Abstract
Hemagglutination of erythrocytes is a common property of Staphylococcus epidermidis strains, which is related to adherence and biofilm formation and may be essential for the pathogenesis of biomaterial-associated infections caused by S. epidermidis. In three independent biofilm-producing, hemagglutination-positive S. epidermidis isolates, interruption of the icaADBC operon essential for polysaccharide intercellular adhesin (PIA) synthesis by Tn917 insertions led to a hemagglutination-negative phenotype. An immunoglobulin G fraction of antiserum to PIA greatly reduced hemagglutination. Purified PIA led to a 64-fold decrease of hemagglutination titers of these strains; however, it did not mediate hemagglutination by itself. These observations define PIA as the hemagglutinin of S. epidermidis or at least as its major functional component.
In recent years, coagulase-negative staphylococci, mostly Staphylococcus epidermidis, have emerged as major nosocomial pathogens due to frequent infections associated with implanted medical devices and nosocomial sepsis (3, 11, 20). It is believed that the formation of adherent multilayered biofilms embedded into a glycocalyx composed of exopolysaccharides on implanted devices is essential for the pathogenesis of S. epidermidis infections.
Biofilm formation apparently proceeds in two phases (reviewed in reference 12). Most S. epidermidis strains are competent for primary attachment, which involves specific surface proteins or a capsular polysaccharide adhesin (PSA) (12). We recently described a polysaccharide intercellular adhesin (PIA), which plays an essential role in the second phase of S. epidermidis biofilm accumulation by mediating cell-to-cell adhesion and is expressed by the majority of biofilm-producing clinical S. epidermidis isolates (13–15). Two isogenic biofilm-negative Tn917 transposon insertion mutants, M10 and M11, derived from a biofilm-producing S. epidermidis strain, did not express detectable amounts of PIA (14). Structural analysis of the purified PIA revealed a linear polysaccharide composed of β-1,6-linked 2-deoxy-2-amino-d-glucopyranosyl residues (16). Of these, 80 to 85% are N-acetylated, whereas the rest are non-N-acetylated and positively charged. A minor component of PIA in addition contained negative charges introduced by modification with phosphate and succinate groups (16). The icaADBC gene locus of S. epidermidis contains genes essential for PIA synthesis (5, 9). An accumulation-associated protein was described; however, its function in biofilm accumulation is not yet known (10).
Recently, the ability of S. epidermidis to mediate hemagglutination of erythrocytes of different species was shown to be associated with the ability to adhere to plastic and to produce biofilm and therefore may be important for the pathogenesis of S. epidermidis infections (19, 21). The hemagglutinating activity could be extracted from the bacterial cells, and preliminary characterization revealed that the hemagglutinin was a polysaccharide of yet undefined nature (21). As there was a striking similarity in the quantitative relation of the amounts of biofilm formed by individual strains and the hemagglutination titers and the amounts of PIA produced, the functional relation between PIA and the hemagglutinin of S. epidermidis was investigated (13, 19, 21).
(Part of this work will appear in the doctoral theses of J.R., H.R., and T.M., Universitätskrankenhaus Eppendorf, Hamburg, Germany.)
Biofilm-producing S. epidermidis 1457, 9142, 8400, RP62A, and SE-5 and biofilm-negative S. epidermidis 5179 (15, 19) as well as isogenic biofilm-negative Tn917 transposon insertion mutants M10 and M11 (14), which are impaired in the accumulative phase of biofilm production due to abolished intercellular adhesion caused by failure of synthesis of PIA, and isogenic biofilm-negative transductants 9142-M10, 9142-M11, and 1457-M11 (14) have been described. Staphylococcus carnosus TM300 (7), kindly provided by Friedrich Götz, University of Tübingen, Tübingen, Germany, and Escherichia coli DH5α (4) were used as hosts in molecular cloning experiments.
