Abstract
The role of nitric oxide (NO) in lipopolysaccharide (LPS)-induced hepatic injury was studied in d-galactosamine (d-GalN)-sensitized mice. The inducible isoform of NO synthase (iNOS) was immunohistochemically detected on hepatocytes around blood vessels in livers of mice injected with d-GalN and LPS not on hepatocytes in mice injected with d-GalN or LPS alone, although mRNA for iNOS was found in those mice. Nitrotyrosine (NT) was also found in livers of mice injected with d-GalN and LPS. The localization of NT was consistent with that of iNOS, and the time courses of NT and iNOS expression were almost the same. Expression of iNOS and NT was detected exclusively in the hepatic lesions of mice injected with d-GalN and LPS. Anti-tumor necrosis factor alpha neutralizing antibody inhibited iNOS and NT expression and hepatic injury. The results suggested that NO from iNOS may play a role in LPS-induced hepatic injury on d-GalN-sensitized mice as an experimental endotoxic shock model.
Nitric oxide (NO) exhibits a wide range of important functions in vivo, acting as a relaxing factor mediating vasodilation, a neuronal messenger molecule, and a major regulatory molecule and principal cytotoxic mediator of the immune system (3, 7, 18). The signal (messenger) molecule NO is synthesized by constitutively expressed NO synthase (NOS) for short periods of time. The killer (cytotoxic) molecule NO is synthesized by an inducible isoform of NOS (iNOS) that, once expressed, produces NO for long periods of time (18). It appears paradoxical that NO can both act as a physiological intercellular messenger and display cytotoxic activity in vivo (18). Cytotoxicity usually correlates with NO produced by iNOS (18). Further, NO and superoxide rapidly react to yield peroxynitrite (1, 3, 27), a potent oxidant which reacts with proteins, lipid, and DNA (3, 18, 31). Some of the biochemical and toxicological effects of NO are suggested to occur via peroxynitrite because of the relatively poor reactivity of NO (29, 32). Peroxynitrite is a highly reactive species which causes nitration and hydroxylation of tyrosine and tryptophan as well as DNA injury (3, 27, 31). Nitrotyrosine (NT) is a convenient in vivo marker of peroxynitrite (2, 5). The formation of NO, especially peroxynitrite, has been implicated in the pathophysiology of numerous inflammation diseases, including sepsis (14, 15).
Bacterial lipopolysaccharide (LPS) is present on the outer membranes of all gram-negative bacteria and causes the systemic inflammatory response syndrome and septic shock, which finally develop to multiorgan failure. Sensitization with d-galactosamine (d-GalN) greatly increases the sensitivity of animals to LPS and augments the lethal activity of LPS (10, 11). LPS-induced lethality is characterized by liver failure, accompanied by severe hepatic injuries. The lethal effect of LPS in d-GalN-sensitized mice is usually considered an experimental model for clinical endotoxic shock or septic shock (11). Previously it was reported that LPS-induced hepatic injury is caused by hepatocyte apoptosis, mediated mainly by tumor necrosis factor alpha (TNF-α) (20, 24). However, there are no reports on the participation of NO in the LPS-induced hepatic injury of d-GalN-sensitized mice. In the present study, we examined whether and how NO participates in LPS-induced hepatic injury in d-GalN-sensitized mice as an experimental endotoxic shock model.
MATERIALS AND METHODS
Mice.
Female BALB/c mice were purchased from Japan SLC (Hamamatsu, Japan) and used at approximately 7 weeks of age.
Reagents.
Rabbit polyclonal immunoglobulin G (IgG) antibodies to iNOS and NT were obtained from Upstate Biotechnology, Inc., Lake Placid, N.Y., and Affinity Bioreagents Inc., Neshanic Station, N.J., respectively. As a second antibody, horseradish peroxidase-conjugated affinity-purified goat anti-rabbit IgG was purchased from BioSource International (Camarillo, Calif.). Rabbit polyclonal antibody neutralizing murine TNF-α, hamster antibody neutralizing gamma interferon or interleukin-1, and murine recombinant TNF-α were purchased from Genzyme (Cambridge, Mass.) and used as recommended by the manufacturer.
LPS and administration.
The LPS preparation extracted by the phenol-water method from Escherichia coli O111:B4 was obtained from Difco Laboratories, Detroit, Mich. Mice were injected intraperitoneally (i.p.) with d-GalN (20 mg) and LPS (1 μg). Three to four mice per experimental group were sacrificed 6 h after injection unless otherwise stated, and the livers were removed. Treatment with d-GalN and LPS led to greater than 80% lethality in mice.
