Abstract
Background
The endocannabinoid 2-Arachidonyl glycerol (2-AG) exerts dose-related anti-nociceptive effects, which are potentiated by the related but inactive 2-palmitoyl glycerol (2-PG) and 2-linoleoyl glycerol (2-LG). This potentiation of analgesia and other in vivo measures was described as the “entourage effect”. We investigated this effect on TRPV1 signalling in cultured dorsal root ganglion (DRG) nociceptors.
Methods
Adult rat DRG neurons were cultured in medium containing NGF and GDNF at 37°C. 48 h later cultures were loaded with 2 µM Fura2AM for calcium imaging, and treated with 2-AG, 2-PG and 2-LG, individually or combined, for 5 min, followed by 1 µMol capsaicin. The amplitude and latency of capsaicin responses were measured (N=3–7 rats, controls N=16), and analysed.
Results
In controls, 1 µMol capsaicin elicited immediate calcium influx in a subset of neurons, with average latency of 1.27 ± 0.2 s and amplitude of 0.15 ± 0.01 Units. 2-AG (10–100 µMol) elicited calcium influx in some neurons. In the presence of 2-AG (0.001–100 µMol), capsaicin responses were markedly delayed in 64% neurons by up to 320 s (P<0.001). 2-PG increased capsaicin response latency at 0.1 nMol-100 µMol (P<0.001), in 60% neurons, as did 2-LG at 0.1–100 µMol (P<0.001), in 76% neurons. Increased capsaicin response latency due to 2-AG and 2-PG was sensitive to the CB2 but not to the CB1 receptor antagonist. Combined application of 1 µMol 2-AG, 5 µMol 2-PG and 10 µMol 2-LG, also resulted in significantly increased capsaicin response latency up to 281.5 ± 41.5 s (P<0.001), in 96% neurons, that was partially restored by the CB2, but not the CB1 antagonist.
Conclusion
2-AG, 2-LG and 2-PG significantly delayed TRPV1 signalling in the majority of capsaicin-sensitive DRG neurons, that was markedly increased following combined application. Further studies of these endocannabinoids are required to identify the underlying mechanisms.
Keywords: entourage effect, endocannabinoids, DRG neurons, nociception, analgesia
Plain Language Summary
Cannabinoids are compounds derived from the cannabis plant, that are recognized to have a variety of beneficial medicinal properties, especially pain relief. These compounds have been studied extensively for treating chronic pain, which affects 1 in 15 individuals worldwide. The analgesic drugs available currently do not provide adequate pain relief and have several undesirable side effects. In this study we have examined the cannabinoid substances produced within the body itself, called endocannabinoids, for their pain relieving properties. These are 2-Arachidonyl glycerol (2-AG), 2-Palmitoyl glycerol (2-PG) and 2-linoleoyl glycerol (2-LG), that have been found to be produced following tissue injury. We have assessed their individual and additive effects in blocking responses to painful stimuli by measuring their ability to block responses to capsaicin (the hot ingredient of chilli peppers), applied to sensory nerve cells (neurons) grown in a dish. Our findings suggest that the pain signals generated by capsaicin application in untreated neurons, are blocked for several minutes in the presence of individually applied 2-AG, 2-PG and 2-LG, in a large proportion of capsaicin-sensitive neurons. Further, this effect was found to be additive, as a greater proportion of neurons were blocked when all three endocannabinoids were applied together. This effect is likely to prevent or significantly reduce the pain signals from being transmitted to the spinal cord and brain and being perceived as pain. Our findings increase understanding of underlying pain mechanisms, and may lead to the development of novel drugs for treating chronic pain.
Introduction
The endocannabinoid system comprised of the cannabinoid receptors CB1 and CB2, their ligands, and their metabolic enzymes influences several physiological processes including long-term potentiation (LTP), and analgesia.1 The endocannabinoid 2-arachidonoylglycerol (2-AG), was identified in the canine gut,2,3 brain,4,5 mouse neuroblastoma cells,6 and found to be present in the brain at 170 times the level of the other endocannabinoid anandamide.
2-AG isolated from the spleen, brain and gut is accompanied by several 2-acyl-glycerol esters, two major ones being 2-linoleoyl-glycerol (2-LG) and 2-palmitoyl-glycerol (2-PG), that are reported not to bind to cannabinoid receptors, nor inhibit adenylyl cyclase via CB1 or CB2.3,7 2-AG evokes a tetrad of behaviours in mice, comprising inhibition of motor behaviour, immobility on a ring, analgesia on a hot plate and hypothermia, similar to the tetrad of effects elicited by the phytocannabinoid ∆9 Tetrahydrocannabinol (THC).3,8 The effects caused by 2-AG were reported to be significantly potentiated by the two esters 2-PG and 2-LG, and the potentiation was described as the entourage effect.3,7
While the entourage effect is likely to be CNS mediated, the role of peripheral nociceptors in the analgesic component requires clarification. Neurons in the dorsal root ganglia (DRG), are primary sensory neurons, that detect and relay a variety of modality specific stimuli from their environment, to the CNS for perception. This is a heterogeneous neuronal population comprised of subgroups expressing a variety of receptors and neuropeptides. These include polymodal nociceptors that detect noxious stimuli, via the activation of specific receptors, leading to the perception of pain. The Transient receptor potential vanilloid subtype 1 (TRPV1, previously known as VR1) ion channel belongs to the TRP superfamily of receptors,9 that is involved in the detection of noxious stimuli.10,11 TRPV1 is highly expressed in DRG neurons, especially the small diameter nociceptive neurons,12–14 and the expression and sensitivity of TRPV1 are increased in clinical conditions of chronic pain.15–18 TRPV1 is activated by noxious stimuli, such as heat (temperatures above 43°C), and capsaicin, the pungent ingredient of chilli peppers. Its sensitivity is enhanced in the presence of the key neurotrophic factors, nerve growth factor (NGF),12,14,19 and glial cell line-derived neurotrophic factor (GDNF).20 The endogenous activators of TRPV1 include lipoxygenase products, 12- and 15-(S)-hydroperoxyeicosatetraenoic acids, 5- and 15-(S)-hydroxyeicosatetraenoic acids, and leukotriene B4.21 The endocannabinoids 2-AG and anandamide also activate TRPV1 expressed by peptidergic sensory nerve endings in blood vessels, to mediate vasodilation.22–24
Cannabinoid agonists are known to act via the G-protein coupled receptors (GPCR) CB1 and CB2, to influence pain signalling via intracellular mechanisms. Ligand binding at CB1 and CB2 receptors, inhibits the activity of the intracellular effector adenylyl cyclase via pertussis toxin (PTX)-sensitive Gi/Go-subunits.3,25 The resulting reduction in intracellular cAMP, leads to diminished neuronal responses,26,27 as cAMP is essential for maintaining the phosphorylation and sensitivity of TRPV1.28,29 Thus, TRPV1 modulation by cannabinoids in cultured adult rat DRG neurons can provide a measure of their analgesic effect. Cyclosporine is the calcineurin (phosphatase) inhibitor. Following calcium influx, the levels of intracellular calcineurin are elevated, leading to TRPV1 dephosphorylation and desensitization. In the presence of cyclosporine, calcineurin is inhibited and TRPV1 remains sensitized.28 Cyclosporine is thus used to determine if the mechanism of 2-AG mediated TRPV1 desensitization is due to dephosphorylation.
Information regarding the synthesis of the 2 acyl glycerols is available, though their physiological roles are partly understood. Receptor stimulation leads to hydrolysis of plasma membrane bound phosphatidylinositol 4,5-bisphosphate (PIP2), with the formation of diacylglycerol (DAG), and inositol triphosphate (IP3).30 DAG is converted to 2-AG by the action of diacylglycerol lipase (DAGL). 2-AG can be synthesized via activation of a wide variety of receptors including G protein coupled receptors (GPCRs), and growth factor receptors.31 Phospholipase C (PLC) activation with bradykinin and ATP, also results in the generation of 2-AG in the DRG.23 There is evidence that calcium influx leads to 2-AG synthesis in N18TG2 cells, without PLC activation.32 2-AG is also formed in hippocampal neurons following high frequency stimulation, in the presence of calcium, and sensitive to the presence of tetrodotoxin.5 The digestion of dietary triacylglycerols in the intestine, results in the formation of 2-AG which plays a major role in feeding and gut motility.33 Similarly, 2-AG, 2-LG and 2-OG were reported to be synthesized in TRPV1 transfected HEK cells following heat and capsaicin stimulation,34 indicating a role in nociception. Further, as 2-AG can elicit calcium transients in transfected CHO cells, via PLC coupled signalling,35 it is possible that cannabinoid mediated calcium influx can contribute to neuronal desensitization, similar to the desensitizing effects of capsaicin,12,36 and the phytocannabinoids CBD, CBG and THC.37
In this study we have used a previously established in vitro model of neuronal sensitization, of DRG neurons cultured in the presence of the key neurotrophic factors (NTFs), nerve growth factor (NGF) and glial cell-line derived neurotrophic factor (GDNF).14,26 The model is based on the role of NGF and GDNF in maintaining the nociceptive phenotype of sensory neurons,38–41 the increased levels of NGF and GDNF in injured human peripheral nerves and ganglia,39,42 and in tissues from clinical conditions of pain.43–45 Further, local and systemic administration of NGF induced thermal and mechanical hyperalgesia,40,46 and NGF and GDNF treatment sensitized cultured DRG neurons.14,20 In this study, we report the modulatory effects of the endocannabinoids 2-AG, 2-LG and 2-PG on capsaicin responses, in a functional assay, using calcium imaging.
