Abstract
Background
Hepatocellular carcinoma (HCC) is the second leading cause of cancer-related mortality worldwide. It is a highly heterogeneous disease with poor prognosis and limited treatment options, which highlights the need for reliable biomarkers. This study aims to explore molecular markers that allow stratification of HCC and may lead to better prognosis and treatment prediction.
Materials and methods
We studied 20 candidate genes (HCC hub genes, potential drug target genes, predominant somatic mutant genes) retrieved from literature and public databases with potential to be used as the molecular markers. We analysed expression of the genes by RT-qPCR in 30 HCC tumour and adjacent non-tumour paired samples from Vietnamese patients. Fold changes in expression were then determined using the 2−∆∆CT method, and unsupervised hierarchical clustering was generated using Cluster v3.0 software.
Results
Clustering of expression data revealed two subtypes of tumours (proliferative and normal-like) and four clusters for genes. The expression profiles of the genes TOP2A, CDK1, BIRC5, GPC3, IGF2, and AFP were strongly correlated. Proliferative tumours were characterized by high expression of the c-MET, ARID1A, CTNNB1, RAF1, LGR5, and GLUL1 genes. TOP2A, CDK1, and BIRC5 HCC hub genes were highly expressed (> twofold) in 90% (27/30), 83% (25/30), and 83% (24/30) in the tissue samples, respectively. Among the drug target genes, high expression was observed in the GPC3, IGF2 and c-MET genes in 77% (23/30), 63% (19/30), and 37% (11/30), respectively. The somatic mutant Wnt/ß-catenin genes (CTNNB1, GLUL and LGR5) and TERT were highly expressed in 40% and 33% of HCCs, respectively. Among the HCC marker genes, a higher percentage of tumours showed GPC3 expression compared to AFP expression [73% (23/30) vs. 43% (13/30)].
Conclusion
The custom panel and molecular markers from this study may be useful for diagnosis, prognosis, biomarker-guided clinical trial design, and prediction of treatment outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12920-022-01386-7.
Keywords: Hepatocellular carcinoma, HCC, Hepatitis B virus, Vietnam, Biomarker, Prognosis
Introduction
Hepatocellular carcinoma (HCC) is the second most common cause of cancer-related mortality worldwide, with two-thirds of cases occurring in Asia [1]. The hepatitis B (HBV) and hepatitis C (HCV) viruses are the major etiological agents for HCC globally [2], and in particular HBV-induced HCC is common with increasing numbers of cases in East Asia and Africa [3]. Vietnam has a high prevalence of HBV infections, ranging from 10 to 20% in the general population and 20–40% among injecting drug users and HIV-positive patients [4]. Prognosis of HCC remains poor because of the underlying chronic liver disease; late diagnosis, often at advanced stages of disease [5].
HCC is heterogeneous and classified into four Barcelona Clinic Liver Cancer (BCLC) stages (BCLC-A: early HCC, BCLC-B: intermediate HCC, BCLC-C: advanced HCC, BCLC-D: terminal HCC) for optimal clinical management using the staging depending on tumour burden, liver function (Child–Pugh score), and overall health [6, 7]. HCC is routinely diagnosed by blood tests (alanine transaminase [ALT], aspartate transaminase [AST], total bilirubin and direct bilirubin), liver biopsy, imaging, and serum alpha-fetoprotein (AFP) and albumin levels. HCC is heterogeneous, which is associated with poor prognostic outcomes because it is more difficult to diagnose accurately. Heterogeneity of HCC tumours are known to occur at different levels; firstly within the population and secondly within tumours of the same patient [8]. Recently, rapid progress has been made in understanding the heterogeneity of HCC by molecular subclassification using molecular and genetic markers [9]. HCC tumours are divided into two major phenotypic classes: the proliferation class and the non-proliferation class [10, 11], which have been identified on the basis of transcriptomic dysregulations and genetic alterations closely related to risk factors, pathologic features, and prognosis. This proliferation class is associated with HBV infection and has a poor clinical outcome. These are also subdivided into several molecular subclasses based on transcriptional profile, as discussed in extensively in several articles [11–16]. This classification would allow identification of predictive biomarkers and facilitate targeted treatments.
The pathogenesis of HCC is a multistep process involving progressive accumulation of genetic alterations in distinct oncogenes. Progression of HCC begins with chronic inflammation caused by viral (HBV, HCV) or non-viral factors that are known to alter the liver microenvironment. As a result, the liver cells increase the production of cytokines, reactive oxygen and nitrogen species that mediate liver injury and trigger the liver's regenerative response. This regeneration predisposes the hepatocyte cells to a variety of genetic alterations at the genomic and transcriptional levels leading to HCC [17–20]. The most commonly altered genetic variants in HCC include mutations of the TERT promoter, CTNNB1, TP53, RB1, CCNA2, CCNE1, PTEN, ARID1A, ARID2, RPS6KA3 or NFE2L2, CCND1, FGF19, VEGFA, MYC and MET [21, 22], leading to changes in TERT, Wnt/β-catenin, p53/p21 and RB1, AKT-mTOR, RAS-MAPK, VEGF/VEGFR, MET, IGF, ARID1A/ARID1B/ARID2 pathways. Understanding the pathways and genes in HCC plays an important role in cancer prognosis and prediction of treatment success through stratification of cases for personalized treatment.
Despite some recent success in the development of new drugs approved for treatment of advanced HCC [23], several clinical trials have failed or shown only modest improvement in overall survival [23]. Further novel drug combinations and treatment regimens with first- and second-line drugs are currently under investigation [24]. As HCC is clinically and molecularly very heterogeneous, the use of biomarkers may guide personalized treatment strategies in clinical trials, leading to more favourable therapeutic outcomes. Recent developments in biomarker-driven therapies show some promise. To identify biomarkers associated with heterogeneous HCC tumours, several studies and multiple insilico computational bioinformatics analysis [25–29] using publicly available high-throughput HCC datasets have identified critical genes and pathways [17–20]. Some of the recent markers identified in the studies include; Cell division cycle-associated protein-3 (CDCA3) [30], ribophorins (RPNs), ARID1A [31]. These molecular markers of HCC enable to predict prognosis and to develop a new rationale for targeted therapeutic strategies. To date, several specific markers and key pathways involved in the HCC development have been identified potentially to assist early diagnosis, to predict prognosis and molecular targeted therapies of HCC tumours have been extensively reviewed. Recent developments in biomarker-driven therapies show some promises have been reviewed [32, 33]. For example, in a phase Ib/II HCC trial, patients with high MET mRNA expression showed a threefold increase in progression-free survival compared to patients with low c-MET expression when given a combination of the anti-VEGFR-2 mAb ramucirumab plus the anti-MET mAb emibetuzumab [34]. The gene expression profiles are used to identify differentially expressed genes (DEGs) during cancer progression [35, 36], which enables the identification of biomarkers for the prognosis, diagnosis, and targeted therapy of tumours [37].
To understand the heterogeneity of HCC and identify appropriate molecular markers, the present study examined a panel of genes including HCC hub, drug target, and somatic mutation genes with the potential to serve as meaningful biomarkers in prognosis and prediction of therapeutic success.
Materials and methods
Study population
The clinical and diagnostic characteristics of the study group are summarized in Table 1. To determine gene expression, pairs of HCC tumour (T) and adjacent non-tumour (NT) tissue specimens were obtained from 30 HCC patients who underwent surgery at the Vietnam National Cancer Hospital, Tan Trieu, Ha Dong in Hanoi in 2018. All patients were negative for anti-HCV and anti-HIV antibodies, nor had they a history of drug abuse. Liver function tests including ALT, AST, total bilirubin and direct bilirubin, albumin and prothrombin were quantified. HCC patients were categorized according to the BCLC staging system [38, 39]. All blood and T and NT tissue samples were stored at − 80 °C until further use.
