Abstract
Background
The aim of our study was to evaluate the curative effect and safety of stereotactic body radiation therapy (SBRT) in treating hepatocellular carcinoma (HCC) patients with inferior vena cava (IVCTT) and right atrial tumor thrombus (RATT).
Methods
This retrospective study included fifteen advanced HCC patients with IVCTT and RATT who were treated with SBRT between 2013 and 2020. The prescribed dose delivered to the tumor was 45–50 Gy/7–10 fx. We report their treatment responses according to survival time and toxicities.
Results
For these patients, the median follow-up time was 15 months (2–52 months). Local tumor control rates of the treated area were 80% at the time of death or at the last follow-up. The 6-month, 12-month, 18-month and 24-month OS rates were 80.0%, 60.0%, 33.3% and 26.7%, respectively. None of these patients died from the toxicity outcomes and complications of SBRT.
Conclusion
SBRT is an effective option for advanced HCC patients with IVCTT and RATT.
Keywords: SBRT, Advanced HCC, IVCTT, RATT, Targeted therapy, Immunotherapy
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide and the third most common cause of cancer-related death [1]. As the disease progresses, a small number of patients develop inferior vena cava tumor thrombosis and right atrial extension, which may result in pulmonary embolism and cardiac tamponade and shorten the survival time of patients.
Previous studies have shown stereotactic body radiation therapy (SBRT) to be a safe treatment, and it also brings satisfactory prognosis, especially for patients with inoperable or recurrent HCC [2–4]. Moreover, SBRT can also be used as palliative care for prolonging the survival time of advanced patients, which includes patients with metastases or vascular invasion.
This retrospective study on prognosis in advanced HCC patients with inferior vena cava (IVCTT) and right atrial tumor thrombus (RATT) treated with SBRT provides some insights for clinical treatment.
Materials and methods
Patient selection
We conducted a retrospective review of fifteen HCC patients with IVCTT and RATT treated with SBRT at the Fifth Medical Center of Chinese PLA General Hospital between September 2013 and December 2020. All patients voluntarily received SBRT treatment and signed informed consent forms.
Radiation therapy and follow-up study
All enrolled patients were administered stereotactic body radiation therapy (CyberKnife, Accuray, USA). The oncologist contoured the gross tumor volume (GTV, including inferior vena cava and right atrial tumor thrombus) and organs at risk (OARs, including the heart, lungs, liver, stomach and spinal cord, etc.). Planning target volume (PTV) expanded 3–5 mm of GTV and avoided the OARs. The prescribed dose delivered to the PTV was 45–50 Gy in 7–10 fractions. The normal tissue dose was within the dose constraints (Table 1), which refer to AAPM TG-101 report [5]. After treatment with SBRT, these patients were followed up every 3 months until June 2022. The follow-up items included laboratory results, chest and abdominal CT/MRI, brain CT/MRI, and PET-CT examination if necessary.
Table 1.
