Abstract
Antisera generated against each of the nine known chemotypes of Salmonella lipopolysaccharide (LPS) core were characterized in order to delineate cross-reactive epitopes and define the bases for their accessibility. Strongly cross-reactive epitopes were associated with three chemotypes: Ra and Rb4, which recognized α-GlcNAc-1→2-α-Glc, and Rd1, which recognized l-α-d-heptose-1→7-l-α-d-heptose. Both these disaccharides and the more weakly cross-reactive α-Gal-1→6-α-Glc terminal in Rb3 LPS represent branch points along the core oligosaccharide. Therefore, branch points in endotoxin core oligosaccharides may generally be cross-reactive.
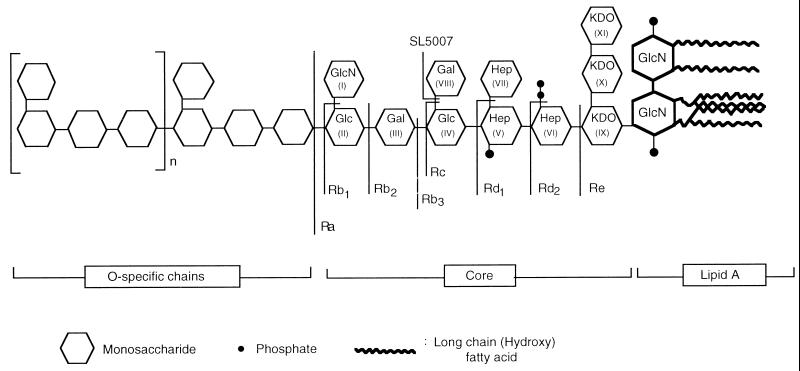
Members of the family Enterobacteriaceae cause a wide variety of human and animal diseases, including gram-negative sepsis, food poisoning, and typhoid fever. The increasing incidence of these diseases has refocused attention on the need for vaccines that would cross-protect against the numerous serotypes of enteric bacteria. Lipopolysaccharide (LPS), an amphipathic moiety present on the bacterial surface (Fig. 1), is both a major virulence factor and an important target for protective immune responses. The large number of studies on the role of LPS in cross-protective immunity have mainly been done in Salmonella infection models due, in part at least, to the availability of mutants expressing nearly all possible forms of truncated LPS. These studies have shown that anti-LPS responses which develop during infection or by vaccination with smooth bacteria are highly protective but are directed mainly against the structurally hypervariable O-antigenic domain (Fig. 1) (19, 20).
FIG. 1.
Schematic structures of Salmonella LPSs with core-defective chemotypes.
Unlike the O antigen, the core region of LPS is highly conserved; the >2,000 serotypes of Salmonella share only two closely related core types (16, 17, 30). Based on this fact, rough bacterial strains, which expose core epitopes, have been extensively investigated as cross-protective immunogens. The results have, however, been inconsistent with some studies demonstrating protection against challenge with virulent smooth organisms (4, 21, 22, 28, 37), while others observed no protective effects (13, 23, 29, 31). An explanation for these dichotomous findings has not been possible due to a lack of knowledge about the identities of core determinants that elicit cross-reactive responses. Despite these contradictions in experimental findings, there is evidence that anticore antibodies protect in clinical settings, as determined in recent studies which show that high levels of natural anti-LPS core antibodies correlate with reduced incidence of complications after surgery and better outcomes from infection (1, 9, 11, 12, 15). The specific core structures and epitopes associated with the protective effects, however, remain to be delineated.
One approach to elicitation or augmentation of cross-protective anti-LPS responses would be to map all cross-reactive epitopes present in the different core types of enteric organisms and to combine these in a composite vaccine. In this regard, it is of interest that two cross-reactive epitopes represented by the disaccharides α-GlcNAc-1→2-α-Glc (24) and l-α-d-heptose-1→7-l-α-d-heptose (25), have been identified in the complete Salmonella LPS core of chemotype Ra. The aim of this study was to map all other cross-reactive epitopes present in this core type. Knowledge of the identities of all cross-reactive epitopes in this moiety not only would help resolve the controversy generated by previous contradictory findings but also would enable the elucidation of features common to such epitopes. Such common features may then be used for putative identification of cross-reactive elements in other core types, such as those of Escherichia coli, for which complete sets of mutants expressing truncated forms are not available for epitope mapping purposes.
