Abstract
Background
After initially recommending palivizumab (PVZ), a monoclonal antibody against respiratory syncytial virus (RSV) for all infants 29 to 32 weeks at birth if <6 months age at season start, the American Academy of Pediatrics (AAP) and Canadian Paediatric Society (CPS) guidelines were revised. British Columbia was the only jurisdiction in North America to restrict eligibility for this group to those with additional risk factors, long before the change in national recommendations.
Objectives
To determine the risk for first season RSV admission for 29 to 32-week gestational age (GA) infants admitted to Victoria Neonatal Intensive Care Unit (NICU) that either received or were denied PVZ prophylaxis.
Methods
Descriptive cohort study of infants eligible for prophylaxis according to earlier CPS guidelines. Instead, BC guidelines for prophylaxis were applied and data for Vancouver Island infants were collected over 10 consecutive RSV seasons.
Results
We followed 423 infants. Three hundred and thirty-six (79%) did not receive prophylaxis, of which 10 (3.0%; 95% confidence interval [CI] 1.4% to 5.4%) had an RSV hospitalization before the end of April during their first RSV season versus 3 admissions from 87 (3.5%; 95% CI 0.7% to 10%) infants who received prophylaxis.
Conclusions
Our risk factor approach to RSV prophylaxis for infants born at 29 to 32 weeks GA resulted in a low (average incidence=3.1%) rate of RSV hospitalization. Our approach would offer considerable cost savings to RSV prophylaxis programs that continue to offer routine prophylaxis beyond 28/29 weeks GA at birth.
Keywords: Immunization, Palivizumab, Prematurity, Respiratory syncitial virus
Background
Twenty-one years ago, the Impact-RSV multicentre randomized study (1) on monthly winter palivizumab (PVZ) prophylaxis found that the respiratory syncytial virus (RSV) hospitalization rate for study enrolled premature infants up to 35 weeks gestation age (GA) without chronic lung disease (CLD) and less than 6 months age at season start was 8% in the control arm and 2% in the prophylaxis arm. Although the difference was statistically significant, due to the prohibitive cost of PVZ no jurisdiction implemented funded universal prophylaxis to this entire group of infants; for many years in the USA and Canada, only the 29 to 32-week GA subgroup were recommended for routine prophylaxis and a risk factor approach was developed for infants 33 to 35 weeks GA at birth (2,3).
In 2014 and 2015 respectively, American Academy of Pediatrics (AAP) and Canadian Paediatric Society (CPS) guidelines were amended to no longer recommend prophylaxis to infants without CLD born after 28+6 (4) or 29+6 (5) weeks GA. There has been significant pushback over the new guidelines (6), with two US studies showing an increased rate of RSV admission in the first 3 months of life in infants born at 29 to 32 weeks GA following the change in AAP guidelines (7,8). In Canada, there remains a significant discrepancy between provinces on eligibility for prophylaxis.
The British Columbia (BC) RSV Prophylaxis Committee determines eligibility criteria for provincially funded PVZ prophylaxis. Our criteria have historically been determined independently from CPS guidelines (Table 1) (9) and until 2015, were more restrictive. While the Canadian registry of palivizumab (CARESS) (10) monitored outcomes of infants receiving prophylaxis, we attempted to follow infants at risk but not eligible, as well as infants receiving abbreviated courses to a maximum of three or four doses (11). Since 2003, BC has used a risk factor approach for determining eligibility for PVZ for infants 29 to 32 weeks GA at birth, thereby restricting prophylaxis to less than a quarter of infants who would have been automatically eligible elsewhere in North America.
Table 1.
