Abstract
Activation of the extracellularly regulated kinase (ERK) pathway is part of the early biochemical events that follow lipopolysaccharide (LPS) treatment of macrophages or their infection by virulent and attenuated Salmonella strains. Phagocytosis as well as the secretion of invasion-associated proteins is dispensable for ERK activation by the pathogen. Furthermore, the pathways used by Salmonella and LPS to stimulate ERK are identical, suggesting that kinase activation might be solely mediated by LPS. Both stimuli activate ERK by a mechanism involving herbimycin-dependent tyrosine kinase(s) and phosphatidylinositol 3-kinase. Phospholipase D activation and stimulation of protein kinase C appear to be intermediates in this novel pathway of MEK/ERK activation.
Salmonellae are enteric pathogens that cause a variety of diseases, from localized gastroenteritis to more severe systemic, host-specific illnesses like typhoid fever. The ability to cross the intestinal epithelial barrier is an important determinant of Salmonella virulence and involves the entry into several cell types, including enterocytes, M cells, and macrophages. Several bacterial genes involved in internalization have been described (15), some of which are necessary for the uptake into epithelial cells as well as into phagocytic cells (4).
Among the most stimulating new developments in this area is the discovery of a host cell-stimulated bacterial protein secretion system (type III secretion system), necessary for the export and, in some cases, translocation of bacterial proteins into the host cells (reviewed in reference 8). The genes coding for the proteins involved in type III secretion are present in a number of distantly related gram-negative pathogens such as Yersinia, Shigella, and Salmonella as well as Pseudomonas, Xanthomonas, and Erwinia, a plant pathogen. In Salmonella, type III secretion genes are clustered in regions, called Salmonella pathogenicity islands (SPIs), at centisomes 63 (SPI-1) and 31 (SPI-2) on the chromosome (reviewed in reference 17).
The bacterial proteins delivered to the host cell via the type III secretion system trigger signal transduction pathways, leading to a variety of responses (12). Among the components of signal-transducing pathways activated during infection are mitogen-activated protein kinases (MAPKs) and phospholipase C-γ (34). Salmonella-induced membrane ruffling and cytoskeletal rearrangements (15) are a prerequisite for invasion of epithelial cells, and they have been shown to be mediated by the small GTPase Cdc42 (6) but not by Ras or Rho (23).
For Salmonella, as well as for many other facultative intracellular pathogens, the key to a successful infection lies in the outcome of their encounter with the host’s macrophages. Still, much less is known about the molecular mechanisms operating during the interaction of Salmonella typhimurium with macrophages than about the signaling events taking place during epithelial cell invasion. In this study, we focus on the signal transduction events that follow the encounter of the microbe with the macrophage, in particular on the mechanism of activation of the extracellularly regulated kinase (ERK) pathway (for a review, see reference 41).
Cytosolic serine/threonine kinase cascades can be activated by extracellular stimuli as diverse as growth and differentiation factors, inflammatory cytokines, apoptotic stimuli, and cellular stresses in general. Thus, multiple sensors can couple to these intracellular signal transducers. Relevant to this study, ERK can be activated in macrophages by bacterial lipopolysaccharide (LPS) (5, 28, 36, 39, 48) as well as by the gram-negative Yersinia enterocolitica (38). In cultured epithelial cells, ERK activation is stimulated by invasive S. typhimurium (20) and during the attachment of the gram-positive bacterium Listeria monocytogenes (45). ERK also appears to play a role in phagocytosis (46) and intramacrophage survival of the latter pathogen (26). However, little is known about the mechanisms mediating ERK stimulation in these situations.
The focus of this study was to dissect the signaling events that follow the encounter of S. typhimurium with macrophages and to compare them with those taking place upon stimulation of these cells with LPS. We show that MAPK kinase (MEK) and ERK are activated in the very early stages of LPS stimulation and of macrophage infection by virulent Salmonella. Pathogen-induced activation is entirely phagocytosis independent and is mediated by a novel mechanism involving phosphatidylinositol 3-kinase (PI 3-K), phospholipase D (PLD), and protein kinase C (PKC) as intermediates. We detected no differences in the pathways mediating ERK activation by live bacteria and LPS alone. Therefore, this surface molecule may be the only component triggering stimulation of ERK by Salmonella.
MATERIALS AND METHODS
Bacteria.
S. typhimurium LT2 (virulent, wild type) was grown in Luria-Bertani broth (1% Bacto Tryptone, 0.5% yeast extract, 1% sodium chloride) at 37°C overnight under agitation.
Cell culture, infection, and stimulation.
BAC-1.2F5 cells (31) were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum and 20% L-cell conditioned medium as a source of colony-stimulating factor 1 (CSF-1).
Confluent cells (about 5 × 106 cells per 100-mm-diameter tissue culture dish) were cultured for 16 h in medium without CSF-1 and then either stimulated with 1.5 μg of S. typhimurium LPS (Sigma, Vienna, Austria) per ml or infected with bacterial cultures. A multiplicity of infection (MOI [bacteria per macrophage]) of 25 was used. After infection with Salmonella, plates were centrifuged 5 min at 300 × g to synchronize the infection.