Immunohistochemical staining.
The livers from mice injected i.p. with d-GalN and LPS were fixed in 4% formaldehyde. Paraffin sections of the livers were deparaffinized, and the endogenous peroxidase activity was blocked with methanol containing 0.3% hydrogen peroxide for 15 min at room temperature. The sections were washed in 0.01 M phosphate-buffered saline, pH 7.2 (PBS), containing 10% normal horse serum and incubated overnight at 4°C with anti-iNOS antibody (1:500) or anti-NT antibody (1:300). Horseradish-conjugated goat anti-rabbit Ig antibody was used at 1:200 after washing. Immune complexes were detected with a solution of 3,3-diaminobenzidine (0.2 mg/ml) and hydrogen peroxide in 0.05 M Tris-HCl buffer. Sections were counterstained with methyl green. In negative control sections, rabbit antiserum against ovalbumin (1:500) was used as an irrelevant antibody.
Measurement of mRNA levels for iNOS by RT-PCR.
Oligonucleotide primers used for reverse transcription (RT)-PCR, designed based on the DNA sequence, were iNOS-1 (5′-CTGCAGGTCTTTGACGCTCG-3′), iNOS-2 (5′-GTGGAACACAGGGGTGATGC-3′), GAPDH-1 (5′-AGATCCACAACGGATACATT-3′), and GAPDH-2 (5′-TCCCTCAAGATTGTCAGCAA-3′). Total RNA was extracted from livers (100 μg) by a modified guanidinium isothiocyanate method using the RNA extraction reagent Isogen (Nippon Gene, Toyama, Japan) as instructed by the manufacturer. Separate aliquots of total RNA were reverse transcribed into cDNA and subjected to RT-PCR using the Titan One Tube RT-PCR system, PCR nucleotide mix, and RNase inhibitor (Boehringer, Mannheim, Germany). The reaction mixture was amplified with a thermal cycler for 30 min as recommended by the manufacturer. After an initial denaturation step at 94°C for 2 min, each cycle consisted of incubations at 94°C for 30 s, 59°C for 30 s, and 68°C for 2 min. Products were analyzed on a 3% agarose 21 gel (Nippon Gene) and visualized under UV light by staining with ethidium bromide. The iNOS primers used for the RT-PCR included the murine macrophage sequence for iNOS from positions 607 to 1413 (13, 22). The RT-PCRs resulted in the amplification of a single product of the predicted size for the iNOS (807 bp) (13). To ensure that equal amounts of reverse-transcribed RNA were applied to the RT-PCR, the parallel expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was tested at 30 min.
RESULTS
Detection of mRNA levels for iNOS in livers of mice injected with d-GalN and LPS.
Induction of the mRNA for iNOS in livers of mice injected with d-GalN, LPS, or the mixture of d-GalN and LPS was analyzed by RT-PCR. Mice were injected i.p. with d-GalN, LPS, or a mixture of d-GalN and LPS, and livers were removed 3 h after the injection. The PCR products for iNOS were detected in livers of mice injected with of d-GalN, LPS, or the mixture of d-GalN and LPS. RT-PCR yielded amplified products with a length of about 807 bp (Fig. 1), consistent with the base pair size expected from the nucleotide sequence of the iNOS in mouse macrophages (13, 22). However, mRNA for iNOS was undetectable in saline-injected control mice. In addition, the treatments did not alter levels of mRNA for the housekeeping gene GAPDH in livers.
FIG. 1.
Detection of iNOS mRNA in livers of mice injected with d-GalN and LPS. iNOS mRNA products of 807 bp were obtained after amplification using total RNA from mice injected with PBS (lanes 2 and 3), d-GalN (lanes 4 and 5), LPS (lanes 6 and 7), or d-GalN and LPS (lanes 8 and 9). Lane 1, DNA size marker; lanes 2 to 9, mRNA products of iNOS (lanes 3, 5, 7, and 9) and the housekeeping gene GAPDH (lanes 2, 4, 6, and 8). The arrow indicates the size of the expected amplification product of the mRNA for iNOS.
Detection and localization of iNOS in livers of mice injected with d-GalN and LPS.
