ABSTRACT
In Saccharomyces cerevisiae cells, dysfunction of the endoplasmic reticulum (ER), so-called ER stress, leads to conversion of HAC1 mRNA to the spliced form (HAC1i), which is translated into a transcription factor that drastically changes the gene expression profile. This cellular response ultimately enhances ER functions and is named the unfolded protein response (UPR). Artificial evocation of the UPR is therefore anticipated to increase productivity of beneficial materials on and in the ER. However, as demonstrated here, cells constitutively expressing HAC1i mRNA (HAC1i cells), which exhibited a strong UPR even under nonstress conditions, grew considerably slowly and frequently yielded fast-growing and low-UPR progeny. Intriguingly, growth of HAC1i cells was faster in the presence of weak ER stress that was induced by low concentrations of the ER stressor tunicamycin or by cellular expression of the ER-located version of green fluorescent protein (GFP). HAC1i cells producing ER-localized GFP stably exhibited a strong UPR activity, carried a highly expanded ER, and abundantly produced triglycerides and heterogenous carotenoids. We therefore propose that our findings provide a basis for metabolic engineering to generate cells producing valuable lipidic molecules.
IMPORTANCE The UPR is thought to be a cellular response to cope with the accumulation of unfolded proteins in the ER. In S. cerevisiae cells, the UPR is severely repressed under nonstress conditions. The findings of this study shed light on the physiological significance of the tight regulation of the UPR. Constitutive UPR induction caused considerable growth retardation, which was partly rescued by the induction of weak ER stress. Therefore, we speculate that when the UPR is inappropriately induced in unstressed cells lacking aberrant ER client proteins, the UPR improperly impairs normal cellular functions. Another important point of this study was the generation of S. cerevisiae strains stably exhibiting a strong UPR activity and abundantly producing triglycerides and heterogenous carotenoids. We anticipate that our findings may be applied to produce valuable lipidic molecules using yeast cells as a potential next-generation technique.
KEYWORDS: Saccharomyces cerevisiae, endoplasmic reticulum, endoplasmic reticulum stress, lipid synthesis, stress response, unfolded protein response
INTRODUCTION
The endoplasmic reticulum (ER) is a flat, membrane-bound sac commonly carried by eukaryotic cells. After being folded and modified in the ER, ER client proteins are transported to the Golgi apparatus and finally delivered to the cell surface or other cellular compartments. The ER also serves as a site for lipidic molecule biosynthesis. The lipid droplets, in which triglycerides are stored, emerge from the ER (1). Upon ER stress, which frequently accompanies ER accumulation of unfolded proteins, eukaryotic cells change their transcriptome profile. This protective response is called the unfolded protein response (UPR), which, in the budding yeast Saccharomyces cerevisiae, is governed by the transcription factor Hac1 and the ER-located transmembrane endoribonuclease Ire1 (2). As shown in Fig. S1 in the supplemental material, the transcript from the HAC1 gene carries an intron sequence and is named the HAC1u mRNA (“u” stands for uninduced). In ER-stressed cells, Ire1 promotes splicing of the HAC1u mRNA to yield the HAC1i mRNA (“i” stands for induced), which is efficiently translated into Hac1. This system is conserved among a wide variety of yeast and fungal species, including Komagataella phaffii (3).
Hac1 transcriptionally induces a large number of UPR target genes, including those encoding ER-located molecular chaperone BiP (Kar2 protein in yeast), protein-folding enzymes, and enzymes for membrane lipid biogenesis (4–8). Accordingly, artificial and constitutive activation of the UPR is expected to boost the ER, occasionally leading to increased production of secretory proteins in S. cerevisiae or K. phaffi cells (9–11). Moreover, production of the terpenoid nerolidol from a genetically engineered S. cerevisiae strain was modestly increased by constitutive expression of the HAC1i mRNA (12).
However, discrepant findings have been reported regarding the effect of constitutive HAC1i expression on S. cerevisiae growth. According to Valkonen et al. (9), S. cerevisiae cells constitutively expressing the HAC1i mRNA grew almost as fast as a control strain. However, a number of other publications argued that inappropriately activated UPR is harmful for S. cerevisiae cells (13–16).
Here, we reconfirmed that artificial and constitutive expression of the HAC1i mRNA severely retards the growth of S. cerevisiae cells through strong induction of the UPR. Intriguingly, this phenotype was partially rescued by inducing weak ER stress. We also describe potential benefits of a fast-growing and HAC1i-expressing strain for the production of triglycerides and lipidic secondary metabolites.
RESULTS
Growth retardation of HAC1i-expressing cells is partially rescued by mild ER stress stimuli.
The HAC1i gene is a mutant form of the HAC1 gene in which the intron sequence is deleted. Throughout this study, we employed S. cerevisiae strains derived from the S288C-based laboratory standard haploid strain BY4742 (17), which we refer to herein as the wild-type strain (Fig. 1A). As described in Materials and Methods, we further mutagenized an IRE1 gene knockout (ire1Δ) strain to carry the HAC1i mutation on the genomic HAC1 locus. Because the resulting progeny (ire1Δ HAC1i) expressed only the HAC1i mRNA, not the HAC1u mRNA, we refer to them as HAC1i cells (Fig. 1A). In contrast, ire1Δ cells not carrying mutations on the HAC1 locus expressed only the HAC1u mRNA, and we thus call them HAC1u cells (Fig. 1A). These are schematically shown in Fig. S2. In order to confirm that the UPR is constitutively and strongly provoked in HAC1i cells, we monitored the cellular abundance of KAR2 mRNA using the reverse transcription (RT)-quantitative PCR (qPCR) technique (Fig. 1B). While tunicamycin, which is an N-glycosylation-inhibiting antibiotic and serves as a potent ER stressor, strongly induced KAR2 expression in wild-type cells, the cellular abundance of KAR2 mRNA in HAC1i cells was quite high even when they were cultured under nonstress conditions. In contrast, the cellular abundance of KAR2 mRNA in HAC1u cells was lower than that in wild-type cells cultured under nonstress conditions. This is presumably because the UPR is weakly provoked in wild-type cells even without external ER-stressing stimuli.
