, including S. mansoni (3) and T. spiralis (2), suggest that chronic eosinophilia and/or overexpression of IL-5 may, by mechanisms yet to be determined, actually be detrimental to host resistance against some infections. In contrast, in this study we have shown that T. canis does not seem to be either advantaged or disadvantaged in eosinophilic IL-5 transgenic mice. Therefore, eosinophilia may not be a universally beneficial response to all invasive helminths. These results provide support for a reassessment of the importance of innate immune mechanisms (5), particularly those operating in infections with metazoan parasites.
Acknowledgments
This work was supported in part by the Australian Research Council, Adelaide Channel 7 Children’s Research Foundation, and the University of Adelaide Faculties of Science and Medicine.
Thanks to Hans Schoppe and Marjorie Quinn (Department of Pathology, University of Adelaide) and the staff of the Department of Pathology, Institute of Medical and Veterinary Science, Adelaide, for preparation of tissues for histology.
REFERENCES
- 1.Coffman R L, Seymour B W, Hudak S, Jackson J, Rennick D. Antibody to interleukin-5 inhibits helminth-induced eosinophilia in mice. Science. 1989;245:308–310. doi: 10.1126/science.2787531. [DOI] [PubMed] [Google Scholar]
- 1a.Daly, C., and L. A. Dent. Unpublished observations.
- 2.Dent, L. A., C. Daly, A. Geddes, J. Cormie, D. A. Finlay, L. Bignold, P. Hagan, R. M. E. Parkhouse, T. Garate, J. Parsons, and G. Mayrhofer. 1997. Immune responses of IL-5 transgenic mice to parasites and aeroallergens. Mem. Inst. Oswaldo Cruz. 92(Suppl. II):45–54. [DOI] [PubMed]
- 3.Dent L A, Munro G H, Piper K P, Sanderson C J, Finlay D A, Dempster R K, Bignold L P, Harkin D G, Hagan P. Eosinophilic interleukin-5 (IL-5) transgenic mice: eosinophil activity and impaired clearance of Schistosoma mansoni. Parasite Immunol. 1997;19:291–300. doi: 10.1046/j.1365-3024.1997.d01-210.x. [DOI] [PubMed] [Google Scholar]
- 4.Dent L A, Strath M, Mellor A L, Sanderson C J. Eosinophilia in transgenic mice expressing interleukin 5. J Exp Med. 1990;172:1425–1431. doi: 10.1084/jem.172.5.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feardon D T. Seeking wisdom in innate immunity (news and views) Nature. 1997;388:323–324. doi: 10.1038/40967. [DOI] [PubMed] [Google Scholar]
- 6.Folkard S G, Hogarth P J, Taylor M J, Bianco A E. Eosinophils are the major effector cells of immunity to microfilariae in a mouse model of onchocerciasis. Parasitology. 1996;112:323–329. doi: 10.1017/s0031182000065847. [DOI] [PubMed] [Google Scholar]
- 7.Grove D I, Mahmoud A A F, Warren K S. Eosinophils and resistance to Trichinella spiralis. J Exp Med. 1977;145:755–759. doi: 10.1084/jem.145.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herndon F J, Kayes S G. Depletion of eosinophils by anti-IL-5 monoclonal antibody treatment of mice infected with Trichinella spiralis does not alter parasite burden or immunologic resistance to reinfection. J Immunol. 1992;149:3642–3647. [PubMed] [Google Scholar]
- 9.Kassai T. Handbook of Nippostrongylus brasiliensis (Nematode). Slough, United Kingdom: Budapest Commonwealth Agricultural Bureaux; 1982. [Google Scholar]
- 10.Khan W I, Abe T, Ishikawa N, Nawa Y, Yoshimura K. Reduced amount of intestinal mucus by treatment with anti-CD4 antibody interferes with the spontaneous cure of Nippostrongylus brasiliensis-infection in mice. Parasite Immunol. 1995;17:485–491. doi: 10.1111/j.1365-3024.1995.tb00919.x. [DOI] [PubMed] [Google Scholar]
- 11.Korenaga M, Hitoshi Y, Yamaguchi N, Sato Y, Takatsu K, Tada I. The role of interleukin-5 in protective immunity to Strongyloides venezuelensis infection in mice. Immunology. 1991;72:502–507. [PMC free article] [PubMed] [Google Scholar]
