Abstract
The essential amino acid tryptophan (Trp) is metabolized by gut commensals, yielding in compounds that affect innate immune cell functions directly, but also acting on the aryl hydrocarbon receptor (AHR), thus regulating the maintenance of group 3 innate lymphoid cells (ILCs), promoting T helper 17 (TH17) cell differentiation, and interleukin-22 production. In addition, microbiota-derived Trp metabolites have direct effects on the vascular endothelium, thus influencing the development of vascular inflammatory phenotypes. Indoxyl sulfate was demonstrated to promote vascular inflammation, whereas indole-3-propionic acid and indole-3-aldehyde had protective roles. Furthermore, there is increasing evidence for a contributory role of microbiota-derived indole-derivatives in blood pressure regulation and hypertension. Interestingly, there are indications for a role of the kynurenine pathway in atherosclerotic lesion development. Here, we provide an overview on the emerging role of gut commensals in the modulation of Trp metabolism and its influence in cardiovascular disease development.
Keywords: Gut microbiota, Tryptophan metabolism, Endothelium, Vascular inflammation, Hypertension, Atherosclerosis
Introduction
The gut microbiota is the assemblage of microbial communities colonizing the intestinal habitat, with the great majority belonging to the bacterial kingdom, but also comprising fungi, archaea, protists, and viruses (Bäckhed et al. 2012). By influencing nutrient availability, through recognition of conserved microbial patterns via a repertoire of innate immune receptors (i.e., Toll-like receptors, nucleotide-binding oligomerization-like receptors, or retinoic acid-inducible gene-I-like receptors), the regulation of a myriad of host metabolic pathways, but foremost all by the uptake of microbiota-derived metabolites, this microbial ecosystem has evolved into a mutualistic relationship with its host, impacting many traits of host physiology (Turnbaugh et al. 2006; Hooper et al. 2012; Koh and Bäckhed 2020; Bäckhed et al. 2005; Schroeder and Bäckhed 2016). For this reason, organisms have to be viewed as holobionts, including their microbiota affecting many organ systems (Meyer-Albich 1950; Margulis and Fester 1991). This is not only exemplified by the evoked adaptive changes in gut morphology or by recent studies on the gut-liver or gut-brain axis (Bayer et al. 2021; de Vadder et al. 2018; Formes et al. 2021; Aswendt et al. 2021), but also by the role of microbiota and its derived metabolites in cardiovascular disease (Karbach et al. 2016).
The pathophysiology of cardiovascular diseases like hypertension and atherosclerosis is closely linked to vascular inflammation (Daiber et al. 2017; Wenzel et al. 2011). Aryl hydrocarbon receptor (AHR)-signaling is well recognized to contribute to those cardiovascular pathologies by inducing expression of pro-inflammatory interleukin (IL)-1β, IL-8, tumor necrosis factor-alpha (TNF-α) and consecutive foam cell formation (Dahlem et al. 2020; Vogel et al. 2004). The various effects of AHR signaling can be conveyed by many different ligands (Safe et al. 2020). Microbiota-derived L-tryptophan (Trp)-metabolites being among them, pathways involved in their generation and signaling deserve attention (Zelante et al. 2013).
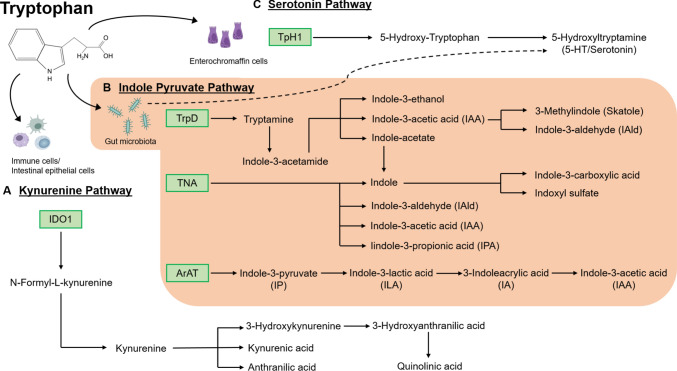
In the intestine, the bulk of nutritional Trp, an essential amino acid that cannot be synthesized de novo by the mammalian host, is metabolized via the kynurenine pathway in immune cells and intestinal epithelial cells, which is initiated by the enzyme indolamine-2,3-dioxygenase-1 (IDO1), converting Trp to N-formyl-L-kynurenine (Taleb 2019) (Fig. 1). Furthermore, in enterochromaffin cells, the enzyme Trp hydroxylase 1 (TpH1) converts a small part of the nutrition-derived Trp into serotonin (5-hydroxytryptamine, 5-HT). A significant proportion of nutritional Trp enters the indole pathway and is metabolized to tryptamine and signaling-active indole metabolites, involving tryptophanase and decarboxylase enzymes of colonizing gut bacteria (Zelante et al. 2013). Metabolomics analyses demonstrated reduced serum levels of Trp and N-acetyltryptophan in conventionally raised (CONV-R) mice relative to germ-free control mice lacking colonization with a gut microbiota, which is due to bacterial tryptophanase activity, the enzyme catalysing the reaction of Trp to indole, pyruvate, and ammonia (Wikoff et al. 2009). In line, the abundance of tryptamine in the feces of CONV-R mice was shown to be increased by 200% as compared to GF controls (Marcobal et al. 2013). In addition, CONV-R mice displayed elevated serotonin serum levels and the indole-metabolites indoxyl sulfate and indole-3-propionic acid could only be detected in CONV-R mice, indicating that the metabolic capacity of the microbiota acts on nutritional Trp, thus interfering with its availability and functional utilization by the host (Wikoff et al. 2009).
Fig. 1.
Metabolic pathways of tryptophan. A Kynurenine pathway in host immune cells and intestinal epithelial cells. B Indole pyruvate pathway performed by gut microbiota. C Serotonin pathway performed by enteroendocrine cells. Key enzymes of the pathways are displayed in green: ArAT aromatic amino acid transaminase, IDO1 indolamine-2,3-dioxygenase-1, TNA tryptophanase, TpH1 tryptophan hydroxylase 1, TrpD tryptophan dehydrogenase
The gut microbiota as a modifier of tryptophan metabolism—implications for intestinal immune function and pathophysiology
Several bacterial species of the gut microbiota were identified that influence Trp metabolism (Table 1). The synthesis of the monoamine tryptamine is catalysed by tryptophan decarboxylase (TrpD), expressed by gut bacterial species, such as members of the genus Clostridia and Lactobacillus (Agus et al. 2018; Williams et al. 2014). Tryptamine is then further processed by tryptophan-2-monooxygenase (TMO) into indole-3-acetamide, which is converted into indole-3-acetic acid (IAA) and subsequently into indole-3-aldehyde (IAld). In an additional sequence of reactions, the bacterial tryptophanase (TNA), which is expressed by many gram-negative and gram-positive bacteria such as Escherichia coli, Clostridium sp., and Bacteroides sp., converts Trp into indole and its derivatives IAld, IAA, and indole-3-propionic acid (IPA). In the aromatic amino acid aminotransferase and indolelactic acid dehydrogenase–dependent pathway, the enzyme aromatic amino acid transaminase (ArAT) converts Trp into indole-3-pyruvate (IP), which is further metabolized into indole-3-lactic acid (ILA) through the enzyme indolelactic acid dehydrogenase (ILDH). Through the phenyl lactate dehydratase gene cluster (fldAIBC), ILA can further be converted into 3-indoleacrylic acid (IA), which is finally converted into indole-3-propionic acid (IPA) (Williams et al. 2014; Wlodarska et al. 2017). Of note, IPA was revealed to increase blood pressure via cardiac and vascular mechanisms but was negatively associated with advanced atherosclerosis (Konopelski et al. 2021; Cason et al. 2017). Interestingly, it has been demonstrated that this metabolite acts on mitochondrial respiration, with chronic exposure resulting in mitochondrial dysfunction in cardiomyocytes but also in hepatic and endothelial cells (Gesper et al. 2021).
Table 1.
Microbiota-derived tryptophan metabolites
| Tryptophan metabolite | Enzyme | Gut microbes | Reference | |
|---|---|---|---|---|
| Phylum level | Species level | |||
| Indole | Tryptophanase (TNA) |
Bacteroides Clostridium Desulfovibrio Enterococcus Escherichia Fusobacterium Haemophilus Peptostreptococcus |
Bac. thetaiotaomicron Bac. ovatus Clo. bifermentans Clo. ghoni Clo. lentoputrescens Clo. limosum Clo. malenomenatum Clo. sordellii Clo. tetani Clo. tetanomorphum Des. vulgaris Ent. faecalis Esc. coli Fus. nucleatum Hae. influenza Pep. asscharolyticus |
Devlin et al. (2016) Elsden et al. (1976) Lee and Lee (2010) Smith et al. (1996) |
|
3-Methyl- indole (Skatole) |
Indoleacetate decarboxylase (IAD) |
Bacteroides Butyrivibrio Clostridium Eubacterium Lactobacillus Megamonas Parabacteroides |
Bac. thetaiotaomicron But. fibrisolvens Clo. bartlettii Clo. scatologenes Clo. drakei Eub. cylindroides Eub. rectale Lac. spp. Meg. hypermegale Par. distasonis |
Honeyfield et al. (1990) Russell et al. (2013) Whitehead et al. (2008) Liu et al. (2018) |
|
Indole-3- acetic acid (IAA) |
Bacterial Tryptophan Monooxygenase |
Bacteroides Bifidobacterium Clostridium Escherichia Eubacterium Parabacteroides Peptostreptococcus |
Bac. thetaiotaomicron Bac. eggerthii Bac. ovatus Bac. fragilis Bif. adolescentis Bif. longum subsp. longum Bif. pseudolongum Clo. bartlettii Clo. difficile Clo. lituseburense Clo. paraputrificum Clo. perfringens Clo. putrefaciens Clo. saccharolyticum Clo. sticklandii Clo. subterminale Esc. coli Eub. hallii Eub. cylindroides Par. distasonis Pep. asscharolyticus |
Elsden et al. (1976) Russell et al. (2013) Smith et al. (1996) |
| 3-Indole- acrylic acid (IA) | Phenyllactate dehydratase (fldBC) |
Clostridium Peptostreptococcus |
Clo. sporogenes Pep. russellii Pep. anaerobius Pep. stomatis |
Dodd et al. (2017) Wlodarska et al. (2017) |
| Indole-3- aldehyde (IAld) | Tryptophanase (TNA) | Lactobacillus |
Lac. acidophilus Lac. murinus Lac. reuteri |
Cervantes-Barragan et al. (2017) Wilck et al. (2017) Zelante et al. (2013) |
| Indole-3- lactic acid (ILA) | Indole-3-lactic acid dehydrogenase (ILDHase) |
Anaerostipes Bacteroides Bifidobacterium Clostridium Escherichia Eubacterium Faecalibacterium Lactobacillus Megamonas Parabacteroides Peptostreptococcus |
Ana. hadrus Ana. caccae Bac. thetaiotaomicron Bac. eggerthii Bac. ovatus Bac. fragilis Bif. adolescentis Bif. bifidum Bif. longum subsp. infantis Bif. longum subsp. longum Bif. pseudolongum Clo. bartlettii Clo. perfringens Clo. sporogenes Clo. saccharolyticum Esc. coli Eub. rectale Eub. cylindroides Fae. prausnitzii Lac. murinus Lac. paracasei Lac. reuteri Meg. hypermegale Par. distasonis Pep. asscharolyticus |
Aragozzini et al. (1979) Dodd et al. (2017) Honoré et al. (2016) Russell et al. (2013) Smith et al. (1996) Wilck et al. (2017) Williams et al. (2014) Wlodarska et al. (2017) |
| Indole-3- propionic acid (IPA) |
Acyl-CoA dehydrogenase (ACD) Phenyllactate dehydratase gene cluster (fldAIBC) |
Clostridium Peptostreptococcus |
Clo. botulinum Clo. caloritolerans Clo. paraputrificum Clo. sporogenes Clo. cadvareris Pep. asscharolyticus Pep. russellii Pep. anaerobius Pep. stomatis |
Dodd et al. (2017) Elsden et al. (1976) Wikoff et al. (2009) Williams et al. (2014) Wlodarska et al. (2017) |
| Tryptamine |
Tryptophan Decarboxylase (TrpD) |
Clostridium Ruminococcus |
Clo. sporogenes Rum. gnavus |
Williams et al. (2014) |
In recent years, it has become evident that the gut microbiota is an important modifier of host physiology and a driver of various organ pathologies (Schroeder and Bäckhed 2016). Based on germ-free mouse models, recent metabolomics analyses identified a myriad of microbiota-derived metabolites that are readily taken up into the bloodstream and impact on remote organ functions (Wu et al. 2019; Lai et al. 2021). Trp was demonstrated to affect both epithelial immunity as well as gut microbial ecology (Hashimoto et al. 2012). Microbiota-derived Trp metabolites interfere with multiple aspects of host physiology. For instance, microbiota-derived indole modulates the secretion of the incretin hormone glucagon-like peptide-1 (GLP-1) from L-cells of the colonic epithelium (Chimerel et al. 2014).
