Abstract
Septic arthritis is a common and feared complication of staphylococcal infections. Staphylococcus aureus produces a number of potential virulence factors including certain adhesins and enterotoxins. In this study we have assessed the roles of cytolytic toxins in the development of septic arthritis by inoculating mice with S. aureus wild-type strain 8325-4 or isogenic mutants differing in the expression of alpha-, beta-, and gamma-toxin production patterns. Mice inoculated with either an alpha- or beta-toxin mutant showed degrees of inflammation, joint damage, and weight decrease similar to wild-type-inoculated mice. In contrast, mice inoculated with either double (alpha- and gamma-toxin-deficient)- or triple (alpha-, beta-, and gamma-toxin-deficient)-mutant S. aureus strains showed lower frequency and severity of arthritis, measured both clinically and histologically, than mice inoculated with the wild-type strain. We conclude that simultaneous production of alpha- and gamma-toxin is a virulence factor in S. aureus arthritis.
Staphylococcus aureus is a major cause of bacterial infections in humans. Serious infections associated with S. aureus bacteremia are osteomyelitis, invasive endocarditis, septic arthritis, and septicemia (12, 21). In the preantibiotic era these infections were often life threatening, and even today they may give rise to death despite treatment with antibiotics. S. aureus strains can produce a number of different components that may contribute to virulence, including surface-associated adhesins, capsular polysaccharides, exoenzymes, and exotoxins. S. aureus produces five different membrane-damaging toxins, four hemolysins (alpha-, beta-, gamma-, and delta-hemolysin) and leucocidin. Alpha-toxin is a pore-forming hemolytic toxin (15) that causes membrane damage to many types of mammalian cells (4). Beta-toxin is Mg2+-dependent sphingomyelinase C, which degrades sphingomylin in the outer phospholipid layer of the erythrocyte membrane. This degradation does not lyse the cell but leaves it vulnerable to a number of other lytic agents (26). The gamma-toxin locus occurs in 99% of S. aureus strains (10). The gamma-toxin locus expresses three proteins, two class S components (HlgA and HlgC) and one class F component (HlgB). Thus, the Hlg locus can express two functional pairs of proteins, HlgA+HlgB and HlgC+HlgB, both of which display proinflammatory effects when injected into the rabbit eye vitreous humor (22, 23). Gamma-toxin has also been proposed to play a role in the pathogenesis of toxic shock syndrome (TSS) together with toxic shock syndrome toxin 1 (TSST-1), since this hemolysin is very frequently found in TSS isolates (8). Many attempts have been made to understand which components of S. aureus are of importance for the development and persistence of infection. Using an animal model of hematogenous S. aureus arthritis, we have assessed the roles of alpha-, beta-, and gamma-toxin on induction and progression of disease.
MATERIALS AND METHODS
Mice.
Male NMRI mice 5 to 8 weeks old were obtained from B&K Universal AB (Sollentuna, Sweden) and maintained in the animal facility of the Department of Rheumatology, Göteborg University, Göteborg, Sweden. They were housed 10 in each cage under standard conditions of light and temperature. They were fed standard laboratory chow and water ad libitum.
Bacterial strains.
We used S. aureus 8325-4, which is a derivative of NCTC 8325 cured of prophages (18). This strain produces significant amounts of alpha-, beta-, gamma-, and delta-hemolysin, lipase hyalonurate lyase, staphylokinase, metalloproteinase, serine proteinase, nuclease, and acid phosphatase. In contrast, production of coagulase and protein A is very low in most complex culture media. The strain does not produce any enterotoxins or TSST-1. Isogenic mutants of 8325-4 differing in hemolysin expression and used in the present study are listed in Table 1. Bacterial mutants contained combinations of mutations affecting alpha-toxin (hla::Emr), beta-toxin (hlb::φ42E), and gamma-toxin (Δhlg::Tcr). Double and triple mutants were constructed by transduction with phage 85. The hlg mutation is a deletion-substitution defective in expression of all three proteins expressed by the hlg locus (23). The production of hemolysins (alpha- and beta-hemolysin) in different mutant strains was performed by growing bacterial cultures in Todd-Hewitt medium at 37°C for 24 h. Briefly, a fixed number of cells was pelleted by centrifugation at 5,856 × g for 10 min. Each culture supernatant was titered for hemolytic activity against rabbit or sheep erythrocytes. The erythrocytes were washed and resuspended in phosphate-buffered saline (PBS) or saline to a final concentration of 1% (vol/vol). Erythrocytes were added to the culture supernatants at equal proportions, and the mixture was incubated at 37°C for 30 min followed by incubation at 4°C for 60 min. The highest dilution giving rise to hemolysis was defined as the hemolytic titer. The results are shown in Table 2 and indicate that the production of, e.g., alpha-toxin is not affected by the defective gene for beta-toxin or vice versa.