FIG 1.
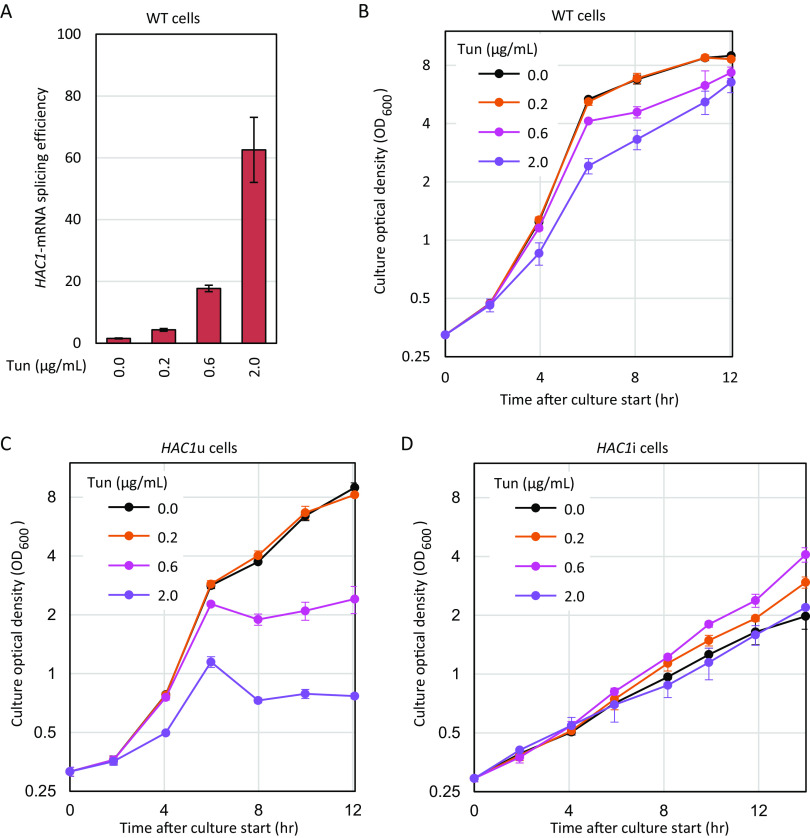
Slow-growing and strong UPR phenotypes of HAC1i cells. (A) IRE1 and HAC1 genotypes of the strains used in this study. (B to D) We cultured cells in liquid SC medium and checked them for KAR2 expression level (B) (RT-qPCR), growth (C), and DNA content (D) (flow cytometry). Tun, cells were treated with 2 μg/mL tunicamycin for 1 h. WT, wild type.
In the experiment shown in Fig. 1C, we monitored the growth of cells in a nutrient-rich synthetic medium (synthetic complete [SC] medium). HAC1i cells exhibited considerably slow growth, while HAC1u cells grew nearly as fast as wild-type cells. However, we detected no large difference in the flow cytometric profiles representing DNA content in HAC1u cells and HAC1i cells (Fig. 1D). We therefore do not attribute the slow-growth phenotype of HAC1i cells to a stage-specific cell cycle delay.
The slow-growing property of HAC1i cells seemed to be unstable. According to our preliminary experiment, growth of HAC1i cells was considerably accelerated when they were successively transferred for passage culturing. In order to demonstrate this issue under controlled experimental conditions, we repeatedly cultured HAC1i cells in liquid medium, as shown in Fig. 2A. In each cycle of the repeated culturing, HAC1i cells were incubated for 24 h, during which time the cells grew well enough to be passaged. As shown in Fig. 2B and Fig. S3, the growth of cells was accelerated alongside the repeated culturing. In order to measure the KAR2 mRNA expression level, RNA samples were extracted from cultures incubated for 12 h, in which the cells seemed to be in the exponentially growing phase (Fig. 2B). As shown in Fig. 2C, the KAR2 mRNA expression level was decreased alongside the repeated culturing. We therefore deduce that HAC1i cells frequently and spontaneously yielded fast-growing progeny that exhibited no or weak UPR. Indeed, we easily isolated fast-growing mutants of HAC1i cells, which are likely to contain missense or nonsense mutations on the HAC1i gene.
FIG 2.
Repeated cultures of HAC1i cells. (A) Procedure for repeated culturing. After every 24 h, we transferred the yeast cultures into new flasks, which were aerobically shaken and checked for optical density for the next 24 h. (B) Growth curves of HAC1i cells in the first and fourth cultures. (C) KAR2 mRNA expression levels in wild-type (WT) and HAC1i cells were determined using the RT-qPCR technique. For RNA extraction from cultures of HAC1i cells, the cells were harvested 12 h after the start of each cycle of the repeated culturing.