The indole metabolite indoxyl sulfate is generated in the liver, where it enters the circulation as an albumin-bound serum molecule. Indoxyl sulfate is known to be harmful to various cell types, such as vascular endothelial cells (Hung et al. 2016). Furthermore, it has been reported that dysbiosis of the intestinal microbiota towards a higher abundance of aerobic indole-producing bacteria (e.g. E. coli) is associated with the accumulation of the nephrotoxin indoxyl sulfate in the serum of uremic patients, which is due to impaired renal secretion (Deguchi et al. 2002; Takayama et al. 2003). The studies by Takayama et al. showed a significant reduction of indoxyl sulfate serum levels in hemodialysis patients orally treated with non-indole-producing bacteria (e.g. Bifidobacterium) by correcting the composition of the gut microbiota (Takayama et al. 2003). In addition, in the gut mucosa, indole metabolites act on the gut epithelial AHR, important to warrant mucosal type 3 innate lymphoid cells (ILC3) and T helper 17 (TH17) immunity (Cella et al. 2009; Kiss et al. 2011; Schiering et al. 2017). While this aspect of mucosal immunity is beneficial to protect from infection, the induction of immunity by Trp metabolites comes with the downside of an enhanced susceptibility to autoimmunity (Sonner et al. 2019; Choi et al. 2020).
Inflammatory bowel disease (IBD) is one example of an autoimmune disease that has been linked to changed Trp metabolism (Roager and Licht 2018). It was found that Trp serum levels are significantly decreased in IBD patients, compared to healthy controls, while Crohn’s disease patients have a more severe reduction compared to ulcerative colitis patients (Nikolaus et al. 2017). Also, IAA was shown to be reduced in fecal samples of IBD patients (Lamas et al. 2016). Furthermore, serum concentration of IPA is reduced in patients suffering from active colitis as compared to healthy individuals (Alexeev et al. 2018). Lastly, orally administered indole or IPA was shown to ameliorate colonic inflammation in mice (Alexeev et al. 2018; Whitfield-Cargile et al. 2016). Furthermore, Trp and its metabolites stimulate AHR activity and induce tumor cell proliferation and tumor escape in colorectal cancer (Venkateswaran et al. 2019; Brandacher et al. 2006).
Microbiota-derived metabolites were demonstrated to protect from autoimmune disease development (Rosser et al. 2020). Interestingly, in the mouse experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis, it was recently demonstrated that the susceptibility to the induction of central nervous system inflammation in IL-17A/F-deficient mice was dependent on the impact that IL-17A exerts on the composition of the gut microbiota (Regen et al. 2021). This highlights the need for a more detailed understanding of how gut commensals interfere with nutritional factors and host metabolism to impact on autoimmune-related disease phenotypes.
In addition to the gut and the nervous system, the vasculature and its endothelial lining are prone to microbiota-dependent inflammatory processes (Karbach et al. 2016; Kiouptsi et al. 2019). Since Trp metabolism is an important determinant of atherogenesis (Metghalchi et al. 2015; Kappel et al. 2020), it is important to understand how the gut microbiota, a known regulator of Trp/indole metabolism, affects vascular inflammatory phenotypes, hypertension, and the development of atherosclerosis.
Microbiota-derived tryptophan metabolites impacting immune mechanisms
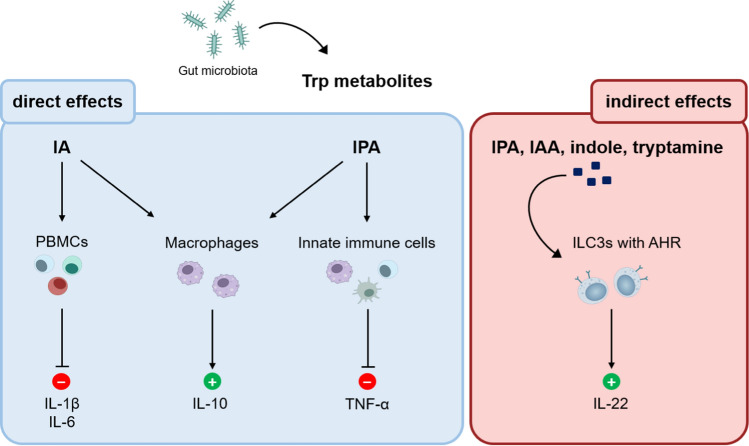
Interestingly, some indole metabolites, such as indole-3-propionic acid (IPA), have direct anti-inflammatory effects on immune cells (Fig. 2) (Wlodarska et al. 2017; Venkatesh et al. 2014). For instance, IPA and 3-Indole-acrylic acid (IA) enhance the production of anti-inflammatory interleukin-10 (IL-10) by macrophages. In addition, IPA reduces the production of pro-inflammatory tumor necrosis factor (TNF) (Wlodarska et al. 2017). Furthermore, IA exerts anti-inflammatory effects by suppressing the expression of the cytokines IL-1β and IL-6 by peripheral blood mononuclear cells (PBMCs) (Fig. 2, left).
Fig. 2.
Effects of Trp metabolites on immune cells. Direct (blue box) and indirect effects (red box) of Trp metabolites on immune cells are highlighted. Promoting effects are marked with a green + , repressing effects with a red – . AHR aryl hydrocarbon receptor, IAA indole-3-acetic acid, IA indoleacrylic acid, IPA indolepropionic acid, ILC3s innate lymphoid cells group 3, IL Interleukin, PBMCs peripheral blood mononuclear cells, Trp tryptophan, TNF tumor necrosis factor
However, Trp/indole metabolites also impact immune phenotypes via indirect signaling mechanisms, predominantly mediated through the AHR (Fig. 2, right). The AHR is a transcription factor, which is especially recognized for its role in xenobiotic metabolism (Hubbard et al. 2015a, b). The AHR is expressed on the surface of group 3 innate lymphoid cells (ILC3s) (Cella et al. 2009; Kiss et al. 2011). AHR activation induces IL-22 production (Fig. 2), which also involves TLR2 signaling (Zelante et al. 2013; Crellin et al. 2010). The cytokine IL-22 regulates intestinal mucosal homeostasis and provides resistance to the fungus Candida albicans and to rotavirus infection (Zelante et al. 2013; Hernandez et al. 2015). The disturbance of the gut microbiota’s ability to generate AHR ligands was associated with the development of IBD (Lamas et al. 2016). Of note, the AHR can be activated by a wealth of different ligands including Trp/indole metabolites. Upon ligand binding, the AHR undergoes conformational changes to enable its transport from the cytosol into the nucleus (Ikuta et al. 1998). In the nucleus, the aryl hydrocarbon receptor nuclear translocator (ARNT) induces the dissociation of the cytoplasmic complex from the AHR leaving the functional heterodimeric transcription factor complex of AHR/ARNT (Reyes et al. 1992). The AHR/ARNT complex subsequently binds to its target sequence in the DNA and recruits further coactivators to remodel the chromatin and transcribe target genes. The AHR can be activated both, by ligands provided through the kynurenine pathway (therefore by the host), and by microbiota-derived ligands originating from the metabolic Trp pathway. Moreover, the AHR can be activated by dietary constituents or by pollutants. Interestingly, activation of the AHR can either have pro- or anti-inflammatory effects, depending on the ligand or the target cell (Gutierrez-Vazquez and Quintana 2018). Indole metabolites (indole, IPA, IAA, and tryptamine) produced by gut microbiota are known to be AHR activators (Zelante et al. 2014).
The AHR interferes with the immune system by inducing IL-22 production, a cytokine of the IL-10 family that stimulates mucosal defence via the induction of antimicrobial peptides (AMPs) (Taleb 2019; Zelante et al. 2013). IL-22 regulates epithelial cell proliferation and production of antimicrobial peptides (AMPs), decreasing the inflammatory potential of commensal bacteria, which has been shown in metabolic syndrome and atherosclerosis (Fatkhullina et al. 2018; Taleb 2019; Wang et al. 2014). In addition to the regulation of mucosal immune responses, the AHR regulates organogenesis, mucosal barrier function, and cell cycle (Kiss et al. 2011; Hubbard et al. 2015a, b; Gronke et al. 2019). AHR-ligands are cleared and detoxified by cytochrome P450 1 (CYP1) enzymes, having a role in feedback regulation. The in vivo relevance of CYP1 enzymes was demonstrated by the constitutive and restricted expression of Cyp1a1 in intestinal epithelial cells, which resulted in the loss of ILC3s and T helper 17 (TH17) cells (Schiering et al. 2017). Hence, gut commensal-derived Trp metabolites interfere with immune regulatory AHR signaling at multiple levels.
Microbiota-derived tryptophan metabolites in vascular inflammation
Endothelial dysfunction precedes atherosclerosis, one of the most severe forms of vascular inflammation. Analysis of vascular dysfunction in germ-free mouse models indicated that the gut microbiota promotes vascular inflammation and oxidative stress (Karbach et al. 2014). Vascular inflammation is frequently associated with autoimmune diseases such as type 2 diabetes (Steven et al. 2019). Notably, gnotobiotic mouse atherosclerosis models and clinical metagenomic shotgun sequencing studies have firmly established the gut microbiota as a relevant modifier of vascular inflammation and atherosclerosis, the primary cause of myocardial infarction (Karbach et al. 2016; Lindskog et al. 2018; Kiouptsi et al. 2019; Jie et al. 2017; Pontarollo et al. 2020). Interestingly, in a rat model, treatment with different antibiotics resulted in shifts of microbial composition and could ameliorate myocardial infarction, which was paralleled by decreased levels of Trp metabolites like kynurenine, indole acetate, indole propionate, and 3-indoxyl sulfate. At the same time, vancomycin treatment increased serotonin levels (Lam et al. 2016). In recent years, several studies demonstrated that the microbiota’s influence on Trp metabolism can impact on vascular inflammation.
Importantly, the indole pathway promotes the development of vascular inflammatory phenotypes. In vitro experiments with endothelial cells demonstrated that indoxyl sulfate can induce oxidative stress and decrease NO production (Dou et al. 2007; Stinghen et al. 2014; Yu et al. 2011). Furthermore, indoxyl sulfate-treatment led to enhanced pro-coagulant properties of endothelial cells since it increased the expression of the coagulation initiator tissue factor in human umbilical vein endothelial cells (HUVECs) along with elevated tissue factor procoagulant activity on their extracellular vesicles (Gondouin et al. 2013). In addition, indoxyl sulfate was demonstrated to inhibit proliferation and wound healing (Dou et al. 2004). Moreover, in vascular smooth muscle cells, this metabolite was shown to induce proliferation (Yamamoto et al. 2006), tissue factor expression and activity (Chitalia et al. 2013), as well as IL-6 expression (Adelibieke et al. 2014), involving AHR signaling. Importantly, these findings also translated into in vivo studies, where indoxyl sulfate enhanced leukocyte recruitment to the vascular wall and enhanced the release of endothelial microparticles (Ito et al. 2016; Faure et al. 2006). In chronic kidney disease patients, elevated levels of serum indoxyl sulfate correlated with aortic calcification (Barreto et al. 2009). However, recent work suggests that indoxyl sulfate may not be the major contributor to vascular dysfunction associated with ischemic acute kidney injury (Nakagawa et al. 2022).