TABLE 1.
S. aureus isogenic mutants, with respect to hemolysin expression, originating from wild-type strain 8325-4 and used in the present study
| S. aureus strain | Genotype | Relevant phenotypea | Source or reference |
|---|---|---|---|
| 8325-4 | Wild type | Hla+ Hlb+ Hlg+ | 18 |
| DU1090 | hla::Emr | Hla− Hlb+ Hlg+ | 20 |
| DU1090(pDU1212) | hla::Emr (hla+) | Hla++ Hlb+ Hlg+ | 20 |
| DU5719 | hlb::φ42E | Hla+ Hlb− Hlg+ | 5 |
| DU5719(pCU1hlb+) | hlb::φ42E (hlb+) | Hla+ Hlb+ Hlg+ | 19 |
| DU5720 | hla::Emrhlb::φ42E | Hla− Hlb− Hlg+ | 5 |
| DU5942 | Δhlg::Tcr | Hla+ Hlb+ Hlg− | This study |
| DU5945 | hla::Emr Δhlg::Tcr | Hla− Hlb+ Hlg− | This study |
| DU5946 | hlb::φ42E Δhlg::Tcr | Hla+ Hlb− Hlg− | This study |
| DU5938 | hlb::φ42E hla::Emr Δhlg::Tcr | Hla− Hlb− Hlg− | This study |
Hla, alpha-hemolysin; Hlb, beta-hemolysin; Hlg, gamma-hemolysin; Hla++, overproduction of Hla.
TABLE 2.
In vitro hemolysin production by S. aureus 8325-4 and its isogenic mutants
| S. aureus strain | Hemolytic titera
|
|
|---|---|---|
| Alpha-toxin | Beta-toxin | |
| 8325-4 | 2,048 | 512 |
| DU1090 | 64 | 512 |
| DU5719 | 2,048 | 16 |
| DU5720 | 64 | 16 |
| DU5942 | 2,048 | 512 |
| DU5945 | 8 | 256 |
| DU5946 | 2,048 | 16 |
| DU5938 | 1 | 1 |
The highest dilution giving rise to lysis of rabbit (alpha-toxin) or sheep (beta-toxin) erythrocytes.
Before experiments, the bacteria were cultured on blood agar plates, containing antibiotics if required, for 24 h and then reincubated on blood agar for another 24 h. The bacteria were harvested and kept frozen at −20°C in PBS containing 5% bovine serum albumin and 10% dimethyl sulfoxide. Before administration, the bacterial solutions were thawed, washed in PBS, and adjusted to appropriate concentrations. The mice were inoculated in the tail vein with 0.2 ml of bacterial solution. Viable counts were used to check the numbers of bacteria in conjunction with each inoculation procedure. In order to test for stability of mutations in vivo after infection of animals, the colonies recovered from organs were plated on antibiotic-free agar plates and then scored for antibiotic resistance.
Experimental protocols.