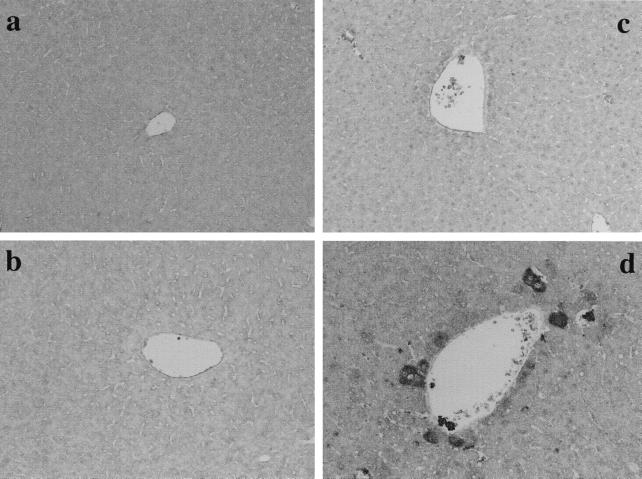
In the experiments described above, the administration of d-GalN, LPS, or the mixture of d-GalN and LPS resulted in induction of the mRNA for iNOS. The expression and localization of iNOS in livers of the mice were studied immunohistochemically with anti-iNOS antibody (Fig. 2). Mice were injected i.p. with d-GalN, LPS, or the mixture of d-GalN and LPS, and livers were removed 6 h after injection. Positive staining was detected exclusively in livers of mice injected with the mixture of d-GalN and LPS. No significant staining was detected in livers of mice injected with d-GalN or LPS alone, although the mRNA for iNOS was found by RT-PCR. The expression of iNOS was detected mainly around blood vessels in livers of mice injected with d-GalN and LPS. Hepatocytes and some vascular endothelial cells were stained positively.
FIG. 2.
Detection of iNOS in livers of mice injected with d-GalN and LPS. Mice were injected i.p. with PBS (a), d-GalN (b), LPS (c), or the mixture of d-GalN and LPS (d), and livers were removed 6 h after injection. Liver sections were immunohistochemically stained by anti-iNOS antibody. Magnification, ×200.
Detection and localization of NT in livers of mice injected with d-GalN and LPS.
Since NO probably exerts its toxic effects via peroxynitrite and peroxynitrite causes the nitration of tyrosine (2, 5), we performed immunohistochemical staining to detect NT as a convenient in vivo marker of peroxynitrite. Mice were injected i.p. with d-GalN, LPS, or the mixture of d-GalN and LPS, and livers were removed 6 h after injection. The result is shown in Fig. 3. NT was detected only in livers from mice injected with the mixture of d-GalN and LPS. Positively stained cells were hepatocytes and vascular endothelial cells around blood vessels of those mice. NT was not found in livers of mice injected with d-GalN or LPS alone. The localization of NT corresponded to that of iNOS.
FIG. 3.
Detection of NT in livers of mice injected with d-GalN and LPS. Mice were injected i.p. with PBS (a), d-GalN (b), LPS (c), or the mixture of d-GalN and LPS (d), and livers were removed 6 h after injection. Liver sections were immunohistochemically stained by anti-NT antibody. Magnification, ×200.
Time course of iNOS and NT expression in livers of mice injected with d-GalN and LPS.
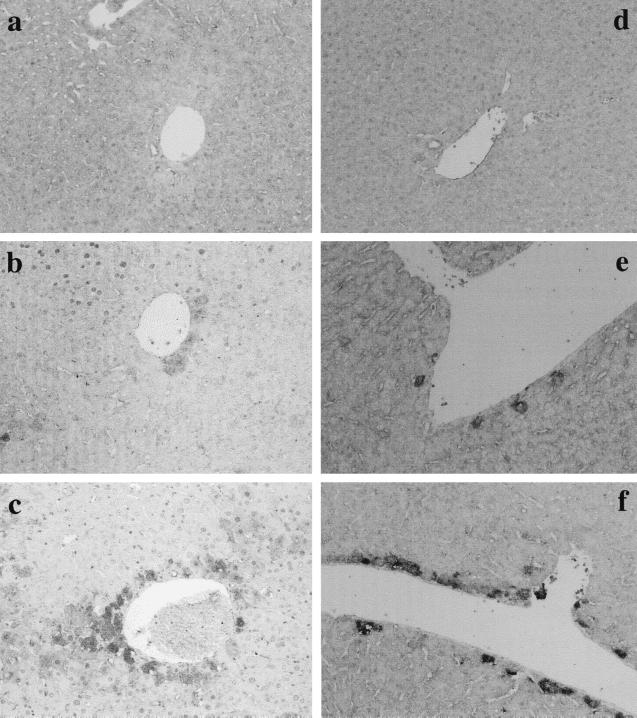
The time course of iNOS and NT expression was monitored in livers of mice injected with d-GalN and LPS. The result is shown in Fig. 4. Weak expression of iNOS around blood vessels in the livers was found 3 h after the injection. Staining of iNOS was much more apparent 6 h after injection, and positive staining was found diffusely around blood vessels. Similarly, expression of NT was detected 3 h and increased up to 6 h after injection. NT was also detected around blood vessels. The staining pattern of NT was almost the same as that of iNOS.