The bacterial strains used in this study were all Salmonella strains and have been described in previous publications (26, 33, 35). These strains express smooth LPS (sLPS) or rough LPS of different chemotypes and serological specificities as follows: IS2 (AO), SL3201 (BO), SL3622 (BO), SL2824 (C1O), SL4388 (C4O), IS78 (EO), SN57 (Ra), TV119 (Ra), SL733 (Rb1), TV161 (Rb2), TV148 (Rb3), SL805 (Rc), SL1032 (Rd1), SL1181 (Rd2), SL1102 (Re), and SL5007 (hereby designated chemotype Rb4). The strains were cultivated as described before (24) and either were heat killed and used as immunizing antigens or were used for LPS extraction. Antisera were generated against each core chemotype (Fig. 1) by immunization of groups of 10 TO mice (Harlan Olac, London, United Kingdom) by a regimen comprising six intraperitoneal injections administered at weekly intervals. Immunization was begun with an initial dose of 108 heat-killed bacterial bodies; this was doubled at each subsequent inoculation so that the last dose contained ca. 3 × 109 bacterial bodies. Mice were bled after the fourth, fifth, and sixth injections, and sera from each group were pooled. The sera were then characterized for reactivity with LPS and glycoconjugates by enzyme-linked immunosorbent assay (ELISA) and immunoblotting as described before (24, 25). Briefly, Maxisorp ELISA plates (Nunc, Roskilde, Denmark) were coated either with glycoconjugates (1 μg/ml) by adsorption in 0.05 M carbonate buffer (pH. 9.6) or with LPS (2 μg/well) by chloroform-ethanol evaporation. The plates were blocked (1 h at 37°C with 0.5% bovine serum albumin [BSA] and 0.025% gelatin in 0.05 M carbonate buffer, pH. 9.6) and washed three times (0.15 M NaCl, 0.05% Tween 20). ELISA was then continued with peroxidase-labelled rabbit anti-mouse polyvalent immunoglobulins (Dakopatts, Glostrup, Denmark) as the conjugate and o-phenylenediamine HCl as the substrate. For immunoblot analyses, samples of sLPS (7.5 μg) or rough LPS (2.5 μg) were resolved in denaturing sodium dodecyl sulfate (SDS)–15% polyacrylamide gels and transferred electrophoretically (120 mA, 12 h) to nitrocellulose membranes. They were then tested for reactivity with sera by using the same conjugates as in ELISA but diaminobenzidine-H2O2 (Sigma, St. Louis, Mo.) as a substrate system.