Three separate in vivo experiments were performed. In the first, wild-type S. aureus 8325-4 producing alpha-, beta-, and gamma-toxin, the alpha-toxin-deficient mutant DU1090, DU1090(pDU1212 hla+), and DU5938 lacking production of alpha-, beta-, and gamma-toxin were used. Fifteen mice per group were inoculated intravenously with 2 × 107 to 3 × 107 CFU of each isogenic strain per mouse. The mice were regularly weighed and examined for arthritis and general appearance until sacrifice by cervical dislocation at 24 days after inoculation. In the second experiment, 10 mice were inoculated with 108 CFU of wild-type strain 8325-4 per mouse and 10 mice received the same amount of the triple-mutant strain DU5938. The mice were monitored for 21 days until sacrifice. In the third experiment, the mice were inoculated with 1.6 × 108 CFU of S. aureus 8325-4 per mouse (n = 13) or with mutant strains DU5938 (Hla− Hlb− Hlg−) (n = 13), DU5719 (Hla+ Hlb− Hlg+) (n = 12), DU5719(pCU1hlb+) (Hla+ Hlb+ Hlg+) (n = 12), DU5720 (Hla− Hlb− Hlg+) (n = 13), DU5942 (Hla+ Hlb+ Hlg−) (n = 13), DU5945 (Hla− Hlb+ Hlg−) (n = 13), and DU5946 (Hla+ Hlb− Hlg−) (n = 12). The mice were regularly weighed and evaluated for arthritis at regular intervals, by a blinded observer, until sacrifice. Four groups of mice were, due to clinical outcome, selected for further examination. Sera were collected at day 21 and stored at −20°C until analysis. Upon sacrifice, kidneys and one standard pair of paws (right ankle and wrist) were examined for bacterial infection. The other pair of paws was used for histopathological examination.
Clinical evaluation of arthritis.
All mice were examined individually. Limbs were inspected visually at regular intervals. Arthritis was defined as visible erythema and/or swelling of at least one joint. Clinical evaluation was carried out with a system in which macroscopic inspection yielded a score of 0 to 3 points for each limb (0, normal appearance; 1, mild swelling and/or erythema; 2, moderate swelling and erythema; 3, marked swelling and erythema). The arthritic index was constructed by adding the scores from all four limbs for each animal, as previously described (1).
Histopathologic examination.
Two standard pairs of limbs (left fore and hind) were collected from each mouse. Paraformaldehyde fixation, decalcification, paraffin embedding, and tissue cutting were performed. Tissue sections were stained with hematoxylin and eosin, and the joints were studied by a blinded observer with regard to synovial hypertrophy, defined as synovial membrane thickness of more than two cell layers (6), and cartilage and bone destruction. Histological scoring was based upon the degree of synovial hypertrophy and degradation of cartilage and/or bone. Scores were 1 point for mild, 2 points for moderate, and 3 points for severe synovial hypertrophy and joint damage.
Bacteriologic examination of infected animals.
In the third experiment, at 21 days after inoculation of S. aureus the talocrural and radiocarpal joints of the right pair of limbs were dissected aseptically, and samples were obtained by sterile sticks and cultured on agar plates for 48 h. To avoid false-positive results due to contamination, an isolate was considered positive when more than 20 S. aureus colonies were present (7). The kidneys were aseptically removed, homogenized, and diluted to appropriate concentrations in PBS. One hundred microliters of homogenate was then transferred to agar plates and incubated for 24 to 48 h at 37°C, and the number of CFU was determined. Bacteria were tested for catalase and coagulase activity as well as for antibiotic resistance, which is associated with the mutation pattern (Table 1).
Analysis of IL-6 levels.
The murine hybridoma cell line B9, which is dependent on interleukin-6 (IL-6) for growth, was used to determine the serum IL-6 levels (6, 16). B9 cells were seeded into microtiter plates (5,000 cells/well), and dilutions of the serum samples were added to the wells. After a 68-h incubation, [3H]thymidine (Radiochemical Centre, Amersham, United Kingdom) was added; 6 h later, the cells were harvested. The results were compared with a recombinant IL-6 standard. B9 cells were previously shown not to react with several recombinant cytokines, including IL-1α, IL-1β, IL-2, IL-3, IL-5, granulocyte-macrophage colony-stimulating factor, tumor necrosis factor alpha (TNF-α), and gamma interferon. There was only weak reactivity with IL-4 (16).
Statistical analysis.
Statistical evaluations were made by the Mann-Whitney U test or the chi-square test with Yates correction. All values are reported as means ± standard errors of the means.
RESULTS
Clinical course of infection.
