SUMMARY
Background:
Topotecan is cytotoxic to glioma cells but clinically ineffective due to drug delivery limitations. Systemic delivery is limited by toxicity and insufficient brain penetrance while convection-enhanced delivery has been restricted to a single treatment of limited duration. To address this, we engineered a subcutaneously implanted catheter-pump system capable of repeated, chronic convection-enhanced delivery of topotecan into the brain and tested its safety and biological effects in recurrent glioblastoma patients.
Methods:
We performed a phase 1b, single-center open label clinical trial at Columbia University Irving Medical Center (New York, NY, USA). Eligible patients were ≥18 years of age with solitary, histologically confirmed recurrent glioblastoma demonstrating radiographic progression following surgery, radiation and chemotherapy, and Karnofsky Performance Status ≥ 70. Five patients had catheters stereotactically implanted into glioma-infiltrated peritumoral brain and connected to subcutaneously implanted pumps that infused 146 uM topotecan 200 uL/hour over 48 hours followed by a 5–7 day therapy holiday between infusions, with four total infusions. After the fourth infusion, the pump was removed and tumor resected. Analyses were carried out in an intention-to-treat fashion. Primary endpoint of the study was safety of the treatment regimen as defined by presence of serious adverse events.
Findings:
In all 5 patients recruited between January 22, 2018 and July 8, 2019, chronic convection-enhanced delivery of topotecan was successfully completed safely, and was well tolerated without significant complications. The only grade 3 adverse event related to treatment was intraoperative a supplemental motor area syndrome (1 [20%] of 5 patients in the treatment group), and there were no grade 4 adverse events. Other serious adverse events were related to surgical resection and not the treatment. Median follow-up was 12 (10–17) months from pump explant. Post-treatment tissue analysis demonstrated that topotecan effectively eliminated proliferating tumor cells across all 5 patients. The trial, now closed, was registered with ClinicalTrials.gov (NCT03154996).
Interpretation:
With this small patient cohort, we showed that chronic convection-enhanced delivery of topotecan is a potentially safe and effective therapy for recurrent glioblastoma. Analysis of MRI-localized biopsies collected before and after treatment, integrated for the first time, to our knowledge, into a human glioma trial, provided a unique tissue-based assessment of treatment response without the need for large patient numbers. This novel delivery of topotecan overcomes current limitations in delivery and treatment response assessment for glioblastoma and is potentially applicable for other anti-glioma agents or other CNS diseases.
Funding:
US National Institutes of Health, The William Rhodes and Louise Tilzer Rhodes Center for Glioblastoma, the Michael Weiner Glioblastoma Research Into Treatment Fund, the Gary and Yael Fegel Foundation and The Khatib Foundation.
INTRODUCTION
Glioblastoma, the most common primary brain malignancy, is uniformly fatal despite conventional therapy with surgery, radiation, and chemotherapy, underscoring the need for more effective treatments(1). Promising anti-glioma drugs have failed clinically because of limitations in drug delivery(2, 3). Topotecan (TPT) is a topoisomerase inhibitor that effectively kills proliferating glioma cells but is clinically impractical as a systemically delivered chemotherapeutic because of systemic toxicity and insufficient brain penetrance(4)(5). We previously demonstrated the safety and feasibility of short-term, single-dose convection-enhanced delivery (CED) in a clinical trial with TPT for patients with refractory malignant gliomas(6). CED is a method of local-regional drug infusion that delivers high concentrations of therapeutic compounds directly into the brain through a surgically implanted thin cannula attached to a microinfusion pump. Because glioblastomas are locally invasive, rarely metastasize, and usually recur within two centimeters of the original resection margin(7), local drug delivery strategies have the potential to impact patient survival and provide insight into the direct effects of chemotherapy on the tumor microenvironment(8, 9). Drugs are infused at flow rates that generate a positive hydrostatic pressure to distribute the infusate by bulk flow through the interstitial space. Because it circumvents blood-brain barrier limitations and avoids systemic toxicity, CED provides a pharmacokinetic advantage that is several orders of magnitude greater for maximizing drug levels in a targeted region of the brain compared to conventional diffusion-driven systemic methods such as oral or intravenous delivery(9–12). However, to the extent that CED relies on an external catheter and bedside pump, the inherent infection risks impose a restriction that limits treatment to only a single infusion of insufficient duration to achieve meaningful clinical results. Sustained chronic delivery with multiple treatment cycles is important therapeutically because topoisomerase poisons such as TPT, like most chemotherapies, are cytotoxic to cycling cells in the S-phase where only a small percentage of glioma cells reside at any given time(13, 14). Preclinical studies in a rat glioma model with locally delivered TPT demonstrated improved survival when the infusion duration was extended to allow more tumor cells to cycle through the vulnerable S-phase(15). Therefore, a chronic local TPT delivery technique for gliomas that circumvents blood brain barrier limitations, avoids systemic toxicity and provides unlimited drug regimens to achieve sustained effective intratumoral drug levels is critically needed.
To achieve this, we engineered a subcutaneously implanted catheter-pump system similar to one effectively used in Parkinson patients(16). After successful preclinical modelling in a large animal(17, 18), we devised a prospective clinical trial for patients with refractory glioblastoma using chronic CED with a refillable pump subcutaneously implanted in the abdomen for prolonged and repeated intracerebral infusions of high-dose TPT chemotherapy. The goals of this clinical trial were to test, for the first time, to our knowledge, in humans, the clinical utility and safety of TPT by chronic CED in glioblastoma patients using a subcutaneous pump/catheter construct. Given the unique nature of this clinical trial which incorporated both a novel device and the off-label use of a drug, the FDA provided approval for a limited number of patients, which precluded us from measuring treatment efficacy with conventional response parameters such as survival or tumor progression. Therefore, we devised a novel trial protocol design that included the procurement of tissue both immediately before and after treatment to facilitate a direct tissue-based assessment of treatment response. This unprecedented analysis enabled us to demonstrate antitumor treatment effects within our limited number of patients and overcome challenges in conventional response assessment in glioma clinical trials which currently relies on ambiguous radiographic and clinical endpoints, or analysis of postmortem tissue that is confounded by the effects of recurrent disease(19–20).
METHODS
Study design and participants
This was an investigator-initiated, single-arm, single center, non-randomised phase 1b clinical trial performed at NY-Presbyterian/Columbia University Irving Medical Center. All patients gave written informed consent to the protocol approved by the Columbia University Irving Medical Center Institutional Review Board (protocol # AAAQ9520) and the Food and Drug Administration. The Principal Investigator (JNB) was the sponsor of the Investigational New Drug approval from the Food and Drug Administration.
