TO THE EDITOR
Autosomal dominant (AD) hyper-IgE syndrome (HIES) is caused by STAT3 dominant-negative mutations, whereas loss-of-function mutations of ZNF341, which governs STAT3 activity through transcriptional mechanisms, or DOCK8 cause autosomal recessive (AR) HIES1,2. HIES patients present early-onset allergy, including features of severe atopic dermatitis, thought to result at least in part from increased Th2 cytokines. Topical corticosteroids and calcineurin inhibitors, associated with oral antihistamines are the main treatment, whereas immunosuppressive agents are not recommended given a higher risk of infection.
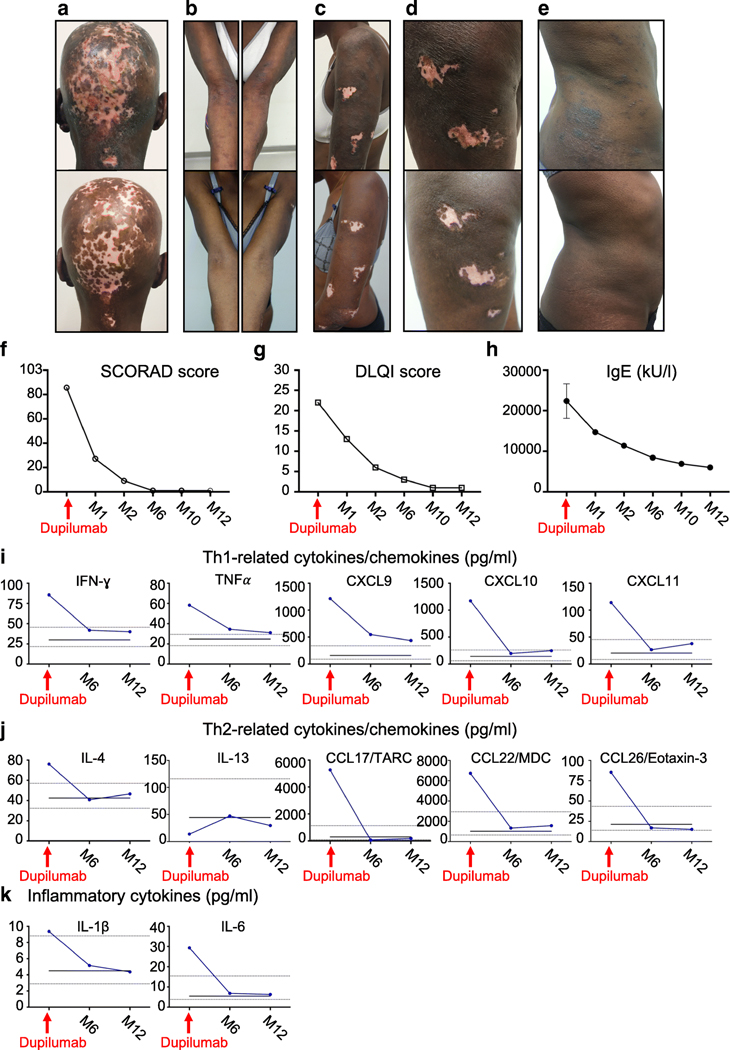
We report the successful treatment of severe atopic dermatitis in a 48-year-old patient with AR ZNF341 deficiency. This patient’s major complaint was chronic, severe, intractable, generalized pruritus. She had confluent papules on her shoulders, flank, abdomen and thighs, multiple excoriated, achromic and atrophic lesions, predominantly on her scalp, arms, legs and abdomen, fissures and lichenification of the inner elbows, and atrophic scars due to life-long scratching (Figure 1A–E). She applied ultra-high potency class I topical corticosteroids daily, and topical tacrolimus, which were ineffective. Standardized questionnaires revealed severe atopic dermatitis (SCORAD score of 85.5/103), with a profound effect on quality of life (DLQI score of 22/30).
Figure 1.