Dose constraints for various critical organs and delivered dose during designing radiation therapy plans in this study
| Dose constraint during designing plans | Delivered dose in this study | |||
|---|---|---|---|---|
| Serial tissue | Threshold dose (Gy) | Max point dose (Gy) | Delivered dose (Gy) Mean (range) |
Delivered max point dose (Gy) Mean (range) |
| Heart (< 15 cc) | 32 Gy | 38 Gy | 20.72 (16.58–24.02) | 31.30 (26.16–36.72) |
| Lung (1000 cc/1500 cc) | 13.5 Gy/12.5 Gy |
9.47 (7.22–12.75)/ 7.40 (5.95–9.45) |
||
| Liver (700 cc/mean dose) | 21 Gy/22 Gy |
16.54 (11.70–20.95)/ 18.15 (12.94–21.85) |
||
| Stomach (< 10 cc) | 18 Gy | 32 Gy | 16.23 (13.96–18.18) | 24.80 (21.08–27.60) |
| Duodenum (< 5 cc/ < 10 cc) | 18 Gy/12.5 Gy | 32 Gy/32 Gy |
15.91 (14.61–17.19)/ 10.47 (9.33–11.78) |
20.75 (18.63–23.14) |
| Spinal cord (< 0.35 cc) | 23 Gy | 30 Gy | 20.27 (18.47–23.49) | 22.84 (19.86–26.22) |
| Trachea and large bronchus (< 4 cc) | 16.6 Gy | 40 Gy | 9.14 (6.39–12.58) | 24.92 (21.44–30.09) |
| Bronchus-smaller airways (< 0.5 cc) | 21 Gy | 33 Gy | 15.45 (12.74–19.25) | 20.77(17.99–26.10) |
Results
The median age was 51 years old (32–69 years old). There were 14 hepatitis B patients (93.3%) and 1 hepatitis C patient (6.7%). Ten patients had Child–Pugh A classification (66.7%) and 5 had Child–Pugh B classification (33.3%). Two patients were ALBI grade 1 (13.33%), 9 were ALBI Grade 2 (60.0%).and 4 were ALBI Grade 3 (26.70%). The AFP level ranged from 12 ng/ml to 39,231 ng/ml. All patients previously received other treatments for liver lesions, such as TACE, targeted therapy or SBRT. The median follow-up period was 15 months (2–52 months). During the follow-up period after SBRT, we found that 11 patients’ GTV lesions shrank, 3 enlarged and 1 showed no change. By June 2022, 14 patients had died (Table 2). The 6-month, 12-month, 18-month and 24-month OS rates were 80.0%, 60.0%, 33.3% and 26.7%, respectively (Fig. 1). A classic case is shown in Fig. 2.
Table 2.
Baseline characteristics, treatment details, and follow-up of the study population
| The details of patients before SBRT | Treatment protocols | Follow-up and review outcome | Survival outcome | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Gender | Age | AFP (ng/ml) | With virus hepatitis infection (B/C/None) | Child–Pugh Classification | ALBI grade | ECOG PS score | Previous treatments | Extrahepatic metastasis | Prescribed doses | Combination treatments | Other active lesions (time to progression) | Targeted lesion progression after SBRT (Y/N) | Follow-up period (months) | Living/dead | Cause of death |
| 1 | Male | 51 | 143 | Hepatitis B virus infection | A6 | 2 | 1 | Liver: TACE, Lenvatinib |
1. Lung 2.Mediastinal lymph node metastasis |
50 Gy/10fx | None |
1. Lung: Progression (uncontrolled) 2. Mediastinal lymph node metastasis: Progression (uncontrolled) 3. Liver: Progression (3 months) |
N | 8 | Dead | Respiratory failure due to lung metastases |
| 2 | Male | 54 | 2837 | Hepatitis B virus infection | A6 | 2 | 1 | Liver: Hepatic Resection, Sorafenib | None | 45 Gy/9fx | None | Lung: New lesions (13 months) | N | 15 | Dead | Infectious shock due to hepatic failure |
| 3 | Male | 62 | 12 | Hepatitis B virus infection | B7 | 3 | 1 | Liver: Radiofrequency ablation, TACE |
1. Lung 2. Kidney |