Estimated life expectancy for enrolled patients with recurrent glioblastoma was not pre-specified by the protocol but expected to be 3–7 months based on the available literature(21). Eligible patients were ≥18 years of age with previously histologically confirmed malignant glioma (WHO grade III-IV) treated with surgical resection, temozolomide chemotherapy, and external beam radiation showing clinical and radiographic evidence of recurrent glioblastoma. Additional eligibility criteria included Karnofsky performance status ≥ 70 and a solitary stereotactically-accessible supratentorial contrast-enhancing tumor localized to a region < 32 cc in volume on pre-enrollment MRI, with histopathological confirmation of recurrent glioblastoma at the time of catheter placement. Basic laboratory tests, including but not limited to serum chemistry, complete blood count, coagulation studies, and pregnancy test (if applicable), were also all required prior to enrollment. There were no explicit restrictions on exclusion of patients based on prior treatments or comorbidities. Patients were recruited from the neuro-oncology practices at Columbia University Irving Medical Center and approved at the weekly Brain Tumor Board. All detailed information can be found in the published study protocol.
Procedures
Trial design is depicted in Supplemental Figure S1 (web appendix page 5). Treatment consisted of multiple infusions of TPT through a surgically placed intracerebral catheter connected to a subcutaneously implanted pump followed by radical resection of the tumor and removal of the pump/catheter four weeks later (Fig. S1A–B). A preoperative MRI was performed to optimize selection of localized tumor biopsies and catheter trajectory. Multiple stereotactic biopsies of the tumor and infiltrated brain tissue were collected through small twist drill holes for comprehensive immunohistopathologic and molecular analyses, as well as for intraoperative histopathologic assessment to verify the diagnosis of recurrent glioblastoma. A 1.5 mm outer diameter silastic Spetzler lumbar shunt catheter (Integra, Plainsboro, NJ) was stereotactically positioned between the contrast enhancing tumor and the margin of the planned surgical resection to be performed at the end of the 4-week treatment (Fig. S1B). This catheter placement strategy was designed to maximize drug delivery into the peritumoral brain tissue. The catheter was connected to silastic tubing that was subcutaneously tunneled and connected to a SynchroMed II infusion pump (Medtronic, Minneapolis, MN) implanted in the abdomen. The infusate was prepared by the research pharmacy and consisted of 1:100 gadolinium (Gadavist, GE Healthcare, Marlborough, MA) plus 146 uM TPT (Hycamtin, GlaxoSmithKline, research Triangle Park, NC). TPT is stable for at least 10 days at 37° C(18).
Four treatment pulses were given consisting of 146 μM of TPT infused over 48 hours at 200 uL/hour followed by a 5–7 day treatment holiday. The TPT dose was derived from our previous Phase I clinical trial(6). Rationale for 48-hour pulsatile infusion was derived from preclinical porcine studies demonstrating that the largest relative gains in volume of distribution of infusate occur within the first 24–48 hours before declining as infusions reach a steady state(17). Gadolinium was co-infused during the first and fourth pulse and T1-weighted MRI was used to monitor the volume of distribution and backflow (Fig. S1C). Basic laboratory data, including serum chemistries and hematology were taken at relative intervals. Adverse events are defined as in protocol section 10, Adverse events are to be recorded irrespective of causality, and each event will be described by its severity (mild, moderate, severe, life-threatening). If at any time the principal investigator determines that the dose must be modified for patient safety they may make the following modifications: decrease active pulse rate, decrease active pulse duration, increase or decrease resting duration between active pulses to less than 5 or greater than 7 days. One patient had their dose and infusion time adjusted, as described in the Results section. Criteria for removal of patients from the study are found in protocol section 8.5, and include intercurrent illness, unacceptable adverse events (per discretion of PI), patient withdrawal, or no recurrent tumor present histologically on pre-CED biopsy.
Immediately after completion of the fourth treatment pulse a final MRI was performed after which the patient underwent multiple radiographically-localized stereotactic-guided biopsies of tumor, tumor margin and surrounding invaded brain tissue followed by surgical tumor resection along with removal of the pump and catheter system (Fig. S1D). An average of 7 biopsies were taken during catheter/pump implantation; an average of 11 biopsies were taken during pump removal and surgical resection. Tissue analysis included immunohistochemistry and RNA sequencing to analyze treatment effects and MALDI-MS for drug level analysis.
3T MR imaging was performed pre-operatively and on post-operative day 0 or 1 following catheter implantation to confirm catheter placement prior to infusion initiation. For the first infusion, MRIs were collected ~8 hours, ~14 hours, ~24 hours, and ~48 hours after the start of infusion, and ~8 hours, ~14 hours, and ~24 hours after completion. For the remaining infusions, MRIs were collected immediately prior to and ~48 hours after the start of each infusion. After the first two patients were treated, the protocol was amended and 18F-FDG PET imaging was performed on Patients 3, 4 and 5 pre-operatively, ~48 hours after start of pulse 1, and ~24 hours after start of pulse 4.
Outcomes
The primary endpoint of this study was to establish the safety of prolonged CED of TPT in patients with recurrent glioblastoma. The primary safety endpoints were reviewed by the Data Safety Monitoring Committee of the Herbert Irving Comprehensive Cancer Center of Columbia University. Independent monitoring was also provided by the Cancer Center’s Clinical Protocol and Data Management Compliance Core. Safety and adverse events were assessed through daily neurological examinations during the treatment period and at continued periodic time points following treatment: 1–2 weeks, 4–6 weeks and then every 1–3 months. QoL and neurocognitive testing using Cognitive Stability Index, FACIT, and PROMIS were immediately performed before and after each treatment pulse for all patients (22–23).
Secondary endpoints per the study protocol included measurement of steady state volume of drug distribution as measured on volumetric MRI, correlation of intraparenchymal TPT concentration with contrast enhancement intensity on MRI, as well as time to tumor progression/recurrence, and time to death. Additionally, tissue-based analyses were performed on pre- and post-treatment MRI-localized biopsies to characterize biological effects of treatment.
Statistical analysis
Clinical toxicity (defined by a grade 2 serious adverse event) was projected to be ≤ 5% at 30 days. A clinical toxicity rate that exceeded 20% was considered unacceptable for this procedure, which is the rationale for a minimum of 5 patients for the present study who were assessed. The trial was ended after 5 patients since there were no serious adverse events. A truncated Sequential Probability Ratio Test was used to determine whether the rate of toxicity exceeded our target. We used correlation analysis to determine the relationship between gadolinium signal intensity and distribution on MRI and the direct measurement of TPT levels. The power calculations were framed to assess our ability to detect these correlations. All statistical analysis was performed at a significance level of p < 0.05. QoL survey data was analyzed using descriptive statistics, and figures were generated in Prism. All bioinformatic tissue analysis, including of the RNA sequencing data, was performed in programming language R (version 4.1.2), and is described in detail in the supplementary methods (web appendix page 2). Briefly, 86 MRI localized biopsies taken pre- and post-CED underwent RNA extraction, and subsequent sequencing using the Illumina TruSeq/NovaSeq pipeline, and aligned, mapped, and quantified using the Kallisto pipeline. RNA count data from all biopsies were processed and normalized using R package “DESeq2”, and pre- and post- treatment biopsies were compared across all patients via differential gene expression analysis using package “DESeq2” (24). Gene set enrichment analysis was performed using GSEA desktop version 4.1.0 (25).