Table 1.
Baseline characteristics of 30 hepatocellular carcinoma (HCC) patients
| Characteristics | n (%) |
|---|---|
| Age (years) | |
| < 40 | 3 (10) |
| 40–60 | 17 (57) |
| > 60 | 10 (33) |
| Gender | |
| Male | 25 (83) |
| Female | 5 (17) |
| Aetiologies | |
| Hepatitis B virus (HBV) | 26 (87) |
| Non-HBV/HCV | 4 (13) |
| Barcelona Clinic Liver Cancer (BCLC) classification | |
| Stage A | 1 (3) |
| Stage B | 29 (97) |
| Metastasis | |
| Yes | 2 (7) |
| No | 28 (93) |
| Size of tumor (cm) | |
| < 3 | 1 (3) |
| 3–5 | 14 (47) |
| ≥ 5 | 15 (50) |
| Number of tumors | |
| 1 | 23 (77) |
| 2 | 4 (13) |
| ≥ 3 | 3 (10) |
| Clinical parameters | Median (range) |
| Alpha Fetoprotein (ng/mL) | 20 [2–19,724] |
| Carbohydrate Antigen 19-9 (U/L) | 3 [0.7–13] |
| Carcinoembryonic Antigen (ng/mL) | 12 [0.6–104] |
| HBV-DNA | NA |
| White blood cells (× 103/mL) | 7 [4–46] |
| Red blood cells (× 106/mL) | 5 [3–8] |
| Platelets (× 103/mL) | 185 [2–391] |
| Aspartate amino transferase (IU/mL) | 37 [11–258] |
| Alanine amino transferase (IU/mL) | 67 [17–242] |
| Total Bilirubin (µmol/L) | 12 [5–72] |
| Direct Bilirubin (µmol/L) | 4 [2–49] |
| Prothrombin (% of standard) | 94 [39–115] |
| Protein (g/L) | 75 [63–84] |
| Albumin (g/L) | 41 [5–49] |
IU International unit, NA Not analysed
Serum levels assessment of AFP, CEA and CA 19-9
Serum levels of the markers AFP, CEA, and CA19-9 were measured using the ARCHITECT AFP reagent kit, Cat. No. 03P3625 (Abbott Ireland Diagnostics, Ireland), ARCHITECT CEA reagent kit, Cat. No. 07K6832 (Abbott Ireland Diagnostics, Ireland), and ARCHITECT CA 19-9XR Reagent Kit, Cat. No. 2K91 (Abbott GMBH & Co. Germany) respectively on the ARCHITECT i2000sr—an automated immunoassay analyser (Abbott Diagnostics, Abbott Park, IL, USA) according to the manufacturer's protocol.
Genes selection criteria for the qPCR panel
The custom qPCR panel included 20 genes (Table 2) retrieved from scientific literature with biomarker potential for diagnosis, prognosis, and prediction of treatment outcomes. Specifically, genes belonging to the categories of HCC hub genes (prognosis and diagnostic potential), drug target genes and somatic mutated genes were considered. Another inclusion criterion was that we considered genes whose expression was significantly upregulated in at least 30–40% of HCCs (www.proteinatlas.org).
Table 2.
Hepatocellular carcinoma qPCR panel genes: diagnosis, prognosis, and treatment prediction
| Genes | Pathway | Criteria for selection | Source |
|---|---|---|---|
| HCC hub genes | |||
| TOP2A (DNA topoisomerase 2-alpha) | Cell cycle | Poor prognosis and overall survival | [26, 27, 37, 40] |
| CDK1 (cyclin-dependent kinase 1) | Cell cycle | Poor prognosis and overall survival | [37] |
| BIRC5 (baculoviral IAP repeat-containing 5) | Cell cycle | Poor prognosis and overall survival | [41] |
| CDC20 (cell division cycle protein 20 homolog) | Cell cycle | Poor prognosis and overall survival | [26, 37] |
| Drug target genes | |||
| GPC3 (glypican-3) | Extracellular molecule | Diagnostic marker and drug target | [42, 43] |
| IGF2 (insulin-like growth factor 2) | AKT/mTOR; RAS/MAPK | Drug target | [44, 45] |
| c-MET (c-MET proto-oncogene, receptor tyrosine kinase) | AKT/mTOR; RAS/MAPK | Drug target | [44, 46] |
| IDH1 (Isocitrate dehydrogenase 1) | TCA cycle | Drug target | [47] |
| MCL1 (myeloid cell leukemia-1) | Apoptotic | Drug target | [48] |
| MDM4 (MDM4 regulator of P53) | P53 | Drug target | [49] |
| RAF (proto-oncogene c-RAF) | Ras/RAF | Drug target | [50] |
| VEGFA (vascular endothelial growth factor A) | VEGF | Drug target | [51] |
| PD-L1 (programmed cell death ligand 1) | Immune checkpoint | Drug target | [44] |
| Somatic mutated and/ drug target genes | |||
| CTNNB1 (catenin beta 1) [GLUL1 (glutamine synthetase) and LGR5 (G-protein–coupled receptor)] | Wnt/ß-catenin | Somatic mutations, drug target and overall survival | [52–54] |
| TERT (telomerase reverse transcriptase) | Telomere maintenance | Somatic mutations, drug target and overall survival | [52, 55] |
| ARID1A (AT-rich interactive domain-containing protein 1A) | Epigenetic modifier | Somatic mutations | [52, 56] |
| TP53 (tumor protein p53) | Cell cycle | Somatic mutations | [52, 57] |
| Housekeeping and marker genes | |||
| AFP (α-fetoprotein) | NA | HCC serum diagnostic marker | NA |
| GAPDH (glyceraldehyde 3-phosphate dehydrogenase), ACTB (actin beta) and TBP (TATA-binding protein) | NA | Housekeeping genes | NA |
NA Not applicable
RNA isolation and real-time qPCR
Total RNA was extracted from approximately 5 mm3 of HCC tumor or adjacent normal liver tissue using 1 mL TRIzol™ reagent (Thermo Fisher Scientific, Waltham, MA, USA). Next, the tissues were homogenized with a Dounce homogenizer, and RNA was isolated according to the manufacturer’s instructions. The RNA pellet was resuspended in 30–50 µL of RNA-free water, depending on the pellet's size. Next, quality and quantity were assessed using a Nanodrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). For cDNA synthesis, one microgram of total RNA from each sample was reverse transcribed in 20 µL reaction using the RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. The cDNA was diluted to 1:10 with nuclease-free water and subjected to quantitative real-time PCR.
The quantitative real-time PCR was performed with the LightCycler 480 Instrument II (Roche, Basel, Switzerland) in 96 well plates using a 10 µL final volume containing 0.3 µmol/L of forward and reverse primers, 2 µL of diluted cDNA and 1X SensiFAST™ SYBR® No-ROX Kit (Bioline, Memphis, TN, USA). PCR cycle parameters were initial denaturation at 94 °C for 2 min, 40 cycles at 95 °C for 10 s, and 60 °C for 25 s, with the fluorescence signal acquired at the end of each cycle. A melting curve analysis was performed at the end of the amplification. All 23 genes (20 genes under investigation plus 3 housekeeping genes) were quantified in duplicates in 96 well plates having a couple of non-template reactions with ACTB primers. The primer sequences used are given in the Additional file 1: Table S1.
