For allogeneic hematopoietic cell transplantation candidates lacking a suitable human leukocyte antigen (HLA)-identical sibling, 8/8 HLA allele-matched unrelated donors (URDs) remain the gold standard.1 However, many patients in the increasingly diverse United States population2 lack 8/8 URDs.3 Mismatched adult donor and cord blood (CB) grafts are increasingly important to ensure donors for all, although the extent to which alternative donors extend access is not established. Also, disparities in donor characteristics are likely, given, for example, that non-Europeans (those with full or part non-European ancestry) have less access to CB3,4 grafts, and African patients have fewer haploidentical donors.5
We evaluated the extent to which alternative donors facilitate the provision of allografts and their graft characteristics by recipient ancestry in consecutive patients 19 to 65 years old over a 6-year period. Our hypothesis was that there are ongoing disparities in the provision of any alternative donor and provision of “optimal” alternative donors. Patient ancestry was defined as previously described6 and “optimal” donors according to published criteria (see below). During the study period (January 2016 to April 2021), in the absence of a suitable HLA-identical sibling, 8/8 URDs had priority, followed by double unit CB grafts (often preferred for patients <60 years) or haploidentical donors. Mismatched (5-7/8) URDs have been used more recently. For adult alternative donors, the youngest suitable readily available donor was usually chosen, especially recently.7 Unit quality, CD34+/total nucleated cell dose, and 8/8 HLA-allele match-grade guided CB graft selection.8 To determine trends over time, we compared early (January 2016 to January 2018 [25 months]), middle (February 2018 to February 2020 [25 months]), and pandemic (March 2020 to April 2021 [14 months]) periods.
We studied 601 adults (median age, 53 years; range, 19-65); 587 (98%) had hematologic malignancies (including 117 with acute leukemia on clinicaltrials.gov NCT02677064). Over one-third (n = 226 [38%]) were non-European (72 African, 56 Asian, 57 White Hispanic, 10 Middle Eastern, and 31 mixed non-European), whereas 375 (62%) were European. Overall, just over half (n = 340 [57%]) received 8/8 URDs. The remainder received CB (n = 139 [23%]), haploidentical (n = 69 [11%]), or 5 to 7/8 URD (n = 44 [7%]) grafts. Very few patients (n = 9 [1.5%]) were deemed to have “no graft” at the time of donor evaluation, although almost all were non-European (6 African, 2 White Hispanic, and 1 mixed European).
Of transplanted Europeans, most (263/374 [70%]) received 8/8 URDs compared with only one-third of transplanted non-Europeans (77/218 [35%]; P < .001). Only a minority of Europeans (111/374 [30%]) received HLA-disparate grafts (60 CB, 29 haploidentical, and 22 5 to 7/8 URD). Of non-Europeans, two-thirds (141/218 [65%]) received HLA-disparate grafts (79 CB, 40 haploidentical, and 22 5 to 7/8 URD). Of the 66 transplanted African patients, few (13/66 [20%]) received 8/8 URDs; 27 received CB, 16 haploidentical, and 10 5 to 7/8 URD transplants. When analyzing by period, the relative proportions of patients receiving 8/8 URD, CB, and haploidentical transplants remained unchanged over time for both Europeans and non-Europeans. However, increasing the use of 5 to 7/8 URDs (4% from January 2016 to January 2018, 8% from February 2018 to February 2020, and 14% from March 2020 to April 2021) decreased the “no graft” incidence to 1% of patients recently.