The Data Safety Monitoring Committee of the Herbert Irving Comprehensive Cancer Center of Columbia University provided oversight. The trial was registered with ClinicalTrials.gov (NCT03154996).
Role of the funding source
The study was funded by the National Institutes of Health which had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
RESULTS
Six patients were enrolled in the study between January 22, 2018 and July 8, 2019. One patient was excluded because histopathological analysis of the pretreatment biopsy showed no recurrent tumor. Basic demographic and clinical data of enrolled patients are summarized in Table 1. Three male and two female patients (median (IQR) age = 56 (48–57) years) were treated at a median (IQR) of 11 (7–16) months from the time of initial diagnosis. All patients had biopsy-proven IDH1 wild-type glioblastoma by current WHO criteria. Detailed pathology descriptions and treatment history are provided in Supplementary Table S1C–D (web appendix page 13–14).
Table 1:
Basic clinical and pathological data from the five patients who completed the study a.
| Patient | Sex | Age at enrollment | Race | Initial diagnosis | Tumor location | Tumor volume | IDH1 status | MGMT promoter methylation | Survival from enrollment (months) |
|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | M | 34 | Caucasian | Anaplastic astrocytoma Grade III b | R, Frontal | 1.9 mL | wt | Unmethylated | 12 |
| Patient 2 | F | 58 | Caucasian | GBM, IDH-wildtype | R, Temporal | 12.9 mL | wt | Unmethylated | 17 |
| Patient 3 | M | 61 | Caucasian | GBM, IDH-wildtype | L, Frontal | 5.1 mL | wt | Methylated | 10 |
| Patient 4 | M | 56 | Caucasian | GBM, IDH-wildtype | R, Temporal | 18.0 mL | wt | Unmethylated | 5 |
| Patient 5 | F | 51 | Caucasian | GBM, IDH-wildtype | R, Frontal | 4.1 mL | wt | Methylated | 25 |
See Supplemental Table S1A (webappendix page 12) for more information.
At recurrence, this patient’s tumor showed histopathological and molecular features diagnostic for GBM, IDH-wildtype
Median survival for all 5 patients was 12 (10–17) months from time of enrollment and median overall survival from time of initial diagnosis was 23 (21–28) months. Median follow-up was 12 (10–17) months from pump explant. Five (100%) of five patients eventually succumbed to tumor progression, and there were no known treatment-related deaths. Radiographic changes related to CED and surgical resection precluded a reliable determination of time to tumor progression based on RANO criteria(19–20) (Supplementary Fig. S2A–B, web appendix page 5).
Overall, TPT by chronic CED was generally well tolerated and complications were uncommon and transient. Importantly, no significant systemic complications occurred (Supplementary Fig. S3, web appendix page 6). Regarding the most common treatment complaints, five (100%) of five had pain at the incision site, three (60%) of five had fatigue, and two (40%) of five had headache, all symptoms grade 1 or 2 (Table 2). Patient 1 had worsening of a baseline supplementary motor area syndrome during the initial infusion pulse which improved over the ensuing treatment holiday and the remaining infusions were given at a 50% reduced infusion rate without further incident. Because patient 1 also had a seizure between the 2nd and 3rd infusion pulse, the 3rd infusion was reduced to 24 hours as a precaution given the patient’s pre-treatment baseline seizure disorder. All 4 (80%) other patients completed treatment protocol as described. Patient 1 also developed transient SIADH which resolved with fluid restriction. Furthermore, patient 1 developed a lower extremity deep venous thrombosis during the treatment period, which was attributed to glioma associated coagulopathy (26) Supplementary Table S1D (web appendix page 19). An IVC filter was placed with no further complications.
Table 2:
Selected treatment and non-treatment related adverse eventsc.
| Grade | Type | Percent (number) of patients |
|---|---|---|
| 1–2 | Pain at incision site | 100% (5) |
| Fatigue | 60% (3) | |
| Headache | 40% (2) | |
| Stroke c | 20% (1) | |
| 3 | Intraoperative stroke d | 20% (1) |
| Supplementary Motor Area (SMA) syndrome e | 20% (1) | |
| 4 | No Grade 4–5 adverse events reported | 0% (0) |
| 5 |
Full list of symptoms pre- and post-trial for each patient can be found in supplemental table S1D (webappendix page 19)
This adverse reaction is not directly related to the treatment delivery, but instead the surgical resection – however, it affected the patient’s postoperative Quality of Life (QoL) and performance outcomes. See webappendix pages 23–26
This adverse reaction is not directly related to the treatment delivery. Patient had arm and face weakness five days after completion of treatment due to a middle cerebral artery stroke which was attributed to a radiographically confirmed stenosis of the M2 branch from prior radiation therapy. See webappendix page 23
Led to temporary dose reduction for this patient
All subjects were ambulatory and maintained their baseline Karnofsky Performance Score (KPS) throughout treatment (Supplementary Fig. S2C, web appendix page 7). Patient 1 had a transient decrease in KPS to as low as 70 due to a transient supplementary motor area syndrome, however KPS returned to baseline 90 at the end of Pulse 4. Quality of life (QoL) testing as measured by the FACT-Brain QoL assessment, the FACIT Fatigue assessment or the PROMIS Global Health Measure showed no meaningful changes (Supplementary Fig. S2D–G). SAEs in the follow-up period appear in relation to surgical resection or underlying disease but unrelated to the TPT treatment are reported in the web appendix (page 3). Trial was ended as expected after completion by a minimum of five eligible patients.
Using the MRI signal of co-infused gadolinium, chronic CED resulted in a large and stable volume of distribution for all patients (mean maximal volume of distribution = 20.4 (10.5) mL) (Fig. 1A) with mean (SD) time to peak volume after start of infusion of 43.1 (11.5) hours (Fig. 1B). At the time of maximal volume of distribution, the mean ratio of the volume of distribution to volume of infusion was 2.37 (Supplementary Table S2, web appendix page 27). Backflow was a small fraction (8.8%) of total infused volume (maximum backflow volume: mean (SD)= 1.8 (2.1) mL) (Supplementary Fig. S4, web appendix page 8).
Figure 1: Chronic CED of TPT achieves large and stable volumes of distribution.
