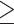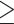Abstract
Background
Obesity adversely influences the central nervous system and cognitive functions. However, the relationship between various obesity indicators and cognitive performance remains controversial. It is unclear which obesity indicator is more relevant to cognitive impairment.
Methods
The Taiwan Biobank (TWB) administered the Chinese version of the Mini-Mental State Examination (MMSE) to 30,697 participants (12,094 males and 18,603 females) aged 60 to 70 years. A total of 3,454 (11.25%) individuals with MMSE < = 24 were classified as having poor cognitive performance. This cross-sectional study investigates the associations of five obesity indicators with cognitive performance. Five separate logistic regression models were fitted for males and another five for females. Covariates adjusted in all models included age, smoking status, drinking status, regular exercise, chronic disease status (diabetes, cardiovascular diseases, heart diseases, stroke, or Parkinson’s disease), depression status, blood pressure level, total cholesterol, fasting glucose, and educational attainment. The five obesity indicators included body mass index (BMI), body fat percentage (BFP), waist circumference (WC), hip circumference (HC), and waist-hip ratio (WHR).
Results
Abdominal obesity defined by WHR was significantly associated with poor cognitive performance. Male WHR > = 0.90 had a higher risk of poor cognitive performance than male WHR < 0.90 (odds ratio [OR] = 1.233; p = 0.007); female WHR > = 0.85 had an increased risk of poor cognitive performance compared with female WHR < 0.85 (OR = 1.221; p = 3.9E-4). HC and general obesity (defined by BMI and BFP) were not significantly associated with cognitive performance.
Conclusion
The results consistently agreed that preventing abdominal obesity is associated with better cognitive performance in both males and females.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12877-022-03457-x.
Keywords: Abdominal obesity, Dementia, Mini-mental state examination
Background
The pace of population aging is increasing. According to the World Health Organization, the number of individuals over age 60 was 1 billion in 2019, estimated to be up to 1.4 billion by 2030 and 2.1 billion by 2050. For aged people, maintaining healthy cognitive function is essential for their quality of life.
Cognitive impairment is defined as difficulties in learning, remembering, concentrating, and making decisions [1]. It decreases the quality of life of aged people and is a critical risk factor for dementia [2, 3]. A tool commonly used to evaluate cognitive impairment in clinical and research settings is the Mini-Mental State Examination (MMSE) [4, 5]. MMSE was developed by Folstein et al. in 1975 [5], containing 11 items and 30 answers. Each answer is scored as one point; therefore, an MMSE test result ranges from 0 to 30. A higher MMSE score is linked to a better cognitive function. MMSE evaluates the performance of orientation, attention, calculation, memory, language, and visual-spatial skills [6].
Some studies have shown that obesity plays a critical role in the decline of cognitive function [7, 8]. Obesity and its comorbidities are risk factors for impaired cognitive performance and neurodegenerative diseases such as dementia [7, 9]. Accumulating evidence has shown that obesity adversely influences the central nervous system and cognitive functions, including learning, attention, decision making, and executive performance [10].
Despite increasing evidence of the relationship between obesity and cognitive decline, the associations of body mass index (BMI) with cognitive function are somewhat controversial [11]. Among several obesity indicators, BMI is the most commonly used indicator in previous investigations [12, 13]. It has been reported that the association between BMI and the risk of cognitive impairment is a U-shaped curve rather than a linear trend [14–16]. Some studies have shown that the overweight category is associated with the lowest risk of cognitive decline [8, 15], where ‘overweight’ indicated a BMI ranging from 23 kg/m2 to 27 kg/m2 [15] or from 24 kg/m2 to 28 kg/m2 [8]. Other studies have shown that a BMI ranging from 18.5 kg/m2 to 23 kg/m2 corresponds to the lowest cognitive impairment risk [17]. Persisting controversies remain in the association of BMI with the risk of cognitive impairment.
Moreover, some studies have also shown that a high body fat percentage (BFP) is associated with worse performance in cognitive control [18]. Abdominal obesity (indicated by a larger waist circumference [WC] or waist-hip ratio [WHR]) is associated with reduced cognitive scores [19–22] and a faster rate of cognitive decline [19, 23]. It remains unclear which obesity indicators are more relevant to cognitive functions.
Individuals with abdominal obesity (indicated by WC or WHR) present higher C-reactive protein and IL-6 concentrations [24]. Abdominal obesity, rather than general obesity, is specifically linked to these inflammatory markers [24]. Moreover, increased C-reactive protein and IL-6 are associated with dementia, identified by a meta-analysis combining seven observational studies (5,717 participants and 746 dementia cases) [25].
Given a large sample size of MMSE test results (30,697 TWB participants), I investigated the relationship between cognitive performance and five obesity indicators, including BMI, BFP, WC, hip circumference (HC), and WHR. In addition to statistical analysis, the biological plausibility of the results will be further discussed.
Methods
Taiwan Biobank
Since October 2012, the TWB has recruited over 100,000 community-based volunteers in Taiwan to build a biobank. Information regarding genomics and lifestyle factors was collected from each participant [26]. After signing informed consent, participants underwent physical examinations and provided urine and blood samples. Trained health professionals gathered lifestyle factors through face-to-face interviews with each participant. The obesity indicators and MMSE measurements were administered by professional nurses or medical laboratory scientists. The interviews and physical examinations for each participant were performed on the same day. The de-identified individual-level data are not publicly downloadable but are available upon application to the TWB (https://www.twbiobank.org.tw/new_web/).
By February 2021, TWB had recruited 132,720 participants aged 30 to 70 years, wherein 30,740 participants were 60 to 70 years old. A total of 30,716 of the 30,740 individuals agreed to participate in the evaluation of MMSE [27–29], including 12,108 males and 18,608 females. The body height and weight of each individual were recorded. BMI was then calculated as body weight in kilograms divided by squared body height in meters. Because body height and weight (and BMI) were basic physical examination items, I removed 14 males and 5 females without the BMI data. Through this step, 30,697 participants remained in this study, wherein 12,094 were males and 18,603 were females.