In contrast to indoxyl sulfate, protective effects were described for indole-3-propionic acid (IPA). IPA could induce pregnane-X-receptor (PXR) expression in the aorta of mice and diminished endothelial-dependent vasodilator responsiveness (Venu et al. 2019). In the context of intestinal inflammation, IPA increased IL-10 receptor (IL-10R) levels in intestinal epithelial cells (Alexeev et al. 2018) and showed an anti-inflammatory effect in murine bone marrow-derived macrophages (Wlodarska et al. 2017). Although IPA administration alone does not seem to be sufficient to protect Western diet-fed mice from detrimental cardiometabolic effects on liver and vasculature (Lee et al. 2020), other in vivo studies suggest that IPA can reduce inflammation (Zhao et al. 2019; Du et al. 2021).
Similar to IPA, the Trp metabolite IAld was also capable of increasing IL-10R expression in vitro and serum levels of both compounds are reduced in mice with active inflammation (Alexeev et al. 2018). In mouse models, IAld treatment resulted in a reduction of the type I interferon response, as shown for graft-versus-host disease and CNS inflammation (Langan, et al. 2021; Swimm et al. 2018; Rothhammer et al. 2016). These observed effects led to the discussion of IAld as a potential treatment option for pulmonary infections with an inflammatory component like aspergillosis (Puccetti et al. 2021). Of note, IAA, another Trp metabolite of the indole pathway produced in bacteria like Clostridium (Elsden et al. 1976), exerted anti-angiogenic effects in HUVECs treated with vascular endothelial growth factor (VEGF) via AHR-signaling (Langan et al. 2021) and was able to inhibit VEGF receptor-2 (VEGFR2) and endothelial nitric oxide synthase (eNOS) phosphorylation in HUVEC cultures (Cerezo et al. 2019). Furthermore, IAA, together with tryptamine, decreased the expression of pro-inflammatory cytokines in cultured macrophages and reduced inflammatory effects of TNF-α in hepatocytes (Krishnan et al. 2018). When mice were fed with a HFD, IDO1 knockout mice had less white adipose tissue, showed lower adiposity, and had lower plasma leptin and LPS levels than WT mice on the same diet (Laurans et al. 2018). Additionally, their livers weighed less, accumulated less lipids and showed lower macrophage infiltration. Overall, this resulted in a lower inflammatory status in the adipose tissue, which was paralleled by higher levels of IAA and lower levels of kynurenine in the intestines of IDO1 knockout mice. Mechanistically, elevations in IAA levels in those mice correlated with elevated IL-22 and IL-17 levels, which led to the hypothesis of IDO1 deletion having a protective function in obesity, insulin sensitivity, and intestinal permeability.
Interestingly, the Indole pathway, through the production of tryptamine by the genera Lactobacillus and Clostridium, was observed to induce serotonin release from enterochromaffin cells in the gut and potentiate inhibitory effects of serotonin on cells in the brain (Takaki et al. 1985; Zucchi et al. 2006). Importantly, the strains Lactococcus lactis subsp. cremoris (MG 1363), Lactococcus lactis subsp. lactis (IL1403), Lactobacillus plantarum (FI8595), Streptococcus thermophilus (NCFB2392), Escherichia coli K-12, Morganella morganii (NCIMB, 10,466), Klebsiella pneumoniae (NCIMB, 673) and Hafnia alvei (NCIMB, 11,999) were described to produce serotonin directly (O’Mahony et al. 2015). Moreover, the presence of spore-forming gut bacteria was shown to elevate host serotonin levels (Yano et al. 2015). Accordingly, in the study by Wikoff et al. it was reported that germ-free mice had a 2.8-fold decrease in plasma serotonin as well as elevated tryptophan levels as compared to conventionalized mice (Wikoff et al. 2009). As serotonin was reported to induce neutrophil degranulation, thus worsening thromboinflammation, the connection between microbiota derived metabolites and serotonin might also be implicated in vascular inflammation (Mauler et al. 2019). Moreover, serotonin is important for central cardiovascular regulation (Ramage and Villalón 2008) and the microbiota was identified to modulate serotonin levels in the brain (Clarke et al. 2013). The ability of Trp metabolites such as IAA and IPA to cross the blood brain barrier might also indicate a central role for those metabolites (Gao et al. 2020).
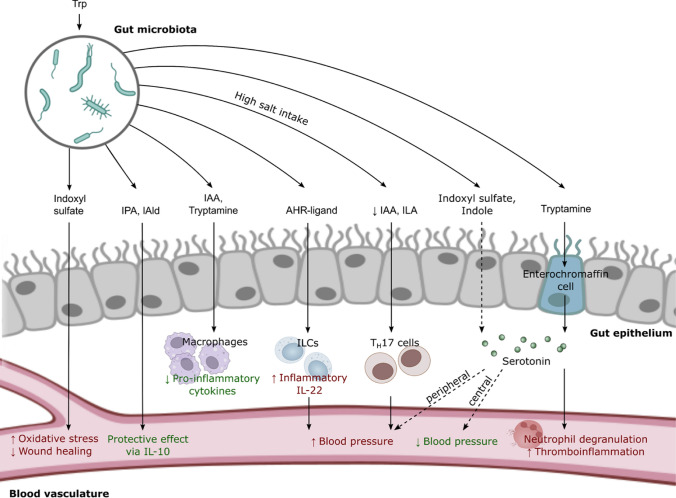
The presented literature outlines various roles for Trp metabolites in vascular inflammation (Fig. 3). Indoxyl sulfate was shown to promote a procoagulant state in vitro as well as endothelial dysfunction in vivo. IPA, IAld and IAA on the other hand are predominantly reported to exert anti-inflammatory responses. Serotonin release also seems to be modulated by the microbiota-derived Trp metabolite Tryptamine, which might affect cardiovascular disease development. Yet, conclusive mechanisms by which these metabolites operate remain to be elucidated and would be highly beneficial for their further characterization. This is also highlighted by conflicting reports on the role of IDO1 in these pathologies, as not only direct effects of its substrates have to be considered, but also their absence and shifts in other regulatory pathways. Nevertheless, Trp metabolites impact on vascular inflammation, which has been firmly linked to hypertension (Wenzel et al. 2011).
Fig. 3.
Gut microbiota-derived tryptophan metabolites regulate vascular physiology and disease. Dietary Trp can be converted into various metabolites by the gut microbiota. Indoxyl sulfate induces oxidative stress and inhibits wound healing of the endothelium. Tryptamine initiates the production of serotonin from enterochromaffin cells. Local effects of serotonin include the degranulation of neutrophils, which exacerbates thromboinflammation. Indoxyl sulfate and indole may regulate blood pressure via the serotonin signaling pathway, which acts either through central or peripheral mechanisms. Anti-inflammatory effects are evoked via IPA and lAld following IL‑10 production, or via IAA and tryptamine resulting in a decreased release of pro-inflammatory cytokines from macrophages. Blood pressure is regulated by a variety of AHR ligands that influence intestinal immune cells. Dysbalance of AHR ligands such as IAA or ILA can act on ILCs or TH17 cells to alter the blood pressure and promote hypertension. Abbreviations IAA indole-3-acetic acid, AHR aryl hydrocarbon receptor, ILA indole-3-lactic acid, IPA indole-3-propionic acid, IAld indole-3-aldehyde, ILC innate lymphoid cell, IL interleukin, TH T-helper cell, Trp tryptophan
Microbiota-derived tryptophan metabolites in hypertension
Hypertension is a common and serious medical condition that can lead to cardiovascular disease. Elevated blood pressure significantly increases the risks of several organ pathologies in heart, brain, or kidney, leading to major complications such as congestive heart failure, cerebral haemorrhage, and renal failure (Doyle 1991; Price and Kasner 2014). Approximately 1.28 billion adults worldwide are affected by hypertension, with an estimated 46% of all affected individuals being unaware of their condition (World Health Organization 2021; https://www.who.int/news-room/fact-sheets/detail/hypertension).
High blood pressure can be caused by several factors: The non-influenceable risk factors, such as genetic predisposition, age or pre-existing diseases like diabetes or kidney diseases, and the influenceable risk factors, which can be affected by lifestyle and environment (Oliveras and La Sierra 2014; Ondimu et al. 2019). In particular physical inactivity, high alcohol and tobacco consumption, stress, obesity, and unhealthy diets, such as the Western diet (including high salt and sugar intake, hyper‑consumption of saturated fats and trans-fats and low levels of nutrition-dense foods like fruits and vegetables), increase the risk of high blood pressure (Narkiewicz 2006; Ondimu et al. 2019; Ruivo and Alcântara 2012; Ozemek et al. 2018). Hypertension is thus related to food intake, which in turn is closely linked to microbiota (Derer et al. 2017; Rothschild et al. 2018). For example, high salt intake affects the gut microbiome by depleting particular bacterial species such as Lactobacillus murinus. The presence of Lactobacillus has been shown to prevent the formation of TH17 cells and consequently reduce hypertension in mice. Lactobacillus depletion due to increased salt intake was accompanied by decrease in the Trp metabolites IAA and ILA, suggesting a link between Trp metabolites and cardiovascular health (Wilck et al. 2017). Furthermore, gut microbiota drives hypertension via the blood pressure hormone angiotensin‑II by promoting vascular inflammatory processes mediated by monocyte chemoattractant protein‑1 (MCP-1, CCL2) and IL‑17 (Karbach et al. 2016). In this study, germ-free housing conditions resulted in the protection against angiotensin II-induced hypertension and associated organ damage.
Since diet is known to influence the composition of the gut microbiome (Derer et al. 2017), it is not surprising that recent mechanistic studies linked the microbiota to hypertension. Most interestingly, hypertension was found to be transferable by fecal microbiota transplant (FMT) into the germ-free mouse model (Li et al. 2017), demonstrating a causal role of the microbiota. The presence or absence of different gut microbes was associated with high blood pressure. For instance, the absence of gram-negative bacteria such as Klebsiella, Parabacteroides, Desulfovibrio, and Prevotella has been linked to elevated blood pressure (Verhaar et al. 2020). Moreover, people affected by hypertension were reported to have a reduced abundance of the genus Oscillibacter and Lactobacillus (Dan et al. 2019), microbes associated with Trp metabolism (Roager and Licht 2018; Chen et al. 2019). Therefore, microbiota-derived Trp metabolites seem to play a role in blood pressure regulation.
The effects of cardiac pressure overload on gut dysbiosis were studied in a transverse aortic constriction (TAC)-model. Within this study, elevated blood pressure was found to decrease Lactobacillus species involved in gut homeostasis and Trp metabolism (Carrillo-Salinas et al. 2020; Larigot et al. 2018). Moreover, during the TAC-induced cardiac pressure overload a reduction in the cardiac expression of the AHR, a known receptor for Trp ligands, was found in CONV-R mice but not in mice lacking the microbiome (Carrillo-Salinas et al. 2020). This supports the conclusion that hypertension is influenced by microbiota-derived Trp metabolites. Besides that, diet-derived AHR ligands promote the production of the inflammatory mediator IL-22 in the gastrointestinal tract (Lee et al. 2012), which in turn leads to increased blood pressure and endothelial dysfunction (Ye et al. 2017). This indicates the importance of the local activity of certain Trp metabolites.
