Abstract
Thrombocytopenia, anasarca, fever, reticulin fibrosis, renal insufficiency, and organomegaly (TAFRO) syndrome is a heterogeneous entity manifesting with a constellation of symptoms described above that can occur in the context of idiopathic multicentric Castleman disease (iMCD) as well as infectious diseases, malignancies, and rheumatologic disorders. iMCD-TAFRO is an aggressive subtype of iMCD with TAFRO syndrome and often hyper-vascularized lymph nodes. Since we proposed diagnostic criteria of iMCD-TAFRO in 2016, we have accumulated new insights on the disorder and additional cases have been reported worldwide. In this systematic review and cohort analysis, we established and validated a definition for iMCD-TAFRO. First, we searched PubMed and Japan Medical Abstracts Society databases using the keyword “TAFRO” to extract cases. Patients with possible systemic autoimmune diseases and hematologic malignancies were excluded. Our search identified 54 cases from 50 articles. We classified cases into 3 categories: 1) iMCD-TAFRO (TAFRO syndrome with lymph node histopathology consistent with iMCD), 2) possible iMCD-TAFRO (TAFRO syndrome with no lymph node biopsy performed / no other co-morbidities), and 3) TAFRO without iMCD or other co-morbidities (TAFRO syndrome with lymph node histopathology not consistent with iMCD or other comorbidities). Based on the findings, we propose an international definition requiring 4 clinical criteria (thrombocytopenia, anasarca, fever/hyperinflammatory status, organomegaly), renal dysfunction or characteristic bone marrow findings, and lymph node features consistent with iMCD. The definition was validated with an external cohort (the ACCELERATE Natural History Registry). The present international definition will facilitate a more precise and comprehensive approach to the diagnosis of iMCD-TAFRO.
Introduction
In 2010, Takai et al. first reported a series of cases exclusively in Japan with a constellation of non-specific clinical symptoms, including thrombocytopenia (T), anasarca (A), fever (F), reticulin fibrosis or renal insufficiency (R), and organomegaly (O)1. Since it was first described, a number of cases of TAFRO syndrome have been reported worldwide2-10. This heterogeneous clinical entity can occur in the context of infectious diseases, malignancies, rheumatologic disorders, and idiopathic multicentric Castleman disease (iMCD). Multicentric Castleman disease (MCD) is a rare heterogenous systemic disorder characterized by systemic inflammation, multicentric lymphadenopathy with characteristic histopathological features, and organ dysfunction due to elevated pro-inflammatory cytokines including interleukin-6 (IL-6)11-16. Although MCD can be caused by uncontrolled human herpes virus 8 (HHV-8) infection in immunocompromised patients17, approximately 50% of MCD cases are human immunodeficiency virus (HIV)-negative and HHV-8-negative MCD and have an unknown etiology (idiopathic MCD; iMCD)18,19.
We previously reported that patients with both iMCD and TAFRO had uniform clinical features and pathological findings with hyper-vascular proliferation in lymph nodes as well as myelofibrosis and megakaryocyte hyperplasia in bone marrow13,20. Thereafter, iMCD patients with TAFRO (iMCD-TAFRO) symptoms have been considered to have an aggressive clinical subtype of iMCD19. iMCD patients who do not meet the criteria for TAFRO often have elevated platelet counts, hypergammaglobulinemia, and a less aggressive course; these cases are described as iMCD not otherwise specified (iMCD-NOS). While anti-interleukin-6 therapy is recommended first-line for iMCD-TAFRO and iMCD-NOS patients, it is helpful to distinguish these subgroups as iMCD-TAFRO is typically more aggressive and additional therapies are often needed in patients who may not have sufficient time to wait for a clinical response.
While elevated serum alkaline phosphatase (ALP) without hyperbilirubinemia and transaminase elevation or the lack of polyclonal hypergammaglobulinemia can also help to distinguish iMCD-TAFRO from iMCD-NOS, no specific disease markers have been found to date. Due to the lack of specific disease markers,21 poor understanding of pathophysiology, rapid clinical deterioration at the onset of disease, intense thrombocytopenia, and small volume lymphadenopathy making lymph node biopsy challenging, the diagnosis of iMCD-TAFRO has been extremely difficult22. It is also imperative to exclude potential differential diagnoses that can have similar clinical presentations including fever of unknown origin, hyperinflammatory status, anasarca, and other common features (Figure 1). Although several diagnostic criteria have been proposed for iMCD and for TAFRO13-15,23, no consensus definition has been established for iMCD-TAFRO based on international data despite its aggressive clinical presentation and high mortality. The purpose of this study is to establish an up-to-date international definition of iMCD-TAFRO based on a comprehensive clinicopathological review of iMCD-TAFRO cases from the literature and a natural history registry.
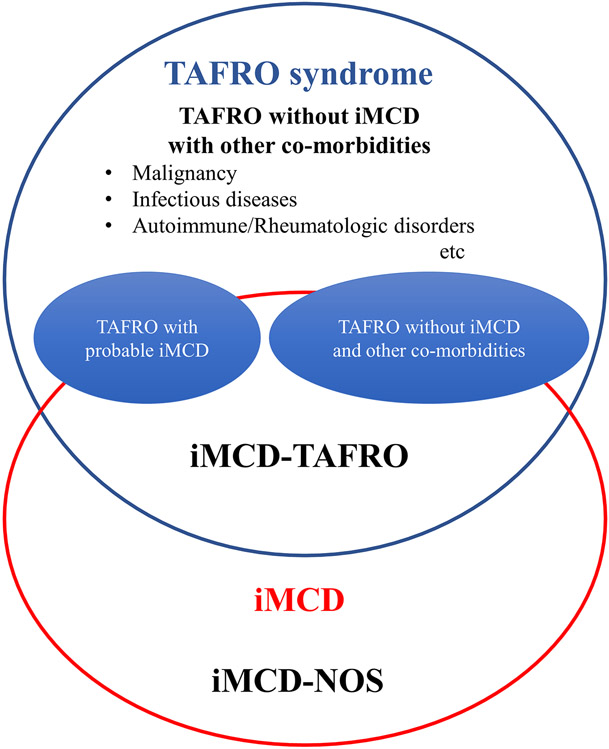
Figure 1: Concepts of TAFRO syndrome and iMCD-TAFRO.
TAFRO syndrome is a heterogenous clinical entity with a constellation of non-specific clinical symptoms including thrombocytopenia (T), anasarca (A), fever (F), reticulin fibrosis or renal insufficiency (R), and organomegaly (O). Due to its heterogeneity, TAFRO syndrome includes various clinical conditions such as malignancies, rheumatologic disorders, infections, and POEMS syndrome. The figure conceptualizes five different classifications related to TAFRO syndrome and iMCD-TAFRO. The present study included cases with iMCD-TAFRO, TAFRO with possible iMCD without lymph node biopsy and other co-morbidities (TAFRO with possible iMCD), and TAFRO without histologically proven iMCD and other co-morbidities (TAFRO without iMCD and other co-morbidities). Attention needs to be paid not to confuse TAFRO syndrome and iMCD-TAFRO.