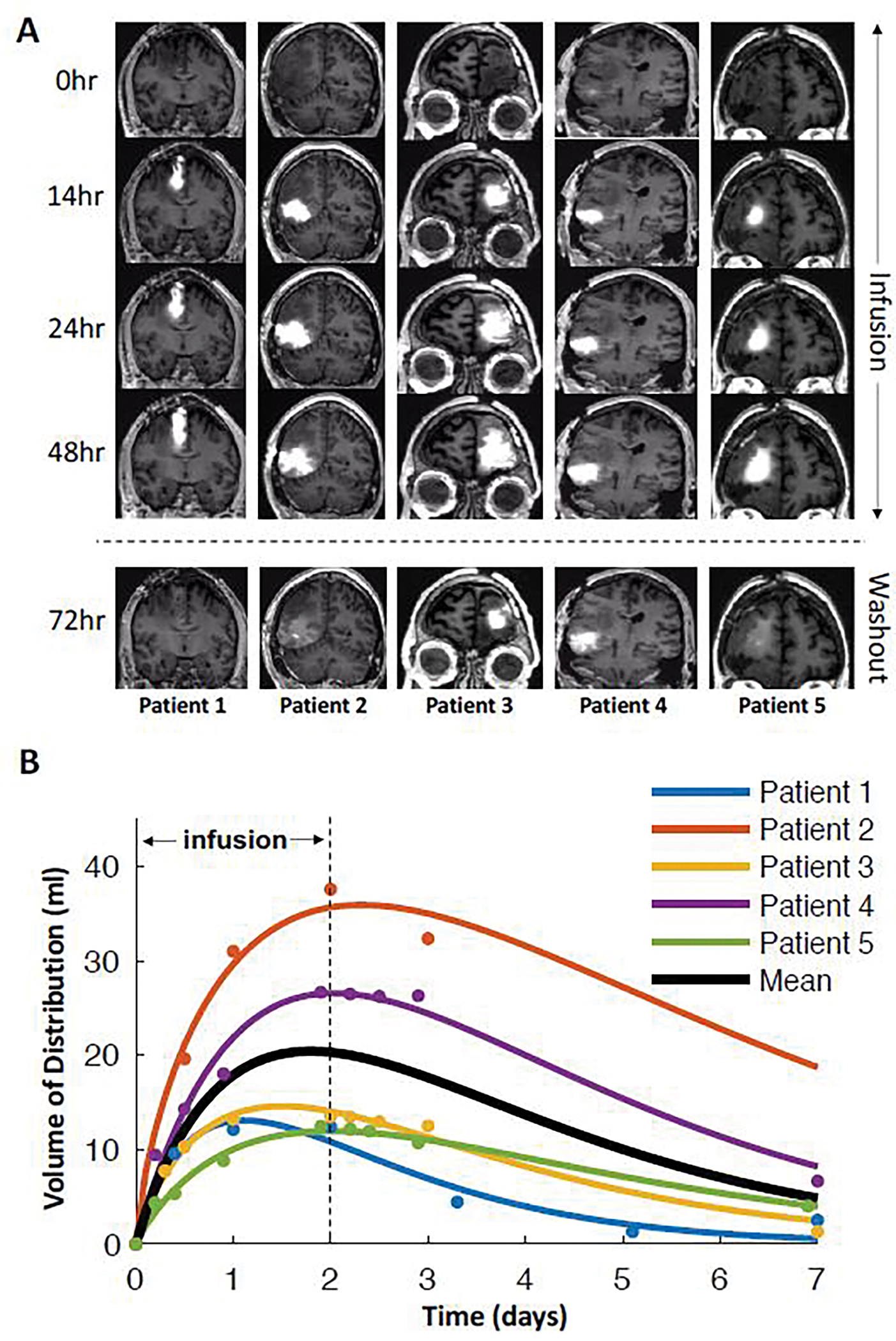
A: Each patient was infused over the course of 48 hours followed by a 5–7 day washout period before the next infusion. All five patients showed large changes in volume of distribution. B: The estimated volume of distribution of the infused contrast was plotted as a function of time and fit to a gamma function for each patient. The solid black line represents the mean time course across subjects, which peaked at 43.1 hours with a mean volume of 20.4 mL.
Eleven of 12 biopsies analyzed with mass spectrometric imaging (MALDI-MSI) had detectable levels of TPT with maximum pixel values above the LOD (3.2 uM) (Supplemental Fig. S5A–B, web appendix page 8) and average TPT concentrations ranging from 1.1μM to 30μM (mean (SD) = 8.9 (2.3) μM). Micromolar concentrations of TPT were present up to 6.5 centimeters (range: 1.1–6.5) from the catheter tip and in all biopsies taken within the maximum volume of gadolinium distribution (Supplemental Fig. S5C).
Tissue collected from patients pre- and post-treatment was analyzed by immunohistochemistry to determine the effects of TPT on tumor cells (Fig. 2A–B). Tumor burden was assessed by staining for SOX2, a highly pervasive glioma cell marker that is expressed by the vast majority of tumor cells in glioblastoma(27). The SOX2 labeling index was significantly decreased in post-CED biopsies compared to pre-CED biopsies (18.5% vs. 25.8%; p = 0.031). Additionally, Ki67 proliferation index was significantly lower in post-CED biopsies compared to pre-CED biopsies (1.4% vs. 3.9%; p = 0.011).
Figure 2: Chronic CED of TPT targets proliferating tumor populations and shifts tumor phenotype.
