Abstract
Untreated congenital toxoplasmosis (CT) remains an important cause of neurologic and ocular disease worldwide. However, infants may not have signs and symptoms their physicians recognize, leading to delayed diagnosis and missed opportunities for treatment. We describe a pair of twins diagnosed with CT at 11 months of age following incidental detection of leukocoria in one twin.
Keywords: congenital toxoplasmosis, hydrocephalus, TORCH, leukocoria, macrocephaly, pyrimethamine, sulfadiazine allergy
Introduction.
Congenital toxoplasmosis (CT) is a preventable and treatable parasitic disease caused by Toxoplasma gondii. Those infected have an increased risk over time of new or recurrent chorioretinal lesions and vision loss, intellectual disability, developmental delays, hydrocephalus, seizure, and motor abnormalities1–5. Prenatal treatment of infected mothers significantly decreases risk of fetal infection, clinical signs, and long-term sequelae. Currently, prenatal screening for toxoplasmosis is not universal in the US. Thus, when a mother is not screened and an infant’s symptoms go unrecognized, diagnosis and treatment of CT can be delayed, leading to risk of later ocular and neurologic morbidity. Herein, we describe a pair of dizygotic twins with late diagnosis of CT after incidental detection of leukocoria in one of the twins and challenges in their management.
Case Report.
Dizygotic twins were born at 38 weeks 3 days gestational age via spontaneous vaginal delivery to a 32-year-old G1P2 previously healthy mother. Mother was negative for HIV, Hepatitis B surface antigen, gonorrhea, chlamydia and syphilis and was rubella immune. She was group-B-Streptococcus positive and received adequate intrapartum antibiotic prophylaxis. Maternal prenatal clinical history was unremarkable, without any unexplained flu-like illness during pregnancy or within 3 months before conception. Prenatal ultrasounds were unremarkable for both twins. Mother was born in the United States and resided there throughout pregnancy. Mother owned a dog but denied cat or other animal exposures, changing cat litter, consumption of unpasteurized milk, raw meat/shellfish, or unwashed produce, consumption of well water or gardening during pregnancy.
Twin A (boy):
Apgar scores at birth were 9 and 9. Birth weight was 2.24 kg (less than 1st percentile); length was 48 cm (16th percentile) and occipito-frontal circumference (OFC) was 34 cm (34th percentile). His early perinatal and postnatal course were unremarkable, and he passed his newborn Automated Auditory Brainstem Response (AABR) assessment. At 9 months of age, the mother incidentally noted a “white pupil” on the left eye on a photograph (Fig 1A) prompting ophthalmologic evaluation for possible leukocoria, which revealed a macular scar characteristic of Toxoplasma infection. The right eye was normal. At 10 months of age, he developed shuddering attacks which resolved; electroencephalogram at this time was normal. Over the course of his infancy, his OFC rose progressively beginning from the 34th percentile at delivery, to the 50th percentile at 2 months of age, to the 79th percentile at 6 months of age and to the 96th percentile at 10 months of age (not noted until he was 11 months of age) (Figure 1B). He was first evaluated by a pediatric infectious diseases physician at 11 months of age, after the recognition of the macular scar. Neonatal Toxoplasma serology panel at 11 months of age (done at the “Dr. Jack S. Remington Laboratory for Specialty Diagnostics”) demonstrated positive Toxoplasma IgG (Dye test: 1:2048), high IgG avidity, negative Toxoplasma IgM and IgA ELISA, and an equivocal differential agglutination test (AC/HS test: 100/400). The diagnosis of CT was established based on the persistence of positive Toxoplasma IgG at 11 months of age, with a mother who also has serum Toxoplasma Dye test 1:512 and Toxoplasma IgG avidity that was equivocal; Toxoplasma IgM that was negative, and equivocal differential agglutination AC/HS test result), consistent with the possibility of an acute Toxoplasma infection acquired during gestation. HIV Ag/Ab were negative and RPR was non-reactive. Abdominal ultrasound was normal, without intrahepatic calcifications.
Figure 1. Twin boy clinical presentation and imaging findings.
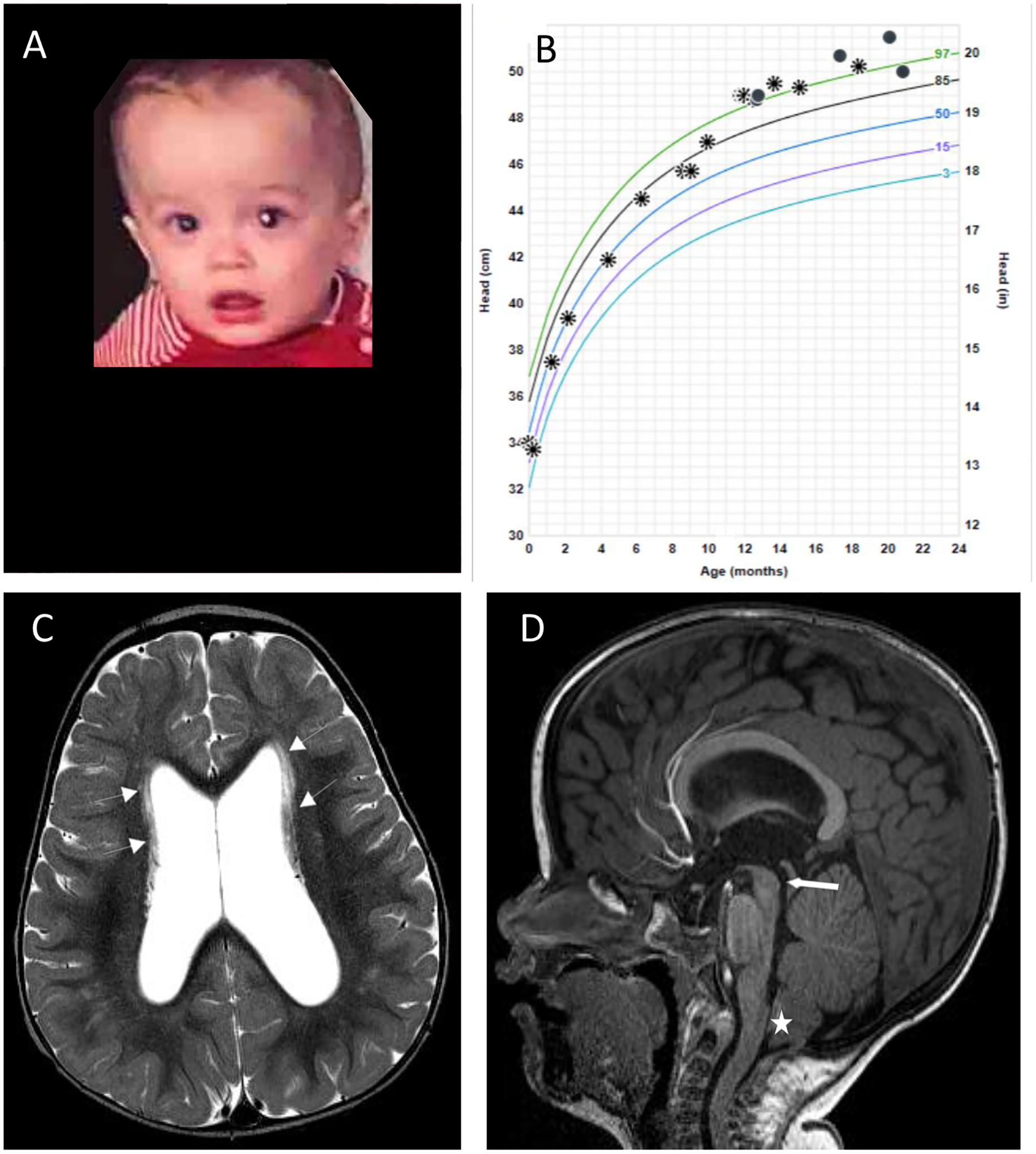
(A) Incidental leukocoria noticed at 9 months of age. (B) Occipitofrontal circumference crossing percentiles; asterisks and circles represent 2 different locations where measurements were taken. (C) Axial T2 image from brain MRI study showing ventriculomegaly and linear periventricular hyperintensity (white arrows) along the bodies of the lateral ventricles. (D) Midline sagittal T1 image from MRI study showing macrocephaly, narrow but patent cerebral aqueduct (white thick arrow) and mild Chiari deformity (white star)
Magnetic resonance imaging (MRI) of the brain with and without gadolinium at 11 months of age demonstrated mild ventriculomegaly with enlargement of the lateral and third ventricles, with a narrowed, but patent, inferior cerebral aqueduct. There were also nonspecific linear T2 hyperintensities in the periventricular, subcortical, and deep white matter of unclear significance, and a Chiari I malformation (6 mm), without syringohydromyelia. There were no calcifications, intracranial mass lesions, focal mass effect or midline shift. Cerebrospinal fluid (CSF) examination was normal (2 white blood cells (WBC)/μL, 11 red blood cells (RBC)/μL, protein 19 mg/dL, glucose 43 mg/dL). He was started anti-Toxoplasma therapy at 11 months of age with pyrimethamine, sulfadiazine, and leucovorin6 and tolerated therapy.
Serial OFC measurements rose above the 97th percentile at 12 months of age and thereafter were approximately stable along this percentile curve (Fig 1b). At 16 months of age, a repeat MRI demonstrated mild to moderate ventriculomegaly, with a slight interval decrease in the prominence of the temporal horns. Paucity of the posterior periventricular white matter was noted at that time. Nonspecific linear T2 hyperintensities were seen again, which were slightly more conspicuous. At 17 months of age, general neurologic evaluation and assessment of developmental milestones revealed slight expressive language delay. At 20 months of age, expressive language delay was improving with speech therapy. He has otherwise remained asymptomatic without signs or symptoms of increased intracranial pressure at the time of this report.
Twin B (girl):
Apgar scores at birth were 9 and 9. Birth weight was 2.62 kg (8th percentile), length was 48 cm (27th percentile) and OFC was 33 cm (23rd percentile). Her perinatal and postnatal course were unremarkable and she passed her AABR. Evaluation for CT was initiated after her twin brother was diagnosed with CT at 11 months of age, as above. Neonatal Toxoplasma serology panel at that time demonstrated positive Toxoplasma IgG (Dye test: 1:2048), high IgG avidity, negative Toxoplasma IgM and IgA and an equivocal differential agglutination AC/HS test (50/400). Similar to her brother, these results were consistent with the diagnosis of CT. Over the course of her infancy, her OFC rose from the 23rd percentile at birth, to >98th percentile at 11 months of age (Fig 2A). Ophthalmologic fundoscopic evaluation was normal at diagnosis. HIV Ag/Ab and RPR were non-reactive. Abdominal ultrasound was normal.
Figure 2. Twin girl clinical presentation and complications.
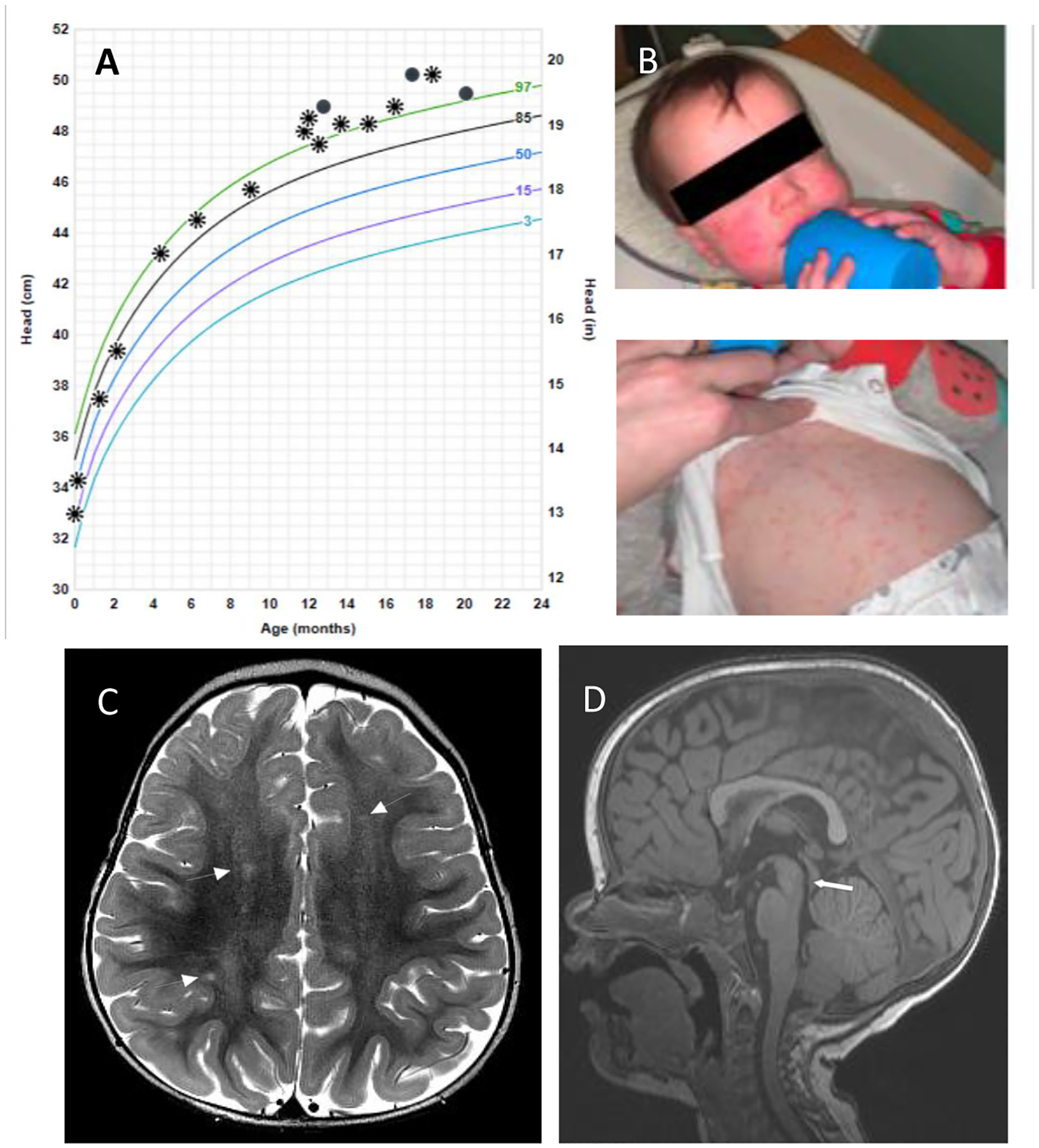
(A) Occipitofrontal circumference crossing percentiles; asterisks and circles represent 2 different locations where measurements were taken. (B) Immediate-type hypersensitivity cutaneous reaction to sulfadiazine after 10 days of treatment with pyrimethamine, sulfadiazine and leucovorin. (C) Axial T2 image from Brain MRI study showing nonspecific T2 hyperintensities (white arrows) in the bilateral high frontal and parietal white matter. (D) Midline sagittal T1 image from MRI study showing macrocephaly, with patent cerebral aqueduct (white thick arrow). There was no ventriculomegaly in this case.
MRI of the brain without and with gadolinium at 11 months, demonstrated a few foci of nonspecific T2 hyperintensities within the bilateral fronto-parietal white matter of unclear significance. There was no ventriculomegaly, intracranial mass lesion, mass effect or midline shift. CSF examination at 11 months was within normal limits (1 WBC/μL, 10 RBC/μL, protein 20 mg/dL, glucose 43 mg/dL).
She was started on anti-Toxoplasma therapy at 11 months of age with pyrimethamine, sulfadiazine and leucovorin. However, 10 days after starting treatment, she developed a whole body, pruritic maculopapular rash consistent with a hypersensitivity reaction (Fig 2B); this resolved after discontinuation of sulfadiazine. Sulfa-desensitization was deferred, and parents elected to continue with pyrimethamine/leucovorin despite discussion about consideration of combination therapy with azithromycin in substitution for sulfadiazine. At 17 months of age, OFC was at the 99th percentile, and general neurologic and developmental milestones assessment demonstrated overall normal development with the exception of a slight expressive language delay. At 20 months of age, OFC remained stable above the 97th percentile, and expressive language delay was improving with speech therapy. She has otherwise remained asymptomatic at the time of this report.
Written consent for publication, consent for release for recording or filming and HIPAA authorization was obtained from the twins’ parents as advised by our institutional Office of Compliance and Privacy. Ethics institutional review board approval was waived in light of case report of 5 or fewer patients.
Discussion.
We present a pair of twin infants with late diagnosis of CT, who had different presentations, clinical and imaging findings. Incidental detection of leukocoria in one twin subsequently led to identification of a unilateral macular chorioretinal scar characteristic of toxoplasmosis, subsequent confirmed diagnosis of CT and recognition of an ongoing progressive macrocephaly in both twins. In contrast to the more symptomatic presentation in twin A, twin B notably had no evidence of ventriculomegaly or ocular disease despite the presence of macrocephaly. Moreover, only twin A demonstrated mild ventriculomegaly despite macrocephaly in both twins. There was no evidence of active chorioretinitis and CSF examination was normal for both twins. No other medical problems were reported. Because of the progressive macrocephaly, ventriculomegaly in Twin A, expressive language delay in both children, and T2 weighted abnormalities which may represent active disease, the twins are being treated. Optimal duration of treatment is unknown and will be established by clinical course aiming for 12 months of treatment of their possibly active disease.
The diagnosis of CT in infants beyond 11 months of age is established by the persistence of positive Toxoplasma IgG at this age in infants whose mother was also Toxoplasma IgG positive7. Congenital infections, particularly CT, should be in the differential diagnosis of leukocoria. Other ophthalmologic signs and symptoms that may be seen with CT include nystagmus, strabismus, microphthalmia, cataract8 and decreased visual acuity and/or blurry vision9. New toxoplasmic chorioretinal lesions have been reported in more than 70% of children with CT diagnosed after (and therefore not treated during) their first year of life5; these may be prevented and vision preserved with early treatment.
While leukocoria was not noted until 9 months of age, both twins did have a progressive increase in OFC beginning at 6 months of age, crossing multiple percentile lines on their growth curves over several months, noted retrospectively. Progressive increase in OFC crossing percentiles in any child should raise suspicion for development of hydrocephalus or intracranial mass lesion and should trigger urgent neuroimaging evaluation. When seen in the context of CT, obstructive hydrocephalus must specifically be ruled out.
The reported incidence of hydrocephalus in infants with CT varies between 2.4% in France, to up to 68% (67/99) in the US (infants born to untreated bothers).10,11. Hydrocephalus has traditionally been attributed to cerebral aqueduct obstruction associated with inflammation12. However, the National Collaborative Chicago based Congenital Toxoplasmosis Study (NCCCTS) has found hydrocephalus in CT not only due to cerebral aqueduct obstruction, but also unilateral or bilateral obstruction of the foramina of Monro, mixed aqueductal and foraminal obstruction, and in up to 21%, with no obvious evidence of intraventricular obstruction. Murine models suggest that the latter pattern may be attributable to intraventricular or leptomeningeal inflammation causing impaired CSF resorption, though this is not fully understood13. Leptomeningeal inflammation has also been proposed as the cause for high failure rates with endoscopic third ventricle ventriculostomy for aqueductal obstruction. The pathogenesis of the ventriculomegaly observed in twin A is most consistent with the non-obstructive pattern. It is possible that the periventricular T2 hyperintensities noted on MRI may be inflammatory/peri-vasculitic lesions of active CT14 There is a unique predilection of T. gondii for the brain periaqueductal and periventricular areas and basal ganglia in congenitally infected infants15. Ventriculomegaly may thus also be due to ex vacuo dilatation of the lateral and third ventricles (e.g normal pressure hydrocephalus)16 in the context of parenchymal volume loss, though those cases more often present with microcephaly. In the case of twin A, because ventricular dilation was mild, OFC had stabilized, developmental milestones were followed closely, and the child was largely clinically asymptomatic, we deferred neurosurgical intervention in favor of medical therapy. His most recent brain MRI at 14.5 months of age, 3.5 months after the initial study, has not demonstrated any progressive structural changes.
Optimal duration of treatment of late presenting T. gondii in infants has not been determined. Given the relative immaturity of the infant’s immune system during the first two years of life17, we consider that treatment of such infants should be continued for a total duration of 12 months. Pharmacologically, CT is typically treated with both pyrimethamine and sulfadiazine, which act synergistically against T. gondii with a combined activity eightfold greater than the additive effect only, as measured in vitro18. Although sulfonamide hypersensitivity reactions have been described in older children with CT (e.g. children with CT treated later in life for reactivation of toxoplasmic chorioretinitis19) and in adults; such reactions in infants under one year of age as that observed in twin B, are extremely rare19,20; and only isolated cases have been reported21. In cases of sulfa-drug reactions limiting continuation of therapy, as it occurred in twin B, the optimal therapeutic alternative regimen for CT remains unknown. There are no published data on the use of combination regimens other than pyrimethamine/sulfadiazine (or sulfadoxine which is not used in the United States)/folinic acid for treating infants with CT. CT experts in France, due to potential concerns for possible future shortage of first line anti-Toxoplasma medications, proposed the use of pyrimethamine plus azithromycin as an alternative regiment for the treatment of infants with CT22. However, long-term outcomes with alternative regimens are unknown, particularly in infants who initiate Toxoplasma therapy outside of the antenatal and neonatal period.
In conclusion, we present a pair of dizygotic twins with late diagnosis of CT to increase awareness about clinical presentations of infants with CT at this age and raise index of suspicion for infants presenting similarly with leukocoria, progressive macrocephaly, and language delays. Universal prenatal screening for toxoplasmosis in the US could have prevented and/or ameliorated neurologic sequelae in these twins. If CT diagnosis is delayed during early infancy, it is important to treat active disease, and it may be important to even initiate therapy up to a year of age to try to prevent long-term ocular and neurologic complications. For an asymptomatic infant, optimal duration when treatment is begun late in the first year of life is unknown.
Acknowledgments:
Funding from Thrasher Foundation(RMc), Samuel, ManmCornwell, Morel, Rooney families, Kiphard Foundation and Taking Out Toxo(RMc).
Funding source:
There was no funding or sponsor for this study
Disclosure of Funding:
Dr. McGuire is supported by the NIH (NINDS) K23 NS094069. Dr. McLeod is funded by the Thrasher Children’s Foundation, Global /Local Health Kiphartt Foundation award
Abbreviations:
- CSF
cerebrospinal fluid
- CT
congenital toxoplasmosis
- MRI
magnetic resonance imaging
- OCF
occipitofrontal circumference
- ANC
absolute neutrophil count
Footnotes
Conflicts of Interest: Dr. Del Valle Mojica, Dr. Montoya, Dr. McGuire, Dr. Palma, Dr. Shekdar, Dr. Contopoulos-Ioannidis have no conflicts of interest to declare. Dr. McLeod prepared a literature review on the use of spiramycin to prevent congenital toxoplasmosis with required reimbursement per Sunshine laws by Sanofi Pasteur.
Prior presentation: This data has not been published nor has been under consideration for publication elsewhere.
Reprints: No reprints are requested.
REFERENCES
- 1.Contopoulos-Ioannidis Despina, Montoya JG. Principles and Practice of Pediatric Infectious Diseases. 5th Edition. Elsevier; 2017. 1688 p. [Google Scholar]
- 2.Koppe JG, Rothova A. Congenital toxoplasmosis. A long-term follow-up of 20 years. Int Ophthalmol. 1989. Dec;13(6):387–90. [DOI] [PubMed] [Google Scholar]
- 3.Koppe JG, Meenken C. Congenital toxoplasmosis, later relapses and treatment. Acta Paediatr Oslo Nor 1992. 1999. Jun;88(6):586–8. [DOI] [PubMed] [Google Scholar]
- 4.McLeod R, Lee D, Clouser F, Boyer K. Toxoplasmosis in the Fetus and Newborn Infant. In: Stevenson DK, Cohen RS, Sunshine P, editors. Neonatology: Clinical Practice and Procedures [Internet]. New York, NY: McGraw-Hill Education; 2015. [cited 2020 Dec 22]. Available from: accesspediatrics.mhmedical.com/content.aspx?aid=1109796692 [Google Scholar]
- 5.Phan L, Kasza K, Jalbrzikowski J, Noble AG, Latkany P, Kuo A, et al. Longitudinal study of new eye lesions in children with toxoplasmosis who were not treated during the first year of life. Am J Ophthalmol. 2008. Sep;146(3):375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maldonado YA, Read JS, COMMITTEE ON INFECTIOUS DISEASES. Diagnosis, Treatment, and Prevention of Congenital Toxoplasmosis in the United States. Pediatrics. 2017. Feb;139(2). [DOI] [PubMed] [Google Scholar]
- 7.Pomares C, Montoya JG. Laboratory Diagnosis of Congenital Toxoplasmosis. J Clin Microbiol. 2016. Oct;54(10):2448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arun V, Noble AG, Latkany P, Troia RN, Jalbrzikowski J, Kasza K, et al. Cataracts in congenital toxoplasmosis. J AAPOS Off Publ Am Assoc Pediatr Ophthalmol Strabismus. 2007. Dec;11(6):551–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melamed J, Eckert GU, Spadoni VS, Lago EG, Uberti F. Ocular manifestations of congenital toxoplasmosis. Eye Lond Engl. 2010. Apr;24(4):528–34. [DOI] [PubMed] [Google Scholar]
- 10.Wallon M, Peyron F, Cornu C, Vinault S, Abrahamowicz M, Kopp CB, et al. Congenital toxoplasma infection: monthly prenatal screening decreases transmission rate and improves clinical outcome at age 3 years. Clin Infect Dis Off Publ Infect Dis Soc Am. 2013. May;56(9):1223–31. [DOI] [PubMed] [Google Scholar]
- 11.Olariu TR, Remington JS, McLeod R, Alam A, Montoya JG. Severe congenital toxoplasmosis in the United States: clinical and serologic findings in untreated infants. Pediatr Infect Dis J. 2011. Dec;30(12):1056–61. [DOI] [PubMed] [Google Scholar]
- 12.Neuberger I, Garcia J, Meyers ML, Feygin T, Bulas DI, Mirsky DM. Imaging of congenital central nervous system infections. Pediatr Radiol. 2018. Apr;48(4):513–23. [DOI] [PubMed] [Google Scholar]
- 13.Stahl W, Kaneda Y. Pathogenesis of murine toxoplasmic hydrocephalus. Parasitology. 1997. Mar;114 (Pt 3):219–29. [DOI] [PubMed] [Google Scholar]
- 14.Hermes G, Ajioka JW, Kelly KA, Mui E, Roberts F, Kasza K, et al. Neurological and behavioral abnormalities, ventricular dilatation, altered cellular functions, inflammation, and neuronal injury in brains of mice due to common, persistent, parasitic infection. J Neuroinflammation. 2008. Oct 23;5:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel DV, Holfels EM, Vogel NP, Boyer KM, Mets MB, Swisher CN, et al. Resolution of intracranial calcifications in infants with treated congenital toxoplasmosis. Radiology. 1996. May;199(2):433–40. [DOI] [PubMed] [Google Scholar]
- 16.McLone D, Frim D, Penn R, Swisher CN, Heydemann P, Boyer KM, et al. Outcomes of hydrocephalus secondary to congenital toxoplasmosis. J Neurosurg Pediatr. 2019. Sep 6;1–8. [DOI] [PubMed] [Google Scholar]
- 17.Lewis David B., Wilson Christopher B.. Chapter 4: Developmental Immunology and Role of Host Defenses in Fetal and Neonatal Susceptibility to Infection. In: Infectious Diseases of the Fetus and Newborn Infant. 7th ed. Elsevier; [Google Scholar]
- 18.Eyles DE, Coleman N. Synergistic effect of sulfadiazine and daraprim against experimental toxoplasmosis in the mouse. Antibiot Chemother Northfield Ill. 1953. May;3(5):483–90. [PubMed] [Google Scholar]
- 19.McLeod R, Khan AR, Noble GA, Latkany P, Jalbrzikowski J, Boyer K, et al. Severe sulfadiazine hypersensitivity in a child with reactivated congenital toxoplasmic chorioretinitis. Pediatr Infect Dis J. 2006. Mar;25(3):270–2. [DOI] [PubMed] [Google Scholar]
- 20.Teil J, Dupont D, Charpiat B, Corvaisier S, Vial T, Leboucher G, et al. Treatment of Congenital Toxoplasmosis: Safety of the Sulfadoxine-Pyrimethamine Combination in Children Based on a Method of Causality Assessment. Pediatr Infect Dis J. 2016. Jun;35(6):634–8. [DOI] [PubMed] [Google Scholar]
- 21.Rioualen S, Dufau J, Flatres C, Lavenant P, Misery L, Roué J-M. [DRESS complicated by hemophagocytic lymphohistiocytosis in an infant treated for congenital toxoplasmosis]. Ann Dermatol Venereol. 2017. Dec;144(12):784–7. [DOI] [PubMed] [Google Scholar]
- 22.Personal communication of Dr Jose G. Montoya with Dr Francois Peyron.