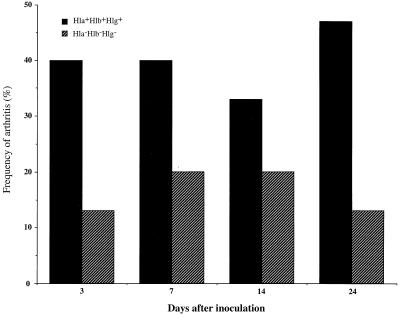
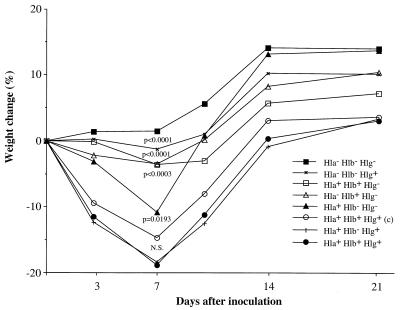
In the first experiment, wild-type S. aureus 8325-4, the isogenic alpha-hemolysin mutant DU1090, mutant DU1090 carrying the complementing hla+ plasmid pDU1212 overproducing alpha-hemolysin, and the triple-toxin-defective mutant DU5938 were used. The mice were inoculated at day 0 with 2 × 107 to 3 × 107 CFU/mouse. They were weighed at regular intervals and examined for the appearance of arthritis. Mice that were inoculated with wild-type strain 8325-4 displayed from day 7 on a significant decrease in weight (P < 0.05) compared to mice inoculated with the triple-mutant strain. The mice inoculated with either the alpha-toxin mutant or with the alpha-toxin-overexpressing strain displayed an intermediate decrease of weight compared to the other two groups. In addition, these two isogenic strains did not differ with respect to the induction or progression of arthritis (day 14, Hla− Hlb+ Hlg+, 33% of mice, versus Hla++ Hlb+ Hlg+, 20%; day 24, Hla− Hlb+ Hlg+, 20% of mice, versus Hla++ Hlb+ Hlg+, 20%). The frequency of arthritis was in general quite low. Whereas 6 of 15 mice (40%) inoculated with the wild-type strain displayed arthritis, only 3 of 15 mice (20%) receiving the triple-mutant strain did so at 1 week after inoculation (Fig. 1). Also, the severity of arthritis was more pronounced in the group inoculated with the wild-type strain than in the group inoculated with the triple mutant (day 7, 0.47 ± 0.17 versus 0.20 ± 0.11; day 24, 0.60 ± 0.19 versus 0.13 ± 0.09). To verify the importance of hemolysins in S. aureus arthritis, the experiment was repeated with a larger inoculum (108 CFU/mouse) of wild-type S. aureus and the triple mutant (n = 10/group). One mouse in the 8325-4-inoculated group died during the course of the experiment, while all of those inoculated with the mutant strain survived. The mice inoculated with the wild-type strain had significantly more pronounced weight decrease than the mice inoculated with the triple-mutant strain (P of <0.01 from day 3 to day 14; P of <0.05 at day 21). Also, the frequency and severity of arthritis were higher in mice inoculated with the wild-type strain than in mice inoculated with the triple mutant (frequency of arthritis at day 21, 89% versus 40%; severity of arthritis, 1.89 ± 0.49 versus 0.80 ± 0.33). When results from experiments 1 and 2 regarding arthritis were pooled, both the frequency and the severity of arthritis reached statistical significance (P < 0.02) at days 21 to 24. In order to assess which toxin is a virulence factor in the development and persistence of infection, we performed a third in vivo experiment with various toxin mutants. The mice were inoculated intravenously with 1.6 × 108 CFU/mouse. Two of the mice inoculated with wild-type S. aureus died during the experiment, while none of the mice inoculated with strain DU5945 (Hla− Hlb+ Hlg−), DU5946 (Hla+ Hlb− Hlg−), or DU5720 (Hla− Hlb− Hlg+) died. In each of the remaining groups, one mouse died. The mice inoculated with the wild-type strain and with the Hla+ Hlb− Hlg+ mutant strain lost significantly more weight than those inoculated with other strains within the first week of the experiment (Fig. 2). In contrast, mice inoculated with the triple mutant did not significantly change their weight (Fig. 2). One week after inoculation, the frequencies of arthritis were 69 and 67% in the wild-type-inoculated group and the pCU1 hlb+-restored DU5719-inoculated group, respectively. Mice inoculated with DU5719 (Hla+ Hlb− Hlg+) showed a frequency of arthritis of 42% at 1 week after inoculation. Within 3 weeks of inoculation of bacteria, mice in all three groups [wild-type 8325-4, DU5719(pCU1 hlb+), and DU5719] showed severe arthritis in the majority of cases (day 21, 1.09 ± 0.34, 1.46 ± 0.37, and 1.36 ± 0.34, respectively). Mice inoculated with the triple mutant and the double mutant (Hla− Hlb+ Hlg−) had frequencies of arthritis of 38 and 23%, respectively. Also, the severity of arthritis was least pronounced in mice inoculated with the Hla− Hlb+ Hlg− mutant (P of <0.05 at day 7 compared to wild-type 8325-4). The other mutants gave rise to intermediate frequency and severity of arthritis.