Next, we monitored the effect of ER stress exposure on the growth of wild-type, HAC1u, and HAC1i cells. As with KAR2 mRNA expression, HAC1 mRNA splicing in wild-type cells serves as a sharp barometer of the cellular UPR level. Figure 3A shows that different concentrations of tunicamycin induced HAC1 mRNA splicing to different levels in wild-type cells. Consistently, the growth of wild-type cells was moderately retarded by tunicamycin in a concentration-dependent manner (Fig. 3B). HAC1u cells were hypersensitive to tunicamycin because they were unable to provoke the UPR (Fig. 3C). Conversely and interestingly, tunicamycin accelerated the growth of HAC1i cells when applied at a low concentration of 0.2 or 0.6 μg/mL (Fig. 3D). However, HAC1i cells treated with a higher concentration of tunicamycin (2.0 μg/mL) grew almost as fast as those cultured under nonstress conditions (Fig. 3D). Therefore, tunicamycin appears to exert two contrasting effects on HAC1i cells. At low concentrations, tunicamycin predominantly assists growth of HAC1i cells, but this positive effect is counteracted by the toxicity associated with higher tunicamycin concentrations.
FIG 3.
Growth of cells exposed to various concentrations of tunicamycin. (A) Wild-type (WT) cells were cultured in the presence of the indicated concentrations of tunicamycin and checked for HAC1 mRNA splicing. (B to D) At time zero, tunicamycin was added at the indicated concentrations to the cultures, the optical densities of which were then monitored.
Tunicamycin totally inhibits N-glycosylation of newly synthesized proteins, possibly causing undesired side effects when applied in cultures for a long duration. We then examined if an ER stress stimulus other than tunicamycin could also increase the rate of growth of HAC1i cells. Overexpression of ER-localized proteins is known to provoke the UPR. Therefore, we transformed S. cerevisiae cells with a plasmid for expression of an ER-localized green fluorescent protein (GFP), named eroGFP (18), under the control of the strong TDH1 promoter. As expected, HAC1 mRNA splicing was moderately induced in wild-type cells expressing eroGFP (Fig. 4A). While the growth of wild-type cells did not seem to be affected by the eroGFP expression, HAC1i cells grew faster when expressing eroGFP (Fig. 4B). We failed to obtain HAC1u cells carrying the eroGFP expression plasmid, probably because HAC1u cells cannot grow even under mild ER stress conditions.
FIG 4.
Acceleration of growth of HAC1i cells by eroGFP expression. (A) Wild-type (WT) cells carrying empty vector pRS315 (EV) or eroGFP expression plasmid pLEU2-eroGFP were checked for HAC1 mRNA splicing. (B) Growth of WT cells and HAC1i cells carrying pRS315 (EV) or pLEU2-eroGFP (eroGFP) was monitored.
Further characterization of HAC1i cells expressing eroGFP toward their application for production of lipidic molecules.
In the experiment shown in Fig. 5, HAC1i cells expressing eroGFP, here called HAC1i/eroGFP cells, were subjected to repeated culturing as shown in Fig. 2A. Even after repeated culturing, HAC1i/eroGFP cell grew at an almost unchanged rate (Fig. 5A) and still exhibited high KAR2 mRNA expression (Fig. 5B). In other words, the high UPR activity of HAC1i cells was stably maintained when they were expressing eroGFP. We then focused on HAC1i/eroGFP cells to demonstrate their potential for future applications.
FIG 5.
Repeated cultures of HAC1i/eroGFP cells. HAC1i cells carrying the eroGFP expression plasmid pLEU2-eroGFP were repeatedly cultured as shown in Fig. 2A and were checked for growth (A) and KAR2 mRNA expression level (B). In panel B, wild-type (WT) cells not carrying plasmids were also assayed. For RNA extraction from cultures of HAC1i/eroGFP cells, the cells were harvested 12 h after the start of each cycle of the repeated culturing.
In the experiment shown in Fig. 6, wild-type cells expressing eroGFP and HAC1i/eroGFP cells were observed under a confocal laser scanning microscope. While we observed the typical ER shape—namely, the double rings, which represent the cortical ER and the nuclear envelope—in wild-type cells (Fig. 6A), the ER in HAC1i cells seemed to be expanded in the cytoplasm (Fig. 6B). Moreover, we often observed punctate and bright dots of eroGFP in HAC1i cells (Fig. 6B). On the other hand, ER stress induction by tunicamycin did not seem to largely change the ER morphology of wild-type cells (Fig. 6C).
FIG 6.
Enlargement of the ER in HAC1i/eroGFP cells. Wild-type (WT) cells (A and C) and HAC1i cells (B) carrying eroGFP expression plasmid pLEU2-eroGFP were observed under a laser scanning microscope. In panel C, wild-type cells were stressed by 2 μg/mL tunicamycin for 1 h before observation. An example of the expanded ER is indicated by a yellow arrowhead. An example of punctate dots of eroGFP is indicated by a red arrowhead.