Abbreviation: iMCD-TAFRO, TAFRO clinical subtype of idiopathic multicentric Castleman disease; iMCD-NOS, idiopathic multicentric Castleman disease not otherwise specified.
Methods
Literature search and selection criteria
We performed a systematic literature review of iMCD-TAFRO to extract data on clinical features including thrombocytopenia, anasarca, fever, renal dysfunction and organomegaly, laboratory data, lymph node size, and histopathological characteristics. To identify cases for this review, we searched for articles published in PubMed and Japan Medical Abstracts Society databases, which includes manuscripts published in Japanese journals, as of May 2019, with the term “TAFRO”. We also screened the reference lists of the retrieved articles to find any eligible cases. Duplicate publications and non-peer reviewed articles were excluded at the first stage by reviewing abstracts. Using the strategy, we extracted 65 case report or case series articles including 75 patients with iMCD-TAFRO. We excluded 21 cases for the following reasons: if cases showed decreases in both complement component 3 and 4 that could be suggestive of systemic lupus erythematosus (SLE, 8 cases); if cases were diagnosed or associated with Sjögren syndrome (SjS, 4 cases); if patients had suspected POEMS syndrome with positive λ light chain restricted monoclonal protein or were diagnosed as diffuse large B-cell lymphoma (DLBCL) during the clinical courses (4 cases); or if patients had any findings that were suggestive of other differential diagnoses including cytoplasmic anti-neutrophil cytoplasmic antibody, human herpesvirus 8-DNA or Epstein-Barr virus (EBV) in the bone marrow, extremely elevated immunoglobulin-G (IgG), and positive deposits of immunoglobulin-A and M (IgA, IgM) as well as C3 in the kidney biopsy specimen (5 cases). As a result, 54 cases were included in this study (Figure 1). Two independent investigators (N.I., and Y.N.) performed the search and confirmed that the 54 cases had none of the exclusion criteria. We further classified the 54 cases into 3 categories; iMCD-TAFRO (Group 1: cases with TAFRO syndrome with lymph node histopathology which is consistent with iMCD), TAFRO with possible iMCD (Group 2: cases with TAFRO syndrome with no lymph node biopsy performed and no other co-morbidities), and TAFRO without iMCD or other co-morbidities (Group 3: cases with TAFRO syndrome with lymph node histopathology not consistent with iMCD, but no other comorbidities identified). Serum alkaline phosphatase (ALP) values of the Japanese articles were converted to the International Federation of Clinical Chemistry and Laboratory Medicine values (global standard) from the Japan Society of Clinical Chemistry values by multiplying 0.35 as appropriate24. We judged that the data were missing if any specific variables were not available in the articles.
Statistical analysis
We calculated summary statistics for different variables by tabulation including percentages. We used the Wilcoxon test to compare the continuous variables between Group 1 and Group 2. The Fisher’s exact test was used to compare the categorical data between the two groups. The threshold for significance was defined as the p-value < 0.05. Due to the small number of cases in Group 3, statistical analysis to compare the differences between Group 1 or 2 and Group 3 were deferred. All statistical analyses were conducted with JMP Version 15.1 (SAS Institute, Cary, NC, USA).
Results
Clinical and Radiological Features
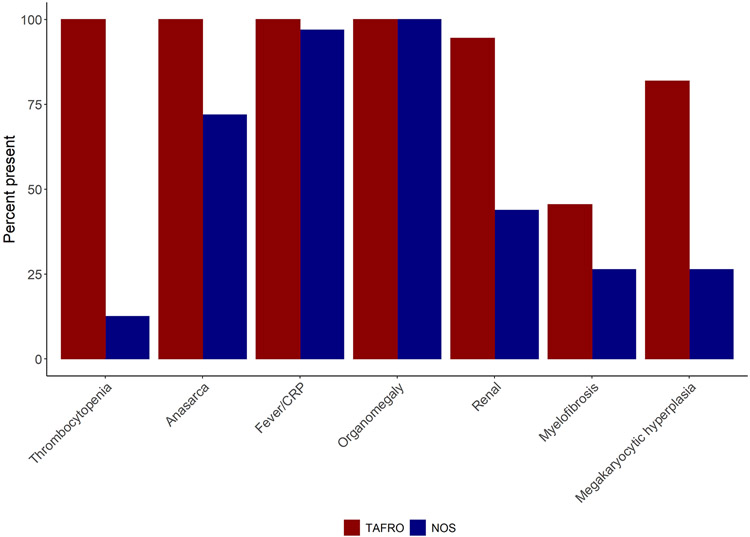
Supplementary Figure 1 describes our search and article selection strategy. Our systematic review identified 175 articles published between January 2013 and May 2019 about iMCD-TAFRO. Of the 75 patients identified, 21 patients were excluded from the study as noted above. Demographics as well as chief clinical and radiological features of the 54 patients are presented in Supplementary Table 1 according to the following groups: cases with TAFRO syndrome and lymph node histopathology consistent with iMCD (Group 1), cases with TAFRO syndrome with no lymph node biopsy performed and no other co-morbidities (Group 2), and cases with TAFRO syndrome with lymph node histopathology that is not consistent with iMCD, but no other comorbidities identified (Group 3). The median ages of patients in the three groups were 54 years, 66 years, and 47 years, respectively, with a slight male predominance overall. Asian patients were predominant but our cohorts included 8 Caucasian and 1 Hispanic patients. Thrombocytopenia (T of TAFRO), defined as a platelet level of less than 10 x 104/μL, upon the pre-treatment nadir was prevalent in all cases. All the cases had some types of anasarca on computed tomography (CT) or fluorodeoxyglucose-positron emission tomography/CT (FDG-PET/CT) (A). Pleural effusion and ascites were more common than subcutaneous edema or pericardial fluid. Regarding fever and inflammation (F), all patients had either fever of more than 37.5°C or CRP of more than 2.0 mg/dL. Of the 47 patients with recorded minimum pre-treatment estimated glomerular filtration rate (eGFR), 27/35 (77.1%) in Group 1, 8/10 (80.0%) in Group 2, and 1/2 (50.0%) in Group 3 had eGFR less than 60 mL/min/1.73 m2. Of note, 11/41 (26.9%) in Group 1, 6/11 (54.5%) in Group 2 and 1/2 (50.0%) in Group 3 underwent hemodialysis therapy (R) during their clinical courses. Reticulin fibrosis and megakaryocyte hyperplasia were found on bone marrow biopsy in about 80-90% of the cases. All patients had some form of organomegaly (O), which we defined as hepatomegaly, splenomegaly, and/or small volume lymphadenopathy. Lymphadenopathy was present in all cases in Group 1; hepatomegaly and splenomegaly were present in approximately half of the cases in Group 1. Hepatomegaly was noted in only 2/11 (18.2%) of the cases in Group 2. Only 1 patient in Group 1 had pulmonary involvement (bilateral parenchymal ground-glass opacities and interlobular septal thickening).