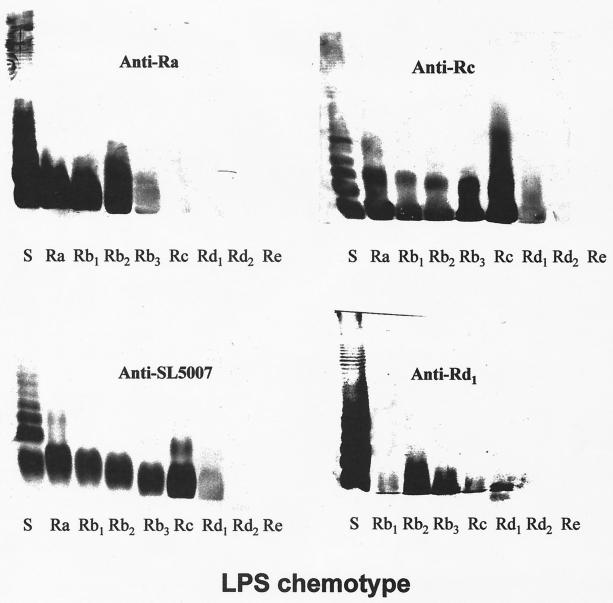
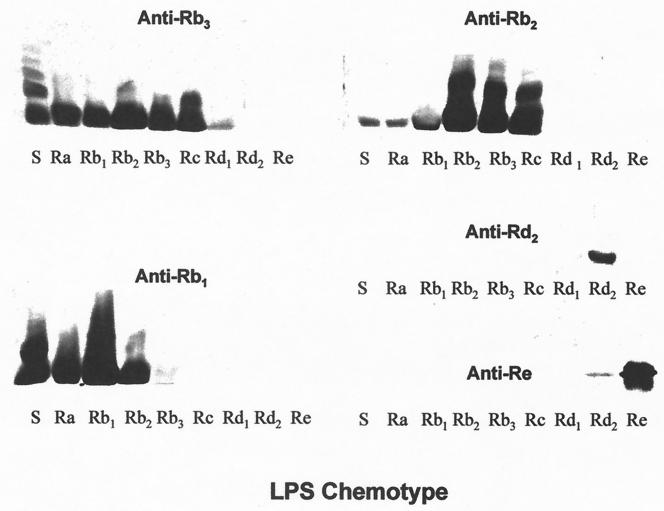
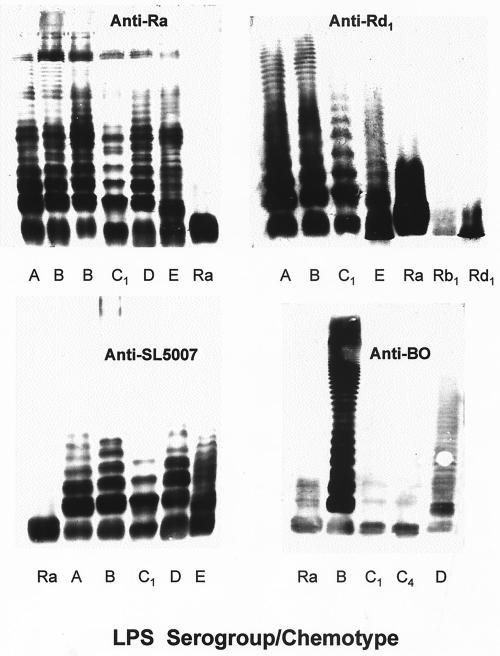
All nine anticore sera showed similarly strong reactivities with their respective homologous LPSs (ELISA end point titers = 24,300 to 72,900) but differed greatly in their reactivities with other core chemotypes in immunoblots. Five sera (anti-Ra, anti-Rb4, anti-Rb3, anti-Rc, and anti-Rd1) demonstrated broad reactivity with rough LPS, strongly recognizing four or more different core chemotypes (Fig. 2 and 3). Two others, anti-Rb1 and anti-Rb2, had a somewhat narrower spectrum of recognition, reacting strongly with their homologous LPSs as well as the immediately adjacent chemotypes (Fig. 3). However, anti-Rb2 also demonstrated a weaker recognition for two additional chemotypes. A third group of sera, anti-Rd2 and anti-Re, reacted with their respective homologous chemotypes only. A comparison of the reactivity profiles of sera raised against the deep-core chemotypes Rd2 and Re and those raised against more distal core determinants (Ra, Rb1, Rb2, Rb3, Rc, and Rd1) suggested a dichotomy in the recognition of LPS by these sera. None of the anti-Ra, anti-Rb1, anti-Rb2, anti-Rb3, anti-Rc, or anti-Rd1 sera reacted with Rd2 and Re LPS, showing that they lacked antibodies directed against deep-core determinants. Likewise, anti-Rd2 and anti-Re were nonreactive with all of the more distal core chemotypes, Ra and Rd1. These findings suggest that deep-core epitopes are completely masked by distal sugar residues.
FIG. 2.
Immunoblotting of sera against Salmonella LPS chemotypes resolved by SDS-polyacrylamide gel electrophoresis.
FIG. 3.
Immunoblotting of sera against Salmonella LPS chemotypes resolved by SDS-polyacrylamide gel electrophoresis.