BFP is the percentage of total fat mass over total body mass. It was measured by bioelectrical impedance analysis (BIA) according to a Tanita body composition analyzer BC-420MA (Tanita Corp., Tokyo, Japan). However, 613 of the 12,094 males (5.1%) and 1,010 of the 18,603 females (5.4%) did not undergo BIA. Therefore, the inference for BFP was based on 29,074 participants, i.e., 94.7% of the 30,697 participants.
WC is the circumference of the midpoint between the iliac crest and the lowest rib (29). HC was the largest circumference around the buttocks in a standing position. Both WC and HC were measured with a nonelastic tape. WHR was then a dimensionless ratio of WC to HC. Among the 30,697 participants, only one female missed WC, HC, and WHR measurements. This female participant was still included in the analysis for BMI and BFP.
Mini-mental state examination
The MMSE evaluates the performance of orientation, attention, calculation, memory, language, and visual-spatial skills [6]. An MMSE score of 24 or lower (adjusted by age and educational attainment [30]) is regarded as likely cognitive impairment. In contrast, a score higher than 24 generally indicates no cognitive impairment [31]. MMSE has been translated into Chinese and is widely used to diagnose dementia or cognitive impairment in many Chinese-speaking areas. It presented acceptable validity and reliability in measuring cognitive functions [27–29].
A previous study showed satisfactory agreement on MMSE scores, given a high interrater correlation coefficient of 0.998 [32]. Moreover, an MMSE score 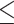 17 was appropriate to define suspected dementia. Based on a Chinese sample, the sensitivity and specificity according to this cutoff value (17) were 1.00 and 0.89, respectively [32]. Dementia is usually diagnosed when cognitive impairment has become severe enough to compromise occupational and social functioning [33]. However, in the TWB, only 40 males (0.3% of the 12,094 males) and 157 females (0.8% of the 18,603 females) had an MMSE score
17 was appropriate to define suspected dementia. Based on a Chinese sample, the sensitivity and specificity according to this cutoff value (17) were 1.00 and 0.89, respectively [32]. Dementia is usually diagnosed when cognitive impairment has become severe enough to compromise occupational and social functioning [33]. However, in the TWB, only 40 males (0.3% of the 12,094 males) and 157 females (0.8% of the 18,603 females) had an MMSE score 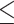 17.
17.
To reach a desirable statistical power, I here investigated the associations of obesity indicators with “poor cognitive performance” (i.e., MMSE score 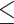 24). An MMSE score ranges from 0 to 30, with a score > 24 indicating basically no cognitive impairment [31]. Therefore, I defined a score
24). An MMSE score ranges from 0 to 30, with a score > 24 indicating basically no cognitive impairment [31]. Therefore, I defined a score 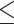 24 as poor cognitive performance, whereas a score > 24 was defined as normal. Here, I used the term “poor cognitive performance” instead of “cognitive impairment” because, in this stage, the MMSE score was original and had not been adjusted by age or educational attainment [30]. Age and educational attainment were adjusted in subsequent logistic regression.
24 as poor cognitive performance, whereas a score > 24 was defined as normal. Here, I used the term “poor cognitive performance” instead of “cognitive impairment” because, in this stage, the MMSE score was original and had not been adjusted by age or educational attainment [30]. Age and educational attainment were adjusted in subsequent logistic regression.
Covariates adjusted in all models
Ten covariates adjusted in all models included age, smoking status, drinking status, regular exercise, chronic disease status, depression status, blood pressure level, total cholesterol, fasting glucose, and educational attainment. These ten covariates were chosen a priori because they were commonly adjusted in studies of MMSE [34].
Smoking was defined as a subject who had smoked for at least 6 months and had not quit smoking when his or her phenotypes were examined. Drinking was defined as a subject having a weekly intake of more than 150cc of alcohol for at least 6 months and having not stopped drinking when his or her phenotypes were measured.
Regular exercise was defined as engaging in 30min of exercise three times a week. Exercise included leisure-time activities such as walking, brisk walking, jogging, swimming, dancing, mountain climbing, and cycling. Chronic disease status was defined as whether an individual had been diagnosed with diabetes, cardiovascular diseases, heart diseases, stroke, or Parkinson’s disease. Depression status was defined as the diagnosis of depression.
Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured twice in a sitting position with a 5-minute rest interval. Blood pressure level was then obtained by the average of these four measurements of BP (i.e., two from SBP and two from DBP). Serum fasting glucose was obtained with a Hitachi LST008 analyzer (Hitachi High-Technologies, Tokyo, Japan) after a fast of at least 6h.
The educational attainment of each participant was surveyed through a face-to-face interview with TWB researchers. It was recorded as a number ranging from 1 to 7, with 1 indicating “illiterate”, 2 indicating “no formal education but literate”, 3 indicating “primary school graduate”, 4 indicating “junior high school graduate”, 5 indicating “senior high school graduate”, 6 indicating “college graduate”, and 7 indicating “master’s or higher degree”. All regression models coded educational attainment as an integer ranging from 1 to 7.
Definition of obesity
This cross-sectional study assesses the associations of five obesity indicators with cognitive performance. The five obesity indicators and the MMSE score of each participant were measured on the same day (from October 2012 to February 2021). According to Taiwan’s Ministry of Health and Welfare, general obesity can be defined by BMI and/or BFP. BMI levels were categorized into four groups: underweight (BMI < 18.5 kg/m2), healthy weight (18.5 kg/m2 < = BMI < 24 kg/m2), overweight (24 kg/m2 < = BMI < 27 kg/m2), and obese (BMI > = 27 kg/m2). Concerning the criterion of BFP, males with BFP > = 25% or females with BFP > = 30% were classified into the obesity group.
According to Taiwan’s Ministry of Health and Welfare, abdominal obesity can be defined either way: (1) WC 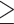 90 cm for males or WC
90 cm for males or WC 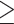 80 cm for females; (2) WHR
80 cm for females; (2) WHR 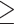 0.90 for males or WHR
0.90 for males or WHR 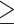 0.85 for females. Here, I assessed the associations of both definitions of abdominal obesity with poor cognitive performance.