In the small intestine, the essential amino acid Trp is converted into indole and its related derivatives such as IAA, IPA and ILA by various intestinal bacteria (listed in Table 1) (DeMoss and Moser 1969; Donia and Fischbach 2015; Konopelski and Ufnal 2018). Several studies indicate an effect of indole and other Trp-derived indole-compounds on gastrointestinal and circulatory system function, including conditions such as hypertension (Hubbard et al. 2015a, b; Huć et al. 2018). In rats, colonic indole has been reported to increase portal blood pressure, thereby affecting processes of intestinal inflammation and hemostasis, most likely via influencing the function of the gut-vascular barrier (Huć et al. 2018; Jaworska et al. 2017). The gut-vascular barrier was described as a permeable barrier for small molecules whose permeability can be influenced by microbiota such as Salmonella typhimurium (Spadoni et al. 2015). According to this, portal hypertension increases the permeability of the microbiota-derived indoles from the intestine into the blood circulation (Huć et al. 2018).
Targeting the gut-vascular barrier is only one possible way how Trp metabolites can influence the circulatory system. The regulation of blood pressure underlies a variety of mechanisms mediated peripheral and central by the brain (Dampney et al. 2002; Guyton et al. 1972), in which microbiota-derived metabolites may also play a role (Tomasova et al. 2016; Verhaar et al. 2020). For instance, the Trp metabolites indole and indole sulfate are able to influence blood pressure via both pathways. Huć et al. discovered the effect of the two metabolites on the circulatory system in rats (Huć et al. 2018). Therefore, indole and indole sulfate were administered intravenously and into the cerebroventricular system at different concentrations to investigate their central and peripheral effects on heart rate and blood pressure. Upon intravenous administration, both indole and indoxyl sulfate increased mean arterial blood pressure at different concentrations. However, significant changes in heart rate occurred with indoxyl sulfate treatment. During cerebroventricular administration of the two metabolites, a significant decrease in mean arterial blood pressure as well as a decrease in heart rate were observed with indole treatment but not with indoxyl sulfate. This highlights the regulatory role of the microbiota-derived Trp metabolites on peripheral and central blood pressure mechanisms (Huć et al. 2018; Gao et al. 2020). In particular, indole and indoxyl sulfate affect arterial blood pressure via the serotonin signaling pathway since pre-treatment with serotonin-receptor blockers such as ondansetron and pizotifen inhibited the hemodynamic effects of indole and indoxyl sulfate (Huć et al. 2018).
Overall, there is multiple evidence linking Trp and its microbiota-derived metabolites with blood pressure regulation and hypertension (Fig. 3). Besides central and peripheral regulation by microbiota-derived Trp metabolites via the serotonin pathway, local activation of AHR signalling by indole metabolites in the gut, but also impaired gut-vascular barrier function could be involved.
Microbiota-derived tryptophan metabolites in atherosclerosis
Despite the use of cholesterol-lowering therapies to reduce atherosclerosis, nearly 18 million people die each year from CVD (Roth et al. 2017). Atherosclerosis is a chronic inflammatory disease, affecting both large and medium sized arteries, initiated by vascular inflammation, increased endothelial cell permeability, and intimal low-density lipoprotein (LDL) cholesterol accumulation (Theodorou and Boon 2018). Overgrowth of atherosclerotic plaques in coronary arteries can result in myocardial ischemia, which leads to myocardial cell death and acute myocardial infarction (AMI) (Thygesen et al. 2018; Ibanez et al. 2018).
Inflammation is considered a key driver of arterial thrombotic events (Ross 1999; Hansson 2005; Libby et al. 2009). Several amino acid metabolic pathways were identified as checkpoints for the control of inflammation-related mechanisms. For example, the branched-chain amino acids (leucine, isoleucine, and valine) have been shown to promote endothelial cell dysfunction by increasing production of reactive oxygen species (ROS) and inflammation (Zhenyukh et al. 2018). In particular, Trp metabolites were implicated in cardiovascular disease (Nitz et al. 2019; Kappel et al. 2020). For example, in clinical studies on advanced atherosclerosis, Trp was negatively associated and the kynurenine/Trp ratio was positively associated with advanced atherosclerosis (Pedersen et al. 2015). In the apolipoprotein E (Apoe)-deficient mouse atherosclerosis model, antibiotic treatment resulted in increased atherosclerosis, connected to a loss of intestinal microbiome diversity and alterations in microbial metabolic functional capacity with a major impact on the host serum metabolome (Kappel et al. 2020). Pathways that were modulated by antibiotics and connected to atherosclerosis included diminished tryptophan and disturbed lipid metabolism. In support of the role of reduced microbial tryptophan biosynthesis in antibiotics-induced atherosclerosis, supplementation of tryptophan in the diet was able to partially reduce atherosclerotic lesion size in antibiotics-treated Apoe-deficient mice.
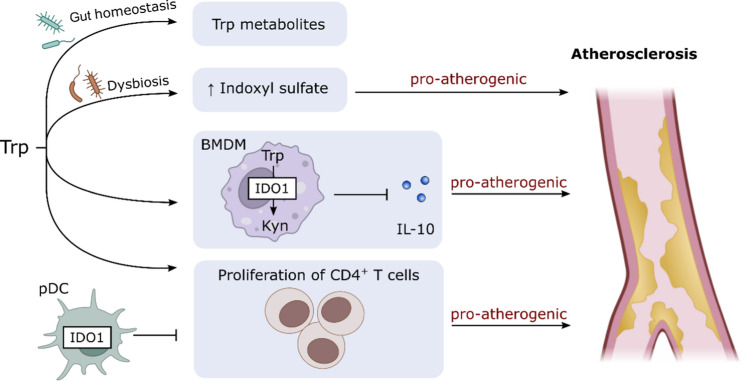
IDO is a rate limiting enzyme implicated in Trp catabolism via the kynurenine pathway (Higuchi and Hayaishi 1967; Yamamoto, and Hayaishi 1967). During inflammation, IDO is up-regulated mostly in macrophages and dendritic cells by pro-inflammatory stimuli, such as IFN-γ (Chon et al. 1996). Daissormont et al. reported a protective effect of plasmacytoid dendritic cells (pDCs) in a model of atherosclerosis, proposing that pDC depletion was accompanied by increased CD4+ T cell proliferation, interferon-γ expression by splenic T cells, and plasma interferon-γ levels. Lymphoid tissue plasmacytoid dendritic cells from atherosclerotic mice showed increased IDO expression and IDO blockage abrogated the pDC suppressive effect on T-cell proliferation (Daissormont et al. 2011). In vascular inflammation and atherosclerosis, IDO1 activity in bone marrow-derived macrophages was proposed to have adverse effects by inhibition of the anti-inflammatory cytokine IL-10 via kynurenic acid mediated activation of a cAMP-dependent pathway and inhibition of Erk1/2 phosphorylation (Metghalchi et al. 2015). As macrophages are crucially involved in vascular inflammation and the pathogenesis of atherosclerosis (Shirai et al. 2015), changes in IDO1 activity might play a role in these diseases as well. A recent study on Apoe-deficient mice suggested that IDO1 protects against the development of atherosclerotic lesions as Indo-knockout mice deficient in Apoe had an increased lesion area, increased macrophage and CD4+ T cell content in their atherosclerotic lesions, and reduced IL-10 production in B cells (Fig. 4) (Cole et al. 2015). Moreover, by depriving T cells of Trp, IDO activity was hypothesized to regulate T cell-related immunity (Fallarino et al. 2006) and therefore to decrease vascular inflammation and the progression of atherosclerosis (Niinisalo et al. 2010). Indeed, recent work indicates that IDO is critically involved in the regulation of intestinal Trp metabolism, thus impacting on the microbiota-dependent control of metabolic disease (Laurans et al. 2018).
Fig. 4.
Dysbalance in tryptophan (Trp) metabolism promotes atherosclerosis. Gut dysbiosis increases the production of indoxyl sulfate which may contribute to atherogenesis. Absence of microbiota promotes Trp involvement in the kynurenine pathway. BMDMs process Trp via IDO1 into Kyn metabolites. The decreased release of IL-10 has a pro-atherogenic effect. Accumulation of CD4+ T cells is crucial for the progression of atherosclerosis and is promoted by Trp. pDCs are known for their protective role in atherosclerosis by inhibiting CD4+ T cell proliferation. Abbreviations: BMDM bone marrow-derived macrophage, CD cluster of differentiation, IDO1 indolamine-2,3-dioxygenase-1, IL interleukin, Kyn kynurenine, pDC plasmacytoid dendritic cell, Trp tryptophan
In patients with coronary artery disease, IDO activity was associated with worse cardiovascular outcome (Pedersen et al. 2011, 2015; Eussen et al. 2015). Furthermore, in patients with end-stage renal disease, IDO activity was linked to atherosclerosis progression (Pawlak et al. 2009). In line, kynurenine supplementation was associated with an aggravation of cardiac function as well as a deleterious cardiac remodeling as revealed by lower capillary number, increased infarct size and interstitial fibrosis. IDO inhibitor treatment, as well as total and specific endothelial deletion of IDO, was recently shown to protect against the deleterious cardiac effects of MI-induced ischemia (Melhem et al. 2021). In contrast, kynurenine supplementation, a major IDO-related metabolite, abolished the protective effects of IDO deficiency in this setting. Hence, the inhibition of IDO activity might represent a novel potential therapeutic strategy to mitigate ischemic cardiac injury.
Of note, dysbiosis of the gut microbiota has been shown to have an adverse role in the development of atherosclerosis by increasing the production of indoxyl sulfate from indole and promoting the progression of chronic kidney disease (CKD) (Poesen et al. 2016). Due to the reduced clearance capability of the kidneys, indoxyl sulfate is accumulated and contributes to characteristic phenotypes of atherosclerosis such as endothelial dysfunction (Dou et al. 2004) and coronary calcification (Adijiang et al. 2008).
Taken together, these recent results from clinical and experimental studies highlight the importance of mechanistic investigations on the microbe-host interactions interfering with Trp metabolism, which may contribute to atherosclerosis and its end-stage complications.
Concluding remarks
The metabolic capacity of the gut microbiota is increasingly recognized to impact on cardiometabolic and cardiovascular disease phenotypes (Kiouptsi et al. 2020; Vieira-Silva et al. 2020). Dependent on the nutritional availability of the essential amino acid Trp, bacteria of the gut microbiota influence host serotonin biosynthesis in enterochromaffin cells and certain members of this microbial ecosystem are even capable to directly provide serotonin to their host (Yano et al. 2015; O’Mahony et al. 2015). Indeed, the microbiota-induced elevation of serotonin levels by spore forming bacteria might contribute to vascular inflammation, most likely by its impact on neutrophil function (Mauler et al. 2022). Another Trp-dependent pathway modulated by the gut microbiota is the indole pathway, which acts via the AHR, thereby impacting mucosal ILC3 and TH17 immunity (Cella et al. 2009; Kiss et al. 2011; Schiering et al. 2017). Although the Trp catabolic kynurenine pathway is metabolically independent of the enzymatic repertoire of the gut microbiota, still this pathway underlies the regulation by microbiota-regulated type I interferons (Chon et al. 1996; Schaupp et al. 2020). Of note, there is ample evidence for a protective role of the gut microbiota in atherosclerotic lesion development, which is for instance mediated by its cholesterol-lowering effects (Pontarollo et al. 2020). Importantly, in mouse atherosclerosis models, a protective role of dietary tryptophan has been demonstrated (Kappel et al. 2020).
In conclusion, there is comprehensive experimental and clinical evidence for the involvement of microbiota-related Trp metabolites in vascular inflammation, blood pressure regulation and cardiovascular disease development. Hence, future work should focus on the pro- and anti-inflammatory effects of microbiota-derived Trp metabolites and their direct and indirect impact on host immune cells. Another future challenge is to dissect effects arising from microbiota-produced Trp metabolites from the various influences of endogenously produced metabolites of the kynurenine pathway. Furthermore, using well-defined cell-type specific mouse models, it will be interesting to define how these pathways interfere to regulate host (patho)physiology on the molecular and cellular level.