In selected experiments, actin polymerization was blocked by pretreatment with 10 μM cytochalasin B (30 min; Sigma). Tyrosine kinases were inhibited by pretreatment with herbimycin A (1 μg/ml, 4 h; Sigma). Activation of PI 3-K was blocked by pretreatment with wortmannin (100 nM, 20 min; Sigma). Inhibition of the Rho family of small GTPases (RhoA, Rac1, and Cdc42) was performed by a 60-min preincubation with toxin B (from Clostridium difficile [1]) at a final concentration of 10 or 100 ng/ml, as indicated. Inhibition of phosphatidylcholine (PC)-PLC activity was determined by preincubating the cells for 60 min with 10 or 180 μM xanthogenate tricyclodecan-9-yl (D609; Alexis Biochemicals, Laufelfingen, Switzerland). PLD was inhibited by the addition of 1% 1-butanol to the cells at the time of infection or stimulation with LPS. The phosphatidic acid (PA) phosphohydrolase was inhibited by pretreating the macrophages with propranolol (250 μM, 10 min; Sigma). PKC was inhibited by treating the cells with 10 μM bisindoleylmaleimide (BIM; Calbiochem, San Diego, Calif.) for 60 min prior to stimulation (5). Furthermore, in selected experiments, down-modulation of PKC was performed by treating cultures with 5 μM tetradecanoylphorbol 13-acetate (TPA; Sigma) in dimethyl sulfoxide (14 mM, final concentration; Sigma) for 24 h. Dimethyl sulfoxide alone had no effect on the phosphorylation state of the kinases (data not shown). MEK activation was inhibited by 60-min pretreatment with 50 μM PD98059 (Calbiochem).
Phagocytosis assay.
Colony counting assays were performed to assess phagocytosis of S. typhimurium. Briefly, cells (0.05 × 106) were seeded in 96-well plates and infected (multiplicity of infection [MOI] of 25). Cells were allowed to phagocytose for 30 min and then washed three times with phosphate-buffered saline (PBS). Fresh medium containing 50 μg of gentamicin per ml was added and the cells were incubated for an additional 60 min to kill residual extracellular bacteria. Thereafter, cells were lysed in PBS supplemented with 0.5% sodium deoxycholate. Serial dilutions of the lysates were prepared in PBS and plated onto LB agar plates. Colonies were allowed to develop for 18 h before counting. Assays were carried out in triplicate.
Cell lysis and Western blotting.
Cells were lysed in solubilization buffer (10 mM Tris base, 50 mM sodium chloride, 30 mM sodium pyrophosphate, 50 mM sodium fluoride, 1% Triton X-100 [pH 7.0]) supplemented with 1 mM phenylmethylsulfonyl fluoride, 100 μM sodium vanadate, 1 mM dithiothreitol, and protease inhibitors (aprotinin [3 μg/ml], pepstatin [0.5 μg/ml], and leupeptin [0.5 μg/ml]). For immunoblotting, 30 to 40 μg of whole-cell lysates was separated by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. After blocking (8 to 16 h at 4°C in TTBS (10 mM Tris-HCl [pH 8.0] 150 mM NaCl, 0.1% Tween 20) supplemented with 4% bovine serum albumin (fraction V; Sigma), the membranes were probed with the appropriate primary antibodies diluted in 1% bovine serum albumin in TTBS prior to incubation with peroxidase-conjugated secondary antibodies and detection by an enhanced chemiluminescence system (Pierce, Rockford, Ill.). The primary antibodies used in this study recognized ERK1/2 and MEK1/2 (Transduction Laboratories, Lexington, Ky.) or their phosphorylated forms selectively (anti-pERK Tyr204 and anti-pMEK Ser271/222; New England BioLabs, Schwalbach, Germany).
In-gel kinase assay.
Five to 15 μg of whole-cell lysates was separated on 10% polyacrylamide gels containing 0.2 mg of myelin basic protein (MBP; Sigma) per ml as a substrate for ERK1/2. After electrophoresis, the gels were denatured in 6 M guanidine hydrochloride, renatured, and assayed for kinase activity as described previously (5).
RESULTS
Wortmannin-sensitive activation of MEK/ERK is an early event in Salmonella- and LPS-induced signal transduction.
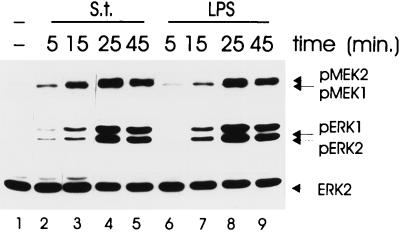
Quiescent BAC-1.2F5 cells were infected with Salmonella or stimulated with LPS for different time periods. The activation state of the kinases was assessed in whole-cell lysates by immunoblotting with antibodies that specifically recognize the phosphorylated, activated form of each enzyme (Fig. 1). All kinases were activated by Salmonella infection of BAC-1.2F5 cells with activation/inactivation kinetics comparable to those induced by LPS. For both stimuli, peak activation occurred after 25 min (Fig. 1, lanes 4 and 8) and then decayed. Inactivation was complete by 1 h, and no further changes were observed over a period of 4 h (data not shown). The anti-pMEK antibody recognized the activated forms of both MEK1 and MEK2, which appear as a doublet particularly well discernible on shorter exposures of the membrane (Fig. 1, upper panel). In this experiment, the amounts of ERK1 and ERK2 phosphorylated following LPS stimulation or infection with Salmonella appeared to be comparable. In general, however, the pERK1 band was stronger than the pERK2 one, albeit to different degrees (Fig. 2 and 4 to 6). The kinetics of Salmonella- and LPS-induced ERK activation were confirmed by in-gel kinase assays using MBP as a substrate (data not shown).
FIG. 1.
Effects of S. typhimurium and LPS on the activity of MEK and ERK. Quiescent BAC-1.2F5 cells were either infected with S. typhimurium (S.t.; MOI of 25) or stimulated with LPS (1.5 μg/ml) at 37°C for different times prior to solubilization. The presence of phosphorylated, active forms of MEK and ERK was detected by immunoblotting with the corresponding antibodies. An anti-ERK blot is shown as a loading control.