Data analysis
The fold change gene expression between the tumour (T) and adjacent non-tumour (NT) pair was determined using the relative quantification 2−∆∆CT method [58]. The fold change with ± two-fold to the transcript levels between T and adjacent NT was considered differentially regulated. For having a better control of the results, mean cycle threshold (Ct) of the three reference genes was taken for the normalization: Ct [ref] = mean (Ct [GAPDH], Ct [ACTB], Ct [TBP]). Correlations between genes were measured using Pearson correlation (r) and differences between groups were determined by either two-tailed t-test or ANOVA. The gene expression heatmap was generated using the Cluster v3.0 software (http://bonsai.hgc.jp/~mdehoon/software/cluster/software.html) [59]. The normalized gene-expression profiles of 30 samples were subjected to unsupervised hierarchical clustering for similarities in expression data. Hierarchical clustering was applied to both rows and columns. The output file was visualized in JavaTreeview (https://sourceforge.net/projects/jtreeview/) [60].
Results
Patients baseline characteristics and blood indices
Paired samples of T and NT tissues from 29 patients with BCLC-B classification and one patient with BCLC-A classification were analysed for the expression of the genes under investigation. The baseline characteristics of the patients are summarized in Table 1. Ninety percent were > 40 years of age at the time of HCC diagnosis, and the mean age of study participants was 57 ± 12 years; 83% were men. Chronic HBV infection was the predominant incidental factor of HCC development (87%). The remaining cases (13%) were due to unknown non-HBV and non-HCV factors. Among other risk factors, liver cirrhosis was found in 23% of patients, and smoking/alcohol consumption was observed in 57% of patients. Tumours in the right liver lobe (67%) were more frequent than in the left liver lobe (33%). Large (≥ 5 cm) tumours were detected in 50% of the cohort, an intermediate size (3–5 cm) in 47% and one patient (3%) had a tumour of < 3 cm diameter. Blood tests showed elevated ALT (> 40 U/L) and AST (> 56 U/L) in 30% and 13% of patients respectively, elevated total (> 17 µmol/L) and direct bilirubin (> 5 µmol/L) levels in 17% and lowered platelet counts (< 150 × 103/mL) in 20% of the cohort.
Identification of molecular HCC subtypes by gene expression profiling
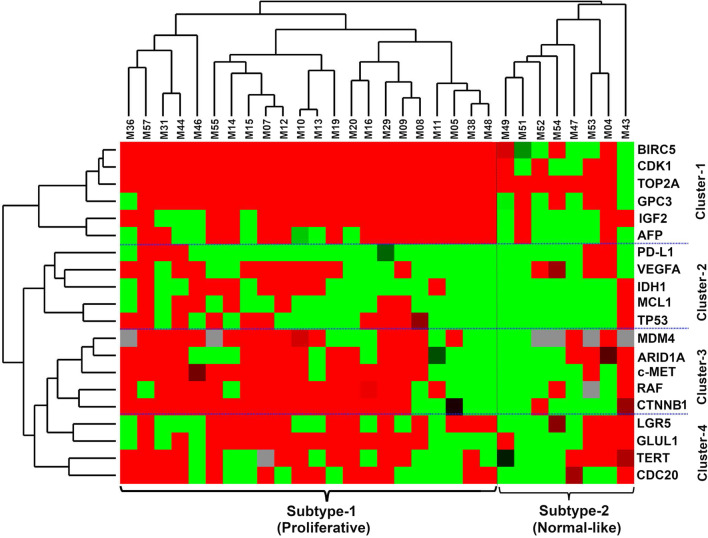
Expression patterns of 20 genes were analysed in 30 paired tumour and non-tumour (T and NT) samples by RT-qPCR. Unsupervised hierarchical clustering using Manhattan distance and complete linkage revealed two subtypes of tumours (Fig. 1). Of the two subtypes, subtype-1 (proliferative) showed higher expression of genes compared to subtype-2 (normal-like). The proliferative subtype included 22 tumour samples compared eight for the normal-like subtypes.
Fig. 1.
Heatmap shows gene expression Z-scores of differentially expressed mRNA of 20 genes in 30 paired HCC tumour samples. Clustering shows two subtypes of tumours (proliferative and normal-like) and four clusters of genes (Cluster-1 to Cluster-4)
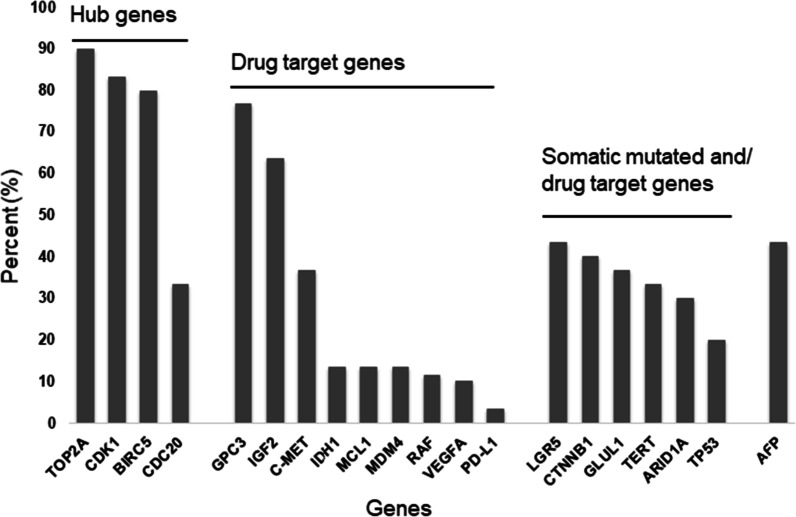
Clustering of the genes revealed four clusters. The hub genes TOP2A, CDK1, and BIRC5 formed the top cluster with expression in 80–90% of the tumours. In addition to the hub genes, GPC3, IGF2, and AFP also showed uniform expression in both the proliferative, but not in the normal-like subtype. Expression of AFP showed a good correlation with the corresponding AFP serum levels (r:0.63, p < 0.001). The second cluster comprised of the PD-L1, VEGFA, IDH1, MCL1 and TP53 genes, which were diffusely upregulated in the proliferative subtype. The third cluster contained MDM4, ARID1A, c-MET, RAF, and CTNNB1, which were strongly upregulated in the proliferative subtype. The last cluster consisted of LGR5, GLUL1, TERT and CDC20, which were diffusely expressed in all subtypes. The bar graph represents the percentage of tumours from the highest to the lowest number of hub genes, drug target genes, and common somatic mutant genes that had > twofold gene expression (Fig. 2).
Fig. 2.
Percentage of HCC tumours in which gene expression levels of the 20 genes were > twofold overexpressed between tumour and non-tumour pairs
Correlation between genes
The expression profiles of genes in the first cluster across all tumours (TOP2A, CDK1, BIRC5, GPC3, IGF2 and AFP) were strongly correlated. The correlations between TOP2A and CDK1 (r:0.77, p < 0.0001), CDK1 and BIRC5 (r;0.77, p < 0.0001) and TOP2A and BIRC5 (r:0.65, p < 0.0001) were significant. The correlations between GPC3 and IGF2 (r:0.61, p < 0.0001) and between GPC3 and AFP (r:0.78, p < 0.00001) and between GPC3 and IGF2 (r:0.55, p < 0.0001) were also significant.