49 Gy/7fx | None |
1. Lung: uncontrolled 2. Liver: progression (1 month) 3. Kidney: uncontrolled |
Y | 6 | Dead | Hepatic encephalopathy due to hepatic failure |
| 4 | Male | 48 | 4856 | Hepatitis B virus infection | A6 | 2 | 1 | Liver: TACE | Retroperitoneal lymph node metastasis | 45 Gy/9fx | Sorafenib |
1.Retroperitoneal lymph node metastasis: uncontrolled 2. Liver: Progression (3 months) |
N | 16 | Dead | Infectious shock due to hepatic failure |
| 5 | Male | 50 | 956 | Hepatitis B virus infection | A6 | 2 | 0 | Liver: Hepatic resection | None | 50 Gy/10fx | Lenvatinib | Liver: Progression (7 months) | N | 14 | Dead | Unknown causes |
| 6 | Female | 46 | 1517 | Hepatitis B virus infection | A6 | 2 | 0 | Liver: TACE |
1. Lung 2. Retroperitoneal lymph node metastasis |
50 Gy/10fx | None |
1. Lung: uncontrolled 2. Retroperitoneal lymph node metastasis: No change 3.Liver: Progression (1 months) |
N | 9 | Dead | Infectious shock due to hepatic failure |
| 7 | Male | 47 | 29 | Hepatitis B virus infection | B7 | 3 | 1 | Liver: Radiofrequency ablation, TACE |
1. Lung 2. Intracranial metastases |
50 Gy/10fx | Lenvatinib |
1. Liver: uncontrolled 2. Lung: uncontrolled 3. intracranial metastases: uncontrolled |
Y | 2 | Dead | Renal failure due to hepatic failure |
| 8 | Male | 45 | 189 | Hepatitis B virus infection | A5 | 1 | 0 | Liver: SBRT | Lung | 49 Gy/7fx | Sorafenib |
1. Lung (uncontrolled) 2. Bone: New lesions (9 months), with radiotherapy as additional treatment for pain relief |
N | 17 | Dead | Respiratory failure due to lung metastases |
| 9 | Male | 69 | 1210 | Hepatitis B virus infection | A5 | 2 | 0 | Liver: SBRT, TACE | None | 49 Gy/7fx | Sorafenib | Lung: New lesions (21 months), with Lenvatinib and toripalimab as additional treatment | N | 52 | Dead | Infectious shock due to hepatic failure |
| 10 | Male | 58 | 2435 | Hepatitis B virus infection | A5 | 1 | 1 | Liver: Hepatic resection, Sorafenib | None | 50 Gy/10fx | Lenvatinib and toripalimab | Liver: Progression (1 months) | N | 45 | Dead | Upper gastrointestinal bleeding |
| 11 | Male | 32 | 323 | Hepatitis B virus infection | B7 | 2 | 1 | Liver: TACE, Sorafenib | Mesenteric lymph node metastasis | 49 Gy/7fx | Lenvatinib |
1. Mesenteric lymph node metastasis: uncontrolled 2. Liver: progression (1 month) |
Y | 2 | Dead | Upper gastrointestinal bleeding |
| 12 | Female | 57 | 39,231 | Hepatitis B virus infection | B7 | 3 | 0 | Liver: TACE | Lung | 45 Gy/9fx | Toripalimab | Lung: New lesions (12 months) | N | 23 | Dead | Upper gastrointestinal bleeding |
| 13 | Male | 64 | 2139 | Hepatitis C virus infection | A6 | 2 | 1 | Liver: SBRT, TACE | Retroperitoneal lymph node metastasis | 45 Gy/9fx | Lenvatinib and toripalimab |
Retroperitoneal lymph node metastasis: no change Liver: New lesion (12 months) |
N | 21 | Dead | Upper gastrointestinal bleeding |
| 14 | Male | 48 | 176 | Hepatitis B virus infection | A6 | 2 | 1 | Liver: SBRT, TACE | None | 45 Gy/9fx | Toripalimab |
Lung: New lesions (21 months) Liver: New lesion (15 months), add TACE |
N | 21 | Living | |
| 15 | Female | 68 | 5679 | Hepatitis B virus infection | B7 | 3 | 0 | Liver: TACE | None | 50 Gy/10fx | Lenvatinib |
Liver: Progression (5 months) Lung: New lesion (2 months), add toripalimab |
N | 11 | Dead | Unknown causes |
Fig. 1.