FIG. 4.
Time course of iNOS and NT expression in livers of mice injected with d-GalN and LPS. Mice were injected i.p. with the mixture of d-GalN and LPS, and livers were removed 0 (a and d), 3 (b and e), and 6 (c and f) h after injection. Liver sections were stained immunohistochemically by anti-iNOS antibody (a to c) or anti-NT antibody (d to f). Magnification, ×200.
Histology of hepatic injury in mice injected with d-GalN and LPS.
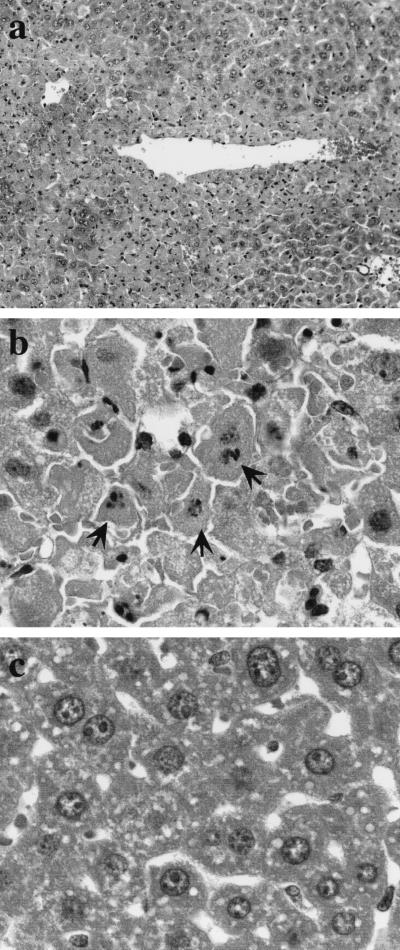
LPS-induced hepatic injury of d-GalN-sensitized mice was histologically inspected in livers of mice injected with d-GalN and LPS. Mice were injected with d-GalN, LPS, and the mixture of d-GalN and LPS, and livers were removed from live mice 12 h after the injection. Hematoxylin and eosin-stained liver sections are shown in Fig. 5. Histological changes were found only in livers of mice injected with d-GalN and LPS, not in livers of mice injected with d-GalN or LPS alone. Vascular walls in livers of those mice became thin and were in part destroyed. Vascular endothelial cells exhibited damage, accompanied by hemorrhage into the parenchymal region. In particular, hepatocytes around blood vessels were massively damaged (Fig. 5a); some of the nuclei were fragmented and condensed (Fig. 5b). Hepatic lesions in mice injected with d-GalN and LPS corresponded to the localization of iNOS and NT.
FIG. 5.
Histology of hepatic injury in mice injected with d-GalN and LPS. Mice were injected i.p. with the mixture of d-GalN and LPS, and livers were removed from live mice 12 h after the injection. Liver sections from mice injected with d-GalN and LPS (a and b) or saline alone (c) were stained with hematoxylin and eosin. Note hepatic injuries around a blood vessel (a) and fragmented nuclei of hepatocytes (arrows in panel b). Magnifications: a, ×200; b and c, ×1000.
Role of TNF-α in the induction of iNOS and NT in livers of mice injected with d-GalN and LPS.