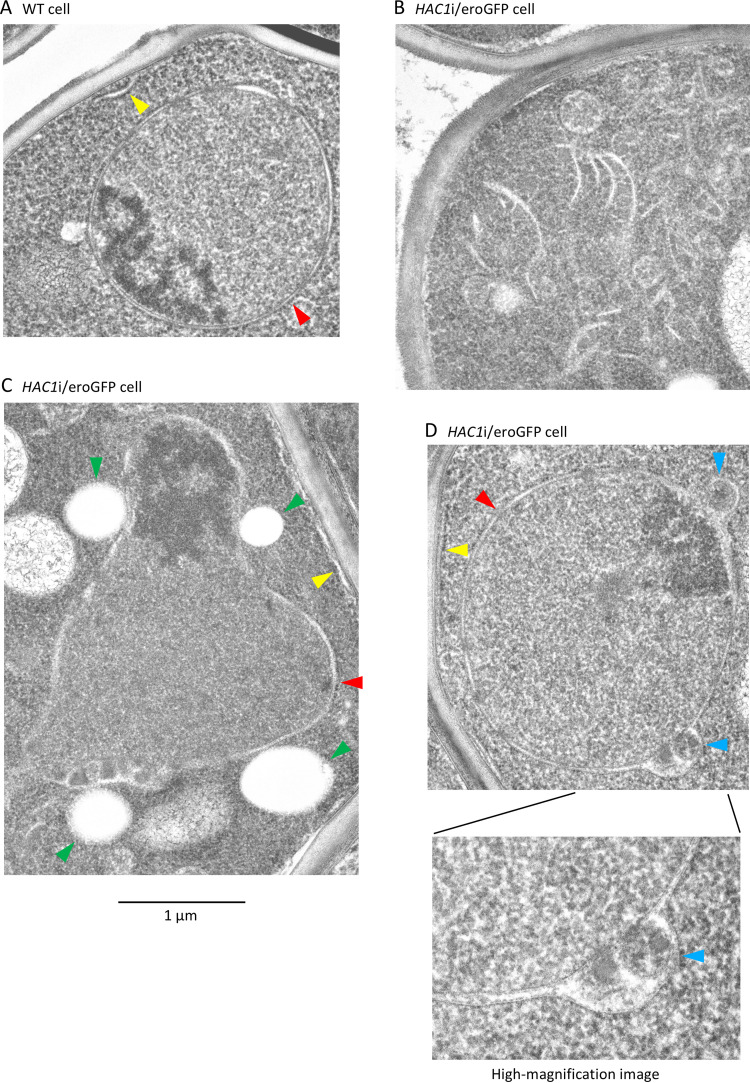
Intracellular morphology was then observed in greater detail by transmission electron microscopy. In wild-type cells, round nuclei were wrapped by a sheet of the nuclear envelope, and the cortical ER was located alongside the cell surface (Fig. 7A). However, in contrast, we observed a expanded ER-like structure (flat sacs) in the cytoplasm of HAC1i/eroGFP cells (Fig. 7B), and their nuclei were frequently deformed (Fig. 7C). In addition, unlike that of wild-type cells, the outer nuclear membrane of HAC1i/eroGFP cells often developed humps (Fig. 7D). The humps appeared to be filled with unidentified materials, which presumably include eroGFP.
FIG 7.
Deformed intracellular structure in HAC1i/eroGFP cells. Transmission electron micrographs of wild-type (WT) cells (A) and HAC1i cells carrying eroGFP expression plasmid pLEU2-eroGFP (B to D) are shown. Arrowheads indicate the following: red arrowheads, nuclear envelope; yellow arrowheads, cortical ER; green arrowheads, lipid droplets; blue arrowheads, humps of the nuclear envelope not observed in wild-type cells.
Transmission electron microscopy also suggested that HAC1i/eroGFP cells carry large and abundant lipid droplets (Fig. 7C). We confirmed this observation by staining cells with Nile red, which is a lipid droplet marker. Under the fluorescence microscope employed here, we could not detect bright fluorescent signals from HAC1i/eroGFP cells unless they were stained with Nile red (Fig. S4). In other words, this microscopy allowed us to observe the lipid droplets but not the eroGFP fluorescence. As shown in Fig. 8A, the lipid droplets seemed more apparent in HAC1i/eroGFP cells than in wild-type cells. We then biochemically monitored the cellular contents of triglycerides and found that HAC1i/eroGFP cells contain triglycerides approximately 6-fold more abundantly than wild-type cells (Fig. 8B).
FIG 8.
High production of lipidic molecules in HAC1i/eroGFP cells. (A and B) Wild-type (WT) cells and HAC1i cells carrying eroGFP expression plasmid pLEU2-eroGFP were stained with Nile red for observation under a conventional fluorescence microscope (A) (red fluorescence of Nile Red) or checked for intracellular triglyceride contents (B). (C) Cells transformed with empty vector pRS316 or with plasmid YEplac195-YB/I/BTS1, which was used for heterologous expression of the β-carotene biosynthesis genes, were checked for intracellular triglyceride contents. The results are indicated as values relative to those of WT cells carrying YEplac195-YB/I/BTS1.
According to Kim et al. (19), enlargement of the ER leads to an increase in terpenoid production in S. cerevisiae cells. As mentioned in the Discussion, terpenoid production in yeast is a promising next-generation biotechnology. In the present study, using carotenoids as a model, we thus monitored the productivity of heterologous terpenoid in HAC1i/eroGFP cells. In the experiment shown in Fig. 8C, we transformed wild-type cells and HAC1i/eroGFP cells with a plasmid for heterologous expression of Xanthophyllomyces dendrorhous genes encoding enzymes for the β-carotene biosynthesis pathway (20). As expected, HAC1i/eroGFP cells contained more abundant carotenoids than wild-type cells.
DISCUSSION
As shown in the present study, artificial and constitutive HAC1i expression strongly induces the UPR and severely retards the growth of S. cerevisiae cells. This issue is not confined to BY4742-based strains, since similar phenomena have been observed in other strain backgrounds (13, 21). Consistently, growth of S. cerevisiae cells is retarded by improper activation of Ire1, which also inappropriately induces the UPR (16, 22). In wild-type cells, inappropriate UPR is avoided, since the activity of Ire1 and cellular abundance of Hac1 are tightly controlled in multiple ways (2). It should also be noted that as shown in Fig. S1 in the supplemental material, the HAC1u mRNA hardly provokes the UPR.