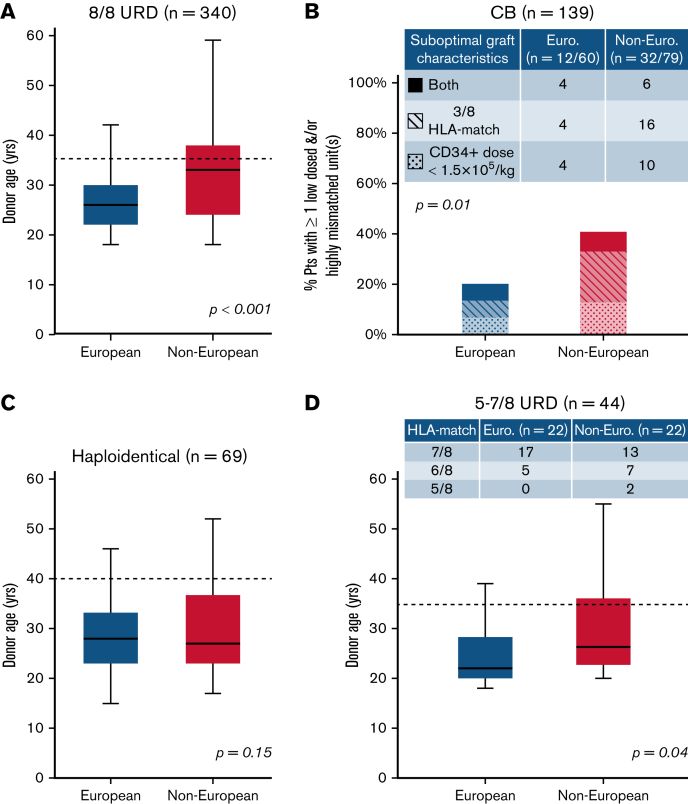
We then analyzed the provision of “optimal” donors defined as 8/8 URDs ≤35 years,1,7,9 double unit CB grafts with each unit of optimal quality, a CD34+ dose ≥1.5 × 105/kg, and ≥4/8 HLA allele-match,7,10,11 haploidentical donors ≤40 years without high titer (mean fluorescence intensity >5000) recipient donor-specific HLA antibodies12, 13, 14, 15, 16 or 5 to 7/8 URDs <35 years (per ClinicalTrials.gov NCT04904588 ACCESS trial). Figure 1 shows comparisons of “optimal” donor characteristics by patient ancestry and donor type. For 8/8 URD recipients, compared with Europeans, non-Europeans had older donors (median, 33 years vs 26 years; P < .001) (Figure 1A) with more than double the proportion over 35 years (29/77 [38%] vs 42/263 [16%]). White Hispanic (7/17 [41%]) and African (11/13 [85%]) patients had the highest proportions of 8/8 URDs over 35 years. For CB recipients, non-Europeans (32/79 [41%], most commonly White Hispanic or African) were more likely than Europeans (12/60 [20%]) to receive a graft with 1 or both units having a low cell dose and/or <4/8 HLA-match (P = .01) (Figure 1B). For haploidentical transplants, although donor age by ancestry was not different (median, 28 years vs 27 years; P = .15) (Figure 1C), more non-Europeans had donors over 40 years (6/40 [15%] vs 2/29 [7%]). Finally, for 5 to 7/8 URD recipients, compared with Europeans, non-Europeans had older donors (median, 27 vs 22 years; P = .04) (Figure 1D) with almost twice the proportion ≥35 years (6/22 [27%] vs 3/22 [14%]).
Figure 1.
Comparison of characteristics that fulfill “optimal graft” criteria in European and non-European transplant recipients by stem cell source. (A) 8/8 URD (n = 340), (B) CB (n = 139), (C) haploidentical donor (n = 69), and (D) 5 to 7/8 URD (n = 44). Compared with Europeans, non-European patients were more likely to receive (A) older 8/8 URDs, (B) CB grafts that were more likely to be low-dosed and/or highly mismatched, (C) similarly aged haploidentical donors (although a higher percentage of non-Europeans had donors >40 years), and (D) older 5 to 7/8 URDs. Also, non-European 5 to 7/8 URD recipients received more HLA-mismatched grafts (compared with European mismatched URD recipients) (D). Box plots (A-B,D) present age medians (solid horizontal lines), IQR (boxes), and range (bars). Dotted lines reflect “optimal” donor age cutoffs for adult donors: 35 years for 8/8 and 5 to 7/8 URDs (A,D), and 40 years for haploidentical donors (C). P values were generated by Student t tests (A,C-D) and chi-squared tests (B).