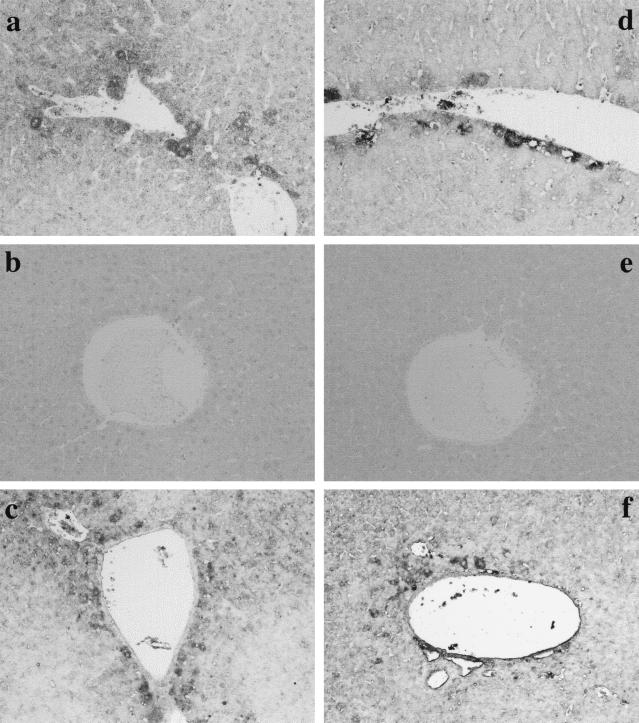
A major feature of LPS-induced hepatic injury of d-GalN-sensitized mice is increased sensitivity to TNF-mediated effects (19, 24). Previously we reported that hepatocyte injury in mice injected with d-GalN and LPS was inhibited by anti-TNF-α antibody and that recombinant TNF and d-GalN produced similar hepatic injuries (24). Based on those findings, we studied the participation of TNF-α in induction of iNOS and NT (Fig. 6). Mice were injected with anti-TNF-α antibody (50 μg) together with d-GalN and LPS. Anti-TNF-α antibody significantly blocked the induction of iNOS and NT, suggesting the participation of TNF-α. Next, mice were injected with TNF-α (50 ng) and d-GalN to confirm the involvement of TNF-α. The administration of recombinant TNF-α and d-GalN caused the expression of iNOS and NT around blood vessels, implicating TNF-α in LPS-induced expression of iNOS and NT. In addition, anti-gamma interferon or anti-interleukin-1 antibody (50 μg) did not block the expression of iNOS and NT (data not shown).
FIG. 6.
Role of TNF-α in the induction of iNOS and NT in livers of mice injected with d-GalN and LPS. Mice were injected i.p. with d-GalN and LPS (a and d), d-GalN and LPS together with anti-TNF-α antibody (b and e), or d-GalN and TNF-α (c and f); livers were removed 6 h after injection. Liver sections were immunohistochemically stained by anti-iNOS antibody (a to c) or anti-NT antibody (d to f). Magnification, ×200.
DISCUSSION
In this study, we demonstrated that the administration of d-GalN and LPS to mice induced the expression of iNOS and NT in livers. iNOS and NT expression was detected at the sites of LPS-induced hepatic injury in d-GalN-sensitized mice. Further, iNOS and NT expression preceded hepatic injury. Neutralization of TNF-α with the antibody inhibited iNOS and NT expression as well as LPS-induced hepatic injury, suggesting that the expression of iNOS and NT was closely associated with hepatic injury in livers of mice injected with d-GalN and LPS. On the other hand, iNOS and NT expression was not detected in mice injected with LPS or d-GalN alone, although RT-PCR analysis indicated that mRNA for iNOS may have been marginally transcribed in those cases. Considering that the killer molecule NO is synthesized by iNOS (18), it was reasonable that no significant hepatic lesions were produced in livers of mice injected with LPS or d-GalN alone. Killer NO molecules produced by iNOS may be important in the development of LPS-induced hepatic injury in d-GalN-sensitized mice. To our knowledge, the induction of iNOS and production of NO and NT in LPS-induced hepatic injury of d-GalN-sensitized mice have not been investigated, although an animal model of sepsis caused by gram-positive organisms has recently been developed (30).
A number of studies have examined the relationship between NO production and tissue injury (23, 26, 29, 34–36). The toxicological effect of NO seems to occur via peroxynitrite because of the relatively poor reactivity of NO and the formation of NT at sites of enhanced NO synthesis (29, 32). Peroxynitrite, a potent oxidant formed from NO and superoxide, causes the nitration of tyrosine (3). Therefore, NT is a convenient in vivo marker of peroxynitrite (2, 5). Since NT was detected at the sites of LPS-induced hepatic injury, peroxynitrite appeared to participate in hepatic injury in mice injected with d-GalN and LPS. NO and superoxide may be produced by vascular endothelial cells and circulating leukocytes, and the reaction forming peroxynitrite may occur around blood vessels, possibly in association with the development of hepatic injury around blood vessels.