0.85 for females. Here, I assessed the associations of both definitions of abdominal obesity with poor cognitive performance.
Statistical analysis
Males and females are different in body composition and risks of cognitive impairment [35]. Females generally have a larger BFP than males, as this is the case in the current study (Table 1). Moreover, females showed a significant higher prevalence of cognitive impairment than males after age 75 (62.7% vs. 45.4%, P-value < 0.005) [35]. To explore sex-specific obesity indicators associated with cognitive performance, I analyzed male and female individuals with separate logistic regression models. With the logistic regression model, the status of cognitive performance (poor if MMSE 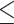 24 vs. normal if MMSE > 24) was regressed on BMI/BFP/WC/WHR categories in separate logistic regression models (Because HC has not been used to define obesity, it was not investigated in this step). In all models, I adjusted for the ten covariates mentioned above, including age, smoking status, drinking status, regular exercise, chronic disease status, depression status, blood pressure level, total cholesterol, fasting glucose, and educational attainment.
24 vs. normal if MMSE > 24) was regressed on BMI/BFP/WC/WHR categories in separate logistic regression models (Because HC has not been used to define obesity, it was not investigated in this step). In all models, I adjusted for the ten covariates mentioned above, including age, smoking status, drinking status, regular exercise, chronic disease status, depression status, blood pressure level, total cholesterol, fasting glucose, and educational attainment.
Table 1.
Basic characteristics of the 30,697 TWB participants
| Males | Females | P-value 1 | |
|---|---|---|---|
| Total | 12,094 (39.4%) | 18,603 (60.6%) | |
| Age (years) | 64.2 2.9 2.9 |
63.8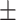 2.8 2.8 |
< 0.001 |
| BMI (kg/m2) | 24.9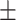 3.1 3.1 |
24.0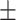 3.5 3.5 |
< 0.001 |
|
Underweight (yes or no) BMI < 18.5 kg/m2 |
169 (1.4%) | 563 (3.0%) | < 0.001 |
|
Healthy weight (yes or no) 18.5 < = BMI < 24 kg/m2 |
4,632 (38.3%) | 9,788 (52.6%) | < 0.001 |
|
Overweight (yes or no) 24 < = BMI < 27 kg/m2 |
4,622 (38.2%) | 5,027 (27.0%) | < 0.001 |
|
Obesity (yes or no) BMI > = 27 kg/m2 |
2,671 (22.1%) | 3,225 (17.4%) | < 0.001 |
| Body fat percentage (%) | 22.4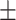 5.1 5.1 |
32.9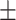 6.1 6.1 |
< 0.001 |
Obesity defined by BFP (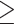 25% for males; 25% for males; 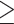 30% for females) 30% for females) |
3,382 (29.5% among 11,481 males with BFP) | 12,238 (69.6% among 17,593 females with BFP) | < 0.001 |
| Waist circumference (cm) | 88.0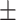 8.5 8.5 |
83.3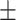 9.6 9.6 |
< 0.001 |
Abdominal obesity defined by WC (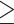 90 cm for males; 90 cm for males; 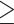 80 cm for females) 80 cm for females) |
4,899 (40.5%) | 11,623 (62.5%) | < 0.001 |
| Hip circumference (cm) | 95.8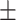 5.8 5.8 |
94.5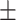 6.8 6.8 |
< 0.001 |
| Waist-hip ratio | 0.92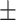 0.05 0.05 |
0.88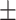 0.07 0.07 |
< 0.001 |
Abdominal obesity defined by WHR (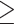 0.90 for males; 0.90 for males; 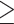 0.85 for females) 0.85 for females) |
7,630 (63.1%) | 12,319 (66.2%) | < 0.001 |
| Drinking (yes or no) 2 | 1,408 (11.6%) | 236 (1.3%) | < 0.001 |
| Smoking (yes or no) 3 | 1,624 (13.4%) | 192 (1.0%) | < 0.001 |
| Regular exercise (yes or no) 4 | 7,767 (64.2%) | 11,564 (62.2%) | < 0.001 |
| Status of chronic diseases 5 | 3,990 (33.0%) | 4,889 (26.3%) | < 0.001 |
| Status of depression | 327 (2.7%) | 861 (4.6%) | < 0.001 |
| MMSE score | 27.6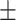 2.3 2.3 |
27.3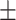 2.6 2.6 |
< 0.001 |
| Poor cognitive performance (MMSE score < = 24) | 1,054 (8.7%) | 2,400 (12.9%) | < 0.001 |
| Education | |||
| Refuse to answer this question | 2 (0.02%) | 8 (0.04%) | 0.351 |
| Illiterate | 8 (0.07%) | 168 (0.90%) | < 0.001 |
|
No formal education but literate |
7 (0.06%) | 41 (0.22%) | < 0.001 |
| Primary school graduate | 1,106 (9.14%) | 3,456 (18.58%) | < 0.001 |
| Junior high school graduate | 1,053 (8.71%) | 2,623 (14.10%) | < 0.001 |
| Senior high school graduate | 3,202 (26.48%) | 6,156 (33.09%) | < 0.001 |
| College graduate | 5,570 (46.05%) | 5,466 (29.39%) | < 0.001 |
| Master’s or higher degree | 1,146 (9.47%) | 685 (3.68%) | < 0.001 |
Data are presented in n (%) or mean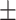 SD.
SD.
1P-value of testing the mean difference between males and females, based on the proportion test (for BMI categories: underweight, healthy weight, overweight, and obese; drinking; smoking; regular exercise; status of chronic diseases; status of depression; MMSE score < = 24) or the two-sample t-test (for other variables). For P-values less than 0.001, I reported it as “< 0.001”
2 Drinking was defined as a person having a weekly intake of more than 150 mL of alcohol for at least 6 months and having not stopped drinking at the time he or she participated in the TWB.
3 Smoking was defined as a person who had smoked cigarettes for at least 6 months and had not quit smoking at the time he or she participated in the TWB.