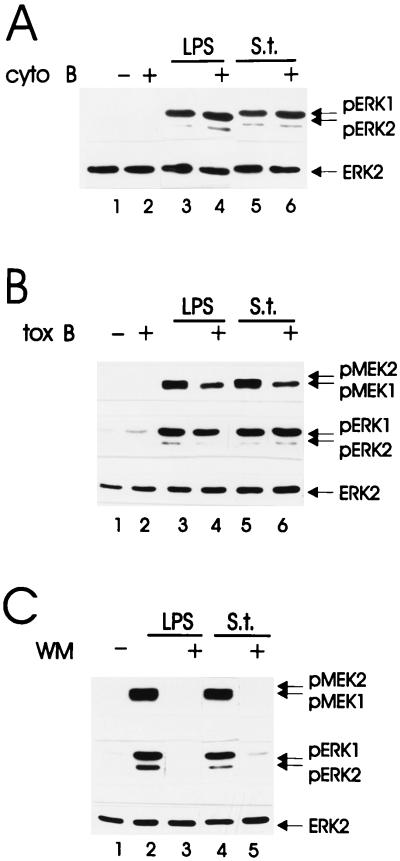
FIG. 2.
Effects of phagocytosis inhibitors on Salmonella- and LPS-induced kinase activity. Cells were treated with cytochalasin B (cyto B; 10 μM, 30 min) (A), with toxin B (tox B; 100 ng/ml, 60 min) (B), or with wortmannin (WM; 100 nM, 20 min) (C) before infection with Salmonella (S.t.; MOI of 25) or stimulation with LPS (1.5 μg/ml) for 15 min. The presence of the phosphorylated, active forms of MEK and ERK was detected by immunoblotting with the corresponding antibodies. An anti-ERK blot is shown as a loading control.
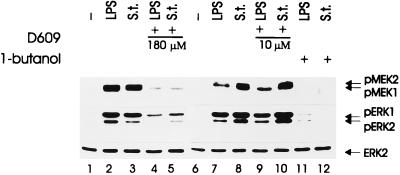
FIG. 4.
Effects of treatment with phospholipase inhibitors on Salmonella- and LPS-induced kinase activity. Quiescent BAC-1.2F5 cells were pretreated with the PC-PLC inhibitor D609 at the final concentration 180 μM (lanes 4 and 5) or 10 μM (lanes 9 and 10) for 60 min before infection with Salmonella (S.t.; MOI of 25) or stimulation with LPS (1.5 μg/ml) for 15 min. To assess the contribution of PLD to kinase activation, infection with Salmonella and stimulation with LPS were carried out in the presence of PLD inhibitor 1-butanol (1%; lanes 11 and 12). The presence of phosphorylated MEK and ERK was detected by immunoblotting with the corresponding antibodies. An anti-ERK blot is shown as a loading control.
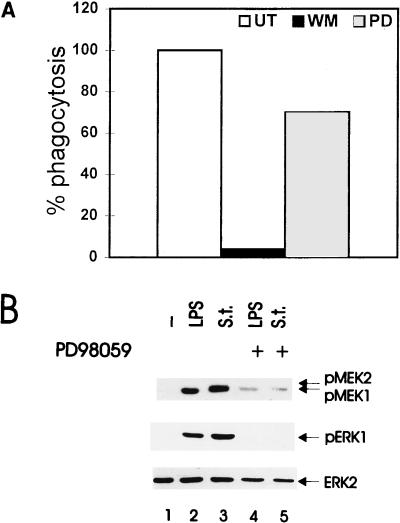
FIG. 6.
Effects of MEK inhibitor PD98059 on the ability of BAC-1.2F5 macrophages to phagocytose Salmonella (A) and on Salmonella- and LPS-stimulated activation of ERK (B). (A) Macrophages were either pretreated with PD98059 (PD; 50 μM, 60 min) or wortmannin (WM; 100 nM, 20 min) or left untreated (UT). Cells were infected with Salmonella (MOI of 25) and allowed to phagocytose for 30 min. Cells were then washed three times with PBS, and fresh medium containing gentamicin (50 μg/ml) was added for a further 60-min incubation to kill residual extracellular bacteria. Phagocytosis was assessed as described in Materials and Methods. Assays were carried out in triplicate. The phagocytic activity of untreated macrophages was set at 100%. (B) Quiescent BAC-1.2F5 cells were pretreated with PD98059 (50 μM, 60 min) and either infected with Salmonella (S.t.; MOI of 25) or stimulated with LPS (1.5 μg/ml) for 15 min prior to solubilization. The presence of phosphorylated MEK and ERK was detected by immunoblotting with the corresponding antibodies. An anti-ERK blot is shown as a loading control.
The time frame investigated (5 to 45 min) allows phagocytosis of Salmonella by the macrophages. We therefore examined whether phagocytosis per se would be able to activate the ERK pathway. Phagocytosis of latex beads of the approximate size of the bacteria stimulated none of the kinases studied (data not shown). However, the mechanisms involved in the phagocytosis of bacteria might differ from those involved in the phagocytosis of inert particles. In support of this possibility, both heat-killed Salmonella and E. coli K-12 triggered kinase activation (data not shown).
We therefore analyzed the effects of inhibitors of specific signal transduction pathways on Salmonella phagocytosis. As a positive control for inhibition of phagocytosis, we used cytochalasin B, which destroyed the actin cytoskeleton and totally abrogated phagocytosis (Table 1). Toxin B, which glucosylates and inactivates the small GTPases Cdc42, Rac and Rho (1), completely inhibited phagocytosis at a concentration of 100 ng/ml, while 10 ng/ml reduced it to 50 to 60% (Table 1); the PI 3-K inhibitor wortmannin, which blocks epithelial cell invasion by L. monocytogenes but not by Salmonella (21), also fully blocked phagocytosis (Table 1).
TABLE 1.
Effects of chemical inhibitors on the phagocytosis of S. typhimurium by BAC-1.2F5 cells
| Pretreatment of cells | Phagocytosis (%) ± SD |
|---|---|
| None | 100 |
| Toxin B | |
| 10 ng/ml | 56 ± 9.0 |
| 100 ng/ml | 5 ± 4.0 |
| Wortmannin (100 ng/ml) | 3 ± 1.5 |
| Cytochalasin B (10 μM) | 2 ± 1.0 |
Macrophages were infected with Salmonella (MOI of 25). Phagocytosis was assessed as described in Materials and Methods. Untreated macrophages phagocytosed 55% (± 11) of the original inoculum. The phagocytic activity of untreated macrophages was set as 100%.