Author contributions
Initial drafting of the manuscript: N.P., M.M., S.B., Z.G., M.P.K, C.R.; final editing of the manuscript: A.M., F.M., T.R., C.R.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was funded by an intramural project grant from the Naturwissenschaftlich-Medizinisches Forschungszentrum (NMFZ), a project grant from the Wilhelm Sander Stiftung, and a project grant from the Boehringer Ingelheim Foundation (cardio consortium “novel and neglected cardiovascular risk factors”) to C.R.. C.R. acknowledges funding from the Forschungsinitiative Rheinland-Pfalz and ReALity. C.R. was awarded a Fellowship of the Gutenberg Research College at the Johannes Gutenberg-University Mainz.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Research involving human and animals participants
No research involving humans or animals was carried out.
Informed consent
Not relevant for the current study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nadja Paeslack, Maximilian Mimmler and Stefanie Becker authors contributed equally.
References
- Adelibieke Y, Yisireyili M, Ng H-Y, Saito S, Nishijima F, Niwa T. Indoxyl sulfate induces IL-6 expression in vascular endothelial and smooth muscle cells through OAT3-mediated uptake and activation of AhR/NF-κB pathway. Nephron Exp Nephrol. 2014;128:1–8. doi: 10.1159/000365217. [DOI] [PubMed] [Google Scholar]
- Adijiang A, Goto S, Uramoto S, Nishijima F, Niwa T. Indoxyl sulphate promotes aortic calcification with expression of osteoblast-specific proteins in hypertensive rats. Nephrol Dial Transplant. 2008;23:1892–1901. doi: 10.1093/ndt/gfm861. [DOI] [PubMed] [Google Scholar]
- Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23:716–724. doi: 10.1016/j.chom.2018.05.003. [DOI] [PubMed] [Google Scholar]
- Alexeev EE, Lanis JM, Kao DJ, Campbell EL, Kelly CJ, Battista KD, Gerich ME, Jenkins BR, Walk ST, Kominsky DJ, et al. Microbiota-derived indole metabolites promote human and murine intestinal homeostasis through regulation of interleukin-10 receptor. Am J Pathol. 2018;188:1183–1194. doi: 10.1016/j.ajpath.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragozzini F, Ferrari A, Pacini N, Gualandris R. Indole-3-lactic acid as a tryptophan metabolite produced by Bifidobacterium spp. Appl Environ Microbiol. 1979;38(3):544–546. doi: 10.1128/aem.38.3.544-546.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aswendt M, Green C, Sadler R, Llovera G, Dzikowski L, Heindl S, Gomez de Agüero M, Diedenhofen M, Vogel S, Wieters F, et al. The gut microbiota modulates brain network connectivity under physiological conditions and after acute brain ischemia. iScience. 2021;24:103095. doi: 10.1016/j.isci.2021.103095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- Bäckhed F, Fraser CM, Ringel Y, Sanders ME, Sartor RB, Sherman PM, Versalovic J, Young V, Finlay BB. Defining a healthy human gut microbiome: current concepts, future directions, and clinical applications. Cell Host Microbe. 2012;12:611–622. doi: 10.1016/j.chom.2012.10.012. [DOI] [PubMed] [Google Scholar]
- Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4:1551–1558. doi: 10.2215/CJN.03980609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer F, Dremova O, Khuu MP, Mammadova K, Pontarollo G, Kiouptsi K, Soshnikova N, May-Simera HL, Endres K, Reinhardt C. The interplay between nutrition, innate immunity, and the commensal microbiota in adaptive intestinal morphogenesis. Nutrients. 2021 doi: 10.3390/nu13072198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandacher G, Perathoner A, Ladurner R, Schneeberger S, Obrist P, Winkler C, Werner ER, Werner-Felmayer G, Weiss HG, Göbel G, et al. Prognostic value of indoleamine-2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res. 2006;12:1144–1151. doi: 10.1158/1078-0432.CCR-05-1966. [DOI] [PubMed] [Google Scholar]
- Carrillo-Salinas FJ, Anastasiou M, Ngwenyama N, Kaur K, Tai A, Smolgovsky SA, Jetton D, Aronovitz M, Alcaide P. Gut dysbiosis induced by cardiac pressure overload enhances adverse cardiac remodeling in a T cell-dependent manner. Gut Microbes. 2020;12:1–20. doi: 10.1080/19490976.2020.1823801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason CA, Dolan KT, Sharma G, Tao M, Kulkarni R, Helenowski IB, Doane BM, Avram MJ, McDermott MM, Chang EB, et al. Plasma microbiome-modulated indole- and phenyl-derived metabolites associate with advanced atherosclerosis and postoperative outcomes. J Vasc Surg. 2018;68:1552–1562. doi: 10.1016/j.jvs.2017.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JKM, Doherty JM, Mills JC, Colonna M. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature. 2009;457:722–725. doi: 10.1038/nature07537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerezo AB, Labrador M, Gutiérrez A, Hornedo-Ortega R, Troncoso AM, Garcia-Parrilla MC. Anti-VEGF signalling mechanism in HUVECs by melatonin, serotonin, hydroxytyrosol and other bioactive compounds. Nutrients. 2019 doi: 10.3390/nu11102421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes-Barragan L, Chai JN, Tianero MD, Di Luccia B, Ahern PP, Merriman J, Cortez VS, Caparon MG, Donia MS, Gilfillan S, et al. Lactobacillus reuteri induces gut intraepithelial CD4+CD8αα+ T cells. Science. 2017;357:806–810. doi: 10.1126/science.aah5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Chen D-Q, Liu J-R, Zhang J, Vaziri ND, Zhuang S, Chen H, Feng Y-L, Guo Y, Zhao Y-Y. Unilateral ureteral obstruction causes gut microbial dysbiosis and metabolome disorders contributing to tubulointerstitial fibrosis. Exp Mol Med. 2019;51:1–18. doi: 10.1038/s12276-019-0234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimerel C, Emery E, Summers DK, Keyser U, Gribble FM, Reimann F. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep. 2014;9:1202–1208. doi: 10.1016/j.celrep.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitalia VC, Shivanna S, Martorell J, Balcells M, Bosch I, Kolandaivelu K, Edelman ER. Uremic serum and solutes increase post-vascular interventional thrombotic risk through altered stability of smooth muscle cell tissue factor. Circulation. 2013;127:365–376. doi: 10.1161/CIRCULATIONAHA.112.118174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S-C, Brown J, Gong M, Ge Y, Zadeh M, Li W, Croker BP, Michailidis G, Garrett TJ, Mohamadzadeh M, et al. Gut microbiota dysbiosis and altered tryptophan catabolism contribute to autoimmunity in lupus-susceptible mice. Sci Transl Med. 2020 doi: 10.1126/scitranslmed.aax2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chon SY, Hassanain HH, Gupta SL. Cooperative role of interferon regulatory factor 1 and p91 (STAT1) response elements in interferon-gamma-inducible expression of human indoleamine 2,3-dioxygenase gene. J Biol Chem. 1996;271:17247–17252. doi: 10.1074/jbc.271.29.17247. [DOI] [PubMed] [Google Scholar]
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18:666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- Cole JE, Astola N, Cribbs AP, Goddard ME, Park I, Green P, Davies AH, Williams RO, Feldmann M, Monaco C. Indoleamine 2,3-dioxygenase-1 is protective in atherosclerosis and its metabolites provide new opportunities for drug development. Proc Natl Acad Sci U S A. 2015;112:13033–13038. doi: 10.1073/pnas.1517820112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crellin NK, Trifari S, Kaplan CD, Cupedo T, Spits H. Human NKp44+IL-22+ cells and LTi-like cells constitute a stable RORC+ lineage distinct from conventional natural killer cells. J Exp Med. 2010;207:281–290. doi: 10.1084/jem.20091509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlem C, Kado SY, He Y, Bein K, Wu D, Haarmann-Stemmann T, Kado NY, Vogel CFA. AHR signalng interacting with nutritional factors regulating the expression of markers in vascular inflammation and atherogenesis. Int J Mol Sci. 2020;21:8287. doi: 10.3390/ijms21218287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiber A, Steven S, Weber A, Shuvaev VV, Muzykantov VR, Laher I, Li H, Lamas S, Münzel T. Targeting vascular (endothelial) dysfunction. Br J Pharmacol. 2017;174:1591–1619. doi: 10.1111/bph13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daissormont ITMN, Christ A, Temmerman L, Sampedro Millares S, Seijkens T, Manca M, Rousch M, Poggi M, Boon L, van der Loos C, et al. Plasmacytoid dendritic cells protect against atherosclerosis by tuning T-cell proliferation and activity. Circ Res. 2011;109:1387–1395. doi: 10.1161/CIRCRESAHA.111.256529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dampney RAL, Coleman MJ, Fontes MAP, Hirooka Y, Horiuchi J, Li YW, Polson JW, Potts PD, Tagawa T. Central mechanisms underlying short- and long-term regulation of the cardiovascular system. Clin Exp Pharmacol Physiol. 2002;29:261–268. doi: 10.1046/j.1440-1681.2002.03640.x. [DOI] [PubMed] [Google Scholar]
- Dan X, Mushi Z, Baili W, Han L, Enqi W, Huanhu Z, Shuchun L. Differential analysis of hypertension-associated intestinal microbiota. Int J Med Sci. 2019;16:872–881. doi: 10.7150/ijms.29322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vadder F, Grasset E, Mannerås Holm L, Karsenty G, Macpherson AJ, Olofsson LE, Bäckhed F. Gut microbota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc Natl Acad Sci USA. 2018;115:6458–6463. doi: 10.1073/pnas.1720017115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi T, Ohtsuki S, Otagiri M, Takanaga H, Asaba H, Mori S, Terasaki T. Major role of organic anion transporter 3 in the transport of indoxyl sulfate in the kidney. Kidney Int. 2002;61:1760–1768. doi: 10.1046/j.1523-1755.2002.00318.x. [DOI] [PubMed] [Google Scholar]
- DeMoss RD, Moser K. Tryptophanase in diverse bacterial species. J Bacteriol. 1969;98:167–171. doi: 10.1128/jb.98.1.167-171.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derer S, Lehnert H, Sina C, Wagner AE. Modulation der intestinalen Mikrobiota durch Ernährungsinterventionen. Internist. 2017;58:435–440. doi: 10.1007/s00108-017-0217-0. [DOI] [PubMed] [Google Scholar]
- Devlin AS, Marcobal A, Dodd D, Nayfach S, Plummer N, Meyer T, Pollard KS, Sonnenburg JL, Fischbach MA. Modulation of a circulating uremic solute via rational genetic manipulation of the gut microbiota. Cell Host Microbe. 2016;20:709–715. doi: 10.1016/j.chom.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd D, Spitzer MH, van Treuren W, Merrill BD, Hryckowian AJ, Higginbottom SK, Le A, Cowan TM, Nolan GP, Fischbach MA, et al. A gut bacterial pathway metabolizes aromatic amino acids into nine circulating metabolites. Nature. 2017;551:648–652. doi: 10.1038/nature24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donia MS, Fischbach MA. Human Microbiota. Small molecules from the human microbiota. Science. 2015;349:1254766. doi: 10.1126/science.1254766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou L, Bertrand E, Cerini C, Faure V, Sampol J, Vanholder R, Berland Y, Brunet P. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004;65:442–451. doi: 10.1111/j.1523-1755.2004.00399.x. [DOI] [PubMed] [Google Scholar]