Phage transduction by using S. epidermidis phage 48, kindly provided by V. T. Rosdahl, Statens Seruminstitut, Copenhagen, Denmark, was performed as described previously (14, 18). Biofilm production by S. epidermidis strains grown in Trypticase soy broth (TSB) (Becton Dickinson, Cockeysville, Md.) was determined with a semiquantitative adherence assay by using 96-well tissue culture plates (Nunc, Roskilde, Denmark) (2, 15). Bacterial extracts of S. epidermidis strains grown in TSB on plastic tissue culture plates were prepared by sonication (15). Concentration of PIA in bacterial extracts was determined by a specific coagglutination assay (13, 15, 16).
Pulsed-field gel electrophoresis (PFGE) was performed essentially as described (14, 23). Chromosomal and plasmid DNA was isolated and digested with restriction enzymes (Pharmacia, Freiburg, Germany) followed by Southern analysis with [32P]dCTP-labeled plasmid pTV1ts as described previously (14).
Tn917-containing EcoRI-fragments of M10 and M11 were cloned directly in S. carnosus by protoplast transformation by using pT181mcs as a vector and selecting for erythromycin-resistant transformants (erythromycin concentration, 10 μg/ml) (7, 8). Cloned DNA fragments were subcloned by using pBluescript II SK (Stratagene, La Jolla, Calif.) as a vector in E. coli DH5α. Sequences of the transposon insertion sites of M10 and M11 were obtained by using oligonucleotides 5′-GGC CTT GAA ACA TTG GTT TAG TGG G-3′ and 5′-CTC ACA ATA GAG AGA TGT CAC CG-3′, which are complementary to the 5′ and 3′ junctions of Tn917 (24), with the Sequenase version 2.0 kit (United States Biochemical, Cleveland, Ohio) (4).
Antisera were raised in rabbits against whole cells of the biofilm-negative, PIA-negative S. epidermidis 5179 (anti-5179) and the biofilm-producing, PIA-positive S. epidermidis 1457 (anti-1457) grown in TSB on tissue culture plates by serial intravenous injection of formalin-fixed cells (15). In addition, an antiserum which was raised against purified PIA was used (9). Normal rabbit serum was used as a control (Gibco BRL, Eggenstein, Germany). Rabbit immunoglobulin G (IgG) fractions were prepared from the sera by affinity chromatography on a protein A-Sepharose CL-4B column (Pharmacia, Uppsala, Sweden) (6). Protein concentrations were determined by the method of Bradford (1), and IgG preparations were stored at −20°C until used.
Antistaphylococcal antibodies in the respective IgG fractions were titrated by analysis of serial dilutions by an immunofluorescence assay with the biofilm-producing, PIA-positive S. epidermidis 1457 and the biofilm-negative, PIA-negative S. epidermidis 1457-M11 as antigens (15). In addition, the contents of anti-PIA antibodies in the antisera were determined by a coagglutination assay (15) prepared with Staphylococcus aureus Cowan I and the corresponding antisera by using a preparation of purified PIA as antigen.
PIA was purified from S. epidermidis 1457 by Q-Sepharose anion exchange chromatography as described, and the major polysaccharide fraction I was used (16). The hexosamine concentration of the purified polysaccharide was determined by colorimetric assay (16).
To assess hemagglutination bacteria were grown in TSB in plastic tissue culture dishes for 22 h at 37°C (15). The medium was aspirated, and the cells were scraped from the surface into 12 ml of phosphate-buffered saline. After passage through a 23-gauge needle the bacterial suspension was adjusted to an optical density at 578 nm (OD578) of 1.0. The hemagglutination assay was performed with 96-well (U-shaped) microtiter plates (Greiner, Nürtingen, Germany) by using sheep erythrocytes (Sigma, Deisenhofen, Germany) essentially as described (19). For assessment of the effects of different rabbit IgG fractions on hemagglutination titers, IgG was added to the bacteria in each well of the microtiter plates at a final concentration of 75 μg/ml. After 1 h at room temperature, erythrocytes were added and hemagglutination was assessed as described above. Bacteria incubated in phosphate-buffered saline without added IgG served as a control. To assess the effect of purified PIA on hemagglutination, PIA was added to the bacteria at a concentration of 9 μg/ml of hexosamine. Hemagglutination titers were evaluated as described above. Bacteria incubated in parallel in phosphate-buffered saline without added PIA served as a control.