We next tested the effect of Salmonella infection on the ERK pathway in macrophages pretreated with the phagocytosis inhibitors (Fig. 2). LPS stimulation was used as a control, to assess whether the differences observed were related to phagocytosis inhibition or rather to the blockage of the corresponding signal transduction pathway.
Pretreatment with cytochalasin B (Fig. 2A) did not suppress LPS- or Salmonella-mediated activation of ERK. Toxin B treatment did not alter the stimulation of MEK1/ERK by LPS treatment or by infection (Fig. 2B). Notably, though, toxin B selectively suppressed the LPS- or Salmonella-mediated phosphorylation of MEK2, suggesting that MEK1 and MEK2 are regulated independently and that full activation of the ERK in this situation can be sustained by MEK1 alone (Fig. 2B). Wortmannin, on the other hand, severely blunted Salmonella- and LPS-stimulated activation of MEK/ERK (Fig. 2C). The effects of wortmannin on LPS- and Salmonella-induced MEK and ERK activation were specific, since the drug failed to inhibit activation of these kinases by the macrophage-specific growth factor CSF-1 (22). These data indicate that phagocytosis and ERK activation by Salmonella are independent events and that PI 3-K is involved in both. PI 3-K also played a role in LPS-mediated ERK stimulation. Together with the fact that phagocytosis is dispensable for the stimulation of ERK by Salmonella, these data suggested that LPS played a major role in kinase stimulation by live bacteria. To investigate this possibility further, we directly compared the contribution of different signaling pathways to ERK activation by either stimulus.
The tyrosine kinase inhibitor herbimycin A decreases Salmonella- and LPS-mediated ERK activation.
Herbimycin-dependent kinases have previously been implicated in the activation of PI 3-K (19) and ERK (36) by LPS in monocytes. To investigate whether this mechanism played a role in ERK activation by Salmonella, we treated BAC-1.2F5 cells with herbimycin A (1 μg/ml) for 4 h before infection or LPS stimulation. LPS- and Salmonella-mediated MEK and ERK activation was efficiently reduced by herbimycin A (Fig. 3). The effect of herbimycin A on ERK activation was specific, since other signaling pathways initiated by Salmonella were unaffected by pretreatment with the drug (35).
FIG. 3.
Effects of herbimycin A treatment on Salmonella- and LPS-stimulated activation of ERK. Quiescent BAC-1.2F5 cells were treated with herbimycin A (1 μg/ml, 4 h) before infection with Salmonella (S.t.; MOI of 25) or stimulation with LPS (1.5 μg/ml) 15 min prior to solubilization. The activity of ERK was measured by an in-gel kinase assay, MBP as a substrate.
Phospholipase inhibitors affect Salmonella- and LPS-mediated activation of the ERK pathway.
PC-PLC is activated in macrophages by mitogenic signals (49) and by inflammatory cytokines (40). Furthermore, the PC-PLC inhibitor D609 decreases LPS-mediated stimulation of Raf, MEK, and ERK (5). To investigate whether phospholipase activation was important for the stimulation of ERK by Salmonella, we treated quiescent BAC-1.2F5 cells with D609 prior to either infection or LPS stimulation of the cells.
D609 pretreatment (180 μM, 60 min) reduced the Salmonella- and LPS-mediated MEK/ERK activation significantly, consistent with our previous report (5) (Fig. 4; compare lanes 2 and 3 and lanes 4 and 5). At the concentration used, however, D609 might also partially inhibit PLD (16). Importantly, the activation of PLD by LPS in monocytes has been reported previously (32).
To distinguish between PC-PLC and PLD inhibition, we used a much lower concentration of D609 (10 μM) which inhibits specifically PC-PLC (Fig. 4). Furthermore, we stimulated or infected the cells in the presence of 1% 1-butanol to block PLD (Fig. 4). At this concentration, 1-butanol efficiently competes with the endogenous PLD substrates (7).
Neither of the inhibitors had any effect on the basal level of kinase activation (data not shown). Low concentrations of D609 did not inhibit MEK/ERK stimulation by LPS and Salmonella (Fig. 4; compare lanes 7 and 8 with lanes 9 and 10); 1-butanol, in contrast, completely abrogated MEK/ERK activation by either stimulus. As a control for the specificity of inhibition, we tested the effect of 1-butanol on the activation of the related JNKs. These kinases are reportedly activated by LPS stimulation of macrophages. Infection with Salmonella as well as LPS treatment stimulated JNK phosphorylation in BAC-1.2F5 cells, and in neither case was the stimulation affected by 1-butanol pretreatment (data not shown). Thus, PLD but not PC-PLC plays a major role in MEK/ERK activation selectively.
PKC is involved in Salmonella- and LPS-mediated activation of the ERK pathway.
BAC-1.2F5 cells express the PKC isoforms δ, ɛ, and ζ, and LPS reportedly increases PKC activation of both diacylglycerol (DAG)-dependent and -independent PKC species in monocytes (28). To determine whether PKC isoenzymes were involved in kinase activation by Salmonella and LPS, BAC-1.2F5 cells were treated with the PKC inhibitor BIM prior to stimulation with Salmonella or LPS. BIM inhibited MEK/ERK activation by either Salmonella or LPS (Fig. 5A).
FIG. 5.