4 Regular exercise was defined as performing 30min of exercise three times a week. Exercise included leisure-time activities such as swimming, jogging, cycling, mountain climbing, dancing, and weight training
5 Status of chronic diseases was defined as whether an individual had diabetes, cardiovascular diseases, heart diseases, stroke, or Parkinson’s disease
As a sensitivity analysis, obesity indicators were also treated as continuous scales in the regression models. The cognitive performance status was regressed on the z-score transformation of each obesity indicator separately while adjusting for the same ten covariates. Through the z-score transformation, the odds ratios (ORs) were independent of the units of various obesity indicators (kg/m2 for BMI; % for BFP; cm for WC and HC). Although HC has not been used to define obesity, it is the denominator of WHR. Therefore, it was also investigated in this step. The effect sizes of various obesity indicators can be compared directly. Because the obesity indicators were investigated in separate regression models, I adjusted multiple testing with the false discovery rate (FDR) control. P-values with Benjamini-Hochberg FDR [36] < 5% were considered statistically significant.
Some studies defined cognitive performance as poor if MMSE 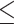 25 vs. normal if MMSE > 25 [37, 38]. As another sensitivity analysis, I performed all the abovementioned analyses according to this cutoff (
25 vs. normal if MMSE > 25 [37, 38]. As another sensitivity analysis, I performed all the abovementioned analyses according to this cutoff (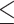 25 vs. > 25). All analyses were performed using R statistical software (version 4.1.1; The R Project for Statistical Computing; http://www.r-project.org/).
25 vs. > 25). All analyses were performed using R statistical software (version 4.1.1; The R Project for Statistical Computing; http://www.r-project.org/).
Results
Basic characteristics
Table 1 summarizes the characteristics of 12,094 males (39.4%) and 18,603 females (60.6%). Except for BFP, male participants, on average, had larger values of the obesity indicators than did female participants. The percentages of alcohol consumption, cigarette smoking, and regular exercise were higher in males than females. Males, on average, had higher educational attainment than females. A total of 55.5% of males and 33.1% of females had college or higher degrees. Regarding the outcome measure, fewer males were categorized as having poor cognitive performance (MMSE score 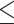 24) than females (8.7% vs. 12.9%, Table 1).
24) than females (8.7% vs. 12.9%, Table 1).
BMI/BFP/WC/WHR categories and cognitive performance
Most male participants had healthy weights (38.3%, 18.5 kg/m2 < = BMI < 24 kg/m2) or were overweight (38.2%, 24 kg/m2 < = BMI < 27 kg/m2), whereas only 1.4% of males were underweight (BMI < 18.5 kg/m2). In contrast, more female participants had healthy weights (52.6%, Table 1).
By treating the healthy weight category as the reference group, none of the four BMI categories presented significantly different risks of poor cognitive performance (Table 2). The underweight group showed the lowest risk (odds ratio [OR] < 1.0), whereas the obesity group (BMI > = 27 kg/m2) presented the highest risk, although these ORs were not significantly different from 1.0. Generally, the risks of poor cognitive performance increased with BMI levels, and this result was consistently observed in males and females (Table 2).
Table 2.
Odds ratio of poor cognitive performance for various BMI (or BFP, WC, WHR) categories compared to the healthy BMI (or BFP, WC, WHR) group ( P -values with false discovery rates < 5% are shown in bold)
| Definition of obesity | Male participants | Female participants | ||||
|---|---|---|---|---|---|---|
| Odds ratio 1 | 95% C.I. | P-value | Odds ratio1 | 95% C.I. | P-value | |
| General obesity defined by BMI 2 | ||||||
|
Underweight (yes vs. no) BMI < 18.5 kg/m2 |
0.822 | [0.406, 1.508] | 0.554 | 0.814 | [0.563, 1.146] | 0.254 |
|
Overweight (yes vs. no) 24 kg/m2 < = BMI < 27 kg/m2 |
1.128 | [0.963, 1.322] | 0.135 | 1.031 | [0.920, 1.155] | 0.600 |
|
Obesity (yes vs. no) BMI > = 27 kg/m2 |
1.158 | [0.966, 1.385] | 0.111 | 1.099 | [0.966, 1.250] | 0.150 |
|
General obesity defined by BFP (yes vs. no) 3 BFP BFP |
1.111 | [0.955, 1.290] | 0.171 | 1.035 | [0.920, 1.165] | 0.569 |
|
Abdominal obesity defined by WC (yes vs. no) 4 WC WC |
1.168 | [1.018, 1.340] | 0.027 | 1.214 | [1.091, 1.351] | 3.9E-4 |
|
Abdominal obesity defined by WHR (yes vs. no) 5 WHR WHR |
1.233 | [1.061, 1.436] | 0.007 | 1.221 | [1.094, 1.364] | 3.9E-4 |
1 In all logistic regression models, I adjusted for ten covariates: age, smoking status (yes vs. no), drinking status (yes vs. no), regular exercise (yes vs. no), chronic disease status (yes vs. no), depression status (yes vs. no), blood pressure level, total cholesterol, fasting glucose, and educational attainment (1, 2, …, or 7)
2 Reference group: the healthy weight group (18.5 kg/m2 < = BMI < 24 kg/m2).
3 Reference group: BFP < 25% for males; BFP < 30% for females
4 Reference group: WC < 90 cm for males; WC < 80 cm for females
5 Reference group: WHR < 0.90 for males; WHR < 0.85 for females
The mean BFP in females was 32.9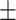 6.1 (%), and 69.6% of female participants were categorized as obese according to the BFP criterion (Table 1). Fortunately, although a large proportion of female participants had a BFP > = 30%, female BFP > = 30% was not a significant risk factor for poor cognitive performance (OR = 1.035, p = 0.569, Table 2).
6.1 (%), and 69.6% of female participants were categorized as obese according to the BFP criterion (Table 1). Fortunately, although a large proportion of female participants had a BFP > = 30%, female BFP > = 30% was not a significant risk factor for poor cognitive performance (OR = 1.035, p = 0.569, Table 2).