Hemagglutination of isogenic biofilm-negative S. epidermidis transposon insertion mutants.
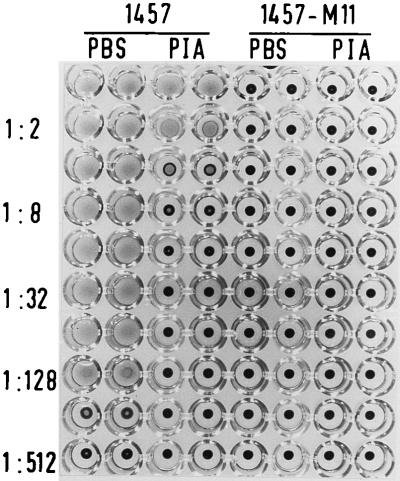
Three biofilm-producing S. epidermidis clinical isolates, 1457, 9142, and 8400, were hemagglutination positive with titers of 1:64 to 1:128 (Table 1). The different banding patterns obtained after SmaI digestion in PFGE indicated that these strains were genetically independent clones (data not shown). In addition to the already-obtained transductants 9142-M10, 9142-M11, and 1457-M11 (14), the transposon insertions of the isogenic biofilm-negative transposon mutants M10 and M11 were transferred into S. epidermidis 1457 and 8400, respectively. The fragment patterns of these transductants obtained in PFGE were identical to the patterns of the corresponding wild-type strains (data not shown). Southern hybridization by using radioactively labeled plasmid pTV1ts as the probe proved that the Tn917 insertions of mutants M10 and M11 were integrated into a fragment of approximately 60 kb in the respective transductants (data not shown). In contrast to the different biofilm-producing wild-type strains, all transductants exhibited a completely biofilm-negative phenotype, and in bacterial extracts of the transductants, PIA could not be detected at all (Table 1). Comparison in the hemagglutination assay revealed that all biofilm-negative transductants were completely hemagglutination negative (Table 1; see also Fig. 3).
TABLE 1.
Hemagglutination and PIA production of transductants with transposon insertions of mutants M10 and M11 in different wild-type strains
| Strain | (OD570)a | PIAb (reciprocal titer) | Hemagglutinationc (reciprocal titer) |
|---|---|---|---|
| 1457 | 2.469 | 1024 | 128 |
| 1457-M10 | 0.044 | 0 | 0 |
| 1457-M11 | 0.045 | 0 | 0 |
| 9142 | 1.836 | 512 | 64 |
| 9142-M10 | 0.014 | 0 | 0 |
| 9142-M11 | 0.020 | 0 | 0 |
| 8400 | 2.080 | 1024 | 64 |
| 8400-M10 | 0.056 | 0 | 0 |
| 8400-M11 | 0.054 | 0 | 0 |
| SE-5 | 2.224 | 1024 | 128 |
| RP62A | 1.798 | 1024 | 256 |
Strains were grown in TSB in 96-well tissue culture plates, the plates were washed with phosphate-buffered saline, cells were fixed with Bouin’s fixative, and adherent bacterial biofilms were stained with gentian violet. Biofilm-producing strains were arbitrarily assigned to have a mean OD570 greater than 0.1.
PIA expression was determined in extracts of bacteria grown in TSB by coagglutination. A coagglutination reagent specific for PIA was prepared by sensitizing S. aureus Cowan I with PIA-specific absorbed antiserum.
Hemagglutination was assessed as described in Materials and Methods.
FIG. 3.
Inhibition of hemagglutination by purified PIA. Serial dilutions of the biofilm-producing, PIA-positive S. epidermidis 1457 and the isogenic biofilm-negative PIA-negative transductant 1457-M11 were applied to the wells of a microtiter plate. To each well purified PIA was added at a concentration of 9 μg/ml (PIA). Control wells did not contain PIA and contained only phosphate-buffered saline (PBS). The hemagglutination titers were assessed as described in Materials and Methods.