Recent studies demonstrate that NO and peroxynitrite cause either necrosis or apoptosis in a variety of cell types (4, 9, 21, 28). It appears that sustained exposure to low levels of NO or peroxynitrite causes apoptosis, whereas sudden exposure to high concentrations of peroxynitrite or NO results in cell necrosis (31). Of particular interest was whether hepatic injury in livers of mice injected with d-GalN and LPS was due to apoptosis or necrosis. Previously we reported the DNA ladder pattern in agarose gel electrophoresis and fragmented nuclei in livers of mice injected with d-GalN and LPS, suggesting hepatocyte apoptosis (24). The present study also demonstrated fragmented and condensed nuclei of hepatocytes around blood vessels. It was suggested that LPS-induced hepatic injury in the system may be due to NO- and peroxynitrite-mediated apoptosis. On the other hand, antiapoptotic effects of NO were reported (7, 8, 18). In vivo antiapoptotic effects of NO are probably indirect effects of NO as a consequence of its improvement of blood circulation in vivo in tissue injury (18). In the present study, it was unlikely that NO production by iNOS exerted an antiapoptotic effect because anti-TNF-α antibody blocked both iNOS and NT expression and hepatic injury.
Previously we reported that TNF is a key molecule in LPS-induced hepatic injury of d-GalN-sensitized mice (24). In fact, simultaneous injection of anti-TNF-α antibody with d-GalN and LPS prevented LPS-induced hepatic injury of d-GalN-sensitized mice and lethality (24). In the present study, anti-TNF-α antibody blocked the expression of iNOS and NT, and the injection of recombinant TNF-α into d-GalN-sensitized mice significantly induced the expression of iNOS and NT. TNF-α was suggested to play a critical role in LPS-induced NO production, although it is known that interleukin-1 and platelet-activating factor can induce iNOS (12, 33). Interleukin-1- or gamma interferon-specific antibody did not block LPS-induced hepatic injury, which suggested that (i) TNF-α may be primarily responsible for hepatic injury and (ii) TNF-α-induced NO production may participate in the development of hepatic injury. It was recently reported that the lethal dose of LPS in d-GalN-sensitized mice correlated with in vitro TNF-α and NO production in a macrophage cell line treated with the same LPS (6), which also supported our conclusions derived from the present study.
Recently we demonstrated the expression of inducible heat shock protein 70 (HSP70) in livers of mice injected with d-GalN and LPS (25). The localization of inducible HSP70 was consistent with that of iNOS expression in this study. It was reported that NO potently stimulates the induction of the heat shock proteins HSP70 and HSP32 (16, 17). HSP induction confers resistance to subsequent apoptotic stimuli (7). NO makes some hepatocytes undergo apoptosis via peroxynitrite-mediated DNA damage, whereas it makes other hepatocytes survive through the production of HSPs. Possibly some hepatocytes, which did not exhibit sufficient autoprotective stress response to LPS stimuli, underwent apoptotic cell death. It is of interest to investigate how LPS-induced NO production may regulate cell death and stress responses.
ACKNOWLEDGMENTS
This work was supported by a grant from the Ministry of Education, Science and Culture of Japan.
We thank K. Takahashi for technical assistance.
REFERENCES
- 1.Beckman J S, Beckman T W, Chen J, Marshall P A, Freeman B A. Apparent hydroxy radical production by peroxynitrite: implication for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckman J S, Ye Y Z, Anderson J, Chen J, Accavetti M A, Tarpey M, White C R. Extensive nitration of protein tyrosines observed in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe-Seyler. 1994;375:81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- 3.Beckman J S, Koppenol W H. Nitric oxide, super oxide, and peroxynitrite: the good, the bad, and the ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 4.Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton S. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with NMDA or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci USA. 1995;92:7162–7166. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crow J P, Ischiropoulos H. Detection and quantitation of nitrotyrosine residues in proteins: in vivo marker of peroxynitrite. Methods Enzymol. 1996;269:185–194. doi: 10.1016/s0076-6879(96)69020-x. [DOI] [PubMed] [Google Scholar]