Materials and Methods
Neuronal Cultures
Bilateral DRG from all levels were micro-dissected from freshly euthanized adult female Wistar rats (Charles River UK Ltd, Margate, Kent, UK), with approvals from the Animal Welfare Ethical Review Body, Imperial College, and following UK Home Office approved procedures, in keeping with the 3Rs ARRIVE guidelines, and the Animal Scientific Procedures Act (ASPA 1986). DRG were collected in Ham’s F12 medium containing penicillin and streptomycin (100 µg/mL each), and enzyme digested in Ham’s F12 medium containing 0.2% collagenase and 0.5% dispase for 3 hours at 37°C, as previously described.26 Enzyme digested tissue was triturated in modified BSF2 medium [containing 2% HIFCS, 0.1 mg/mL transferrin, 60 ng/mL progesterone, 0.16 μg/mL sodium selenite, 3 mg/mL bovine serum albumin (BSA), penicillin/streptomycin 100 μg/mL each, 16 μg/mL putrescine, 10 μg/mL insulin], soybean trypsin inhibitor and DNAse to obtain a neuronal suspension. 150,000–200,000 neurons were obtained from each rat, and diluted in medium; 200 µL cell suspension containing 8000–10,000 neurons was plated in the central well of 15–20 glass bottom MatTek dishes (MatTek Corp, USA), coated with poly-l-lysine and laminin (20 μg/mL each). 40 minutes later after the cells had attached, 2 ml BSF2 medium supplemented with 100 ng/mL of NGF and 50 ng/mL GDNF were added to all culture dishes and incubated at 37°C in a humidified environment of 5% CO2 in air. 24 hours later 5 µMol Ara C was added to all dishes to eliminate non-neuronal cells.
Functional Studies
48 hours after plating, calcium imaging was used to determine the effect of acute 2-AG, 2-LG or 2-PG application on the capsaicin sensitivity of DRG neurons loaded with 2 µmol/L Fura2 AM (Life Technologies, Paisley, UK). Studies were conducted in HEPES buffered phenol-red free Hanks Balanced Salt Solution (HBSS) containing 0.1% Bovine Serum Albumin (BSA), at 37°C in a humidified environment. Individual cells under study were highlighted as regions of interest for measuring the mean ratios of bound to unbound calcium within the area of interest. Neuron cultures were alternately excited at 340 and 380 nm λex wavelengths on an inverted Nikon microscope (Diaphot 300; Nikon, UK Ltd, Kingston upon Thames, Surrey, UK). Images of 15−20 neurons in each experiment were captured every 2 seconds in each of three channels − brightfield, 340 and 380 nm λex/510 λem, and recordings of mean intracellular changes in bound/unbound Ca2+ ratio were obtained before, during and after the addition of cannabinoids and capsaicin. This provided baseline recordings as well as intracellular changes in Ca2+ levels in response to added compounds. Responses to capsaicin stimuli, were measured as the maximum change in the 340/380 λex nm ratio from baseline in individual neurons. Cells were uniformly loaded with the dye and no intracellular compartmentalization of the loaded dye was observed. Images were acquired with a Hamamatsu Orca CCD Camera and analysed with AQM Advance Kinetic imaging software.
The effect of 2-acyl glycerols on TRPV1 activation was determined by applying 2-PG, 2-LG, 2-AG, individually or combined in a 5:10:1 ratio respectively, 5 minutes prior to capsaicin application. To identify the mechanism of action of the 2-acyl glycerols, after the capsaicin response, the medium was washed out, and the neurons were allowed to rest for 45 minutes, to recover from capsaicin-induced desensitization. This was followed by application of the CB1 or CB2 antagonist, or the MAGL inhibitor JZL184, or cyclosporine, followed by the same 2-acyl glycerol applied previously, and capsaicin after 5 min. Control capsaicin responses were obtained in the presence of vehicle (0.1% DMSO), and characterized by a rapid increase in 340/380 ratio from the baseline, sustained for more than 13 minutes. The amplitudes of capsaicin responses were measured in individual neurons as the difference between baseline and peak response more than 20% from the baseline. Latency was measured as the time taken for increase in 340/380 ratio after addition of capsaicin, for each condition, averaged and normalized to the control average. Data are presented as mean ± s.e.m., and the Mann−Whitney test in Graphpad Prism software was used for statistical analysis, for comparison between rats. *P<0.05 was considered statistically significant, **P<0.01, and ***P<0.001. “N” indicates the number of rats, and “n” the number of neurons from which data are derived.
2-Arachidonyl glycerol (#1298, Tocris Bioscience, UK), and 2-Palmitoyl glycerol (Cayman Chemicals #17882), were prepared as 100 mMol/L stock solutions in DMSO. 2-Linoleoyl-glycerol (#62260, Cayman, UK), was obtained as a 15 mMol/L solution in acetonitrile. The acetonitrile was evaporated under nitrogen gas, as instructed by the manufacturer and reconstituted to 30 mM in DMSO. Capsaicin (Sigma, UK), was dissolved in ethanol at 100 mMol/L concentration. All stock solutions were aliquoted and stored at −20°C. Intermediate cannabinoid dilutions were freshly prepared at 1000x final concentration in DMSO, and capsaicin at 500x in ethanol. JZL184 was purchased from Tocris (#3836). CB1 receptor antagonist (SR141716 Rimonabant #SML0800), CB2 receptor antagonist (SR144528 #SML1899), and all other chemicals were obtained from Sigma-Aldrich UK unless otherwise stated.
Results
Vehicle Controls
In vehicle treated neurons, 1 μMol capsaicin elicited immediate and sustained calcium influx, with average latency (time to increase in baseline after adding capsaicin) of 1.86 ± 0.2 seconds, and amplitude (340/380 ratio change measured as the difference between peak response and baseline), of 0.16 ± 0.01. The elevated intracellular calcium was maintained for up to 13 minutes (Figure 1A).
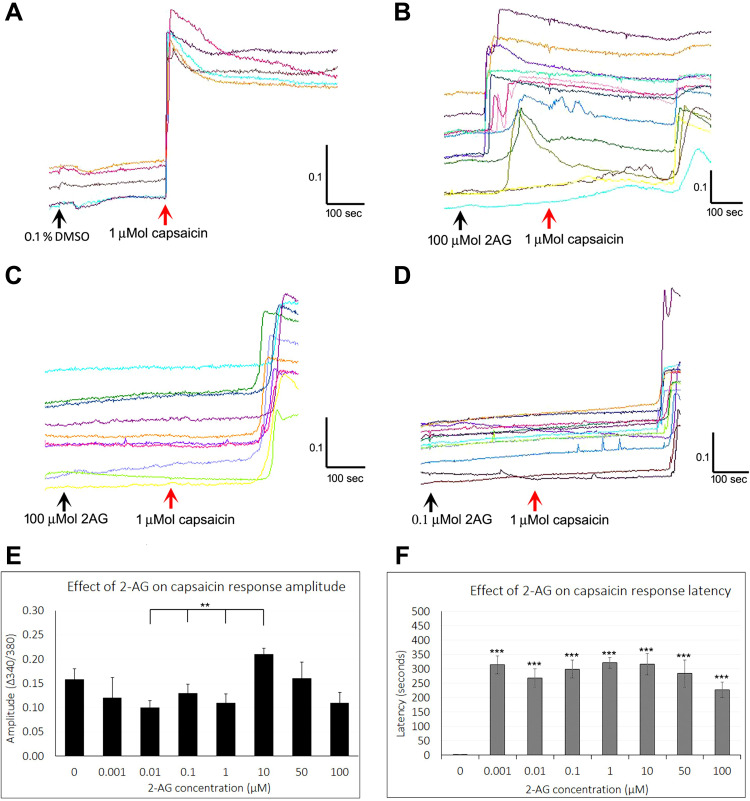
Figure 1.
Effect of 2-AG on capsaicin responses. Sample traces from individual neurons showing stable baseline in the presence of vehicle, and rapid increase in intracellular calcium, sustained over several minutes, in response to application of 1 µMol capsaicin (A). Similar traces showing calcium influx in response to added 100 µMol 2-AG, followed by delayed response to capsaicin (B). Delayed capsaicin responses in the presence of 100 µMol 2-AG (C), and 0.1 µMol 2-AG (D). Black arrows indicate application of vehicle (A), or 2-AG (B–D), and red arrow indicates capsaicin application. Scale bars indicate time in seconds (X axis), and 340/380 calcium ratio for response amplitude (Y axis). Graph showing capsaicin response amplitude in the presence of 2-AG at a range of concentrations (n.s. compared with control, but significantly different at 0.01, 0.1 and 1 µMol 2-AG compared with the response amplitude at 10 µMol 2-AG (**P<0.01)) (E). Graph showing highly significant delay in capsaicin responses by several minutes in the presence of 2-AG at all the concentrations tested (***P<0.001) (F).