Characterization of Tn917 insertion sites of mutants M10 and M11.
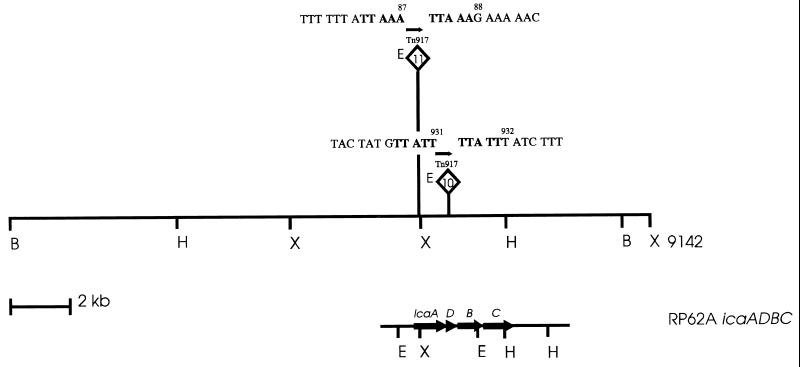
To investigate which genes are inactivated by the Tn917 insertions of M10 and M11, chromosomal EcoRI fragments of 6.8 and 5.8 kb, respectively, containing insertions of Tn917 in mutants M10 and M11, were cloned in S. carnosus. When the cloned DNA fragments of mutants M10 and M11 were used as probes, hybridization with 7.6-kb and 4.3-kb XbaI fragments and with 2.2-kb and 0.8-kb EcoRI fragments, respectively, was observed with chromosomal DNA of biofilm-producing S. epidermidis 9142 (data not shown). In contrast, both probes hybridized with a HindIII fragment of identical size (13 kb). Using the cloned probes and two AvaI fragments corresponding to the 5′ (erm) and the 3′ junctions of Tn917 (24), mapping of the transposon insertions was performed with restriction enzymes XbaI, HindIII, and BglII (Fig. 1). Nucleotide sequence analysis revealed that mutants M10 and M11 had their Tn917 insertions at nucleotides 931 and 87, respectively, of the coding sequence of the icaA gene of icaADBC (Fig. 1) (5, 9).
FIG. 1.
Mapping of transposon insertions of biofilm-negative mutants M10 and M11. The flanking DNA sequences of Tn917 are indicated; transpositions at nucleotides 931 and 87 of the icaA gene were accompanied by 5-bp duplications, indicated in bold letters. E, erythromycin resistance gene erm; arrow, direction of transcription of erm. Restriction sites: B, BglII; H, HindIII; X, XbaI; and E, EcoRI. The region containing the transposon insertions in S. epidermidis 9142 is aligned to the homologous icaADBC operon of S. epidermidis RP62A (5, 9).
Inhibition of hemagglutination by PIA-specific antibodies.
To investigate whether PIA is specifically involved in hemagglutination of S. epidermidis, inhibition of hemagglutination by PIA-specific antibodies was evaluated. Therefore, IgG was prepared from several different antisera. An antiserum raised against purified PIA which specifically reacted in an immunofluorescence assay only with the PIA-positive S. epidermidis 1457 and not with the isogenic PIA-negative S. epidermidis 1457-M11 was used (Table 2). Anti-5179 reacted with staphylococcal cells irrespective of PIA production and did not contain any PIA-specific antibody (Table 2). Anti-1457 reacted with staphylococcal cells and in addition contained specific anti-PIA antibodies (Table 2). Normal rabbit serum, which did not contain detectable amounts of antistaphylococcal antibodies, was used as a control (Table 2).
TABLE 2.