FIG. 1.
Frequency of arthritis after inoculation with 2 × 107 to 3 × 107 CFU of S. aureus wild-type strain 8325-4 Hla+ Hlb+ Hlg+ (n = 15) or its isogenic triple-mutant, DU5938 Hla− Hlb− Hlg− (n = 15).
FIG. 2.
Changes in body weight of mice after inoculation with S. aureus wild-type strain 8325-4 Hla+ Hlb+ Hlg+ or any of its isogenic mutants differing in hemolysin secretion patterns (12 or 13 in each group). c, the Hla+ Hlb− Hlg+ strain complemented with the wild-type hlg gene. P values refer to comparisons with the wild-type strain. N.S., not significant.
Histopathology.
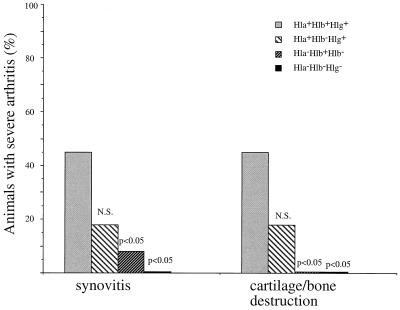
Having in mind the clinical outcome of arthritis, we decided to histopathologically analyze joints in mice inoculated with DU5938 (Hla− Hlb− Hlg−) and DU5945 (Hla− Hlb+ Hlg−) (slight weight decrease, low-level arthritis), as well as DU5719 (Hla+ Hlb− Hlg+) and wild-type 8325-4 (more-frequent arthritis, greater weight decrease). The left front and hind paws were analyzed in each mouse irrespective of the macroscopic appearance of arthritis. All of the mice inoculated with wild-type 8325-4 or DU5719 (Hla+ Hlb− Hlg+) showed signs of moderate or severe synovitis. Also, erosivity of bone and cartilage was more pronounced in these two groups. Moreover, 45% of mice inoculated with the wild-type strain, and 18% of those inoculated with DU5719 (Hla+ Hlb− Hlg+), had severe arthritis with severe destruction of cartilage and/or subcondral bone (Fig. 3). Thirty-three percent of mice inoculated with the DU5938 triple mutant and 15% of those inoculated with DU5945 (Hla− Hlb+ Hlg−) showed a total absence of synovitis or erosions. The majority of the remaining mice in these two groups showed signs of mild synovitis and/or cartilage or bone erosion. Only 8 and 15%, respectively, of these mice displayed moderate arthritis with erosivity.
FIG. 3.
Histopathological changes in joints of NMRI mice inoculated with S. aureus wild-type strain 8325-4 Hla+ Hlb+ Hlg+ or its isogenic mutants DU5945 Hla− Hlb+ Hlg−, DU5719 Hla+ Hlb− Hlg+, or DU5938 Hla− Hlb− Hlg−. P values refer to comparison with the wild-type strain. N.S., not significant. Severe synovitis and erosivity equal scores of more than 2 points, as described in Materials and Methods.
Bacterial load.
In order to assess whether the expression of toxins is of importance for the staphylococcal ability to persist in different organs, we assessed the bacterial load in kidneys and joints at 21 days after infection with S. aureus. There were no significant differences in bacterial numbers in kidneys or joints (data not shown).
The stabilities of mutants in kidney isolates were 93% for the DU5938 triple mutant and 96% for DU5945 (Hla− Hlb+ Hlg−), as assessed by antibiotic resistance patterns.
Serum IL-6.