Laboratory Findings
Chief laboratory findings of the included patients are summarized in Supplementary Table 2. Serum immunoglobulin levels were in the low- to mid-ranges in the recorded cases Groups 1 and 2. All the recorded iMCD-TAFRO cases had serum IgG levels less than 2,000 mg/dL. Elevated serum ALP was also noted in the patient population with the median ALP level of 209 U/L (range: 58.5-736 U/L) and 189 U/L (range: 78.1-503 U/L) in Group 1 and 2, respectively. Despite the increase in ALP, serum transaminase levels remained generally normal. Only a small proportion of cases had highly elevated serum lactate dehydrogenase (LDH) levels with 21/25 (84.0%) and 9/10 (90.0%) in Group 1 and 2 having LDH ≤400 U/L. Regarding autoantibodies, some of the patients had positive antinuclear antibody (ANA), rheumatoid factor (RF), anti-Sjögren-syndrome-related antigen A (SS-A) antibody, and anti-Sjögren-syndrome-related antigen B (SS-B) antibody, although none of them satisfied classification criteria for systemic autoimmune diseases. It should be noted that a few patients with iMCD-TAFRO had strongly positive serum SS-A and/or SS-B levels. None of them had positive anti-double strand DNA (dsDNA) antibody, cytoplasmic-antineutrophil cytoplasmic antibody (C-ANCA), or perinuclear-antineutrophil cytoplasmic antibody (P-ANCA).
Biopsy Site of the iMCD-TAFRO Patients
Data about biopsy, a critically important procedure to diagnose iMCD-TAFRO, are summarized in Supplementary Table 3. Histological features of iMCD-TAFRO are shown in Supplementary Figure 2. Nearly all of the patients had a bone marrow biopsy (48/54: 88.9%) and 79.6% had a lymph node biopsy (43/54). Other biopsy sites included kidney, liver, skin, intestine, adrenal gland, or pleura. Of the 52 cases with detailed records about biopsy sites, 46/52 (84.6%) received biopsy from more than one organ. Of note, lymph node biopsy is the only procedure that can confirm a diagnosis of iMCD.
Definition for iMCD-TAFRO
Based on the context of previous research25 and the findings of the current study, we propose an international definition for iMCD-TAFRO (Table 1). This definition requires at least 4 clinical criteria (thrombocytopenia, anasarca, fever/hyperinflammatory status, and organomegaly [hepatomegaly, splenomegaly, +/− small volume lymphadenopathy], or TAFO), renal dysfunction or pathological feature in bone marrow, such as reticulin fibrosis (R) or megakaryocyte hyperplasia as well as pathological criteria (iMCD histopathological features in lymph node) and exclusion criteria. Tables 2-3 summarize and compare iMCD-TAFRO/TAFRO syndrome criteria and iMCD criteria proposed to date, respectively. To evaluate the applicability of this definition of iMCD-TAFRO in an independent cohort, we interrogated the patient-powered arm of the ACCELERATE Natural History Registry26 to identify the proportion of iMCD patients who would meet the proposed definition [TAFRO + iMCD-consistent lymph node histopathology according to an expert panel + exclusion of overlapping conditions as well as additional clinical and pathological criteria]; Table 4 and Figure 2]. Among the 68 pathology-reviewed, expert-confirmed cases of iMCD in ACCELERATE that primarily come from the United States, 36 cases would meet the proposed criteria for iMCD-TAFRO. Importantly, all iMCD patients with thrombocytopenia also had anasarca. In this cohort, there were four patients with thrombocytopenia but did not meet TAFRO criteria. Two met TARO criteria but not F criteria, though both had elevated ESR, suggesting inflammation, and two met TAFO criteria but not R criteria, though neither had a bone marrow report to confirm the presence of reticulin fibrosis or hyperplasia. Given that these are real-world data, it is possible that all four of these patients may have met TAFRO if the necessary criteria were measured at the time of diagnosis. Additional clinical criteria of elevated alkaline phosphatase (mean: 171.1 (SD: 99.7) U/L) and low to normal gammaglobulin/IgG levels (mean gammaglobulin: 1.54 (0.94) g/dL; mean IgG: 1286 (958.6) mg/dL) were also confirmed in this cohort. Though mildly elevated LDH (<2x upper limit of normal) was considered as additional clinical criteria and there was a higher relative proportion of iMCD-TAFRO patients with elevated LDH, it was less than 25%, so it was not included in the final definition. The interrogation of this independent cohort suggests that the above definition which was primarily established based on data from Japan is appropriate for identifying iMCD-TAFRO patients internationally.
Table 1.