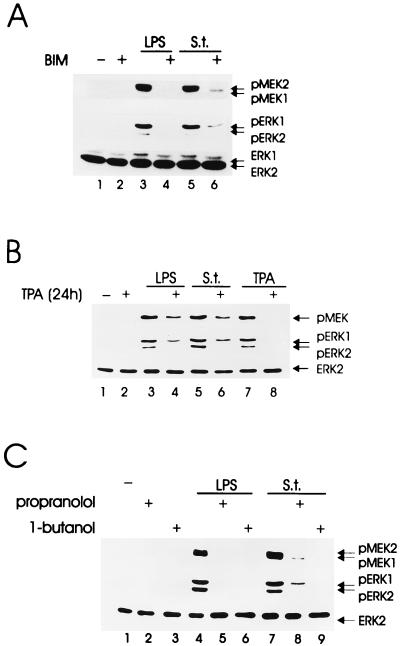
Effects of the PKC inhibitor BIM (A), of chronic TPA treatment (B), and of the PA-phosphohydrolase inhibitor propanolol (C) on Salmonella- and LPS-induced kinase activity. Quiescent BAC-1.2F5 cells were treated with the PKC inhibitor BIM (10 μM, 60 min) or incubated with TPA (5 μM, 24 h) to down-regulate DAG-dependent PKC isoforms. In panel C, propanolol (250 μM, 10 min) was used to inhibit DAG generation via the PLD-PA phosphohydrolase pathway. Cells were subsequently infected with Salmonella (S.t.; MOI of 25) or stimulated with LPS (1.5 μg/ml) for 15 min. In panel B, short stimulation with TPA (5 μM, 15 min) was used as a positive control for the down-regulation of DAG-dependent PKC isoforms. The presence of phosphorylated MEK and ERK was detected by immunoblotting with the corresponding antibodies. An anti-ERK blot is shown as a loading control.
DAG-dependent PKCs would be the natural targets of the DAG generated by PLD, and they are necessary for LPS-mediated MEK/ERK activation (5). To test whether DAG-dependent PKC isoforms were also required for Salmonella-mediated MEK/ERK activation, we down-regulated these enzymes by pretreating BAC-1.2F5 cells with TPA for 24 h. MEK/ERK activation in response to both LPS and Salmonella was impaired in TPA-treated cells. As a control for PKC depletion, TPA-treated cells were unable to activate MEK and ERK when restimulated with the phorbol ester for a short time period (Fig. 5B).
Together with the experiment shown in Fig. 4, the experiments shown in Fig. 5A and B strongly suggest that DAG generated via the PLD-PA phosphohydrolase pathway is needed for the activation of the MEK/ERK pathway via PKC. We tested this hypothesis by pretreating BAC-1.2F5 with the PA phosphohydrolase inhibitor propanolol. Propanolol pretreatment (250 μM, 10 min) completely blocked MEK/ERK activation by both LPS and Salmonella. This experiment clearly identifies DAG generated by the PLD-PA phosphohydrolase pathway as the second messenger lipid necessary for ERK activation in our system.
The MEK phosphorylation inhibitor PD98059 does not block phagocytosis.
The results reported above show that PI 3-K inhibition blocks both Salmonella phagocytosis and ERK activation. ERK activation by Salmonella is, however, phagocytosis independent. MEK/ERK activation has recently been implicated in epithelial cell invasion by L. monocytogenes. To investigate whether this signal transduction pathway played a role in Salmonella phagocytosis by BAC-1.2F5 cells, we pretreated the macrophages with PD98059, a specific inhibitor of MEK phosphorylation (2). PD98059 efficiently blocked MEK and ERK phosphorylation stimulated by either LPS or Salmonella (Fig. 6B). In contrast to wortmannin, which blocks ERK activation, PD98059 did not affect the ability of BAC-1.2F5 cells to phagocytose the bacteria (Fig. 6A). Thus, Salmonella phagocytosis and ERK activation are entirely independent phenomena.
DISCUSSION
Salmonellosis is one of the emerging bacterial diseases, and the number of cases has been increasing steadily in the past few years. Although the interaction of the bacterium with the host’s macrophages is a key event in the early phases of infection, little is known about the signaling events taking place during the interaction of this pathogen with macrophages.
In this study, we compared the mechanisms of activation of the ERK pathway by S. typhimurium and by soluble LPS. We find that both inflammatory stimuli activate ERK by identical pathways involving PI 3-K and PLD as novel intermediates. Our results further imply that LPS is very likely responsible for ERK stimulation by Salmonella.
Phagocytosis-independent ERK activation is part of the early biochemical response to LPS and Salmonella.
MEK and ERK were phosphorylated and activated in response to LPS treatment or Salmonella infection of BAC-1.2F5 macrophages. The kinetics of activation of the kinases were identical and peaked 25 min after infection (Fig. 1). Salmonella engendered activation kinetics slightly faster than LPS stimulation. This led us to test whether phagocytosis played a role in kinase activation. This seemed particularly likely in view of the fact that a significant portion of the ERKs are associated with the cytoskeleton (37).
Cytochalasin B, toxin B, and wortmannin completely inhibited phagocytosis of Salmonella. This is in agreement with previous observations indicating that Rho family GTPases and PI 3-K are necessary for these processes (3, 9, 18, 33).
Neither cytochalasin B nor toxin B had any effect on ERK activation in response to Salmonella or LPS. Wortmannin, in contrast, significantly reduced activation of ERK by both stimuli, indicating that this effect was not secondary to phagocytosis inhibition. We therefore concluded that phagocytosis is not necessary for kinase activation by the bacterium (Fig. 2).
A wortmannin-sensitive pathway mediates the stimulation of MEK/ERK by LPS and Salmonella.