The correlation between TOP2A and CDK1 remained significant in both the tumour subtypes (r:0.58, p:0.004; r:0.79, p:0.02 in proliferative, and normal-like subtypes, respectively). The correlation between TOP2A and BIRC5 (r:0.57, p:0.006), TOP2A and GPC3 (r:0.48, p:0.02) was significant in the proliferative, but not in the normal-like subtype. Expression data for MDM4 were available for 24 of the 30 tumours and its correlation with TP53 was significant in proliferative (r:0.52, p:0.04), but not in normal-like (r:0.36, p:0.5). In the second gene cluster (IDH1, VEGFA, MCL1, PD-L1, TP53), statistically significant correlations were observed in proliferative subtype for VEGFA and IDH1 (r:0.42, p:0.05), VEGFA and MCL1 (r:0.43. p:0.05), VEGFA and TP53 (r:0.52, p:0.01), MCL1 and IDH1 (r:0.57, p < 0.01), PD-l1 and TP53 (r:0.57, p < 0.01). In the normal-like subtype, correlations were weak between genes and not statistically significant. In the third cluster, the correlations between c-MET and ARID1A (r:0.74, p < 0.01), c-MET and CTNNB1 (r:0.71, p < 0.01), CTNNB1 and ARID1A (r:0.51, p:0.03) and LGR5 and GLUL1 (r: 0.69, p:0.02) were only significant for the proliferative, but not in normal-like subtype. The correlation of TERT with CDC20 was significant in both the subtypes (r:0.60, p < 0.01; r:0.80, p:0.02 in proliferative and normal-like subtypes, respectively).
Comparison of tumour subtypes
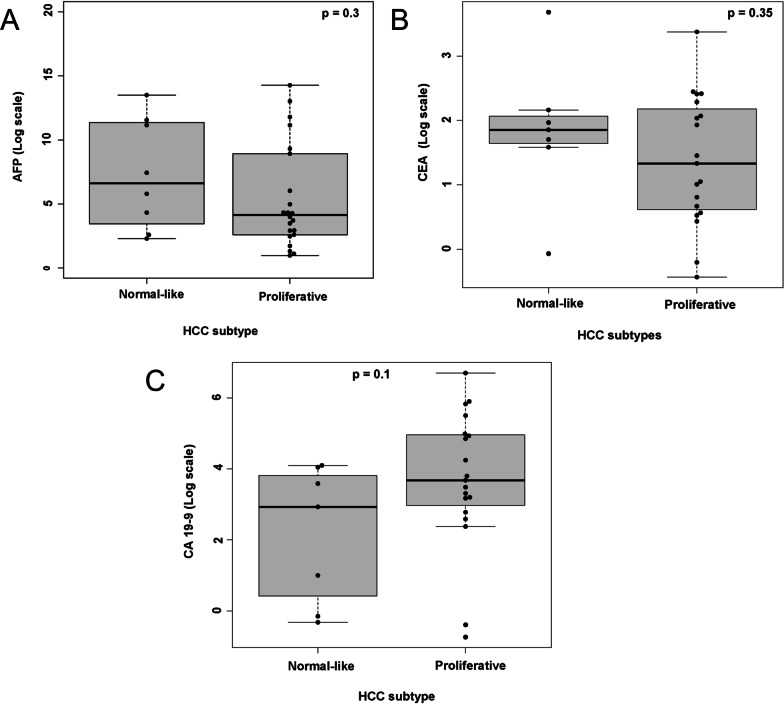
The tumour sizes and serum levels of AFP, CEA and CA19-9 were compared between the proliferative and normal-like subtypes. Comparison of tumour sizes showed that the normal-like subtype showed larger tumour sizes in the normal-like (median [IQR]: 6.5 cm [3.8–8.5]) compared to the proliferative (4.8 cm [3–7]) and the difference was not statistically significant (t-test, two-tailed p:0.19).
Comparison of serum AFP levels revealed higher levels in the normal-like subtype (median 114 ng/ml [16–2462]; (log2-transformed median 6.6 [7–11]) compared with the proliferative (median 16 ng/ml [6–486]; (log2-transformed median 4.1 [3–8])). No statistical difference in serum AFP levels was observed between the groups (p:0.30) (Fig. 3A). The normal-type subtype had higher levels of the serum marker CEA (median 3.6 ng/ml [3–4]; (log2-transformed median 1.8 [1.6–2])) compared to the proliferative subtype (median 2.3 ng/ml [1.5–4]; (log2-transformed median 1.3 [0.6–2])), although the difference between groups was not statistically significant (p:0.35) (Fig. 3B). The proliferative subtype showed higher serum CA19-9 levels (median 13.4 U/L [9–31]; (log2-transformed median 3.7 [3–5])) compared with normal-like (median 7.6 U/L [1.5–14]; (log2-transformed median 2.9 [0.4–4])), but no statistical difference was found between groups (p:0.1) (Fig. 3C).
Fig. 3.
Comparison of serum AFP, CEA and CA19-9 levels (log-transformed) between proliferative and normal-like hierarchical subtypes
Discussion
The intratumoural heterogeneity among the BCLC-B patients is a major impediment in effective HCC therapy and renders long-term prognoses difficult and largely unreliable. The expression profile of key mutated genes and molecular classification of HCC and a subclassification for BCLC-B into four stages (B1-B4) has been proposed for better patient management. However, contradictory results from validation studies have shown a greater need of improved scoring systems and clinically relevant biomarkers [61–65]. To address the issue of biomarkers, our study investigated the expression pattern of important genes in HCC tumours and assessed their utility as biomarkers.
By using hierarchical clustering of gene expression data our study revealed two tumour subtypes: a proliferative and normal-like subtype. The proliferative subtype showed mutually exclusive expression patterns, except for the hub genes GPC3, IGF2 and AFP. This observation is in agreement with findings published previously [44, 66, 67]. The proliferative subtype accounts for 50% of HCC cases and is associated with a highly aggressive phenotype and poor outcome [8]. In our data, the proliferative subtype showed higher expression of the c-MET, ARID1A, CTNNB1, RAF1, MADM4, LGR5, GLUL1, TERT and CDC20 genes. Based on the expression profiles it is plausible that the RAS/MAPK, MET, AKT/mTOR and liver-Wnt pathways are responsible for cellular plasticity in the proliferative subtype [8, 44]. The normal-like subtype consisted of eight tumours and showed a scattered expression of hub genes (except TOP2A), drug-target genes and somatically mutated genes without any pattern, perhaps an indication that tissue analysed was mixed with normal tissue.
The higher expression of the hub genes TOP2A, CDK1 and BIRC5 in all samples indicates active cell division and proliferation. The selection of hub genes for the present study was based on their strong association with poor prognosis and overall survival in HCC patients [26, 27, 37, 40]. The strong correlation of TOP2A with CDK1 and BIRC5 as well as an earlier report showed that TOP2A could substitute other hub genes to be a good biomarker [37]. Moreover, TOP2A is associated with tumour grades, HBV infection, and vascular invasion [29]. In addition to expression of the hub genes, we noticed that GPC3 and IGF2 were expressed in 65–75% and AFP in more than 40% of the tumour samples. Our findings of GPC3 expression in 75% of HCC cases confirms earlier reports of its utility as a biomarker [42, 68]. GPC3 enhances cell proliferation through the Wnt/β-catenin pathway and is associated with a poor prognosis [42, 68]. IGF-2 expression is almost absent in adult hepatic cells, but its upregulation in HCC has been attributed to epigenetic mechanisms [69]. GPC3 and IGF-2 are potential drug targets that have significantly reduced tumour growth and prolonged survival in Phase 1 clinical trials and in animal models respectively [70, 71]. Overall, we show that, in addition to HCC hub genes, GPC3 and IGF-2 may also serve as drug targets and early diagnostic markers for HCC.