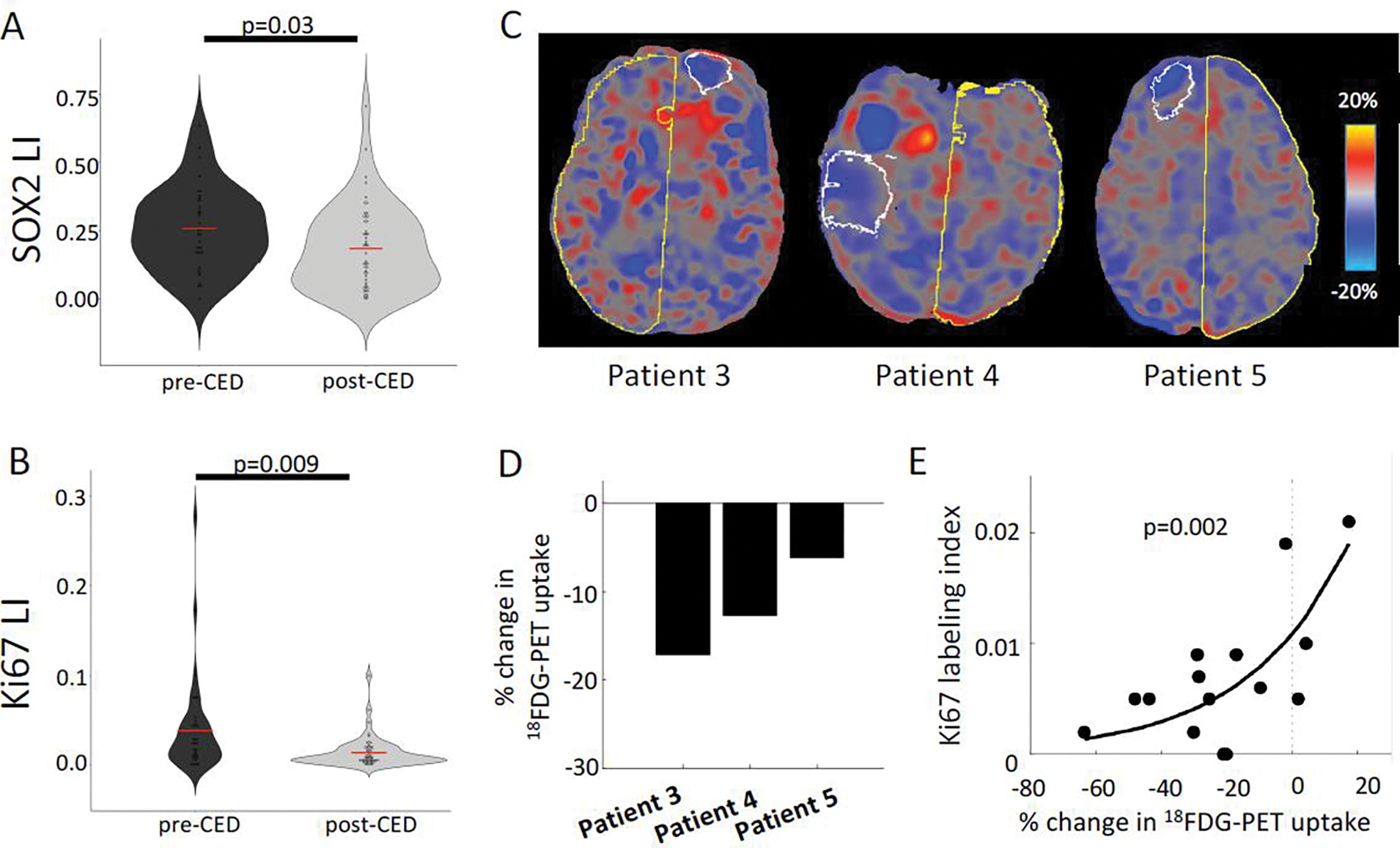
A-B: Violin plot displaying quantification of SOX2 (glioma marker) and Ki67 (proliferation marker) by labeling index across all MRI-localized biopsies from all patients, comparing biopsies taken pre- and post-CED using a student’s T-test (n=86). C: PET scans were performed on the last 3 patients in this series. The difference between post- and pre-infusion PET images were computed and converted to percent signal change. The white outline represents the maximum infused volume; the yellow outline represents the control hemisphere. Blue voxels represent decreases in metabolism, red voxels represent increases in metabolism, and gray voxels represent no change after treatment. D: All three patients showed a large (6.2–17.2%) reduction in metabolism within the infused volume mask. E: The regions treated with TPT demonstrated reduced Ki67 labeling and reduced 18FDG metabolism (n = 14). These two measures were exponentially related, such that, the larger the reduction in metabolism, the lower the proliferation index.
To further characterize the functional impact of chronic infusion of TPT, 18FDG-PET was performed in three patients and glucose metabolic activity was assessed in the treated regions, as defined by maximal volume of the infused gadolinium (Fig. 2C). CED treatment produced a significant reduction in glucose uptake relative to pre-treatment (Patient 3 = −17.2%; Patient 4 = −12.7%; Patient 5 = −6.2%) (Fig. 2D). A comparison of post-CED biopsies showed an inverse exponential correlation between Ki67 proliferation index and the 18FDG-PET uptake (p = 0.0020) (Fig. 2E).
To further understand the effects of TPT, RNA sequencing and differential gene expression analysis were performed on all MRI-localized biopsies with sufficient quality RNA (RIN > 7) which included pre-CED at time of catheter placement (n = 35) and post-treatment at the time of tumor resection (n = 51) (Supplementary Table S3, web appendix page 28). Gene set enrichment analysis (GSEA) demonstrated marked heterogeneity across patients pre- and post-treatment but demonstrated clear patterns of tissue response, with increases in gene signatures for DNA damage, apoptosis, and generation of reactive oxygen species, as well as upregulation of several metabolic programs, including oxidative phosphorylation (Supplemental Fig. 6C, Supplementary Table S4, web appendix pages 10 & 65). Mitotic spindle and other proliferation-associated gene ontologies were significantly decreased across all patients, further demonstrating the impact of chronic TPT infusion on the proliferating tumor cell population. Single-sample gene set variation analysis (GSVA) revealed a significant shift in gene signatures for glioma cell states (28), with samples from with the treatment volume showing a significant decrease in the samples showing highest enrichment for the proliferative or proneural signatures and a significant increase in the samples showing highest enrichment for mesenchymal signatures (p < 0.0001) (Supplemental Fig. S6E–F, web appendix page 10), further described in “additional molecular results” of the web appendix page 4. We also analyzed the effects of chronic CED of TPT on the tumor microenvironment. CD68, a macrophage marker was significantly increased in post-CED biopsies as assessed by immunohistochemistry (17.4% vs. 9.6%; p = 0.020) (Supplemental Fig. S6A). Post-CED biopsies were also positively enriched in pro-inflammatory transcriptional programs (Supplemental Fig. 6C, web appendix page 10). Differential gene expression analysis was performed by pooling biopsies across all patients, and pre-CED versus post-CED comparison demonstrated significant increase in several pro-inflammatory cytokines and other immuno-active markers (Supplemental Fig. S6D, web appendix page 10). Immunostaining with the neuronal marker NeuN showed no significant difference in post-treatment biopsies as compared to pre-treatment biopsies, providing supportive evidence that TPT is not significantly toxic to neurons in the peritumoral invaded brain (post-CED 5.4% vs. pre-CED 4.7%; p = 0.77) (Supplemental Fig. S6B, web appendix page 10).
DISCUSSION
Our successful, safe use of a subcutaneously implanted pump to provide repeated prolonged infusion duration of TPT overcomes a significant shortcoming in the treatment of gliomas. Previous CED trials for glioblastoma used externalized hardware, which restricts treatment to a single infusion of limited duration. We used this system to significantly reduce proliferating tumor cells in refractory glioblastoma patients, delivering multiple cycles of TPT at high concentrations directly into the tumor and surrounding brain over 4 weeks without serious neurological or neurobehavioral events thereby circumventing the limitations associated with traditional systemic delivery with the added benefit of improved quality of life. Using MRI to non-invasively monitor the distribution of co-infused gadolinium as a surrogate for TPT distribution, we demonstrated large and stable volumes of drug distribution effectively targeting peritumoral brain tissue where unresectable invasive tumor cells reside. Comparison of pre- and post-treatment tissue demonstrated consistent responses to TPT after treatment, including significant reduction in proliferating tumor cells and increase in mesenchymal and inflammatory gene signatures. These changes were only seen in biopsies taken within the CED treatment volume, providing further evidence that they represent response to TPT. Larger studies will be needed to determine if chronic CED of TPT will significantly prolong survival or delay recurrence in GBM patients.