- Dou L, Jourde-Chiche N, Faure V, Cerini C, Berland Y, Dignat-George F, Brunet P. The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J Thromb Haemost. 2007;5:1302–1308. doi: 10.1111/j.1538-7836.2007.02540.x. [DOI] [PubMed] [Google Scholar]
- Doyle AE. Hypertension and vascular disease. Am J Hypertens. 1991;4:103S–106S. doi: 10.1093/ajh/4.2.103s. [DOI] [PubMed] [Google Scholar]
- Du L, Qi R, Wang J, Liu Z, Wu Z. Indole-3-Propionic Acid, a functional metabolite of clostridium sporogenes, promotes muscle tissue development and reduces muscle cell inflammation. Int J Mol Sci. 2021 doi: 10.3390/ijms222212435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsden SR, Hilton MG, Waller JM. The end products of the metabolism of aromatic amino acids by Clostridia. Arch Microbiol. 1976;107:283–288. doi: 10.1007/BF00425340. [DOI] [PubMed] [Google Scholar]
- Eussen SJPM, Ueland PM, Vollset SE, Nygård O, Midttun Ø, Sulo G, Ulvik A, Meyer K, Pedersen ER, Tell GS. Kynurenines as predictors of acute coronary events in the Hordaland Health Study. Int J Cardiol. 2015;189:18–24. doi: 10.1016/j.ijcard.2015.03.413. [DOI] [PubMed] [Google Scholar]
- Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176:6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- Fatkhullina AR, Peshkova IO, Dzutsev A, Aghayev T, McCulloch JA, Thovarai V, Badger JH, Vats R, Sundd P, Tang H-Y, et al. An interleukin-23-interleukin-22 axis regulates intestinal microbial homeostasis to protect from diet-Induced atherosclerosis. Immunity. 2018;49:943–957.e9. doi: 10.1016/j.immuni.2018.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure V, Dou L, Sabatier F, Cerini C, Sampol J, Berland Y, Brunet P, Dignat-George F. Elevation of circulating endothelial microparticles in patients with chronic renal failure. J Thromb Haemost. 2006;4:566–573. doi: 10.1111/j.1538-7836.2005.01780.x. [DOI] [PubMed] [Google Scholar]
- Formes H, Bernardes JP, Mann A, Bayer F, Pontarollo G, Kiouptsi K, Schäfer K, Attig S, Nikolova T, Hofmann TG, et al. The gut microbiota instructs the hepatic endothelial cell transcriptome. iScience. 2021;24:103092. doi: 10.1016/j.isci.2021.103092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao K, Mu C-L, Farzi A, Zhu W-Y. Tryptophan metabolism: A link between the gut microbiota and brain. Adv Nutr. 2020;11:709–723. doi: 10.1093/advances/nmz127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesper M, Nonnast ABH, Kumowski N, Stoehr R, Schuett K, Marx N, Kappel BA. Gut-derived metabolite indole-3-propionic acid modulates mitochondrial function in cardiomyocytes and alters cardiac function. Front Med (Lausanne) 2021;8:648259. doi: 10.3389/fmed.2021.648259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondouin B, Cerini C, Dou L, Sallée M, Duval-Sabatier A, Pletinck A, Calaf R, Lacroix R, Jourde-Chiche N, Poitevin S, et al. Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney Int. 2013;84:733–744. doi: 10.1038/ki.2013.133. [DOI] [PubMed] [Google Scholar]
- Gronke K, Hernandez P, Zimmermann J, Klose CSN, Kofoed-Branzk M, Guendel F, Witkowski M, Tizian C, Amann L, Schumacher F, et al. Interleukin-22 protects intestinal stem cells against genotoxic stress. Nature. 2019;566:249–253. doi: 10.1038/s41586-019-0899-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Vázquez C, Quintana FJ. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity. 2018;48:19–33. doi: 10.1016/j.immuni.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton AC, Coleman TG, Cowley AW, Scheel KW, Manning RD, Norman RA. Arterial pressure regulation. Am J Med. 1972;52:584–594. doi: 10.1016/0002-9343(72)90050-2. [DOI] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, Sigl V, Hanada T, Hanada R, Lipinski S, et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature. 2012;487:477–481. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández PP, Mahlakoiv T, Yang I, Schwierzeck V, Nguyen N, Guendel F, Gronke K, Ryffel B, Hoelscher C, Dumoutier L, et al. Interferon-λ and interleukin 22 act synergistically for the induction of interferon-stimulated genes and control of rotavirus infection. Nat Immunol. 2015;16:698–707. doi: 10.1038/ni.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi K, Hayaishi O. Enzymic formation of d-kynurenine from d-tryptophan. Arch Biochem Biophys. 1967;120:397–403. doi: 10.1016/0003-9861(67)90256-1. [DOI] [PubMed] [Google Scholar]
- Honeyfield DC, Carlson JR. Effect of indoleacetic acid and related indoles on lactobacillus sp. strain 11201 growth, indoleacetic acid catabolism, and 3-methylindole formation. Appl Environ Microbiol. 1990;56:1373–1377. doi: 10.1128/aem.56.5.1373-1377.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré AH, Aunsbjerg SD, Ebrahimi P, Thorsen M, Benfeldt C, Knøchel S, Skov T. Metabolic footprinting for investigation of antifungal properties of Lactobacillus paracasei. Anal Bioanal Chem. 2016;408:83–96. doi: 10.1007/s00216-015-9103-6. [DOI] [PubMed] [Google Scholar]
- Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard TD, Murray IA, Bisson WH, Lahoti TS, Gowda K, Amin SG, Patterson AD, Perdew GH. Adaptation of the human aryl hydrocarbon receptor to sense microbiota-derived indoles. Sci Rep. 2015;5:1–13. doi: 10.1038/srep12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard TD, Murray IA, Perdew GH. Indole and tryptophan metabolism: Endogenous and dietary routes to Ah receptor activation. Drug Metab Dispos. 2015;43:1522–1535. doi: 10.1124/dmd.115.064246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huc T, Konop M, Onyszkiewicz M, Podsadni P, Szczepańska A, Turło J, Ufnal M. Colonic indole, gut bacteria metabolite of tryptophan, increases portal blood pressure in rats. Am J Physiol Regul Integr Comp Physiol. 2018;315:R646–R655. doi: 10.1152/ajpregu.00111.2018. [DOI] [PubMed] [Google Scholar]
- Huć T, Nowinski A, Drapala A, Konopelski P, Ufnal M. Indole and indoxyl sulfate, gut bacteria metabolites of tryptophan, change arterial blood pressure via peripheral and central mechanisms in rats. Pharmacol Res. 2018;130:172–179. doi: 10.1016/j.phrs.2017.12.025. [DOI] [PubMed] [Google Scholar]
- Hung SC, Kuo KL, Huang HL, Lin CC, Tsai TH, Wang CH, Chen JW, Lin SJ, Huang PH, Tarng DC. Indoxyl sulfate suppresses endothelial progenitor cell-mediated neovascularization. Kidney Int. 2016;89(3):574–585. doi: 10.1016/j.kint.2015.11.020. [DOI] [PubMed] [Google Scholar]
- Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- Ikuta T, Eguchi H, Tachibana T, Yoneda Y, Kawajiri K. Nuclear localization and export signals of the human aryl hydrocarbon receptor. J Biol Chem. 1998;273:2895–2904. doi: 10.1074/jbc.273.5.2895. [DOI] [PubMed] [Google Scholar]
- Ito S, Osaka M, Edamatsu T, Itoh Y, Yoshida M. Crucial role of the aryl hydrocarbon receptor (AhR) in indoxyl sulfate-induced vascular inflammation. J Atheroscler Thromb. 2016;23:960–975. doi: 10.5551/jat.34462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworska K, Huc T, Samborowska E, Dobrowolski L, Bielinska K, Gawlak M, Ufnal M. Hypertension in rats is associated with an increased permeability of the colon to TMA, a gut bacteria metabolite. PLoS ONE. 2017;12:e0189310. doi: 10.1371/journal.pone.0189310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jie Z, Xia H, Zhong S-L, Feng Q, Li S, Liang S, Zhong H, Liu Z, Gao Y, Zhao H, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8:1–12. doi: 10.1038/s41467-017-00900-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappel BA, de Angelis L, Heiser M, Ballanti M, Stoehr R, Goettsch C, Mavilio M, Artati A, Paoluzi OA, Adamski J, et al. Cross-omics analysis revealed gut microbiome-related metabolic pathways underlying atherosclerosis development after antibiotics treatment. Mol Metab. 2020;36:100976. doi: 10.1016/j.molmet.2020.100976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbach S, Wenzel P, Waisman A, Munzel T, Daiber A. eNOS uncoupling in cardiovascular diseases–the role of oxidative stress and inflammation. Curr Pharm Des. 2014;20:3579–3594. doi: 10.2174/13816128113196660748. [DOI] [PubMed] [Google Scholar]
- Karbach SH, Schönfelder T, Brandão I, Wilms E, Hörmann N, Jäckel S, Schüler R, Finger S, Knorr M, Lagrange J, et al. Gut microbiota promote angiotensin II-induced arterial hypertension and vascular dysfunction. J Am Heart Assoc. 2016 doi: 10.1161/JAHA.116.003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiouptsi K, Jäckel S, Pontarollo G, Grill A, Kuijpers MJE, Wilms E, Weber C, Sommer F, Nagy M, Neideck C, et al. The microbiota promotes arterial thrombosis in low-density lipoprotein receptor-deficient mice. mBio. 2019 doi: 10.1128/mBio.02298-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiouptsi K, Pontarollo G, Todorov H, Braun J, Jäckel S, Koeck T, Bayer F, Karwot C, Karpi A, Gerber S, et al. Germ-free housing conditions do not affect aortic root and aortic arch lesion size of late atherosclerotic low-density lipoprotein receptor-deficient mice. Gut Microbes. 2020;11:1809–1823. doi: 10.1080/19490976.2020.1767463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss EA, Vonarbourg C, Kopfmann S, Hobeika E, Finke D, Esser C, Diefenbach A. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science. 2011;334:1561–1565. doi: 10.1126/science.1214914. [DOI] [PubMed] [Google Scholar]
- Koh A, Bäckhed F. From association to causality: the role of the gut microbiota and its functional products on host metabolism. Mol Cell. 2020;78:584–596. doi: 10.1016/j.molcel.2020.03.005. [DOI] [PubMed] [Google Scholar]
- Konopelski P, Ufnal M. Indoles - gut bacteria metabolites of tryptophan with pharmacotherapeutic potential. Curr Drug Metab. 2018;19:883–890. doi: 10.2174/1389200219666180427164731. [DOI] [PubMed] [Google Scholar]
- Konopelski P, Chabowski D, Aleksandrowicz M, Kozniewska E, Podsadni P, Szczepanska A, Ufnal M. Indole-3-propionic acid, a tryptophan-derived bacterial metabolite, increases blood pressure via cardiac and vascular mechanisms in rats. Am J Physiol Regul Integr Comp Physiol. 2021;321:R969–R981. doi: 10.1152/ajpregu.00142.2021. [DOI] [PubMed] [Google Scholar]