The proliferative subtype in our study was characterised by high expression of the c-MET, ARID1A, CTNNB1, RAF1, MDM4, LGR5 and GLUL1 genes. Of these, c-MET along with hepatocyte growth factor (HGF) activates RAS-ERK and PI3K-AKT pathways, strengthening tumour aggressiveness with poor prognosis [72–74]. ARID1A has a context-dependent oncogenic and tumour suppressive function in HCC. A higher expression as seen in our study indicates oncogenic activity, supposedly mediated through cytochrome P450 and oxidative stress [75]. RAF1 is a proto-oncogene that encodes MAP3 kinase and triggers cell proliferation through the ERK signalling pathway. Higher expression of RAF1 is associated with resistance to sorafenib, a tyrosine-kinase inhibitor and a standard drug for the treatment of advanced HCC [76]. CTNNB1 encodes beta-catenin which is essential for the canonical Wnt pathway; higher expression of this gene in HCC is mediated through exonic mutations and epigenetic factors [77, 78]. LGR5 encodes a member of the G-protein coupled receptor superfamily and is believed to promote HCC metastasis formation by inducing epithelial-mesenchymal transition. GLUL1 catalyses the synthesis of glutamine from glutamate and ammonium in the liver tissue and is tightly controlled by Wnt/β-catenin signalling. Mutations in CTNNB1 activate the pathway leading to higher levels of GLUL1 and LGR5 [79, 80]. Overexpression of GLUL1 sensitizes HCCs to sorafenib, indicating the relevance of GLUL1 as a potential biomarker for the stratification of patients regarding treatment with sorafenib [81]. The other two genes that showed expression in proliferative subtype tumours are TERT and CDC20. TERT is responsible for maintaining telomere length in tumours and re-activated in HCCs primarily through mutations in promoter region [82]. Studies have shown a good concordance between mutations in CTNNB1 and the TERT promoter in HCCs, indicating that inhibitors targeting Wnt/β-catenin and TERT could be beneficial in HCC therapy [83]. CDC20 regulates cell division through its interaction with anaphase-promoting complex/cyclosome (APC/C) and its overexpression is associated with poor prognosis [84].
Serum AFP, CA19-9, and CEA are used as preoperative tumour markers [85]. Especially, AFP is commonly used in clinical practice to diagnose HCC and various tumours [86–88]. In combination with AFP, serum markers CA19-9 and CEA are being used to improve the diagnostic and prognostic performance of HCC patients. In this study, comparing serum AFP levels between the tumour subtypes showed that the normal-like subtype showed trend with higher AFP serum levels compared to the proliferative. However, it was statistically insignificant. This contrasts with published results, namely that the proliferative subtype has higher AFP levels [44]. Interestingly, we observed a statistically significant positive correlation between AFP expression and AFP serum levels across all subtypes. Higher AFP levels promote HCC cell growth by activating the NF-κB pathway, in addition to suppressing the Fas/FADD-mediated apoptotic pathway [89, 90]. Higher AFP levels showed an association with higher metastasising activities and post-operative recurrence rates. In addition, we did not detect significant differences in serum levels of CA19-9 and CEA between proliferative and normal HCC subtypes.
Conclusion
Taken together our study has shown two main subtypes in BCLC-B classified tumours. We have demonstrated that a molecular classification of HCC can be achieved through a gene panel using RT-PCR. This approach enables patient stratification based on gene expression profiles for targeted personalized treatment. The genes c-MET, ARID1A, CTNNB1 and RAF1 showing an association with the proliferative subtype in our study may be used as molecular markers for subtype determination, and hub genes can be applied for HCC diagnosis. However, large prospective, well-designed follow-up studies are required to evaluate these marker genes for clinical applications. Besides being a retrospective study, with a rather small sample size our major limitation has been lacking longitudinal follow-ups of patients for a comprehensive survival analysis.
Supplementary Information
Additional file 1. Table S1. List of genes and primers.
Acknowledgements
We thank all study subjects for their participation. The authors acknowledge Mr. Bui Dinh Tung, and Ms. Bui Thuy Linh for their experimental support.
Abbreviations
- HCC
Hepatocellular carcinoma
- HBV
Hepatitis B virus
- HCV
Hepatitis C virus
- RT-qPCR
Real-time quantitative PCR
- BCLC
Barcelona Clinic Liver Cancer
- T
Tumour
- NT
Non-tumour
- ALT
Alanine transaminase
- AST
Aspartate transaminase
- AFP
Alpha fetoprotein
- CEA
Carbohydrate antigen 19-9
- CA 19-9
Carcinoembryonic antigen
- HGF
Hepatocyte growth factor
- ANOVA
Analysis of variance
Author contributions
SRP, NXH, and TPV designed the study and the qPCR panel. HVT and NXH recruited patients and collected samples. SRP, LTKL and DPG performed the experiments. NXH, SR and SRP performed the statistical analyses and interpreted data. SRP, SR, and NXH wrote the first draft. TPV, CGM and PGK revised the manuscript. NXH, TPV, LHS, MHB, NLT contributed to materials and reagents. All authors agreed with the results and conclusions. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. NXH acknowledges a Grant from the Vietnam National Foundation for Science and Technology Development (NAFOSTED, Grant Number: 108.02-2018.315). TPV acknowledges Grants from the German Federal Ministry of Education and Research (BMBF01DP19006A, BMBF01DP17047).
Availability of data and materials
The datasets generated and/or analysed during the current study are available in the Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/), [GEO Submission (GSE191298)].
Declarations
Ethics approval and consent to participate
The study was approved by the institutional review board of the 108 Military Central Hospital and the 103 Military Hospital of the Vietnam Military Medical University, Hanoi, Vietnam. All experiments were performed in accordance with relevant guidelines and regulations. Informed written consent was obtained from the participants in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Srinivas Reddy Pallerla and Nghiem Xuan Hoan are contributed equally to this work.
Le Huu Song and Thirumalaisamy P. Velavan shared senior authors.
Contributor Information
Srinivas Reddy Pallerla, Email: srinivas-reddy.pallerla@uni-tuebingen.de.
Nghiem Xuan Hoan, Email: nghiemxuanhoan@108-icid.com.