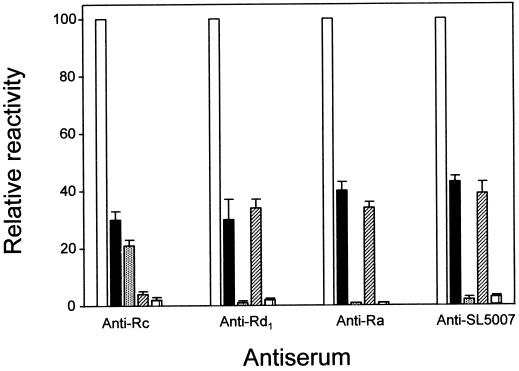
Blotted against sLPS of serogroup B, anti-Ra revealed a ladder-like pattern of bands consistent with ability to bind LPS molecules substituted by O chains (Fig. 2), as previously demonstrated for this antiserum (25). Three other sera, anti-Rb4, anti-Rc, and anti-Rd1, similarly reacted with sLPS molecules, while the rest recognized either none (anti-Re and anti-Rd2) or at the most three bands in sLPS (Fig. 3). These latter sera thus contained only antibodies directed at core moieties inaccessible in LPS molecules with long O-chains substitutions. The recognition of long-chained LPS molecules by anti-Rb4, anti-Rc, and anti-Rd1 could be due to antibodies directed at accessible core epitopes or to O-specific antibodies generated by such other mechanisms as polyclonal activation of B cells. To further delineate the applicable mechanisms, the sera were compared for reactivity in ELISA against sLPS of two different serospecificities and their corresponding O-specific glycoconjugates. The serogroup B-specific glycoconjugate, designated AM-PAA (O:4 specific), was prepared by copolymerization of a haptenic glycoside and acrylamide (5), while that of serogroup C1, CO-BSA (O:7 specific), comprised a dodecasaccharide from Salmonella O:6,7 polysaccharide (serogroup C1) covalently coupled to BSA (8). The results (Fig. 4) showed that while anti-Rc reacted well with both serogroup B LPS and AM-PAA, it was poorly reactive with both serogroup C1 LPS and CO-BSA. This pattern of reactivity is consistent with the presence in anti-Rc of group B O-specific antibodies and the lack of both group C1 O-specific and cross-reactive core-specific antibodies. It may, therefore, be deduced that anti-Rc reacted with sLPS only because it contained O-specific antibodies of serogroup B. Unlike anti-Rc, the sera anti-Ra, anti-Rb4, and anti-Rd1 reacted with group B as well as C1 LPS and failed to react with the glycoconjugates. These results are consistent with a lack of O-specific antibodies in these sera and thus reactivity with long-chained LPS molecules via recognition of core epitopes. The cross-reactivity of these three sera with sLPSs of different serospecificities was subsequently visualized directly by electrophoretic resolution and immunoblotting (Fig. 5). Unlike these anticore sera, anti-BO (generated against a smooth Salmonella strain of serogroup B) showed differential reactivities with sLPSs of different serospecificities (Fig. 5). It showed strong reactivity with sLPS molecules of serogroup B (O:4,5,12), weak reactivity with those of serogroup D (O:9,12), and a lack of reactivity with those of serogroups C1 (O:6,7) and C4 (O:6,7,14). These findings conform to expectation, since LPSs of serogroups B and D have O factor 12 but share no determinants with LPSs of serogroups C1 and C4.
FIG. 4.
Relative reactivities in ELISA of four anticore sera with sLPS and glycoconjugates of serogroups B and C1. The bars represent LPS from the immunizing strain (□), serogroup B-specific LPS (■), serogroup B-specific glycoconjugate (░⃞), serogroup C1-specific LPS (▨), and serogroup C1-specific glycoconjugate (□).
FIG. 5.
Immunoblotting of sera raised against three rough mutants and one smooth strain against smooth Salmonella LPSs of different serological specificities.
The presence of serogroup B O-specific antibodies in anti-Rc serum is not surprising since strain SL805, used for its generation, is a galE mutant derived from a smooth strain of Salmonella typhimurium. While galE mutants normally make rough LPS of the Rc chemotype, they are known to switch their LPS phenotype and make some sLPS in the presence of even trace amounts of galactose (10). This unique ability to switch between the rough and smooth phenotypes is the basis for both their reduced virulence and usefulness as live vaccines (27, 32). It seems likely, therefore, that the cross-protective effects that have been observed with Rc strains have resulted from the immune response either to shared O-specific determinants or to other cross-protective antigens, such as outer membrane proteins (18).
The data strongly suggests that the cross-reactivity of anti-Rd1 with sLPS molecules was mediated by antibodies directed against the disaccharide l-α-d-heptose-1→7-l-α-d-heptose (residues VII to V in Fig. 1) of the inner core domain. The lack of cross-reactive antibodies in both anti-Rc and anti-Rd2 sera supports this deduction by showing that such antibodies are not generated once this disaccharide is lost or replaced by an additional sugar moiety. Moreover, the disaccharide has been shown to be the epitope of a broadly reactive monoclonal antibody (25). It is likewise deduced that the epitope recognized by anti-Rb4 lies in the terminal core disaccharide, α-GlcNAc-1→2-α-Glc, based on the facts that anti-Ra recognizes this disaccharide (24) and that Rb4 differs from Ra LPS only in lacking the α-Gal1→6 (VIII) branch residue. The latter residue appears to contribute, though only slightly, to cross-reactivity, as indicated by the ability of anti-Rb3 to recognize sLPS molecules containing short O chains. Therefore, taken together, the results show that only three chemotypes, Ra, Rb4, and Rd1, elicit core-specific antibodies which bind LPS molecules with long O-chain substitutions.