International Definition of iMCD-TAFRO
| 1. Definite iMCD-TAFRO Criteria |
| 1.1 Clinical Criteria (all 4 required) |
| ➢ Thrombocytopenia (T): Pre-treatment nadir platelet level ≤ 10 x 104/μL |
| ➢ Anasarca (A): Pleural effusion, ascites, or subcutaneous edema with CT scan |
| ➢ Fever or hyperinflammatory status (F): Fever ≥ 37.5°C of unknown etiology or CRP ≥ 2.0 mg/dL |
| ➢ Organomegaly (O): Small volume lymphadenopathy in two or more regions, hepatomegaly, or splenomegaly on CT scan |
| 1.2 Pathological Criteria (required) |
| ➢ Lymph node consistent with iMCD: Must be consistent with histopathologic features of the International iMCD Diagnostic Criteria† |
| In brief, atrophic germinal centers, concentric rings of mantle zone cells, and interfollicular hypervascularization or plasmacytosis. Negative for light chain restriction and HHV-8. |
| 1.3 Additional Clinical and Pathological Criteria (at least 1 of the following required) |
| ➢ Renal insufficiency (R): Pre-treatment eGFR ≤ 60 mL/min/1.73 m2 , creatinine >1/1 mg/dL (female)/ >1.3 mg/dL (male), or renal failure necessitating hemodialysis. |
| ➢ TAFRO-consistent bone marrow: Reticulin fibrosis (R) or megakaryocytic hyperplasia, without evidence of an alternative diagnosis |
| 1.4 Exclusion Criteria (required): see below |
| 1.5 Supportive Clinical Criteria (not required but strongly supportive) |
| ➢ Renal insufficiency (R): Pre-treatment eGFR ≤ 60 mL/min/1.73 m2 , creatinine >1/1 mg/dL (female)/ >1.3 mg/dL (male), or renal failure necessitating hemodialysis. |
| ➢ TAFRO-consistent bone marrow: Reticulin fibrosis (R) or megakaryocytic hyperplasia, without evidence of an alternative diagnosis |
| ➢ Absence of polyclonal hypergammaglobulinemia (immunoglobulin G ≤ 1.2x upper limit of normal by nephelometry |
| ➢ Elevated alkaline phosphatase with mild to no elevation in bilirubin and transaminases |
| 2. Probable iMCD-TAFRO Criteria: All 4 Clinical Criteria and Additional Clinical and Pathological Criteria met, but Pathological Criteria not able to be assessed because no lymph node biopsy was performed or an insufficient specimen was obtained |
| 3. TAFRO syndrome, not iMCD-TAFRO: All 4 Clinical Criteria and Renal insufficiency (R) met, but lymph node biopsy was not consistent with iMCD OR an exclusion criteria diagnosis was made |
| Exclusion Criteria - Must rule out the following diseases |
| ➢ Infectious diseases - including the below but not limited to: |
| 1. HHV-8 |
| 2. EBV-associated lymphoproliferative disorders |
| 3. Acute HIV infection |
| 4. Tuberculosis |
| 5. COVID-19 cytokine storm syndrome |
| ➢ Autoimmune/rheumatologic diseases: |
| 1. Systemic lupus erythematosus |
| 2. Sjögren syndrome |
| 3. Rheumatoid arthritis |
| 4. Adult-onset Still disease |
| 5. Juvenile idiopathic arthritis |
| 6. IgG ≥ 3,400 mg/dL (suggestive of autoimmune diseases or plasma cell dyscrasias) |
| 7. Primary hemophagocytic lymphohistiocytosis |
| ➢ Malignancy - including the below but not limited to: |
| 1. Malignant lymphoma |
| 2. Multiple myeloma |
| 3. Metastatic cancer |
| 4. POEMS syndrome |
Abbreviations: CRP, C-reactive protein; CT, computed tomography; eGFR, estimated glomerular filtration rate; HHV-8, human herpesvirus-8.
International, evidence-based consensus diagnostic criteria for HHV-8–negative/idiopathic multicentric Castleman disease. Blood. 2017 Mar 23;129(12):1646-1657.
Table 2.
Comparison of iMCD-TAFRO and TAFRO Syndrome Criteria/Definition to Date
| Inclusion Criteria | Exclusion Criteria | |||
|---|---|---|---|---|
| Required Histopathological Criteria |
Required Clinical Criteria | Other Criteria | ||
| Iwaki et al. for iMCD-TAFRO (2016) a | (Mandatory)
|
(Mandatory)
|
(Need 1 or more)
|
|
| Masaki et al. for TAFRO syndrome (2020) b |
|
(Mandatory)
|
(Need 2 or more)
|
|
| Present Definition of iMCD-TAFRO | (Mandatory for definite diagnosis)
|
(Mandatory)
|
(At least 1 of the following required)
|
|
Abbreviations: ALP, alkaline phosphatase; AOSD, adult-onset Still disease; BM, bone marrow; COVID-19-CSS, COVID-19 cytokine storm syndrome; CRP, C-reactive protein; CT, computed tomography; EBV, Epstein-Barr virus; eGFR, estimated glomerular filtration rate; HHV-8, human herpesvirus-8; HIV, human immunodeficiency virus; IgG, immunoglobulin G; JIA, Juvenile idiopathic arthritis; LN, lymph node; ML; malignant lymphoma; MM, multiple myeloma; RA, rheumatoid arthritis; SjS, Sjögren syndrome; SLE, systemic lupus erythematosus; TB, tuberculosis; TTP/HUS, thrombotic thrombocytopenic purpura/hemolytic uremic syndrome
2019 Updated diagnostic criteria and disease severity classification for TAFRO syndrome. Int J Hematol 111(1): 155-158.
Clinicopathologic analysis of TAFRO syndrome demonstrates a distinct subtype of HHV-8-negative multicentric Castleman disease. Am J Hematol 91(2): 220-226.
International, evidence-based consensus diagnostic criteria for HHV-8–negative/idiopathic multicentric Castleman disease. Blood. 2017 Mar 23;129(12):1646-1657.
Table 3.
Diagnostic Criteria for iMCD or CD
| Inclusion Criteria | Exclusion Criteria | ||
|---|---|---|---|
| Major Criteria | Minor Criteria | ||
| Fajgenbaum et al. (2017) a | (Mandatory)
|
(need 2 or more of 11 with at least 1 laboratory criterion) Laboratory
|
|
| Fujimoto et al. for CD (2018) b | (Mandatory)
|
N/A |
|
Abbreviations: CD, Castleman disease; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; HHV-8, human herpesvirus-8; HIV, human immunodeficiency virus; IgG4-RD, IgG4-related diseases; LN, lymph node; UCD, unicentric Castleman disease
International, evidence-based consensus diagnostic criteria for HHV-8–negative/idiopathic multicentric Castleman disease. Blood. 2017 Mar 23;129(12):1646-1657.
Tentative diagnostic criteria and disease severity classification for Castleman disease: A report of the research group on Castleman disease in Japan. Mod Rheumatol 28(1): 161-167.
Table 4.