Abdominal obesity can be defined by WC and/or WHR. Different from general obesity (indicated by BMI or BFP), abdominal obesity was significantly associated with poor cognitive performance, especially that defined by WHR. The ORs were 1.233 (95% CI = 1.061 ~ 1.436, p = 0.007) and 1.221 (95% CI = 1.094 ~ 1.364, p = 3.9E-4) for male WHR > = 0.90 and female WHR > = 0.85, respectively (Table 2). Moreover, the OR was 1.214 (95% CI = 1.091 ~ 1.351, p = 3.9E-4) for female WC > = 80 cm (Table 2).
Continuous BMI/BFP/WC/HC/WHR values and cognitive performance
In addition to assessing the associations of cognitive performance with various BMI/BFP/WC/WHR categories, I also treated the five obesity indicators as continuous scales in the association analysis. The z-score transformation was performed on each obesity measure before fitting the logistic regression. Table 3 shows the ORs of poor cognitive performance by increasing one standard deviation (SD) of each obesity indicator. Again, WHR provided the most significant association with cognitive performance. For males, an increase of one SD in WHR (0.05) was significantly associated with an increase in the risk of poor cognitive performance (OR = 1.107, 95% CI = 1.033 ~ 1.187, p = 4.0E-3). For females, every SD increase in WHR (0.07) was significantly associated with an increase in the risk of poor cognitive performance (OR = 1.136, 95% CI = 1.081 ~ 1.194, p = 5.9E-7), and every SD increase in WC (9.6 cm) was also significantly associated with an increase in the risk of poor cognitive performance (OR = 1.109, 95% CI = 1.055 ~ 1.165, p = 4.3E-5, Table 3).
Table 3.
Odds ratio of poor cognitive performance by increasing one SD of each obesity indicator ( P -values with false discovery rates < 5% are shown in bold)
| Male participants | Female participants | |||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% C.I. | P-value | Odds ratio | 95% C.I. | P-value | |
| BMI 1 | 1.068 | [0.998, 1.142] | 0.057 | 1.059 | [1.008, 1.112] | 0.023 |
| Body fat percentage 1 | 1.078 | [1.003, 1.158] | 0.042 | 1.053 | [0.999, 1.110] | 0.055 |
| Waist circumference 1 | 1.067 | [0.997, 1.142] | 0.061 | 1.109 | [1.055, 1.165] | 4.3E-5 |
| Hip circumference 1 | 1.007 | [0.941, 1.076] | 0.849 | 1.028 | [0.979, 1.078] | 0.266 |
| Waist-hip ratio 1 | 1.107 | [1.033, 1.187] | 4.0E-3 | 1.136 | [1.081, 1.194] | 5.9E-7 |
1 The z-score transformation was performed on each obesity measure before fitting the logistic regression. In all logistic regression models, I adjusted for ten covariates: age, smoking status (yes vs. no), drinking status (yes vs. no), regular exercise (yes vs. no), chronic disease status (yes vs. no), depression status (yes vs. no), blood pressure level, total cholesterol, fasting glucose, and educational attainment (1, 2, …, or 7)
Some studies used another MMSE cutoff to define cognitive performance, i.e., poor if MMSE 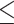 25 vs. normal if MMSE > 25 [37, 38]. The results corresponding to this cutoff also agreed that abdominal obesity is more relevant to cognitive performance than general obesity (Supplementary Materials, Tables S1-S2).
25 vs. normal if MMSE > 25 [37, 38]. The results corresponding to this cutoff also agreed that abdominal obesity is more relevant to cognitive performance than general obesity (Supplementary Materials, Tables S1-S2).
Discussion
This study showed that WHR was most relevant to cognitive performance among five commonly used obesity indicators. The results consistently agreed that preventing abdominal obesity is associated with better cognitive performance in both sexes. WC is a straightforward measurement for abdominal obesity. However, WC alone may underestimate the risk of abdominal obesity for smaller people. In contrast, WHR adjusts WC with HC, becoming a better health indicator than WC in some situations [39–41]. For example, based on 1,189 adults aged 70–79 years at baseline, 12-year all-cause mortality was associated with WHR rather than WC or BMI [39].
This study has two significant strengths: the availability of various obesity indicators and a much larger sample size for MMSE results in a single study (30,697 individuals) compared with previous studies [8, 42]. Cognitive decline can start from age 45 [43]. Although these 30,697 individuals (aged 60 to 70 years) were relatively young considering cognitive impairment, identifying the obesity indicator most associated with cognitive performance at age 60–70 can help prevent cognitive impairment later.
Obesity indicators were treated as continuous scales (Table 3) and several categories (Table 2) according to Taiwan’s Ministry of Health and Welfare recommendations. Results consistently agreed that, among the five obesity indicators, WHR was most relevant to cognitive performance. WC was generally the second indicator that was associated with cognitive performance. These results highlighted the importance of abdominal obesity to the risk of cognitive impairment.
Both the results from males and females showed the tendency of increased risk of poor cognitive performance given elevated BMI levels, but these associations were not statistically significant (Table 2). Due to this nonsignificant result, the associations of obesity with cognitive function may be overlooked. This study illustrates that abdominal obesity, rather than general obesity, is associated with cognitive performance.
According to the criteria of Taiwan’s Ministry of Health and Welfare, many female participants were in the general obesity category according to BFP (69.6%, Table 1) and abdominal obesity by WHR (66.2%). However, only abdominal obesity by WHR was significantly associated with an increased risk of poor cognitive performance (OR = 1.221, 95% CI = 1.094 ~ 1.364, p = 3.9E-4, Table 2). In contrast, general obesity by BFP was found to be of little relevance to poor cognitive performance (OR = 1.035, 95% CI = 0.920 ~ 1.165, p = 0.569). A large WHR is a threat to cognitive health.