It is of interest that the idea of using rough mutants as broadly cross-protective vaccines gained popularity with the report (4) that equine antisera raised against a Salmonella strain of the Ra chemotype protected mice against challenge with a virulent strain of Klebsiella pneumoniae. However, E. coli J5 (Rc) and Salmonella minnesota R595 (Re) have been used in most investigations of cross-protection by rough mutants in the belief that the immunodominant epitopes in these chemotypes are the most conserved among gram-negative organisms. Passive immunization with J5 was reported to protect experimental animals against the toxic effects of LPS (2, 6) as well as against lethal gram-negative bacteremia (37, 38). In a clinical trial, human antiserum raised against J5 was found to reduce mortality in patients with gram-negative bacteremia (36). Other studies have likewise reported that active or passive immunization with S. minnesota R595 protected against challenge with endotoxins or virulent bacteria in both experimental animals (3, 21, 35) and human volunteers (7). The failure of many other studies to demonstrate similar protective effects after immunization with J5 or R595 (14, 23, 29, 31) led to a >20-year-old controversy. The results of the present systematic analysis of immunization by core chemotypes clearly show that Rc and Re strains do not elicit cross-reactive LPS-specific antibodies. Therefore, the reported protective effects of immunization with J5 or R595 could not have resulted from an adaptive immune response to LPS core determinants and must be attributed to other mechanisms.
Two lines of evidence show that cross-reactive epitopes reside only at branch points along the core oligosaccharide. Firstly, all four LPS chemotypes that elicited antibodies reactive with sLPS molecules have branch points at the terminal nonreducing ends of the chains. Secondly, none of the five chemotypes (Rb1, Rb2, Rc, Rd2, and Re) which terminate other than at branch points elicited cross-reactive antibodies. However, it appears that secondary factors, such as overall conformation of the core moiety, modulate the extent to which a particular branch residue is accessible, as exemplified by the fact that the Rb3 chemotype elicited cross-reactive antibodies which recognized only low-molecular-weight chains.
REFERENCES
- 1.Bennett-Guerrero E, Ayuso L, Hamilton-Davies C, White W D, Barclay G R, Smith P K, King S A, Muhlbaier L H, Newman M F, Mythen M G. Relationship of preoperative antiendotoxin core antibodies and adverse outcomes following cardiac surgery. JAMA. 1997;277:646–650. [PubMed] [Google Scholar]
- 2.Braude A I, Ziegler E J, McCutchan J A, Douglas H. Immunization against nosocomial infection. Am J Med. 1981;70:463–466. doi: 10.1016/0002-9343(81)90789-0. [DOI] [PubMed] [Google Scholar]
- 3.Bruins S C, Stumacher R, Johns M A, McCabe W R. Immunization with R mutants of Salmonella minnesota. III. Comparison of the protective effect of immunization with lipid A and the Re mutant. Infect Immun. 1977;17:16–20. doi: 10.1128/iai.17.1.16-20.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chedid L, Parant M, Parant F, Boyer F. A proposed mechanism for natural immunity to enterobacterial pathogens. J Immunol. 1968;100:292–301. [PubMed] [Google Scholar]
- 5.Chernyak A Y, Weintraub A, Kochetov N K, Lindberg A A. The β-configuration of the rhamnosidic linkage in Salmonellaserogroups C2 and C3 lipopolysaccharide is important for the immunochemistry of the O antigen 8. Mol Immunol. 1993;30:887–893. doi: 10.1016/0161-5890(93)90012-z. [DOI] [PubMed] [Google Scholar]
- 6.Davis C E, Zigler E J, Arnold K F. Neutralization of meningococcal endotoxin by antibody to core glycolipid. J Exp Med. 1978;147:1007–1017. doi: 10.1084/jem.147.4.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeMaria A, Jr, Johns M A, Berberich H, McCabe W R. Immunization with rough mutants of Salmonella minnesota: initial studies in human subjects. J Infect Dis. 1988;158:301–311. doi: 10.1093/infdis/158.2.301. [DOI] [PubMed] [Google Scholar]