Effect of 2-AG
Application of 2-AG at 0.1, 1, 5, 10 µMol concentrations resulted in occasional short transient spikes in some neurons. More sustained calcium influx was observed in some neurons, with a delay after adding 2-AG at 5, 10, 25 and 100 µMol. Sample traces of 2-AG mediated calcium influx are shown in Figure 1B, and data are summarized in Table 1 and Figure 2A.
Table 1.
2-AG Mediated Calcium Influx
| Concentration | µMol | 1 µMol Capsaicin | 25 µMol 2-AG | 50 µMol 2-AG | 100 µMol 2-AG |
|---|---|---|---|---|---|
| Amplitude | Mean ± sem | 0.16 ± 0.01 | 0.089 ± 0.005 | 0.11 ± 0.03 | 0.15 ± 0.02 |
| N | Rats | 16 | 4 | 3 | 7 |
| Total no. | Neurons | 176 | 19 | 21 | 52 |
Notes: Table showing amplitude of responses to 2-AG compared with responses to 1 µM capsaicin.
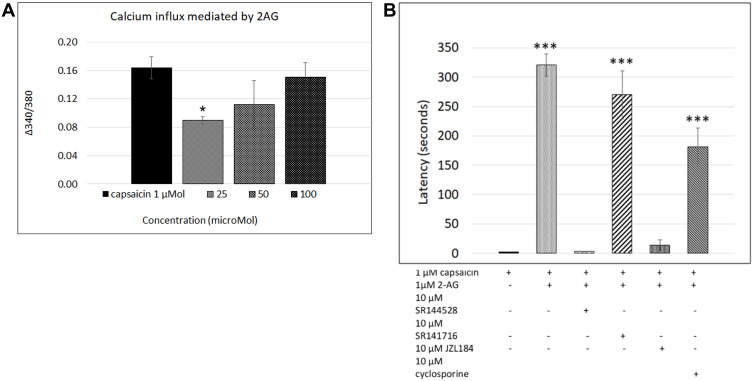
Figure 2.
2-AG mediated calcium influx. Graph showing responses to 1 µMol capsaicin (1st bar), compared with dose-related responses to application of 2-AG (bars 2, 3, 4) *P<0.05 (A). Graph showing latency of capsaicin responses in control neurons (1st bar, N= 16), and its significant increase due to 2-AG (2nd bar, N=10). This increased latency was sensitive to the CB2 receptor antagonist (3rd bar, N= 3), but not to CB1 receptor antagonist (4th bar, N=5). The presence of the MAGL inhibitor JZL184 reversed the increased latency (5th bar, N= 3), but the phosphatase inhibitor cyclosporine had no effect (6th bar, N= 3). ***P<0.001 (B).
Capsaicin responses were diminished in neurons demonstrating calcium influx in response to 2-AG. The overall amplitude of subsequent capsaicin responses was similar to controls, and there was a significant difference in capsaicin response amplitudes in the presence of 2-AG at 0.01, 0.1 and 1 µM, compared with 10 µM 2-AG (**P<0.01) (Figure 1E). The predominant effect of 2-AG was significantly increased latency of capsaicin responses (Figure 1B–D and F) i.e. capsaicin responses were completely suppressed in the presence of 0.001, 0.01, 0.1, 1, 10, 30, 50 and 100 µMol 2-AG, for a duration of up to 320.5 seconds (5.5 minutes after adding capsaicin) (***P<0.001), after which calcium influx was observed. Capsaicin response latency was restored to control values after washout of medium, and reapplication of capsaicin. 64% capsaicin-sensitive neurons demonstrated significantly delayed responses (456/712 neurons), while the remaining neurons showed normal response latency.
The number of neurons demonstrating calcium influx in response to 2-AG, and amplitude of 2-AG mediated calcium influx increased with increasing 2-AG concentration (Figure 2A). Increased capsaicin response latency due to 2-AG was sensitive to the presence of the CB2 receptor antagonist, but not the CB1 antagonist (Figure 2B). The presence of the MAGL inhibitor JZL184 (N=4), reversed the capsaicin response delay mediated by 2-AG in 41.8% neurons, but cyclosporine (N=3) had no effect (Figure 2B). The effects of 2-AG on capsaicin response amplitude and latency are summarized in Table 2, and Figure 2B shows the effects of the CB1 and CB2 receptor antagonists, JZL184 and the calcineurin inhibitor, cyclosporine.
Table 2.
Effect of 2-AG on Capsaicin Response Latency and Amplitude
| 2-AG Concentration | µMol | 0 | 0.001 | 0.01 | 0.1 | 1 | 10 | 50 | 100 |
|---|---|---|---|---|---|---|---|---|---|
| Capsaicin Response Amplitude | 340/380 Ratio mean ± sem | 0.16 ± 0.01 | 0.14 ± 0.04 | 0.11 ± 0.01 | 0.1 ± 0.01 | 0.11 ± 0.01 | 0.21 ± 0.01 | 0.16 ± 0.03 | 0.11 ± 0.02 |
| Capsaicin Response Latency | Seconds mean ± sem | 1.77 ± 0.3 | 313.8 ± 31.5 | 268.4 ± 31.9 | 298.3 ± 31.3 | 320.5 ± 19.3 | 316.1 ± 36.7 | 283.4 ± 47 | 226.2 ± 26.3 |
| N | Rats | 16 | 4 | 4 | 4 | 10 | 4 | 4 | 9 |
| Total no. | Neurons | 176 | 41 | 45 | 40 | 99 | 43 | 38 | 97 |
Notes: Data showing increased capsaicin response latency in the presence of 2-AG from 0.001 to 100 µMol. Effects on capsaicin response amplitude were similar to control values.
Effect of 2-PG
While control neurons showed immediate and sustained responses to capsaicin (Figure 3A), capsaicin responses were delayed by several minutes in the presence of 2-PG at a range of concentrations (Figure 3B–D and F). Few neurons responded with calcium influx to 2-PG application, and the responses were either transient or sustained only for a short time, and having smaller amplitude compared with capsaicin responses (Figure 3B and C).
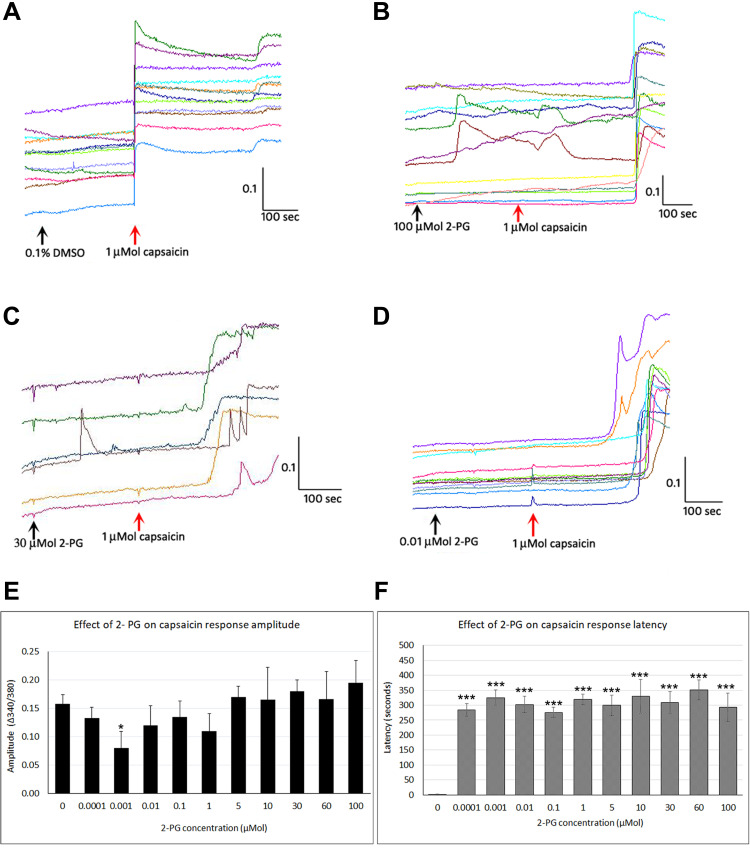
Figure 3.
Effect of 2-PG on capsaicin responses. Control neurons showing immediate responses to capsaicin application in individual neurons (red arrow) (A). Addition of 100 µMol 2-PG elicited transient calcium influx, and delayed subsequent capsaicin responses (B). Capsaicin response latency was increased in the presence of 30 µMol 2-PG (C), and 0.01 µMol 2-PG (D). Capsaicin response amplitude was significantly reduced at 0.001 µM (*P<0.05), but changes at other concentrations were not significant compared with control (E). 2-PG application significantly delayed capsaicin responses at all concentrations tested (***P<0.001) (F). Black arrows indicate application of vehicle (A) or 2-PG, and red arrow indicates point of capsaicin application (B–D). Scale bars: x axis indicates time in seconds, and y axis indicates intracellular 340/380 calcium ratio.