Risk factors used to determine eligibility for prophylaxis for infants 29+0–34+6 in BC
| Season | Risk factors | Notes |
|---|---|---|
| 2009–2010 | ||
| Male gender | ||
| Birth Weight <10% | Three or more risk factors | |
| Will attend day care | Born after 1 September | |
| Preschool aged siblings attending day care | Maximum five doses | |
| More than five in household | ||
| Two or more smokers in household | ||
| Resides in remote location | ||
| 2010–2011 | ||
| Male gender | ||
| Birth Weight <10% | Three or more risk factors | |
| Will attend day care | Born after 1 September | |
| Any sibling <5 years in household | Maximum five doses | |
| More than five in household | ||
| Two or more smokers in household | ||
| 2011–2012 | ||
| Male gender | ||
| Birth Weight <10% | Three or more risk factors | |
| Will attend day care | Discharged on or after 1 November | |
| Any sibling <5 years in household | Maximum three doses | |
| More than five in household | ||
| Two or more smokers in household | ||
| Reside in remote location | ||
| Girls not receiving human milk | ||
| 2012–2019 | ||
| Male or (female + no human milk) | Score 8 | |
| Birth Weight <10% | Score 8 | |
| Will attend day care | Score 22 | |
| Any sibling <5 years in household | Score 14 (12 for 2012 season) | |
| More than five in household | Score 12 | |
| Two or more smokers in household | Score 8 | |
| Reside in remote location | Score 10 | |
| Gestation at birth 29–30+6 | Score 10 | |
| Discharged Nov/March | Score 10 | |
| Discharged December/January/February | Score 20 | |
| Total Score 42 or more and discharged after 1 October | ||
| Maximum three doses |
The west coast of BC has a temperate climate. The RSV season typically lasts from mid-November through to the end of March. Vancouver Island has a population of approximately 800,000 or 17% of the province of BC. Infants born at 29 to 32 weeks GA are usually admitted to Victoria General Hospital Neonatal Intensive Care Unit (NICU), the only level 3 unit on the island. For those infants 29 to 32 weeks GA discharged from our NICU, we were able to track RSV admissions to all Vancouver Island hospitals from 2009 through 2019.
Methods
For RSV seasons 2009 to 2010 through 2018 to 2019, the PVZ eligibility of infants 29 +0/7 through 32 + 6/7 weeks GA, born between June (May for first two seasons) and March of the following year and admitted to Victoria NICU were determined, using BC criteria in place at the time (Table 1). Names and identifiers were entered into a local database at discharge. RSV positive admissions to all hospitals on Vancouver Island were monitored for matches using the Vancouver Island Health Authority computer network. At each season end, data on RSV hospitalizations was transferred to a spreadsheet which included both PVZ eligible and non - eligible (using BC criteria) infants. Each infant was followed until the end of April for one season only. Written consent was not obtained as the study involved anonymous data collection as part of an ongoing audit.
Until 2011, BC prophylaxis guidelines used a date of birth cut-off of 1 September to determine PVZ eligibility. For seasons 2012 to 2013 onwards, BC guidelines were modified to determine eligibility for this group by creating a fillable portable document format (pdf) application file which calculated a risk score from scores for month of discharge from hospital NICU (12,13) (Table 1) as well as other risk factors described in the published literature (14,15).
RSV testing was performed for all infant respiratory admissions on the island. For seasons 2009/2010 and later, enzyme immunoassay (EIA) testing was used. Results were confirmed by BC Centre for Disease Control (BC CDC). After Jan 2013, the island laboratories switched to Polymerase Chain Reaction (PCR) testing. Any positive result from either the hospital laboratory or BC CDC on an inpatient was counted as an RSV hospitalization. Families were also invited to contact one of the authors, should their child require admission for respiratory illness.
Results
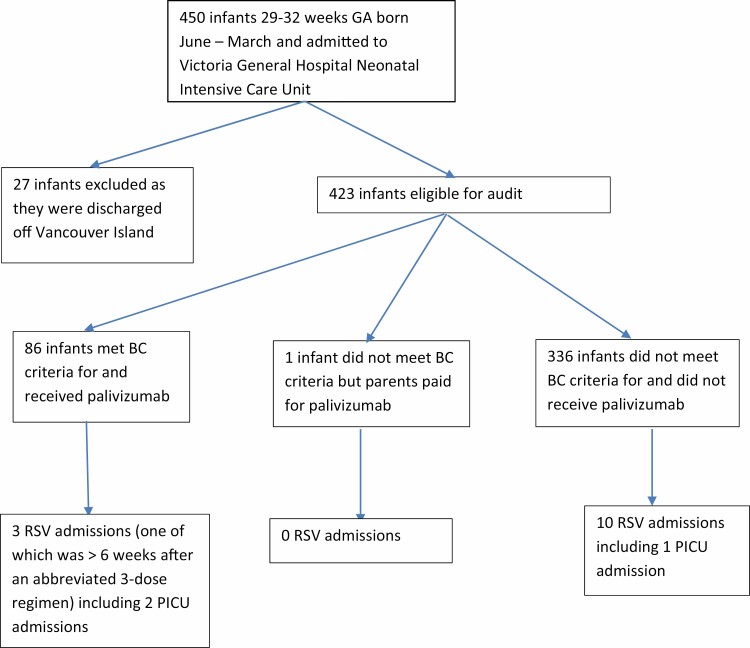
There were 450 infants born between 1 June (1 May for 2009 to 2011 seasons) and 31 March of the following year admitted to our NICU and potentially eligible for audit. Twenty-seven infants could not be tracked, leaving 423 infants who were followed for possible RSV hospitalization, until the end of April.