Nevertheless, according to some previous publications by others, constitutive HAC1i expression in S. cerevisiae cells results in somewhat different outcomes. Valkonen et al. (9) reported that S. cerevisiae cells constitutively expressing the HAC1i mRNA grew as fast as normal cells. Moreover, Qu et al. (12) described a HAC1i-expressing strain in which the UPR was induced only slightly. We speculate that in these studies, the expression level of the HAC1i mRNA may have been insufficiently high for induction of a strong UPR. It is also possible that the HAC1i-expressing strains carried unnoticed mutations to grow fast and suppress the UPR. In our hands, such mutants easily appeared and became dominant in repeated cultures of HAC1i cells.
Here, we found that the growth of HAC1i cells was accelerated by mild ER stress induced by a low concentration of tunicamycin or by eroGFP expression. From the viewpoint of application usages, the fast-growing property of HAC1i/eroGFP cells should be beneficial for the rapid production of desired materials. Moreover, unlike HAC1i cells, HAC1i/eroGFP cells stably exhibited a strong UPR even after long-term culturing. This is probably because, since HAC1i/eroGFP cells grew fast, their mutants in which the UPR was compromised were hardly given an opportunity to become dominant in cultures of HAC1i/eroGFP cells. In addition, eroGFP causes ER stress, albeit weakly, leading to growth inhibition of no- or low-UPR mutants of HAC1i/eroGFP cells.
Hac1 directly or indirectly induces the expression of hundreds of UPR target genes, which includes those encoding ER-located molecular chaperones and factors for ER-associated protein degradation (ERAD) (4, 5). Fordyce et al. (23) reported that Hac1 binds to two distinct promoter elements. Moreover, Hac1 also yields long undecoded transcript isoforms, leading to the decreased production of certain proteins (24). Therefore, it is difficult to determine the exact reason why HAC1i cells grew considerably slowly.
One possible explanation for the harmful effect of constitutive HAC1i expression is that the UPR target genes are highly and unwantedly transcribed and translated, leading to unnecessary consumption of cellular resources to produce mRNAs and proteins. In addition to this idea, our finding that mild ER stress stimuli accelerate the growth of HAC1i cells raised another hypothesis suggesting that the inappropriate upregulation of ER machineries in unstressed HAC1i cells damages normal proteins because of the lack of unfolded proteins. Excess molecular chaperones may inhibit protein folding in the ER. It is also possible that improperly upregulated ERAD degrades normal proteins. On the other hand, mild ER stress stimuli may generate unfolded proteins shielding normal proteins. In HAC1i/eroGFP cells, eroGFP seemed to form large aggregates in the ER, which may sequester unnecessary ER proteins that were responsible for the growth retardation of HAC1i cells.
Schuck et al. (7) reported that the ER expands with the UPR in S. cerevisiae cells. However, here we were hardly able to observe ER expansion in tunicamycin-treated wild-type cells. In contrast, HAC1i/eroGFP cells had enlarged ERs with complicated morphologies. Therefore, it is likely that a strong and chronic UPR is required to significantly expand the ER. The deformation of nuclei in HAC1i/eroGFP cells is probably a consequence of the ER expansion, since nuclei are also deformed in S. cerevisiae cells upon impairment of COPII vesicle formation on the ER (25).
As described so far, HAC1i/eroGFP cells stably exhibited a high UPR, leading to the enlargement of the ER. We therefore anticipate that HAC1i/eroGFP cells can be used as the basis of genetic engineering tools for high-yield production of beneficial materials on and in the ER. As shown here, HAC1i/eroGFP cells carried abundant and large lipid droplets. Moreover, they contained a considerably larger amount of triacylglycerol than did wild-type cells. These findings seem to be consistent with the commonly accepted view that lipid droplets derive from the ER (1). Nevertheless, wide-range proteome and metabolome analyses may be required to elucidate the molecular basis by which the unregulated UPR elevates triacylglycerol productivity.
Kim et al. (19) described the involvement of the ER in the heterogenous production of terpenoids in S. cerevisiae cells. Moreover, Qu et al. (12) reported that HAC1i expression enhances heterogenous production of a sesquiterpene compound, nerolidol, albeit modestly. In agreement with these findings, here we demonstrated that when transformed with a plasmid for heterogenous β-carotene production, HAC1i/eroGFP cells accumulate a drastically higher amount of carotenoids than do wild-type cells. We therefore speculate that the productivity of lipidic molecules may be totally enhanced in HAC1i/eroGFP cells. It should be also noted that triacylglycerol productivity and heterogenous carotenoid productivity are highly correlated in S. cerevisiae cells (26).
Figure 9 represents a model to explain our findings shown in this study. The constitutively high UPR transcriptionally induces a number of proteins, which include ER-located molecular chaperones, ERAD factors, and enzymes for membrane lipid biogenesis, in HAC1i cells (4, 5). Inappropriately induced molecular chaperones and/or ERAD factors damage normal proteins, leading to growth retardation of HAC1i cells. However, ER stress stimuli lead to ER accumulation of malfolded proteins, which shield normal proteins. Since lipid biogenesis is upregulated even under weak ER stress conditions in HAC1i cells, these cells produce lipidic molecules abundantly, albeit while growing quickly.
FIG 9.
Model to explain our observations obtained from this study. See the text for details.