Induction of phosphorylation and activation of herbimycin A-sensitive kinases by LPS in macrophages has been reported previously (43, 48). More precisely, these kinases have been implicated in PI 3-K (19) and ERK (36) activation by LPS in these cells. Herbimycin A and wortmannin completely blocked LPS- and Salmonella-mediated activation of MEK/ERK (Fig. 2 and 4). Inhibition of PI 3-K by wortmannin has been reported to prevent ERK activation by G-protein-coupled receptors (reviewed in reference 44), by integrins (25), by the platelet-derived growth factor (10, 30) and insulin (13) receptor tyrosine kinases, and by the receptors for interleukin-2 (24) and alpha interferon (47). Little, however, is known about the signaling events occurring between PI 3-K activation and ERK activation in these pathways. Among the PI 3-K downstream targets are the protein kinases PKB and p70S6K (reviewed in reference 11); furthermore, PI 3-K participates in the activation of PKC isoenzymes α, β, δ, ɛ, and ζ (reviewed in reference 11). In particular, DAG-dependent PKC isoforms become activated as a result of PI 3-K-mediated stimulation of PLD-dependent phosphatidylcholine hydrolysis (42). Both PLD (32) and PKC (28) are activated by stimulation of monocytes with LPS. In our case, the PLD inhibitor 1-butanol and the PA phosphohydrolase inhibitor propanolol abrogate stimulation of MEK/ERK by LPS and Salmonella (Fig. 4 and 5C). These experiments show that DAG generated by the PLD-PA phosphohydrolase pathway, and not PLD-derived PA, is the second messenger lipid needed in ERK activation. Furthermore, both the PKC inhibitor BIM and TPA-mediated down-regulation of PKC blocked MEK/ERK activation by LPS (5) and Salmonella (Fig. 5A and B). These results (Fig. 4 and 5) suggest that DAG-sensitive PKC isoenzyme(s) activated in a PLD-dependent manner function downstream of PI 3-K in LPS- and Salmonella-mediated stimulation of the MEK/ERK module. Such a pathway would resemble the one described previously by Staendaert et al. (42).
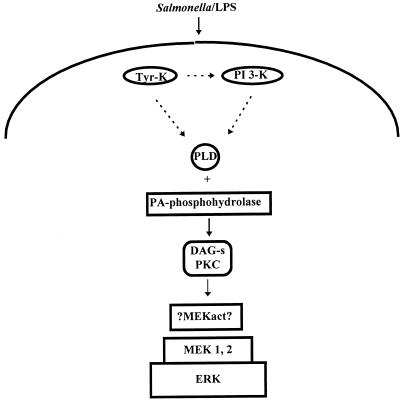
It should be noted, however, that the existence of a PKC-dependent pathway of PLD activation has been postulated in LPS-treated monocytes (32). The PKC isoforms involved were Ca2+-dependent, DAG-dependent enzymes, which are not expressed in BAC-1.2F5 cells. Furthermore, for this mechanism to function in our cells, we would have to postulate either that LPS stimulates DAG generation involving different phospholipases or that the enzymes upstream of PLD are DAG independent. We cannot formally exclude that such a pathway is operating in our system; however, we currently favor the working model depicted in Fig. 7.
FIG. 7.
Mechanisms of cytosolic kinase activation following macrophage stimulation with LPS or infection with Salmonella: a working model. LPS and Salmonella stimulate the MEK/ERK module by a common mechanism, involving activation of one or more herbimycin-sensitive tyrosine kinases (Tyr-K) and of PI 3-K. PI 3-K stimulates MEK/ERK via activation of PLD and of DAG-sensitive PKC (DAG-s PKC). Activation of PI 3-K, PLD, and PKC by LPS has been demonstrated previously (19, 28, 32). The nature of the intermediates laying between PKC and MEK is unknown. This scheme represents the working hypothesis that we currently favor. Alternative models are possible and are discussed in the text. Solid arrows, steps in the pathway for which experimental evidence is provided in the paper; dashed arrows, speculative steps in the pathway.
At present, we have no data about the identity of the downstream targets of PKC activity. A role for Raf-1 in MEK/ERK activation seems unlikely, since LPS-mediated activation of these kinases has been shown to be Raf-1 independent in BAC-1.2F5 cells (5).
PI 3-K (3) and PLD (27) have been implicated in the process of phagocytosis. However, the MEK phosphorylation inhibitor PD98059, which abrogates MEK/ERK activation by LPS and Salmonella, had no effect on phagocytosis. These results indicate that the signal transduction pathways leading to phagocytosis and to MEK/ERK activation diverge downstream of PI 3-K and PLD activation. This is in contrast with the situation in epithelial cell invasion by L. monocytogenes, which requires both PI 3-K (21) and MEK/ERK activation (46).
The identical effects of all the inhibitors tested on ERK stimulation by Salmonella and LPS strongly suggest that the latter molecule by itself may be responsible for kinase activation by the live bacteria.
The data reported here extend our understanding of LPS and Salmonella signal transduction and show for the first time that a pathway comprising PI 3-K, PLD, and PKC leads to ERK activation as part of the response of macrophages to these inflammatory stimuli.
ACKNOWLEDGMENTS
We thank Klaus Aktories (University of Freiburg) for his gift of C. difficile toxin B. We are indebted to Thomas Decker (Vienna Biocenter) for critically reading the manuscript and to Angelika Kranawetter for helping with the preparation of figures.
This work was supported by grant P10766-MED from the Austrian Research Fund (to M.B.) and by grant 70008/2 -Pr/4/95 from the Austrian Federal Ministry of Science, Transport and the Arts (to A.G.).