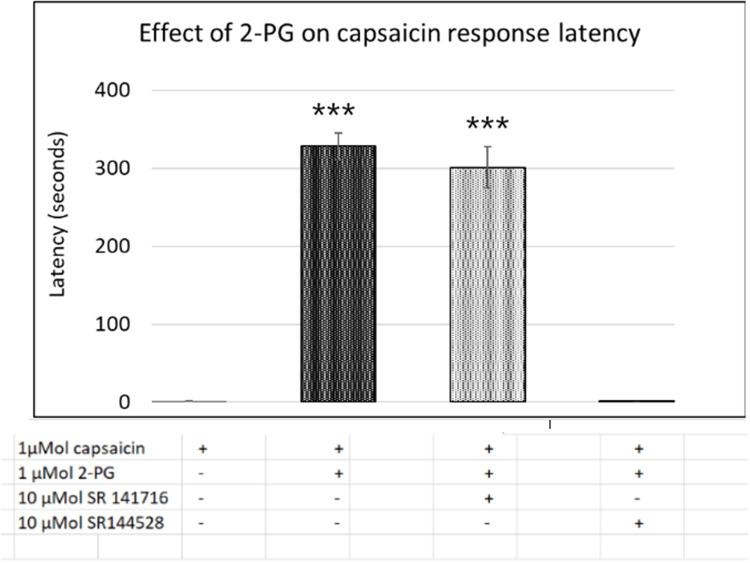
Capsaicin response amplitude in 2-PG treated neurons was largely similar to controls, except at 0.001 µMol 2-PG (*P<0.05) (Figure 3E and Table 3). 60% capsaicin-sensitive neurons demonstrated significantly delayed responses to capsaicin application (335/561 neurons), in the presence of 2-PG at 0.0001, 0.001, 0.01, 0.1, 1, 5, 10, 30, 50 and 100 µMol concentrations (Figure 3F and Table 3). Capsaicin response latency was restored after washout. 2-PG mediated delay in capsaicin responses was not affected by the CB1 receptor antagonist, but was reversed by the CB2 receptor antagonist (Figure 4).
Table 3.
Effect of 2-PG on Capsaicin Responses
| 2-PG Concentration | µMol | 0 | 0.0001 | 0.001 | 0.01 | 0.1 | 1 | 5 | 10 | 30 | 60 | 100 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Capsaicin Response Amplitude | 340/380 Ratio mean ± sem | 0.16 ± 0.01 | 0.13 ± 0.01 | 0.08 ± 0.02 | 0.12 ± 0.03 | 0.13 ± 0.02 | 0.11 ± 0.03 | 0.17 ± 0.01 | 0.16 ± 0.05 | 0.18 ± 0.01 | 0.16 ± 0.04 | 0.19 ± 0.03 |
| Capsaicin Response Latency | Seconds mean ± sem | 1.77 ± 0.3 | 284.7 ± 21 | 331.6 ± 25 | 302.6 ± 27 | 272.5 ± 17 | 328.2 ± 16 | 299.2 ± 33 | 404.9 ± 57 | 254 ± 36 | 351 ± 33 | 347 ± 47 |
| N | Rats | 16 | 3 | 3 | 4 | 5 | 6 | 7 | 6 | 6 | 3 | 6 |
| Total no. | Neurons | 176 | 19 | 16 | 26 | 49 | 35 | 88 | 66 | 70 | 42 | 68 |
Notes: Data showing the increased capsaicin response latency in the presence of 2-PG from 0.0001 to 100 µMol. Effects on capsaicin response amplitude were similar to control values.
Figure 4.
Capsaicin response latency in control neurons (1st bar, N=16), was increased in the presence of 2-PG (2nd bar, N=6). The 2-PG mediated increase in capsaicin response latency was unaffected by the CB1 receptor antagonist (3rd bar, N=5), but was reversed by the CB2 receptor antagonist (4th bar, N=6). ***P<0.001 compared with control.
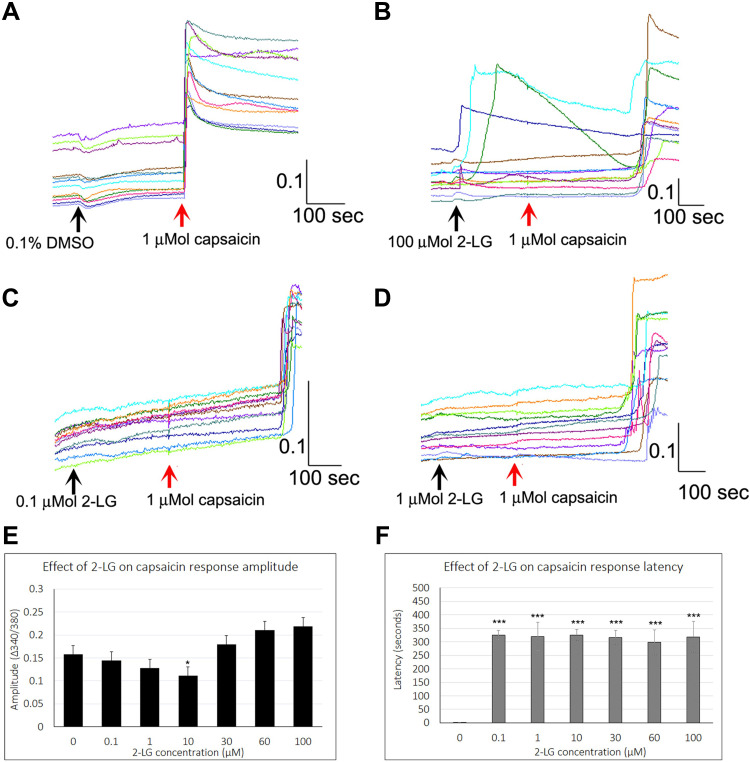
Effect of 2-LG
Control capsaicin responses in the absence of 2-LG were instantaneous, rapid and sustained (Figure 5A), while the presence of 2-LG modulated capsaicin response amplitude and latency. No calcium influx was observed with application of 0.1–10 μMol 2-LG, but 30, 60 and 100 μMol 2-LG elicited calcium influx (Figure 5B). In other neurons, capsaicin responses were significantly delayed in the presence of 2-LG at different doses (Figure 5C and D). Dose-related reduction in response amplitude was observed in the presence of 2-LG, but this was significant only at 10 µM 2-LG, and capsaicin response amplitudes increased at higher 2-LG concentrations (Figure 5E). 76% capsaicin-sensitive neurons showed delayed responses (521/688 neurons), regardless of 2-LG mediated calcium influx (Figure 5F and Table 4). 2-LG mediated delay in capsaicin responses was unaffected by the presence of the CB1 or CB2 receptor antagonists (Figure 6).
Figure 5.
Effect of 2-LG on capsaicin responses. Control neurons showing stable baseline in the presence of vehicle, with immediate and sustained responses to capsaicin in individual neurons (red arrow) (A). Addition of 100 µMol 2-LG elicited calcium influx in some neurons, and delayed subsequent capsaicin responses (B). Sample traces showing increased capsaicin response latency in the presence of 0.1 µMol 2-LG (C), and 1 µMol 2-LG (D). The amplitude of capsaicin responses was significantly reduced in the presence of 10 µM 2-LG (*P<0.05), but not at other concentrations (E). Delayed latency of capsaicin responses was highly significant (***P<0.001), at all concentrations of 2-LG tested (F). Black arrows indicate application of vehicle (A) or 2-LG, and red arrow indicates point of capsaicin application. Scale bars: x axis indicates time in seconds, and y axis indicates intracellular 340/380 calcium ratio.
Table 4.
Effect of 2-LG on Capsaicin Responses
| 2-LG Concentration | µMol | 0 | 0.1 | 1 | 10 | 30 | 60 | 100 |
|---|---|---|---|---|---|---|---|---|
| Capsaicin Response Amplitude | 340/380 Ratio mean ± sem | 0.16 ± 0.01 | 0.14 ± 0.02 | 0.12 ± 0.02 | 0.11 ± 0.02 | 0.179 ± 0.02 | 0.21 ± 0.02 | 0.21 ± 0.01 |
| Capsaicin Response Latency | Seconds mean ± sem | 1.77 ± 0.3 | 324.7 ± 18 | 319.5 ± 52 | 325 ± 21 | 316.4 ±26 | 303 ± 46 | 318 ± 57 |
| N | Rats | 16 | 4 | 6 | 10 | 6 | 4 | 4 |
| Total no. | Neurons | 176 | 37 | 35 | 100 | 76 | 54 | 47 |
Notes: Data showing the effect of 2-LG on amplitude (340/380 ratio), of capsaicin responses was similar to controls. Latency (seconds) of capsaicin responses was significantly increased in the presence of 2-LG at concentrations ranging from 0.1 to 100 µM, that was unaffected by the presence of the CB1 or CB2 antagonists.
Figure 6.
Capsaicin response latency in control neurons (1st bar, N=16), was significantly increased in the presence of 2-LG (2nd bar, N=6). The 2-LG mediated increase in capsaicin response latency was unaffected by the CB1 receptor antagonist (3rd bar, N=6), and the CB2 receptor antagonist (4th bar, N=6). ***P<0.001 compared with control.