Effective treatment response with TPT was possible by modifying prior CED constructs to facilitate chronic delivery with potential unlimited regimens modeled after a similar protocol in Parkinson patients(16). The advantage of an implantable CED pump allows for percutaneous refilling and the capacity for sequential or simultaneous treatment algorithms using one or more drugs while maximizing distribution volume. The pump/tubing/catheter construct that we utilized was improvised from a variety of different commercial sources and was designed to be used with a skill set common to neurosurgeons. The tested system has numerous features designed to ease its clinical use: 1) self-contained subcutaneously implanted hardware to avoid infection and facilitate chronic use; 2) implanted microinfusion pump with a reservoir containing the treatment drug which can be percutaneously refilled or emptied with a needle and syringe; 3) wireless programming pump technology to modify flow rate; 4) simple flexible thin bore catheter which can be implanted precisely with stereotactic guidance and can accommodate flow rates within the therapeutic range without significant backflow; 5) incorporation of a technique for co-infusing gadolinium to monitor volume of delivery in real time using a safe and reliable non-invasive methodology; and 6) safety profile suitable for use in an out-patient setting. Improvements in the CED construct are a source of ongoing study by our lab and others including catheter design, use of multiple catheters, as well as improvements in pump technology, monitoring software and stereotactic technologies. Future studies will be needed to optimize infusion variables and treatment indications including potentially eliminating the need for tumor resection.
While validating the safety and feasibility of chronic CED, the major limitation of our study was that we were underpowered and lacked a comparison group for determination of definitive survival benefit. Despite this, our unique treatment protocol provides a novel broad clinical framework to study the effects of locally-delivered therapy at the tissue-based level, using patients as their own controls. Performing a series of pre- and post-therapy MRIs and PET scans, as well as taking dozens of MRI-localized biopsies upon implantation and removal of the CED pump-catheter system, enable patient-specific measurements of drug delivery and treatment response. MRI localized biopsies taken before treatment at pump implantation can potentially allow tailoring of the treatment regimen to each patient’s tumor, and even facilitate combination therapies to target distinct glioma subpopulations. Biopsies taken immediately after therapy can provide an unparalleled view into mechanisms of tumor resistance and recurrence from which new therapeutic approaches can be designed.
Additionally, because the blood brain barrier is bypassed, new classes of drugs and targeted compounds can be exploited including high molecular weight compounds, proteins, viruses, liposomes, nanoparticles and other biologics that would not be feasible with systemic delivery due to toxicity or metabolic breakdown. This is a useful and strategic approach to study the tissue-specific effects of novel therapies to the brain in a direct clinical setting with ever greater utility, not only for gliomas but other non-neoplastic CNS diseases as well(16, 29, 30).
In summary, we present the results of a phase 1b clinical trial in which we show that chronic convection-enhanced delivery of topotecan is a safe and feasible therapeutic approach for patients with recurrent glioblastoma. Analysis of MRI-localized biopsies collected before and after treatment provided a unique tissue-based assessment of treatment response without the need for large patient numbers. This novel drug delivery strategy and innovative clinical trial framework overcomes current limitations in delivery and treatment response assessment in glioma.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
PubMed was searched for preclinical animal studies and human clinical trials using topotecan or convection-enhanced delivery for gliomas using the search term “((glioma[Title/Abstract]) OR (glioblastoma[Title/Abstract])) AND ((topotecan[Title/Abstract]) OR (convection-enhanced delivery[Title/Abstract]))” from 01/01/1996–12/31/2018. 372 articles were reviewed, and studies that included human or animal studies were included, with review papers and non-English language publications excluded. Topotecan is cytotoxic to glioma cells but was ineffective with systemic delivery in a Phase II clinical trial. Although topotecan with convection-enhanced delivery produced tumor regression in a Phase I clinical trial for malignant glioma, its reliance on external pumps and catheters limited treatment to a single infusion for a short period of time to minimize infection risks. Evidence from pre-clinical animal studies showed that chronic infusion strategies that prolong infusion duration provide survival benefits for topotecan and could be effective in humans if technological obstacles could be overcome.
Added value of this study
To achieve intratumoral delivery of topotecan at high concentrations and for unlimited duration, we developed a subcutaneously implanted pump/catheter system, and tested it prospectively for the first time in human glioma patients, to our knowledge. By delivering topotecan, an antiproliferative chemotherapeutic, for multiple cycles over a four-week period, we were able to demonstrate the clinical safety and therapeutic efficacy of direct interstitial topotecan delivery into the tumor and peritumoral brain tissue. The treatment protocol incorporated a unique method for collecting tissue specimens immediately before and after treatment to provide an unprecedented opportunity to directly analyze treatment effects in tissue for the first time to our knowledge in a human glioma trial. The clinical utility of this treatment was further enhanced by MR imaging of co-infused gadolinium that demonstrated broad drug distribution into the brain where unresectable invaded tumor cells reside and are responsible for recurrence. Tissue analysis confirmed topotecan treatment effectiveness by demonstrating the significant reductions of proliferating tumor cells without toxicity to neurons.
Implications of all the available evidence
Despite conventional therapy, glioblastoma is a rapidly fatal disease and new treatment strategies are desperately needed. Since treatment failure with chemotherapeutics such as topotecan is primarily due to limitations in drug delivery, our ability to use an implantable catheter/pump to deliver drugs at high concentrations for extended periods of time has the potential to transform our approach to brain tumor therapy. While we demonstrate its success in glioma patients using topotecan, this paradigm has broad clinical applicability as it is adaptable for multi-drug regimens as well as new classes of drugs including high molecular weight compounds, proteins, viruses, liposomes, nanoparticles, and other biologics that would not be feasible with systemic delivery. This treatment strategy can also be applied to other central nervous system diseases where therapeutic effectiveness is limited by drug delivery. Additionally, as used here to demonstrate the effectiveness of our treatment strategy, our unique approach of collecting and analyzing pre- and post-treatment tissue provides not only insight into effects for individual patients, but a novel methodology for overall response assessment in glioma clinical trials.
Acknowledgements
This research was funded in part by NIH/NCI R01CA161404 (JNB) and NIH/NINDS R01NS103473 (PC, PAS, JNB), Also funded in part through the NIH/NCI Cancer Center Support Grant P30CA013696 as well as the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1TR001873. ABL was supported in part by grants 5P30CA013696–43 and 5UG1CA189960–04 from the NCI, as well as. The work was partly funded by the Advanced Technologies-National Center for Image Guided Therapy (AT-NCIGT) NIH P41EB028741 (NYRA) and MIT/Mayo Physical Science Oncology Center for Drug Distribution and Drug Efficacy in Brain Tumors NIH U54 CA210180 (NYRA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We also acknowledge support from the William Rhodes and Louise Tilzer Rhodes Center for Glioblastoma (JNB, ABL). We would like to acknowledge the Khatib Foundation (JNB), the Gary and Yael Fegel Foundation (JNB), the Michael Weiner Glioblastoma Research Into Treatment Fund (ABL), the Irving Institute for Clinical and Translational Research, the Herbert Irving Comprehensive Cancer Center Molecular and Pathology Core, and the patients, nurses, technicians and physicians who have made this work possible. A medical writer or editor was not used for this manuscript.