Antibody titers of different IgG preparations used
| Serum | Antigen used in immuno-fluorescence assay
|
Coagglutination with purified PIA (titer) | |
|---|---|---|---|
| 1457 (PIA positive) (titer) | 1457-M11 (PIA negative) (titer) | ||
| Anti-PIA | 1:128 | <1:32 | 1:1024 |
| Normal rabbit serum | <1:32 | <1:32 | Negative |
| Anti-5179 (PIA-negative) | 1:512 | 1:512 | Negativea |
| Anti-1457 (PIA-positive) | 1:1024 | 1:1024 | 1:256 |
As a coagglutination reagent prepared with anti-5179 displayed autoagglutination, probably by cross-reaction with S. aureus, the antiserum was diluted 1:50 in phosphate-buffered saline and absorbed with an overnight culture of the biofilm-negative, PIA-negative S. epidermidis 5179 before preparation of the coagglutination reagent. A coagglutination reagent prepared with anti-PIA serum absorbed in parallel displayed a titer of 1:512.
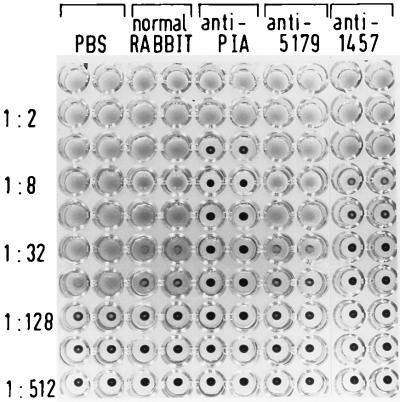
With S. epidermidis 1457 only marginal differences in hemagglutination titers were observed with normal rabbit serum and anti-5179 antiserum as compared to controls incubated in phosphate-buffered saline (Fig. 2). In contrast, a 32-fold-lower hemagglutination titer was observed with the specific anti-PIA antiserum (Fig. 2). Similarly, the anti-1457 antiserum significantly inhibited hemagglutination (Fig. 2). Similar results were obtained with the biofilm-producing and hemagglutination-positive S. epidermidis 9142, 8400, RP62A, and SE-5 (Table 3). Therefore, specific antibody against PIA inhibits hemagglutination of S. epidermidis.
FIG. 2.
Inhibition of hemagglutination by different IgG preparations. Serial dilutions of biofilm-producing PIA-positive S. epidermidis 1457 were applied to each well of a microtiter plate. Each well contained (at concentrations of 75 μg/ml) the IgG preparation of normal rabbit serum (normal RABBIT), antiserum raised against purified PIA (anti-PIA), antiserum raised against whole cells of the biofilm-negative, PIA-negative S. epidermidis 5179 (anti-5179), and antiserum raised against whole cells of the biofilm-producing, PIA-positive S. epidermidis 1457 (anti-1457). Control wells contained no IgG and only phosphate-buffered saline (PBS). The hemagglutination titers were assessed as described in Materials and Methods.
TABLE 3.
Inhibition of S. epidermidis hemagglutination by IgG specific for the PIA
| Strain | Hemagglutination (reciprocal titers)
|
||||
|---|---|---|---|---|---|
| Phosphate-buffered saline | Normal IgG | Anti-PIA | Anti-5179 | Anti-1457 | |
| 9142 | 32 | 16 | 1 | 16 | 4 |
| 8400 | 64 | 32 | 2 | 32 | 4 |
| SE-5 | 128 | 64 | 2 | 64 | 4 |
| RP62A | 256 | 128 | 4 | 128 | 16 |
Inhibition of hemagglutination by purified PIA.
Attempts to reconstitute hemagglutination of erythrocytes by purified PIA were not successful at concentrations up to 18 μg/ml (data not shown). In addition, purified PIA did not lead to hemagglutination of the biofilm-negative, hemagglutination-negative S. epidermidis 1457-M11 (Fig. 3). In contrast, purified PIA, at a concentration of 9 μg/ml, decreased the hemagglutination titer of S. epidermidis 1457 64-fold (Fig. 3). Similar inhibition of hemagglutination by purified PIA was observed with S. epidermidis 9142, 8400, RP62A, and SE-5 (Table 4). Apparently, purified PIA significantly interferes with hemagglutination of S. epidermidis.
TABLE 4.