- 6.Denlinger L C, Garis K A, Sommer J A, Guadarrama A G, Proctor R A, Bertics P J. Nuclear translocation of NF-κB in lipopolysaccharide-treated macrophages fails to correspond to endotoxicity: evidence suggesting a requirement for a gamma interferon-like signal. Infect Immun. 1998;66:1638–1647. doi: 10.1128/iai.66.4.1638-1647.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dimmeler S, Zeiher A M. Nitric oxide and apoptosis: another paradigm for the double-edged role of nitric oxide. Nitric Oxide Biol Chem. 1997;1:275–281. doi: 10.1006/niox.1997.0133. [DOI] [PubMed] [Google Scholar]
- 8.Dimmeler S, Haendeler J, Nehls M, Zeiher A M. Suppression of apoptosis by nitric oxide via inhibition of ICE-like and CPP32-like proteases. J Exp Med. 1997;185:601–608. doi: 10.1084/jem.185.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Estevez A G, Radi R, Barbeito L, Shin J T, Thompson J A, Beckman J S. Peroxynitrite-induced cytotoxicity in PC12 cells: evidence for an apoptotic mechanism differentially modulated by neurotrophic factors. J Neurochem. 1995;65:1543–1550. doi: 10.1046/j.1471-4159.1995.65041543.x. [DOI] [PubMed] [Google Scholar]
- 10.Galanos C, Freudenberg M A, Katschinski T, Salomao R, Mossmann H, Kumazawa Y. Tumor necrosis factor and host response to endotoxin. In: Ryan J L, Morrison D C, editors. Bacterial endotoxic lipopolysaccharides. Vol. 2. Boca Raton, Fla: CRC Press; 1992. pp. 75–104. [Google Scholar]
- 11.Galanos C, Freudenberg M A, Reutter W. Galactosamine-induced sensitization to the lethal effects of endotoxin. Proc Natl Acad Sci USA. 1979;76:5939–5943. doi: 10.1073/pnas.76.11.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutierrez H H, Pitt B R, Schwarz M, Watkins S C, Lowenstein C, Caniggia I, Chumley P, Freeman B A. Pulmonary alveolar epithelial inducible NO synthase gene expression: regulation by inflammatory mediators. Am J Physiol. 1995;268:L501–L508. doi: 10.1152/ajplung.1995.268.3.L501. [DOI] [PubMed] [Google Scholar]
- 13.Hattori Y, Szabo C, Gross S S, Thiermermann C, Vane J R. Lipid A and the lipid A analogue anti-tumour compound ONO-4007 induce nitric oxide synthase in vitro and in vivo. Eur J Pharmacol. 1995;291:83–90. doi: 10.1016/0922-4106(95)90128-0. [DOI] [PubMed] [Google Scholar]
- 14.Johnson M L, Billiar T R. Roles of nitric oxide in surgical infection and sepsis. World J Surg. 1998;22:187–196. doi: 10.1007/s002689900368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamisaki Y, Wada K, Ataka M, Yamada Y, Nakamoto K, Ashida K, Kishimoto Y. Lipopolysaccharide-induced increase in plasma nitrotyrosine concentrations in rats. Biochim Biophys Acta. 1997;28:24–28. doi: 10.1016/s0925-4439(97)00052-5. [DOI] [PubMed] [Google Scholar]
- 16.Kim Y-M, Bergonia H, Lancaster J R. Nitrogen oxide-induced autoprotection in isolated rat hepatocytes. FEBS Lett. 1995;374:228–232. doi: 10.1016/0014-5793(95)01115-u. [DOI] [PubMed] [Google Scholar]
- 17.Kim Y-M, de Vera M E, Watkins S C, Billiar T R. Nitric oxide protects cultured rat hepatocytes form tumor necrosis factor-alpha-induced apoptosis by inducting heat shock protein 70 expression. J Biol Chem. 1997;272:1402–1411. doi: 10.1074/jbc.272.2.1402. [DOI] [PubMed] [Google Scholar]
- 18.Kroncke K-D, Fehsel K, Kolb-Bachofen V. Nitric oxide: cytotoxicity versus cytoprotection—how, why, when and where? Nitric Oxide Biol Chem. 1997;1:107–120. doi: 10.1006/niox.1997.0118. [DOI] [PubMed] [Google Scholar]
- 19.Lehman V, Freudenberg M A, Galanos C. Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and d-galactosamine-treated mice. J Exp Med. 1987;163:657–663. doi: 10.1084/jem.165.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leist M, Gantner F, Bohlinger I, Tiegs G, Germann P G, Wendel A. Tumor necrosis factor-induced hepatocyte apoptosis precedes liver failure in experimental murine shock models. Am J Pathol. 1995;146:1220–1234. [PMC free article] [PubMed] [Google Scholar]