References
- 1.Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Pineros M, Znaor A, Bray F. Cancer statistics for the year 2020: an overview. Int J Cancer. 2021;149:778–789. doi: 10.1002/ijc.33588. [DOI] [PubMed] [Google Scholar]
- 2.Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, Kudo M, Johnson P, Wagner S, Orsini LS, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35(9):2155–2166. doi: 10.1111/liv.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nguyen VT. Hepatitis B infection in Vietnam: current issues and future challenges. Asia Pac J Public Health. 2012;24(2):361–373. doi: 10.1177/1010539510385220. [DOI] [PubMed] [Google Scholar]
- 5.Park JW, Kim YJ, Kim DY, Bae SH, Paik SW, Lee YJ, Kim HY, Lee HC, Han SY, Cheong JY, et al. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: the phase III STAH trial. J Hepatol. 2019;70(4):684–691. doi: 10.1016/j.jhep.2018.11.029. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. doi: 10.1038/nrdp.2016.18. [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(3):329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 8.Barcena-Varela M, Lujambio A. The endless sources of hepatocellular carcinoma heterogeneity. Cancers (Basel) 2021;13(11):2621. doi: 10.3390/cancers13112621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raja A, Haq F. Molecular classification of hepatocellular carcinoma: prognostic importance and clinical applications. J Cancer Res Clin Oncol. 2022;148(1):15–29. doi: 10.1007/s00432-021-03826-w. [DOI] [PubMed] [Google Scholar]
- 10.Calderaro J, Ziol M, Paradis V, Zucman-Rossi J. Molecular and histological correlations in liver cancer. J Hepatol. 2019;71(3):616–630. doi: 10.1016/j.jhep.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Rebouissou S, Nault JC. Advances in molecular classification and precision oncology in hepatocellular carcinoma. J Hepatol. 2020;72(2):215–229. doi: 10.1016/j.jhep.2019.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Lee JS, Heo J, Libbrecht L, Chu IS, Kaposi-Novak P, Calvisi DF, Mikaelyan A, Roberts LR, Demetris AJ, Sun Z, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006;12(4):410–416. doi: 10.1038/nm1377. [DOI] [PubMed] [Google Scholar]
- 13.Boyault S, Rickman DS, de Reynies A, Balabaud C, Rebouissou S, Jeannot E, Herault A, Saric J, Belghiti J, Franco D, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology. 2007;45(1):42–52. doi: 10.1002/hep.21467. [DOI] [PubMed] [Google Scholar]
- 14.Chiang DY, Villanueva A, Hoshida Y, Peix J, Newell P, Minguez B, LeBlanc AC, Donovan DJ, Thung SN, Sole M, et al. Focal gains of VEGFA and molecular classification of hepatocellular carcinoma. Cancer Res. 2008;68(16):6779–6788. doi: 10.1158/0008-5472.CAN-08-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoshida Y, Nijman SM, Kobayashi M, Chan JA, Brunet JP, Chiang DY, Villanueva A, Newell P, Ikeda K, Hashimoto M, et al. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009;69(18):7385–7392. doi: 10.1158/0008-5472.CAN-09-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cancer Genome Atlas Research Network Electronic address wbe, Cancer Genome Atlas Research N: Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell. 2017;169(7):1327–1341. doi: 10.1016/j.cell.2017.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar M, Zhao X, Wang XW. Molecular carcinogenesis of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: one step closer to personalized medicine? Cell Biosci. 2011;1(1):5. doi: 10.1186/2045-3701-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31(4):339–346. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- 19.Hussain SP, Schwank J, Staib F, Wang XW, Harris CC. TP53 mutations and hepatocellular carcinoma: insights into the etiology and pathogenesis of liver cancer. Oncogene. 2007;26(15):2166–2176. doi: 10.1038/sj.onc.1210279. [DOI] [PubMed] [Google Scholar]
- 20.Yeh SH, Chen PJ, Shau WY, Chen YW, Lee PH, Chen JT, Chen DS. Chromosomal allelic imbalance evolving from liver cirrhosis to hepatocellular carcinoma. Gastroenterology. 2001;121(3):699–709. doi: 10.1053/gast.2001.27211. [DOI] [PubMed] [Google Scholar]
- 21.Dhanasekaran R, Nault JC, Roberts LR, Zucman-Rossi J. Genomic medicine and implications for hepatocellular carcinoma prevention and therapy. Gastroenterology. 2019;156(2):492–509. doi: 10.1053/j.gastro.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tornesello ML, Buonaguro L, Izzo F, Buonaguro FM. Molecular alterations in hepatocellular carcinoma associated with hepatitis B and hepatitis C infections. Oncotarget. 2016;7(18):25087–25102. doi: 10.18632/oncotarget.7837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Llovet JM, Montal R, Villanueva A. Randomized trials and endpoints in advanced HCC: role of PFS as a surrogate of survival. J Hepatol. 2019;70(6):1262–1277. doi: 10.1016/j.jhep.2019.01.028. [DOI] [PubMed] [Google Scholar]
- 24.Huang A, Yang XR, Chung WY, Dennison AR, Zhou J. Targeted therapy for hepatocellular carcinoma. Signal Transduct Target Ther. 2020;5(1):146. doi: 10.1038/s41392-020-00264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoon JK, Choi JY, Rhee H, Park YN. MRI features of histologic subtypes of hepatocellular carcinoma: correlation with histologic, genetic, and molecular biologic classification. Eur Radiol. 2022;32(8):5119–5133. doi: 10.1007/s00330-022-08643-4. [DOI] [PubMed] [Google Scholar]
- 26.Chen Z, Chen J, Huang X, Wu Y, Huang K, Xu W, Xie L, Zhang X, Liu H. Identification of potential key genes for hepatitis B virus-associated hepatocellular carcinoma by bioinformatics analysis. J Comput Biol. 2019;26(5):485–494. doi: 10.1089/cmb.2018.0244. [DOI] [PubMed] [Google Scholar]
- 27.Li N, Li L, Chen Y. The identification of core gene expression signature in hepatocellular carcinoma. Oxid Med Cell Longev. 2018;2018:3478305. doi: 10.1155/2018/3478305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao X, Wang X, Zhang S. Bioinformatics identification of crucial genes and pathways associated with hepatocellular carcinoma. Biosci Rep. 2018;38(6). [DOI] [PMC free article] [PubMed]
- 29.Li L, Lei Q, Zhang S, Kong L, Qin B. Screening and identification of key biomarkers in hepatocellular carcinoma: evidence from bioinformatic analysis. Oncol Rep. 2017;38(5):2607–2618. doi: 10.3892/or.2017.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Chen S, Wang G, Li S, Qin X. CDCA3 is a novel prognostic biomarker associated with immune infiltration in hepatocellular carcinoma. Biomed Res Int. 2021;2021:6622437. doi: 10.1155/2021/6622437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng Y, Tang X, Li C, Su Y, Wang X, Li N, Zhang A, Jiang F, Wu C. ARID1A is a prognostic biomarker and associated with immune infiltrates in hepatocellular carcinoma. Can J Gastroenterol Hepatol. 2022;2022:3163955. doi: 10.1155/2022/3163955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altundag O. Recent advances in systemic therapy for hepatocellular carcinoma. Exp Clin Transpl. 2022. [DOI] [PubMed]
- 33.Cucarull B, Tutusaus A, Rider P, Hernaez-Alsina T, Cuno C, Garcia de Frutos P, Colell A, Mari M, Morales A. Hepatocellular carcinoma: molecular pathogenesis and therapeutic advances. Cancers (Basel) 2022;14(3):621. doi: 10.3390/cancers14030621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harding JJ, Zhu AX, Bauer TM, Choueiri TK, Drilon A, Voss MH, Fuchs CS, Abou-Alfa GK, Wijayawardana SR, Wang XA, et al. A phase Ib/II study of ramucirumab in combination with emibetuzumab in patients with advanced cancer. Clin Cancer Res. 2019;25(17):5202–5211. doi: 10.1158/1078-0432.CCR-18-4010. [DOI] [PubMed] [Google Scholar]