A previous study used the same criterion for WHR (male WHR 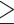 0.90; female WHR
0.90; female WHR 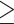 0.85) to define abdominal obesity, and the investigators also found a significant association between abdominal obesity and cognitive impairment (OR = 1.532, 95% CI = 1.037~2.263, p = 0.032) [8]. This result was in line with the results shown here. However, the current work has three strengths over the previous study [8]. First, this work was based on a much larger sample size (30,697) than that of the previous research (1,100) [8]. Second, more obesity indicators were assessed (five) than in the previous research (only BMI and WHR) [8]. Finally, I performed an analysis within each sex stratum. A recent study has shown that aging is associated with different obesity indicators in males and females [44]. I performed a sex-specific analysis to clarify whether this is also the case in cognitive performance.
0.85) to define abdominal obesity, and the investigators also found a significant association between abdominal obesity and cognitive impairment (OR = 1.532, 95% CI = 1.037~2.263, p = 0.032) [8]. This result was in line with the results shown here. However, the current work has three strengths over the previous study [8]. First, this work was based on a much larger sample size (30,697) than that of the previous research (1,100) [8]. Second, more obesity indicators were assessed (five) than in the previous research (only BMI and WHR) [8]. Finally, I performed an analysis within each sex stratum. A recent study has shown that aging is associated with different obesity indicators in males and females [44]. I performed a sex-specific analysis to clarify whether this is also the case in cognitive performance.
Abdominal obesity indicates excess truncal (particularly visceral) fat [45], which specifically increases the risk of developing insulin resistance [46]. Insulin resistance further drives metabolic syndromes and cognitive declines [47]. By analyzing the MMSE results of more than 30,000 TWB individuals, this study confirmed the link between abdominal obesity and poor cognitive performance. Abdominal obesity is a risk factor for poor cognitive performance independent of age, educational attainment, smoking, drinking, regular exercise, depression, blood pressure level, total cholesterol, fasting glucose, and chronic diseases (diabetes, cardiovascular diseases, heart diseases, stroke, or Parkinson’s disease).
Finally, the main limitation of this work is that it is a cross-sectional study, and the associations observed here cannot be explained as causality. Residual confounding and reverse causation are possible. Furthermore, the lack of association of BMI (or BFP) with poor cognitive performance could be because of insufficient power. Medication information was not collected by the TWB, and therefore it was not adjusted in the analyses. An even more extensive study will be needed to replicate these results.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material: Table S1. Odds ratio of “poor cognitive performance” (MMSE ≤ 25) for various BMI (or BFP, WC, WHR) categories compared to the healthy BMI (or BFP, WC, WHR) group (P-values with false discovery rates < 5% were shown in bold)
Table S2. Odds ratio of “poor cognitive performance” (MMSE ≤ 25) by increasing one SD of each obesity indicator (P-values with false discovery rates < 5% were shown in bold)
Acknowledgements
The author would like to thank the TWB for approving my application to access the data.
Abbreviations
- BFP
body fat percentage.
- BMI
body mass index.
- FDR
false discovery rate.
- MMSE
Mini-Mental State Examination.
- HC
hip circumference.
- TWB
Taiwan Biobank.
- WC
waist circumference.
- WHR
waist-hip ratio.
Author contributions
Wan-Yu Lin is a professor at the Institute of Epidemiology and Preventive Medicine & Master of Public Health Degree Program, College of Public Health, National Taiwan University.
Wan-Yu Lin conceived the study design, applied for the TWB data, developed the analysis tool, performed the analyses, interpreted the results, and wrote the manuscript.
Funding
This study was supported by the National Science and Technology Council of Taiwan (grant number MOST 111-2314-B-002-099 to Wan-Yu Lin).
Data availability
The individual-level TWB data supporting this study’s findings are available upon application to TWB (https://www.twbiobank.org.tw/new_web/). TWB approved my application to access the data on February 18, 2020 (application number: TWBR10810-07; principal investigator: Wan-Yu Lin).
Declarations
Ethics approval and consent to participate
TWB was approved by the Institutional Review Board on Biomedical Science Research/IRB-BM, Academia Sinica, and by the Ethics and Governance Council of TWB, Taiwan. Written informed consent was obtained from each participant and the study was carried out in accordance with institutional requirements and the principles of the Declaration of Helsinki. This study further received approval from the Research Ethics Committee of National Taiwan University Hospital (NTUH-REC no. 201805050RINB).
Consent for publication
Not Applicable.
Competing interests
The author declares that she has no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1. Fredriksen-Goldsen KI, Jen S, Bryan AEB, Goldsen J: Cognitive Impairment, Alzheimer’s Disease, and Other Dementias in the Lives of Lesbian, Gay, Bisexual and Transgender (LGBT) Older Adults and Their Caregivers: Needs and Competencies. J Appl Gerontol 2018, 37(5):545–569. [DOI] [PMC free article] [PubMed]
- 2. Plassman BL, Langa KM, McCammon RJ, Fisher GG, Potter GG, Burke JR, Steffens DC, Foster NL, Giordani B, Unverzagt FW et al: Incidence of dementia and cognitive impairment, not dementia in the United States. Ann Neurol 2011, 70(3):418–426. [DOI] [PMC free article] [PubMed]