Several techniques have been developed to genetically engineer S. cerevisiae cells to modify metabolic flux in order to increase the production of fatty acids that may be used as biofuels in the future (27). Moreover, metabolic engineering is also ongoing for heterogenous production of terpenoids from yeast cells (19, 28). We expect that higher-utility strains will be created by combining genetic modifications that directly alter metabolic pathways with those developed in our present study. The genetic manipulation that we propose here is a simple method that drastically and stably changes cellular properties by only introducing the HAC1i mutation and inducing mild ER stress. We think that other means besides eroGFP expression can also be used to induce mild ER stress for this purpose. Therefore, this method can be easily applied to other yeast species for which genetic manipulation techniques are still at a primary level.
MATERIALS AND METHODS
Yeast strains and plasmids.
We used the S. cerevisiae S288C-based standard laboratory strain BY4742 (MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 [17]) as the wild-type strain. The KanMX-based ire1Δ deletion strain (BY4742 ire1::kanMX4 [29]) was obtained from Euroscarf (http://www.euroscarf.de/).
We constructed HAC1u cells and HAC1i cells as described previously (5). In brief, BY4742 ire1::kanMX4 cells were transformed with plasmid pRS306-partialHAC1i, which carries a partial fragment of the HAC1i gene. The genomic HAC1 locus of the resulting transformant carried the unmutagenized HAC1 gene (HAC1u gene) and a partial fragment of the HAC1i gene, and the URA3 gene was interposed between these two genes. We then obtained ura3 progeny from the transformant through URA3 counterselection using 5-fluoroorotic acid (5-FOA) (30). Because of the pop-out homologous recombination, the ura3 progeny did not contain genomically integrated pRS306-partialHAC1i and carried only one copy of the HAC1 gene (either the HAC1u gene or the HAC1i mutant gene) on the HAC1 locus.
The eroGFP expression module (TDH1 promoter-eroGFP coding gene-CYC1 terminator) on plasmid pPM28 (18) was transferred to the centromeric vector pRS315 (the LEU2 marker) (31), and the resulting plasmid was named pLEU2-eroGFP. We used the URA3 marker 2 μ-based episomal plasmid YEplac195-YB/I/BTS1 for expression of the Xanthophyllomyces dendrorhous carotenoid biosynthesis genes (20). In addition to pRS315, a URA3 marker plasmid pRS316 (31) served as an empty vector control.
Yeast growth and stress exposure.
SC medium contained 2% glucose, 0.66% Difco yeast nitrogen base (without amino acids; BD Biosciences, Franklin Lakes, NJ, USA), and a wide variety of amino acid and vitamin supplements (30). To culture cells containing a plasmid, we modified SC medium for auxotrophic selection. The optical density at 600 nm (OD600) of cultures was measured using a Bio-Rad SmartSpec 3000 spectrophotometer (Bio-Rad, Hercules, CA, USA). S. cerevisiae cells were grown at 30°C in aerobically shaken SC cultures. Precultures were diluted to an OD600 of 0.30 to 0.35, and cells were harvested at the fast-growing phase. Tunicamycin (Sigma-Aldrich, St. Louis, MO, USA) was prepared as a stock solution at 2 mg/mL in dimethyl sulfoxide.
RNA analysis.
Total RNA samples were extracted, treated with DNase I, and subjected to oligo(dT)-primed reverse transcription (RT) in accordance with our previous publication (16). To analyze the KAR2 mRNA abundance, we analyzed the cDNA samples by real-time quantitative PCR (qPCR) as described by Tran et al. (8). The TAF10 primer sets served as the internal control. In accordance with our previous publication (32), we also analyzed the cDNA samples through qPCR with the HAC1i-specific primer set and the primer set that totally amplifies the total HAC1 species (HAC1i plus HAC1u). Then, the HAC1 mRNA splicing efficiency was calculated as the relative abundance of the HAC1i mRNA against that of the total HAC1 species. The cloned HAC1i gene was used for correction of the calculation.
Fluorescence microscopy.
We observed GFP fluorescence in yeast cells under a laser scanning microscope (SP8 Falcon; Leica Microsystems, Wetzlar, Germany) with an HC PL APO 100×/1.40 oil STED white objective lens. Cells were illuminated by a 489-nm white light laser, and emission photons were collected by an internal Leica HyD hybrid detector with a spectral window of 500 to 600 nm.
For the staining of lipid droplets, cells (approximately equivalent to an OD600 of 1.0) were suspended in 200 μL phosphate-buffered saline (PBS), into which 2 μL Nile red solution (100 μg/mL acetone; Fujifilm Wako Chemicals, Miyazaki, Japan) and 2 μL dimethyl sulfoxide were added. We then observed the cells under a Keyence BZ-9000 nonconfocal fluorescence microscope (Keyence, Osaka, Japan) with the CFI Plan Apochromat λ100×H objective lens (Nikon, Tokyo, Japan) and the preset fluorescence filters for visualization of Texas red fluorescence.
Flow cytometry.
We fixed the cells in ice-cold 70% ethanol and resuspended them with 50 mM sodium citrate. Cells were then treated with 0.1 mg/mL RNase A at 37°C for 2 h and stained with 4 μg/mL propidium iodide. We detected the fluorescence signal by using an Accuri C6 flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) through the FL2 channel.
Triglyceride measurement.
We suspended cells (equivalent to an OD600 of 80) in a solution composed of 200 μL water, 730 μL methanol, and 270 μL chloroform and disrupted them by agitation with glass beads (425 to 600 μm; Sigma-Aldrich, St. Louis, MO, USA). After the addition of 300 μL water and 400 μL chloroform to the lysates, organic layers were obtained, dried under vacuum, and subjected to the measurement of triglyceride amounts using the LabAssay triglyceride kit (Fujifilm Wako Chemicals, Miyazaki, Japan).
Carotenoid measurement.