REFERENCES
- 1.Aktories K. Rho proteins: targets for bacterial toxins. Trends Microbiol. 1997;5:282–288. doi: 10.1016/S0966-842X(97)01067-6. [DOI] [PubMed] [Google Scholar]
- 2.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 3.Araki N, Johnson M T, Swanson J A. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betts J, Finlay B B. Identification of Salmonella typhimurium invasiveness loci. Can J Microbiol. 1992;38:852–857. doi: 10.1139/m92-138. [DOI] [PubMed] [Google Scholar]
- 5.Buscher D, Hipskind R A, Krautwald S, Reimann T, Baccarini M. Ras-dependent and -independent pathways target the mitogen-activated protein kinase network in macrophages. Mol Cell Biol. 1995;15:466–475. doi: 10.1128/mcb.15.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen L M, Hobbie S, Galan J E. Requirement of CDC42 for Salmonella-induced cytoskeletal and nuclear responses. Science. 1996;274:2115–2118. doi: 10.1126/science.274.5295.2115. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y-G, Siddhanta A, Austin C D, Hammond S M, Sung T-C, Frohman M A, Morris A J, Shields D. Phospholipase D stimulates release of nascent secretory vesicles from the trans-Golgi network. J Cell Biol. 1997;138:495–504. doi: 10.1083/jcb.138.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collazo C M, Galan J E. The invasion-associated type-III protein secretion system in Salmonella—a review. Gene. 1997;192:51–59. doi: 10.1016/s0378-1119(96)00825-6. [DOI] [PubMed] [Google Scholar]
- 9.Cox D, Chang P, Zhang Q, Reddy P G, Bokoch G M, Greenberg S. Requirements for both Rac1 and Cdc42 in membrane ruffling and phagocytosis in leukocytes. J Exp Med. 1997;186:1487–1494. doi: 10.1084/jem.186.9.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duckworth B C, Cantley L C. Conditional inhibition of the mitogen-activated protein kinase cascade by wortmannin. Dependence on signal strength. J Biol Chem. 1997;272:27665–27670. doi: 10.1074/jbc.272.44.27665. [DOI] [PubMed] [Google Scholar]
- 11.Duronio V, Scheid M P, Ettinger S. Downstream signaling events regulated by phosphatidylinositol 3-kinase activity. Cell Signalling. 1998;10:233–239. doi: 10.1016/s0898-6568(97)00129-0. [DOI] [PubMed] [Google Scholar]
- 12.Finlay B B, Cossart P. Exploitation of mammalian host cell functions by bacterial pathogens. Science. 1997;276:718–725. doi: 10.1126/science.276.5313.718. [DOI] [PubMed] [Google Scholar]
- 13.Flynn A, Proud G. Insulin-stimulated phosphorylation of initiation factor 4E is mediated by the MAP kinase pathway. FEBS Lett. 1996;389:162–166. doi: 10.1016/0014-5793(96)00564-9. [DOI] [PubMed] [Google Scholar]
- 14.Galan J E. Molecular genetic bases of Salmonella entry into host cells. Mol Microbiol. 1996;20:263–271. doi: 10.1111/j.1365-2958.1996.tb02615.x. [DOI] [PubMed] [Google Scholar]
- 15.Ginocchio C C, Olmsted S B, Wells C L, Galan J E. Contact with epithelial cells induces the formation of surface appendages on Salmonella typhimurium. Cell. 1994;76:717–724. doi: 10.1016/0092-8674(94)90510-x. [DOI] [PubMed] [Google Scholar]
- 16.Gratas C, Powis G. Inhibition of phospholipase D by agents that inhibit cell growth. Anticancer Res. 1993;13:1239–1244. [PubMed] [Google Scholar]
- 17.Groisman E A, Ochman H. How Salmonella became a pathogen. Trends Microbiol. 1997;5:343–349. doi: 10.1016/S0966-842X(97)01099-8. [DOI] [PubMed] [Google Scholar]
- 18.Hackam D J, Rotstein O D, Schreiber A, Zhang W, Jr, Grinstein S. Rho is required for the initiation of calcium signaling and phagocytosis by Fcγ receptors in macrophages. J Exp Med. 1997;186:955–966. doi: 10.1084/jem.186.6.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrera-Velit P, Reiner N E. Bacterial lipopolysaccharide induces the association and coordinated activation of p53/56lyn and phosphatidylinositol 3-kinase in human monocytes. J Immunol. 1996;156:1157–1165. [PubMed] [Google Scholar]
- 20.Hobbie S, Chen L M, Davis R J, Galan J E. Involvement of mitogen-activated protein kinase pathways in the nuclear responses and cytokine production induced by Salmonella typhimurium in cultured intestinal epithelial cells. J Immunol. 1997;159:5550–5559. [PubMed] [Google Scholar]
- 21.Ireton K, Payrastre B, Chap H, Ogawa W, Sakaue H, Kasuga M, Cossart P. A role for phosphoinositide 3-kinase in bacterial invasion. Science. 1996;274:780–782. doi: 10.1126/science.274.5288.780. [DOI] [PubMed] [Google Scholar]
- 22.Jesenberger, V., and M. Baccarini. Unpublished results.
- 23.Jones B D, Paterson H F, Hall A, Falkow S. Salmonella typhimurium induces membrane ruffling by a growth factor-receptor-independent mechanism. Proc Natl Acad Sci USA. 1993;90:10390–10394. doi: 10.1073/pnas.90.21.10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karnitz L M, Burns L A, Sutor S L, Blenis J, Abraham R T. Interleukin-2 triggers a novel phosphatidylinositol 3-kinase-dependent MEK activation pathway. Mol Cell Biol. 1995;15:3049–3057. doi: 10.1128/mcb.15.6.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King W G, Mattaliano M D, Chan T O, Tsichlis P N, Brugge J S. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation. Mol Cell Biol. 1997;17:4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kugler S, Schuller S, Goebel W. Involvement of MAP-kinases and -phosphatases in uptake and intracellular replication of Listeria monocytogenes in J774 macrophage cells. FEMS Microbiol Lett. 1997;157:131–136. doi: 10.1111/j.1574-6968.1997.tb12763.x. [DOI] [PubMed] [Google Scholar]
- 27.Kusner D J, Hall C F, Schlesinger L S. Activation of phospholipase D is tightly coupled to the phagocytosis of Mycobacterium tuberculosis or opsonized zymosan by human macrophages. J Exp Med. 1996;184:585–595. doi: 10.1084/jem.184.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu M K, Herrera-Velit P, Brownsey R W, Reiner N E. CD14-dependent activation of protein kinase C and mitogen-activated protein kinases (p42 and p44) in human monocytes treated with bacterial lipopolysaccharide. J Immunol. 1994;153:2642–2652. [PubMed] [Google Scholar]
- 29.Lundberg, U., U. Vinatzer, D. Berdnik, A. von Gabain, and M. Baccarini. Growth-phase regulated induction of Salmonella-induced macrophage apoptosis correlates with the transient expression of SPI-1 genes. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 30.Marra F, Pinzani M, DeFranco R, Laffi G, Gentilini P. Involvement of phosphatidylinositol 3-kinase in the activation of extracellular signal-regulated kinase by PDGF in hepatic stellate cells. FEBS Lett. 1995;376:141–145. doi: 10.1016/0014-5793(95)01261-0. [DOI] [PubMed] [Google Scholar]
- 31.Morgan C J, Pollard W, Stanley E R. Isolation and characterization of a cloned growth factor dependent macrophage cell line, BAC-1.2F5. J Cell Physiol. 1987;130:420–427. doi: 10.1002/jcp.1041300316. [DOI] [PubMed] [Google Scholar]
- 32.Natarajan V, Iwamoto G K. Lipopolysaccharide-mediated signal transduction through phospholipase D activation in monocytic cell lines. Biochim Biophys Acta. 1994;1213:14–20. doi: 10.1016/0005-2760(94)90216-x. [DOI] [PubMed] [Google Scholar]
- 33.Ninomiya N, Hazeki K, Fukui Y, Seya T, Okada T, Hazeki O, Ui M. Involvement of phosphatidylinositol 3-kinase in Fc gamma receptor signaling. J Biol Chem. 1994;269:22732–22737. [PubMed] [Google Scholar]
- 34.Pace J, Hayman M J, Galan J E. Signal transduction and invasion of epithelial cells by S. typhimurium. Cell. 1993;72:505–514. doi: 10.1016/0092-8674(93)90070-7. [DOI] [PubMed] [Google Scholar]
- 35.Procyk, K. J., and M. Baccarini. Unpublished results.