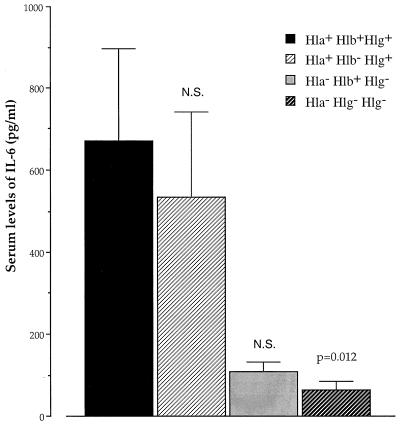
To assess the inflammatory response to infection with the staphylococcal mutants, serum IL-6 levels were analyzed at 21 days after inoculation with 1.6 × 108 CFU of S. aureus. Mice inoculated with the wild-type strain displayed significantly higher levels (P = 0.012) of IL-6 in serum than mice inoculated with the triple mutant. Mice inoculated with the DU5719 (Hla+ Hlb− Hlg+) mutant displayed almost the same levels of IL-6 as the wild-type strain (Fig. 4).
FIG. 4.
Levels of IL-6 in serum at 21 days after inoculation with 1.6 × 108 CFU of either wild-type S. aureus 8325-4 Hla+ Hlb+ Hlg+ (n = 11), DU5938 Hla− Hlb− Hlg− (n = 12), DU5945 Hla− Hlb+ Hlg− (n = 13), or DU5719 Hla+ Hlb− Hlg+ (n = 11). P values refer to comparisons with the Hla+ Hlb+ Hlg+ wild-type strain. N.S., not significant.
DISCUSSION
In the present study we have demonstrated the role of hemolysins in the pathogenesis of septic arthritis by using S. aureus mutants with different toxin production patterns. In the first experiment we concluded that alpha-toxin is of a minor importance in the induction and progression of septic arthritis, since mice had the same frequency of arthritis irrespective of the level of alpha-toxin expression by the inoculated strains. In contrast, Gemmel et al. (11) suggested recently that alpha-toxin might play a major role in the pathogenesis of septic arthritis. However, the S. aureus strains used in that study were both alpha- and beta-toxin deficient, and no control for beta-toxin alone was provided. Thus, the conclusions obtained by Gemmel et al. (11) might be premature.
We further assessed the roles of beta- and gamma-toxin. Our experiments revealed that the wild-type S. aureus strain 8325-4, as well as the DU5719(pCU1 hlb+) complemented strain, gave rise to severe arthritis in the great majority of animals and to infection-associated weight decrease. Also, the DU5719 (Hla+ Hlb− Hlg+) mutant showed a similar pattern of infection-triggered pathology. Interestingly, the Hla− Hlb+ Hlg− mutant did not give rise to severe arthritis or weight decrease. Judging from the above results, one can conclude either that concerted action of alpha- and gamma-toxin gives rise to virulence or that beta-toxin has protective properties. We believe that the first hypothesis is the most probable, since restoration of beta-toxin production in the presence of alpha- and gamma-toxin gave rise to severe arthritis and weight decrease of approximately the same magnitude as the wild-type, triple-positive strain. These clinical results were also confirmed by the fact that the mice inoculated with S. aureus DU5719 (Hla+ Hlb− Hlg+) showed greater systemic inflammation, mirrored by the levels of IL-6 (Fig. 4). IL-6 is known as an activator of osteoclasts, and its release can consequently increase damage of joints during the arthritic process (13). Mice inoculated with the triple mutant and the Hla− Hlb+ Hlg− strain showed, despite similar in vivo bacterial persistence, clearly lower levels of this cytokine in serum, indicating lesser inflammation. Indeed, these mutants gave rise to a significantly lower frequency of severe arthritis (Fig. 3).
How would alpha- and gamma-toxin contribute to the severity of arthritis? It is established that alpha-toxin promotes the adherence of neutrophils to endothelial cells (17), an important step in the early inflammatory reaction. It is also known that alpha-toxin causes the release of large amounts of IL-1β from cultured cells (3). Along with IL-6 and TNF-α, IL-1β is a proinflammatory cytokine that causes joint damage both directly, by activating osteoclasts, and indirectly, by triggering synovial macrophages to produce proinflammatory mediators, e.g., TNF-α. Alpha-toxin and gamma-toxin are pore-forming toxins. Alpha-toxin binds most efficiently to phosphatidylcholine and sphingomyelin, while gamma-hemolysin binds preferentially to phosphatidylinositol; thus, it is conceivable that these hemolysins act in similar ways, giving rise to pore formation, and that the combined action of the two hemolysins can affect cell integrity in a profound way, leading to a severe disease outcome (27). Pore-forming bacterial toxins are known to trigger the release of various inflammatory mediators, such as synovial phospholipase A2, prostaglandin I2, platelet activating factor, leukotriene B4, and nitric oxide (2, 9, 14, 24, 25). All of these mediators may contribute to the final outcome of septic arthritis.