Footnotes
Declaration of interests
JNB has a consulting agreement with Theracle, Inc. and held the Sponsor-Investigator IND for this study. NYRA receives support from EMD Serono and Bruker Daltonics. FMI has obtained grants or contracts through Columbia from Merck, BMS, Roche, Sapience, Novocure, Celldex, Tocagen, Forma, Celldex, and Northwest Biotherapeutics, is in consulting agreements with Novocure, Regeneron, Tocagen, Alexion Pharmaceuticals, Abbvie, Guidepoint Global, Merck, Kiyatec, PPD, Massive Bio, Medtronic, MimiVax, Gennao Bio, and Xcures, has two US provisional patent applications (No. 62/739,617 and 63/062,805) through Columbia, received support for meetings and travel from Roche and Oncoceutics, and participates on advisory boards of Mimivax and Northwest Biotherapeutics. AMi is in consulting agreements and on Advisory Board of Regeneron. PAS receives consulting fees from Wilson Sonsini and EpiCypher, received payment from AstraZeneca for honorarium for seminar, and royalties from Guardant Health through Harvard University. SZ is now the Pediatric Oncology lead at Bristol Myers Squibb. ABL receives consulting fees and/or personal financial support for honoraria or meetings from Affinia, Bioclinica, Elsevier, Fondazion AIRC, NCI, Novocure, Sapience, Leal, Abbott, AbbVie, Clinical Education Alliance, MJH Healthcare, Novartis, Northwest Biotherapeutics, Oligonation, Pfizer, RTOG Foundation, ASCO, Bayer, FDA, Forma, Karyopharm, QED, GCAR, Matheson Foundation, NBTS, SNO, VBI vaccines, and is on advisory boards of Abbvie, Bayer, Chimerix, Forma, Karyopharm, Novocure, Orbus, QED, and Vivacitas. All other authors report no conflicts of interest. No authors are employees of WHO, IARC, or PAHO.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Eleonora F. Spinazzi, Department of Neurological Surgery, Columbia University Irving Medical Center, New York, NY, USA.
Michael G. Argenziano, Department of Neurological Surgery, Columbia University Irving Medical Center, New York, NY, USA.
Pavan S. Upadhyayula, Department of Neurological Surgery, Columbia University Irving Medical Center, New York, NY, USA.
Matei A. Banu, Department of Neurological Surgery, Columbia University Irving Medical Center, New York, NY, USA.
Justin A. Neira, Department of Neurological Surgery, Columbia University Irving Medical Center, New York, NY, USA.
Dominique M.O. Higgins, Department of Neurological Surgery, Columbia University Irving Medical Center, New York, NY, USA
Peter B. Wu, Department of Neurological Surgery, UCLA Geffen School of Medicine, Los Angeles, CA, USA
Brianna Pereira, Department of Pathology and Cell Biology, Columbia University Irving Medical Center, New York, NY, USA.
Aayushi Mahajan, Department of Neurological Surgery, Columbia University Irving Medical Center, New York, NY, USA.
Nelson Humala, Department of Neurological Surgery, Columbia University Irving Medical Center, New York, NY, USA.
Osama Al-Dalahmah, Department of Pathology and Cell Biology, Columbia University Irving Medical Center, New York, NY, USA.
Wenting Zhao, Department of System Biology, Columbia University Irving Medical Center, New York, NY, USA.
Akshay V. Save, Department of Neurological Surgery, NYU Grossman School of Medicine, New York, NY, USA
Brian JA Gill, Department of Neurological Surgery, Columbia University Irving Medical Center, New York, NY, USA.
Deborah M. Boyett, Department of Neurological Surgery, Columbia University Irving Medical Center, New York, NY, USA
Tamara Marie, Department of Neurological Surgery, Columbia University Irving Medical Center, New York, NY, USA.
Julia L Furnari, Department of Neurological Surgery, Columbia University Irving Medical Center, New York, NY, USA.
Tejaswi D. Sudhakar, Department of Neurological Surgery, Columbia University Irving Medical Center, New York, NY, USA
Sylwia A. Stopka, Department of Neurosurgery and Radiology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
Michael S. Regan, Department of Neurosurgery, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
Vanessa Catania, Department of Neurosurgery, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Laura Good, Department of Neurological Surgery, Columbia University Irving Medical Center, New York, NY, USA.
Stergios Zacharoulis, Department of Pediatrics, Columbia University Irving Medical Center, New York, NY, USA.
Meenu Behl, Department of Radiology, Columbia University Irving Medical Center, New York, NY, USA.
Petros Petridis, Department of Neurological Surgery, Columbia University Irving Medical Center, New York, NY, USA.
Sachin Jambawalikar, Department of Radiology, Columbia University Irving Medical Center, New York, NY, USA.
Akiva Mintz, Department of Radiology, Columbia University Irving Medical Center, New York, NY, USA.
Angela Lignelli, Department of Radiology, Columbia University Irving Medical Center, New York, NY, USA.
Nathalie Y.R. Agar, Department of Neurosurgery and Radiology, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA; Department of Cancer Biology, Dana-Farber Cancer Institute Boston, MA, USA.
Peter A. Sims, Department of System Biology, Columbia University Irving Medical Center, New York, NY, USA.
Mary R. Welch, Division of Neuro-Oncology, Department of Neurology and the Herbert Irving Comprehensive Cancer Center, Columbia University Vagelos College of Physicians and Surgeons and New York-Presbyterian Hospital, New York, NY, USA.
Andrew B. Lassman, Division of Neuro-Oncology, Department of Neurology and the Herbert Irving Comprehensive Cancer Center, Columbia University Vagelos College of Physicians and Surgeons and New York-Presbyterian Hospital, New York, NY, USA.
Fabio M. Iwamoto, Division of Neuro-Oncology, Department of Neurology and the Herbert Irving Comprehensive Cancer Center, Columbia University Vagelos College of Physicians and Surgeons and New York-Presbyterian Hospital, New York, NY, USA.
Randy S. D’Amico, Department of Neurosurgery, Lenox Hill Hospital, New York, NY, USA.
Jack Grinband, Departments of Radiology and Psychiatry, Columbia University Irving Medical Center, New York, NY, USA.
Peter Canoll, Department of Pathology and Cell Biology, Columbia University Irving Medical Center, New York, NY, USA.