- 8.Ekwall E, Svenson S B, Lindberg A A. Identification of Salmonellaof serogroup C1 by immunofluorescence and co-agglutination with antiserum against an oligosaccharide-protein conjugate. J Med Microbiol. 1982;15:173–180. doi: 10.1099/00222615-15-2-173. [DOI] [PubMed] [Google Scholar]
- 9.Freeman R, Gould F K. Prevention of fever and gram negative infections after open heart surgery by antiendotoxin. Thorax. 1985;40:846–848. doi: 10.1136/thx.40.11.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukasawa T, Nikaido H. Galactose-sensitive mutants of Salmonella. II. Bacteriolysis induced by galactose. Biochim Biophys Acta. 1961;48:470–483. doi: 10.1016/0006-3002(61)90045-2. [DOI] [PubMed] [Google Scholar]
- 11.Goldie A S, Fearon K C H, Ross J A, Barclay G R, Jackson R E, Grant I S, Ramsay G, Blyth A S, Howie J C. Natural cytokine antagonists and endogenous antiendotoxin core antibodies in sepsis syndrome. JAMA. 1995;274:172–177. [PubMed] [Google Scholar]
- 12.Gould F K, Harvey J A, Dytrych J K. Antibody to endotoxin is associated with decreased frequency of postoperative infection. Am J Obstet Gynecol. 1989;160:317–319. doi: 10.1016/0002-9378(89)90433-x. [DOI] [PubMed] [Google Scholar]
- 13.Greisman S E, Johnston C A. Evidence against the hypothesis that antibodies to the inner core of lipopolysaccharide in antisera raised by immunization with enterobacterial deep-rough mutants confer broad-spectrum protection during Gram-negative bacterial sepsis. J Endotoxin Res. 1997;4:123–153. [Google Scholar]
- 14.Greisman S E, Johnston C A. Failure of antisera to J5 and R595 rough mutants to reduce endotoxemic lethality. J Infect Dis. 1988;157:54–64. doi: 10.1093/infdis/157.1.54. [DOI] [PubMed] [Google Scholar]
- 15.Hamilton-Davies C, Barclay G R, Cardigan R A, McDonald S J, Purdy G, Machin S J, Webb A R. Relationship between endotoxin immune status and outcome following cardiac valve replacement surgery. Chest. 1995;112:1189–1196. doi: 10.1378/chest.112.5.1189. [DOI] [PubMed] [Google Scholar]
- 16.Holst O, Brade H. Chemical structure of the core region of lipopolysaccharides. In: Morrison D C, Ryan J L, editors. Bacterial endotoxic lipopolysaccharides. 1. Molecular biochemistry and cellular biology. Boca Raton, Fla: CRC Press; 1992. pp. 135–170. [Google Scholar]
- 17.Jansson P-E, Lindberg A A, Lindberg B, Wollin R. Structural studies of the hexose region of the core in lipopolysaccharides from Enterobacteriaceae. Eur J Biochem. 1981;115:571–577. doi: 10.1111/j.1432-1033.1981.tb06241.x. [DOI] [PubMed] [Google Scholar]
- 18.Kuusi N, Nurminen M, Saxen H, Valtonen M, Mäkelä P H. Immunization with major outer membrane proteins in experimental salmonellosis of mice. Infect Immun. 1979;25:857–862. doi: 10.1128/iai.25.3.857-862.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindberg A A, LeMinor L. Serology of Salmonella. Methods Microbiol. 1984;15:1–141. [Google Scholar]
- 20.Lyman M B, Stocker B A D, Roantree R J. Evaluation of the immune response directed against the Salmonellaantigenic factors O4,5 and O9. Infect Immun. 1979;26:956–965. doi: 10.1128/iai.26.3.956-965.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCabe W R. Immunization with R mutants of S. minnesota. I. Protection against challenge with heterologous Gram negative bacilli. J Immunol. 1972;108:601–610. [PubMed] [Google Scholar]
- 22.Muotiala A, Hovi M, Makela P H. Protective immunity in mouse salmonellosis: comparison of smooth and rough live and killed vaccines. Microb Pathog. 1989;6:51–60. doi: 10.1016/0882-4010(89)90007-7. [DOI] [PubMed] [Google Scholar]
- 23.Ng A-K, Chen C-L H, Chang C-M, Nowotny A. Relationship of structure to function in bacterial endotoxins: serologically cross-reactive components and their effect on protection of mice against some Gram-negative infections. J Gen Microbiol. 1976;94:107–116. doi: 10.1099/00221287-94-1-107. [DOI] [PubMed] [Google Scholar]