To summarize, our findings suggest that hemolysins, especially a combination of alpha- and gamma-toxin, are important in the development and progression of S. aureus-induced arthritis.
ACKNOWLEDGMENTS
We thank Margareta Verdrengh and Theresa Hogan for excellent technical assistance.
This work was supported by grants from the Göteborg Medical Society, the Swedish Association against Rheumatism, King Gustaf V’s 80 Years Foundation, the Swedish Medical Research Council, the Nanna Svartz Foundation, the A.-G. Crafoord Foundation, the University of Göteborg, the Börje Dahlin Foundation, Wellcome Trust (grant no. 041823), and the A. M. E. Wolffs Foundation.
REFERENCES
- 1.Abdelnour A, Arvidson S, Bremell T, Rydén C, Tarkowski A. The accessory gene regulator (agr) controls Staphylococcus aureus virulence in a murine arthritis model. Infect Immun. 1993;61:3879–3885. doi: 10.1128/iai.61.9.3879-3885.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauldry S A, Wooten R E, Bass D A. Activation of cytosolic phospholipase A2 in permeabilized human neutrophils. Biochim Biophys Acta. 1996;1299:223–234. doi: 10.1016/0005-2760(95)00207-3. [DOI] [PubMed] [Google Scholar]
- 3.Bhakdi S, Muhly M, Korom S, Hugo F. Release of interleukin-1β associated with potent cytocidal action of staphylococcal alpha-toxin on human monocytes. Infect Immun. 1989;57:3512–3519. doi: 10.1128/iai.57.11.3512-3519.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhakdi S, Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev. 1991;55:733–751. doi: 10.1128/mr.55.4.733-751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bramley A J, Patel A H, O’Reilly M, Foster R, Foster T J. Roles of alpha-toxin and beta-toxin in virulence of Staphylococcus aureus for the mouse mammary gland. Infect Immun. 1989;57:2489–2494. doi: 10.1128/iai.57.8.2489-2494.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bremell T, Abdelnour A, Tarkowski A. Histopathological and serological progression of experimental Staphylococcus aureus arthritis. Infect Immun. 1992;60:2976–2985. doi: 10.1128/iai.60.7.2976-2985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bremell T, Lange S, Yacoub A, Rydén C, Tarkowski A. Experimental Staphylococcus aureus arthritis in mice. Infect Immun. 1991;59:2615–2623. doi: 10.1128/iai.59.8.2615-2623.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clyne M, De Azavedo J, Carlson E, Arbutnott J. Production of gamma-hemolysin and lack of production of alpha-hemolysin by Staphylococcus aureus strains associated with toxic shock syndrome. J Clin Microbiol. 1988;26:535–539. doi: 10.1128/jcm.26.3.535-539.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durkin J P, Shier W T. Staphylococcal delta toxin stimulates endogenous phospholipase A2 activity and prostaglandin synthesis in fibroblasts. Biochim Biophys Acta. 1981;663:467–479. doi: 10.1016/0005-2760(81)90175-2. [DOI] [PubMed] [Google Scholar]
- 10.Fink-Barbancon V, Prévost G, Piémont Y. Improved purification of leucocidin from Staphylococcus aureus and toxin distribution among hospital strains. Res Microbiol. 1991;142:75–85. doi: 10.1016/0923-2508(91)90099-v. [DOI] [PubMed] [Google Scholar]
- 11.Gemmel C G, Goutcher S C, Reid R, Sturrock R D. Role of certain virulence factors in a murine model of Staphylococcus aureus arthritis. J Med Microbiol. 1997;46:208–213. doi: 10.1099/00222615-46-3-208. [DOI] [PubMed] [Google Scholar]
- 12.Goldenberg D L, Reed J I. Bacterial arthritis. N Engl J Med. 1985;312:764–771. doi: 10.1056/NEJM198503213121206. [DOI] [PubMed] [Google Scholar]