Cells (equivalent to an OD600 of 25) were suspended in 200 μL water, broken by agitation with 200 μL glass beads (425 to 600 μm; Sigma-Aldrich, St. Louis, MO, USA), and mixed with 500 μL methanol containing 0.2% pyrogallol and 250 μL 60% (wt/vol) potassium hydroxide. The mixture was then incubated at 70°C for saponification and lipidic components were extracted by using 500 μL n-hexane, the total colored carotenoid concentration of which was estimated by measuring the optical absorbance at 449 nm.
Transmission electron microscopy.
All transmission electron microscopy procedures were performed by Tokai Electron Microscopy. In brief, cells were sandwiched between copper disks and quickly frozen in liquid propane at −175°C. For freeze substitution, the samples were stored in acetone and 2% water containing 1% tannic acid and 2% glutaraldehyde at −80°C for 2 days. The fixation was performed in acetone containing 2% osmium tetroxide at room temperature for 60 min. The samples were then embedded into Quetol 651 resin (Nisshin EM, Tokyo, Japan). Ultrathin sections (70 nm) were stained with a lead stain solution (Sigma-Aldrich) at room temperature for 3 min and observed under a transmission electron microscope (JEM-1400Plus; JEOL Ltd., Tokyo, Japan) at an acceleration voltage of 100 kV. Digital images were taken with a charge-coupled device camera (EM-14830RUBY2; JEOL Ltd., Tokyo, Japan).
Statistical analysis.
Basically, we analyzed three independent clones of the same genotype and express the resulting values as the averages and standard deviations from triplicate biological replicates.
Data availability.
All of the original data that we used to prepare Fig. 1 to 8 are available on reasonable request to the corresponding author.
ACKNOWLEDGMENTS
We deeply thank Hiroshi Takagi (Nara Institute of Science and Technology) for a wide variety of support.
This work was funded by a research grant to Y.K. from the Noda Institute for Scientific Research.
We declare that there are no conflicts of interest.
Y.K. and P.T.M.N. developed the theoretical framework. P.T.M.N. and Y.I.-K. constructed the yeast strains and performed the experiments. Y.K. designed the experiments and completed the manuscript.
Footnotes
Supplemental material is available online only.
Contributor Information
Yukio Kimata, Email: kimata@bs.naist.jp.
Isaac Cann, University of Illinois at Urbana-Champaign.
REFERENCES
- 1.Graef M. 2018. Lipid droplet-mediated lipid and protein homeostasis in budding yeast. FEBS Lett 592:1291–1303. 10.1002/1873-3468.12996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le QG, Kimata Y. 2021. Multiple ways for stress sensing and regulation of the endoplasmic reticulum-stress sensors. Cell Struct Funct 46:37–49. 10.1247/csf.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fauzee YNBM, Taniguchi N, Ishiwata-Kimata Y, Takagi H, Kimata Y. 2020. The unfolded protein response in Pichia pastoris without external stressing stimuli. FEMS Yeast Res 20:foaa053. 10.1093/femsyr/foaa053. [DOI] [PubMed] [Google Scholar]
- 4.Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P. 2000. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 101:249–258. 10.1016/s0092-8674(00)80835-1. [DOI] [PubMed] [Google Scholar]
- 5.Kimata Y, Ishiwata-Kimata Y, Yamada S, Kohno K. 2006. Yeast unfolded protein response pathway regulates expression of genes for anti-oxidative stress and for cell surface proteins. Genes Cells 11:59–69. 10.1111/j.1365-2443.2005.00921.x. [DOI] [PubMed] [Google Scholar]
- 6.Graf A, Gasser B, Dragosits M, Sauer M, Leparc GG, Tüchler T, Kreil DP, Mattanovich D. 2008. Novel insights into the unfolded protein response using Pichia pastoris specific DNA microarrays. BMC Genomics 9:390. 10.1186/1471-2164-9-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuck S, Prinz WA, Thorn KS, Voss C, Walter P. 2009. Membrane expansion alleviates endoplasmic reticulum stress independently of the unfolded protein response. J Cell Biol 187:525–536. 10.1083/jcb.200907074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tran DM, Ishiwata-Kimata Y, Mai TC, Kubo M, Kimata Y. 2019. The unfolded protein response alongside the diauxic shift of yeast cells and its involvement in mitochondria enlargement. Sci Rep 9:12780. 10.1038/s41598-019-49146-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valkonen M, Penttilä M, Saloheimo M. 2003. Effects of inactivation and constitutive expression of the unfolded-protein response pathway on protein production in the yeast Saccharomyces cerevisiae. Appl Environ Microbiol 69:2065–2072. 10.1128/AEM.69.4.2065-2072.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gasser B, Maurer M, Gach J, Kunert R, Mattanovich D. 2006. Engineering of Pichia pastoris for improved production of antibody fragments. Biotechnol Bioeng 94:353–361. 10.1002/bit.20851. [DOI] [PubMed] [Google Scholar]
- 11.Raschmanová H, Weninger A, Knejzlík Z, Melzoch K, Kovar K. 2021. Engineering of the unfolded protein response pathway in Pichia pastoris: enhancing production of secreted recombinant proteins. Appl Microbiol Biotechnol 105:4397–4414. 10.1007/s00253-021-11336-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qu Z, Zhang L, Zhu S, Yuan W, Hang J, Yin D, Tang X, Zheng J, Wang Z, Sun J. 2020. Overexpression of the transcription factor HAC1 improves nerolidol production in engineered yeast. Enzyme Microb Technol 134:109485. 10.1016/j.enzmictec.2019.109485. [DOI] [PubMed] [Google Scholar]