Jeffrey N. Bruce, Department of Neurological Surgery, Columbia University Irving Medical Center, New York, NY, USA.
Data Sharing
All individual participant data that underlie the results reported in this article, after de-identification (text, tables, figures, and appendices) will be available immediately following publication (no end date) upon reasonable request to the corresponding author jnb2@cumc.columbia.edu. The study protocol can be found in the web appendix page 68.
REFERENCES
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96. [DOI] [PubMed] [Google Scholar]
- 2.Aldape K, Brindle KM, Chesler L, Chopra R, Gajjar A, Gilbert MR, et al. Challenges to curing primary brain tumours. Nat Rev Clin Oncol. 2019;16(8):509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacus MO, Daryani VM, Harstead KE, Patel YT, Throm SL, Stewart CF. Pharmacokinetic Properties of Anticancer Agents for the Treatment of Central Nervous System Tumors: Update of the Literature. Clin Pharmacokinet. 2016;55(3):297–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman HS, Kerby T, Fields S, Zilisch JE, Graden D, McLendon RE, et al. Topotecan treatment of adults with primary malignant glioma. The Brain Tumor Center at Duke. Cancer. 1999;85(5):1160–5. [PubMed] [Google Scholar]
- 5.Shen J, Carcaboso AM, Hubbard KE, Tagen M, Wynn HG, Panetta JC, et al. Compartment-specific roles of ATP-binding cassette transporters define differential topotecan distribution in brain parenchyma and cerebrospinal fluid. Cancer research. 2009;69(14):5885–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruce JN, Fine RL, Canoll P, Yun J, Kennedy BC, Rosenfeld SS, et al. Regression of recurrent malignant gliomas with convection-enhanced delivery of topotecan. Neurosurgery. 2011;69(6):1272–9; discussion 9–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hochberg FH, Pruitt A. Assumptions in the radiotherapy of glioblastoma. Neurology. 1980;30(9):907–11. [DOI] [PubMed] [Google Scholar]
- 8.Bastiancich C, Malfanti A, Preat V, Rahman R. Rationally designed drug delivery systems for the local treatment of resected glioblastoma. Adv Drug Deliv Rev. 2021;177:113951. [DOI] [PubMed] [Google Scholar]
- 9.D’Amico RS, Aghi MK, Vogelbaum MA, Bruce JN. Convection-enhanced drug delivery for glioblastoma: a review. J Neurooncol. 2021;151(3):415–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994;91(6):2076–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan RJ, Synold T, Mamelak A, Lim D, Al-Kadhimi Z, Twardowski P, et al. Plasma and cerebrospinal fluid pharmacokinetics of topotecan in a phase I trial of topotecan, tamoxifen, and carboplatin, in the treatment of recurrent or refractory brain or spinal cord tumors. Cancer Chemother Pharmacol. 2010;66(5):927–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morrison PF, Laske DW, Bobo H, Oldfield EH, Dedrick RL. High-flow microinfusion: tissue penetration and pharmacodynamics. Am J Physiol. 1994;266(1 Pt 2):R292–305. [DOI] [PubMed] [Google Scholar]
- 13.Feeney GP, Errington RJ, Wiltshire M, Marquez N, Chappell SC, Smith PJ. Tracking the cell cycle origins for escape from topotecan action by breast cancer cells. Br J Cancer. 2003;88(8):1310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang FL, Wang P, Liu YH, Liu LB, Liu XB, Li Z, et al. Topoisomerase I inhibitors, shikonin and topotecan, inhibit growth and induce apoptosis of glioma cells and glioma stem cells. PLoS One. 2013;8(11):e81815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez KA, Tannenbaum AM, Assanah MC, Linskey K, Yun J, Kangarlu A, et al. Convection-enhanced delivery of topotecan into a PDGF-driven model of glioblastoma prolongs survival and ablates both tumor-initiating cells and recruited glial progenitors. Cancer Res. 2011;71(11):3963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gill SS, Patel NK, Hotton GR, O’Sullivan K, McCarter R, Bunnage M, et al. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9(5):589–95. [DOI] [PubMed] [Google Scholar]
- 17.D’Amico RS, Neira JA, Yun J, Alexiades NG, Banu M, Englander ZK, et al. Validation of an effective implantable pump-infusion system for chronic convection-enhanced delivery of intracerebral topotecan in a large animal model. J Neurosurg. 2019:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonabend AM, Stuart RM, Yun J, Yanagihara T, Mohajed H, Dashnaw S, et al. Prolonged intracerebral convection-enhanced delivery of topotecan with a subcutaneously implantable infusion pump. Neuro Oncol. 2011;13(8):886–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen PY, Chang SM, Van den Bent MJ, Vogelbaum MA, Macdonald DR, Lee EQ. Response Assessment in Neuro-Oncology Clinical Trials. J Clin Oncol. 2017;35(21):2439–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–72. [DOI] [PubMed] [Google Scholar]
- 21.van Linde ME, Brahm CG, de Witt Hamer PC, Reijneveld JC, Bruynzeel AME, Vandertop WP, et al. Treatment outcome of patients with recurrent glioblastoma multiforme: a retrospective multicenter analysis. J Neurooncol. 2017;135(1):183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erlanger D, Feldman D, Kaplan D, Theodoracopulos A. Development and validation of the cognitive stability index, a Web-based protocol for monitoring change in cognitive function. Archives of Clinical Neuropsychology. 2000;15(8):693–4. [Google Scholar]
- 23.Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health and Quality of Life Outcomes. 2003;1(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perry JR. Thromboembolic disease in patients with high-grade glioma. Neuro Oncol. 2012;14 Suppl 4:iv73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan J, Levitin HM, Frattini V, Bush EC, Boyett DM, Samanamud J, et al. Single-cell transcriptome analysis of lineage diversity in high-grade glioma. Genome Med. 2018;10(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al-Dalahmah O, Argenziano M, Boyett D, Save A, Mahajan A, Khan F, et al. Re-convolving the compositional landscape of primary and recurrent glioblastoma using single nucleus RNA sequencing. bioRxiv. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barua NU, Miners JS, Bienemann AS, Wyatt MJ, Welser K, Tabor AB, et al. Convection-enhanced delivery of neprilysin: a novel amyloid-beta-degrading therapeutic strategy. J Alzheimers Dis. 2012;32(1):43–56. [DOI] [PubMed] [Google Scholar]
- 30.Lonser RR, Walbridge S, Murray GJ, Aizenberg MR, Vortmeyer AO, Aerts JM, et al. Convection perfusion of glucocerebrosidase for neuronopathic Gaucher’s disease. Ann Neurol. 2005;57(4):542–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All individual participant data that underlie the results reported in this article, after de-identification (text, tables, figures, and appendices) will be available immediately following publication (no end date) upon reasonable request to the corresponding author jnb2@cumc.columbia.edu. The study protocol can be found in the web appendix page 68.