From the 423 infants followed, 13 (3.1%; 95% confidence interval [CI] 1.6% to 5.2%) had an RSV+ admission (Figure 1).
Figure 1.
Palivizumab and RSV admission status of infants 29–32 weeks gestational age cared for at Victoria General Hospital during birth hospitalization.
As per BC guidelines (Table 1), 336 island infants did not receive PVZ prophylaxis (even though eligible by pre-2014 AAP/CPS guidelines). From these infants, there were 10 RSV+ admissions (3.0%; 95% CI 1.4% to 5.4%). There was equal distribution of admissions across the study period at 0 to 2 cases per year. There was one Paediatric Intensive Care (PICU) admission. No infant required ventilation.
Eighty-seven infants received PVZ prophylaxis, including one child who did not qualify according to BC guidelines at that time, (parents paid privately for PVZ) and another child who qualified under our guidelines for Chronic Lung Disease (CLD). From this group, there were three admissions (3.7%; 95% CI 0.7% to 10%), all of whom qualified according to BC guidelines. Two of the admissions occurred within 4 weeks of the previous dose. The third infant was admitted 6 weeks after the third and final dose. Two were admitted to PICU. One child required ventilation. All survived. Another child (not counted) was hospitalized for RSV in May.
Discussion
To our knowledge, BC is the only jurisdiction in North America which has consistently restricted access to PVZ prophylaxis for infants 29 to 32 weeks GA to those with additional risk factors. Until 2014, both the CPS and the AAP guidelines recommended universal prophylaxis for this group (if <6 months age at season start), with selection of more mature infants (up to 35 weeks gestation) by applying risk factors (2,3,15). Other developed countries remain more restrictive (16,17). BC eligibility criteria evolved with changes based on committee consensus and ongoing literature review. In 2012, we introduced a risk score which included month of NICU discharge home (12), since validated as a significant risk factor for RSV admission (18). The AAP and CPS revised their guidelines in 2014 and 2015 (4,5). However, the new national recommendations are controversial (6) and at the time of writing, most Canadian provinces have still not adopted the new CPS guidelines. There is ongoing debate regarding the risk of RSV admission in this group, as well as a lack of consensus over the risk threshold at which prophylaxis should be given.
Our rate of 3% RSV + admissions for the majority of 29-32 week gestation infants that were denied PVZ prophylaxis over the last 10 seasons is far lower than the 8% rate in the IMPACT study control arm (1) but similar to an audit of RSV hospital admissions for the whole of BC over three consecutive seasons (19) obtained by using anonymous hospital discharge diagnosis data.