Inhibition of S. epidermidis hemagglutination by purified PIA
| Strain | Hemagglutination (reciprocal titer)
|
|
|---|---|---|
| Phosphate-buffered saline | PIA | |
| 9142 | 64 | 1 |
| 8400 | 64 | 2 |
| SE-5 | 128 | 2 |
| RP62A | 256 | 4 |
Interruption of the icaADBC operon at nucleotides 931 and 87 of the icaA gene in mutants M10 and M11 led to a biofilm-negative and hemagglutination-negative phenotype together with complete abolition of PIA synthesis after transfer of the corresponding Tn917 insertions in three independent S. epidermidis wild-type strains. M10 and M11 are the only biofilm-negative S. epidermidis transposon insertion mutants yet described that contain Tn917 insertions within the coding sequence of biosynthetic genes responsible for the synthesis of PIA (5, 9). In contrast, the mutant Mut2 described by Heilmann et al. (8, 9) has a Tn917 insertion 72 bp proximal of the ATG start codon of the icaA gene, whereas the insertion site of Mut2a is nearly 1 kb proximal to the icaA translational start site, which probably leads to polar inhibition of the expression of PIA synthetic genes.
The suggested specific function of PIA in hemagglutination was corroborated by analysis of the effects of anti-PIA antibodies on hemagglutination. Only antibodies specific for PIA significantly inhibited hemagglutination of several different S. epidermidis strains (Fig. 2; Table 3). This inhibition of hemagglutination is specifically caused by interaction of antibody with PIA and not merely by steric hindrance by binding of antibody to the bacterial cell surface leading to interference with a still undefined hemagglutinin, because anti-5179, which contains high titers of antistaphylococcal antibodies but no anti-PIA antibodies, only marginally inhibited hemagglutination, similarly to normal rabbit serum. To further ascertain the specific function of PIA in hemagglutination of S. epidermidis, we investigated the effect of the purified PIA on hemagglutination. Purified PIA decreased the hemagglutination titers of five independent hemagglutination-positive S. epidermidis strains. The amount of PIA used corresponds to an approximately 10- to 100-fold excess of exogenously added purified PIA compared to the amount of PIA produced by the S. epidermidis cells used in the hemagglutination assay. This indicates that purified PIA as used in our study competitively inhibits hemagglutination.
Published evidence regarding the properties of the crude hemagglutinin extracted from S. epidermidis, including its sensitivity to periodate oxidation and degradation by glycosidases containing β-N-acetylglucosaminidase activity, molecular size as determined by ultrafiltration, and dependence of expression on the glucose concentration of the growth medium, is consistent with the idea that PIA is the active compound (15, 19, 21). In addition, the identity of PIA with the hemagglutinin clearly explains the similar quantitative relation of the amounts of biofilm formed by individual strains and the hemagglutination titers and the amounts of PIA produced by different clinical S. epidermidis isolates (13, 19, 21).
In the present study we could not reconstitute hemagglutination using purified PIA. Several reasons could account for that observation. First, extraction of PIA from S. epidermidis by sonication may alter the configuration of the polysaccharide chains, rendering them inactive as a hemagglutinin but still functional as a competitive inhibitor. Second, in hemagglutination inhibition experiments, we used polysaccharide I of PIA, which contains exclusively positive charges due to non-N-acetylated glucosamine residues in the polysaccharide chain (16). Polysaccharide II, which is a minor component of PIA accounting for approximately 15% of the polysaccharide, contains negative charges introduced by modification of the polysaccharide by phosphate and succinate residues (16). The mechanism of hemagglutination mediated by PIA could be related to synergistic interactions of the differentially charged polysaccharide species with the negatively charged surface of the erythrocyte. In contrast, as purified PIA inhibits hemagglutination, PIA might interact with a putative specific receptor for the hemagglutinin on the erythrocyte surface. At present we cannot completely exclude the possibility that a component in addition to PIA is necessary for the functional activity of the hemagglutinin of S. epidermidis. However, it seems highly unlikely that polysaccharides completely unrelated to PIA are involved in hemagglutination, as the icaADBC operon contains only a single gene homologous to a glycosyltransferase and no additional synthetic genes related to sugar precursor biosynthesis (5, 9) whose interruption could lead to impaired synthesis of a polysaccharide different from PIA. It is interesting that in a recent report it was proposed that PSA, which in its purified form was reported to contain 54% hexoses, 20% amino sugars, and 10% uronic acids and as specific sugars 22% galactose, 15% glucosamine, and 5% galactosamine (25), is in fact an acidic polysaccharide that is very similar to PIA and composed exclusively from β-1,6-linked glucosamine residues containing succinate and acetate and that its production is dependent on the icaADBC locus (17). As detailed data for the structural analysis of this potential variant of PIA have not yet been reported, differentiation of this polysaccharide from polysaccharide II of PIA awaits further studies.