- Krishnan S, Ding Y, Saedi N, Choi M, Sridharan GV, Sherr DH, Yarmush ML, Alaniz RC, Jayaraman A, Lee K. Gut microbiota-derived tryptophan metabolites modulate inflammatory response in hepatocytes and macrophages. Cell Rep. 2018;23:1099–1111. doi: 10.1016/j.celrep.2018.03.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Liu C-W, Yang Y, Hsiao Y-C, Ru H, Lu K. High-coverage metabolomics uncovers microbiota-driven biochemical landscape of interorgan transport and gut-brain communication in mice. Nat Commun. 2021;12:1–16. doi: 10.1038/s41467-021-26209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam V, Su J, Hsu A, Gross GJ, Salzman NH, Baker JE. Intestinal microbial metabolites are linked to severity of myocardial infarction in rats. PLoS ONE. 2016;11:e0160840. doi: 10.1371/journal.pone.0160840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamas B, Richard ML, Leducq V, Pham H-P, Michel M-L, Da Costa G, Bridonneau C, Jegou S, Hoffmann TW, Natividad JM, et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 2016;22:598–605. doi: 10.1038/nm.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langan D, Perkins DJ, Vogel SN, Moudgil KD. Microbiota-derived metabolites, indole-3-aldehyde and indole-3-acetic acid, differentially modulate innate cytokines and stromal remodeling processes associated with autoimmune arthritis. Int J Mol Sci. 2021 doi: 10.3390/ijms22042017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larigot L, Juricek L, Dairou J, Coumoul X. AhR signaling pathways and regulatory functions. Biochim Open. 2018;7:1–9. doi: 10.1016/j.biopen.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurans L, Venteclef N, Haddad Y, Chajadine M, Alzaid F, Metghalchi S, Sovran B, Denis RGP, Dairou J, Cardellini M, et al. Genetic deficiency of indoleamine 2,3-dioxygenase promotes gut microbiota-mediated metabolic health. Nat Med. 2018;24:1113–1120. doi: 10.1038/s41591-018-0060-4. [DOI] [PubMed] [Google Scholar]
- Lee J-H, Lee J. Indole as an intercellular signal in microbial communities. FEMS Microbiol Rev. 2010;34:426–444. doi: 10.1111/j.1574-6976.2009.00204.x. [DOI] [PubMed] [Google Scholar]
- Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, Nukaya M, Mantovani A, Kopan R, Bradfield CA, Newberry RD, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol. 2012;13:144–151. doi: 10.1038/ni.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DM, Ecton KE, Trikha SRJ, Wrigley SD, Thomas KN, Battson ML, Wei Y, Johnson SA, Weir TL, Gentile CL. Microbial metabolite indole-3-propionic acid supplementation does not protect mice from the cardiometabolic consequences of a Western diet. Am J Physiol Gastrointest Liver Physiol. 2020;319:G51–G62. doi: 10.1152/ajpgi.00375.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, Wu S, Liu W, Cui Q, Geng B, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5:1–19. doi: 10.1186/s40168-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindskog Jonsson A, Caesar R, Akrami R, Reinhardt C, Fåk Hållenius F, Borén J, Bäckhed F. Impact of gut microbiota and diet on the development of atherosclerosis in Apoe-/- mice. Arterioscler Thromb Vasc Biol. 2018;38:2318–2326. doi: 10.1161/ATVBAHA.118.311233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Wei Y, Liu X, ZhouY JL, Yin J, Wang F, Hu Y, Nanjaraj Urs AN, Liu Y, et al. Indoleacetate decarboxylase is a glycyl radical enzyme catalysing the formation of malodorant skatole. Nat Commun. 2018;9:4224. doi: 10.1038/s41467-018-06627-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcobal A, Kashyap PC, Nelson TA, Aronov PA, Donia MS, Spormann A, Fischbach MA, Sonnenburg JL. A metabolomic view of how the human gut microbiota impacts the host metabolome using humanized and gnotobiotic mice. ISME J. 2013;7:1933–1943. doi: 10.1038/ismej.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulis L, Fester R (ed.) (1991) Symbiosis as a source of evolutionary innovation. Speciation and morphogenesis. Conference on Symbiosis as a Source of Evolutionary Innovation. Cambridge, Mass.: MIT Pr [PubMed]
- Mauler M, Herr N, Schoenichen C, Witsch T, Marchini T, Härdtner C, Koentges C, Kienle K, Ollivier V, Schell M, et al. Platelet serotonin aggravates myocardial ischemia/reperfusion injury via neutrophil degranulation. Circulation. 2019;139:918–931. doi: 10.1161/CIRCULATIONAHA.118.033942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauler M, Schanze N, Krauel K, Schoenichen C, Glatzki F, Poeschl S, Stallmann D, Mezger J, Gauchel N, Sharipova D, et al. Peripheral serotonin lacks effects on endothelial adhesion molecule expression in acute inflammation. J Thromb Haemost. 2022;20:222–229. doi: 10.1111/jth.15541. [DOI] [PubMed] [Google Scholar]
- Melhem NJ, Chajadine M, Gomez I, Howangyin K-Y, Bouvet M, Knosp C, Sun Y, Rouanet M, Laurans L, Cazorla O, et al. Endothelial cell indoleamine 2, 3-dioxygenase 1 alters cardiac function after myocardial infarction through kynurenine. Circulation. 2021;143:566–580. doi: 10.1161/CIRCULATIONAHA.120.050301. [DOI] [PubMed] [Google Scholar]
- Metghalchi S, Ponnuswamy P, Simon T, Haddad Y, Laurans L, Clément M, Dalloz M, Romain M, Esposito B, Koropoulis V, et al. Indoleamine 2,3-dioxygenase fine-tunes immune homeostasis in atherosclerosis and colitis through repression of interleukin-10 production. Cell Metab. 2015;22:460–471. doi: 10.1016/j.cmet.2015.07.004. [DOI] [PubMed] [Google Scholar]
- Meyer-Abich A (1950) Beiträge zur Theorie der Evolution der Organismen. Leiden: Brill
- Nakagawa K, Tanaka R, Donouchi M, Kanda M, Kamada S, Kobuchi S, Tawa M, Matsumura Y, Ohkita M. Vascular endothelial dysfunction in the thoracic aorta of rats with ischemic acute kidney injury: contribution of indoxyl sulfate. Oxid Med Cell Longev. 2022 doi: 10.1155/2022/7547269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkiewicz K. Diagnosis and management of hypertension in obesity. Obes Rev. 2006;7:155–162. doi: 10.1111/j.1467-789X.2006.00226.x. [DOI] [PubMed] [Google Scholar]
- Niinisalo P, Oksala N, Levula M, Pelto-Huikko M, Järvinen O, Salenius J-P, Kytömäki L, Soini JT, Kähönen M, Laaksonen R, et al. Activation of indoleamine 2,3-dioxygenase-induced tryptophan degradation in advanced atherosclerotic plaques: Tampere vascular study. Ann Med. 2010;42:55–63. doi: 10.3109/07853890903321559. [DOI] [PubMed] [Google Scholar]
- Nikolaus S, Schulte B, Al-Massad N, Thieme F, Schulte DM, Bethge J, Rehman A, Tran F, Aden K, Häsler R, et al. Increased tryptophan metabolism is associated with inflammatory bowel diseases. Gastroenterology. 2017;153:1504–1516.e2. doi: 10.1053/j.gastro.2017.08.028. [DOI] [PubMed] [Google Scholar]
- Nitz K, Lacy M, Atzler D. Amino acids and their metabolism in atherosclerosis. Arterioscler Thromb Vasc Biol. 2019;39:319–330. doi: 10.1161/ATVBAHA.118.311572. [DOI] [PubMed] [Google Scholar]
- Oliveras A, La Sierra A, de, Resistant hypertension: patient characteristics, risk factors, co-morbidities and outcomes. J Hum Hypertens. 2014;28:213–217. doi: 10.1038/jhh.2013.77. [DOI] [PubMed] [Google Scholar]
- O'Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- Ondimu DO, Kikuvi GM, Otieno WN (2019) Risk factors for hypertension among young adults (18–35) years attending in Tenwek Mission Hospital, Bomet County, Kenya in 2018. Pan Afr Med J 10.11604/pamj.2019.33.210.18407 [DOI] [PMC free article] [PubMed]
- Ozemek C, Laddu DR, Arena R, Lavie CJ. The role of diet for prevention and management of hypertension. Curr Opin Cardiol. 2018;33:388. doi: 10.1097/HCO.0000000000000532. [DOI] [PubMed] [Google Scholar]
- Pawlak K, Domaniewski T, Mysliwiec M, Pawlak D. The kynurenines are associated with oxidative stress, inflammation and the prevalence of cardiovascular disease in patients with end-stage renal disease. Atherosclerosis. 2009;204:309–314. doi: 10.1016/j.atherosclerosis.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Pedersen ER, Midttun Ø, Ueland PM, Schartum-Hansen H, Seifert R, Igland J, Nordrehaug JE, Ebbing M, Svingen G, Bleie Ø, et al. Systemic markers of interferon-γ-mediated immune activation and long-term prognosis in patients with stable coronary artery disease. Arterioscler Thromb Vasc Biol. 2011;31:698–704. doi: 10.1161/ATVBAHA.110.219329. [DOI] [PubMed] [Google Scholar]
- Pedersen ER, Tuseth N, Eussen SJPM, Ueland PM, Strand E, Svingen GFT, Midttun Ø, Meyer K, Mellgren G, Ulvik A, et al. Associations of plasma kynurenines with risk of acute myocardial infarction in patients with stable angina pectoris. Arterioscler Thromb Vasc Biol. 2015;35:455–462. doi: 10.1161/ATVBAHA.114.304674. [DOI] [PubMed] [Google Scholar]
- Poesen R, Windey K, Neven E, Kuypers D, De Preter V, Augustijns P, D’Haese P, Evenepoel P, Verbeke K, Meijers B. The influence of CKD on colonic microbial metabolism. J Am Soc Nephrol. 2016;27:1389–1399. doi: 10.1681/ASN.2015030279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontarollo G, Kiouptsi K, Reinhardt C (2020) A holobiont view on thrombosis: unravelling the microbiotas influence on arterial thrombus growth. Microb Cell 7:28–31. 10.15698/mic2020.01.704 [DOI] [PMC free article] [PubMed]
- Price RS, Kasner SE (2014) Chapter 12 - Hypertension and hypertensive encephalopathy. In: José Biller and José M. Ferro (ed.): Handbook of Clinical Neurology: Neurologic Aspects of Systemic Disease Part I, Bd. 119: Elsevier: 161–167. https://www.sciencedirect.com/science/article/pii/B9780702040863000126
- Puccetti M, Gomes Dos Reis L, Pariano M, Costantini C, Renga G, Ricci M, Traini D, Giovagnoli S. Development and in vitro-in vivo performances of an inhalable indole-3-carboxaldehyde dry powder to target pulmonary inflammation and infection. Int J Pharm. 2021;607:121004. doi: 10.1016/j.ijpharm.2021.121004. [DOI] [PubMed] [Google Scholar]
- Ramage AG, Villalón CM. 5-hydroxytryptamine and cardiovascular regulation. Trends Pharmacol Sci. 2008;29:472–481. doi: 10.1016/j.tips.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Regen T, Isaac S, Amorim A, Núñez NG, Hauptmann J, Shanmugavadivu A, Klein M, Sankowski R, Mufazalov IA, Yogev N, et al. IL-17 controls central nervous system autoimmunity through the intestinal microbiome. Sci Immunol. 2021 doi: 10.1126/sciimmunol.aaz6563. [DOI] [PubMed] [Google Scholar]
- Reyes H, Reisz-Porszasz S, Hankinson O. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science. 1992;256:1193–1195. doi: 10.1126/science.256.5060.1193. [DOI] [PubMed] [Google Scholar]
- Roager HM, Licht TR. Microbial tryptophan catabolites in health and disease. Nat Commun. 2018;9:1–10. doi: 10.1038/s41467-018-05470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Rosser EC, Piper CJM, Matei DE, Blair PA, Rendeiro AF, Orford M, Alber DG, Krausgruber T, Catalan D, Klein N, et al. Microbiota-derived metabolites suppress arthritis by amplifying aryl-hydrocarbon receptor activation in regulatory B cells. Cell Metab. 2020;31:837–851.e10. doi: 10.1016/j.cmet.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, Ahmed M, Aksut B, Alam T, Alam K, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. 2017;70:1–25. doi: 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, Chao C-C, Patel B, Yan R, Blain M, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016;22:586–597. doi: 10.1038/nm.4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi D, Costea PI, Godneva A, Kalka IN, Bar N, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555:210–215. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- Ruivo JA, Alcântara P. Hipertensão arterial e exercício físico. Rev Port Cardiol. 2012;31:151–158. doi: 10.1016/j.repc.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Russell WR, Duncan SH, Scobbie L, Duncan G, Cantlay L, Calder AG, Anderson SE, Flint HJ. Major phenylpropanoid-derived metabolites in the human gut can arise from microbial fermentation of protein. Mol Nutr Food Res. 2013;57:523–535. doi: 10.1002/mnfr.201200594. [DOI] [PubMed] [Google Scholar]