(A-E) Comparative photographs of the patient’s skin lesions, taken before dupilumab initiation (upper panels) and after one year of treatment (lower panels). From left to right, photographs of the patient’s head, forearms, left shoulder, left thigh and right flank. (F-G) Standardized SCORAD (Scoring Atopic Dermatitis) and DLQI (Dermatology Life Quality Index) scores at dupilumab initiation and at multiple time points on treatment. (H) Serum IgE levels before dupilumab initiation (mean of 7 separate samples) and at multiple time points on treatment. K-L) Levels of cytokines and chemokines in the patient’s serum, determined by LUMINEX methods, at dupilumab initiation and after six and 12 months on treatment. The black line represents the mean value for 10 healthy non-atopic individuals tested in parallel at a single time point. The dot lines represent the range of the values obtained for the 10 healthy individuals. The values are given in pg/ml. TARC: Thymus- and activation-regulated chemokine; MDC: Macrophage-derived chemokine.
Dupilumab, which blocks the alpha chain of the interleukin-4 receptor, was recently licensed for the treatment of severe atopic dermatitis in adults and in adolescents from the age of 12 years in most countries3,4. Our patient received an initial dose of 600 mg dupilumab, followed by subcutaneous injections of 300 mg dupilumab at two-week intervals. At each visit, she completed the DLQI and SCORAD questionnaires, and gave a serum sample.
The patient reported rapid improvement after three injections, with strong decreases in DLQI and SCORAD scores (Figure 1F,G). She stopped applying topical treatment after three months. Continuous treatment during one year led to complete response and was well tolerated. No further scratching-induced lesions were observed. The papulonodular lesions progressively disappeared, as did skin inflammation in previously excoriated areas (Figure 1A–E). This improvement was accompanied by a progressive decrease in total serum IgE, and allergen-specific IgE (Figure 1H, and Supplemental Figure 1). Likewise, the serum concentrations of chemokines involved in leukocyte trafficking in the skin including Th2-driven chemokines like TARC and Eotaxin-3, and inflammatory cytokines normalized over time (Figure 1 I–K).
This observation suggests that Th2-mediated IL-4 and/or IL-13 signaling plays a central role in atopic dermatitis in HIES. Dupilumab should be considered for patients with AD STAT3 or AR ZNF341 deficiency, and possibly other primary immunodeficiencies in which Th2-mediated atopic disorders are suspected or have been demonstrated, such as AR DOCK8 deficiency and X-linked WAS deficiency5.
Supplementary Material
Supplemental figure Serum allergen-specific IgE levels before (black bars) and 4 months (red bars) after dupilumab initiation, measured by ImmunoCAP ISAC chip. ISU-E: ISAC standardized unit for IgE.
Funding source
This work was supported by grants from the French National Research Agency (ANR) (grant HGDIFD no. ANR-14-CE15-0006-01; grant EURO-CMC no. ANR-14-RARE-0005-02 to Anne Puel; the National Institute of Allergy and Infectious Diseases (grant no. R01AI127564 and P01AI061093 to Jean-Laurent Casanova). Romain Lévy was supported by the INSERM-Bettencourt program.
Footnotes
Conflict of interest
The authors declare having no conflict of interest.
Ethic committee approval
Informed consent was obtained from the patient to participate in this study, in accordance with World Medical Association rules, the Helsinki Declaration, and EU directives.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Béziat V. et al. A recessive form of hyper-IgE syndrome by disruption of ZNF341-dependent STAT3 transcription and activity. Sci. Immunol 3, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frey-Jakobs S. et al. ZNF341 controls STAT3 expression and thereby immunocompetence. Sci. Immunol 3, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck LA et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N. Engl. J. Med 371, 130–139 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Simpson EL et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N. Engl. J. Med 375, 2335–2348 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Lyons JJ & Milner JD Primary atopic disorders. J. Exp. Med 215, 1009–1022 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure Serum allergen-specific IgE levels before (black bars) and 4 months (red bars) after dupilumab initiation, measured by ImmunoCAP ISAC chip. ISU-E: ISAC standardized unit for IgE.