Effect of Combined 2PG+2LG+2AG
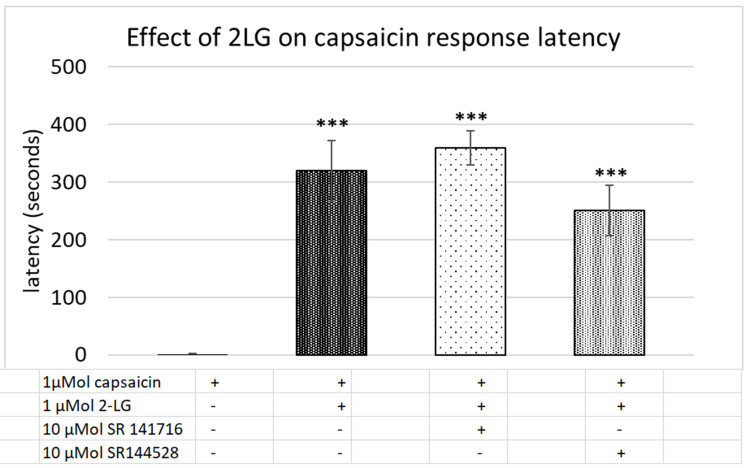
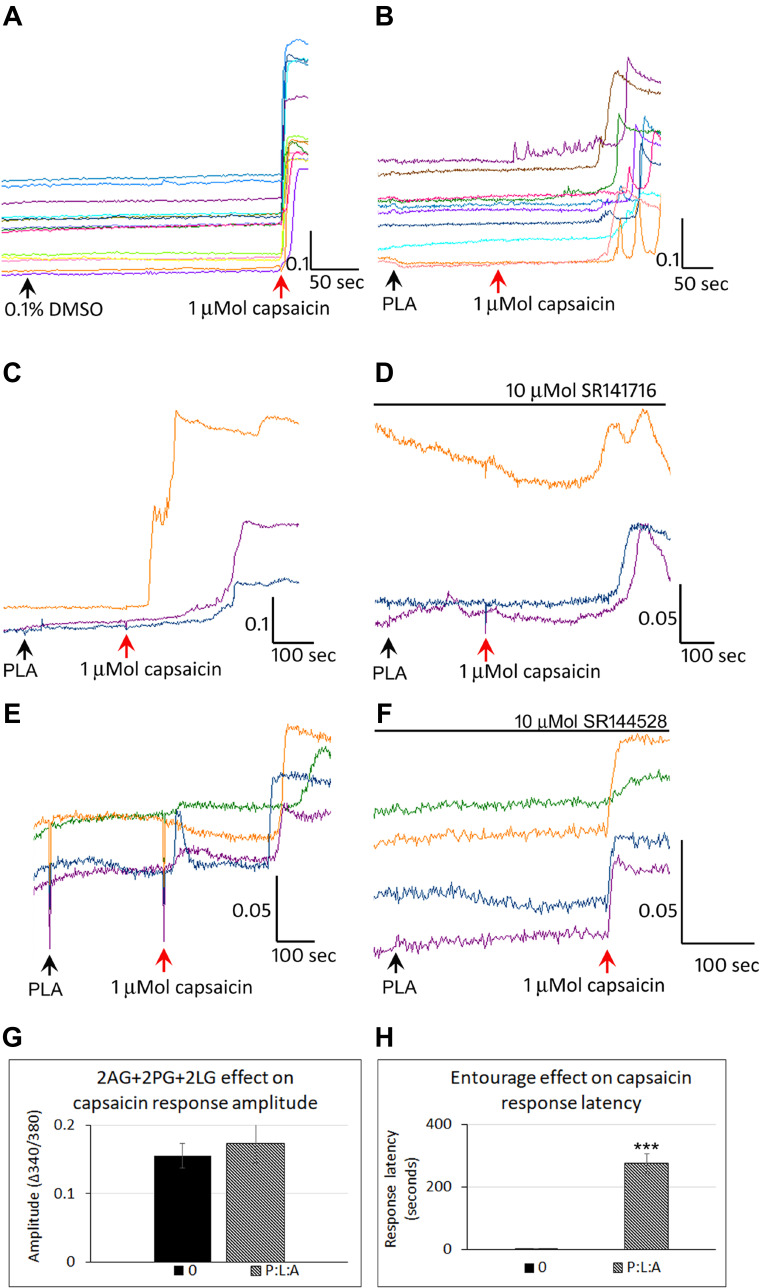
Control neurons showed a stable baseline in the presence of vehicle, and immediate responses to capsaicin application (Figure 7A). Application of combined 2-PG, 2-LG, 2-AG (PLA), in the ratio 5:10:1 (5 µMol 2-PG + 10 µMol 2-LG + 1 µMol 2-AG) had similar effects of delayed capsaicin responses, as the individually applied 2-AG, 2-PG and 2-LG (Figure 7B). Majority of capsaicin-sensitive neurons had delayed responses compared with those having normal response latency. Increased latency of capsaicin responses due to PLA (Figure 7C), was unaffected by the presence of the CB1 antagonist (Figure 7D), (N=4). PLA mediated increased latency of capsaicin responses (Figure 7E), was reversed by the CB2 antagonist in 65.7% neurons (N=3) (Figure 7F). There was no significant effect of PLA on the amplitude of capsaicin responses, compared with control (Figure 7G). The average latency of capsaicin responses was significantly increased to 274.4 seconds (N=8, ***p<0.001) in the presence of the combined PLA compared with control (Figure 7H). The combined application (PLA), affected almost all (96%) capsaicin-sensitive neurons as compared with individually applied 2-AG, 2-LG or 2-PG (Figure 8).
Figure 7.
Effect of combined PLA on capsaicin responses. Control neurons showed a stable baseline in the presence of vehicle, and immediate responses to capsaicin application (A). In the presence of combined 2-AG + 2-PG + 2-LG (PLA), capsaicin responses were delayed by several minutes (B). PLA induced delay in capsaicin responses (C), were not affected by the CB1 antagonist SR141716 (D). PLA induced delay in capsaicin responses (E), was abolished in the presence of the CB2 antagonist SR144528 (F). Graphs showing lack of effect of PLA on capsaicin response amplitude (G), and increased latency compared with control (H) (***P<0.001).
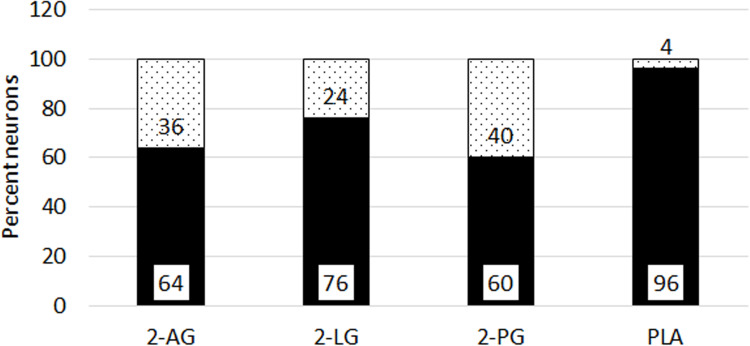
Figure 8.
Proportion of neurons with delayed responses. Graph showing percentage capsaicin sensitive neurons with delayed responses (black bars), and not delayed responses (grey bars), in the presence of individual 2-AG (N=11, 712 neurons), 2-LG (N=17, 688 neurons), and 2-PG (N=9, 561 neurons). Combined PLA (N=7, 178 neurons) showed the maximum proportion of neurons with delayed responses.
Discussion
The endocannabinoid 2-AG was reported to elicit a tetrad of behavioural effects comprising immobility, absence of spontaneous movement, hypothermia and antinociception,3,7 similar to those mediated by THC.8 The effects elicited by 2-AG were further reported to be potentiated by the related and inactive 2-PG and 2-LG, termed the entourage effect.7 This phenomenon is likely to include motor and sensory signalling pathways, with the antinociception observed in the delayed tail flick test involving peripheral sensory neurons. Hence we addressed the interaction of the 2-AG, 2-PG and 2-LG with the noxious pain receptor TRPV1, which is expressed in DRG neurons and has been extensively studied as a target for neuropathic pain.
We sought to determine the effects of the 2 acylglycerols, 2-AG, 2-PG and 2-LG applied individually and combined, on TRPV1 signalling in a model of neuronal hypersensitivity. The results of our study show that at high micromolar concentrations, 2-AG, 2-PG and 2-LG, applied individually, evoked calcium influx in a subset of DRG neurons, and reduced responses to capsaicin applied subsequently. However, in 65% capsaicin-sensitive neurons, application of 2-AG completely suppressed capsaicin responses for a duration of 4–6 minutes after application, after which responses were observed. Similar effects were observed with 2-PG delaying responses in 60% capsaicin-sensitive neurons, and with 2-LG in 76% capsaicin-sensitive neurons. A synergistic effect or additive effect of the combined PLA was observed in the increased proportion of capsaicin-sensitive neurons (96%), that demonstrated delayed responses. The effect of increased latency due to 2-AG, 2-PG and 2-LG was reversible, as capsaicin response latency was normalized after washout of medium, and in the absence of the endocannabinoids. The CB2 antagonist was effective in normalizing the capsaicin responses latency in 65.7% neurons treated with combined PLA, in agreement with reports of 2-AG being a full agonist at CB2.47 Our results reflect the interaction between the cannabinoid and TRPV1 receptors, and indicate that other mechanisms may also be responsible for the observed effects.