- Safe S, Jin U-H, Park H, Chapkin RS, Jayaraman A. Aryl hydrocarbon receptor (AHR) ligands as selective AHR modulators (SAhRMs) Int J Mol Sci. 2020;21:6654. doi: 10.3390/ijms21186654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaupp L, Muth S, Rogell L, Kofoed-Branzk M, Melchior F, Lienenklaus S, Ganal-Vonarburg SC, Klein M, Guendel F, Hain T, et al. Microbiota-induced type I interferons instruct a poised basal state of dendritic cells. Cell. 2020;181:1080–1096.e19. doi: 10.1016/j.cell.2020.04.022. [DOI] [PubMed] [Google Scholar]
- Schiering C, Wincent E, Metidji A, Iseppon A, Li Y, Potocnik AJ, Omenetti S, Henderson CJ, Wolf CR, Nebert DW, et al. Feedback control of AHR signalling regulates intestinal immunity. Nature. 2017;542:242–245. doi: 10.1038/nature21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder BO, Bäckhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med. 2016;22:1079–1089. doi: 10.1038/nm.4185. [DOI] [PubMed] [Google Scholar]
- Shirai T, Hilhorst M, Harrison DG, Goronzy JJ, Weyand CM. Macrophages in vascular inflammation–From atherosclerosis to vasculitis. Autoimmunity. 2015;48:139–151. doi: 10.3109/08916934.2015.1027815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EA, Macfarlane GT. Enumeration of human colonic bacteria producing phenolic and indolic compounds: effects of pH, carbohydrate availability and retention time on dissimilatory aromatic amino acid metabolism. J Appl Bacteriol. 1996;81:288–302. doi: 10.1111/j.1365-2672.1996.tb04331.x. [DOI] [PubMed] [Google Scholar]
- Sonner JK, Keil M, Falk-Paulsen M, Mishra N, Rehman A, Kramer M, Deumelandt K, Röwe J, Sanghvi K, Wolf L, et al. Dietary tryptophan links encephalogenicity of autoreactive T cells with gut microbial ecology. Nat Commun. 2019;10:1–14. doi: 10.1038/s41467-019-12776-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A, Caprioli F, Bottiglieri L, Oldani A, Viale G, et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science. 2015;350:830–834. doi: 10.1126/science.aad0135. [DOI] [PubMed] [Google Scholar]
- Steven S, Frenis K, Oelze M, Kalinovic S, Kuntic M, Bayo Jimenez MT, Vujacic-Mirski K, Helmstädter J, Kröller-Schön S, Münzel T, et al. Vascular inflammation and oxidative stress: Major triggers for cardiovascular disease. Oxid Med Cell Longev. 2019;2019:7092151. doi: 10.1155/2019/7092151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinghen AEM, Chillon J-M, Massy ZA, Boullier A. Differential effects of indoxyl sulfate and inorganic phosphate in a murine cerebral endothelial cell line (bEnd. 3) Toxins (basel) 2014;6:1742–1760. doi: 10.3390/toxins6061742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swimm A, Giver CR, DeFilipp Z, Rangaraju S, Sharma A, Ulezko Antonova A, Sonowal R, Capaldo C, Powell D, Qayed M, et al. Indoles derived from intestinal microbiota act via type I interferon signaling to limit graft-versus-host disease. Blood. 2018;132:2506–2519. doi: 10.1182/blood-2018-03-838193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaki M, Mawe GM, Barasch JM, Gershon MD, Gershon MD. Physiological responses of guinea-pig myenteric neurons secondary to the release of endogenous serotonin by tryptamine. Neuroscience. 1985;16:223–240. doi: 10.1016/0306-4522(85)90059-4. [DOI] [PubMed] [Google Scholar]
- Takayama F, Taki K, Niwa T. Bifidobacterium in gastro-resistant seamless capsule reduces serum levels of indoxyl sulfate in patients on hemodialysis. Am J Kidney Dis. 2003;41(3 Suppl 1):S142–S145. doi: 10.1053/ajkd.2003.50104. [DOI] [PubMed] [Google Scholar]
- Taleb S. Tryptophan dietary impacts gut barrier and metabolic diseases. Front Immunol. 2019;10:2113. doi: 10.3389/fimmu.2019.02113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodorou K, Boon RA. Endothelial cell metabolism in atherosclerosis. Front Cell Dev Biol. 2018;6:82. doi: 10.3389/fcell.2018.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD. Fourth universal definition of myocardial infarction. J Am Coll Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- Tomasova L, Dobrowolski L, Jurkowska H, Wróbel M, Huc T, Ondrias K, Ostaszewski R, Ufnal M. Intracolonic hydrogen sulfide lowers blood pressure in rats. Nitric Oxide. 2016;60:50–58. doi: 10.1016/j.niox.2016.09.007. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP, Qiu Z, Maher L, Redinbo MR, Phillips RS, et al. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity. 2014;41:296–310. doi: 10.1016/j.immuni.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkateswaran N, Lafita-Navarro MC, Hao Y-H, Kilgore JA, Perez-Castro L, Braverman J, Borenstein-Auerbach N, Kim M, Lesner NP, Mishra P, et al. MYC promotes tryptophan uptake and metabolism by the kynurenine pathway in colon cancer. Genes Dev. 2019;33:1236–1251. doi: 10.1101/gad.327056.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venu PVK, Saifeddine M, Mihara K, Tsai Y-C, Nieves K, Alston L, Mani S, McCoy KD, Hollenberg MD, Hirota SA. The pregnane X receptor and its microbiota-derived ligand indole 3-propionic acid regulate endothelium-dependent vasodilation. Am J Physiol Endocrinol Metab. 2019;317:E350–E361. doi: 10.1152/ajpendo.00572.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaar BJH, Prodan A, Nieuwdorp M, Muller M. Gut microbiota in hypertension and atherosclerosis: a review. Nutrients. 2020;12:2982. doi: 10.3390/nu12102982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira-Silva S, Falony G, Belda E, Nielsen T, Aron-Wisnewsky J, Chakaroun R, Forslund SK, Assmann K, Valles-Colomer M, Nguyen TTD, et al. Statin therapy is associated with lower prevalence of gut microbiota dysbiosis. Nature. 2020;581:310–315. doi: 10.1038/s41586-020-2269-x. [DOI] [PubMed] [Google Scholar]
- Vogel CFA, Sciullo E, Matsumura F. Activation of inflammatory mediators and potential role of ah-receptor ligands in foam cell formation. Cardiovasc Toxicol. 2004;4:363–373. doi: 10.1385/CT:4:4:363. [DOI] [PubMed] [Google Scholar]
- Wang X, Ota N, Manzanillo P, Kates L, Zavala-Solorio J, Eidenschenk C, Zhang J, Lesch J, Lee WP, Ross J, et al. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature. 2014;514:237–241. doi: 10.1038/nature13564. [DOI] [PubMed] [Google Scholar]
- Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, Karbarch SH, Schwenk M, Yogev N, Schulz E, Oelze M, Grabbe S, Jonuleit H, Becker C, Daiber A, Waisman A, Münzel T. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation. 2011;124:1370–1381. doi: 10.1161/CIRCULATIONAHA.111.034470. [DOI] [PubMed] [Google Scholar]
- Whitehead TR, Price NP, Drake HL, Cotta MA. Catabolic pathway for the production of skatole and indoleacetic acid by the acetogen Clostridium drakei, Clostridium scatologenes, and swine manure. Appl Environ Microbiol. 2008;74:1950–1953. doi: 10.1128/AEM.02458-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Cargile CM, Cohen ND, Chapkin RS, Weeks BR, Davidson LA, Goldsby JS, Hunt CL, Steinmeyer SH, Menon R, Suchodolsky JS, et al. The microbiota-derived metabolite indole decreases mucosal inflammation and injury in a murine model of NSAID enteropathy. Gut Microbes. 2016;7:246–261. doi: 10.1080/19490976.2016.1156827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci U S A. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, Haase S, Mähler A, Balogh A, Markó L, et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature. 2017;551:585–589. doi: 10.1038/nature24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BB, van Benschoten AH, Cimermancic P, Donia MS, Zimmermann M, Taketani M, Ishihara A, Kashyap PC, Fraser JS, Fischbach MA. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe. 2014;16:495–503. doi: 10.1016/j.chom.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarska M, Luo C, Kolde R, d'Hennezel E, Annand JW, Heim CE, Krastel P, Schmitt EK, Omar AS, Creasey EA, et al. Indoleacrylic acid produced by commensal peptostreptococcus species suppresses inflammation. Cell Host Microbe. 2017;22:25–37.e6. doi: 10.1016/j.chom.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2021) Hypertension. https://www.who.int/news-room/fact-sheets/detail/hypertension. Accessed 15 December 21
- Wu W-K, Chen C-C, Liu P-Y, Panyod S, Liao B-Y, Chen P-C, Kao H-L, Kuo H-C, Kuo C-H, Chiu THT, et al. Identification of TMAO-producer phenotype and host-diet-gut dysbiosis by carnitine challenge test in human and germ-free mice. Gut. 2019;68:1439–1449. doi: 10.1136/gutjnl-2018-317155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Hayaishi O. Tryptophan pyrrolase of rabbit intestine. D- and L-tryptophan-cleaving enzyme or enzymes. J Biol Chem. 1967;242(22):5260–5266. doi: 10.1016/S0021-9258(18)99420-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Tsuruoka S, Ioka T, Ando H, Ito C, Akimoto T, Fujimura A, Asano Y, Kusano E. Indoxyl sulfate stimulates proliferation of rat vascular smooth muscle cells. Kidney Int. 2006;69:1780–1785. doi: 10.1038/sj.ki.5000340. [DOI] [PubMed] [Google Scholar]
- Yano JM, Yu K, Donaldson GP, Shastri GG, Ann P, Ma L, Nagler CR, Ismagilov RF, Mazmanian SK, Hsiao EY. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Ji Q, Liu J, Liu L, Huang Y, Shi Y, Shi L, Wang M, Liu M, Feng Y, et al. Interleukin 22 promotes blood pressure elevation and endothelial dysfunction in angiotensin II-treated mice. J Am Heart Assoc. 2017 doi: 10.1161/JAHA.117.005875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Kim YJ, Kang D-H. Indoxyl sulfate-induced endothelial dysfunction in patients with chronic kidney disease via an induction of oxidative stress. Clin J Am Soc Nephrol. 2011;6:30–39. doi: 10.2215/CJN.05340610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelante T, Iannitti RG, Cunha C, de Luca A, Giovannini G, Pieraccini G, Zecchi R, D'Angelo C, Massi-Benedetti C, Fallarino F, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity. 2013;39:372–385. doi: 10.1016/j.immuni.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Zelante T, Iannitti RG, Fallarino F, Gargaro M, de Luca A, Moretti S, Bartoli A, Romani L. Tryptophan feeding of the IDO1-AhR axis in host-microbial symbiosis. Front Immunol. 2014;5:640. doi: 10.3389/fimmu.2014.00640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z-H, Xin F-Z, Xue Y, Hu Z, Han Y, Ma F, Zhou D, Liu X-L, Cui A, Liu Z, et al. Indole-3-propionic acid inhibits gut dysbiosis and endotoxin leakage to attenuate steatohepatitis in rats. Exp Mol Med. 2019;51:1–14. doi: 10.1038/s12276-019-0304-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhenyukh O, González-Amor M, Rodrigues-Diez RR, Esteban V, Ruiz-Ortega M, Salaices M, Mas S, Briones AM, Egido J. Branched-chain amino acids promote endothelial dysfunction through increased reactive oxygen species generation and inflammation. J Cell Mol Med. 2018;22:4948–4962. doi: 10.1111/jcmm.13759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucchi R, Chiellini G, Scanlan TS, Grandy DK. Trace amine-associated receptors and their ligands. Br J Pharmacol. 2006;149:967–978. doi: 10.1038/sj.bjp.0706948. [DOI] [PMC free article] [PubMed] [Google Scholar]