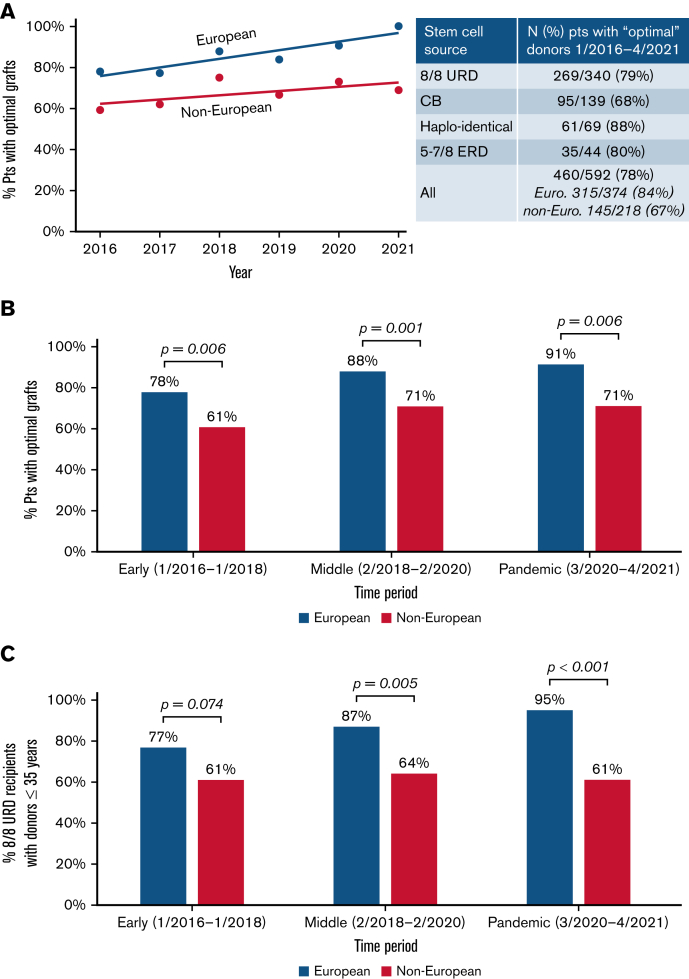
As shown in Figure 2A (inset), while 78% of transplanted patients received an “optimal” donor overall, this was more likely in Europeans than non-Europeans (84% vs 67%; P < .001). White Hispanic and African patients had the lowest proportions with “optimal” grafts. When analyzed over time, either by year (Figure 2A) or time period (Figure 2B), the disparity between the ancestry groups is not improving. Notably, when evaluating European 8/8 URD recipients (n = 263), the proportion with optimal grafts (ie, donor ≤35 years) has increased over time to 95%, whereas of the 77 non-European 8/8 URD recipients, only 61% received a graft from a young donor, and this is not improving (Figure 2C).
Figure 2.
Provision of “optimal” adult donors/CB grafts over time by recipient ancestry. The likelihood that non-Europeans received an “optimal” graft did not improve. Results by (A) year of transplantation are shown, (B) by period for all transplanted patients, and (C) for 8/8 URD recipients only. Lines displayed in (A) show linear models of best fit through the data. The P values in (B-C) were generated by chi-squared tests.
In our real-world experience, 8/8 URD access for non-Europeans is not improving, and prioritizing younger donors is possible for nearly all European, but not non-European, patients. CB, haploidentical, and 5 to 7/8 URD grafts increasingly provide “donors for all” with the use of HLA-mismatched URDs, greatly reducing the likelihood of “no graft.” However, within each donor type, there are persistent disparities in the provision of an “optimal” graft, with non-Europeans, particularly White Hispanic and African patients, being the most at risk for suboptimal grafts.
We acknowledge for each donor type, “optimal” graft definitions will evolve. Additional specifications for 8/8 URDs may consider HLA-DP match,17,18 next-generation sequencing HLA-matching,19 or minor antigen mismatches.20 For CB, an improved understanding of the relative importance of CD34+ cell dose vs 8/8 HLA allele-match and the safety of 3/8 HLA-matched or single-unit transplants is needed.8,11 For haploidentical donors, donor relationship,15 age, and HLA-matching refinements21 could be considered. For ≤7/8 URDs, the number and loci of HLA mismatches, mismatches with high peptide divergence,22 and the HLA-B leader genotype may be relevant.23 Most importantly, timely adult donor availability is critical, especially for acute leukemia patients for whom an optimal donor facilitates transplantation in the time required for best patient care. We are now analyzing the speed to allograft in acute myelogenous leukemia patients,24 and future studies should consider time to donor availability.
Our analysis cannot address which donor type may be the best in a given setting. However, importantly, when considering whether one stem cell source is better than another, either for an individual or in analyses comparing outcomes by donor type, the specific graft characteristics should be considered. Also, for patients with >1 potential stem cell source,25 we propose transplant centers implement selection algorithms that consider “optimal donor” definitions instead of a rigid hierarchy of one source over another. For example, for patients without 8/8 HLA-matched donors, selecting young mismatched adult donors over low-dose CB grafts or high-dose CB grafts over older mismatched adult donors (or those with delayed availability) may be appropriate. Moreover, improving transplantation approaches using all donor types will be required to optimize therapy for all, regardless of ancestry.