Taken together, the observations that S. epidermidis mutants that are impaired in PIA synthesis by a defined genetic manipulation are hemagglutination negative and that specific anti-PIA antibodies and purified PIA inhibit hemagglutination define PIA as the hemagglutinin of S. epidermidis or at least as its major functional component. As these results were corroborated by results obtained with several independent clinical S. epidermidis isolates, including the reference strains RP62A and SE-5, it seems reasonable to generalize this conclusion to other hemagglutination-positive S. epidermidis strains.
Acknowledgments
We thank Vibeke T. Rosdahl, Statens Serum Institute, Copenhagen, Denmark, for providing phages and propagating strains and Friedrich Götz, Molekulare Genetik, University of Tübingen, Germany, for bacterial strains and plasmids and for suggesting oligonucleotide primers useful for sequence analysis of DNA-flanking Tn917 insertions. We thank Peter Schäfer for critically reading the manuscript. The photographic work of C. Schlüter is gratefully acknowledged.
This work was supported in part by a grant of the Deutsche Forschungsgemeinschaft to D. M.
REFERENCES
- 1.Bradford M M. A rapid and sensitive method for quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 2.Christensen G D, Simpson W A, Younger J J, Baddour L M, Barrett F F, Melton D M, Beachey E H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985;22:996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christensen G D, Baldassarri L, Simpson W A. Colonization of medical devices by coagulase-negative staphylococci. In: Bisno A L, Waldvogel F A, editors. Infections associated with indwelling medical devices. 2nd ed. Washington, D.C: American Society of Microbiology; 1994. pp. 45–78. [Google Scholar]
- 4.Feucht H H, Zöllner B, Polywka S, Laufs R. Study on reliability of commercially available hepatitis C virus antibody test. J Clin Microbiol. 1995;33:620–624. doi: 10.1128/jcm.33.3.620-624.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerke C, Kraft A, S̈ßmuth R, Schweitzer O, Götz F. Characterization of the N-acetylglucosaminyltransferase activity involved in the biosynthesis of the Staphylococcus epidermidis polysaccharide intercellular adhesin. J Biol Chem. 1998;273:18586–18593. doi: 10.1074/jbc.273.29.18586. [DOI] [PubMed] [Google Scholar]
- 6.Goding J W. Conjugation of antibodies with fluorochromes: modification to the standard methods. J Immunol Methods. 1976;13:215–226. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- 7.Götz F, Schumacher B. Improvements of protoplast transformation in Staphylococcus carnosus. FEMS Microbiol Lett. 1987;40:285–288. [Google Scholar]
- 8.Heilmann C, Gerke C, Perdreau-Remington F, Götz F. Characterization of Tn917 insertion mutants of Staphylococcus epidermidis affected in biofilm formation. Infect Immun. 1996;64:277–282. doi: 10.1128/iai.64.1.277-282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heilmann C, Schweitzer O, Gerke C, Vanittanakom N, Mack D, Götz F. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol Microbiol. 1996;20:1083–1091. doi: 10.1111/j.1365-2958.1996.tb02548.x. [DOI] [PubMed] [Google Scholar]
- 10.Hussain M, Herrmann M, von Eiff C, Perdreau-Remington F, Peters G. A 140-kilodalton extracellular protein is essential for the accumulation of Staphylococcus epidermidis strains on surfaces. Infect Immun. 1997;65:519–524. doi: 10.1128/iai.65.2.519-524.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kloos W E, Bannerman T L. Update on clinical significance of coagulase-negative staphylococci. Clin Microbiol Rev. 1994;7:117–140. doi: 10.1128/cmr.7.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mack, D. Molecular mechanisms of Staphylococcus epidermidis biofilm formation. J. Hosp. Infect., in press. [DOI] [PubMed]