- 13.Green J, Schotland S, Sella Z, Kleeman C R. Interleukin-6 attenuates agonist-mediated calcium mobilization in murine osteoblastic cells. J Clin Investig. 1994;93:2340–2350. doi: 10.1172/JCI117239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimminger F, Rose F, Sibelius U, Meinhardt M, Pötzsch B, Spriesterbach R, Bhakdi S, Suttorp N, Seeger W. Human endothelial cell activation and mediator release in response to the bacterial exotoxins Escherichia coli hemolysin and staphylococcal α-toxin. J Immunol. 1997;159:1909–1916. [PubMed] [Google Scholar]
- 15.Harshman S, Boquet P, Duflot E, Alouf J E, Montecucco C, Papini E. Staphylococcal α-toxin: a study of membrane penetration and pore formation. J Biol Chem. 1989;264:14978–14984. [PubMed] [Google Scholar]
- 16.Helle M, Boeije L, Aarden L. Functional discrimination between interleukin 6 and interleukin 1. Eur J Immunol. 1988;18:1525–1540. doi: 10.1002/eji.1830181010. [DOI] [PubMed] [Google Scholar]
- 17.Krüll M, Dold C, Hippenstiel S, Rosseau S, Lohmeyer J, Suttorp N. Escherichia coli hemolysin and Staphylococcus aureus α-toxin potently induce neutrophil adhesion to cultured human endothelial cells. J Immunol. 1996;157:4133–4140. [PubMed] [Google Scholar]
- 18.Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967;33:155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- 19.O’Callaghan R J, Callegan M C, Moreau J M, Green L C, Foster T J, Hartford O M, Engel L S, Hill J M. Specific roles of alpha-toxin and beta-toxin during Staphylococcus aureus corneal infection. Infect Immun. 1997;65:1571–1578. doi: 10.1128/iai.65.5.1571-1578.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Reilly M, De Azavedo J C S, Kennedy S, Foster T J. Inactivation of the alpha-hemolysin gene of Staphylococcus aureus 8325-4 by site directed mutagenesis and studies on the expression of hemolysins. Microb Pathog. 1986;1:125–138. doi: 10.1016/0882-4010(86)90015-x. [DOI] [PubMed] [Google Scholar]
- 21.Sheagren J N. Staphylococcus aureus. The persistent pathogen. N Engl J Med. 1984;310:1368–1373. doi: 10.1056/NEJM198405243102107. [DOI] [PubMed] [Google Scholar]
- 22.Siqueira J A, Speeg-Schatz C, Freitas F I S, Sahel J, Monteil H. Channel-forming leucotoxins from Staphylococcus aureus cause severe inflammatory reactions in a rabbit eye model. J Med Microbiol. 1997;46:486–494. doi: 10.1099/00222615-46-6-486. [DOI] [PubMed] [Google Scholar]
- 23.Supersac G, Piémont Y, Kubina M, Prévost G, Foster T J. Assessment of the role of gamma-toxin in experimental endophthalmitis using a hlg-deficient mutant of Staphylococcus aureus. Microb Pathog. 1998;24:241–251. doi: 10.1006/mpat.1997.0192. [DOI] [PubMed] [Google Scholar]
- 24.Suttorp N, Fuhrmann M, Tannert-Otto S, Grimminger F, Bhakdi S. Pore-forming bacterial toxins potently induce release of nitric oxide in porcine endothelial cells. J Exp Med. 1993;178:337–341. doi: 10.1084/jem.178.1.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suttorp N, Seeger W, Zucker-Reimann J, Roka L, Bhakdi S. Mechanisms of leukotriene generation in polymorphonuclear leukocytes by staphylococcal alpha-toxin. Infect Immun. 1987;55:104–110. doi: 10.1128/iai.55.1.104-110.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thelestam M. Modes of membrane damaging action of staphylococcal toxins. In: Easmon C S F, Adlam C, editors. Staphylococci and staphylococcal infections. Vol. 2. London, England: Academic Press Ltd.; 1983. pp. 705–744. [Google Scholar]
- 27.Tomita T, Kamio Y. Molecular biology of pore-forming cytolysins from Staphylococcus aureus, and α- and γ-hemolysins and leukocidin. Biosci Biotechnol Biochem. 1997;61:565–572. doi: 10.1271/bbb.61.565. [DOI] [PubMed] [Google Scholar]