- 35.Shi YX, Zhu T, Zou T, Zhuo W, Chen YX, Huang MS, Zheng W, Wang CJ, Li X, Mao XY, et al. Prognostic and predictive values of CDK1 and MAD2L1 in lung adenocarcinoma. Oncotarget. 2016;7(51):85235–85243. doi: 10.18632/oncotarget.13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang B, Li C, Zhao J. Identification of key pathways and genes in colorectal cancer using bioinformatics analysis. Med Oncol. 2016;33(10):111. doi: 10.1007/s12032-016-0829-6. [DOI] [PubMed] [Google Scholar]
- 37.Zhu Q, Sun Y, Zhou Q, He Q, Qian H. Identification of key genes and pathways by bioinformatics analysis with TCGA RNA sequencing data in hepatocellular carcinoma. Mol Clin Oncol. 2018;9(6):597–606. doi: 10.3892/mco.2018.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diaz-Gonzalez A, Reig M, Bruix J. Treatment of hepatocellular carcinoma. Dig Dis. 2016;34(5):597–602. doi: 10.1159/000445275. [DOI] [PubMed] [Google Scholar]
- 39.Cillo U, Vitale A, Grigoletto F, Farinati F, Brolese A, Zanus G, Neri D, Boccagni P, Srsen N, D’Amico F, et al. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J Hepatol. 2006;44(4):723–731. doi: 10.1016/j.jhep.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 40.Wei Z, Liu Y, Qiao S, Li X, Li Q, Zhao J, Hu J, Wei Z, Shan A, Sun X, et al. Identification of the potential therapeutic target gene UBE2C in human hepatocellular carcinoma: an investigation based on GEO and TCGA databases. Oncol Lett. 2019;17(6):5409–5418. doi: 10.3892/ol.2019.10232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang H, Zhang X, Cai XY, Wen DY, Ye ZH, Liang L, Zhang L, Wang HL, Chen G, Feng ZB. From big data to diagnosis and prognosis: gene expression signatures in liver hepatocellular carcinoma. PeerJ. 2017;5:e3089. doi: 10.7717/peerj.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo M, Zhang H, Zheng J, Liu Y. Glypican-3: A New Target for Diagnosis and Treatment of Hepatocellular Carcinoma. J Cancer. 2020;11(8):2008–2021. doi: 10.7150/jca.39972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neureiter D, Stintzing S, Kiesslich T, Ocker M. Hepatocellular carcinoma: therapeutic advances in signaling, epigenetic and immune targets. World J Gastroenterol. 2019;25(25):3136–3150. doi: 10.3748/wjg.v25.i25.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. Genetic Landscape and biomarkers of hepatocellular carcinoma. Gastroenterology. 2015;149(5):1226–1239. doi: 10.1053/j.gastro.2015.05.061. [DOI] [PubMed] [Google Scholar]
- 45.de Bono J, Lin CC, Chen LT, Corral J, Michalarea V, Rihawi K, Ong M, Lee JH, Hsu CH, Yang JC, et al. Two first-in-human studies of xentuzumab, a humanised insulin-like growth factor (IGF)-neutralising antibody, in patients with advanced solid tumours. Br J Cancer. 2020;122(9):1324–1332. doi: 10.1038/s41416-020-0774-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang H, Rao B, Lou J, Li J, Liu Z, Li A, Cui G, Ren Z, Yu Z. The function of the HGF/c-Met axis in hepatocellular carcinoma. Front Cell Dev Biol. 2020;8:55. doi: 10.3389/fcell.2020.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okoye-Okafor UC, Bartholdy B, Cartier J, Gao EN, Pietrak B, Rendina AR, Rominger C, Quinn C, Smallwood A, Wiggall KJ, et al. New IDH1 mutant inhibitors for treatment of acute myeloid leukemia. Nat Chem Biol. 2015;11(11):878–886. doi: 10.1038/nchembio.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hird AW, Tron AE. Recent advances in the development of Mcl-1 inhibitors for cancer therapy. Pharmacol Ther. 2019;198:59–67. doi: 10.1016/j.pharmthera.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 49.Jochemsen AG. Reactivation of p53 as therapeutic intervention for malignant melanoma. Curr Opin Oncol. 2014;26(1):114–119. doi: 10.1097/CCO.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 50.Adnane L, Trail PA, Taylor I, Wilhelm SM. Sorafenib (BAY 43-9006, Nexavar), a dual-action inhibitor that targets RAF/MEK/ERK pathway in tumor cells and tyrosine kinases VEGFR/PDGFR in tumor vasculature. Methods Enzymol. 2006;407:597–612. doi: 10.1016/S0076-6879(05)07047-3. [DOI] [PubMed] [Google Scholar]
- 51.Liu L, Qin S, Zheng Y, Han L, Zhang M, Luo N, Liu Z, Gu N, Gu X, Yin X. Molecular targeting of VEGF/VEGFR signaling by the anti-VEGF monoclonal antibody BD0801 inhibits the growth and induces apoptosis of human hepatocellular carcinoma cells in vitro and in vivo. Cancer Biol Ther. 2017;18(3):166–176. doi: 10.1080/15384047.2017.1282019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.An P, Xu J, Yu Y, Winkler CA. Host and viral genetic variation in HBV-related hepatocellular carcinoma. Front Genet. 2018;9:261. doi: 10.3389/fgene.2018.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang W, Skanderup AJ, Lee CG. Advances in genomic hepatocellular carcinoma research. Gigascience. 2018;7(11):giy135. doi: 10.1093/gigascience/giy135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ruden M, Puri N. Novel anticancer therapeutics targeting telomerase. Cancer Treat Rev. 2013;39(5):444–456. doi: 10.1016/j.ctrv.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 55.Pez F, Lopez A, Kim M, Wands JR, Caron de Fromentel C, Merle P. Wnt signaling and hepatocarcinogenesis: molecular targets for the development of innovative anticancer drugs. J Hepatol. 2013;59(5):1107–1117. doi: 10.1016/j.jhep.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 56.Niu ZS, Niu XJ, Wang WH. Genetic alterations in hepatocellular carcinoma: an update. World J Gastroenterol. 2016;22(41):9069–9095. doi: 10.3748/wjg.v22.i41.9069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schulze K, Nault JC, Villanueva A. Genetic profiling of hepatocellular carcinoma using next-generation sequencing. J Hepatol. 2016;65(5):1031–1042. doi: 10.1016/j.jhep.2016.05.035. [DOI] [PubMed] [Google Scholar]
- 58.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 59.de Hoon MJ, Imoto S, Nolan J, Miyano S. Open source clustering software. Bioinformatics. 2004;20(9):1453–1454. doi: 10.1093/bioinformatics/bth078. [DOI] [PubMed] [Google Scholar]
- 60.Saldanha AJ. Java Treeview—extensible visualization of microarray data. Bioinformatics. 2004;20(17):3246–3248. doi: 10.1093/bioinformatics/bth349. [DOI] [PubMed] [Google Scholar]
- 61.Muller L, Hahn F, Mahringer-Kunz A, Stoehr F, Gairing SJ, Foerster F, Weinmann A, Galle PR, Mittler J, Pinto Dos Santos D, et al. Immunonutritive scoring in patients with hepatocellular carcinoma undergoing transarterial chemoembolization: Prognostic nutritional index or controlling nutritional status score? Front Oncol. 2021;11:696183. doi: 10.3389/fonc.2021.696183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Golfieri R, Bargellini I, Spreafico C, Trevisani F. Patients with Barcelona Clinic Liver Cancer stages B and C hepatocellular carcinoma: time for a subclassification. Liver Cancer. 2019;8(2):78–91. doi: 10.1159/000489791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bolondi L, Burroughs A, Dufour JF, Galle PR, Mazzaferro V, Piscaglia F, Raoul JL, Sangro B. Heterogeneity of patients with intermediate (BCLC B) Hepatocellular Carcinoma: proposal for a subclassification to facilitate treatment decisions. Semin Liver Dis. 2012;32(4):348–359. doi: 10.1055/s-0032-1329906. [DOI] [PubMed] [Google Scholar]
- 64.Ha Y, Shim JH, Kim SO, Kim KM, Lim YS, Lee HC. Clinical appraisal of the recently proposed Barcelona Clinic Liver Cancer stage B subclassification by survival analysis. J Gastroenterol Hepatol. 2014;29(4):787–793. doi: 10.1111/jgh.12452. [DOI] [PubMed] [Google Scholar]