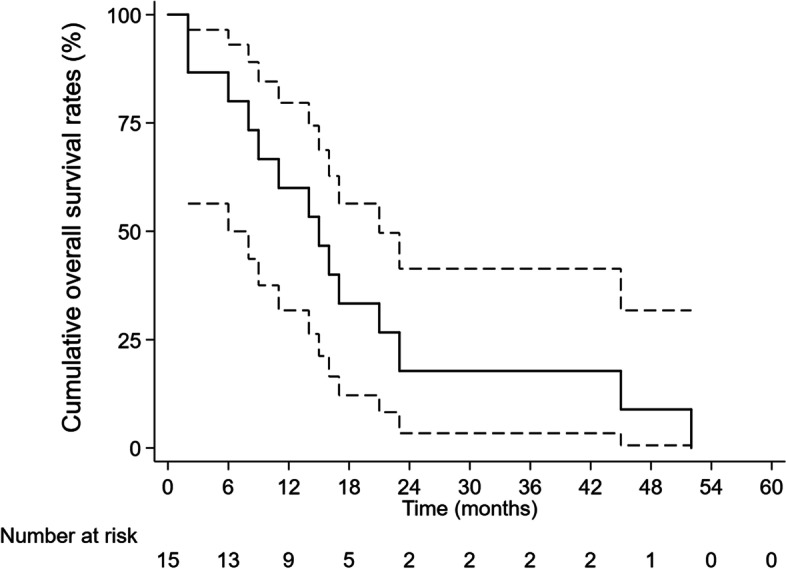
The overall survival curve in fifteen patients
Fig. 2.
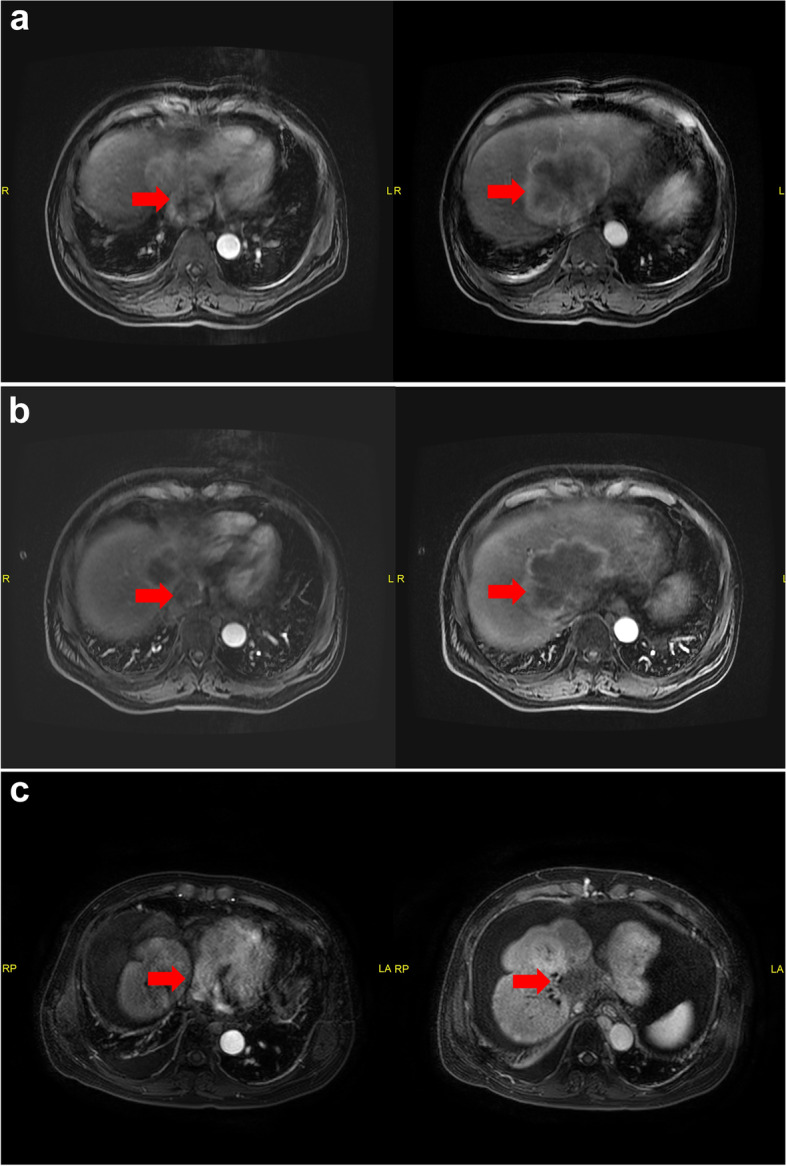
The classic patient (Case 9) with IVCTT and RATT received SBRT. January 2018: The primary upper-abdominal MRI scan with lesions a MRI scan in May 2018 b and March 2022 c after SBRT. The patient died of infectious shock due to hepatic failure in May 2022
Toxicity and complications
All patients in our study had liver cirrhosis of different degrees and portal hypertension, which could lead to esophageal and gastric varices. Three patients died of upper gastrointestinal bleeding within two years of SBRT. Patients 11 received emergency gastroscopy for gastrointestinal bleeding, which was confirmed to be gastric vein rupture and obvious varicose manifestation at the bleeding site. Patient 12 and 13 received gastroscopy regularly and no ulcer was found, but obvious varicose manifestations was observed. By reviewing their treatment plans, 10 cc and maximum point dose to stomach of patient 12 were 17.60 Gy and 25.50 Gy, respectively. Those of patient 13 were 16.50 Gy and 25.64 Gy, respectively. Both plans were under dose constraints value. In addition, these two patients died over 20 months after SBRT. Thus we concluded that their gastrointestinal bleeding was related to liver cirrhosis.