- 3. Pais R, Ruano L, O PC, Barros H: Global Cognitive Impairment Prevalence and Incidence in Community Dwelling Older Adults-A Systematic Review. Geriatrics (Basel) 2020, 5(4). [DOI] [PMC free article] [PubMed]
- 4. Arevalo-Rodriguez I, Smailagic N, Roque-Figuls M, Ciapponi A, Sanchez-Perez E, Giannakou A, Pedraza OL, Bonfill Cosp X, Cullum S: Mini-Mental State Examination (MMSE) for the early detection of dementia in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev 2021, 7:CD010783. [DOI] [PMC free article] [PubMed]
- 5. Folstein MF, Folstein SE, McHugh PR: “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975, 12(3):189–198. [DOI] [PubMed]
- 6. Baker FA, Bloska J, Braat S, Bukowska A, Clark I, Hsu MH, Kvamme T, Lautenschlager N, Lee YC, Smrokowska-Reichmann A et al: HOMESIDE: home-based family caregiver-delivered music and reading interventions for people living with dementia: protocol of a randomised controlled trial. Bmj Open 2019, 9(11):e031332. [DOI] [PMC free article] [PubMed]
- 7. Dye L, Boyle NB, Champ C, Lawton C: The relationship between obesity and cognitive health and decline. P Nutr Soc 2017, 76(4):443–454. [DOI] [PubMed]
- 8. Hou QT, Guan Y, Yu WH, Liu XT, Wu LH, Xiao MZ, Lu Y: Associations between obesity and cognitive impairment in the Chinese elderly: an observational study. Clinical Interventions in Aging 2019, 14:367–373. [DOI] [PMC free article] [PubMed]
- 9. Nguyen JC, Killcross AS, Jenkins TA: Obesity and cognitive decline: role of inflammation and vascular changes. Front Neurosci 2014, 8:375. [DOI] [PMC free article] [PubMed]
- 10. O’Brien PD, Hinder LM, Callaghan BC, Feldman EL: Neurological consequences of obesity. Lancet Neurol 2017, 16(6):465–477. [DOI] [PMC free article] [PubMed]
- 11. Zhang T, Yan R, Chen Q: Body mass index, waist-to-hip ratio and cognitive function among Chinese elderly: a cross-sectional study (vol 8, e022055, 2018). Bmj Open 2018, 8(11). [DOI] [PMC free article] [PubMed]
- 12. Yuan YM, Li J, Zhang N, Fu PP, Jing ZY, Yu CT, Zhao D, Hao WT, Zhou CC: Body mass index and mild cognitive impairment among rural older adults in China: the moderating roles of gender and age. Bmc Psychiatry 2021, 21(1). [DOI] [PMC free article] [PubMed]
- 13. Garcia-Ptacek S, Faxen-Irving G, Cermakova P, Eriksdotter M, Religa D: Body mass index in dementia. Eur J Clin Nutr 2014, 68(11):1204–1209. [DOI] [PubMed]
- 14. Luchsinger JA, Patel B, Tang MX, Schupf N, Mayeux R: Measures of adiposity and dementia risk in elderly persons. Arch Neurol 2007, 64(3):392–398. [DOI] [PMC free article] [PubMed]
- 15. Deschamps V, Astier X, Ferry M, Rainfray M, Emeriau JP, Barberger-Gateau P: Nutritional status of healthy elderly persons living in Dordogne, France, and relation with mortality and cognitive or functional decline. Eur J Clin Nutr 2002, 56(4):305–312. [DOI] [PubMed]
- 16. Kuo HK, Jones RN, Milberg WP, Tennstedt S, Talbot L, Morris JN, Lipsitz LA: Cognitive function in normal-weight, overweight, and obese older adults: an analysis of the Advanced Cognitive Training for Independent and Vital Elderly cohort. J Am Geriatr Soc 2006, 54(1):97–103. [DOI] [PMC free article] [PubMed]
- 17. Wang F, Zhao M, Han Z, Li D, Zhang S, Zhang Y, Kong X, Sun N, Zhang Q, Lei P: Association of body mass index with amnestic and non-amnestic mild cognitive impairment risk in elderly. Bmc Psychiatry 2017, 17(1):334. [DOI] [PMC free article] [PubMed]
- 18. Huang T, Chen Z, Shen L, Fan X, Wang K: Associations of Cognitive Function with BMI, Body Fat Mass and Visceral Fat in Young Adulthood. Medicina (Kaunas) 2019, 55(6). [DOI] [PMC free article] [PubMed]
- 19. Anand SS, Friedrich MG, Lee DS, Awadalla P, Despres JP, Desai D, de Souza RJ, Dummer T, Parraga G, Larose E et al: Evaluation of Adiposity and Cognitive Function in Adults. JAMA Netw Open 2022, 5(2):e2146324. [DOI] [PMC free article] [PubMed]
- 20. Zeki Al Hazzouri A, Haan MN, Whitmer RA, Yaffe K, Neuhaus J: Central obesity, leptin and cognitive decline: the Sacramento Area Latino Study on Aging. Dement Geriatr Cogn Disord 2012, 33(6):400–409. [DOI] [PMC free article] [PubMed]
- 21. Kesse-Guyot E, Andreeva VA, Touvier M, Jeandel C, Ferry M, Hercberg S, Galan P, Grp SVMr: Overall and abdominal adiposity in midlife and subsequent cognitive function. J Nutr Health Aging 2015, 19(2):183–189. [DOI] [PubMed]
- 22. Kanaya AM, Lindquist K, Harris TB, Launer L, Rosano C, Satterfield S, Yaffe K, Health ABCS: Total and regional adiposity and cognitive change in older adults: The Health, Aging and Body Composition (ABC) study. Arch Neurol 2009, 66(3):329–335. [DOI] [PMC free article] [PubMed]
- 23. West NA, Haan MN: Body adiposity in late life and risk of dementia or cognitive impairment in a longitudinal community-based study. J Gerontol A Biol Sci Med Sci 2009, 64(1):103–109. [DOI] [PMC free article] [PubMed]
- 24. Hermsdorff HHM, Zulet MA, Puchau B, Martinez JA: Central Adiposity Rather Than Total Adiposity Measurements Are Specifically Involved in the Inflammatory Status from Healthy Young Adults. Inflammation 2011, 34(3):161–170. [DOI] [PubMed]
- 25. Koyama A, O’Brien J, Weuve J, Blacker D, Metti AL, Yaffe K: The role of peripheral inflammatory markers in dementia and Alzheimer’s disease: a meta-analysis. J Gerontol A Biol Sci Med Sci 2013, 68(4):433–440. [DOI] [PMC free article] [PubMed]
- 26. Chen CH, Yang JH, Chiang CWK, Hsiung CN, Wu PE, Chang LC, Chu HW, Chang J, Song IW, Yang SL et al: Population structure of Han Chinese in the modern Taiwanese population based on 10,000 participants in the Taiwan Biobank project. Human Molecular Genetics 2016, 25(24):5321–5331. [DOI] [PMC free article] [PubMed]
- 27. PK Yip, YI Shyu, SI Liu, JY Lee, CF Chou, RC CHEN, SH Lin, Chen R: An epidemiological survey of dementia among elderly in an urban district of Taipei. Acta Neurol Sinica 1992, 1:347–354.