- 24.Nnalue N A. α-GlcNAc-1→2-α-Glc, the Salmonella homologue of a conserved lipopolysaccharide motif in the Enterobacteriaceae, elicits broadly cross-reactive antibodies. Infect Immun. 1998;66:4389–4396. doi: 10.1128/iai.66.9.4389-4396.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nnalue N A, Lind S M, Lindberg A A. The disaccharide l-α-d-heptose1→7-l-α-d-heptose→ of the inner core domain of Salmonellalipopolysaccharide is accessible to antibody and is the epitope of a broadly reactive monoclonal antibody. J Immunol. 1992;149:2722–2728. [PubMed] [Google Scholar]
- 26.Nnalue N A, Lindberg A A. Salmonella choleraesuisstrains deficient in O antigen remain fully virulent for mice by parenteral inoculation but are avirulent by oral administration. Infect Immun. 1990;58:2493–2501. doi: 10.1128/iai.58.8.2493-2501.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nnalue N A, Stocker B A D. Tests of the virulence and live-vaccine efficacy of auxotrophic and galE derivatives of Salmonella choleraesuis. Infect Immun. 1987;55:955–962. doi: 10.1128/iai.55.4.955-962.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nys M, Cloes J M, Demonty J, Joassin L. Protective effects of polyclonal sera and monoclonal antibodies active on Salmonella minnesotaRe595 lipopolysaccharide during experimental endotoxemia. J Infect Dis. 1990;162:1087–1095. doi: 10.1093/infdis/162.5.1087. [DOI] [PubMed] [Google Scholar]
- 29.Peter G, Chernow M, Keating M H, Ryff J C, Zinner S H. Limited protective effect of rough mutant antisera in murine Escherichia colibacteremia. Infection. 1982;10:228–232. doi: 10.1007/BF01666916. [DOI] [PubMed] [Google Scholar]
- 30.Tsang R S W, Schlect S, Aleksic S, Chan K H, Chau P Y. Lack of the a-1,2-linked N-acetyl-d-glucosamine epitope in the outer core structures of lipopolysaccharides from certain O serogroups and subspecies of Salmonella enterica. Res Microbiol. 1991;142:521–533. doi: 10.1016/0923-2508(91)90185-d. [DOI] [PubMed] [Google Scholar]
- 31.van Dijk W C, Verbrugh H A, van Erne-van der Tol M E, Peters R, Verhoef J. Escherichia coliantibodies in opsonisation and protection against infection. Med Microbiol. 1981;14:381–389. doi: 10.1099/00222615-14-4-381. [DOI] [PubMed] [Google Scholar]
- 32.Wahdan M H, Serie C, Cerisier Y, Sallam S, Germanier R. A controlled field trial of live S. typhiTy 21a oral vaccine against typhoid: three-year results. J Infect Dis. 1982;145:292–295. doi: 10.1093/infdis/145.3.292. [DOI] [PubMed] [Google Scholar]
- 33.Wilkinson R G, Gemski P, Stocker B A D. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J Gen Microbiol. 1972;70:527–554. doi: 10.1099/00221287-70-3-527. [DOI] [PubMed] [Google Scholar]
- 34.Wollin R. Ph.D. thesis. Stockholm, Sweden: Karolinska Institute; 1989. [Google Scholar]
- 35.Young L S, Stevens P, Ingram J. Functional role of antibody against core glycolipid of Enterobacteriaceae. J Clin Investig. 1975;56:850–861. doi: 10.1172/JCI108164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ziegler E J, McCutchan J A, Fierer J, Glauser M P, Sadoff J C, Douglas H, Braude A I. Treatment of Gram-negative bacteremia and shock with human antiserum to a mutant Escherichia coli. N Engl J Med. 1982;307:1225–1230. doi: 10.1056/NEJM198211113072001. [DOI] [PubMed] [Google Scholar]
- 37.Ziegler E J, McCutchan J A, Douglas H, Braude A I. Prevention of lethal Pseudomonas bacteremia with epimerase deficient E. coliantiserum. Trans Assoc Am Physicians. 1975;88:101–108. [PubMed] [Google Scholar]
- 38.Ziegler E J, Douglas H, Sherman J E, Davis C E, Braude A I. Treatment of E. coli and Klebsiella bacteremia in agranulocytic animals with antiserum to a UDP-GAL epimerase-deficient mutant. J Immunol. 1973;111:433–438. [PubMed] [Google Scholar]