While previous in vivo studies found 2-LG and 2-PG to be inactive individually, their combination was reported to enhance the antinociceptive effects of 2-AG, by further delaying responses in the mouse tail flick test.3,7 We observed that 2-PG and 2-LG exerted similar effects as 2-AG, by inhibiting TRPV1 for several minutes after being applied individually, and in combination, thus delaying capsaicin responses. Our results showed that while 2-AG and 2-PG delayed capsaicin responses in similar proportions of neurons, 2-LG delayed responses in a higher proportion of neurons (76%). Combined application of PLA further increased the proportion of neurons demonstrating delayed responses to 96% capsaicin-sensitive neurons, suggesting synergistic effects between 2-AG, 2-PG and 2-LG. In our recent study, the phytocannabinoids CBG, CBD and THC when applied in combination exerted synergistic effects greater than their individual effects, on capsaicin response amplitude.37
Noxious stimuli such as heat or capsaicin are known to induce a state of diminished responsiveness to a repeated stimulus,48 that lasts for 30–40 minutes after the first stimulus. This state of desensitization, also known as tachyphylaxis, has been characterized to depend on calcium influx and elevation of intracellular protein phosphatase calcineurin.28,29,49, TRPV1 phosphorylation by cAMP dependent protein kinase A was found to be responsible for TRPV1 sensitization.50 However, in the present study the calcineurin inhibitor cyclosporine was ineffective in reversing the delayed capsaicin responses due to 2-AG. As 2-AG formation results from noxious stimulation, it is likely to contribute to tachyphylaxis, providing negative feedback, to prevent further stimulation by a different mechanism.
Our findings showed that in neurons treated with combined PLA, capsaicin response latency was restored to control values in 65.7% neurons in the presence of the CB2 antagonist, suggesting that CB2 receptors may be involved in mediating the observed effects. In the CNS, 2-AG is derived from post-synaptic neurons for retrograde inhibition of presynaptic neurons.51 However, 2-AG formation in DRG neurons follows noxious stimulation, and PLC activation, PIP2 hydrolysis and the formation of DAG and IP3,23 which are downstream of receptor activation. In DRG neurons, receptor antagonists are likely to be effective when the 2 acyl glycerols are released and exert autocrine or paracrine effects, by stimulating surface receptors.
We also found that the MAGL inhibitor JZL184 eliminated the delay in capsaicin responses due to 2-AG in 41.8% neurons, while it would be expected to enhance the effect of 2-AG by preventing its degradation. This stimulatory effect is due to the elevated levels of 2-AG by MAGL inhibition, that are likely to activate TRPV1. Similar effects were reported for anandamide due to FAAH inhibitors, and a dual FAAH-TRPV1 blocker provided antihyperalgesic effects in a model of osteoarthritis pain.52 The mono acyl glycerols (2-AG, 2-PG, 2-LG, 2-OG), are rapidly metabolized by the mono acyl glycerol lipase (MAGL), leading to the formation of arachidonic acid and glycerol, and the antinociceptive effects of MAGL inhibitors such as JZL184 have been previously described.31 The central terminals of DRG neurons express MAGL distributed in the superficial dorsal horn, where co-expression of MAGL with CGRP positive peptidergic primary afferents, is consistent with a role in regulating nociception.53 While acute MAGL inhibition elevates 2-AG levels and has antinociceptive effects, chronic inhibition significantly augmented nociceptive behaviour in models of acute somatic and visceral pain. This observation led to the conclusion that complete inhibition of MAGL activity has no beneficial effect, but leads to exacerbation of pain via desensitization of CB1 receptors.54,55
Our findings indicate interaction between TRPV1 and cannabinoid receptors, which are co-expressed in a large proportion of DRG neurons,26,56 with the potential to cause cross-desensitization. A rat model of neuropathic pain showed CB1R expression in 76–83% of nociceptive DRG neurons following spinal nerve ligation, accompanied by significant increase in the levels of both anandamide and 2-AG.57 CB1, CB2 and TRPV1 receptors are expressed in injured human DRG neurons, with 61% neurons positive for both CB2 and TRPV1, 29% for TRPV1 alone and 10% for CB2 alone. Further, co-expression of CB1 and CB2 immunostaining was present in 38.7% small/medium diameter neurons, 21.4% were CB1 positive, and 14.9 were negative for both,26 providing a point of convergence of signalling pathways involving CB1, CB2 and TRPV1.
Cannabinoid-mediated analgesia is intricately linked to TRPV1, and the expression of CB2 receptors in peripheral nerve, human neuropathic tissues, and the desensitizing effect of CB2 agonists at the TRPV1 receptor has been previously described in pain models and human tissues.26,58–62 The analgesic efficacy of 2-AG comparable to morphine, was reported in a mouse model of bone cancer pain, via a CB2R dependent mechanism.63
The diacyl glycerols (DAGs) are the most important precursors of 2-AG, formed from the hydrolysis of membrane bound phosphoinositol bisphosphate (PIP2), via phospholipase C (PLC) activation, or hydrolysis of phosphatidic acid (PA).5,32,64,65 Diacyl glycerol lipase (DAGL), metabolises DAG to form 2-AG,6,66 in a variety of cell types. In mouse neuroblastoma cells, 2-AG formation and release was observed after ionomycin induced calcium influx,6,67 and 2-AG levels were elevated by thrombin in DRG neurons.68
2-AG dose-dependently raised intracellular Ca2+ in TRPV1HEK cells and desensitized the cells to capsaicin, that was significantly enhanced by PEA.69 Our results showed similar dose-dependent increase in calcium influx in the presence of high micromolar doses of 2-AG, followed by diminished capsaicin responses. More recently, TRPV1 activation in transfected HEK cells by heat or capsaicin, resulted in the production of 2-AG, 2-LG and 2-oleoyl glycerol (2-OG).34 Thus 2-AG formation results from activation of the nociceptive signalling pathways, with the likely role of providing negative feedback, or desensitization.
Our results are in agreement with previous studies reporting 2-AG mediated inhibition of depolarization-induced calcium influx in NG108-15 cells.70 Similar inhibitory effects of 2-AG were reported in the CNS, via retrograde suppression of synaptic transmission,66 and suppression of capsaicin mediated calcium influx via CB2R in retinal ganglion neurons.71 Our findings further show that the accompanying 2-PG and 2-LG, exerted similar effects of delayed capsaicin responses as 2-AG at a wide range of concentrations, in the majority of capsaicin-sensitive neurons, and also evoked calcium influx at higher concentrations. We have previously reported similar dual effects of TRPV1 inhibition at low concentrations and its activation with high concentrations of the phytocannabinoid CBD.27 Our findings show that the endocannabinoids 2-AG, 2-PG and 2-LG exert desensitizing effects in DRG neurons, contributing to a lipid-mediated peripheral gating mechanism.72
The metabolic products of 2-AG, 2-PG and 2-LG, which are omega-3 (n-3) polyunsaturated fatty acids (PUFA – linolenic acid, arachidonic acid, oleic acid and docosahexaenoic acid) have also been shown to inhibit TRPV1 activation by endovanilloids in transfected HEK293 cells and sensory neurons.73,74 However, under inflammatory conditions, oxygenation of PUFAs by cyclooxygenase (COX), leads to formation of eicosanoids (prostaglandins) that can be pronociceptive.75 Further studies are required to determine the precise mechanisms involved.
Conclusions
The 2-acyl glycerols, 2-AG, 2-PG and 2-LG individually and combined, desensitized and delayed TRPV1 activation over a wide range of concentrations in DRG neurons, that is likely to underlie their antinociceptive effects. Combined PLA application increased the proportion of capsaicin-sensitive neurons that were desensitized, compared with individually applied 2-AG, 2-PG or 2-LG. Higher micromolar concentrations elicited calcium influx, with involvement of the CB2 receptors in DRG neurons.
Disclosure
MHS received a grant to support this research and he is a consultant at Curaleaf International. BP is Chief Scientific Officer (employee) at Curaleaf International. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors report no other conflicts of interest in this work.