Conflict-of-interest disclosure: B.G. has received research funding from Actinium Pharmaceuticals and serves on the data and safety monitoring board for Synthetic Biologics, Inc. A.S. serves as a consultant at the Scientific Advisory Board of ExCellThera. S.A.G. has served as a consultant for Amgen, Actinium, Celgene, Johnson & Johnson, Jazz Pharmaceutical, Takeda, Novartis, Kite, and Spectrum Pharma and has received research funding from Amgen, Actinium, Celgene, Johnson & Johnson, Miltenyi, and Takeda. M.-A.P. has received honoraria from Abbvie, Bellicum, Bristol-Myers Squibb, Incyte, Kite (Gilead), Merck, Novartis, Nektar Therapeutics, and Takeda; serves on data and safety monitoring boards for Servier, Cidara Therapeutics, and Medigene; serves on the scientific advisory boards of MolMed and NexImmune; and has received research support for clinical trials from Incyte, Kite (Gilead), and Miltenyi Biotec. C.S.S. has served as a paid consultant on advisory boards for Juno Therapeutics, Sanofi-Genzyme, Spectrum Pharmaceuticals, Novartis, Genmab, Precision Biosciences, Kite (Gilead), Celgene, Gamida Cell, and GlaxoSmithKline; and has received research funds for clinical trials from Juno Therapeutics, Celgene, Precision Biosciences, and Sanofi-Genzyme. B.C.S. served as a paid consultant for Hansa Biopharma and Gamida Cell; received research funds from Miltenyi Biotec and Celgene. I.P. has received research funding from Merck and serves as a member of a Data and Safety Monitoring Board for ExcellThera. J.N.B. has received consultancy payments from Gamida Cell and the New York Blood Center and an unrestricted research grant from Merck. The remaining authors declare no competing financial interests.
Acknowledgments
We thank the Memorial Sloan-Kettering Cancer Center transplant coordinators for performing detailed patient ancestral evaluations and their diligent work in securing donors.
This research was workshopped as part of the American Society for Transplantation and Cellular Therapy Clinical Research Training Course and was supported in part by the National Cancer Institute P30 CA008748.
Contribution: W.B.F. and J.N.B. designed the study, assembled and analyzed the data, and wrote the manuscript; E.D., S.C., K.A.N., and S.Q. maintained the patient database and provided data; J.F. performed the statistical analyses; W.B.F., B.G., C.C., S.A.G., A.A.J., R.J.L., E.P., M.-A.P., D.M.P., B.C.S., C.S.S., R.T., J.W.Y., A.S., I.P., and J.N.B. provided patient care; and all authors interpreted the data, reviewed and edited the manuscript, and approved the submitted version of the manuscript.
Footnotes
For original data, please contact barkerj@mskcc.org.
References
- 1.Dehn J, Spellman S, Hurley CK, et al. Selection of unrelated donors and cord blood units for hematopoietic cell transplantation: guidelines from the NMDP/CIBMTR. Blood. 2019;134(12):924–934. doi: 10.1182/blood.2019001212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United States Census Bureau. National population by characteristics: 2010-2019. https://www.census.gov/data/tables/time-series/demo/popest/2010s-national-detail.html Available at:
- 3.Barker JN, Boughan K, Dahi PB, et al. Racial disparities in access to HLA-matched unrelated donor transplants: a prospective 1312-patient analysis. Blood Adv. 2019;3(7):939–944. doi: 10.1182/bloodadvances.2018028662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker JN, Mazis CM, Devlin SM, et al. Evaluation of cord blood total nucleated and CD34+ cell content, cell dose, and 8-allele HLA match by patient ancestry. Biol Blood Marrow Transplant. 2020;26(4):734–744. doi: 10.1016/j.bbmt.2019.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kosuri S, Wolff T, Devlin SM, et al. Prospective evaluation of unrelated donor cord blood and haploidentical donor access reveals graft availability varies by patient ancestry: practical implications for donor selection. Biol Blood Marrow Transplant. 2017;23(6):965–970. doi: 10.1016/j.bbmt.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker JN, Byam CE, Kernan NA, et al. Availability of cord blood extends allogeneic hematopoietic stem cell transplant access to racial and ethnic minorities. Biol Blood Marrow Transplant. 2010;16(11):1541–1548. doi: 10.1016/j.bbmt.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker JN, Devlin SM, Naputo KA, et al. High progression-free survival after intermediate intensity double unit cord blood transplantation in adults. Blood Adv. 2020;4(23):6064–6076. doi: 10.1182/bloodadvances.2020003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kollman C, Spellman SR, Zhang MJ, et al. The effect of donor characteristics on survival after unrelated donor transplantation for hematologic malignancy. Blood. 