Applicability of the Present Definition of iMCD-TAFRO to the ACCELERATE Natural History Registry Cohort
| iMCD patients in ACCELERATE, who are confirmed by an expert panel as meeting iMCD diagnostic criteria (pathology consistent, multicentric lymphadenopathy, clinical criteria, and exclusionary criteria)* |
(N = 68) | ||
|---|---|---|---|
| TAFRO, N(%) |
NOS, N(%) | Combined | |
| Newly proposed TAFRO criteria met (TAFRO) versus not met (NOS), N(%) | 36 (52.9) | 32 (47.1) | 68 |
| Required TAFRO criteria that were met, N(%) | |||
| At least T (thrombocytopenia), N(%)† | 36 (100) | 4 (12.5)* | 40 (58.8) |
| At least A (anasarca/fluid accumulation), N(%) | 36 (100) | 23 (71.9) | 59 (86.8) |
| At least F (fever or elevated CRP), N(%) | 36 (100) | 26 (81.3) | 62 (91.2) |
| At least O (organomegaly: hepatomegaly, splenomegaly, or lymphadenopathy), N% | 36 (100) | 32 (100) | 68 (100) |
| At least T+A, N(%) | 36 (100) | 4 (12.5) | 40 (58.8) |
| At least T+F, N(%) | 36 (100) | 2 (6.3) | 41 (60.3) |
| At least A+F, N(%) | 36 (100) | 19 (59.4) | 55 (80.9) |
| Additional Clinical Criteria, N(%) | |||
| Renal dysfunction (elevated creatinine or decreased eGFR) | |||
| Present, N(%) | 34 (94.4) | 14 (43.8) | 48 (70.6) |
| Absent, N(%) | 2 (5.6) | 18 (56.3) | 20 (29.4) |
| Not recorded, N | 0 | 0 | 0 |
| Fibrosis of bone marrow ^ | |||
| Present, N(%) | 15 (45.5) | 5 (26.3) | 20 (38.5) |
| Absent, N(%) | 18 (54.5) | 14 (73.7) | 32 (61.5) |
| Not recorded, N | 3 | 13 | 16 |
| Megakaryocytic hyperplasia of bone marrow ^ | |||
| Present, N(%) | 27 (81.8) | 5 (26.3) | 32 (61.5) |
| Absent, N(%) | 6 (18.2) | 14 (73.7) | 20 (38.5) |
| Not recorded, N | 3 | 13 | 16 |
| At least one of R (renal dysfunction, fibrosis of bone marrow, megakaryocytic hyperplasia of bone marrow), N (%) | |||
| Present, N(%) | 36 (100.0) | 17 (53.1) | 53 (77.9) |
| Absent, N(%) | 0 (0.0) | 15 (46.9) | 15 (22.1) |
| Not recorded, N | 0 | 0 | 0 |
| Additional laboratory parameters ‡ | |||
| Gammaglobulin (g/dL, ref: <1.7) | |||
| N | 14 | 14 | 28 |
| Mean (SD) | 1.54 (0.94) | 2.87 (2.08) | 2.20 (1.72) |
| > Upper limits normal, N (%) | 4 (28.6) | 8 (57.1) | 12 (42.9) |
| Immunoglobulin G (mg/dL, ref: <1700) | |||
| N | 29 | 24 | 53 |
| Mean (SD) | 1285.7 (958.6) | 2898.6 (2015.5) | 2916.0 (1717.1) |
| > Upper limits normal, N (%) | 6 (20.7) | 16 (66.7) | 22 (41.5) |
| Alkaline phosphatase (U/L, ref: <147) | |||
| N | 36 | 30 | 66 |
| Mean (SD) | 171.1 (99.7) | 133.0 (93.1) | 153.8 (97.9) |
| > Upper limits normal, N (%) | 16 (44.4) | 7 (23.3) | 23 (34.8) |
| Lactate dehydrogenase (U/L, ref: <400) | |||
| N | 34 | 23 | 57 |
| Mean (SD) | 358.9 (327.2) | 200.7 (160.4) | 295.1 (281.6) |
| > Upper limits normal, N (%) | 8 (23.5) | 3 (13.0) | 11 (19.3) |
Abbreviations: ART, ACCELERATE Registry Team; CAS, Certification and Access Subcommittee; CRP, C-reactive protein; NOS, Not Otherwise Specified
ACCELERATE is an international natural history registry of Castleman disease (CD) patients of all subtypes who enroll directly via a web-based portal, in which they electronically provide consent and Health Insurance Portability and Accountability Act authorization to the ACCELERATE Registry Team (ART). The ART collects and extracts all medical records into the study database. Each case undergoes Certification and Access Subcommittee (CAS) review and grading. CAS-approved grade 3 or higher are those who are considered likely to have CD.
If a bone marrow biopsy was performed and myelofibrosis or megakaryocytic hyperplasia was not documented in the pathology report, then it is considered to be absent. It is possible that a reticulin stain was not performed or a description of megakaryocytic hyperplasia was not included though it may have been present.
Due to the nature of real-world data, thrombocytopenia required at least 2 platelet measurements of <100 k/μL to meet thrombocytopenia in this cohort
Closest value to date of diagnosis within +/− 90 days
Figure 2: Performance of the Present Definition to Distinguish iMCD-TAFRO from iMCD-NOS in ACCELERATE Natural History Registry Cohort.
36 cases met the present definition for iMCD-TAFRO, and the rest of the 32 cases were noted as iMCD-NOS among the 68 pathology-reviewed, expert-confirmed cases of iMCD in ACCELERATE that primarily come from the United States. All patients noted as iMCD-TAFRO had thrombocytopenia, anasarca, and fever or elevated CRP, as well as renal dysfunction or reticulin fibrosis or hyperplasia of the bone marrow. Of note, of the four iMCD-NOS patients with low platelets, two met TARO criteria but not F criteria, though both had elevated ESR, suggesting inflammation, and two met TAFO criteria but not R criteria, though neither had a bone marrow report to confirm the presence of reticulin fibrosis or hyperplasia.
Abbreviation: C-reactive protein; iMCD-TAFRO, TAFRO clinical subtype of idiopathic multicentric Castleman disease; iMCD-NOS, idiopathic multicentric Castleman disease not otherwise specified.
Discussion
In the present study, we systematically analyzed and reviewed the clinical, radiological, and laboratory features of 54 published cases either with iMCD-TAFRO, possible iMCD with TAFRO syndrome without lymph node biopsy and other identified co-morbidities, and TAFRO syndrome without having evidence of iMCD on lymph node biopsy without comorbidities, reported between January 2013 and May 2019.
Clinically, our data on international cases support that the characteristic features of iMCD-TAFRO are thrombocytopenia, anasarca, fever and hyperinflammatory status, renal insufficiency, and organomegaly. Clinical criteria need to be sensitive enough not to miss possible iMCD-TAFRO cases. As all patients had pre-treatment platelet levels ≤10 x 104/μL, we adopted the cut-off serum platelet value of 10 x 104/μL as one of the clinical criteria to improve the sensitivity. The cut-off value is compatible with the latest criteria proposed by Masaki et al in 202023. Because all the included cases had some sort of fluid accumulation recognized on imaging studies, anasarca should remain one of the required clinical criteria as in our previous diagnostic criteria. Fever and hyperinflammatory status, which are common first presentations of iMCD-TAFRO, were prevalent in our cases as previously reported22,27 and all cases satisfied the cut-off value of body temperature ≥37.5°C and/or CRP ≥2.0 mg/dL which we incorporated into the criteria in this update. Renal insufficiency, a condition that was noted as one of the minor criteria in the criteria proposed by us in 2016 as well as by Masaki et al.15,23, continues to be important although it was not uniformly present at presentation. Our data suggested that approximately 75% of iMCD-TAFRO cases had mild to moderate renal impairment on presentation; a considerable number of patients required hemodialysis. Though renal dysfunction would have likely developed if given sufficient time in each of these patients, we decided to make renal dysfunction as an optional feature so as to not slow down timely identification of iMCD-TAFRO. However, presence of renal dysfunction is strongly supportive/confirmatory of TAFRO. To determine renal dysfunction, we recommend findings of eGFR≤ 60 mL/min/1.73 m2, creatinine >1.1 mg/dL (female) or >1.3 mg/dL (male), or renal failure necessitating hemodialysis. The combined feature of organomegaly including lymphadenopathy, hepatomegaly, and splenomegaly occurred in all iMCD-TAFRO patients in both cohorts. These data support requiring thrombocytopenia (T), anasarca (A), fever or hyperinflammatory status (F), renal dysfunction (R: or pathological feature in bone marrow), and organomegaly (O; at least small volume lymphadenopathy) for the diagnosis of definite iMCD-TAFRO.
The analysis of laboratory data also indicated that iMCD-TAFRO cases often had additional criteria that can be observed but are not required. We found elevated ALP without other transaminase increases and rarely had marked hypergammaglobulinemia or high LDH levels, which were consistent with previous reports22,28. Also, moderately elevated serum interleukin 6 (IL-6) levels were noted in iMCD-TAFRO patients. Due to the COVID-19 pandemic and an evolving concept of COVID-19 cytokine storm syndrome (COVID-19-CSS) characterized by undue immune response29, there has been a growing interest regarding cytokine-driven diseases30,31. In addition to a direct role of IL-6, recent data support a role for the sIL-6R:sgp130 buffering system in these syndromes. Although elevated serum IL-6 levels may not be diagnostic for iMCD-TAFRO and the etiology is unknown, it is important to recognize that iMCD-TAFRO patients often have hypercytokinemia. Despite the exclusion of possible complications of rheumatologic disorders, our results showed that some iMCD-TAFRO patients still had positive ANA, RF, and anti SS-A and -B antibodies. Among the 21 excluded cases, 8 had significant decreases in complement components, a characteristic finding of SLE, despite the authors diagnosing them as iMCD-TAFRO. Moreover, 4 of the 21 cases had history of or coexisting SjS. These findings highlight that existing iMCD-TAFRO case reports might include patients with undiagnosed autoimmune disorders as suggested previously32-36. Thus, clinicians need to carefully exclude those disorders, and the diagnostic criteria need to be defined to avoid misdiagnosis of autoimmune disorders as iMCD-TAFRO.
Previously, we reported that atrophic germinal centers combined with interfollicular vascular proliferation might be characteristic histopathological findings of iMCD-TAFRO on lymph node biopsy2,13. Despite the necessity of lymph node biopsy and its usefulness to diagnose iMCD-TAFRO, some cases may have severe thrombocytopenia and small volume lymphadenopathy37, which could make a lymph node biopsy difficult or contraindicated. For this reason, the diagnostic criteria proposed by Masaki et al. did not mandate the histopathological analysis for the diagnosis of TAFRO syndrome23. Our review showed that lymph node biopsy was performed in approximately 80% of the cases included in the present study. Given that iMCD cannot be diagnosed without histopathological evidence in lymph node tissue, lymph node biopsy needs to be done whenever possible. Though kidney biopsy would not be able to demonstrate histopathologic features consistent with iMCD-like lymph node tissue, kidney biopsy was performed in 20.4% of the cases, and there has been an increasing number of reports describing kidney biopsy in iMCD-TAFRO38. A recent case series of 7 iMCD-TAFRO patients reported that all patients had endotheliopathy with mesangiolysis and a double contour of the basal membrane with renal biopsy39,40. Furthermore, Mizuno and colleagues suggested that, unlike lymph node biopsy, the findings seen in the kidney specimen might persist even after the introduction of immunosuppressive treatment in their case series40. In the meantime, due to the lack of data, renal pathology findings in iMCD-TAFRO need to be further examined to assess their consistency with lymph node findings. Bone marrow biopsy may also have diagnostic utility, considering the extent of thrombocytopenia observed in our study (the median pre-treatment platelet: 3.7 x 104/μL) and that approximately 90% of the cases had either reticulin fibrosis or increased megakaryocytes in the bone marrow specimen. Bone marrow biopsy is feasible without additional platelet transfusion in most cases and may provide useful information to exclude other differential diagnoses such as hidden hematologic malignancy and infectious diseases41-43. Given that prior definitions have defined the R in TAFRO as either renal dysfunction or reticulin fibrosis, we recommend defining R as either renal dysfunction or reticulin fibrosis (or other bone marrow features) and looking for one of these features as required of iMCD-TAFRO. A previous study reported that adrenomegaly or adrenal ischemia on CT scan could be early signs of TAFRO syndrome44, although it is not clear if these signs are found in those with iMCD-TAFRO. Unfortunately, only one patient in our study had an adrenal biopsy. Further research is needed on the specificity of these findings and the utility of this procedure for the diagnosis of iMCD-TAFRO. Taken together, while lymph node biopsy may not be essential for diagnosing TAFRO syndrome, which is a broad category including iMCD-TAFRO, lymph node histopathological findings are crucial for diagnosis of iMCD-TAFRO. Thus, we propose that a definite diagnosis of iMCD-TAFRO should be made only in the presence of lymph node histopathological analysis consistent with histopathologic features of the International iMCD Diagnostic Criteria15 to secure sufficient specificity and prevent misdiagnosis. While several different criteria have been proposed for the diagnosis of iMCD, iMCD-TAFRO, and TAFRO syndrome as shown in Tables 2-3, our present definition have been based on rigorous reviews of data from patients of different ethnicities and international discussions to establish a precise iMCD-TAFRO diagnostic definition. Ultimately, we hope to identify appropriate treatment strategies for patients with iMCD-TAFRO, who often have poor clinical outcomes and require second or third line therapies with immunosuppressants, cytotoxic agents, or monoclonal antibodies22.
Our study has a few limitations that need to be considered. First, as we analyzed the data of published case reports, there might be publication bias leading to overestimation of the prevalence of clinical symptoms and laboratory data as clinically significant cases may have been more likely to be published. Second, the small number of cases and missing data in a few of the included cases may lessen the precision of our analysis. Third, the data reported from the patient-powered arm of the ACCELERATE Natural History Registry likely does not reflect the proportion of iMCD-TAFRO to iMCD-NOS in the general population. Any patient who has received a pathology report suggestive of Castleman disease can self-enroll in the natural history registry. Previously, it was reported that ACCELERATE is skewed towards more symptomatic and less easily treated patients, who are more likely to seek out resources online26. Accordingly, ACCELERATE is likely biased towards a higher iMCD-TAFRO population than the general iMCD population, as these patients typically have a more severe and unpredictable clinical course and may be more likely to seek out resources. More research is needed to determine the relative proportions of iMCD-TAFRO versus iMCD-NOS and whether iMCD-NOS should be further sub-classified. Finally, some of the patients considered to have iMCD-TAFRO by the studies’ authors in the literature review may have actually had another condition given the challenges of diagnosing iMCD.
Despite the limitations, our systematic review and the validated international definition of iMCD-TAFRO represents an important attempt to improve the way we diagnose this rare and challenging disease entity. Despite the rarity of iMCD-TAFRO, data from the ACCELERATE Natural History Registry (NCT02817997) was used as a validation set. Our validated international definition, highlighting the necessity of histopathological analysis, should help to clear up a possible confusion between TAFRO syndrome and iMCD-TAFRO, and to facilitate a precise and comprehensive approach to diagnosis of iMCD-TAFRO in accordance with the international, evidence-based consensus diagnostic criteria for iMCD.