References
- 1.Pertwee RG. Elevating endocannabinoid levels: pharmacological strategies and potential therapeutic applications. Proc Nutr Soc. 2014;73(1):96–105. doi: 10.1017/S0029665113003649 [DOI] [PubMed] [Google Scholar]
- 2.Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–1949. doi: 10.1126/science.1470919 [DOI] [PubMed] [Google Scholar]
- 3.Mechoulam R, Ben-Shabat S, Hanus L, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50(1):83–90. doi: 10.1016/0006-2952(95)00109-D [DOI] [PubMed] [Google Scholar]
- 4.Sugiura T, Kondo S, Sukagawa A, et al. 2Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215(1):89–97. doi: 10.1006/bbrc.1995.2437 [DOI] [PubMed] [Google Scholar]
- 5.Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates longterm potentiation. Nature. 1995;388(6644):773–778. doi: 10.1038/42015 [DOI] [PubMed] [Google Scholar]
- 6.Bisogno T, Sepe N, Melck D, Maurelli S, De Petrocellis L, Di Marzo V. Biosynthesis, release and degradation of the novel endogenous cannabimimetic metabolite 2-arachidonoylglycerol in mouse neuroblastoma cells. Biochem J. 1997;322(Pt 2):671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Shabat S, Fride E, Sheskin T, et al. An entourage effect: inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur J Pharmacol. 1998;353(1):23–31. doi: 10.1016/S0014-2999(98)00392-6 [DOI] [PubMed] [Google Scholar]
- 8.Dewey W, Harris L, Howes J, et al. Pharmacology of some marijuana constituents and two heterocyclic analogues. Nature. 1970;226:1265–1267. doi: 10.1038/2261265a0 [DOI] [PubMed] [Google Scholar]
- 9.Montell C, Birnbaumer L, Flockerzi V, et al. A unified nomenclature for the superfamily of TRP cation channels. Mol Cell. 2002;9:229–231. doi: 10.1016/S1097-2765(02)00448-3 [DOI] [PubMed] [Google Scholar]
- 10.Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487 [DOI] [PubMed] [Google Scholar]
- 11.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196 [DOI] [PubMed] [Google Scholar]
- 12.Winter J, Bevan S, Campbell EA. Capsaicin and pain mechanisms. Br J Anaesth. 1995;75:157–168. [DOI] [PubMed] [Google Scholar]
- 13.Szallasi A, Nilsson S, Farkas-Szallasi T, Blumberg PM, Hokfelt T, Lundberg JM. Vanilloid (capsaicin) receptors in the rat: distribution in the brain, regional differences in the spinal cord, axonal transport to the periphery, and depletion by systemic vanilloid treatment. Brain Res. 1995;703:175–183. doi: 10.1016/0006-8993(95)01094-7 [DOI] [PubMed] [Google Scholar]
- 14.Anand U, Otto WR, Casula MA, et al. The effect of neurotrophic factors on morphology, TRPV1 expression and capsaicin responses of cultured human DRG sensory neurons. Neurosci Lett. 2006;399(1–2):51–56. doi: 10.1016/j.neulet.2006.01.046 [DOI] [PubMed] [Google Scholar]
- 15.Apostolidis A, Brady CM, Yiangou Y, Davis J, Fowler CJ, Anand P. Capsaicin receptor TRPV1 in urothelium of neurogenic human bladders and effect of intravesical resiniferatoxin. Urology. 2005;65(2):400–405. doi: 10.1016/j.urology.2004.10.007 [DOI] [PubMed] [Google Scholar]
- 16.Akbar A, Yiangou Y, Facer P, Walters JR, Anand P, Ghosh S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008;57(7):923–929. doi: 10.1136/gut.2007.138982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yilmaz Z, Renton T, Yiangou Y, et al. Burning mouth syndrome as a trigeminal small fibre neuropathy: increased heat and capsaicin receptor TRPV1 in nerve fibres correlates with pain score. J Clin Neurosci. 2007;14(9):864–871. doi: 10.1016/j.jocn.2006.09.002 [DOI] [PubMed] [Google Scholar]
- 18.Matthews PJ, Aziz Q, Facer P, Davis JB, Thompson DG, Anand P. Increased capsaicin receptor TRPV1 nerve fibres in the inflamed human oesophagus. Eur J Gastroenterol Hepatol. 2004;16(9):897–902. doi: 10.1097/00042737-200409000-00014 [DOI] [PubMed] [Google Scholar]
- 19.Shu X, Mendell LM. Nerve growth factor acutely sensitizes the response of adult rat sensory neurons to capsaicin. Neurosci Lett. 1999;274(3):159–162. doi: 10.1016/S0304-3940(99)00701-6 [DOI] [PubMed] [Google Scholar]
- 20.Malin SA, Molliver DC, Koerber HR, et al. Glial cell line-derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J Neurosci. 2006;26(33):8588–8599. doi: 10.1523/JNEUROSCI.1726-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang SW, Cho H, Kwak J, Lee S-Y, Kang C-J, Jung J. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. PNAS. 2000;97(11):6155–6160. doi: 10.1073/pnas.97.11.6155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zygmunt PM, Petersson J, Andersson DA, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400(6743):452–457. doi: 10.1038/22761 [DOI] [PubMed] [Google Scholar]
- 23.Zygmunt PM, Ermund A, Movahed P, et al. Monoacylglycerols Activate TRPV1 – a link between Phospholipase C and TRPV1. PLoS One. 2013;8(12):e81618. doi: 10.1371/journal.pone.0081618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akerman S, Kaube H, Goadsby PJ. Anandamide acts as a vasodilator of dural blood vessels in vivo by activating TRPV1 receptors. Br J Pharmacol. 2004;142:1354–1360. doi: 10.1038/sj.bjp.0705896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howlett AC. Cannabinoid inhibition of adenylate cyclase. Biochemistry of the response in neuroblastoma cell membranes. Mol Pharmacol. 1985;27:429–436. [PubMed] [Google Scholar]
- 26.Anand U, Otto WR, Sanchez-Herrera D, et al. Cannabinoid receptor CB2 localisation and agonist-mediated inhibition of capsaicin responses in human sensory neurons. Pain. 2008;138:667–680. doi: 10.1016/j.pain.2008.06.007 [DOI] [PubMed] [Google Scholar]
- 27.Anand U, Jones B, Korchev Y, et al. CBD effects on TRPV1 signaling pathways in cultured DRG neurons. J Pain Res. 2020;13:2269–2278. doi: 10.2147/JPR.S258433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Docherty RJ, Yeats JC, Bevan S, Boddeke HW. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurons from adult rats. Pflugers Arch. 1996;431:828–837. doi: 10.1007/s004240050074 [DOI] [PubMed] [Google Scholar]
- 29.Mohapatra DP, Nau C. Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J Biol Chem. 2005;280(14):13424–13432. doi: 10.1074/jbc.M410917200 [DOI] [PubMed] [Google Scholar]
- 30.Berridge MJ, Irvine RF. Inositol phosphates and cell signalling. Nature. 1989;341:197–205. [DOI] [PubMed] [Google Scholar]
- 31.Hillard CJ. The endocannabinoid signaling system in the CNS: a primer. Int Rev Neurobiol. 2015;125:1–47. doi: 10.1016/bs.irn.2015.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bisogno T, Melck D, De Petrocellis L, Di Marzo V. Phosphatidic acid as the biosynthetic precursor of the endocannabinoid 2-arachidonoylglycerol in intact mouse neuroblastoma cells stimulated with ionomycin. J Neurochem. 1999;72:2113–2119. doi: 10.1046/j.1471-4159.1999.0722113.x [DOI] [PubMed] [Google Scholar]
- 33.DiPatrizio NV. Endocannabinoids in the gut. Cannabis Cannabinoid Res. 2016;1(1):67–77. doi: 10.1089/can.2016.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manchanda M, Leishman E, Sangani K, Alamri A, Bradshaw HB. Activation of TRPV1 by capsaicin or heat drives changes in 2-Acyl glycerols and N-acyl ethanolamines in a time, dose, and temperature dependent manner. Front Cell Dev Biol. 2021;9:611952. doi: 10.3389/fcell.2021.611952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shoemaker JL, Ruckle MB, Mayeux PR, Prather PL. Agonist directed trafficking of response by endocannabinoids acting at CB2 receptors. JPET. 2005;315:828–838. doi: 10.1124/jpet.105.089474 [DOI] [PubMed] [Google Scholar]
- 36.Anand P, Bley K. Topical capsaicin for pain management: therapeuticpotential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br J Anaesth. 2011;107(4):490–502. doi: 10.1093/bja/aer260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anand U, Oldfield C, Pacchetti B, Anand P, Sodergren MH. Dose-related inhibition of capsaicin responses by cannabinoids CBG, CBD, THC and their combination in cultured sensory neurons. J Pain Res. 2021;14:3603–3614. doi: 10.2147/JPR.S336773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molliver DC, Wright DE, Leitner ML, et al. IB-4 binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–861. doi: 10.1016/S0896-6273(00)80966-6 [DOI] [PubMed] [Google Scholar]
- 39.Bar KJ, Saldanha GJ, Kennedy AJ, et al. GDNF and its receptor component ret in injured human nerves and dorsal root ganglia. Neuroreport. 1998;9(1):43–47. doi: 10.1097/00001756-199801050-00009 [DOI] [PubMed] [Google Scholar]