- 65.Weinmann A, Koch S, Sprinzl M, Kloeckner R, Schulze-Bergkamen H, Duber C, Lang H, Otto G, Worns MA, Galle PR. Survival analysis of proposed BCLC-B subgroups in hepatocellular carcinoma patients. Liver Int. 2015;35(2):591–600. doi: 10.1111/liv.12696. [DOI] [PubMed] [Google Scholar]
- 66.Nault JC, Villanueva A. Biomarkers for hepatobiliary cancers. Electronic 1527–3350. [DOI] [PubMed]
- 67.Desert R, Nieto N, Musso O. Dimensions of hepatocellular carcinoma phenotypic diversity. World J Gastroenterol. 2018;24(40):4536–4547. doi: 10.3748/wjg.v24.i40.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nishida T, Kataoka H. Glypican 3-targeted therapy in hepatocellular carcinoma. Cancers (Basel) 2019;11(9):1339. doi: 10.3390/cancers11091339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thomas H. Liver cancer: IGF2—an epigenetic oncodriver in HCC. Nat Rev Gastroenterol Hepatol. 2016;13(11):625. doi: 10.1038/nrgastro.2016.162. [DOI] [PubMed] [Google Scholar]
- 70.Shi D, Shi Y, Kaseb AO, Qi X, Zhang Y, Chi J, Lu Q, Gao H, Jiang H, Wang H, et al. Chimeric antigen receptor-glypican-3 T-cell therapy for advanced hepatocellular carcinoma: results of phase I trials. Clin Cancer Res. 2020;26(15):3979–3989. doi: 10.1158/1078-0432.CCR-19-3259. [DOI] [PubMed] [Google Scholar]
- 71.Martinez-Quetglas I, Pinyol R, Dauch D, Torrecilla S, Tovar V, Moeini A, Alsinet C, Portela A, Rodriguez-Carunchio L, Sole M, et al. IGF2 is up-regulated by epigenetic mechanisms in hepatocellular carcinomas and is an actionable oncogene product in experimental models. Gastroenterology. 2016;151(6):1192–1205. doi: 10.1053/j.gastro.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 72.Garcia-Vilas JA, Medina MA. Updates on the hepatocyte growth factor/c-Met axis in hepatocellular carcinoma and its therapeutic implications. World J Gastroenterol. 2018;24(33):3695–3708. doi: 10.3748/wjg.v24.i33.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Organ SL, Tsao MS. An overview of the c-MET signaling pathway. Ther Adv Med Oncol. 2011;3(1 Suppl):S7–S19. doi: 10.1177/1758834011422556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fujita R, Blot V, Wong E, Stewart C, Lieuw V, Richardson R, Banah A, Villicana J, Timmer A, Coronella J, et al. A novel non-agonist c-Met antibody drug conjugate with superior potency over a c-Met tyrosine kinase inhibitor in c-Met amplified and non-amplified cancers. Cancer Biol Ther. 2020;21(6):549–559. doi: 10.1080/15384047.2020.1737490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun X, Wang SC, Wei Y, Luo X, Jia Y, Li L, Gopal P, Zhu M, Nassour I, Chuang JC, et al. Arid1a has context-dependent oncogenic and tumor suppressor functions in liver cancer. Cancer Cell. 2017;32(5):574–589. doi: 10.1016/j.ccell.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tian N, Wu D, Tang M, Sun H, Ji Y, Huang C, Chen L, Chen G, Zeng M. RAF1 expression is correlated with HAF, a parameter of liver computed tomographic perfusion, and may predict the early therapeutic response to sorafenib in advanced hepatocellular carcinoma patients. Open Med (Wars) 2020;15:167–174. doi: 10.1515/med-2020-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khalaf AM, Fuentes D, Morshid AI, Burke MR, Kaseb AO, Hassan M, Hazle JD, Elsayes KM. Role of Wnt/beta-catenin signaling in hepatocellular carcinoma, pathogenesis, and clinical significance. J Hepatocell Carcinoma. 2018;5:61–73. doi: 10.2147/JHC.S156701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Javanmard D, Najafi M, Babaei MR, Karbalaie Niya MH, Esghaei M, Panahi M, Safarnezhad Tameshkel F, Tavakoli A, Jazayeri SM, Ghaffari H, et al. Investigation of CTNNB1 gene mutations and expression in hepatocellular carcinoma and cirrhosis in association with hepatitis B virus infection. Infect Agent Cancer. 2020;15:37. doi: 10.1186/s13027-020-00297-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lachenmayer A, Alsinet C, Savic R, Cabellos L, Toffanin S, Hoshida Y, Villanueva A, Minguez B, Newell P, Tsai HW, et al. Wnt-pathway activation in two molecular classes of hepatocellular carcinoma and experimental modulation by sorafenib. Clin Cancer Res. 2012;18(18):4997–5007. doi: 10.1158/1078-0432.CCR-11-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Desert R, Rohart F, Canal F, Sicard M, Desille M, Renaud S, Turlin B, Bellaud P, Perret C, Clement B, et al. Human hepatocellular carcinomas with a periportal phenotype have the lowest potential for early recurrence after curative resection. Hepatology. 2017;66(5):1502–1518. doi: 10.1002/hep.29254. [DOI] [PubMed] [Google Scholar]
- 81.Sohn BH, Park IY, Shin JH, Yim SY, Lee JS. Glutamine synthetase mediates sorafenib sensitivity in beta-catenin-active hepatocellular carcinoma cells. Exp Mol Med. 2018;50(1):e421. doi: 10.1038/emm.2017.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jager K, Walter M. Therapeutic Targeting of Telomerase. Genes (Basel) 2016;7(7):39. doi: 10.3390/genes7070039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kotiyal S, Evason KJ. Exploring the interplay of telomerase reverse transcriptase and beta-catenin in hepatocellular carcinoma. Cancers (Basel) 2021;13(16):4202. doi: 10.3390/cancers13164202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li J, Gao JZ, Du JL, Huang ZX, Wei LX. Increased CDC20 expression is associated with development and progression of hepatocellular carcinoma. Int J Oncol. 2014;45(4):1547–1555. doi: 10.3892/ijo.2014.2559. [DOI] [PubMed] [Google Scholar]
- 85.Edoo MIA, Chutturghoon VK, Wusu-Ansah GK, Zhu H, Zhen TY, Xie HY, Zheng SS. Serum biomarkers AFP, CEA and CA19-9 combined detection for early diagnosis of hepatocellular carcinoma. Iran J Public Health. 2019;48(2):314–322. [PMC free article] [PubMed] [Google Scholar]
- 86.Montal R, Andreu-Oller C, Bassaganyas L, Esteban-Fabro R, Moran S, Montironi C, Moeini A, Pinyol R, Peix J, Cabellos L, et al. Molecular portrait of high alpha-fetoprotein in hepatocellular carcinoma: implications for biomarker-driven clinical trials. Br J Cancer. 2019;121(4):340–343. doi: 10.1038/s41416-019-0513-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ahn JC, Teng PC, Chen PJ, Posadas E, Tseng HR, Lu SC, Yang JD. Detection of circulating tumor cells and their implications as a biomarker for diagnosis, prognostication, and therapeutic monitoring in hepatocellular carcinoma. Hepatology. 2021;73(1):422–436. doi: 10.1002/hep.31165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Su F, Weiss NS, Beste LA, Moon AM, Jin GY, Green P, Berry K, Ioannou GN. Screening is associated with a lower risk of hepatocellular carcinoma-related mortality in patients with chronic hepatitis B. J Hepatol. 2021;74(4):850–859. doi: 10.1016/j.jhep.2020.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen T, Dai X, Dai J, Ding C, Zhang Z, Lin Z, Hu J, Lu M, Wang Z, Qi Y, et al. AFP promotes HCC progression by suppressing the HuR-mediated Fas/FADD apoptotic pathway. Cell Death Dis. 2020;11(10):822. doi: 10.1038/s41419-020-03030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li QT, Qiu MJ, Yang SL, Fang X, He XX, Wang MM, Li YN, Xiong ZF, Huang S. Alpha-fetoprotein regulates the expression of immune-related proteins through the NF-kappaB (P65) pathway in hepatocellular carcinoma cells. J Oncol. 2020;2020:9327512. doi: 10.1155/2020/9327512. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table S1. List of genes and primers.
Data Availability Statement
The datasets generated and/or analysed during the current study are available in the Gene Expression Omnibus (GEO, http://www.ncbi.nlm.nih.gov/geo/), [GEO Submission (GSE191298)].