Supplementary Material
Supplementary Figure 1: Search and article selection strategy
Abbreviation: DLBCL, diffuse large B-cell lymphoma; iMCD-TAFRO, TAFRO subtype of idiopathic multicentric Castleman’s disease; SjS, Sjögren syndrome; SLE, systemic lupus erythematosus.
Supplementary Figure 2: Histological features of iMCD-TAFRO
Histological features of iMCD-TAFRO lymph nodes (A-C) and bone marrow (D and E). (A) An involved lymph node shows small, atrophic germinal center. The interfollicular areas demonstrate increased vascularity (HE, 100×); (B) CD138 staining shows scattered plasma cells in the interfollicular areas (CD138 staining, 100×); (C) Prominent, hyalinized blood vessel with plump endothelial cells were observed in the germinal centers and interfollicular zone (HE, 200×); (D) Bone marrow biopsy showed a hypercellular marrow with megakaryocytic hyperplasia. Megakaryocytes were slightly atypical, including micro- and multi-separated nuclear megakaryocytes. (HE, 200×); (E) Silver impregnation staining highlighted mild reticulin fibrosis (Silver impregnation staining, 200×). Abbreviation: iMCD-TAFRO, TAFRO clinical subtype of idiopathic multicentric Castleman disease; HE, hematoxylin and eosin.
Footnotes
Conflict of Interest: DCF has received grant funding from EUSA Pharma and Janssen Pharmaceuticals as well as study drug from Pfizer for a clinical trial. He has two provisional patents pending for the diagnosis and treatment of Castleman disease. The remaining authors declare no conflicts of interest.
References
- 1.Takai K, Nikkuni K, Shibuya H, Hashidate H. [Thrombocytopenia with mild bone marrow fibrosis accompanied by fever, pleural effusion, ascites and hepatosplenomegaly]. Rinsho Ketsueki. 2010;51(5):320–325. [PubMed] [Google Scholar]
- 2.Iwaki N, Sato Y, Takata K, et al. Atypical hyaline vascular-type castleman's disease with thrombocytopenia, anasarca, fever, and systemic lymphadenopathy. J Clin Exp Hematop. 2013;53(1):87–93. [DOI] [PubMed] [Google Scholar]
- 3.Masaki Y, Nakajima A, Iwao H, et al. Japanese variant of multicentric castleman's disease associated with serositis and thrombocytopenia--a report of two cases: is TAFRO syndrome (Castleman- Kojima disease) a distinct clinicopathological entity? J Clin Exp Hematop. 2013;53(1):79–85. [DOI] [PubMed] [Google Scholar]
- 4.Allegra A, Rotondo F, Russo S, et al. Castleman-Kojima disease (TAFRO syndrome) in a Caucasian patient: A rare case report and review of the literature. Blood Cells Mol Dis. 2015;55(3):206–207. [DOI] [PubMed] [Google Scholar]
- 5.Jose FF, Kerbauy LN, Perini GF, et al. A life-threatening case of TAFRO syndrome with dramatic response to tocilizumab, rituximab, and pulse steroids: The first case report in Latin America. Medicine (Baltimore). 2017;96(13):e6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louis C, Vijgen S, Samii K, et al. TAFRO Syndrome in Caucasians: A Case Report and Review of the Literature. Front Med (Lausanne). 2017;4:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Islamoglu Z, Duman AE, Sirin G, et al. TAFRO Syndrome: A Case Report from Turkey and Review of the Literature. Int J Hematol Oncol Stem Cell Res. 2018;12(4):253–259. [PMC free article] [PubMed] [Google Scholar]
- 8.Ma WL, Zhang L, Zhu TN, et al. TAFRO Syndrome - A Specific Subtype of Castleman's Disease in China. Chin Med J (Engl). 2018;131(15):1868–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortiz A, Cardenas P, Peralta M, Rodriguez H, Frederick G, Ortiz J. Neuro-ophthalmological findings in TAFRO syndrome in a patient from South America, a variant of multicentric Castleman's disease. Int Ophthalmol. 2018;38(4):1641–1646. [DOI] [PubMed] [Google Scholar]
- 10.Owattanapanich W, Pholmoo W, Pongpruttipan T, Siritanaratkul N. High proportion of TAFRO syndrome in Thai adult Castleman's disease patients: a 10-year experience. Ann Hematol. 2018;97(6):1019–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawabata H, Takai K, Kojima M, et al. Castleman-Kojima disease (TAFRO syndrome) : a novel systemic inflammatory disease characterized by a constellation of symptoms, namely, thrombocytopenia, ascites (anasarca), microcytic anemia, myelofibrosis, renal dysfunction, and organomegaly : a status report and summary of Fukushima (6 June, 2012) and Nagoya meetings (22 September, 2012). J Clin Exp Hematop. 2013;53(1):57–61. [DOI] [PubMed] [Google Scholar]
- 12.Carbone A, Pantanowitz L. TAFRO syndrome: An atypical variant of KSHV-negative multicentric Castleman disease. Am J Hematol. 2016;91(2):171–172. [DOI] [PubMed] [Google Scholar]
- 13.Iwaki N, Fajgenbaum DC, Nabel CS, et al. Clinicopathologic analysis of TAFRO syndrome demonstrates a distinct subtype of HHV-8-negative multicentric Castleman disease. Am J Hematol. 2016;91(2):220–226. [DOI] [PubMed] [Google Scholar]
- 14.Masaki Y, Kawabata H, Takai K, et al. Proposed diagnostic criteria, disease severity classification and treatment strategy for TAFRO syndrome, 2015 version. Int J Hematol. 2016;103(6):686–692. [DOI] [PubMed] [Google Scholar]
- 15.Fajgenbaum DC, Uldrick TS, Bagg A, et al. International, evidence-based consensus diagnostic criteria for HHV-8-negative/idiopathic multicentric Castleman disease. Blood. 2017;129(12):1646–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakashita K, Murata K, Takamori M. TAFRO syndrome: current perspectives. J Blood Med. 2018;9:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang HW, Pittaluga S, Jaffe ES. Multicentric Castleman disease: Where are we now? Semin Diagn Pathol. 2016;33(5):294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fajgenbaum DC. Novel insights and therapeutic approaches in idiopathic multicentric Castleman disease. Blood. 2018;132(22):2323–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dispenzieri A, Fajgenbaum DC. Overview of Castleman disease. Blood. 2020;135(16):1353–1364. [DOI] [PubMed] [Google Scholar]