- 28. Guo N-W, Liu H-C, Wong P-F, Liao K-K, Yan S-H, Lin K-P, Chang C-Y, Hsu T-C: Chinese Version and Norms of the Mini-Mental State Examination. J Rehab Med 1988, 16:52–59.
- 29. Katzman R, Zhang MY, Ouang Ya Q, Wang ZY, Liu WT, Yu E, Wong SC, Salmon DP, Grant I: A Chinese version of the Mini-Mental State Examination; impact of illiteracy in a Shanghai dementia survey. J Clin Epidemiol 1988, 41(10):971–978. [DOI] [PubMed]
- 30. Magni E, Binetti G, Bianchetti A, Rozzini R, Trabucchi M: Mini-Mental State Examination: a normative study in Italian elderly population. Eur J Neurol 1996, 3(3):198–202. [DOI] [PubMed]
- 31. Pezzotti P, Scalmana S, Mastromattei A, Di Lallo D, Grp PAW: The accuracy of the MMSE in detecting cognitive impairment when administered by general practitioners: A prospective observational study. Bmc Fam Pract 2008, 9. [DOI] [PMC free article] [PubMed]
- 32. Li G, Shen YC, Chen CH, Zhao YW, Li SR, Lu M: An epidemiological survey of age-related dementia in an urban area of Beijing. Acta Psychiatr Scand 1989, 79(6):557–563. [DOI] [PubMed]
- 33. Hugo J, Ganguli M: Dementia and cognitive impairment: epidemiology, diagnosis, and treatment. Clin Geriatr Med 2014, 30(3):421–442. [DOI] [PMC free article] [PubMed]
- 34. Lin E, Kuo PH, Lin WY, Liu YL, Yang AC, Tsai SJ: An association study in the Taiwan Biobank elicits three novel candidates for cognitive aging in old adults: NCAM1, TTC12 and ZBTB20. Aging (Albany NY) 2021, 13(14):18769–18788. [DOI] [PMC free article] [PubMed]
- 35. Wang J, Xiao LD, Wang K, Luo Y, Li XM: Gender Differences in Cognitive Impairment among Rural Elderly in China. Int J Env Res Pub He 2020, 17(10):3724. [DOI] [PMC free article] [PubMed]
- 36. Benjamini Y, Hochberg Y: Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc B 1995, 57:289–300.
- 37. Burdick DJ, Cholerton B, Watson GS, Siderowf A, Trojanowski JQ, Weintraub D, Ritz B, Rhodes SL, Rausch R, Factor SA et al: People with Parkinson’s Disease and Normal MMSE Score Have a Broad Range of Cognitive Performance. Movement Disord 2014, 29(10):1258–1264. [DOI] [PMC free article] [PubMed]
- 38. Kvitting AS, Fallman K, Wressle E, Marcusson J: Age-Normative MMSE Data for Older Persons Aged 85 to 93 in a Longitudinal Swedish Cohort. J Am Geriatr Soc 2019, 67(3):534–538. [DOI] [PMC free article] [PubMed]
- 39. Srikanthan P, Seeman TE, Karlamangla AS: Waist-Hip-Ratio as a Predictor of All-Cause Mortality in High-Functioning Older Adults. Ann Epidemiol 2009, 19(10):724–731. [DOI] [PMC free article] [PubMed]
- 40. Welborn TA, Dhaliwal SS: Preferred clinical measures of central obesity for predicting mortality. Eur J Clin Nutr 2007, 61(12):1373–1379. [DOI] [PubMed]
- 41. Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L et al: Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet 2005, 366(9497):1640–1649. [DOI] [PubMed]
- 42. Guo DD, Zhang X, Zhan CQ, Lin QX, Liu J, Yang QX, Tu J, Ning XJ, Wang JH, Song YJ: Sex Differences in the Association Between Obesity and Cognitive Impairment in a Low-Income Elderly Population in Rural China: A Population-Based Cross-Sectional Study. Front Neurol 2021, 12. [DOI] [PMC free article] [PubMed]
- 43. Singh-Manoux A, Kivimaki M, Glymour MM, Elbaz A, Berr C, Ebmeier KP, Ferrie JE, Dugravot A: Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. BMJ 2012, 344:d7622. [DOI] [PMC free article] [PubMed]
- 44. Lin WY, Wang YC, Teng IH, Liu C, Lou XY: Associations of five obesity metrics with epigenetic age acceleration: Evidence from 2,474 Taiwan Biobank participants. Obesity (Silver Spring) 2021, 29(10):1731–1738. [DOI] [PubMed]
- 45. Cashdan E: Waist-to-Hip Ratio across Cultures: Trade-Offs between Androgen- and Estrogen-Dependent Traits. Curr Anthropol 2008, 49(6):1098–1106.
- 46. Westphal SA: Obesity, abdominal obesity, and insulin resistance. Clin Cornerstone 2008, 9(1):23–29; discussion 30 − 21. [DOI] [PubMed]
- 47. Kim B, Feldman EL: Insulin resistance as a key link for the increased risk of cognitive impairment in the metabolic syndrome. Exp Mol Med 2015, 47. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material: Table S1. Odds ratio of “poor cognitive performance” (MMSE ≤ 25) for various BMI (or BFP, WC, WHR) categories compared to the healthy BMI (or BFP, WC, WHR) group (P-values with false discovery rates < 5% were shown in bold)
Table S2. Odds ratio of “poor cognitive performance” (MMSE ≤ 25) by increasing one SD of each obesity indicator (P-values with false discovery rates < 5% were shown in bold)
Data Availability Statement
The individual-level TWB data supporting this study’s findings are available upon application to TWB (https://www.twbiobank.org.tw/new_web/). TWB approved my application to access the data on February 18, 2020 (application number: TWBR10810-07; principal investigator: Wan-Yu Lin).