The complications related to liver failure occurred in six patients. We carefully reviewed the SBRT plan and confirmed that 700 cc normal liver dose and mean liver dose of these six cases were 16.08 (11.70–19.20) Gy and 18.12 (12.94–21.67) Gy, respectively. In addition, RILD usually occurs within 3 months after radiotherapy. All these six patients died over 6 months after SBRT [6]. In addition, the intrahepatic lesions progressed in all of these patients. Therefore, it was considered that the main cause of death is disease progression rather than toxicities of treatment.
During and after SBRT, no toxicities of Grade ≥ 3 were observed in lungs, esophagus and trachea. It is worth noting that none of the patients in this study had cardiac-related complications or malignant pericardial effusion. We considered that SBRT is treated with noncoplanar radiation, and more radiation angles will better protect the normal organs.
Discussion
Advanced HCC always has vascular invasion, most commonly the portal vein and hepatic veins. Previous studies showed that the incidence of HCC with IVCTT was approximately 3.8% [7], while RATT occurred in approximately 2% of the cases [8]. In general, the median survival time of advanced HCC patients with IVCTT has been shown to be less than 6 months, with no patients surviving beyond 2 years [9], which is even worse in those with RATT. In recent years, the treatment modalities for advanced HCC patients with IVCTT and/or RA include thrombectomy, transhepatic arterial chemotherapy embolization (TACE), targeted therapy and immunotherapy (checkpoint inhibitor of the programmed cell death protein-1, PD-1 inhibitor). However, a few clinical studies have been reported, and the sample size of the studies was small.
Tomita K [10] conducted a study of three patients with thrombi in the inferior vena cava, right atrium and phrenic vein who underwent resection. The results showed that the microscopic surgical margins of the combined resected diaphragms were positive in all cases, and the respective overall postoperative survival was 98.0, 38.9, and 30.9 months. The patients died due to liver cirrhosis, acute heart failure, and hepatocellular carcinoma. Chai Hong Rim reported a meta-analysis and systematic review, and his results showed pooled 1- and 2-year OS rates were 53.6% and 36.9%, respectively [11], which were better than ours. We found that 60.0% (9/15) of patients in our study also had other active metastases, such as lung and/or lymph nodes metastases; therefore, their treatments were mainly palliative care, which may affect their prognosis. In our study, patients 9 and 10 had active tumors located in blood vessels, and their survival times were all more than 3 years.
It is necessary to carry out a multicenter prospective study to explore the efficacy of IVCTT and RATT in HCC patients. The results of our study provide some ideas for clinical work, but further research and confirmation are still needed.
Acknowledgements
We appreciate Ye Lin for her valuable suggestions and editing support.
Authors’ contributions
DZ and QL were involved in drafting the manuscript and revising it critically for important intellectual content, and JS made substantial contributions to the interpretation of the data; DL and JD made substantial contributions to the acquisition of the data; JJ was responsible for the follow-up content. XD was responsible for the final approval of the version to be published. The author(s) read and approved the final manuscript.
Funding
None.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of 302 Hospital of PLA (People’s Liberation Army) and was conducted in accordance with the Declaration of Helsinki and internationally accepted ethical guidelines. During their admission for SBRT treatment, the patients enrolled in this study provided written informed consent for their information to be stored in hospital databases and used for research.
Consent for publication
At the time of enrollment, written informed consent was obtained from the patients for publication of this case report and any accompanying images.
Competing interests
The authors declare no competing financial interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dan Zhang, Qian Li and Dong Li contributed equally to this work.
Contributor Information
Dan Zhang, Email: 15711239551@qq.com.
Qian Li, Email: 55883492@qq.com.
Dong Li, Email: ld_1768@163.com.
Jun Jia, Email: jiaj1234@163.com.
JunQiang Ding, Email: 799435134@qq.com.
Jing Sun, Email: sunjing0801@foxmail.com.
Xuezhang Duan, Email: duanxuezhang2006@163.com.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Huertas A, Baumann AS, Saunier-Kubs F, Salleron J, Oldrini G, Croisã©-Laurent V, Barraud H, Ayav A, Bronowicki JP, Peiffert D. Stereotactic body radiation therapy as an ablative treatment for inoperable hepatocellular carcinoma. Radiother Oncol. 2015;115(2):211–216. doi: 10.1016/j.radonc.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Su TS, Liang P, Lu HZ, Liang J, Gao YC, Zhou Y, Huang Y, Tang MY, Liang JN. Stereotactic body radiation therapy for small primary or recurrent hepatocellular carcinoma in 132 Chinese patients. J Surg Oncol. 2016;113(2):181–187. doi: 10.1002/jso.24128. [DOI] [PubMed] [Google Scholar]
- 4.Andolino DL, Johnson CS, Maluccio M, Kwo P, Tector AJ, Zook J, Johnstone PA, Cardenes HR. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2011;81(4):e447–e453. doi: 10.1016/j.ijrobp.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Benedict SH, Yenice KM, Followill D, Galvin JM, Hinson W, Kavanagh B, Keall P, Lovelock M, Meeks S, Papiez L. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys. 2010;37(8):4078–4101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 6.Pan C, Kavanagh B, Dawson L, Li X, Das S, Miften M, Ten Haken R. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys. 2010;76:S94–100. doi: 10.1016/j.ijrobp.2009.06.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee I, Chung J, Kim H, Yin Y, So Y, Jeon U, Jae H, Cho B, Park J. Extrahepatic collateral artery supply to the tumor thrombi of hepatocellular carcinoma invading inferior vena cava: the prevalence and determinant factors. J Vasc Interv Radiol. 2009;20(1):22–29. doi: 10.1016/j.jvir.2008.09.030. [DOI] [PubMed] [Google Scholar]
- 8.Li S, Jian L, Gang P, Ando K, Ming L. Removal of hepatocellular carcinoma extending into the right atrium with extracorporeal circulation. Hepatogastroenterology. 2012;59(117):1591–1593. doi: 10.5754/hge09440. [DOI] [PubMed] [Google Scholar]
- 9.Ikai I, Yamamoto Y, Yamamoto N, Terajima H, Hatano E, Shimahara Y, Yamaoka Y. Results of hepatic resection for hepatocellular carcinoma invading major portal and/or hepatic veins. Surg Oncol Clin N Am. 2003;12(1):65–75, ix. doi: 10.1016/S1055-3207(02)00082-0. [DOI] [PubMed] [Google Scholar]
- 10.Tomita K, Shimazu M, Takano K, Gunji T, Ozawa Y, Sano T, Chiba N, Abe Y, Kawachi S. Resection of recurrent hepatocellular carcinoma with thrombi in the inferior vena cava, right atrium, and phrenic vein: a report of three cases. World J Surg Oncol. 2020;18(1):138. doi: 10.1186/s12957-020-01914-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rim C, Kim C, Yang D, Yoon W. External beam radiation therapy to hepatocellular carcinoma involving inferior vena cava and/or right atrium: A meta-analysis and systemic review. Radiother Oncol. 2018;129(1):123–129. doi: 10.1016/j.radonc.2018.02.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