- 36.Reimann T, Buscher D, Hipskind R A, Krautwald S, Lohmann-Matthes M L, Baccarini M. Lipopolysaccharide induces activation of the Raf-1/MAP kinase pathway. A putative role for Raf-1 in the induction of the IL-1 beta and the TNF-alpha genes. J Immunol. 1994;153:5740–5749. [PubMed] [Google Scholar]
- 37.Reszka A A, Seger R, Diltz C D, Krebs E G, Fischer E H. Association of mitogen-activated protein kinase with the microtubule cytoskeleton. Proc Natl Acad Sci USA. 1995;92:8881–8885. doi: 10.1073/pnas.92.19.8881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruckdeschel K, Machold J, Roggenkamp A, Stubbier S, Pierre J, Zumbihl R, Liautard J P, Heesemann J, Rouot B. Yersinia enterocolitica promotes deactivation of macrophage mitogen-activated protein kinases extracellular signal-regulated kinase-1/2, p38, and c-Jun NH2-terminal kinase. Correlation with its inhibitory effect on tumor necrosis factor-alpha production. J Biol Chem. 1997;272:15920–15927. doi: 10.1074/jbc.272.25.15920. [DOI] [PubMed] [Google Scholar]
- 39.Sanghera J S, Weinstein S L, Aluwalia M, Girn J, Pelech S L. Activation of multiple proline-directed kinases by bacterial lipopolysaccharide in murine macrophages. J Immunol. 1996;156:4457–4465. [PubMed] [Google Scholar]
- 40.Schutze S, Potthoff K, Machleidt T, Berkovic D, Wiegmann K, Kronke M. TNF activates NF-kappa B by phosphatidylcholine-specific phospholipase C-induced “acidic” sphingomyelin breakdown. Cell. 1992;71:765–776. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- 41.Seger R, Krebs E G. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 42.Standaert M L, Avignon A, Yamada K, Bandyopadhyay G, Farese R V. The phosphatidylinositol 3-kinase inhibitor, wortmannin, inhibits insulin-induced activation of phosphatidylcholine hydrolysis and associated protein kinase C translocation in rat adipocytes. Biochem J. 1996;313:1039–1046. doi: 10.1042/bj3131039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stefanová I, Corcoran M L, Horak E M, Wahl L M, Bolen J B, Horak I D. Lipopolysaccharide induces activation of CD14-associated protein tyrosine kinase p53/56lyn. J Biol Chem. 1993;268:20725–20725. [PubMed] [Google Scholar]
- 44.Sugden P H, Clerk A. Regulation of the ERK subgroup of MAP kinase cascades through G protein-coupled receptors. Cell Signalling. 1997;9:337–351. doi: 10.1016/s0898-6568(96)00191-x. [DOI] [PubMed] [Google Scholar]
- 45.Tang P, Rosenshine I, Finlay B B. Listeria monocytogenes, an invasive bacterium, stimulates MAP kinase upon attachment to epithelial cells. Mol Biol Cell. 1994;5:455–464. doi: 10.1091/mbc.5.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang P, Sutherland C L, Gold M R, Finlay B B. Listeria monocytogenes invasion of epithelial cells requires the MEK-1/ERK-2 mitogen-activated protein kinase pathway. Infect Immun. 1998;66:1106–1112. doi: 10.1128/iai.66.3.1106-1112.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uddin S, Fish E N, Sher D A, Gardziola C, White M F, Platanias L C. Activation of the phosphatidylinositol 3-kinase serine kinase by IFN-alpha. J Immunol. 1997;158:2390–2397. [PubMed] [Google Scholar]
- 48.Weinstein S L, Sanghera J S, Lemke K, DeFranco A L, Pelech S L. Bacterial lipopolysaccharide induces tyrosine phosphorylation and activation of mitogen-activated protein kinases in macrophages. J Biol Chem. 1992;267:14955–14962. [PubMed] [Google Scholar]
- 49.Xu X X, Tessner T G, Rock C O, Jackowski S. Phosphatidylcholine hydrolysis and c-myc expression are collaborating mitogenic pathways activated by colony-stimulating factor 1. Mol Cell Biol. 1993;13:1522–1533. doi: 10.1128/mcb.13.3.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]