2016;127(2):260–267. doi: 10.1182/blood-2015-08-663823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaw BE, Logan BR, Spellman SR, et al. Development of an unrelated donor selection score predictive of survival after HCT: donor age matters most. Biol Blood Marrow Transplant. 2018;24(5):1049–1056. doi: 10.1016/j.bbmt.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Purtill D, Smith K, Devlin S, et al. Dominant unit CD34+ cell dose predicts engraftment after double-unit cord blood transplantation and is influenced by bank practice. Blood. 2014;124(19):2905–2912. doi: 10.1182/blood-2014-03-566216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Politikos I, Davis E, Nhaissi M, et al. American Society for Transplantation and Cellular Therapy Cord Blood Special Interest Group. Guidelines for cord blood unit selection. Biol Blood Marrow Transplant. 2020;26(12):2190–2196. doi: 10.1016/j.bbmt.2020.07.030. [DOI] [PubMed] [Google Scholar]
- 12.McCurdy SR, Fuchs EJ. Selecting the best haploidentical donor. Semin Hematol. 2016;53(4):246–251. doi: 10.1053/j.seminhematol.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Ciurea SO, Shah MV, Saliba RM, et al. Haploidentical transplantation for older patients with acute myeloid leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2018;24(6):1232–1236. doi: 10.1016/j.bbmt.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solomon SR, Aubrey MT, Zhang X, et al. Selecting the best donor for haploidentical transplant: impact of HLA, killer cell immunoglobulin-like receptor genotyping, and other clinical variables. Biol Blood Marrow Transplant. 2018;24(4):789–798. doi: 10.1016/j.bbmt.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Ciurea SO, Al Malki MM, Kongtim P, et al. The European Society for Blood and Marrow Transplantation (EBMT) consensus recommendations for donor selection in haploidentical hematopoietic cell transplantation. Bone Marrow Transplant. 2020;55(1):12–24. doi: 10.1038/s41409-019-0499-z. [DOI] [PubMed] [Google Scholar]
- 16.DeZern AE, Franklin C, Tsai HL, et al. Relationship of donor age and relationship to outcomes of haploidentical transplantation with posttransplant cyclophosphamide. Blood Adv. 2021;5(5):1360–1368. doi: 10.1182/bloodadvances.2020003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pidala J, Lee SJ, Ahn KW, et al. Nonpermissive HLA-DPB1 mismatch increases mortality after myeloablative unrelated allogeneic hematopoietic cell transplantation. Blood. 2014;124(16):2596–2606. doi: 10.1182/blood-2014-05-576041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oran B, Saliba RM, Carmazzi Y, et al. Effect of nonpermissive HLA-DPB1 mismatches after unrelated allogeneic transplantation with in vivo T-cell depletion. Blood. 2018;131(11):1248–1257. doi: 10.1182/blood-2017-07-798751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayor NP, Wang T, Lee SJ, et al. Impact of previously unrecognized HLA mismatches using ultrahigh resolution typing in unrelated donor hematopoietic cell transplantation. J Clin Oncol. 2021;39(21):2397–2409. doi: 10.1200/JCO.20.03643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernández-Viña MA, Klein JP, Haagenson M, et al. Multiple mismatches at the low expression HLA loci DP, DQ, and DRB3/4/55 ssociate with adverse outcomes in hematopoietic stem cell transplantation. Blood. 2013;121(22):4603–4610. doi: 10.1182/blood-2013-02-481945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuchs EJ, McCurdy SR, Solomon SR, et al. HLA informs risk predictions after haploidentical stem cell transplantation with posttransplantation cyclophosphamide. Blood. 2022;139(10):1452–1468. doi: 10.1182/blood.2021013443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crivello P, Arrieta-Bolanos E, He M, et al. Single HLA class I mismatches with high peptide divergence in the graft-versus-host direction are associated with inferior survival after 9/10 HLA-matched UD-HCT: a retrospective study from the CIBMTR. Blood. 2021;138(suppl 1):97. [Google Scholar]
- 23.Petersdorf EW, Stevenson P, Bengtsson M, et al. HLA-B leader and survivorship after HLA-mismatched unrelated donor transplantation. Blood. 2020;136(3):362–369. doi: 10.1182/blood.2020005743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fingrut W, Davis E, Scaradavou A, et al. Analysis of disparities in speed to BMT referral and allograft in 279 adults with AML: non-Europeans have delayed referrals and cord blood can facilitate prompt transplantation regardless of ancestry. Transplant Cell Ther. 2022;28(3):S135–S136. [Google Scholar]
- 25.European Society for Blood and Marrow Transplantation. FACT-JACIE international standards for hematopoietic cellular therapy product collection, processing, and administration, seventh edition 7.0. Standard B6.1.2. https://www.ebmt.org/jacie-standards/7th-edition-effective-june-1st-2018 Available at: