Abstract
Aberrant dopamine system function is thought to contribute to the positive symptoms of schizophrenia. Clinical imaging studies have demonstrated that the largest dopamine abnormality in patients appears to be an increase in presynaptic dopamine activity. Indeed, studies utilizing [18F]DOPA positive emission tomography reliably report increases in presynaptic dopamine bioavailability in patients and may serve as a biomarker for treatment response. The mechanisms contributing to this increased presynaptic activity in human patients is not yet fully understood, which necessitates the use of preclinical models. Dopamine system function can be directly examined in experimental animals using in vivo electrophysiology. One consistent finding from preclinical studies in rodent models used to study schizophrenia-like neuropathology is a 2-fold increase in the number of spontaneously active dopamine neurons in the ventral tegmental area (VTA), termed population activity. We posit that increased striatal dopamine synthesis capacity is attributed to an augmented VTA dopamine neuron population activity. Here, we directly test this hypothesis using [3H]DOPA ex vivo autoradiography, to quantify striatal dopamine synthesis capacity, in the methylazoxymethanol acetate (MAM) model, a validated rodent model displaying neurophysiological and behavioral alterations consistent with schizophrenia-like symptomatologies. Consistent with human imaging studies, dopamine synthesis capacity was significantly increased in dorsal and ventral striatal subregionis, including the caudate putamen and nucleus accumbens, of MAM-treated rats and associated with specific increases in dopamine neuron population activity. Taken together, these data provide a link between mechanistic studies in rodent models and clinical studies of increased presynaptic dopamine function in human subjects.
Keywords: dopamine, MAM, schizophrenia, ventral tegmental area, autoradiography
Introduction
The dopamine hypothesis of schizophrenia is one of the longest standing theories advanced to explain psychosis and hypothesizes that enhanced mesolimbic dopamine transmission underlies positive symptoms of schizophrenia.1–4 Several observations validate this hypothesis including the efficacy of antipsychotics that block dopamine D2 receptors.5 Additionally, dopamine receptor agonists, such as amphetamine, can induce psychosis in the general population,6 while in individuals with schizophrenia they can exacerbate psychosis.2,4 Human imaging studies using single-photon emission computerized tomography or positive emission tomography (PET) demonstrate an increase in dopamine D2 receptor occupancy7–9 and elevated dopamine synthesis capacity10–12 in striatal regions of individuals with schizophrenia. A meta-analysis of these imaging studies13 reported that presynaptic alterations in dopamine synthesis capacity, baseline synaptic dopamine levels, and dopamine release are the largest dopaminergic abnormalities observed in schizophrenia patients. This is significant because current antipsychotic therapies for the treatment of psychosis act on postsynaptic D2 receptors and markers of presynaptic dopamine function may predict treatment response in patients.14
Psychiatric disorders, such as schizophrenia, are inherently human conditions; however, preclinical models are required to examine the mechanisms contributing to disease pathophysiology. Rodent models developed to study schizophrenia-related symptomatology have been used to examine alterations in mesolimbic dopamine neuron activity. One such model is the methylazoxymethanol acetate (MAM) gestational disruption rat which exhibits anatomical, physiological, and behavioral deficits that model observations in patients with schizophrenia.15–17 MAM-treated rats consistently display aberrant dopamine system function and have been extensively used to study circuit-level and molecular mechanisms that underlie symptoms of schizophrenia.17–23 Using in vivo extracellular electrophysiology, 3 parameters of ventral tegmental area (VTA) dopamine neuron activity can be measured: basal firing rate, the proportion of action potentials occurring in bursts, and population activity (a measure of the number of spontaneously active dopamine neurons). Population activity is based on the observation that under normal conditions, approximated 50% of VTA dopamine neurons are spontaneously active in vivo.24 The role of the remaining silent dopamine neurons appears critical, as they provide a gain of function of the dopamine signal.25 Specifically, by regulating the number of spontaneously active dopamine neurons, one can manipulate the magnitude of the dopamine signal25 and it is this gain of function, ie, believed to be dysfunctional in psychosis. Indeed, numerous diverse rodent models used to study schizophrenia-related pathologies, including the MAM model, consistently demonstrate a significant increase in the number of spontaneously active dopamine neurons in the VTA without any observable differences in the average firing rate or pattern of bursting activity.20,22,23,26–30 This pathological increase in VTA dopamine neuron activity results in a loss of this critical gain of function, without which, all stimuli will produce the same, enhanced level of dopamine in target regions. Thus, we posit that an increase in dopamine synthesis capacity is attributable to the increase in the number of spontaneously active dopamine neurons in the VTA.
Here, we directly tested this predicted association using in vivo electrophysiology, to directly measure alterations in VTA dopamine neuron activity, and [3H]DOPA ex vivo autoradiography to measure presynaptic dopamine function in striatal subregions, in the MAM model. We provide evidence that dysfunction of the dopamine system can lead to alterations in presynaptic dopamine synthesis capacity. Specifically, dopamine synthesis capacity is elevated as a result of chronic, but not acute, changed in dopamine neuron activity. Gaining a better understanding of the mechanisms contributing to aberrant dopamine neuron activity is warranted and could help to identify potential therapeutic targets.
Methods
All experiments were performed in accordance with the guidelines outlined in the National Institute of Health’s Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and the Use Committees of UT Health San Antonio and the Department of Veterans Affairs.
Animals
We administered MAM as previously described,15,17 to generate rodents with circuit-level alterations relevant to schizophrenia. In brief, timed pregnant female Sprague-Dawley rats were obtained from Envigo on gestational day 16 and injected with MAM (22 mg/kg diluted in saline, i.p.) or saline (1 ml/kg) on gestational day 17. Male pups were weaned on postnatal day 21 and housed with littermates in groups of 2–3. All experiments were performed on multiple litters of male MAM- and saline-treated rats during adulthood (>8 weeks; 300–500 g).
Dopamine Neuron Electrophysiology
MAM- and saline-treated rats were anesthetized with 8% chloral hydrate (400 mg/kg; i.p.), as this anesthetic does not significantly depress dopamine neuron activity.31 Anesthesia was maintained by supplemental administration of chloral hydrate to maintain suppression of limb withdrawal reflex. Rats were positioned in a stereotaxic apparatus (Kopf), and a core body temperature of 37°C was maintained by a thermostatically controlled heating pad (PhysioSuite; Kent Scientific). Extracellular glass microelectrodes (impedance 6–10 MΩ) were lowered into the VTA (AP −5.3 mm and ML ±0.6 mm from Bregma; DV −6.5 to −9.0 mm ventral of the brain surface). Five to nine vertical passes were made throughout the cell body region of the VTA. Spontaneously active dopamine neurons were recorded for a period of 2–3 minutes and identified with open filter settings (low pass: 30 Hz; high pass: 30 kHz) using previously established criteria.32 We measured 3 parameters of dopamine neuron activity: (1) population activity (expressed as the average number of spontaneously active dopamine neurons encountered per dorsoventral track), (2) basal firing rate, and (3) the proportion of action potentials occurring in bursts (defined as the incidence of spikes with <80 ms between them; termination of the burst is defined by >160 ms between spikes). At the cessation of all electrophysiological recordings, rats were rapidly decapitated, and brains removed for histological verification of electrode tracks.
Ex Vivo Autoradiography
MAM- and saline-treated rats were anesthetized with chloral hydrate (400 mg/kg; i.p.), as detailed above. A core body temperature of 37°C was maintained by a thermostatically controlled heating pad. Rats were positioned in a stereotaxic apparatus. All rats received a systemic injection of the COMT inhibitor, Entacapone (20 mg/kg diluted in saline; i.p.), and the AADC inhibitor, Benserazide (10 mg/kg dissolved in Tween 20 then diluted in saline; i.p.) 30 min prior to the intravenous administration of [3H]DOPA (20 µCi; diluted in 5.7 mM ascorbate; 1 ml/kg). Ninety minutes following [3H]DOPA administration, rats were rapidly decapitated, and brains removed, frozen, coronally sectioned (25 µm) on a cryostat (Leica), and mounted onto slides. Sections were air dried and opposed to single-emulsion autoradiography film (Carestream BioMax MR film) for 12 months. Films were processed in an automated developer (Mini-MED 90; AFP Manufacturing) and uncalibrated optical density was quantified using ImageJ. Specifically, images were converted to 8-bit grayscale and a global uncalibrated OD applied in ImageJ. Areas containing the nucleus accumbens (NAc) or dorsal striatum were outlined and mean gray value analyzed with reference to background.
Acute Manipulations of Dopamine Neuron Activity
Intrahippocampal N-methyl-d-aspartate (NMDA) or TTX were administered to examine the effects of acute changes in dopamine neuron activity on dopamine synthesis capacity. For intracranial drug administration, a 26-gauge guide cannula (Plastics One) was lowered into the vHipp (AP −5.0 mm and ML ±4.8 mm from Bregma; DV −6.5 mm ventral of the brain surface). An internal cannula (Plastics One) extending 1 mm past the end of the guide cannula was used to deliver a unilateral injection of NMDA or TTX at a rate of approximately 0.5 µl/min. After 2 hours, an additional injection of NMDA was administered.
Histological Verification
To verify electrode placement in the VTA (figure 1A), rats were rapidly decapitated at the completion of all electrophysiological experiments. Brains were extracted, fixed for at least 24 hours (4% formaldehyde in saline), and cryoprotected (10% [wt/vol] sucrose in phosphate-buffered saline) until saturated. Brains were coronally sectioned (25 µm) using a cryostat (Leica). Sections containing electrode tracks were mounted onto gelatin-chrome alum-coated slides, stained with neutral red (0.1%) and thionin acetate (0.01%) and cover slipped with DPX mountant.
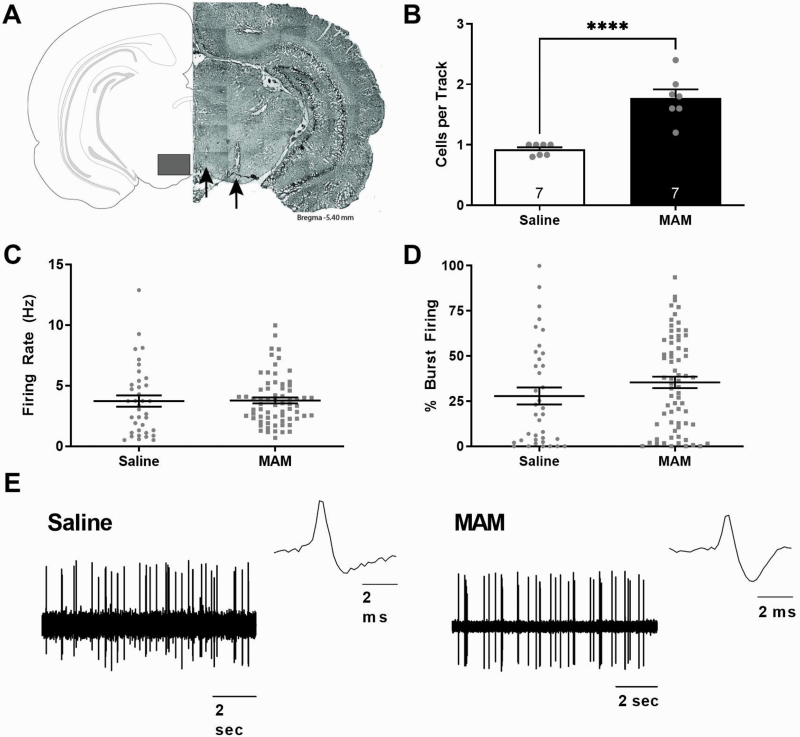
Fig. 1.
Gestational MAM administration produces a significant increase in dopamine neuron population activity in offspring. Representative brain section containing electrodes tracks in the ventral tegmental area (VTA), denoted by the black arrows (A). Three parameters of dopamine activity were measured: (B) dopamine neuron population activity (average number of spontaneously active dopamine neurons per track), (C) average firing rate, and (D) average percent of spikes firing in a burst. Population activity was significantly increased in MAM-treated rats, when compared with saline-treated controls, while the average firing rate and pattern of bursting activity were unchanged in MAM- and saline-treated rats. Representative dopamine neuron traces and action potentials (E). **** denotes significance from saline. P < .0001. Note: MAM, methylazoxymethanol acetate.
Statistical Analysis
Data are represented as the average ± SEM with n values representing the number of animals per experimental group unless otherwise stated. Electrophysiological analysis of dopamine neuron activity was performed using commercially available computer software (Lab Chart 8; ADInstruments Ltd) and plotted with Prism5 (GraphPad Software Inc). Statistical analyses were calculated using SigmaPlot 12.0 (Systat Software Inc). Uncalibrated optical density of films from ex vivo autoradiography experiments were quantified using ImageJ. Autoradiography and electrophysiological data were analyzed by t test or Mann-Whitney Rank Sum Test where appropriate. Significance was determined at P < .05.
Materials
MAM (MRI#0213) was purchased from Midwestern Research Institute. Chloral hydrate (C8383), NMDA (M2362), and DPX mountant for histology (06522) were sourced from Sigma-Aldrich. Entacapone (141533) and Benserazide (20298) were purchased from Cayman Chemical Company. Carestream BioMax MR film (Z350400) was from Sigma and [3H]DOPA (dihydroxyphenylalanine, L-3,4-[ring-2,5,6-3H]; 20–60 Ci/mmol, 0.74–2.22 TBq/mmol, 20–60 Ci/mmol) was from American Radiolabeled Chemicals, Inc (ART 0235-250 µCi).
Results
MAM-Treated Rats Exhibit Aberrant Dopamine System Function
A number of preclinical rat models developed to study schizophrenia-like pathologies consistently display a significant increase in dopamine neuron population activity.20,22,23,26–30 Here, we used in vivo electrophysiology to directly measure alterations in dopamine neuron activity (figure 1). We observed a significant increase in dopamine neuron population activity in MAM-treated rats (n = 7 rats; 1.78 ± 0.14 cells/track; t test; t = −5.849 with 12 degrees of freedom; P < .001) when compared with saline-treated controls (n = 7 rats; 0.92 ± 0.04 cells/track). Further, there were no observable differences in the average firing rates (MAM: n = 68 neurons; 3.78 ± 0.24 Hz; saline: n = 37 neurons; 3.74 ± 0.47 Hz) or bursting pattern (MAM: n = 68 neurons; 35.35 ± 3.16%; saline: n = 37 neurons; 27.81 ± 4.72%) between MAM- and saline-treated rats.
MAM-Treated Rats Display Increased Presynaptic Dopamine System Function
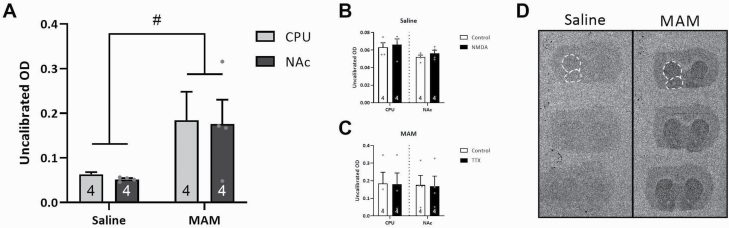
Human studies, using [18F]DOPA PET, have identified an increase in dopamine synthesis capacity, thought to contribute to the dopamine dysfunction in psychosis. Here, we performed a similar approach in rodents, examining presynaptic function with [3H]DOPA ex vivo autoradiography (figure 2A). As predicted, MAM-treated rats exhibited a similar significant increase in dopamine synthesis capacity in the caudate putamen (CPU; n = 4 rats/group; 0.184 ± 0.064 uncalibrated OD) and NAc (n = 4 rats/group; 0.176 ± 0.055 uncalibrated OD; 2-way ANOVA; FStrain(1,15) = 8.407; P = .013) and when compared with saline-treated controls (CPU: n = 4 rats/group; 0.063 ± 0.005 uncalibrated OD; NAc: n = 4 rats/group; 0.052 ± 0.002 uncalibrated OD).
Fig. 2.
Dopamine synthesis capacity in the striatal subregions of MAM-treated rats was significantly elevated. [3H]DOPA ex vivo autoradiography studies indicated a significant increase in dopamine synthesis capacity in the caudate putamen (CPU) and nucleus accumbens (NAc) of MAM-treated rats when compared with saline-treated controls (A), which was not influenced by intrahippocampal injections of NMDA in saline-treated controls to acutely activate the vHipp (B), and TTX in MAM-treated rats to acutely inhibit vHipp activity (C). Representative sections in saline- and MAM-treated rats depicting increased granular accumulation in the striatum of MAM-treated rats (D). White dotted lines indicated subregions that were analyzed. # denotes a significant main effect of MAM. P = .013. Note: MAM, methylazoxymethanol acetate; NMDA, N-methyl-d-aspartate.
Acute Afferent Manipulation of Dopamine Neuron Activity Did Not Alter Presynaptic Dopamine System Function
The ventral hippocampus (vHipp) has been consistently demonstrated to selectively regulate VTA dopamine neuron population activity. MAM-treated rats have been previously shown to display chronic hippocampal hyperactivity, which results in elevated dopamine neuron population activity. Similar increases in dopamine neuron activity are observed upon acute activation of the vHipp in control rats, therefore we examined whether presynaptic dopamine synthesis capacity was altered by acute manipulations of dopamine neuron population activity. Specifically, the vHipp was activated unilaterally in control rats or inhibited in MAM rats (figure 2B and C). Interestingly, acute vHipp manipulation of dopamine neuron population activity did not alter presynaptic dopamine system function in the striatum of saline (CPU: n = 4 rats/group; Control: 0.063 ± 0.005; NMDA: 0.066 ± 0.007 uncalibrated OD; NAc: n = 4 rats/group; Control: 0.052 ± 0.002; NMDA: 0.056 ± 0.004 uncalibrated OD) or MAM rats (CPU: n = 4 rats/group; Control: 0.184 ± 0.064; TTX: 0.180 ± 0.064 uncalibrated OD; NAc: n = 4 rats/group; Control: 0.176 ± 0.055; TTX: 0.169 ± 0.058 uncalibrated OD).
Discussion
Schizophrenia is a complex psychiatric disorder that remains one the leading causes of global health and economic burden.33 The most widely accepted hypothesis of schizophrenia is the dopamine hypothesis, which posits that a pathological increase in subcortical dopamine transmission correlates with positive symptom severity.1–4 Increasing evidence has supported the notion that the largest dopaminergic abnormalities in schizophrenia are presynaptic.10,11,13,34–38 Further, evidence from human imaging studies have further supported the notion that patients with schizophrenia display presynaptic dopamine abnormalities.4,7,10,13,39,40 Presynaptic dopamine synthesis capacity can be assessed using [18F]DOPA, which enters the brain via the large neutral amino acid transport system, is converted into dopamine, and stored in presynaptic vesicles. Using this approach, several studies have demonstrated that dopamine synthesis capacity is increased in patients with schizophrenia and may serve as a biomarker for antipsychotic treatment response.14,40 Such studies provide evidence of presynaptic dopaminergic abnormalities in schizophrenia; however, the mechanisms by which dopamine synthesis capacity is increased remain unknown.
The regulation of dopamine system function is complex; and dopamine neurons in the ventral mesencephalon have been demonstrated to exist in distinct states that are regulated by both intrinsic properties and afferent pathways. In vivo recordings identified 2 inactive states including a hyperpolarized, inactive cell, as well as depolarization block.41,42 Inactive cells can be made to fire by injecting a depolarizing current or by applying an excitatory substance, such as glutamate.43,44 Further, there are single-spike firing and burst-firing active states.32,43–45 Dopamine neurons in vitro exhibit a nonbursting, pacemaker-like activity46,47 and do not exhibit firing patterns (burst and irregular) observed in vivo, likely due to the lack of afferent inputs. Compared with the firing pattern observed in vitro (pacemaker-like), in vivo dopamine neurons fire in a slow, regular pattern or burst fire.43,44
Striatal dopamine transmission is dependent on the distinct activity states of VTA dopamine neurons. First, baseline, single-spike tonic activity provides consistent low-level (5–10 nM) concentrations of dopamine in the NAc.48 Alterations in tonic dopamine transmission occur slowly (seconds to minutes) and these changes in striatal dopamine efflux enable various cognitive, motor, and motivational processes.49,50 In addition, VTA dopamine neurons can enter a phasic, burst-firing activity state, in response to behaviorally relevant stimuli and results in a dramatic increase in striatal dopamine release within milliseconds.51 Indeed, this phasic signal is thought to assign salience to environmental stimuli and guide motivated behavior.52 Finally, the total number of spontaneously active dopamine neurons in the VTA (ie, population activity) is thought to influence tonic dopamine efflux.53
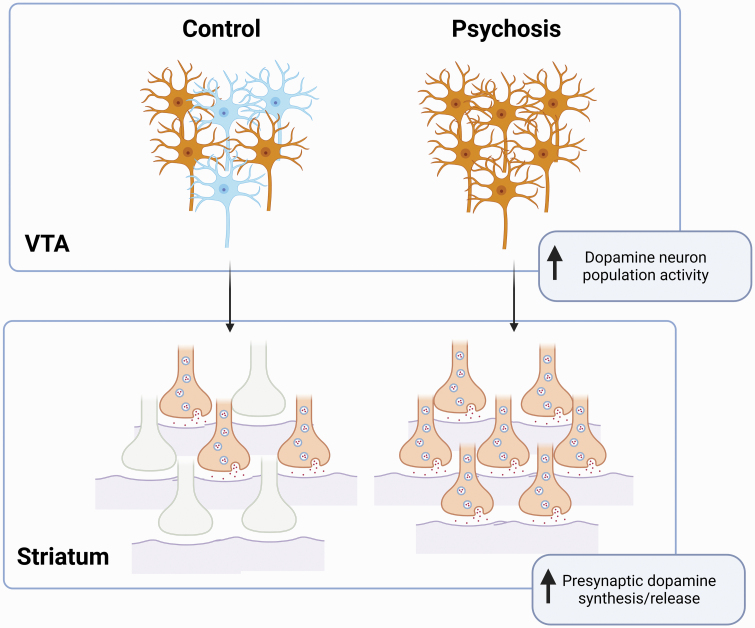
Consistent with data from a diverse array of rodent models used to study schizophrenia-related pathologies, we demonstrate that MAM-treated rats display a significant increase in the number of spontaneously active VTA dopamine neurons without any observable differences in the average firing rate or pattern of bursting activity.20,22,23,26–30 This was associated with an increase in presynaptic dopamine synthesis capacity, determined by [3H]DOPA ex vivo autoradiography within the CPU and NAc. These data mirror clinical findings,13 and add to a significant literature validating the MAM-treated rat as a model of schizophrenia-related pathophysiology. Moreover, these data provide a potential mechanism by which dopamine synthesis capacity is increased (figure 3). Specifically, it is a selective increase in the number of spontaneously active neurons which likely drives increases in presynaptic dopamine synthesis capacity in terminal regions.
Fig. 3.
Dopamine neuron population activity in the ventral tegmental area (VTA) correlates with presynaptic dopamine release in the striatum. Under control conditions, approximately half of the dopamine neurons in the VTA are spontaneously active. In the case of psychosis, a significant increase in VTA population activity, or number of spontaneously active dopamine neurons in the VTA, yields an increase in presynaptic dopamine synthesis capacity/release in the striatum.
To determine the time frame over which dopamine neuron population activity can regulate dopamine synthesis capacity, we modulated an afferent input to the VTA known to selectively regulate this activity state. Previous work from our laboratory and others has demonstrated that increased hippocampal drive to the NAc can increase the number of spontaneously active dopamine neurons in the VTA, through a multisynaptic circuit.20,22,54,55 Specifically, acute activation of the vHipp (using intra-vHipp injections of NMDA) can reliably increase the number of spontaneously active VTA dopamine neurons in rats.25,50 Furthermore, increased glutamatergic drive from the anterior hippocampus (ventral in rodents) is thought to be a key pathophysiological mechanism by which dopamine transmission is increased in schizophrenia.26,56–58 Indeed, reducing hippocampal activity in MAM-treated rats can restore dopamine system function and behavioral deficits.18,22,23,59–61 Interestingly, acute vHipp manipulations did not alter [3H]DOPA autoradiography in either control or MAM-treated rats, suggesting that presynaptic dopamine synthesis capacity is not dynamically regulated by dopamine neuron population activity across relatively short time frames (hours).
Taken together, these data support the notion that aberrant VTA dopamine neuron population activity is associated with elevated presynaptic dopamine synthesis capacity. Importantly, it appears to be the chronic dysregulation of dopamine system function that results in presynaptic alterations. Future studies using animal models should aim to elucidate the molecular mechanisms that may contribute to increased presynaptic dopamine synthesis capacity, such as atypical dopamine catabolism or changes in aromatic l-amino acid decarboxylase (AADC) function following chronic hippocampal hyperactivity. Given that antipsychotic treatments do not reduce dopamine synthesis capacity in patients with schizophrenia,62 it is imperative that we utilize animal models to better understand changes in presynaptic dopamine synthesis capacity in order to develop pharmacological interventions that more directly target presynaptic dopamine abnormalities.
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Contributor Information
Stephanie M Perez, Department of Pharmacology and Center for Biomedical Neuroscience, UT Health San Antonio, San Antonio, TX, USA; South Texas Veterans Health Care System, Audie L. Murphy Division, San Antonio, TX, USA.
Hannah B Elam, Department of Pharmacology and Center for Biomedical Neuroscience, UT Health San Antonio, San Antonio, TX, USA; South Texas Veterans Health Care System, Audie L. Murphy Division, San Antonio, TX, USA.
Daniel J Lodge, Department of Pharmacology and Center for Biomedical Neuroscience, UT Health San Antonio, San Antonio, TX, USA; South Texas Veterans Health Care System, Audie L. Murphy Division, San Antonio, TX, USA.
Funding
This work was supported by Merit Awards #BX004693 (D.J.L.) and #BX004646 (D.J.L.) from the United States Department of Veterans Affairs, Biomedical Laboratory Research and Development Service, and the National Institutes of Health RO1-MH090067 (D.J.L.).
References
- 1.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull. 2009;35(3):549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J Psychopharmacol. 1999;13(4):358–371. [DOI] [PubMed] [Google Scholar]
- 3.Carlsson A, Waters N, Waters S, Carlsson ML. Network interactions in schizophrenia—therapeutic implications. Brain Res Brain Res Rev. 2000;31(2–3):342–349. [DOI] [PubMed] [Google Scholar]
- 4.Abi-Dargham A. Do we still believe in the dopamine hypothesis? New data bring new evidence. Int J Neuropsychopharmacol. 2004;7(suppl 1):S1–S5. [DOI] [PubMed] [Google Scholar]
- 5.Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10(1):79–104. [DOI] [PubMed] [Google Scholar]
- 6.Lieberman JA, Kane JM, Alvir J. Provocative tests with psychostimulant drugs in schizophrenia. Psychopharmacology (Berl). 1987;91(4):415–433. [DOI] [PubMed] [Google Scholar]
- 7.Abi-Dargham A, Gil R, Krystal J, et al. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psychiatry. 1998;155(6):761–767. [DOI] [PubMed] [Google Scholar]
- 8.Laruelle M, D’Souza CD, Baldwin RM, et al. Imaging D2 receptor occupancy by endogenous dopamine in humans. Neuropsychopharmacology. 1997;17(3):162–174. [DOI] [PubMed] [Google Scholar]
- 9.Fujita M, Verhoeff NP, Varrone A, et al. Imaging extrastriatal dopamine D(2) receptor occupancy by endogenous dopamine in healthy humans. Eur J Pharmacol. 2000;387(2):179–188. [DOI] [PubMed] [Google Scholar]
- 10.Egerton A, Chaddock CA, Winton-Brown TT, et al. Presynaptic striatal dopamine dysfunction in people at ultra-high risk for psychosis: findings in a second cohort. Biol Psychiatry. 2013;74(2):106–112. [DOI] [PubMed] [Google Scholar]
- 11.Howes OD, Egerton A, Allan V, McGuire P, Stokes P, Kapur S. Mechanisms underlying psychosis and antipsychotic treatment response in schizophrenia: insights from PET and SPECT imaging. Curr Pharm Des. 2009;15(22):2550–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGowan S, Lawrence AD, Sales T, Quested D, Grasby P. Presynaptic dopaminergic dysfunction in schizophrenia: a positron emission tomographic [18F]Fluorodopa study. Arch Gen Psychiatry. 2004;61(2):134–142. [DOI] [PubMed] [Google Scholar]
- 13.Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69(8):776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howes OD, Whitehurst T, Shatalina E, et al. The clinical significance of duration of untreated psychosis: an umbrella review and random-effects meta-analysis. World Psychiatry. 2021;20(1):75–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore H, Jentsch JD, Ghajarnia M, Geyer MA, Grace AA. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: implications for the neuropathology of schizophrenia. Biol Psychiatry. 2006;60(3):253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lodge DJ, Grace AA. Gestational methylazoxymethanol acetate administration: a developmental disruption model of schizophrenia. Behav Brain Res. 2009;7(204(2):306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lodge DJ. The MAM rodent model of schizophrenia. Curr Protoc Neurosci. 2013;63:9.43.1-9.43.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donegan JJ, Boley AM, Yamaguchi J, Toney GM, Lodge DJ. Modulation of extrasynaptic GABAA alpha 5 receptors in the ventral hippocampus normalizes physiological and behavioral deficits in a circuit specific manner. Nat Commun. 2019;10(1):2819. doi: 10.1038/s41467-019-10800-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lodge DJ, Grace AA. Gestational methylazoxymethanol acetate administration alters proteomic and metabolomic markers of hippocampal glutamatergic transmission. Neuropsychopharmacology. 2012;37(2):319–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27(42):11424–11430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perez SM, Donegan JJ, Lodge DJ. Effect of estrous cycle on schizophrenia-like behaviors in MAM exposed rats. Behav Brain Res. 2019;362:258–265. doi: 10.1016/j.bbr.2019.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez SM, Lodge DJ. Hippocampal interneuron transplants reverse aberrant dopamine system function and behavior in a rodent model of schizophrenia. Mol Psychiatry. 2013;18(11):1193–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez SM, Shah A, Asher A, Lodge DJ. Hippocampal deep brain stimulation reverses physiological and behavioural deficits in a rodent model of schizophrenia. Int J Neuropsychopharmacol. 2013;16(6):1331–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grace AA, Bunney BS. Opposing effects of striatonigral feedback pathways on midbrain dopamine cell activity. Brain Res. 1985;333(2):271–284. [DOI] [PubMed] [Google Scholar]
- 25.Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology. 2006;31(7):1356–1361. [DOI] [PubMed] [Google Scholar]
- 26.Lodge DJ, Grace AA. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci. 2011;32(9):507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez SM, Lodge DJ. Aberrant dopamine D2-like receptor function in a rodent model of schizophrenia. J Pharmacol Exp Ther. 2012;343(2):288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah A, Lodge DJ. A loss of hippocampal perineuronal nets produces deficits in dopamine system function: relevance to the positive symptoms of schizophrenia. Transl Psychiatry. 2013;3(1):e215. doi: 10.1038/tp.2012.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aguilar DD, Chen L, Lodge, DJ. Increasing endocannabinoid levels in the ventral pallidum restores aberrant dopamine neuron activity in the subchronic PCP rodent model of schizophrenia. Int J Neuropsychopharmacol. 2014;18(1):pyu035. doi: 10.1093/ijnp/pyu035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boley AM, Perez SM, Lodge DJ. A fundamental role for hippocampal parvalbumin in the dopamine hyperfunction associated with schizophrenia. Schizophr Res. 2014;157(1–3):238–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114(2):475–492. [DOI] [PubMed] [Google Scholar]
- 32.Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons—1. Identification and characterization. Neuroscience. 1983;10(2):301–315. [DOI] [PubMed] [Google Scholar]
- 33.Rossler W, Salize HJ, van Os J, Riecher-Rossler A. Size of burden of schizophrenia and psychotic disorders. Eur Neuropsychopharmacol. 2005;15(4):399–409. [DOI] [PubMed] [Google Scholar]
- 34.Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry. 1999;46(1):56–72. [DOI] [PubMed] [Google Scholar]
- 35.Hietala J, Syvalahti E, Vuorio K, et al. Presynaptic dopamine function in striatum of neuroleptic-naive schizophrenic patients. Lancet. 1995;346(8983):1130–1131. [DOI] [PubMed] [Google Scholar]
- 36.Dao-Castellana MH, Paillere-Martinot ML, Hantraye P, et al. Presynaptic dopaminergic function in the striatum of schizophrenic patients. Schizophr Res. 1997;23(2):167–174. [DOI] [PubMed] [Google Scholar]
- 37.Lindstrom LH, Gefvert O, Hagberg G, et al. Increased dopamine synthesis rate in medial prefrontal cortex and striatum in schizophrenia indicated by l-(beta-11C) DOPA and PET. Biol Psychiatry. 1999;46(5):681–688. [DOI] [PubMed] [Google Scholar]
- 38.Reith J, Benkelfat C, Sherwin A, et al. Elevated dopa decarboxylase activity in living brain of patients with psychosis. Proc Natl Acad Sci USA. 1994;91(24):11651–11654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howes OD, Montgomery AJ, Asselin MC, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66(1):13–20. [DOI] [PubMed] [Google Scholar]
- 40.Howes OD, Bose SK, Turkheimer F, et al. Dopamine synthesis capacity before onset of psychosis: a prospective [18F]-DOPA PET imaging study. Am J Psychiatry. 2011;168(12):1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bunney BS, Grace AA. Acute and chronic haloperidol treatment: comparison of effects on nigral dopaminergic cell activity. Life Sci. 1978;23(16):1715–1727. [DOI] [PubMed] [Google Scholar]
- 42.Braszko JJ, Bannon MJ, Bunney BS, Roth RH. Intrastriatal kainic acid: acute effects on electrophysiological and biochemical measures of nigrostriatal dopaminergic activity. J Pharmacol Exp Ther. 1981;216(2):289–293. [PubMed] [Google Scholar]
- 43.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J Neurosci. 1984;4(11):2877–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. J Neurosci. 1984;4(11):2866–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bunney BS, Aghajanian GK, Roth RH. Comparison of effects of l-dopa, amphetamine and apomorphine on firing rate of rat dopaminergic neurones. Nat New Biol. 1973;245(143):123–125. [DOI] [PubMed] [Google Scholar]
- 46.Grace AA, Onn SP. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci. 1989;9(10):3463–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ungless MA, Grace AA. Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci. 2012;35(7):422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garris PA, Ciolkowski EL, Pastore P, Wightman RM. Efflux of dopamine from the synaptic cleft in the nucleus accumbens of the rat brain. J Neurosci. 1994;14(10):6084–6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80(1):1–27. [DOI] [PubMed] [Google Scholar]
- 50.Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6(9):968–973. [DOI] [PubMed] [Google Scholar]
- 51.Gonon FG. Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic neurons as studied by in vivo electrochemistry. Neuroscience. 1988;24(1):19–28. [DOI] [PubMed] [Google Scholar]
- 52.Miller JD, Sanghera MK, German DC. Mesencephalic dopaminergic unit activity in the behaviorally conditioned rat. Life Sci. 1981;29(12):1255–1263. [DOI] [PubMed] [Google Scholar]
- 53.Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41(1):1–24. [DOI] [PubMed] [Google Scholar]
- 54.Perez SM, Lodge DJ. Convergent inputs from the hippocampus and thalamus to the nucleus accumbens regulate dopamine neuron activity. J Neurosci. 2018;38(50):10607–10618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Floresco SB, Yang CR, Phillips AG, Blaha CD. Basolateral amygdala stimulation evokes glutamate receptor-dependent dopamine efflux in the nucleus accumbens of the anaesthetized rat. Eur J Neurosci. 1998;10(4):1241–1251. [DOI] [PubMed] [Google Scholar]
- 56.Provenzano FA, Guo J, Wall MM, et al. Hippocampal pathology in clinical high-risk Patients and the onset of schizophrenia. Biol Psychiatry. 2020;87(3):234–242. [DOI] [PubMed] [Google Scholar]
- 57.Schobel SA, Chaudhury NH, Khan UA, et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78(1):81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bossong MG, Antoniades M, Azis M, et al. Association of hippocampal glutamate levels with adverse outcomes in individuals at clinical high risk for psychosis. JAMA Psychiatry. 2019;76(2):199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perez SM, Carreno FR, Frazer A, Lodge DJ. Vagal nerve stimulation reverses aberrant dopamine system function in the methylazoxymethanol acetate rodent model of schizophrenia. J Neurosci. 2014;34(28):9261–9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perez SM, Lodge DJ. New approaches to the management of schizophrenia: focus on aberrant hippocampal drive of dopamine pathways. Drug Des Devel Ther. 2014;8:887–896. doi: 10.2147/DDDT.S42708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21(13):4915–4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jauhar S, Laws KR, McKenna PJ. CBT for schizophrenia: a critical viewpoint. Psychol Med. 2019;49(8):1233–1236. [DOI] [PubMed] [Google Scholar]