- 20.Fajgenbaum DC, Wu D, Goodman A, et al. Insufficient evidence exists to use histopathologic subtype to guide treatment of idiopathic multicentric Castleman disease. Am J Hematol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paydas S Tafro syndrome: Critical review for clinicians and pathologists. Crit Rev Oncol Hematol. 2018;128:88–95. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura Y, Hanayama Y, Fujii N, Kondo E, Otsuka F. Comparison of the clinical characteristics of TAFRO syndrome and idiopathic multicentric Castleman disease in general internal medicine: a 6-year retrospective study. Intern Med J. 2020;50(2):184–191. [DOI] [PubMed] [Google Scholar]
- 23.Masaki Y, Kawabata H, Takai K, et al. 2019 Updated diagnostic criteria and disease severity classification for TAFRO syndrome. Int J Hematol. 2020;111(1):155–158. [DOI] [PubMed] [Google Scholar]
- 24.Yamadate S, Yamazaki H, Araki H, et al. The proposal revised method from JSCC recommended method to traceable to IFCC reference method for the catalytic activity concentrations of alkaline phosphatase in serum. Nihon Rinsho. 2017;46:138–145. [Google Scholar]
- 25.Iwaki N, Fajgenbaum DC, Nabel CS, et al. Clinicopathologic analysis of TAFRO syndrome demonstrates a distinct subtype of HHV-8-negative multicentric Castleman disease. Am J Hematol. 2016;91(2):220–226. [DOI] [PubMed] [Google Scholar]
- 26.Pierson SK, Khor JS, Ziglar J, et al. ACCELERATE: A Patient-Powered Natural History Study Design Enabling Clinical and Therapeutic Discoveries in a Rare Disorder. Cell Reports Medicine. 2020;1(9):100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakai K, Maeda T, Kuriyama A, Shimada N, Notohara K, Ueda Y. TAFRO syndrome successfully treated with tocilizumab: A case report and systematic review. Mod Rheumatol. 2018;28(3):564–569. [DOI] [PubMed] [Google Scholar]
- 28.Coutier F, Meaux Ruault N, Crepin T, et al. A comparison of TAFRO syndrome between Japanese and non-Japanese cases: a case report and literature review. Ann Hematol. 2018;97(3):401–407. [DOI] [PubMed] [Google Scholar]
- 29.Chen LYC, Quach TTT. COVID-19 cytokine storm syndrome: a threshold concept. Lancet Microbe. 2021;2(2):e49–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fajgenbaum DC, June CH. Cytokine Storm. New England Journal of Medicine. 2020;383(23):2255–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McElvaney OJ, Curley GF, Rose-John S, McElvaney NG. Interleukin-6: obstacles to targeting a complex cytokine in critical illness. Lancet Respir Med. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hasegawa E, Sato H, Wada Y, et al. Characterization of patients with systemic lupus erythematosus who meet the diagnostic criteria for TAFRO syndrome. Lupus. 2018;27(3):417–427. [DOI] [PubMed] [Google Scholar]
- 33.Iwanaga N, Harada K, Tsuji Y, et al. TAFRO syndrome with primary Sjogren's syndrome. Nihon Rinsho Meneki Gakkai Kaishi. 2016;39(5):478–484. [DOI] [PubMed] [Google Scholar]
- 34.Edahiro Y, Ichikawa K, Sunami Y, Koike M, Komatsu N. [Autoimmune hemolytic anemia in a patient with TAFRO syndrome]. Rinsho Ketsueki. 2015;56(11):2346–2350. [DOI] [PubMed] [Google Scholar]
- 35.Fujimoto S, Kawabata H, Kurose N, et al. Sjogren's syndrome manifesting as clinicopathological features of TAFRO syndrome: A case report. Medicine (Baltimore). 2017;96(50):e9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tokunaga M, Yamada M, Yoshikawa S, et al. [Systemic lupus erythematosus with marked eosinophilia and clinical features mimicking TAFRO syndrome]. Rinsho Ketsueki. 2018;59(6):688–694. [DOI] [PubMed] [Google Scholar]
- 37.Fujiki T, Hirasawa S, Watanabe S, Iwamoto S, Ando R. Successful treatment by tocilizumab without steroid in a very severe case of TAFRO syndrome. CEN Case Rep. 2017;6(1):105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noda-Narita S, Sumida K, Sekine A, et al. TAFRO syndrome with refractory thrombocytopenia responding to tocilizumab and romiplostim: a case report. CEN Case Rep. 2018;7(1):162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mizuno H, Sekine A, Oguro M, et al. Renal histology in a patient with TAFRO syndrome: a case report. Hum Pathol. 2018;82:258–263. [DOI] [PubMed] [Google Scholar]
- 40.Mizuno H, Sawa N, Watanabe S, et al. The Clinical and Histopathological Feature of Renal Manifestation of TAFRO Syndrome. Kidney Int Rep. 2020;5(8):1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohya E, Mizutani M, Sakaguchi H, Sekine T. Diffuse Large B-cell Lymphoma during Corticosteroid Therapy for TAFRO Syndrome. Intern Med. 2016;55(19):2861–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oka K, Yamane M, Yokota Y, et al. Disseminated Mycobacterium genavense infection mimicking TAFRO syndrome. J Infect Chemother. 2020. [DOI] [PubMed] [Google Scholar]
- 43.Igawa T, Omote R, Sato H, et al. A possible new morphological variant of mantle cell lymphoma with plasma-cell type Castleman disease-like features. Pathol Res Pract. 2017;213(11):1378–1383. [DOI] [PubMed] [Google Scholar]
- 44.Kurokawa R, Gonoi W, Yokota H, et al. Computed tomography findings of early-stage TAFRO syndrome and associated adrenal abnormalities. Eur Radiol. 2020;30(10):5588–5598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Search and article selection strategy
Abbreviation: DLBCL, diffuse large B-cell lymphoma; iMCD-TAFRO, TAFRO subtype of idiopathic multicentric Castleman’s disease; SjS, Sjögren syndrome; SLE, systemic lupus erythematosus.
Supplementary Figure 2: Histological features of iMCD-TAFRO
Histological features of iMCD-TAFRO lymph nodes (A-C) and bone marrow (D and E). (A) An involved lymph node shows small, atrophic germinal center. The interfollicular areas demonstrate increased vascularity (HE, 100×); (B) CD138 staining shows scattered plasma cells in the interfollicular areas (CD138 staining, 100×); (C) Prominent, hyalinized blood vessel with plump endothelial cells were observed in the germinal centers and interfollicular zone (HE, 200×); (D) Bone marrow biopsy showed a hypercellular marrow with megakaryocytic hyperplasia. Megakaryocytes were slightly atypical, including micro- and multi-separated nuclear megakaryocytes. (HE, 200×); (E) Silver impregnation staining highlighted mild reticulin fibrosis (Silver impregnation staining, 200×). Abbreviation: iMCD-TAFRO, TAFRO clinical subtype of idiopathic multicentric Castleman disease; HE, hematoxylin and eosin.