- 21.Lin K, Xue J Y, Nomen M, Spur B, Wong P Y. Peroxynitrite-induced apoptosis in HL-60 cells. J Biol Chem. 1995;270:16487–16490. doi: 10.1074/jbc.270.28.16487. [DOI] [PubMed] [Google Scholar]
- 22.Lyons R C, Orloff G J, Cunningham J M. Molecular cloning and functional expression of an inducible nitric oxide synthase from a murine macrophage cell line. J Biol Chem. 1992;267:6370–6374. [PubMed] [Google Scholar]
- 23.Milano S, Arcoleo F, D’Agostino P, Cillari E. Intraperitoneal injection of tetracyclines protects mice from lethal endotoxemia downregulating inducible nitric oxide synthase in various organs and cytokine and nitrate secretion in blood. Antimicrob Agents Chemother. 1997;41:117–121. doi: 10.1128/aac.41.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morikawa A, Sugiyama T, Kato Y, Koide N, Jiang G Z, Takahashi K, Tamada Y, Yokochi T. Apoptotic cell death in the response of d-galactosamine-sensitized mice to lipopolysaccharide as an experimental endotoxic shock model. Infect Immun. 1996;64:737–738. doi: 10.1128/iai.64.3.734-738.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morikawa A, Kato Y, Sugiyama T, Koide N, Kawai M, Fukada M, Yoshida T, Yokochi T. Altered expression of constitutive type and inducible type heat shock proteins in response of d-galactosamine-sensitized mice to lipopolysaccharide as an experimental endotoxic shock model. FEMS Immunol Med Microbiol. 1998;21:37–45. doi: 10.1111/j.1574-695X.1998.tb01147.x. [DOI] [PubMed] [Google Scholar]
- 26.Numata M, Suzuki S, Miyazawa N, Miyashita A, Nagashima Y, Inoue S, Kaneko T, Okubo T. Inhibition of inducible nitric oxide synthase prevents LPS-induced acute lung injury in dogs. J Immunol. 1998;160:3031–3037. [PubMed] [Google Scholar]
- 27.Pryor W, Squadrito G. The chemistry of peroxynitrite: A product from the reaction of nitric oxide with superoxide. Am J Physiol. 1995;268:L699–L722. doi: 10.1152/ajplung.1995.268.5.L699. [DOI] [PubMed] [Google Scholar]
- 28.Salgo M G, Squadrito G L, Pryor W A. Peroxynitrite causes apoptosis in rat thymocytes. Biochem Biophys Res Commun. 1995;215:1111–1118. doi: 10.1006/bbrc.1995.2578. [DOI] [PubMed] [Google Scholar]
- 29.Sandoval M, Ronzio R A, Muanza D N, Clark D A, Miller M J S. Peroxynitrite-induced apoptosis in epithelial (T84) and macrophage (RAW 264.7) cell lines: effect of legume-derived polyphenols (phytolens) Nitric Oxide Biol Chem. 1997;1:476–483. doi: 10.1006/niox.1997.0160. [DOI] [PubMed] [Google Scholar]
- 30.Sriskandan S, Moyes D, Buttery L K, Wilkinson J, Evans T J, Polak J, Cohen J. The role of nitric oxide in experimental murine sepsis due to pyrogenic exotoxin A-producing Streptococcus pyogenes. Infect Immun. 1998;65:1767–1772. doi: 10.1128/iai.65.5.1767-1772.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Szabo C, Ohshima H. DNA damage induced by peroxynitrite: subsequent biological effects. Nitric Oxide Biol Chem. 1997;1:373–385. doi: 10.1006/niox.1997.0143. [DOI] [PubMed] [Google Scholar]
- 32.Szabo C. DNA strand breakage and activation of polyADP ribosyltransferase: a cytotoxic pathway triggered by peroxynitrite. Free Radical Biol Med. 1996;21:855–869. doi: 10.1016/0891-5849(96)00170-0. [DOI] [PubMed] [Google Scholar]
- 33.Szabo C. Alterations in nitric oxide production in various forms of circulatory shock. New Horiz. 1995;3:2–32. [PubMed] [Google Scholar]
- 34.Wang J F, Gao Y Q, Lippton H, Hyman A, Spitzer J J. The roles of nitric oxide and hydrogen peroxide production in lipopolysaccharide-induced intestinal damage. Shock. 1994;2:185–191. doi: 10.1097/00024382-199409000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Wizemann T M, Gardner C R, Laskin J D, Quinones S, Durham S K, Goller N L, Ohnishi S T, Laskin D L. Production of nitric oxide and peroxynitrite in the lung during acute endotoxemia. J Leukoc Biol. 1994;56:759–768. doi: 10.1002/jlb.56.6.759. [DOI] [PubMed] [Google Scholar]
- 36.Yu S M, Wu J F, Lin T L, Kuo S C. Inhibition of nitric oxide synthase expression by PPM-18, a novel anti-inflammatory agent, in vitro and in vivo. Biochem J. 1997;328:363–369. doi: 10.1042/bj3280363. [DOI] [PMC free article] [PubMed] [Google Scholar]