The risk of first season RSV admission in premature infants without CLD and not receiving prophylaxis is reviewed in the most recent CPS statement (5), by Simoes (20) and remains uncertain. The higher risk for 29 to 35-week gestation infants in the IMPACT study control arm was probably an overestimate of the true risk, due to selection bias in recruitment (1,20) for the study, particularly as more mature infant controls had a higher admission rate than the less mature infants. However, a recent American report found a risk as high as 11.7% for Medicaid infants and 6.3% for commercially insured infants 29 to 30 weeks GA and under 3 months of age (8). Risk of first season RSV admission might be higher in the USA due to wider use of early daycare. In Canada, the PICNIC study (18) found a risk of 3.6% for more mature (33 to 35+6 GA) infants (also far lower than IMPACT controls) with a 10-fold variation in admission risk related to risk factors (early daycare being the most important). Until recently, most 29 to 32-week GA infants received PVZ prophylaxis in both the USA and Canada, creating problems estimating prior risk, as well as determining the relative importance of risk factors. An estimate of the prior risk in the Canadian population might be derived from CARESS data (10) obtained when most provinces were following earlier CPS guidelines, which found an RSV admission rate of 1.25% in treated infants 29 to 32 weeks GA at birth: if one assumes an 80% efficacy for this population, this would imply an RSV admission risk of 6% (1.25%/(1–0.8) for these first season infants if they had not received prophylaxis. However this figure is very sensitive to the efficacy of the product. An efficacy of 75% when applied to CARESS data would give an average prior risk of 5%
As the average risk (assume 5 to 6% for this GA range) with no prophylaxis is the sum of fraction fh (0.21) of eligible infants multiplied by their prior risk, and fraction fl (0.79, as fh+fl=1) of low risk infants multiplied by their risk from this audit, it is possible to calculate the prior risk of our ‘high risk’ infants from our data: our ‘high risk’ infants, who received PVZ would have needed to have a prior risk of 17% for first season RSV admission in order to satisfy the equation: (0.21×prior risk for eligible infants)+(0.79×0.03)=6%. If one assumes a lower average risk of 5% for this population, this would imply a risk of about 12% for our treated infants, if they had not received PVZ. This relatively high risk is supported by anonymous provincial hospital discharge data for the seasons 2013/2014 to 2016/2017, which found that BC infants 29 to 32 weeks at birth who received prophylaxis had a higher rate of RSV admission than infants that did not receive prophylaxis (19) in spite of the drug being 75 to 80% effective at reducing risk.
BC guidelines allow for a maximum of three doses (11) to selected infants without chronic disease, starting near discharge: so, at an average weight of 3 kg, the total drug cost per infant is about $2,000 (assuming a cost of CAN $1,500 per 100 mg PVZ vial and no waste): If the efficacy is 80% and if the RSV admission rate were to be reduced by 10% for our treated cohort (assuming a prior risk of > 12%), this would make PVZ cost effective if the average cost of RSV related hospital admission were $20,000 or more. In contrast, if our untreated cohort had each received five doses of PVZ as per earlier CPS guidelines, instead of no doses, the drug cost alone (with no assumptions required) for reducing our 10 RSV+ island hospitalizations to two would have been approximately $1.2 million, equivalent to $7 million for the entire province, or $150,000 per admission averted.
To our knowledge, this is the first study to show outcome data on markedly restricted use of PVZ in 29 to 32+6 week GA infants approved or denied PVZ through the application of risk factors. The strength of our study is that it was performed over many seasons, with each infant followed by their unique personal health number using the Island hospital network, making us certain of our data. As far as known, all island infant respiratory admissions had RSV testing performed. Limitations include lack of a ‘control’ group (not feasible as this was an audit, not a clinical trial) and our inability to track RSV admissions away from the island.
Conclusions
Our first season RSV+ hospital admission data over the last 10 seasons supports a restrictive approach to RSV prophylaxis for infants 29 to 32 weeks GA. The higher RSV hospitalization rate in our treated cohort suggests that our scoring tool is effective at discriminating infants at higher risk. A risk factor approach to RSV prophylaxis for this GA range may be an optimal strategy.
Acknowledgements
The authors would like to thank Professor Charles C. Taylor, School of Mathematics, University of Leeds, UK for checking math and wording suggestions, Dr. Pascal Lavoie, BC Children’s Hospital for editing suggestions and Dr. Alfonso Solimano, BC Children’s Hospital for collaboration in developing BC guidelines.
Funding: There are no funders to report for this submission.
Potential Conflicts of Interest: RST reports that he is chair of the BC RSV Immunoprophylaxis Committee. This committee determines guidelines for provincial funding for palivizumab prophylaxis. He does not receive any separate remuneration for work on this committee. There are no other disclosures. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Richard S Taylor, University of Victoria, Victoria, British Columbia; NICU, Victoria General Hospital, Victoria, British Columbia.
Margaret H Baker, NICU, Victoria General Hospital, Victoria, British Columbia.
References
- 1. Impact study group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 1998;102:531–7. [PubMed] [Google Scholar]
- 2. Sampson L. CPS Guidelines for preventing respiratory syncytial virus infections. Paediatr Child Health 2009;14(8):521–6.20885804 [Google Scholar]
- 3. Krilov LR, Weiner LB, Yogev R, et al. Modified recommendations for use of palivizumab for prevention of respiratory syncytial virus infections committee on infectious diseases. Pediatrics 2009;124:1694. [DOI] [PubMed] [Google Scholar]
- 4. American Academy of Pediatrics Committee on Infectious Diseases and Bronchiolitis Guidelines Committee. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics 2014;134(2):415–20. [DOI] [PubMed] [Google Scholar]
- 5. Robinson JL, Le Saux N. Canadian Paediatric Society . Preventing hospitalizations for respiratory syncytial virus infection. Paediatr Child Health 2015;20(6):321–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goldstein M, Phillips R, DeVincenzo J, et al. National Perinatal Association 2018 Respiratory Syncytial Virus (RSV) prevention clinical practice guideline: An evidence-based interdisciplinary collaboration. Neonatology Today 2017;12:10. [Google Scholar]
- 7. Anderson EJ, Krilov LR, DeVincenzo JP, Checchia PA, Halasa N, Simoes EA, et al. SENTINEL1: An observational study of respiratory syncytial virus hospitalizations among U.S. Infants born at 29 to 35 weeks' gestational age not receiving immunoprophylaxis. Am J Perinatol. 2017;34(1):51–61. [DOI] [PubMed] [Google Scholar]
- 8. Kong AM, Krilov LR, Fergie J, Goldstein M, Diakun D, Wade SW, et al. The 2014-2015 National Impact of the 2014 American Academy of Pediatrics guidance for respiratory syncytial virus immunoprophylaxis on preterm infants born in the United States. Amer J Perinatol 2018;35(2):192–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Current BC guidelines. http://www.childhealthbc.ca/
- 10. Mitchell I, Paes BA, Li A, Lanctôt KL. CARESS Investigators . CARESS: The Canadian registry of palivizumab. Pediatr Infect Dis J 2011;30(8):651–5. [DOI] [PubMed] [Google Scholar]
- 11. Lavoie PM, Solimano A, Taylor R, et al. Outcomes of respiratory syncytial virus immunoprophylaxis in infants using an abbreviated dosing regimen of palivizumab. JAMA Pediatr 2016;170(2):174–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Solimano A, Lavoie P, Taylor R, Bayzand L. Discharge date is a better predictor of probability of RSV hospital admission than date of birth. Pediatric Academic Societies Meeting; Boston. April 28 to May 1, 2012. Abstract 1516.293, 2012. [Google Scholar]
- 13. Rietveld E, Steyerberg EW, Polder JJ, et al. Passive immunisation against respiratory syncytial virus: A cost-effectiveness analysis. Arch Dis Child 2010;95(7):493–8. [DOI] [PubMed] [Google Scholar]
- 14. Sinha A, Madden J, Ross-Degnan D, Soumerai S, Platt R. Reduced risk of neonatal respiratory infections among breastfed girls but not boys. Pediatrics 2003;112(4):e303. [DOI] [PubMed] [Google Scholar]
- 15. Law BJ, Langley JM, Allen U, et al. The Pediatric Investigators Collaborative Network on Infections in Canada study of predictors of hospitalization for respiratory syncytial virus infection for infants born at 33 through 35 completed weeks of gestation. Pediatr Infect Dis J 2004;23(9):806–14. [DOI] [PubMed] [Google Scholar]
- 16. UK guidelines. http://webarchive.nationalarchives.gov.uk/20130104184116/https://www.wp.dh.gov.uk/immunisation/files/2012/07/chap-27a-dh_130131.pdf (Accessed May 28, 2019).
- 17. Vogel AM, McKinlay MJ, Ashton T, et al. Cost-effectiveness of palivizumab in New Zealand. J Paediatr Child Health 2002;38(4):352–7. [DOI] [PubMed] [Google Scholar]
- 18. Evan Anderson E, DeVincenzo J, Simoes E, et al. Effects of timing of birth hospitalization discharge, birth month, and chronologic age on Respiratory Syncytial Virus (RSV) hospitalizations of US preterm infants not receiving immunoprophylaxis. Abstract/Poster at Pediatric Academic Society (Toronto) 2018. [Google Scholar]
- 19. Sidi S, Claydon J, Solimano A, Taylor R, et al. A risk factors approach is highly effective at selecting 29-34w Gestation infants at risk for first season RSV-related admission in british Columbia – A population study. Paediatrics & Child Health 2019;24(Supplement 2):e17–e17. [Google Scholar]
- 20. Simoes EAF. Immunoprophylaxis of respiratory syncytial virus: Global experience. Respir Res 2002,3(suppl 1):S26–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]