- 13.Mack D, Haeder M, Siemssen N, Laufs R. Association of biofilm production of coagulase-negative staphylococci with expression of a specific polysaccharide intercellular adhesin. J Infect Dis. 1996;174:881–884. doi: 10.1093/infdis/174.4.881. [DOI] [PubMed] [Google Scholar]
- 14.Mack D, Nedelmann M, Krokotsch A, Schwarzkopf A, Heesemann J, Laufs R. Characterization of transposon mutants of biofilm-producing Staphylococcus epidermidis impaired in the accumulative phase of biofilm production: genetic identification of a hexosamine-containing polysaccharide intercellular adhesin. Infect Immun. 1994;62:3244–3253. doi: 10.1128/iai.62.8.3244-3253.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mack D, Siemssen N, Laufs R. Parallel induction by glucose of adherence and a polysaccharide antigen specific for plastic-adherent Staphylococcus epidermidis: evidence for functional relation to intercellular adhesion. Infect Immun. 1992;60:2048–2057. doi: 10.1128/iai.60.5.2048-2057.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mack D, Fischer W, Krokotsch A, Leopold K, Hartmann R, Egge H, Laufs R. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear β-1,6-linked glucosaminoglycan: purification and structural analysis. J Bacteriol. 1996;178:175–183. doi: 10.1128/jb.178.1.175-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McKenney D, Hübner J, Muller E, Wang Y, Goldmann D A, Pier G B. The ica locus of Staphylococcus epidermidis encodes production of the capsular polysaccharide/adhesin. Infect Immun. 1998;66:4711–4720. doi: 10.1128/iai.66.10.4711-4720.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nedelmann M, Sabottke A, Laufs R, Mack D. Generalized transduction for genetic linkage analysis and transfer of transposon insertions in different Staphylococcus epidermidis strains. Zentbl Bakteriol. 1998;287:85–92. doi: 10.1016/s0934-8840(98)80151-5. [DOI] [PubMed] [Google Scholar]
- 19.Rupp M E, Archer G D. Hemagglutination and adherence to plastic by Staphylococcus epidermidis. Infect Immun. 1992;60:4322–4327. doi: 10.1128/iai.60.10.4322-4327.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rupp M E, Archer G D. Coagulase-negative staphylococci: pathogens associated with medical progress. Clin Infect Dis. 1994;19:231–245. doi: 10.1093/clinids/19.2.231. [DOI] [PubMed] [Google Scholar]
- 21.Rupp M E, Sloot N, Meyer H G W, Han J, Gatermann S. Characterization of the hemagglutinin of Staphylococcus epidermidis. J Infect Dis. 1995;172:1509–1518. doi: 10.1093/infdis/172.6.1509. [DOI] [PubMed] [Google Scholar]
- 22.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 23.Schwarzkopf A, Karch H, Schmidt H, Lenz W, Heesemann J. Phenotypical and genotypical characterization of epidemic clumping factor-negative, oxacillin-resistant Staphylococcus aureus. J Clin Microbiol. 1993;31:2281–2285. doi: 10.1128/jcm.31.9.2281-2285.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaw J H, Clewell D B. Complete nucleotide sequence of macrolide-lincosamide-streptogramin B-resistance transposon Tn917 in Streptococcus faecalis. J Bacteriol. 1985;164:782–796. doi: 10.1128/jb.164.2.782-796.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tojo M, Yamashita N, Goldmann D A, Pier G B. Isolation and characterization of a capsular polysaccharide adhesin from Staphylococcus epidermidis. J Infect Dis. 1988;157:713–722. doi: 10.1093/infdis/157.4.713. [DOI] [PubMed] [Google Scholar]