- 13.Mori K, Ogawa N, Kawahara T, Yanagi H, Yura T. 2000. mRNA splicing-mediated C-terminal replacement of transcription factor Hac1p is required for efficient activation of the unfolded protein response. Proc Natl Acad Sci USA 97:4660–4665. 10.1073/pnas.050010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chawla A, Chakrabarti S, Ghosh G, Niwa M. 2011. Attenuation of yeast UPR is essential for survival and is mediated by IRE1 kinase. J Cell Biol 193:41–50. 10.1083/jcb.201008071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubio C, Pincus D, Korennykh A, Schuck S, El-Samad H, Walter P. 2011. Homeostatic adaptation to endoplasmic reticulum stress depends on Ire1 kinase activity. J Cell Biol 193:171–184. 10.1083/jcb.201007077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le QG, Ishiwata-Kimata Y, Phuong TH, Fukunaka S, Kohno K, Kimata Y. 2021. The ADP-binding kinase region of Ire1 directly contributes to its responsiveness to endoplasmic reticulum stress. Sci Rep 11:4506. 10.1038/s41598-021-83890-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brachmann CB, Davies A, Cost GJ, Caputo E, Li J, Hieter P, Boeke JD. 1998. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14:115–132. . [DOI] [PubMed] [Google Scholar]
- 18.Merksamer PI, Trusina A, Papa FR. 2008. Real-time redox measurements during endoplasmic reticulum stress reveal interlinked protein folding functions. Cell 135:933–947. 10.1016/j.cell.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JE, Jang IS, Son SH, Ko YJ, Cho BK, Kim SC, Lee JY. 2019. Tailoring the Saccharomyces cerevisiae endoplasmic reticulum for functional assembly of terpene synthesis pathway. Metab Eng 56:50–59. 10.1016/j.ymben.2019.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Verwaal R, Wang J, Meijnen JP, Visser H, Sandmann G, van den Berg JA, van Ooyen AJ. 2007. High-level production of beta-carotene in Saccharomyces cerevisiae by successive transformation with carotenogenic genes from Xanthophyllomyces dendrorhous. Appl Environ Microbiol 73:4342–4350. 10.1128/AEM.02759-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higashio H, Kohno K. 2002. A genetic link between the unfolded protein response and vesicle formation from the endoplasmic reticulum. Biochem Biophys Res Commun 296:568–574. 10.1016/S0006-291X(02)00923-3. [DOI] [PubMed] [Google Scholar]
- 22.Mathuranyanon R, Tsukamoto T, Takeuchi A, Ishiwata-Kimata Y, Tsuchiya Y, Kohno K, Kimata Y. 2015. Tight regulation of the unfolded protein sensor Ire1 by its intramolecularly antagonizing subdomain. J Cell Sci 128:1762–1772. 10.1242/jcs.164111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fordyce PM, Pincus D, Kimmig P, Nelson CS, El-Samad H, Walter P, DeRisi JL. 2012. Basic leucine zipper transcription factor Hac1 binds DNA in two distinct modes as revealed by microfluidic analyses. Proc Natl Acad Sci USA 109:E3084–E3093. 10.1073/pnas.1212457109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Dalfsen KM, Hodapp S, Keskin A, Otto GM, Berdan CA, Higdon A, Cheunkarndee T, Nomura DK, Jovanovic M, Brar GA. 2018. Global proteome remodeling during ER stress involves Hac1-driven expression of long undecoded transcript isoforms. Dev Cell 46:219–235.e8. 10.1016/j.devcel.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimata Y, Lim CR, Kiriyama T, Nara A, Hirata A, Kohno K. 1999. Mutation of the yeast epsilon-COP gene ANU2 causes abnormal nuclear morphology and defects in intracellular vesicular transport. Cell Struct Funct 24:197–208. 10.1247/csf.24.197. [DOI] [PubMed] [Google Scholar]
- 26.Bu X, Lin JY, Duan CQ, Koffas MAG, Yan GL. 2022. Dual regulation of lipid droplet-triacylglycerol metabolism and ERG9 expression for improved β-carotene production in Saccharomyces cerevisiae. Microb Cell Fact 21:3. 10.1186/s12934-021-01723-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu Y, Zhu Z, Nielsen J, Siewers V. 2019. Engineering Saccharomyces cerevisiae cells for production of fatty acid-derived biofuels and chemicals. Open Biol 9:190049. 10.1098/rsob.190049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma T, Shi B, Ye Z, Li X, Liu M, Chen Y, Xia J, Nielsen J, Deng Z, Liu T. 2019. Lipid engineering combined with systematic metabolic engineering of Saccharomyces cerevisiae for high-yield production of lycopene. Metab Eng 52:134–142. 10.1016/j.ymben.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Giaever G, Nislow C. 2014. The yeast deletion collection: a decade of functional genomics. Genetics 197:451–465. 10.1534/genetics.114.161620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adams A, Gottschling DE, Kaiser CA, Stearns T. 1997. Methods in yeast genetics: a Cold Spring Harbor laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 31.Sikorski RS, Hieter P. 1989. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122:19–27. 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hata T, Ishiwata-Kimata Y, Kimata Y. 2022. Induction of the unfolded protein response at high temperature in Saccharomyces cerevisiae. Int J Mol Sci 23:1669. 10.3390/ijms23031669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download aem.01083-22-s0001.pdf, PDF file, 0.1 MB (59KB, pdf)
Data Availability Statement
All of the original data that we used to prepare Fig. 1 to 8 are available on reasonable request to the corresponding author.