- 12.Lange A M, Yutanawiboonchai W, Scott P, Abraham D. IL-4 and IL-5 dependent protective immunity to Onchocerca volvulus infective larvae in BALB/cBYJ mice. J Immunol. 1994;153:205–211. [PubMed] [Google Scholar]
- 13.Mahmoud A F, Warren K, Peters P. A role for the eosinophil in acquired resistance to Schistosoma mansoni infection as determined by antieosinophil serum. J Exp Med. 1975;142:805–813. doi: 10.1084/jem.142.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller H R P, Jarrett W F H. Immune reactions in mucous membranes. I. Intestinal mast cell response during helminth expulsion in the rat. Immunology. 1971;20:277–287. [PMC free article] [PubMed] [Google Scholar]
- 15.Parsons J C, Coffman R L, Grieve R B. Antibody to interleukin 5 prevents blood and tissue eosinophilia but not liver trapping in murine larval toxocariasis. Parasite Immunol. 1993;15:501–508. doi: 10.1111/j.1365-3024.1993.tb00637.x. [DOI] [PubMed] [Google Scholar]
- 16.Parsons J C, Grieve R B. Effect of egg dose and host genotype on liver trapping in murine larval toxocariasis. J Parasitol. 1990;76:53–58. [PubMed] [Google Scholar]
- 17.Sasaki O, Sugaya H, Ishida K, Yoshimura K. Ablation of eosinophils with anti-IL-5 antibody enhances the survival of intracranial worms of Angiostrongylus cantonensis in the mouse. Parasite Immunol. 1993;15:349–354. doi: 10.1111/j.1365-3024.1993.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 18.Sher A, Coffman R F, Hieny S, Scott P, Cheever A W. Interleukin 5 is required for the blood and tissue eosinophilia but not the granuloma formation induced by infection with Schistosoma mansoni. Proc Natl Acad Sci USA. 1990;87:61–65. doi: 10.1073/pnas.87.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sher A, Coffman R L, Hieny S, Cheever A W. Ablation of eosinophil and IgE responses with anti-IL-5 or anti-IL-4 antibodies fails to affect immunity against Schistosoma mansoni in the mouse. J Immunol. 1990;145:3911–3916. [PubMed] [Google Scholar]
- 20.Strath M, Dent L A, Sanderson C J. Infection of IL5 transgenic mice with Mesocestoides corti induces very high levels of IL5 but depressed production of eosinophils. Exp Hematol. 1992;20:229–234. [PubMed] [Google Scholar]
- 21.Sugane K, Kusama Y, Takamoto M, Tominaga A, Takatsu K. Eosinophilia, IL-5 level and recovery of larvae in IL-5 transgenic mice infected with Toxocara canis. J Helminthol. 1996;70:153–158. doi: 10.1017/s0022149x00015315. [DOI] [PubMed] [Google Scholar]
- 22.Sugaya H, Aoki M, Yoshida T, Takatsu K, Yoshimura K. Eosinophilia and intracranial worm recovery in interleukin-5 transgenic and interleukin-5 receptor α chain-knockout mice infected with Angiostrongylus cantonensis. Parasitol Res. 1997;83:583–590. doi: 10.1007/s004360050302. [DOI] [PubMed] [Google Scholar]
- 23.Takamoto M, Ovington K S, Behm C A, Sugane K, Young I G, Matthaei K I. Eosinophilia, parasite burden and lung damage in Toxocara canis infection in C57Bl/6 mice genetically deficient in IL-5. Immunology. 1997;90:511–517. doi: 10.1046/j.1365-2567.1997.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tominaga A, Takaki S, Koyama N, Katoh S, Matsumoto R, Migata M, Hitoshi Y, Hosoya Y, Yamauchi S, Kanai Y, Miyazaki J-I, Usuku G, Yamamura K-I, Takatsu K. Transgenic mice expressing a B cell growth and differentiation factor gene (interleukin 5) develop eosinophilia and autoantibody production. J Exp Med. 1991;173:429–437. doi: 10.1084/jem.173.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Y, Bao S, Rothwell T L, Husband A J. Differential expression of interleukin-5 mRNA+ cells and eosinophils in Nippostrongylus brasiliensis infection in resistant and susceptible strains of mice. Eur J Immunol. 1996;26:2133–2139. doi: 10.1002/eji.1830260926. [DOI] [PubMed] [Google Scholar]