- 40.Lewin GR, Ritter AM, Mendell LM. Nerve growth factor-induced hyperalgesia in the neonatal and adult rat. J Neurosci. 1993;13(5):2136–2148. doi: 10.1523/JNEUROSCI.13-05-02136.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mendell LM, Albers KM, Davis BM. Neurotrophins, nociceptors and pain, Micsrosc. Res Tech. 1999;45:252–261. doi: [DOI] [PubMed] [Google Scholar]
- 42.Anand P. Neurotrophic factors and their receptors in human sensory neuropathies. Prog Brain Res. 2004;146:477–492. [DOI] [PubMed] [Google Scholar]
- 43.Aloe L, Tuveri MA, Carcassi U, Levi-Montalcini R. Nerve growth factor in the synovial fluid of patients with chronic arthritis. Arthritis Rheum. 1992;35:351–355. doi: 10.1002/art.1780350315 [DOI] [PubMed] [Google Scholar]
- 44.Gillardon F, Eschenfelder C, Ruish RA, Zimmerman M. Increase in neuronal jun immunoreactivity and epidermal NGF levels following UV exposure of rat skin. Neuroreport. 1995;6:1322–1324. doi: 10.1097/00001756-199506090-00023 [DOI] [PubMed] [Google Scholar]
- 45.Lowe E, Anand P, Terenghi G, Williams-Chestnut RE, Sinicropi DV, Osborne JL. Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br J Urol. 1997;79:572–577. [DOI] [PubMed] [Google Scholar]
- 46.Petty BC, Cornblath VR, Adornato BT, et al. The effect of systemically administered recombinant human nerve growth factor in healthy human subjects. Ann Neurol. 1994;36:244–246. doi: 10.1002/ana.410360221 [DOI] [PubMed] [Google Scholar]
- 47.Gonsiorek W, Lunn C, Fan X, Narula S, Lundell D, Hipkin RW. Endocannabinoid 2-arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: antagonism by anandamide. Mol Pharm. 2000;57(5):1045–1050. [PubMed] [Google Scholar]
- 48.Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- 49.Cholewinsky A, Burgess GM, Bevan S. The role of calcium in capsaicin-induced desensitization in rat cultured dorsal root ganglion neurons. Neurosci. 1993;55(4):1015–1023. doi: 10.1016/0306-4522(93)90315-7 [DOI] [PubMed] [Google Scholar]
- 50.Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau IVRW. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/S0896-6273(02)00802-4 [DOI] [PubMed] [Google Scholar]
- 51.Di Marzo V. Endocannabinoid signaling in the brain: biosynthetic mechanisms in the limelight. Nat Neurosci. 2011;14:9–15. doi: 10.1038/nn.2720 [DOI] [PubMed] [Google Scholar]
- 52.Malek N, Mrugala M, Makuch W, et al. A multi-target approach for pain treatment: dual inhibition of fatty acid amide hydrolase and TRPV1 in a rat model of osteoarthritis. Pain. 2015;156(5):890–893. doi: 10.1097/j.pain.0000000000000132 [DOI] [PubMed] [Google Scholar]
- 53.Horvath E, Woodhams SG, Nyilas R, Henstridge CM, Kano M, Sakimura K. Heterogeneous presynaptic distribution of monoacylglycerol lipase, a multipotent regulator of nociceptive circuits in the mouse spinal cord. Eur J Neurosci. 2014;39(3):419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petrenko AB, Yamazaki M, Sakimura K, Kano M, Baba H. Augmented tonic pain-related behavior in knockout mice lacking monoacylglycerol lipase, a major degrading enzyme for the endocannabinoid 2-arachidonoylglycerol. Behav Brain Res. 2014;271:51–58. doi: 10.1016/j.bbr.2014.05.063 [DOI] [PubMed] [Google Scholar]
- 55.Taschler U, Eichmann TO, Radner FPW, et al. Monoglyceride lipase deficiency causes desensitization of intestinal cannabinoid receptor type 1 and increased colonic µ-opioid receptor sensitivity. Br J Pharmacol. 2015;2015(172):4419–4429. doi: 10.1111/bph.13224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahluwalia J, Urban L, Capogna M, Bevan S, Nagy I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neurosci. 2000;100(4):685–688. doi: 10.1016/S0306-4522(00)00389-4 [DOI] [PubMed] [Google Scholar]
- 57.Mitrirattanakul S, Ramakul N, Guerrero AV, et al. Site-specific increases in peripheral cannabinoid receptors and their endogenous ligands in a model of neuropathic pain. Pain. 2006;126(1–3):102–114. doi: 10.1016/j.pain.2006.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Griffin G, Fernando SR, Ross RA, et al. Evidence for the presence of CB2-1ike cannabinoid receptors on peripheral nerve terminals. Eur J Pharmacol. 1997;339:53–61. doi: 10.1016/S0014-2999(97)01336-8 [DOI] [PubMed] [Google Scholar]
- 59.Ständer S, Schmelz M, Metze D, Luger T, Rukwied R. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J Dermatol Sci. 2005;38:177–188. doi: 10.1016/j.jdermsci.2005.01.007 [DOI] [PubMed] [Google Scholar]
- 60.Sagar DR, Kelly S, Mills PJ, O’Shaughnessey CT, Kendall DA, Chapman V. Inhibitory effects of CB1 and CB2 receptor agonists on responses of DRG neurons and dorsal horn neurons in neuropathic rats. Eur J Neurosci. 2005;22:371–379. doi: 10.1111/j.1460-9568.2005.04206.x [DOI] [PubMed] [Google Scholar]
- 61.Wotherspoon G, Fox A, McIntyre P, Colley S, Bevan S, Winter J. Peripheral nerve injury induces cannabinoid receptor 2 protein expression in rat sensory neurons. Neuroscience. 2005;135:235–245. doi: 10.1016/j.neuroscience.2005.06.009 [DOI] [PubMed] [Google Scholar]
- 62.Belvisi MG. (2008) Preclinical assessment of novel therapeutics on the cough reflex: cannabinoid agonists as potential antitussives. Lung. 2008;186(Suppl 1):S66–S69. doi: 10.1007/s00408-007-9028-8 [DOI] [PubMed] [Google Scholar]
- 63.Khasabova IA, Chandiramani A, Harding-Rose C, Simone DA, Seybold VS. Increasing 2-arachidonoyl glycerol signaling in the periphery attenuates mechanical hyperalgesia in a model of bone cancer pain. Pharmacol Res. 2011;64(1):60–67. doi: 10.1016/j.phrs.2011.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Farooqui AA, Rammohan KW, Horrocks LA. Isolation, characterization, and regulation of diacylglycerol lipases from the bovine brain. Ann N Y Acad Sci. 1989;559:25–36. doi: 10.1111/j.1749-6632.1989.tb22596.x [DOI] [PubMed] [Google Scholar]
- 65.Carrier EJ, Kearn CS, Barkmeier AJ, et al. Cultured rat microglial cells synthesize the endocannabinoid 2-arachidonylglycerol, which increases proliferation via a CB2 receptor-dependent mechanism. Mol Pharmacol. 2004;65:999–1007. doi: 10.1124/mol.65.4.999 [DOI] [PubMed] [Google Scholar]
- 66.Tanimura A, Yamazaki M, Hashimotodani Y, et al. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron. 2010;65(3):320–327. doi: 10.1016/j.neuron.2010.01.021 [DOI] [PubMed] [Google Scholar]
- 67.Murataeva N, Straiker A, Mackie K. Parsing the players: 2-arachidonoylglycerol synthesis and degradation in the CNS. Br J Pharmacol. 2014;171:1379–1391. doi: 10.1111/bph.12411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vellani V, Petrosino S, De Petrocellis L, et al. Functional lipidomics. Calcium-independent activation of endocannabinoid/endovanilloid lipid signalling in sensory neurons by protein kinases C and A and thrombin. Neuropharmacol. 2008;55(8):1274–1279. doi: 10.1016/j.neuropharm.2008.01.010 [DOI] [PubMed] [Google Scholar]
- 69.Petrosino S, Schiano Moriello A. The anti-inflammatory mediator palmitoylethanolamide enhances the levels of 2-arachidonoyl-glycerol and potentiates its actions at TRPV1 cation channels. Br J Pharmacol. 2016;173(7):1154–1162. doi: 10.1111/bph.13084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sugiura T, Waku K. 2-Arachidonoylglycerol and the cannabinoid receptors. Chem Phys Lipids. 2000;108(1–2):89–106. doi: 10.1016/S0009-3084(00)00189-4 [DOI] [PubMed] [Google Scholar]
- 71.Jo AO, Noel JM, Lakk M, et al. Mouse retinal ganglion cell signalling is dynamically modulated through parallel anterograde activation of cannabinoid and vanilloid pathways. J Physiol. 2017;595(20):6499–6516. doi: 10.1113/JP274562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Piomelli D, Sasso O. Peripheral gating of pain signals by endogenous analgesic lipids. Nat Neurosci. 2014;17(2):164–174. doi: 10.1038/nn.3612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Matta JA, Miyares RL, Ahern GP. TRPV1 is a novel target for omega-3 polyunsaturated fatty acids. J Physiol. 2007;578(2):397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morales-Lazaro SL, Llorente I, Sierra-Ramirez F, et al. Inhibition of TRPV1 channels by a naturally occurring omega-9 fatty acid reduces pain and itch. Nat Commun. 2016;7:13092. doi: 10.1038/ncomms13092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jang Y, Kim M, Hwang SW. Molecular mechanisms underlying the actions of arachidonic acid-derived prostaglandins on peripheral nociception. J Neuroinflammation. 2020;17:30. doi: 10.1186/s12974-020-1703-1 [DOI] [PMC free article] [PubMed] [Google Scholar]